The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut–Brain Axis Development and Life-Long Health Outcomes
Abstract
1. Introduction
2. Adaptation of Maternal Gut Microbiota to Pregnancy and Fetal Development
3. Maternal Gut Microbiota–Fetal Interaction during Pregnancy: Role of the Placenta
4. Effect of Maternal Gut Microbiota during Pregnancy on Fetal GBA Development
4.1. Impact of Maternal Microbiota during Pregnancy on Fetal CNS
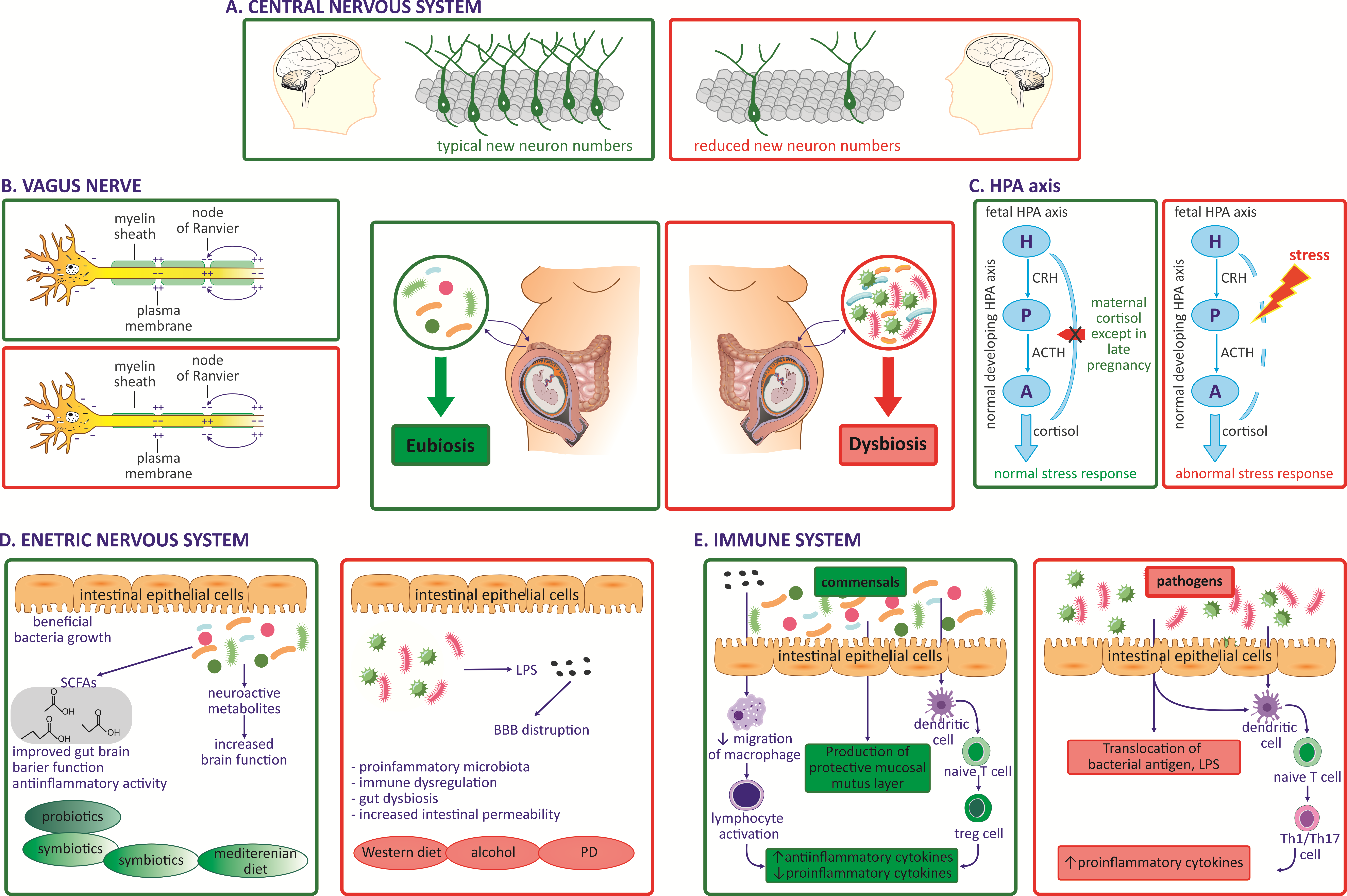
4.2. Impact of Maternal Microbiota during Pregnancy on the Fetal VN
4.3. Impact of Maternal Microbiota during Pregnancy on Fetal Immune System Development
4.4. Impact of Maternal Microbiota during Pregnancy on the Fetal Endocrine System, HPA Axis
4.5. Impact of Maternal Microbiota during Pregnancy on Fetal Gut and ENS Development
5. Factors Affecting Maternal Gut Microbiota during Pregnancy: Gender-Specific Multigenerational Effect
5.1. Obesity during Pregnancy Is Associated with Changes in Maternal Gut Microbiota (Table 1)
| Maternal Conditions during Pregnancy | Maternal Gut Microbiota Dysbiosis | Maternal Health Risks | Fetal/Postnatal Health Issues | References |
|---|---|---|---|---|
| Obesity | Increased ratio of Firmicutes: Bacteroidetes. | Excessive inflammation. Translocation of harmful Gram-negative bacteria to the placenta, vascular dysfunction of placenta. | Fetal growth restriction, impacted neurodevelopment. | [88] |
| Decrease SCFA producing bacteria. | Metabolic syndrome, low grade inflammation, altered endocrine and placental functions | Impaired placental growth, structure, and function; altered growth and development. | [89] | |
| High concentration of Bacteroides, Clostridium, and Staphylococcus. | Increase in inflammatory process, fat storage. | Heavier newborns with increased risk of overweight. | [18] | |
| Increase Firmicutes, Firmicutes to Bacteroides ratio Actinobacteria | Chronic proinflammatory state. | Increased fetal macrosomia and preterm birth, increased neonatal body weight and body fat. | [22] | |
| Diet: High fat diet (HFD) | Decreased alpha diversity | Elevated levels of proinflammatory cytokines, placental dysfunctions. | Impaired fetal DA system, increased neural-tube defect; higher risk of depression, anxiety postnatally. | [91] |
| Increased, Firmicutes to Bacteroides ratio, decreased alpha diversity | Systemic inflammation, autoimmune diseases | Negative Impact on neurodevelopment, neuronal migration, microglia maturation, loss of BBB, increased inflammation. | [40] | |
| Stress | Altered bacterial diversity and composition. | HPA dysregulation | Dysregulation of fetal HPA axis. Altered offspring’s microbiome composition; | [92,93] |
| Inflammation | Increase in Proteobacteria | Maternal immune activation (MIA) | Fetal inflammation; neuronal disorders in childhood. | [54] |
| Infection | Dysbiosis | Increased health risks to the mother | Fetal gut inflammation in utero. | [94] |
| Antibiotics | Increased alpha diversity; altered microbiota at phylum and genus level. | Altered metabolic activity, increased levels of glucose and insulin | Fetal growth and development. | [95,96,97] |
| Antidepressants | Altered microbial diversity, composition | Increase in spontaneous abortions and still birth | Increased risk of congenital heart disease. | [97,98] |
| Age at Conception | Maternal dysbiosis increases with age. | Gestational diabetes, obesity, preeclampsia, Digestive and autoimmune disorders. | Fetal macrosomia, altered development | [12] |
5.2. Diet Is Vital in Regulating Maternal Gut Microbiota before and during Pregnancy (Table 1)
5.3. Stress during Pregnancy Disrupts Maternal Gut Microbiota and Impacts Maternal and Fetal Organisms (Table 1)
5.4. Maternal Inflammation, Infection, Antibiotics, and Antidepressants Negatively Affect Maternal Gut Microbiota during Pregnancy (Table 1)
5.5. Maternal Age at Conception Impacts Maternal Microbial Diversity (Table 1)
5.6. Multigenerational Effect of Altered Maternal Gut Microbiota during Pregnancy
6. Conclusions
7. Future Perspectives
Funding
Conflicts of Interest
References
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X. Gut microbiota: Effect of pubertal status. BMC Microbiol. 2020, 20, 334. [Google Scholar] [CrossRef]
- Korpela, K.; Kallio, S.; Salonen, A.; Hero, M.; Kukkonen, A.K.; Miettinen, P.J.; Savilahti, E.; Kohva, E.; Kariola, L.; Suutela, M.; et al. Gut microbiota develop towards an adult profile in a sex-specific manner during puberty. Sci. Rep. 2021, 11, 23297. [Google Scholar] [CrossRef]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Meijer, B.; Hugenholtz, F.; van der Gaast-de Jongh, C.; Savelkoul, H.F.; de Jonge, M.I.; Faas, M.M.; Boekschoten, M.V.; et al. The Impact of gut microbiota on gender-specific differences in immunity. Front. Immunol. 2017, 8, 754. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy aging and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Nadim, J.A.; Petrosino, J.F.; Costa-Mattioli, M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring Shelly. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef]
- Vuong, H.E.; Pronovost, G.N.; Williams, D.W.; Colley, E.J.L.; Siegler, E.L.; Qiu, A.; Kazantsev, M.; Wilson, C.J.; Rendon, T.; Hsiao, E.Y. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020, 586, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.L.; O’Hely, M.; Jacka, F.N.; Posonby, A.-L.; Symeonides, C.; Loughman, A.; Collier, F.; Vuillermin, P. Maternal prenatal gut microbiota composition predicts child behaviour. eBioMedicine 2021, 68, 103400. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Escherich, T. The Intestinal Bacteria of the Neonate and Breast-Fed Infant. Rev. Infect. Dis. 1989, 11, 352–356. [Google Scholar] [CrossRef]
- Nyangahu, D.D.; Jaspan, H.B. Influence of maternal microbiota during pregnancy on infant immunity. Clin. Exp. Immunol. 2019, 198, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Haroon, N.; Anwari, A.; Malhi, J. Maternal age is correlated with decreased infant gut microbial diversity and change in eating behaviour. UJEMI 2022, 8, 1–12. [Google Scholar]
- Bhatia, P.; Chhabra, S. Physiological and anatomical changes of pregnancy: Implications for anesthesia. Indian J. Anaesth. 2018, 62, 651–657. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 10, 14. [Google Scholar] [CrossRef]
- Miko, E.; Csaszar, A.; Bodi, J.; Kovacs, K. The maternal-fetal gut microbiota axis: Physiological changes, dietary influence, and modulation possibilities. Life 2022, 12, 424. [Google Scholar] [CrossRef]
- Di Simone, N.; Ortiz, A.S.; Specchia, M.; Tersigni, C.; Villa, P.; Gasbarrini, A.; Scambia, G.; S’Ippolito, S. Recent insights on the maternal microbiota; Impact on pregnancy outcomes. Front. Immunol. 2020, 11, 528202. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 2008, 88, 894–899. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Procházková, N.; Falony, G.; Dragsted, L.O.; Licht, T.R.; Raes, J.; Roager, H.M. Advancing human gut microbiota research by considering gut transit time. Gut 2023, 72, 180–191. [Google Scholar] [CrossRef]
- Zakaria, Z.Z.; Al-Rumaihi, S.; Al-Absi, R.S.; Farah, H.; Elamin, M.; Nader, R.; Bouabidi, S.; Suleiman, S.E.; Nasr, S.; Al-Asmakh, M. Physiological changes and interactions between microbiome and the host during pregnancy. Front. Cell. Infect. Microbiol. 2022, 12, 824925. [Google Scholar] [CrossRef]
- Rubini, E.; Schenkelaars, N.; Rousian, M.; Sinclair, K.D.; Wekema, L.; Faas, M.M.; Steegers-Theunissen, R.P.M.; Schoenmakers, S. Maternal obesity during pregnancy leads to derangements in one-carbon metabolism and the gut microbiota: Implications for fetal development and offspring wellbeing. Am. J. Obstet. Gynecol. 2022, 227, 392–400. [Google Scholar] [CrossRef]
- Chen, J.J.; Zeng, B.H.; Li, W.W.; Zhou, C.J.; Fan, S.H.; Cheng, K.E.; Zeng, L.I.; Zheng, P.; Fang, L.; Wei, H.; et al. Effects of gut microbiota on the microRNA and mRNA expression in the hippocampus of mice. Behav. Brain Res. 2017, 322 Pt A, 34–41. [Google Scholar] [CrossRef]
- Gars, A.; Ronczkowski, N.M.; Chassaing, B.; Castillo-Ruiz, A.; Forger, N.G. First Encounters: Effects of the Microbiota on Neonatal Brain Development. Front. Cell. Neurosci. 2021, 15, 682505. [Google Scholar] [CrossRef]
- Goasdoué, K.; Miller, S.M.; Colditz, P.B.; Björkman, S.T. Review: The blood-brain barrier; protecting the developing fetal brain. Placenta 2017, 54, 111–116. [Google Scholar] [CrossRef]
- McGovern, N.; Shin, A.; Low, G.; Low, D.; Duan, K.; Yao, L.J.; Msallam, R.; Low, I.; Shadan, N.B.; Sumatoh, H.R.; et al. Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 2017, 546, 662–666. [Google Scholar] [CrossRef]
- Manrique-Corredor, E.J.; Orozco-Beltran, D.; Lopez-Pineda, A.; Quesada, J.A.; Gil-Guillen, V.F.; Carratala-Munuera, C. Maternal periodontitis and preterm birth: Systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2019, 47, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zakis, D.R.; Paulissen, E.; Kornete, L.; (Marije) Kaan, A.M.; Nicu, E.A.; Zaura, E. The evidence for placental microbiome and its composition in healthy pregnancies: A systematic review. J. Reprod. Immunol. 2022, 149, 103455. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef]
- Kimura, I.; Miyamato, J.; Ohue-Kitano, R.; Watanabe, K.; Hase, K. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef] [PubMed]
- Segarra, M.; Aburto, M.R.; Acker-Palmer, A. Blood–brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef]
- Saunders, N.R.; Liddelow, S.A.; Dziegielewska, K.M. Barrier mechanisms in the developing brain. Front. Pharmacol. 2012, 3, 46. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Ait Ali, L.; D’Aurizio, R.; Rizzo, F.; Saggese, P.; Sanguinetti, E.; Weisz, A.; Pellegrini, M.; Iozzo, P. Fetal cardiac growth is associated with in utero gut colonization. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 170–176. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonization may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef]
- Jena, A.; Montoya, C.A.; Mullaney, J.A.; Dilger, R.N.; Young, W.; McNabb, W.C.; Roy, N.C. Gut-brain axis in the early postnatal years of life: A developmental perspective. Front. Integr. Neurosci. 2020, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef]
- Ratsika, A.; Codagnone, M.C.; O’Mahony, S.; Stanton, C.; Cryan, J.F. Priming for life: Early life nutrition and the microbiota-gut-brain axis. Nutrients 2021, 13, 423. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Di Gesù, C.M.; Matz, L.M.; Buffington, S.A. Diet-induced dysbiosis of the maternal gut microbiome in early life programming of neurodevelopmental disorders. Neurosci. Res. 2021, 168, 3–19. [Google Scholar] [CrossRef]
- Kim, S.W.; Youk, T.; Kim, J. Maternal and neonatal risk factors affecting the occurrence of neurodevelopmental disorders: A population-based nationwide study. Asia Pac. J. Public Health 2022, 34, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, A.; Levitt, P. Fetal, maternal and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 2011, 197, 1–7. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.-L.; JLicinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the Causal Link Between Inflammation and Neurodevelopmental Disorders. Front. Cell Dev. Biol. 2022, 10, 880544. [Google Scholar] [CrossRef] [PubMed]
- Motavaf, M.; Piao, X. Oligodendrocyte Development and Implication in Perinatal White Matter Injury. Front. Cell. Neurosci. 2021, 15, 764486. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut microbiota and the neuroendocrine system. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Ng, P.C. The fetal and neonatal hypothalamic-pituitary-adrenal axis. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 82, F250–F254. [Google Scholar] [CrossRef]
- Leulier, F.; Amélie Joly, A.; de Vadder, F. Microbial Modulation of the Development and Physiology of the Enteric Nervous System. Trends Microbiol. 2021, 29, 686–699. [Google Scholar] [CrossRef]
- Zietek, M.; Celewicz, Z.; Szczuko, M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef]
- Yang, L.L.; Millischer, V.; Rodin, S.; MacFabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J. Neurochem. 2020, 154, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Cerritelli, F.; Frasch, M.G.; Antonelli, M.C.; Viglione, C.; Vecchi, S.; Chiera, M.; Manzotti, A. A Review on the Vagus Nerve and Autonomic NervousSystem During Fetal Development: Searching for Critical Windows. Front. Neurosci. 2021, 15, 721605. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jin, P.; Peng, J.; Zhang, X.; Wong, F.S.; Wen, L. Different immunological responses to early-life antibiotic exposure affecting autoimmune diabetes development in NOD mice. J. Autoimmun. 2016, 72, 47–56. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 1, 1838. [Google Scholar] [CrossRef]
- Momose-Sato, Y.; Sato, K. Development of synaptic networks in the mouse vagal pathway revealed by optical mapping with a voltage-sensitive dye. Eur. J. Neurosci. 2016, 44, 1906–1918. [Google Scholar] [CrossRef]
- Sachis, P.N.; Armstrong, D.L.; Becker, L.E.; Bryan, A.C. Myelination of the human vagus nerve from 24 weeks postconceptional age to adolescence. J. Neuropathol. Exp. Neurol. 1982, 41, 466–472. [Google Scholar] [CrossRef]
- Schneider, U.; Bode, F.; Schmidt, A.; Nowack, S.; Rudolph, A.; Dölker, E.M.; Schlattmann, P.; Götz, T.; Hoyer, D. Developmental milestones of the autonomic nervous system revealed via longitudinal monitoring of fetal heart rate variability. PLoS ONE 2018, 13, e0200799. [Google Scholar] [CrossRef]
- Garzoni, L.; Faure, C.; Frasch, M.G. Fetal cholinergic anti-inflammatory pathway and necrotizing enterocolitis: The brain-gut connection begins in utero. Front. Integr. Neurosci. 2013, 7, 57. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front. Immunol. 2022, 13, 906258. [Google Scholar] [CrossRef]
- Nyangahu, D.D.; Lennard, K.S.; Brown, B.P.; Darby, M.G.; Wendoh, J.M.; Havyarimana, E.; Smith, P.; Butcher, J.; Stintzi, A.; Mulder, N.; et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 2018, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Holladay, S.D.; Smialowicz, R.J. Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ. Health Perspect. 2000, 108, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G.; Lochner, M. The development of intestinallymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009, 2, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Weström, B.; Arévalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.J. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Holmes, E.; Li, J.V.; Marchesi, J.R.; Nicholson, J.K. Gut Microbiota Composition and Activity in Relation to Host Metabolic Phenotype and Disease Risk. Cell Metab. 2012, 16, 559–564. [Google Scholar] [CrossRef]
- McElroy, S.J.; Weitkamp, J.H. Innate Immunity in the Small Intestine of the Preterm Infant. Neoreviews 2011, 12, e517–e526. [Google Scholar] [CrossRef] [PubMed]
- Timm, S.; Schlunssen, V.; Olsen, J.; Ramlau-Hansen, C.H. Prenatal antibiotic and atopic dermatitis among 18-month-old children in the Danish National Birth Cohort. Clin. Exp. Allergy 2017, 47, 929–936. [Google Scholar] [CrossRef]
- Örtqvist, A.K.; Lundholm, C.; Halfvarson, J.; Ludvigsson, J.F.; Almqvist, C. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: A population-based study. Gut 2019, 68, 218–225. [Google Scholar] [CrossRef]
- Matthews, S.G. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Dev. Brain Res. 1998, 107, 123–132. [Google Scholar] [CrossRef]
- Challis, J.R.G.; Matthews, S.G.; Gibb, W.; Lye, S.J. Endocrine and paracrine regulation of birth at term and preterm. Endocr. Rev. 2000, 21, 514–550. [Google Scholar] [CrossRef]
- Kapoor, A.; Dunn, E.; Kostaki, A.; Andrews, M.H.; Matthews, S.G. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J. Physiol. 2006, 572, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.; Gosden, C.; Gardener, D.; Nickson, P.; Martin Hewison, M.; Howie, A.J.; Stewart, P.M. Expression of Type 2 11β-Hydroxysteroid Dehydrogenase and Corticosteroid Hormone Receptors in Early Human Fetal Life. J. Clin. Endocrinol. Metab. 1998, 83, 4490–4497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thliveris, J.A.; Currie, R.W. Observations on the hypothalamo-hypophyseal portal vasculature in the developing human fetus. Am. J. Anat. 1980, 157, 441–444. [Google Scholar] [CrossRef]
- Ackland, J.F.; Ratter, S.J.; Bourne, G.L.; Rees, L.H. Corticotrophin-releasing factor-like immunoreactivity and bioactivity of human fetal and adult hypothalami. J. Endocrinol. 1986, 108, 171–180. [Google Scholar] [CrossRef]
- Mesiano, S.; Jaffe, R.B. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 1997, 18, 378–403. [Google Scholar] [CrossRef]
- Pessa-Morikawa, T.; Husso, A.; Karkkainen, O.; Koistinen, V.; Hanhineva, K.; Livanainen, A.; Niku, M. Maternal microbiota-derived metabolic profile in fetal murin eintestine, brain, and placenta. BMC Microbiol. 2022, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Kwok, L.Y.; Xi, X.; Zhong, Z.; Ma, T.; Xu, H.; Meng, H.; Zhao, F.; Zhang, H. The meconium microbiota shares more features with the amniotic fluid microbiota than the maternal fecal and vaginal microbiota. Gut Microbes 2020, 12, 1794266. [Google Scholar] [CrossRef]
- Chin, A.M.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017, 66, 81–93. [Google Scholar] [CrossRef]
- Lim, A.A.; Nadkarnii, R.R.; Courteau, B.C.; Draper, J.S. Comparison of human and mouse fetal intestinal tissues reveals differential maturation timelines. bioRxiv 2020. [Google Scholar] [CrossRef]
- Touré, A.M.; Landry, M.; Souchkova, O.; Kembel, S.W.; Pilon, N. Gut microbiota-mediated Gene-Environment interaction in the TashT mouse model of Hirschsprung disease. Sci. Rep. 2019, 9, 492. [Google Scholar] [CrossRef]
- Bhatia, A.; Shatanof, R.A.; Bordoni, B. Embryology, Gastrointestinal. In StatPearls; Stat Pearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537172/ (accessed on 10 July 2023).
- Schneider, K.M.; Kim, J.; Bahnsen, K.; Heuckeroth, R.O.; Thaiss, C.A. Environmental perception and control of gastrointestinal immunity by the enteric nervous system. Trends Mol. Med. 2022, 28, 989–1005. [Google Scholar] [CrossRef] [PubMed]
- Rackaityte, E.; Halkias, J.; Fukui, E.M.; Mendoza, V.F.; Hayzelden, C.; Crawford, E.D.; Fujimura, K.E.; Burt, T.D.; Lynch, S.V. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 2020, 26, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Martinez, K.A., II; Romano-Keeler, J.; Zackular, J.P.; Moore, D.J.; Brucker, R.M.; Hooper, C.; Meng, S.; Brown, N.; Mallal, S.; Reese, J.; et al. Bacterial DNA is present in the fetal intestine and overlaps with that in the placenta in mice. PLoS ONE 2018, 13, e0197439. [Google Scholar] [CrossRef] [PubMed]
- Ostrea, E.M., Jr.; Bielawski, D.M.; Posecion, N.C., Jr. Meconium analysis to detect fetal exposure to neurotoxicants. Arch. Dis. Child. 2006, 91, 628–629. [Google Scholar] [CrossRef]
- Yang, H.; Guo, R.; Li, S.; Liang, F.; Tian, C.; Zhao, X.; Long, Y.; Liu, F.; Jiang, M.; Zhang, Y.; et al. Systematic analysis of gut microbiota in pregnant women and its correlations with individual heterogeneity. NPJ Biofilms Microbiomes 2020, 6, 32. [Google Scholar] [CrossRef]
- Gorczyca, K.; Obuchowska, A.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Changes in the Gut Microbiome and Pathologies in Pregnancy. Int. J. Environ. Res. Public Health 2022, 19, 9961. [Google Scholar] [CrossRef]
- Edwards, M.S.; Cunningham, S.A.; Dunlop, A.L.; Corwin, E.J. The maternal gut microbiome during pregnancy. MCM Am. J. Matern. Child Nurs. 2017, 42, 310–317. [Google Scholar] [CrossRef]
- Basak, S.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Maternal obesity and gut microbiota are associated with fetal brain development. Nutrients 2022, 14, 4515. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Ederveen, T.H.A.; Rizzo, F.; Weisz, A.; Collado, M.C.; Muratori, F.; Gross, G.; Alkema, W.; Iozzo, P. Maternal pre-pregnancy overweight and neonatal gut bacterial colonization are associated with cognitive development and gut microbiota composition in pre-school-age offspring. Brain Behav. Immun. 2022, 100, 311–320. [Google Scholar] [CrossRef]
- Jantsch, J.; Tassinari, I.D.; Giovenardi, M.; Bambini-Junior, V.; Guedes, R.P.; de Fraga, L.S. Mood Disorders Induced by Maternal Overnutrition: The Role of the Gut-Brain Axis on the Development of Depression and Anxiety. Front. Cell Dev. Biol. 2022, 10, 795384. [Google Scholar] [CrossRef]
- Jahnke, J.R.; Roach, J.; Azcarate-Peril, M.A.; Thompson, A.L. Maternal precarity and HPA axis functioning shape infant gut microbiota and HPA axis development in humans. PLoS ONE 2021, 16, e0251782. [Google Scholar] [CrossRef]
- Turroni, F.; Rizzo, S.M.; Ventura, M.; Bernasconi, S. Cross-talk between the infant/maternal gut microbiota and the endocrine system: A promising topic of research. Microbiome Res. Rep. 2022, 1, 14. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; Van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Gan, X.P.; Li, F.F.; Zhang, D.Y.; Chen, L.; Cao, Y.N.; Qiu, H.H.; Cheng, D.C.; Zu, J.F.; Liu, W.Y.; et al. Effect of exposure to antibiotics on the gut microbiome and biochemical indexes of pregnant women. BMJ Open Diabetes Res. Care 2021, 9, e002321. [Google Scholar] [CrossRef]
- Lin, J.; Ding, J.; Di, X.; Sun, W.; Chen, H.; Zhang, H. Association between prenatal antibiotics exposure and measures of fetal growth: A repeated-measure study. Ecotoxicol. Environ. Saf. 2022, 244, 114041. [Google Scholar] [CrossRef] [PubMed]
- Dubovicky, M.; Belovicova, K.; Csatlosova, K.; Bogi, E. Risks of using SSRI/SNRI antidepressants during pregnancy and lactation. Interdiscip. Toxicol. 2017, 10, 30–34. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, S.; Li, Y.; Chen, L.; Diao, J.; Li, J.; Wei, J.; Song, X.; Liu, Y.; Shu, J.; et al. Effect of Maternal Antidepressant Use During the Pre-pregnancy/Early Pregnancy Period on Congenital Heart Disease. A Prospective Cohort Study in Central China. Front. Cardiovasc. Med. 2022, 9, 916882. [Google Scholar] [CrossRef]
- Smith, B.L.; Reyes, T.M. Offspring neuroimmune consequences of maternal malnutrition: Potential mechanism for behavioral impairments that underlie metabolic and neurodevelopmental disorders. Front. Neuroendocrinol. 2017, 47, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Milunsky, A.; Jick, H.; Jick, S.S.; Bruell, C.L.; MacLaughlin, D.S.; Rothman, K.J.; Willett, W. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA 1989, 262, 2847–2852. [Google Scholar] [CrossRef]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal prepregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Barrett, H.L.; Gomez-Arango, L.F.; Wilkinson, S.A.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Nitert, M.D. A vegetarian diet is a major determinant of gut microbiota composition in early pregnancy. Nutrients 2018, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Codagnone, M.G.; Stanton, C.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Microbiota and Neurodevelopmental Trajectories: Role of Maternal and Early-Life Nutrition. Ann. Nutr. Metab. 2019, 74 (Suppl. 2), 16–27. [Google Scholar] [CrossRef]
- Kucha, W.; Seifu, D.; Tirsit, A.; Yigeremu, M.; Abebe, M.; Hailu, D.; Tsehay, D.; Genet, S. Folate, Vitamin B12, and Homocysteine Levels in Women with Neural Tube Defect-Affected Pregnancy in Addis Ababa, Ethiopia. Front. Nutr. 2022, 9, 873900. [Google Scholar] [CrossRef] [PubMed]
- Serpeloni, F.; Radtke, K.; de Assis, S.G.; Henning, F.; Nätt, D.; Elbert, T. Grandmaternal stress during pregnancy and DNA methylation of the third generation: An epigenome-wide association study. Transl. Psychiatry 2017, 7, e1202. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Morrison, K.E.; Misic, A.; Weinkopff, T.; Scott, P.; Hunter, C.; Beiting, D.; Bale, T.L. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 2018, 21, 1061–1071. [Google Scholar] [CrossRef]
- Poston, L.; Harthoorn, L.F.; Van der Beek, E.M. Obesity in Pregnancy: Implications for the mother and lifelong health of the child. Pediatr. Res. 2011, 69, 175–180. [Google Scholar] [CrossRef]
- Sherman, M.P.; Zaghouani, H.; Niklas, V. Gut microbiota, the immune system, and the diet influence the neonatal gut brain axis. Pediatr. Res. 2015, 77, 127–135. [Google Scholar] [CrossRef]
- Kuperman, A.A.; Koren, O. Antibiotic use during pregnancy: How bad is it? BMC Med. 2016, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Strain, C.R.; Fouhy, F.; Strain, R.G.; Peterson, V.L.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology 2019, 236, 1671–1685. [Google Scholar] [CrossRef]
- Munoz-Bellido, J.L.; Munoz-Criado, S.; Garcìa-Rodrìguez, J.A. Antimicrobial activity of psychotropic drugs: Selective serotonin reuptake inhibitors. Int. J. Antimicrob. Agents 2000, 14, 177–180. [Google Scholar] [CrossRef]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef]
- Anderson, K.N.; Lind, J.N.; Simeone, R.M.; Bobo, W.V.; Mitchell, A.A.; Riehle-Colarusso, T.; Polen, K.N.; Reefhuis, J. Maternal Use of Specific Antidepressant Medications During Early Pregnancy and the Risk of Selected Birth Defects. JAMA Psychiatry 2020, 77, 1246–1255. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, X.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. eBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Vuong, H.E.; Luna, C.D.G.; Pronovost, G.N.; Aleksandrova, A.A.; Riley, N.G.; Vavilina, A.; McGinn, J.; Rendon, T.; Forrest, L.R.; et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat. Microbiol. 2019, 4, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.D.; Vallès, A.; van Heesch, F.; Afrasiab-Middelman, A.; Roelofs, J.J.; Jonkers, M.; Peeters, E.J.; Korte-Bouws, G.A.; Dederen, J.P.; Kiliaan, A.J.; et al. Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology 2011, 217, 419–432. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wilms, E.; Masclee, A.A.; Smidt, H.; Zoetendal, E.G.; Jonkers, D. Age-dependent changes in GI physiology and microbiota: Time to reconsider? Gut 2018, 67, 2213–2222. [Google Scholar] [CrossRef]
- Barker, D.; Osmond, C. Infant Mortality, Childhood Nutrition, and ischemic heart disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Fusco, S.; Spinelli, M.; Cocco, S.; Ripoli, C.; Mastrodonato, A.; Natale, F.; Rinaudo, M.; Livrizzi, G.; Grassi, C. Maternal Insulin Resistance Multigenerationally Impairs Synaptic Plasticity and Memory via Gametic Mechanisms. Nat. Commun. 2019, 10, 4799. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajdel-Sulkowska, E.M. The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut–Brain Axis Development and Life-Long Health Outcomes. Microorganisms 2023, 11, 2199. https://doi.org/10.3390/microorganisms11092199
Sajdel-Sulkowska EM. The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut–Brain Axis Development and Life-Long Health Outcomes. Microorganisms. 2023; 11(9):2199. https://doi.org/10.3390/microorganisms11092199
Chicago/Turabian StyleSajdel-Sulkowska, Elizabeth M. 2023. "The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut–Brain Axis Development and Life-Long Health Outcomes" Microorganisms 11, no. 9: 2199. https://doi.org/10.3390/microorganisms11092199
APA StyleSajdel-Sulkowska, E. M. (2023). The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut–Brain Axis Development and Life-Long Health Outcomes. Microorganisms, 11(9), 2199. https://doi.org/10.3390/microorganisms11092199






