The Possible Role of Probiotic Supplementation in Inflammation: A Narrative Review
Abstract
1. Introduction
1.1. The Intestinal Microbiota
1.2. Intestinal Eubiosis
1.3. Intestinal Dysbiosis
1.4. Role of Ox-Inflammaging on Intestinal Dysbiosis
2. Materials and Methods
3. Results
3.1. Probiotics and Gut Inflammation and Oxidative Stress
3.2. Probiotics and Urinary Tract Inflammation and Infections
3.3. Probiotics and Cardiovascular Inflammation and Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Livovsky, D.M.; Pribic, T.; Azpiroz, F. Food, Eating, and the Gastrointestinal Tract. Nutrients 2020, 12, 986. [Google Scholar] [CrossRef]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. BioMedicine 2023, 164, 114985. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Stress and the gut: Pathophysiology, clinical consequences, diagnostic approach and treatment options. J. Physiol. Pharmacol. 2011, 62, 591–599. [Google Scholar]
- Lin, D.; Medeiros, D.M. The microbiome as a major function of the gastrointestinal tract and its implication in micronutrient metabolism and chronic diseases. Nutr. Res. 2023, 112, 30–45. [Google Scholar] [CrossRef]
- Stec, A.; Sikora, M.; Maciejewska, M.; Paralusz-Stec, K.; Michalska, M.; Sikorska, E.; Rudnicka, L. Bacterial Metabolites: A Link between Gut Microbiota and Dermatological Diseases. Int. J. Mol. Sci. 2023, 24, 3494. [Google Scholar] [CrossRef]
- Rizzo, M.; Colletti, A.; Penson, P.E.; Katsiki, N.; Mikhailidis, D.P.; Toth, P.P.; Gouni-Berthold, I.; Mancini, J.; Marais, D.; Ruscica, M.; et al. Nutraceutical approaches to non-alcoholic fatty liver disease (NAFLD): A position paper from the International Lipid Expert Panel (ILEP). Pharmacol. Res. 2023, 189, 106679. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Romanidou, G.; Voidarou, C.; Bezirtzoglou, E. Focus on the Gut–Kidney Axis in Health and Disease. Front. Med. 2021, 7, 620102. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; von Haehling, S.; Vinereanu, D.; Bielecka-Dabrowa, A.; Sahebkar, A.; Toth, P.P.; Reiner, Ž.; Wong, N.D.; Mikhailidis, D.P.; et al. Nutraceutical support in heart failure: A position paper of the International Lipid Expert Panel (ILEP). Nutr. Res. Rev. 2020, 33, 155–179. [Google Scholar] [CrossRef]
- Salazar, A.M.; Neugent, M.L.; De Nisco, N.J.; Mysorekar, I.U. Gut-bladder axis enters the stage: Implication for recurrent urinary tract infections. Cell Host Microbe 2022, 30, 1066–1069. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Jha, N.K.; Kesari, K.K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2021, 38, 223–244. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Proctor, L.M. The Human Microbiome Project in 2011 and Beyond. Cell Host Microbe 2011, 10, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, W.R.; Chen, D.; Garud, N.R. Comparative Population Genetics in the Human Gut Microbiome. Genome Biol. Evol. 2022, 14, evab116. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging role of gut microbiota dysbiosis in neuroinflammation and neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar]
- Buccigrossi, V.; Nicastro, E.; Guarino, A. Functions of intestinal microflora in children. Curr. Opin. Gastroenterol. 2013, 29, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ottma, N.; Smidt, H.; de Vos, W.M.; Belzer, C. The function of our microbiota: Who is out there and what do they do? Front. Cell. Infect. Microbiol. 2012, 2, 104. [Google Scholar]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef]
- Zimmer, J.; Lange, B.; Frick, J.-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef]
- Schmiedová, L.; Tomášek, O.; Pinkasová, H.; Albrecht, T.; Kreisinger, J. Variation in diet composition and its relation to gut microbiota in a passerine bird. Sci. Rep. 2022, 12, 3787. [Google Scholar] [CrossRef] [PubMed]
- Rodiño-Janeiro, B.K.; Vicario, M.; Alonso-Cotoner, C.; Pascua-García, R.; Santos, J. A Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv. Ther. 2018, 35, 289–310. [Google Scholar] [CrossRef]
- Eom, T.; Kim, Y.S.; Choi, C.H.; Sadowsky, M.J.; Unno, T. Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease. J. Microbiol. 2018, 56, 189–198. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Engen, P.A.; Dodiya, H.B.; Naqib, A.; Forsyth, C.B.; Green, S.J.; Voigt, R.M.; Kordower, J.H.; Mutlu, E.A.; Shannon, K.M.; Keshavarzian, A. The Potential Role of Gut-Derived Inflammation in Multiple System Atrophy. J. Park. Dis. 2017, 7, 331–346. [Google Scholar] [CrossRef]
- Van de Wiele, T.; Van Praet, J.T.; Marzorati, M.; Drennan, M.B.; Elewaut, D. How the microbiota shapes rheumatic diseases. Nat. Rev. Rheumatol. 2016, 12, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.F.A.; Claassen, E. The mechanistic link between health and gut microbiota diversity. Sci. Rep. 2018, 8, 2183. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Wallace, M.B.; Vazquez-Roque, M.; Bojarski, C.; Schulzke, J.-D. Imaging the Leaky Gut. Gastroenterology 2014, 147, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Shivaji, S. We are not alone: A case for the human microbiome in extra intestinal diseases. Gut Pathog. 2017, 7, 9–13. [Google Scholar] [CrossRef][Green Version]
- Zuccotti, G.V. Probiotics in clinical practise: An overview. J. Int. Med. Res. 2008, 36 (Suppl. S1), 1A–53A. [Google Scholar] [CrossRef]
- Meneghin, F.; Fabiano, V.; Mameli, C.; Zuccotti, G.V. Probiotics and Atopic Dermatitis in Children. Pharmaceuticals 2012, 5, 727–744. [Google Scholar] [CrossRef]
- Available online: https://www.thelancet.com/series/ageing (accessed on 19 January 2019).
- Shintouo, C.M.; Mets, T.; Beckwee, D.; Bautmans, I.; Ghogomu, S.M.; Souopgui, J.; Leemans, L.; Meriki, H.D.; Njemini, R. Is inflammageing influenced by the microbiota in the aged gut? A systematic review. Exp. Gerontol. 2020, 141, 111079. [Google Scholar] [CrossRef]
- Brüünsgaard, H.; Pedersen, B.K. Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. N. Am. 2003, 23, 15–39. [Google Scholar] [CrossRef]
- De Martinis, M.; Di Benedetto, M.C.; Mengoli, L.P.; Ginaldi, L. Senile osteoporosis: Is it an immune-mediated disease? Inflamm. Res. 2006, 55, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W. (Dis)Trust your gut: The gut microbiome in age-related inflammation, health, and disease. Microbiome 2017, 5, 80. [Google Scholar] [CrossRef]
- Miron, N.; Cristea, V. Enterocytes: Active cells in tolerance to food and microbial antigens in the gut. Clin. Exp. Immunol. 2012, 167, 405–412. [Google Scholar] [CrossRef]
- Chassaing, B.; Gewirtz, A.T. Gut Microbiota, Low-grade Inflammation, and Metabolic Syndrome. Toxicol. Pathol. 2013, 42, 49–53. [Google Scholar] [CrossRef]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Health Aging 2018, 4, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017, 21, 455–466. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut Dysbiosis and Detection of “Live Gut Bacteria” in Blood of Japanese Patients With Type 2 Diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Spychala, M.S.; Venna, V.R.; Jandzinski, M.; Doran, S.J.; Durgan, D.J.; Ganesh, B.P.; Ajami, N.J.; Putluri, N.; Graf, J.; Bryan, R.M.; et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 2018, 84, 23–36. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-α Modulation of Intestinal Epithelial Tight Junction Barrier Is Regulated by ERK1/2 Activation of Elk-1. Am. J. Pathol. 2013, 183, 1871–1884. [Google Scholar] [CrossRef]
- Kim, K.-A.; Jeong, J.-J.; Yoo, S.-Y.; Kim, D.-H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Toward, R.; Montandon, S.; Walton, G.; Gibson, G.R. Effect of prebiotics on the human gut microbiota of elderly persons. Gut Microbes 2012, 3, 57–60. [Google Scholar] [CrossRef]
- Biagi, E.; Candela, M.; Turroni, S.; Garagnani, P.; Franceschi, C.; Brigidi, P. Ageing and gut microbes: Perspectives for health maintenance and longevity. Pharmacol. Res. 2013, 69, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zeng, T.; Zinellu, A.; Rubino, S.; Kelvin, D.J.; Carru, C. A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. mSystems 2019, 4, e00325-19. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Zhang, Z.; Yin, G. Effect of Probiotics on the Performance and Intestinal Health of Broiler Chickens Infected with Eimeria tenella. Vaccines 2022, 10, 97. [Google Scholar] [CrossRef]
- Mohsin, M.; Abbas, R.Z.; Yin, G.; Sindhu, Z.-U.-D.; Abbas, A.; Huang, Z.; Aleem, M.T.; Saeed, Z.; Afzal, M.Z.; Ejaz, A.; et al. Probiotics as therapeutic, antioxidant and immunomodulatory agents against poultry coccidiosis. World’s Poult. Sci. J. 2021, 77, 331–345. [Google Scholar] [CrossRef]
- Lin, W.-Y.; Lin, J.-H.; Kuo, Y.-W.; Chiang, P.-F.R.; Ho, H.-H. Probiotics and their Metabolites Reduce Oxidative Stress in Middle-Aged Mice. Curr. Microbiol. 2022, 79, 104. [Google Scholar] [CrossRef]
- Vasquez, E.C.; Pereira, T.M.C.; Peotta, V.A.; Baldo, M.P.; Campos-Toimil, M. Probiotics as Beneficial Dietary Supplements to Prevent and Treat Cardiovascular Diseases: Uncovering Their Impact on Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 3086270. [Google Scholar] [CrossRef]
- Wang, B.-G.; Xu, H.-B.; Xu, F.; Zeng, Z.-L.; Wei, H. Efficacy of oral Bifidobacterium bifidum ATCC 29521 on microflora and antioxidant in mice. Can. J. Microbiol. 2016, 62, 249–262. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.S.; Korber, D.R.; Tanaka, T. Influence of oxygen on NADH recycling and oxidative stress resistance systems in Lactobacillus panis PM1. AMB Express 2013, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Kumar, P.; Banerjee, R.; Basu, M.; Pal, A.; Samanta, M.; Das, S. Lactobacillus acidophilus attenuates Aeromonas hydrophila induced cytotoxicity in catla thymus macrophages by modulating oxidative stress and inflammation. Mol. Immunol. 2016, 75, 69–83. [Google Scholar] [CrossRef]
- Xin, J.; Zeng, D.; Wang, H.; Ni, X.; Yi, D.; Pan, K.; Jing, B. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl. Microbiol. Biotechnol. 2014, 98, 6817–6829. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, Y. Effects of Probiotic Supplementation on Inflammation and Oxidative Stress for Gestational Diabetes: A Meta-Analysis Study. 2023, 227, 106–111. Z. Geburtshilfe Neonatol. 2023, 227, 106–111. [Google Scholar] [CrossRef]
- St-Amant, A.; Bergdahl, A. A systematic review and meta-analysis of randomized controlled trials investigating the effects of probiotics on oxidative stress in healthy adults. Clin. Nutr. ESPEN 2023, 54, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, S.; Esmailzadeh, M.; Sadeghinejad, A.; Shahrbabaki, M.E.; Asadikaram, G.; Nikvarz, N. The Effect of Adjunctive Probiotics on Markers of Inflammation and Oxidative Stress in Bipolar Disorder: A Double-blind, Randomized, Controlled Trial. J. Psychiatr. Pract. 2022, 28, 373–382. [Google Scholar] [CrossRef]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a Probiotic Intake on Oxidant and Antioxidant Parameters in Plasma of Athletes During Intense Exercise Training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef]
- Verdenelli, M.; Silvi, S.; Cecchini, C.; Orpianesi, C.; Cresci, A. Influence of a combination of two potential probiotic strains, Lactobacillus rhamnosus IMC 501® and Lactobacillus paracasei IMC 502® on bowel habits of healthy adults. Lett. Appl. Microbiol. 2011, 52, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Liou, J.-M.; Lu, T.-M.; Lin, Y.-H.; Wang, C.-K.; Pan, T.-M. Effects of Vigiis 101-LAB on a healthy population’s gut microflora, peristalsis, immunity, and anti-oxidative capacity: A randomized, double-blind, placebo-controlled clinical study. Heliyon 2020, 6, e04979. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Tang, P.; Wu, Y.; Zhang, A.; Li, D.; Wang, C.-Z.; Wan, J.-Y.; Yao, H.; Yuan, C.-S. Probiotics fortify intestinal barrier function: A systematic review and meta-analysis of randomized trials. Front. Immunol. 2023, 14, 1143548. [Google Scholar] [CrossRef]
- Foxman, B. Urinary Tract Infection Syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Suskind, A.M.; Saigal, C.S.; Hanley, J.M.; Lai, J.; Setodji, C.M.; Clemens, J.Q. Incidence and Management of Uncomplicated Recurrent Urinary Tract Infections in a National Sample of Women in the United States. Urology 2016, 90, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Beerepoot, M.; Geerlings, S. Non-Antibiotic Prophylaxis for Urinary Tract Infections. Pathogens 2016, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Talan, D.A.; Takhar, S.S.; Krishnadasan, A.; Abrahamian, F.M.; Mower, W.R.; Moran, G.J. Fluoroquinolone-resistant and ex-tended-spectrum b-lactamase producing Escherichia coli infections in patients with pyelonephritis, United States. Emerg. Infect. Dis. 2016, 22, 1594–1603. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Drug Safety Communication: FDA Advises Restricting Fluoroquinolone Antibiotic Use for Certain Uncomplicated Infections; Warns About Disabling Side Effects That Can Occur Together. 2016. Available online: http://www.fda.gov/Drugs/DrugSafety/ucm500143.htm (accessed on 19 January 2019).
- Tamadonfar, K.O.; Omattage, N.S.; Spaulding, C.N.; Hultgren, S.J. Reaching the End of the Line: Urinary Tract Infections. Microbiol. Spectr. 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Scribano, D.; Sarshar, M.; Fettucciari, L.; Ambrosi, C. Urinary tract infections: Can we prevent uropathogenic Escherichia coli infection with dietary intervention? Int. J. Vitam. Nutr. Res. 2021, 91, 391–395. [Google Scholar] [CrossRef]
- Meštrović, T.; Matijašić, M.; Perić, M.; Paljetak, H.; Barešić, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2020, 11, 7. [Google Scholar] [CrossRef]
- Graziani, C.; Laterza, L.; Talocco, C.; Pizzoferrato, M.; Di Simone, N.; D’ippolito, S.; Ricci, C.; Gervasoni, J.; Persichilli, S.; Del Chierico, F.; et al. Intestinal Permeability and Dysbiosis in Female Patients with Recurrent Cystitis: A Pilot Study. J. Pers. Med. 2022, 12, 1005. [Google Scholar] [CrossRef]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Caspani, M.; Mondello, F.; Cresci, A. In vitro evaluation on HeLa cells of protective mechanisms of probiotic lactobacilli against Candida clinical isolates. J. Appl. Microbiol. 2015, 119, 1383–1390. [Google Scholar] [CrossRef]
- E O’Hanlon, D.; Moench, T.R.; A Cone, R. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011, 11, 200. [Google Scholar] [CrossRef]
- Ventolini, G.; Mitchell, E.; Salazar, M. Biofilm formation by vaginal Lactobacillus in vivo. Med Hypotheses 2015, 84, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Verdenelli, M.C.; Cecchini, C.; Coman, M.M.; Silvi, S.; Orpianesi, C.; Coata, G.; Cresci, A.; Di Renzo, G.C. Impact of Probiotic SYNBIO® Administered by Vaginal Suppositories in Promoting Vaginal Health of Apparently Healthy Women. Curr. Microbiol. 2016, 73, 483–490. [Google Scholar] [CrossRef]
- Di Pierro, F.; Polzonetti, V.; Patrone, V.; Morelli, L. Microbiological Assessment of the Quality of Some Commercial Products Marketed as Lactobacillus crispatus-Containing Probiotic Dietary Supplements. Microorganisms 2019, 7, 524. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Reid, G.; Challis, J.R.; Gloor, G.B.; Asztalos, E.; Money, D.; Seney, S.; Bocking, A.D. Effect of Oral Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the Vaginal Microbiota, Cytokines and Chemokines in Pregnant Women. Nutrients 2020, 12, 368. [Google Scholar] [CrossRef]
- Reid, G.; Bruce, A.W.; Taylor, M. Influence of three-day antimicrobial therapy and Lactobacillus vaginal suppositories on recur-rence of urinary tract infections. Clin. Ther. 1992, 14, 11–16. [Google Scholar] [PubMed]
- Čeprnja, M.; Hadžić, E.; Oros, D.; Melvan, E.; Starcevic, A.; Zucko, J. Current Viewpoint on Female Urogenital Microbiome—The Cause or the Consequence? Microorganisms 2023, 11, 1207. [Google Scholar] [CrossRef]
- Beerepoot, M.A.J.; ter Riet, G.; Nys, S.; van der Wal, W.M.; de Borgie, C.A.J.M.; de Reijke, T.M.; Prins, J.M.; Koeijers, J.; Verbon, A.; Stobberingh, E.; et al. Lactobacilli vs Antibiotics to Prevent Urinary Tract Infections. Arch. Intern. Med. 2012, 172, 704–712. [Google Scholar] [CrossRef]
- New, F.J.; Theivendrampillai, S.; Julliebø-Jones, P.; Somani, B. Role of Probiotics for Recurrent UTIs in the Twenty-First Century: A Systematic Review of Literature. Curr. Urol. Rep. 2022, 23, 19–28. [Google Scholar] [CrossRef]
- Stapleton, A.; Au-Yeung, M.; Hooton, T.M.; Fredricks, D.N.; Roberts, P.L.; Czaja, C.A.; Yarova-Yarovaya, Y.; Fiedler, T.; Cox, M.; Stamm, W.E. Randomized, placebocontrolled phase 2 trial of a Lactobacillus crispatus pro-biotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 2011, 52, 1212–1217. [Google Scholar] [CrossRef]
- Koradia, P.; Kapadia, S.; Trivedi, Y.; Chanchu, G.; Harper, A. Probiotic and cranberry supplementation for preventing recurrent uncomplicated urinary tract infections in premenopausal women: A controlled pilot study. Expert Rev. Anti-infective Ther. 2019, 17, 733–740. [Google Scholar] [CrossRef] [PubMed]
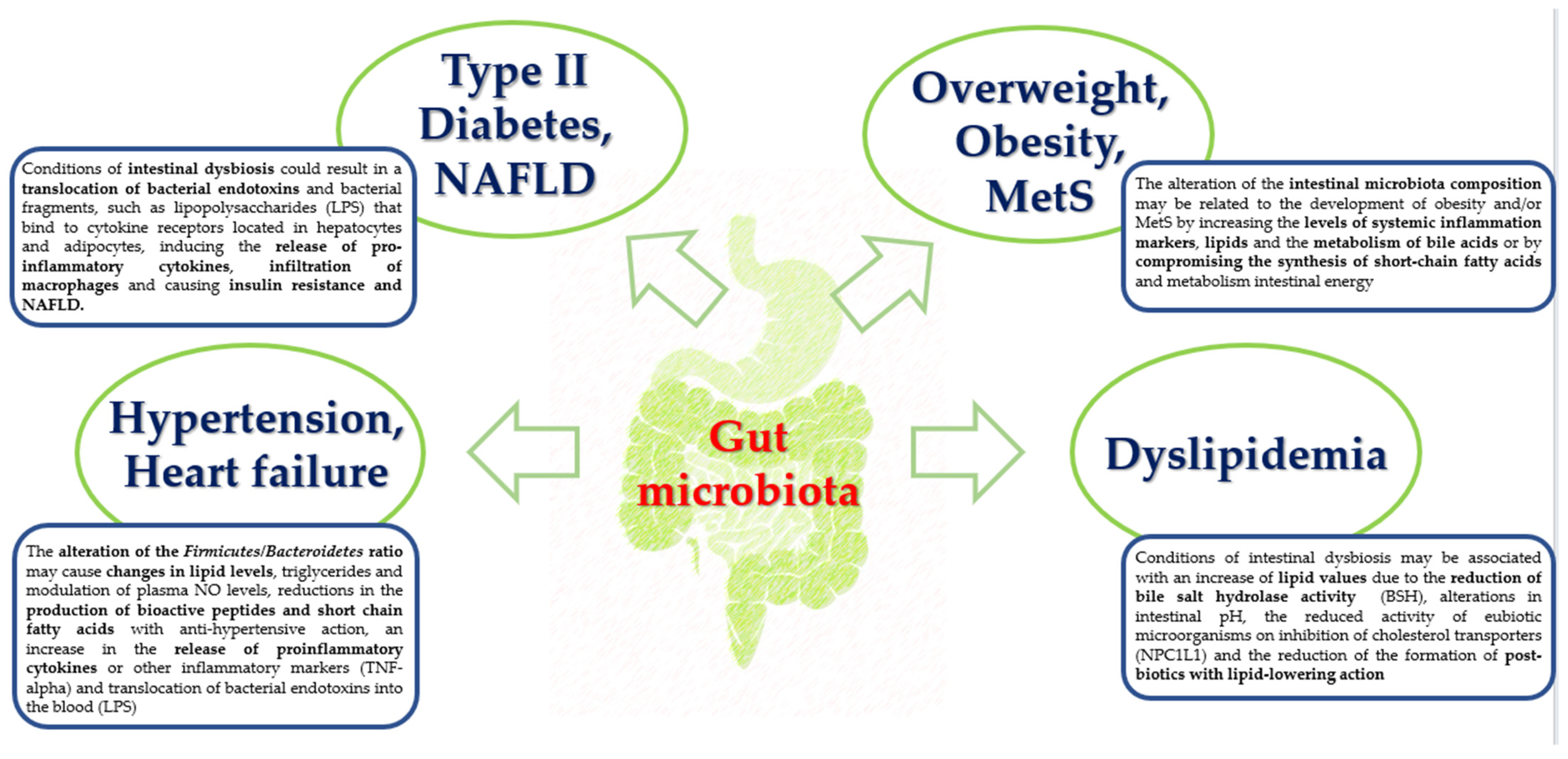
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D'Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and In-flammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, F.; -Or-Rashid, H.; Al Mamun, A.; Rahaman, S.; Islam, M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Sanz, Y.; Rastmanesh, R.; Agostoni, C. Understanding the role of gut microbes and probiotics in obesity: How far are we? Pharmacol. Res. 2013, 69, 144–155. [Google Scholar] [CrossRef]
- Ferrer, M.; Ruiz, A.; Lanza, F.; Haange, S.-B.; Oberbach, A.; Till, H.; Bargiela, R.; Campoy, C.; Segura, M.T.; Richter, M.; et al. Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environ. Microbiol. 2013, 15, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Stefura, T.; Zapała, B.; Gosiewski, T.; Skomarovska, O.; Pędziwiatr, M.; Major, P. Changes in the Composition of Oral and Intestinal Microbiota After Sleeve Gastrectomy and Roux-En-Y Gastric Bypass and Their Impact on Outcomes of Bariatric Surgery. Obes. Surg. 2022, 32, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, E.; Fasoulas, A.; Mantzorou, M.; Giaginis, C. Clinical Evidence on the Potential Beneficial Effects of Probiotics and Prebiotics in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 15898. [Google Scholar] [CrossRef]
- Pendyala, S.; Walker, J.M.; Holt, P.R. A High-Fat Diet Is Associated With Endotoxemia That Originates From the Gut. Gastroenterology 2012, 142, 1100–1101.e2. [Google Scholar] [CrossRef]
- Guigoz, Y.; Doré, J.; Schiffrin, E.J. The inflammatory status of old age can be nurtured from the intestinal environment. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L. Inflammation as the Key Interface of the Medical and Nutrition Universes: A Provocative Examination of the Future of Clinical Nutrition and Medicine. J. Parenter. Enter. Nutr. 2006, 30, 453–463. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef]
- Lartigue, G.; de La Serre, C.B.; Raybould, H.E. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol. Behav. 2011, 105, 100–105. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Lundén, G.O.; Felin, J.; Bäckhed, F. Regulation of Serum Amyloid A3 (SAA3) in Mouse Colonic Epithelium and Adipose Tissue by the Intestinal Microbiota. PLoS ONE 2009, 4, e5842. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.J.; Thomas, D.R.; Kumar, V.B.; Brown, C.; Hager, C.; Hof, M.A.V.; E Morley, J.; Guigoz, Y. Systemic inflammatory markers in older persons: The effect of oral nutritional supplementation with prebiotics. J. Nutr. Health Aging 2007, 11, 475–479. [Google Scholar] [PubMed]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men With Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef]
- Seppo, L.; Kerojoki, O.; Suomalainen, T.; Korpela, R. The effect of a Lactobacillus helveticus lbk-16 h fermented milk on hypertension: A pilot study on humans. Milchwissenschaft 2002, 57, 124–127. [Google Scholar]
- Mizushima, S.; Ohshige, K.; Watanabe, J.; Kimura, M.; Kadowaki, T.; Nakamura, Y.; Tochikubo, O.; Ueshima, H. Randomized controlled trial of sour milk on blood pressure in borderline hy-pertensive men. Am. J. Hypertens. 2004, 17, 701–706. [Google Scholar] [CrossRef]
- Inoue, K.; Shirai, T.; Ochiai, H.; Kasao, M.; Hayakawa, K.; Kimura, M.; Sansawa, H. Blood-pressure-lowering effect of a novel fermented milk containing [gamma]-aminobutyric acid (gaba) in mild hypertensives. Eur. J. Clin. Nutr. 2003, 57, 490–495. [Google Scholar] [CrossRef]
- Dong, J.Y.; Szeto, I.M.; Makinen, K.; Gao, Q.; Wang, J.; Qin, L.-Q.; Zhao, Y. Effect of probiotic fermented milk on blood pressure: A meta-analysis of random-ised controlled trials. Br. J. Nutr. 2013, 110, 1188–1194. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Johansson, M.-L.; Zapolska-Downar, D.; Bukowska, H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 2002, 76, 1249–1255. [Google Scholar] [CrossRef]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of random-ized, controlled trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankow-ska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Rev. Esp. Cardiol. 2016, 69, 1167. [Google Scholar]
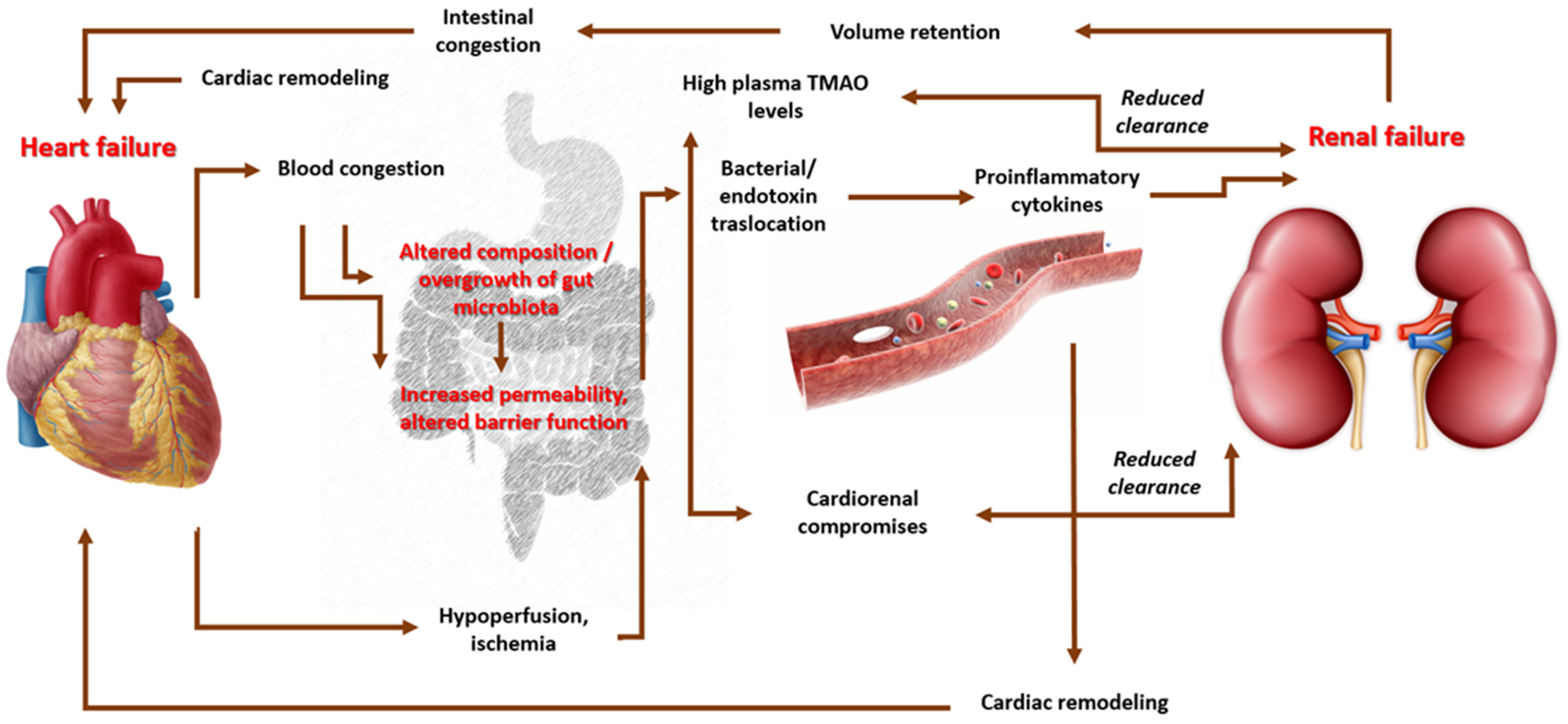
- Sandek, A.; Bjarnason, I.; Volk, H.-D.; Crane, R.; Meddings, J.B.; Niebauer, J.; Kalra, P.R.; Buhner, S.; Herrmann, R.; Springer, J.; et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int. J. Cardiol. 2012, 157, 80–85. [Google Scholar] [CrossRef]
- Krack, A.; Sharma, R.; Figulla, H.R.; Anker, S.D. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur. Heart J. 2005, 26, 2368–2374. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Wilson, T.W.H.; Zeneng, W.; Yiying, F.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite Trimethylamine-N-Oxide in Patients With Heart Failure. J. Am. Coll. Cardiol. 2014, 64, 1908–1914. [Google Scholar]
- Shih, D.M.; Wang, Z.; Lee, R.; Meng, Y.; Che, N.; Charugundla, S.; Qi, H.; Wu, J.; Pan, C.; Brown, J.M.; et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res. 2015, 56, 22–37. [Google Scholar] [CrossRef]
- Hartiala, J.; Bennett, B.J.; Tang, W.H.; Wang, Z.; Stewart, A.F.R.; Roberts, R.; McPherson, R.; Lusis, A.J.; Hazen, S.L.; Allayee, H.; et al. Comparative genome-wide association studies in mice and humans for trimethyl-amine Noxide, a proatherogenic metabolite of choline and L-carnitine. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Costanza, A.C.; Moscavitch, S.D.; Neto, H.C.F.; Mesquita, E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: A randomized, double-blind, placebo-controlled pilot trial. Int. J. Cardiol. 2015, 179, 348–350. [Google Scholar] [CrossRef]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic Administration Attenuates Myocardial Hypertrophy and Heart Failure After Myocardial Infarction in the Rat. Circ. Hear. Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef]
- Masenga, S.K.; Hamooya, B.; Hangoma, J.; Hayumbu, V.; Ertuglu, L.A.; Ishimwe, J.; Rahman, S.; Saleem, M.; Laffer, C.L.; Elijovich, F.; et al. Recent advances in modulation of cardiovascular diseases by the gut microbiota. J. Hum. Hypertens. 2022, 36, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Kaku, N.; Matsumoto, N.; Sasaki, D.; Tsuda, K.; Kosai, K.; Uno, N.; Morinaga, Y.; Tagami, A.; Adachi, S.; Hasegawa, H.; et al. Effect of probiotics on gut microbiome in patients with administration of surgical antibiotic prophylaxis: A randomized con-trolled study. J. Infect. Chemother. 2020, 26, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Cirota, G.G.; Rossini, B.; Lungaro, L.; Di Biase, A.R.; Colecchia, A.; Volta, U.; De Giorgio, R.; Festi, D.; Caio, G. Probiotics, Prebiotics and Other Dietary Supplements for Gut Microbiota Modulation in Celiac Disease Patients. Nutrients 2020, 12, 2674. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Ahmadi, S.; Hayes, J.; Gagliano, J.; Subashchandrabose, S.; Kitzman, D.W.; Becton, T.; Read, R.; Yadav, H. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbi-ome. Sci. Rep. 2018, 8, 12649. [Google Scholar] [CrossRef]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Noguez, C.G.; Cruceño-Casarrubias, C.E.; Pardo, M.E.S.; Bermúdez-Humarán, L.G. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Van Schoor, J. Probiotics and gut health. SA Pharm. Assist. 2020, 20, 32–33. [Google Scholar]
- Murakami, T. Absorption sites of orally administered drugs in the small intestine. Expert Opin. Drug Discov. 2017, 12, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29555994 (accessed on 19 January 2019). [CrossRef] [PubMed]
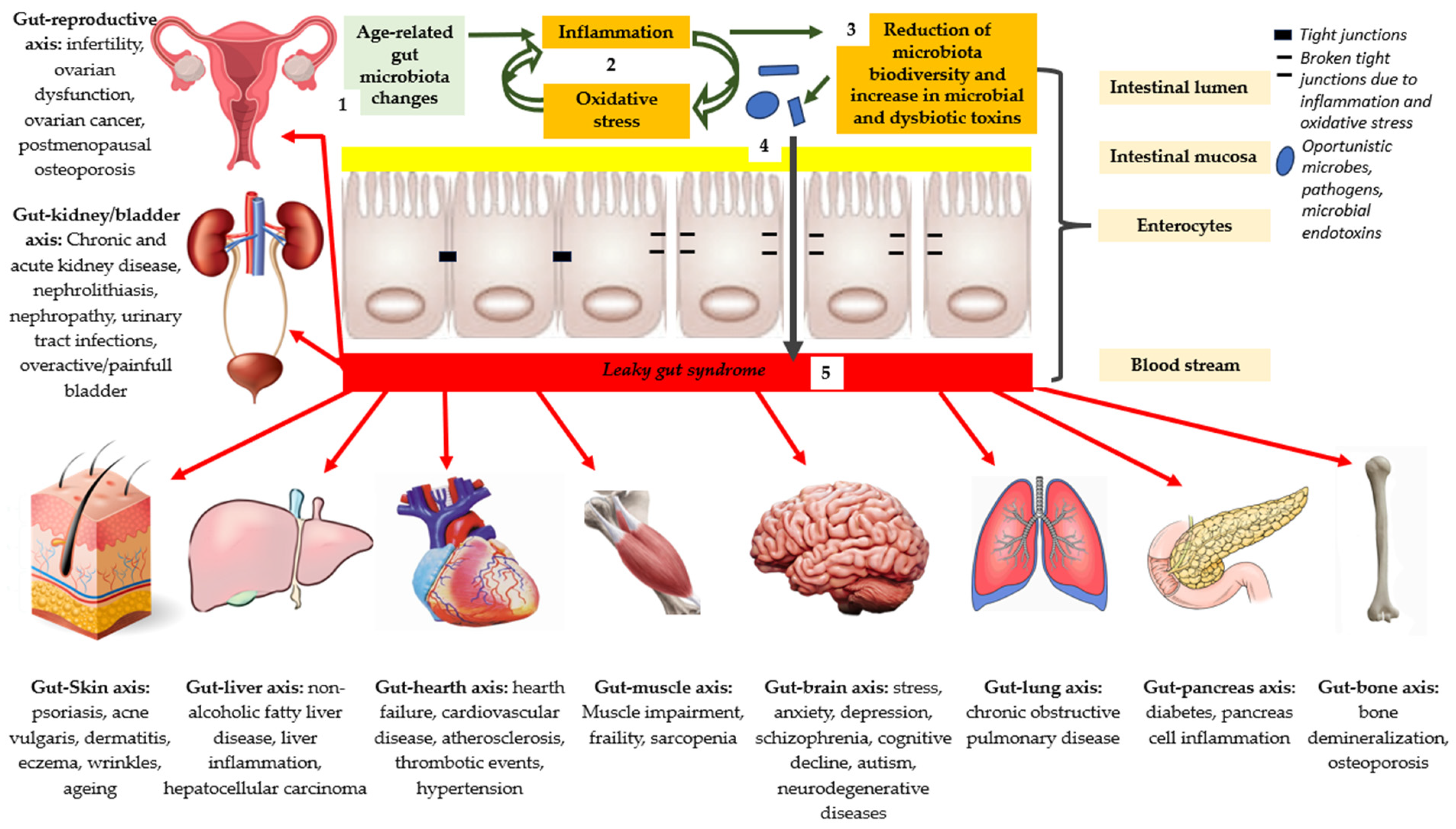

| Digestive Disorders |
| Acute microbial diarrhea Traveler’s diarrhea Antibiotic-therapy-associated diarrhea Clostridium difficile-associated diarrhea Irritable bowel syndrome (IBS) Inflammatory bowel disease (IBD) Helicobacter pylori-associated gastritis |
| Extra-Intestinal Disorders |
| Gut–reproductive axis: infertility, ovarian dysfunction, ovarian cancer, and postmenopausal osteoporosis; Gut–kidney/bladder axis: chronic and acute kidney disease, nephrolithiasis, nephropathy, urinary tract infections, and overactive/painful bladder; Gut–Skin axis: psoriasis, acne vulgaris, dermatitis, eczema, wrinkles, and aging; Gut–liver axis: nonalcoholic fatty liver disease, liver inflammation, and hepatocellular carcinoma; Gut–heart axis: heart failure, cardiovascular disease, atherosclerosis, thrombotic events, and hypertension; Gut–muscle axis: muscle impairment, frailty, and sarcopenia; Gut–brain axis: stress, anxiety, depression, schizophrenia, cognitive decline, autism, and neurodegenerative diseases; Gut–lung axis: chronic obstructive pulmonary disease; Gut–pancreas axis: diabetes and pancreas cell inflammation; Gut–bone axis: bone demineralization and osteoporosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colletti, A.; Pellizzato, M.; Cicero, A.F. The Possible Role of Probiotic Supplementation in Inflammation: A Narrative Review. Microorganisms 2023, 11, 2160. https://doi.org/10.3390/microorganisms11092160
Colletti A, Pellizzato M, Cicero AF. The Possible Role of Probiotic Supplementation in Inflammation: A Narrative Review. Microorganisms. 2023; 11(9):2160. https://doi.org/10.3390/microorganisms11092160
Chicago/Turabian StyleColletti, Alessandro, Marzia Pellizzato, and Arrigo Francesco Cicero. 2023. "The Possible Role of Probiotic Supplementation in Inflammation: A Narrative Review" Microorganisms 11, no. 9: 2160. https://doi.org/10.3390/microorganisms11092160
APA StyleColletti, A., Pellizzato, M., & Cicero, A. F. (2023). The Possible Role of Probiotic Supplementation in Inflammation: A Narrative Review. Microorganisms, 11(9), 2160. https://doi.org/10.3390/microorganisms11092160







