A Scoping Review Evaluating the Current State of Gut Microbiota Research in Africa
Abstract
:1. Introduction
2. Methods
2.1. Scoping Review Questions
2.2. Eligibility Criteria
2.3. Search Strategy
2.3.1. Identifying Search Terms and Sources of Information
2.3.2. Conducting the Searches
2.4. Selection of Sources of Evidence
2.5. Data Charting and Data Items
3. Results
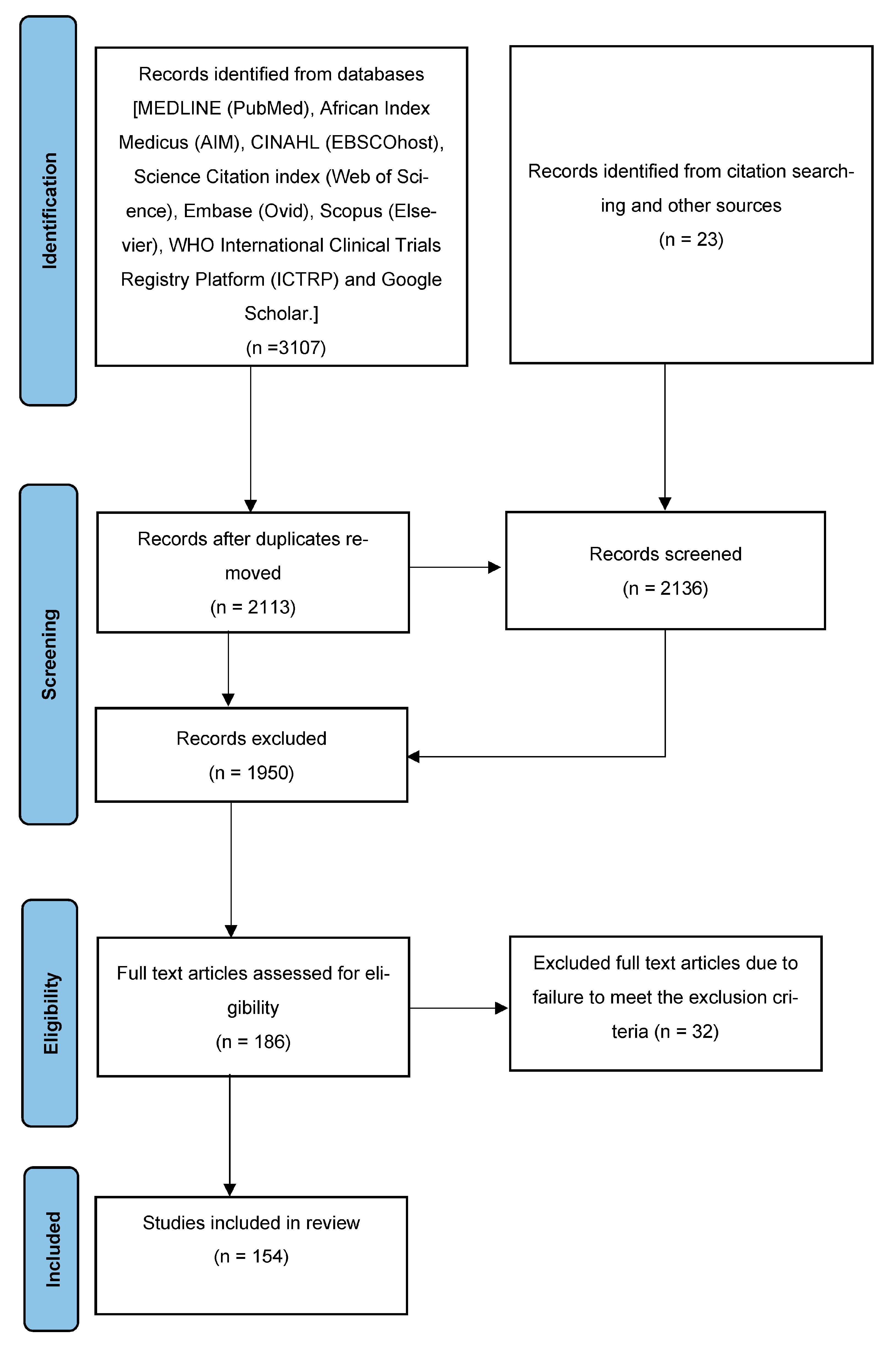
3.1. Study Selection Process
3.2. Description of the Generalized Population from All the Included Studies
3.3. Characteristics of Included Studies
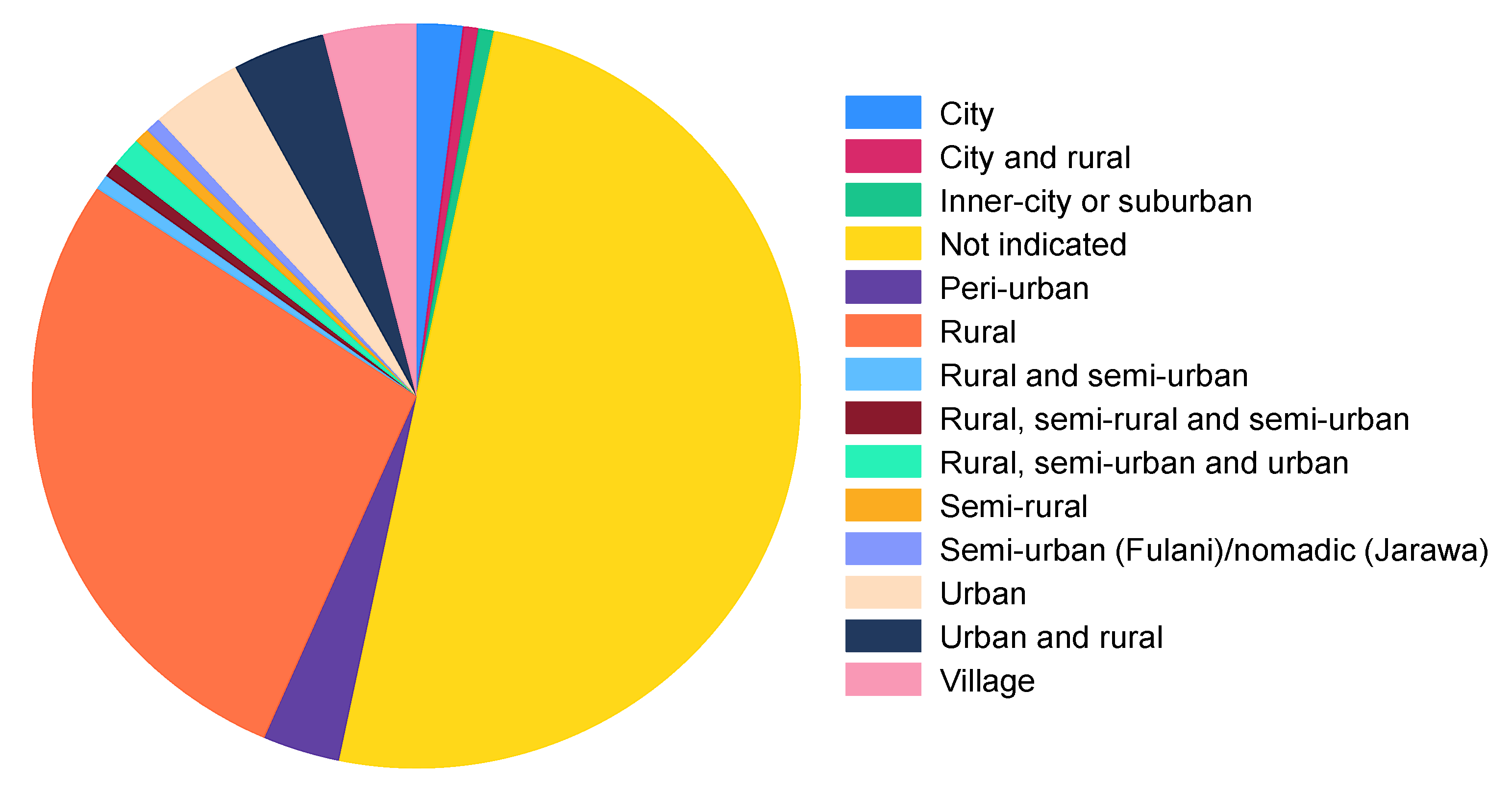
3.3.1. Study Settings
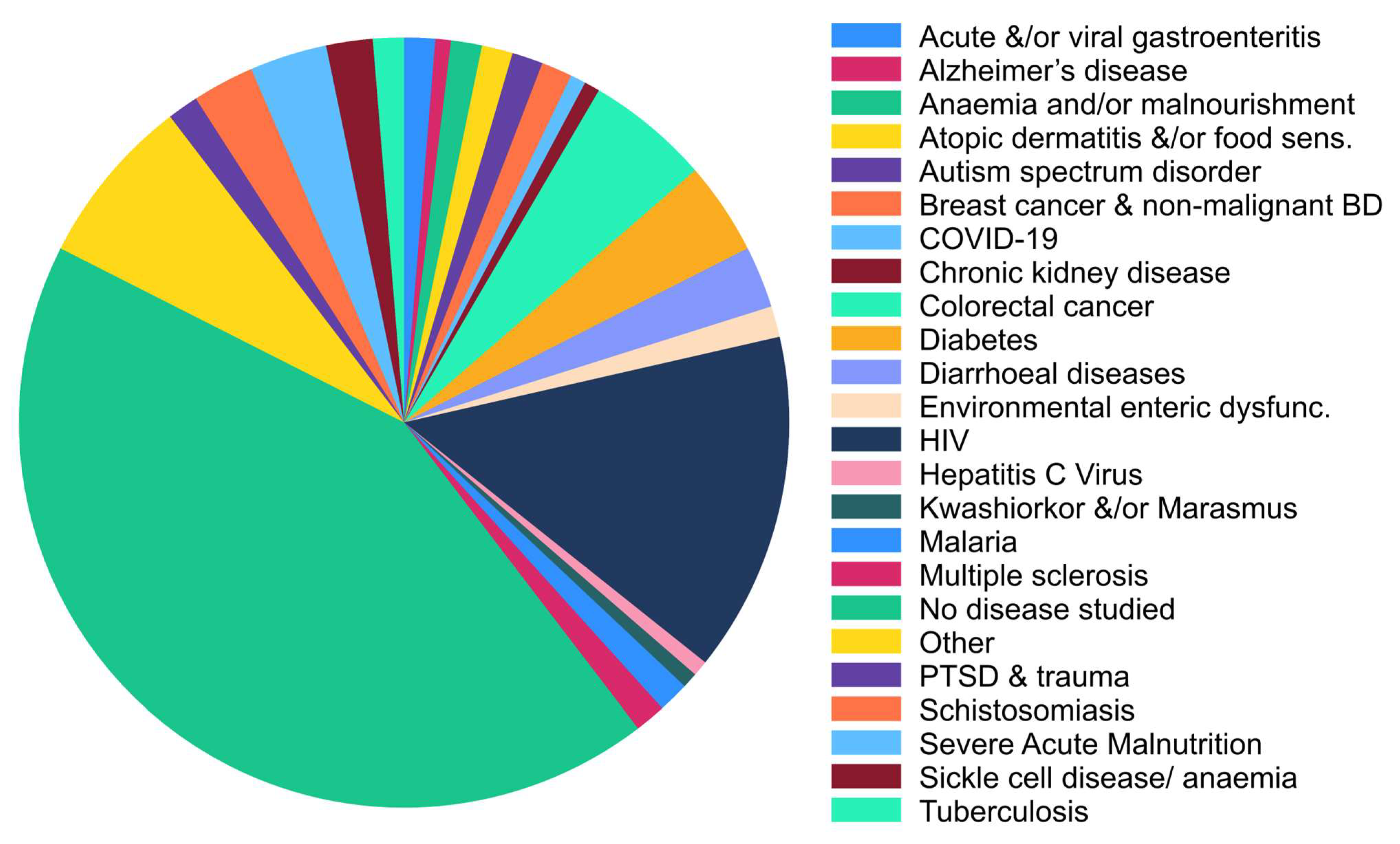
3.3.2. Disease Types
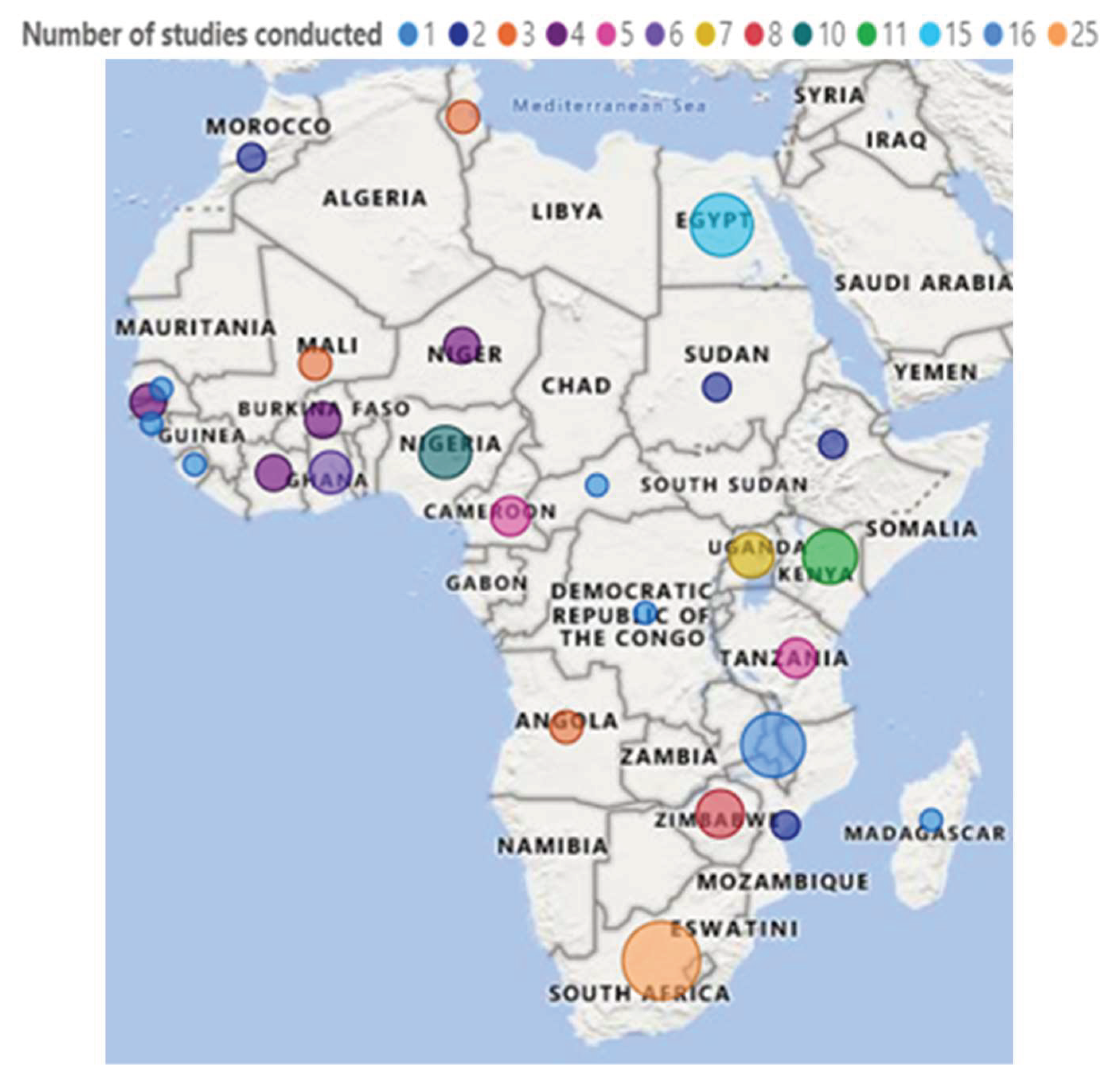
3.3.3. Countries Where the Included Studies Were Conducted
3.3.4. Methods/Techniques Used to Profile Gut Microbiota
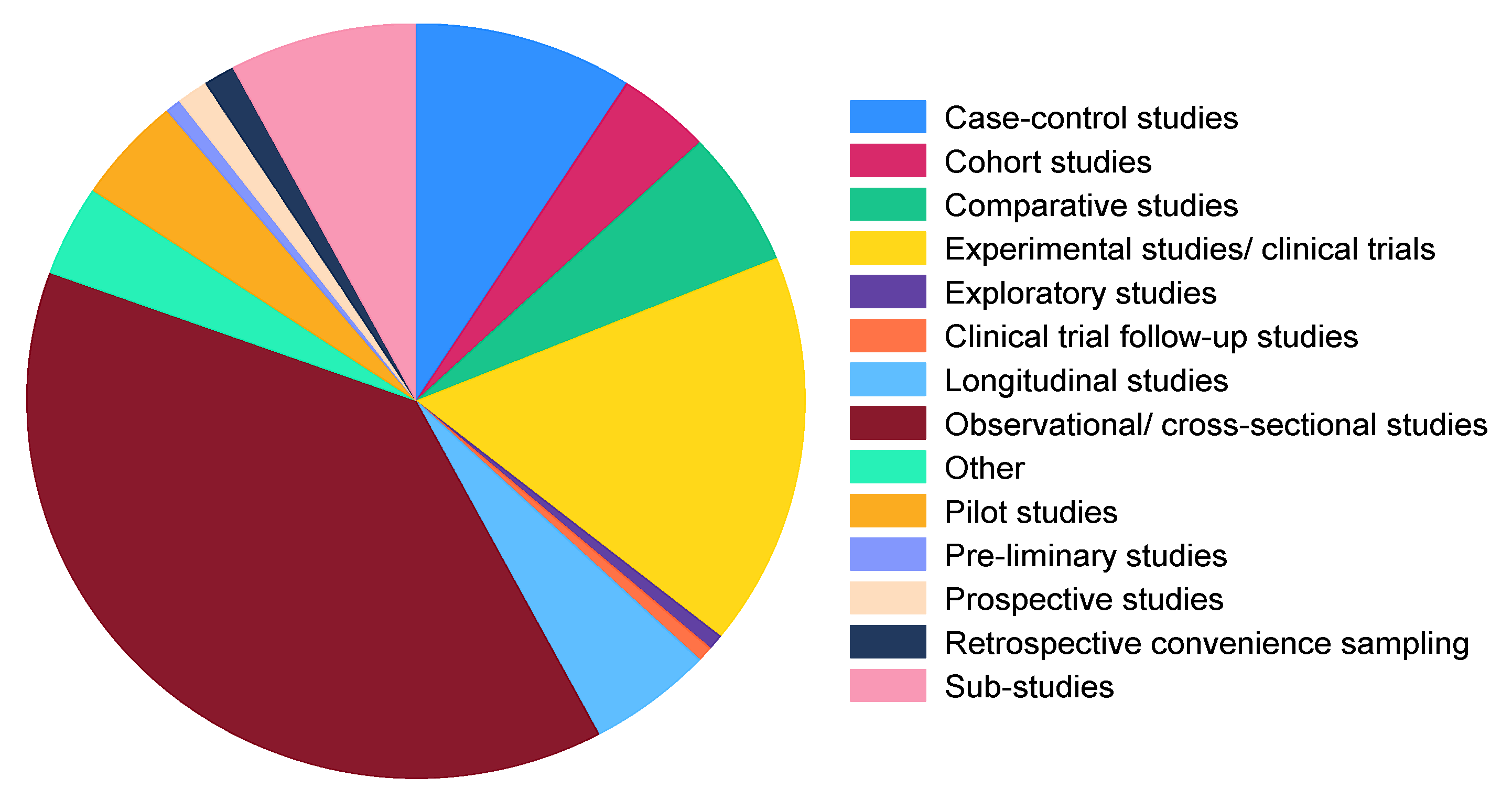
3.3.5. Study Types/Designs
3.3.6. Types of Samples
3.4. Generalized Study Findings
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allali, I.; Abotsi, R.E.; Tow, L.A.; Thabane, L.; Zar, H.J.; Mulder, N.M.; Nicol, M.P. Human microbiota research in Africa: A systematic review reveals gaps and priorities for future research. Microbiome 2021, 9, 241. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Galland, L. The Gut Microbiome and the Brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Wu, Z.; Tian, E.; Chen, Y.; Dong, Z.; Peng, Q. Gut microbiota and its roles in the pathogenesis and therapy of endocrine system diseases. Microbiol. Res. 2023, 268, 127291. [Google Scholar] [CrossRef]
- Islam, M.R.; Arthur, S.; Haynes, J.; Butts, M.R.; Nepal, N.; Sundaram, U. The Role of Gut Microbiota and Metabolites in Obesity-Associated Chronic Gastrointestinal Disorders. Nutrients 2022, 14, 624. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflamm. 2022, 19, 154. [Google Scholar] [CrossRef]
- Ding, R.-X.; Goh, W.-R.; Wu, R.-N.; Yue, X.-Q.; Luo, X.; Khine, W.W.T.; Wu, J.-R.; Lee, Y.-K. Revisit gut microbiota and its impact on human health and disease. J. Food Drug Anal. 2019, 27, 623–631. [Google Scholar] [CrossRef]
- Rosas-Plaza, S.; Hernández-Terán, A.; Navarro-Díaz, M.; Escalante, A.E.; Morales-Espinosa, R.; Cerritos, R. Human Gut Microbiome Across Different Lifestyles: From Hunter-Gatherers to Urban Populations. Front. Microbiol. 2022, 13, 843170. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Oduaran, O.H.; Tamburini, F.B.; Sahibdeen, V.; Brewster, R.; Gómez-Olivé, F.X.; Kahn, K.; Norris, S.A.; Tollman, S.M.; Twine, R.; Wade, A.N.; et al. Gut microbiome profiling of a rural and urban South African cohort reveals biomarkers of a population in lifestyle transition. BMC Microbiol. 2020, 20, 330. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Kamm, M.A.; Colombel, J.-F.; Ng, S.C. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 440–452. [Google Scholar] [CrossRef]
- Gupta, V.; Kumar, R.; Sood, U.; Singhvi, N. Reconciling Hygiene and Cleanliness: A New Perspective from Human Microbiome. Indian J. Microbiol. 2020, 60, 37–44. [Google Scholar] [CrossRef]
- Owino, V.O. Challenges and opportunities to tackle the rising prevalence of diet-related non-communicable diseases in Africa. Proc. Nutr. Soc. 2019, 78, 506–512. [Google Scholar] [CrossRef]
- Nkengasong, J.N.; Tessema, S.K. Africa Needs a New Public Health Order to Tackle Infectious Disease Threats. Cell 2020, 183, 296–300. [Google Scholar] [CrossRef]
- Peters, M.D.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Pheeha, S.M.; Tamuzi, J.L.; Manda, S.; Nyasulu, P.S. Identifying Gut Microbiota Conditions Associated with Disease in the African Continent: A Scoping Review Protocol. Methods Protoc. 2023, 6, 2. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—Regional Office for Africa. The State of Health in the WHO African Region: An Analysis of the Status of Health, Health Services and Health Systems in the Context of the Sustainable Development Goals; World Health Organization—Regional Office for Africa: Brazzaville, Republic of Congo, 2018; Available online: https://apps.who.int/iris/handle/10665/275292 (accessed on 4 July 2023).
- Kamng’Ona, A.W.; Young, R.; Arnold, C.D.; Patson, N.; Jorgensen, J.M.; Kortekangas, E.; Chaima, D.; Malamba, C.; Ashorn, U.; Cheung, Y.B.; et al. Provision of Lipid-Based Nutrient Supplements to Mothers during Pregnancy and 6 Months Postpartum and to Their Infants from 6 to 18 Months Promotes Infant Gut Microbiota Diversity at 18 Months of Age but Not Microbiota Maturation in a Rural Malawian Setting: Secondary Outcomes of a Randomized Trial. J. Nutr. 2020, 150, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Arnold, C.D.; Young, R.R.; Ashorn, P.; Maleta, K.; Fan, Y.-M.; Ashorn, U.; Chaima, D.; Malamba-Banda, C.; Kable, M.E.; et al. Infant gut microbiota characteristics generally do not modify effects of lipid-based nutrient supplementation on growth or inflammation: Secondary analysis of a randomized controlled trial in Malawi. Sci. Rep. 2020, 10, 14861. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Edens, T.J.; Carr, L.; Mutasa, K.; Gough, E.K.; Evans, C.; Geum, H.M.; Baharmand, I.; Gill, S.K.; Ntozini, R.; et al. The gut microbiome and early-life growth in a population with high prevalence of stunting. Nat. Commun. 2023, 14, 654. [Google Scholar] [CrossRef] [PubMed]
- Easton, A.V.; Quiñones, M.; Vujkovic-Cvijin, I.; Oliveira, R.G.; Kepha, S.; Odiere, M.R.; Anderson, R.M.; Belkaid, Y.; Nutman, T.B. The Impact of Anthelmintic Treatment on Human Gut Microbiota Based on Cross-Sectional and Pre- and Postdeworming Comparisons in Western Kenya. mBio 2019, 10, e00519-19. [Google Scholar] [CrossRef]
- Abange, W.B.; Martin, C.; Nanfack, A.J.; Yatchou, L.G.; Nusbacher, N.; Nguedia, C.A.; Kamga, H.G.; Fokam, J.; Kennedy, S.P.; Ndjolo, A.; et al. Alteration of the gut fecal microbiome in children living with HIV on antiretroviral therapy in Yaounde, Cameroon. Sci. Rep. 2021, 11, 7666. [Google Scholar] [CrossRef]
- Brazier, L.; Koumavor, C.K.; Renaud, N.; Prugnolle, F.; Thomas, F.; Ategbo, S.; Engoba, M.; Obengui; Leroy, E.M.; Durand, P.; et al. Evolution in fecal bacterial/viral composition in infants of two central African countries (Gabon and Republic of the Congo) during their first month of life. PLoS ONE 2017, 12, e0185569. [Google Scholar] [CrossRef]
- Naidoo, C.C.; Nyawo, G.R.; Sulaiman, I.; Wu, B.G.; Turner, C.T.; Bu, K.; Palmer, Z.; Li, Y.; Reeve, B.W.; Moodley, S.; et al. Anaerobe-enriched gut microbiota predicts pro-inflammatory responses in pulmonary tuberculosis. Ebiomedicine 2021, 67, 103374. [Google Scholar] [CrossRef]
- Radwan, S.; Gilfillan, D.; Eklund, B.; Radwan, H.M.; El Menofy, N.G.; Lee, J.; Kapuscinski, M.; Abdo, Z. A comparative study of the gut microbiome in Egyptian patients with Type I and Type II diabetes. PLoS ONE 2020, 15, e0238764. [Google Scholar] [CrossRef]
- Schneeberger, P.; Coulibaly, J.T.; Panic, G.; Daubenberger, C.; Gueuning, M.; Frey, J.E.; Keiser, J. Investigations on the interplays between Schistosoma mansoni, praziquantel and the gut microbiome. Parasites Vectors 2018, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.; Boekhorst, J.; Schneeberger, S.; Karanja, S.; Hennet, T.; Zimmermann, M.B. Maternal Human Milk Oligosaccharide Profile Modulates the Impact of an Intervention with Iron and Galacto-Oligosaccharides in Kenyan Infants. Nutrients 2019, 11, 2596. [Google Scholar] [CrossRef]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.M.; Cercamondi, C.I.; Winkler, H.C.; Boekhorst, J.; Moretti, D.; Lacroix, C.; Karanja, S.; Zimmermann, M.B. Iron-containing micronutrient powders modify the effect of oral antibiotics on the infant gut microbiome and increase post-antibiotic diarrhoea risk: A controlled study in Kenya. Gut 2019, 68, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Popovic, A.; Bourdon, C.; Wang, P.W.; Guttman, D.S.; Voskuijl, W.; Grigg, M.E.; Bandsma, R.H.J.; Parkinson, J. Design and application of a novel two-amplicon approach for defining eukaryotic microbiota. Microbiome 2018, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.E.; El Shebini, S.M.; El-Masry, S.A.; Ahmed, N.H.; Kamal, A.N.; Ismail, A.S.; Alian, K.M.; Mostafa, M.I.; Selim, M.; Afify, M.A.S. Brief overview of dietary intake, some types of gut microbiota, metabolic markers and research opportunities in sample of Egyptian women. Sci. Rep. 2022, 12, 17291. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E.; Enos, M.K.; PrayGod, G.; Seney, S.; Macklaim, J.M.; Chilton, S.; Willner, D.; Knight, R.; Fusch, C.; Fusch, G.; et al. Microbiota at Multiple Body Sites during Pregnancy in a Rural Tanzanian Population and Effects of Moringa-Supplemented Probiotic Yogurt. Appl. Environ. Microbiol. 2015, 81, 4965–4975. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Enos, M.K.; Mwanga, J.R.; Changalucha, J.P.; Burton, J.; Gloor, G.B.; Reid, G. Randomized Open-Label Pilot Study of the Influence of Probiotics and the Gut Microbiome on Toxic Metal Levels in Tanzanian Pregnant Women and School Children. mBio 2014, 5, e01580-14. [Google Scholar] [CrossRef]
- Hemmings, S.M.J.; Malan-Müller, S.; van den Heuvel, L.L.; Demmitt, B.A.; Stanislawski, M.A.; Smith, D.G.; Bohr, A.D.; Stamper, C.E.; Hyde, E.R.; Morton, J.T.; et al. The Microbiome in Posttraumatic Stress Disorder and Trauma-Exposed Controls: An Exploratory Study. Psychosom. Med. 2017, 79, 936–946. [Google Scholar] [CrossRef]
- Cooper, P.; Bolton, K.D.; Velaphi, S.; De Groot, N.; Emady-Azar, S.; Pecquet, S.; Steenhout, P. Early Benefits of a Starter Formula Enriched in Prebiotics and Probiotics on the Gut Microbiota of Healthy Infants Born to HIV+ Mothers: A Randomized Double-Blind Controlled Trial. Clin. Med. Insights Pediatr. 2016, 10, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Bramble, M.S.; Vashist, N.; Ko, A.; Priya, S.; Musasa, C.; Mathieu, A.; Spencer, D.A.; Kasendue, M.L.; Dilufwasayo, P.M.; Karume, K.; et al. The gut microbiome in konzo. Nat. Commun. 2021, 12, 5371. [Google Scholar] [CrossRef]
- El-Zawawy, H.T.; Ahmed, S.M.; El-Attar, E.A.; Ahmed, A.A.; Roshdy, Y.S.; Header, D.A. Study of gut microbiome in Egyptian patients with autoimmune thyroid diseases. Int. J. Clin. Pract. 2021, 75, e14038. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.M.; Adel, A.; El-Gendy, A.O.; Essam, T.M.; Aziz, R.K. Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog. 2016, 8, 42. [Google Scholar] [CrossRef]
- Castro-Mejía, J.L.; O’ferrall, S.; Krych, Ł.; O’mahony, E.; Namusoke, H.; Lanyero, B.; Kot, W.; Nabukeera-Barungi, N.; Michaelsen, K.F.; Mølgaard, C.; et al. Restitution of gut microbiota in Ugandan children administered with probiotics (Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12) during treatment for severe acute malnutrition. Gut Microbes 2020, 11, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Eyongabane Ako, S.; Nkenfou, C.N.; Assob, J.N.; Thumamo Pokam, B.; Njopin, C.; Eteneneng Enoh, J.; Nchang, C.F.; Mbanya, G.; Woguia, G.F.; Ngoume Moukoma, Y.F.; et al. Characterization and Profiling of Gut Bacterial Microbiome and Pathobionts among HIV-Negative and HIV-Infected Individuals in Cameroon. medRxiv 2022, 1–21. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2018, 21, 676–681. [Google Scholar] [CrossRef]
- Sulaimon, L.A.; Joel, I.Y.; Sanusi, O.S.; Olanipekun, Q.A.-K.; Haliru, A.M.; Ajanaku, Z.A.; Hassan, A.E.; Zakariyah, I.F.; Ajibade, F.B.; Olajide, M.; et al. Metagenomic analysis of gut microbiota of patients with colorectal cancer at the Federal Medical Centre (FMC), Abeokuta, Ogun State, Nigeria. J. Clin. Oncol. 2023, 41, 195. [Google Scholar] [CrossRef]
- Khedr, E.M.; Omeran, N.; Karam-Allah Ramadan, H.; Ahmed, G.K.; Abdelwarith, A.M. Alteration of Gut Microbiota in Alzheimer’s Disease and Their Relation to the Cognitive Impairment. J. Alzheimer’s Dis. 2022, 88, 1103–1114. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Elhefnawy, A.M.; Azouz, H.G.; Roshdy, Y.S.; Ashry, M.H.; Ibrahim, A.E.; Meheissen, M.A. Study of the gut Microbiome Profile in Children with Autism Spectrum Disorder: A Single Tertiary Hospital Experience. J. Mol. Neurosci. 2020, 70, 887–896. [Google Scholar] [CrossRef]
- Mekky, J.; Wani, R.; Said, S.M.; Ashry, M.; Ibrahim, A.E.; Ahmed, S.M. Molecular characterization of the gut microbiome in egyptian patients with remitting relapsing multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 57, 103354. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Coetzee, V.; Kruger, J.; Potgieter, H.; Buys, E.M. Dysbiosis Signatures of Fecal Microbiota in South African Infants with Respiratory, Gastrointestinal, and Other Diseases. J. Pediatr. 2020, 218, 106–113.e3. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Côte d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Aakko, J.; Grześkowiak, Ł.; Asukas, T.; Päivänsäde, E.; Lehto, K.-M.; Fan, Y.-M.; Mangani, C.; Maleta, K.; Ashorn, P.; Salminen, S. Lipid-based Nutrient Supplements Do Not Affect Gut Bifidobacterium Microbiota in Malawian Infants: A Randomized Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Goosen, C.; Proost, S.; Tito, R.; Baumgartner, J.; Mikulic, N.; Barnabas, S.; Cotton, M.; Zimmermann, M.; Raes, J.; Blaauw, R. Associations between HIV status and the gut microbiota in South African children with low iron stores. Clin. Nutr. ESPEN 2021, 46, S573. [Google Scholar] [CrossRef]
- Sehli, S.; Azami, A.I.; Dini, N.; Habib, N.; Chaouni, B.; Hamdi, S.; Al Idrissi, N.; Amzazi, S.; Allali, I.; Nejjari, C.; et al. Gut Microbiome 16S rRNA Gene Amplicon Taxonomic Profiling of Hospitalized Moroccan COVID-19 Patients. Microbiol. Resour. Announc. 2022, 11, e0025622. [Google Scholar] [CrossRef]
- McMillan, A.; Orimadegun, A.E.; Sumarah, M.W.; Renaud, J.; da Encarnacao, M.M.; Gloor, G.B.; Akinyinka, O.O.; Reid, G.; Allen, S.J. Metabolic derangements identified through untargeted metabolomics in a cross-sectional study of Nigerian children with severe acute malnutrition. Metabolomics 2017, 13, 13. [Google Scholar] [CrossRef]
- Mondot, S.; Poirier, P.; Abou-Bacar, A.; Greigert, V.; Brunet, J.; Nourrisson, C.; Randrianarivelojosia, M.; Razafindrakoto, J.-L.; Morel, E.; Rakotomalala, R.S.; et al. Parasites and diet as main drivers of the Malagasy gut microbiome richness and function. Sci. Rep. 2021, 11, 17630. [Google Scholar] [CrossRef]
- Ugboko, H.; Nwinyi, O.; Oranusi, S. Metagenomic profiling of gut microbiota of diarrhoeic children in Southwest Nigeria. Int. J. Infect. Dis. 2020, 101, 181. [Google Scholar] [CrossRef]
- Budree, S.; Osman, M.; Nduru, P.; Kaba, M.; Zellmer, C.; Claasens, S.; Zar, H. Evaluating the Gut Microbiome in Children with Stunting: Findings from a South African Birth Cohort. In Proceedings of the 2019 Poster Session—Harvard Chan Microbiome in Public Health (HCMPH) Symposium, Boston, MA, USA, 15–19 May 2019. [Google Scholar]
- Van Niekerk, M.; Dunbar, R.; Benycoub, J.; Grathwohl, D.; Labadarios, D. Microbiota Richness and Diversity in a Cohort of Underweight HIV-Positive Children Aged 24–72 Months in Cape Town, South Africa. HIV Med. 2019, 20, 317–337. [Google Scholar] [CrossRef]
- Kitchin, N.; Womersley, J.; Engelbrecht, A.; Marais, A.-S.; de Vries, M.M.; May, P.A.; Seedat, S.; Hemmings, S. TU19. The gut microbiota’s influence in the development of foetal alcohol spectrum disorders. Eur. Neuropsychopharmacol. 2021, 51, e103–e104. [Google Scholar] [CrossRef]
- Iebba, V.; Santangelo, F.; Totino, V.; Pantanella, F.; Monsia, A.; Di Cristanziano, V.; Di Cave, D.; Schippa, S.; Berrilli, F.; D’Alfonso, R. Gut microbiota related to Giardia duodenalis, Entamoeba spp. and Blastocystis hominis infections in humans from Côte d’Ivoire. J. Infect. Dev. Ctries. 2016, 10, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Ragab, S.H.; ElBaky, A.A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch. Med. Sci. 2011, 3, 501–507. [Google Scholar] [CrossRef]
- Jacobson, D.K.; Kagone, T.S.; Meda, N.; Carabin, H.; Honap, T.; Sankaranarayanan, K.; Lewis, C.M. Gut Microbiome Community Composition Is Significantly Influenced by Shared Livingspace in Rural Agriculturalists from Burkina Faso. In Proceedings of the 88th Annual Meeting of the American Association of Physical Anthropologists, Cleveland, OH, USA, 27–30 March 2019. [Google Scholar] [CrossRef]
- El-Sokkary, M.M.A. Molecular characterization of gut microbial structure and diversity associated with colorectal cancer patients in Egypt. Pan Afr. Med. J. 2022, 43, 119. [Google Scholar] [CrossRef] [PubMed]
- Kortekangas, E.; Young, R.; Cheung, Y.B.; Fan, Y.-M.; Jorgensen, J.M.; Kamng’ona, A.W.; Chaima, D.; Ashorn, U.; Dewey, K.G.; Maleta, K.; et al. A Prospective Study on Child Morbidity and Gut Microbiota in Rural Malawi. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 431–437. [Google Scholar] [CrossRef]
- Digitale, J.; Sié, A.; Coulibaly, B.; Ouermi, L.; Dah, C.; Tapsoba, C.; Bärnighausen, T.; Lebas, E.; Arzika, A.M.; Glymour, M.M.; et al. Gut Bacterial Diversity and Growth among Preschool Children in Burkina Faso. Am. J. Trop. Med. Hyg. 2020, 103, 2568–2573. [Google Scholar] [CrossRef]
- Mandal, R.K.; Crane, R.J.; A Berkley, J.; Gumbi, W.; Wambua, J.; Ngoi, J.M.; Ndungu, F.M.; Schmidt, N.W. Longitudinal Analysis of Infant Stool Bacteria Communities Before and After Acute Febrile Malaria and Artemether-Lumefantrine Treatment. J. Infect. Dis. 2019, 220, 687–698. [Google Scholar] [CrossRef]
- Ayeni, F.A.; Biagi, E.; Rampelli, S.; Fiori, J.; Soverini, M.; Audu, H.J.; Cristino, S.; Caporali, L.; Schnorr, S.L.; Carelli, V.; et al. Infant and Adult Gut Microbiome and Metabolome in Rural Bassa and Urban Settlers from Nigeria. Cell Rep. 2018, 23, 3056–3067. [Google Scholar] [CrossRef]
- Salah, M.; Azab, M.; Ramadan, A.; Hanora, A. New Insights on Obesity and Diabetes from Gut Microbiome Alterations in Egyptian Adults. OMICS 2019, 23, 477–485. [Google Scholar] [CrossRef]
- Flygel, T.T.; Sovershaeva, E.; Claassen-Weitz, S.; Hjerde, E.; Mwaikono, K.S.; Odland, J.Ø.; Ferrand, R.A.; McHugh, G.; Gutteberg, T.J.; Nicol, M.; et al. Composition of gut microbiota of children and adolescents with perinatal Human Immunodeficiency Virus infection taking antiretroviral therapy in Zimbabwe. J. Infect. Dis. 2020, 221, 483–492. [Google Scholar] [CrossRef]
- Naudé, P.J.; Claassen-Weitz, S.; Gardner-Lubbe, S.; Botha, G.; Kaba, M.; Zar, H.J.; Nicol, M.P.; Stein, D.J. Association of maternal prenatal psychological stressors and distress with maternal and early infant faecal bacterial profile. Acta Neuropsychiatr. 2020, 32, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kay, G.L.; Millard, A.; Sergeant, M.J.; Midzi, N.; Gwisai, R.; Mduluza, T.; Ivens, A.; Nausch, N.; Mutapi, F.; Pallen, M. Differences in the Faecal Microbiome in Schistosoma haematobium Infected Children vs. Uninfected Children. PLoS Negl. Trop. Dis. 2015, 9, e0003861. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, O.; Rowan, A.D.; Ogedengbe, C.O.; Mshelia, M.B.; Cabral, D.J.; Eze, A.A.; Obaro, S.; Belenky, P. Urogenital schistosomiasis is associated with signatures of microbiome dysbiosis in Nigerian adolescents. Sci. Rep. 2019, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Doumatey, A.P.; Adeyemo, A.; Zhou, J.; Lei, L.; Adebamowo, S.N.; Adebamowo, C.; Rotimi, C.N. Gut Microbiome Profiles Are Associated With Type 2 Diabetes in Urban Africans. Front. Cell. Infect. Microbiol. 2020, 10, 63. [Google Scholar] [CrossRef]
- Di Cristanziano, V.; Farowski, F.; Berrilli, F.; Santoro, M.; Di Cave, D.; Glé, C.; Daeumer, M.; Thielen, A.; Wirtz, M.; Kaiser, R.; et al. Analysis of Human Gut Microbiota Composition Associated to the Presence of Commensal and Pathogen Microorganisms in Côte d’Ivoire. Microorganisms 2021, 9, 1763. [Google Scholar] [CrossRef]
- Dostal, A.; Baumgartner, J.; Riesen, N.; Chassard, C.; Smuts, C.M.; Zimmermann, M.B.; Lacroix, C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: A randomised, placebo-controlled intervention trial in South African children. Br. J. Nutr. 2014, 112, 547–556. [Google Scholar] [CrossRef]
- Nowak, R.G.; Bentzen, S.M.; Ravel, J.; Crowell, T.A.; Dauda, W.; Ma, B.; Liu, H.; Blattner, W.A.; Baral, S.D.; Charurat, M.E. Rectal microbiota among HIV-uninfected, untreated HIV, and treated HIV-infected in Nigeria. Aids 2017, 31, 857–862. [Google Scholar] [CrossRef]
- Elmagzoub, W.A.; Idris, S.M.; Isameldin, M.; Arabi, N.; Abdo, A.; Ibrahim, M.; Khan, A.A.; Tanneberger, F.; Bakhiet, S.M.; Okuni, J.B.; et al. Mycobacterium avium subsp. paratuberculosis and microbiome profile of patients in a referral gastrointestinal diseases centre in the Sudan. PLoS ONE 2022, 17, e0266533. [Google Scholar] [CrossRef]
- Elkholy, A.; Behring, M.; Mohsen, M.; Bajpai, P.; Embaby, A.; Header, D.; Elwafa, R.A.; Saeed, H.; Fouad, M.; Arafat, W.; et al. Absence of Mitsuokella Multacida Isassociated with Early Onset of Colorectal Cancer. In Proceedings of the Annual Meeting of the American Association for Cancer Research, AACR, Philadelphia, PA, USA, 22–24 June 2020. [Google Scholar]
- Arafat, W. P-316 Profile of microbiota is associated with early onset of colorectal cancer in Egyptian and Kenyan patients. Ann. Oncol. 2020, 31, S192. [Google Scholar] [CrossRef]
- Cheung, Y.B.; Xu, Y.; Mangani, C.; Fan, Y.-M.; Dewey, K.G.; Salminen, S.J.; Maleta, K.; Ashorn, P. Gut microbiota in Malawian infants in a nutritional supplementation trial. Trop. Med. Int. Health 2016, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Quaye, E.K.; Adjei, R.L.; Isawumi, A.; Allen, D.J.; Caporaso, J.G.; Quaye, O. Altered Faecal Microbiota Composition and Structure of Ghanaian Children with Acute Gastroenteritis. Int. J. Mol. Sci. 2023, 24, 3607. [Google Scholar] [CrossRef]
- Kamng’ona, A.W.; Young, R.; Arnold, C.D.; Kortekangas, E.; Patson, N.; Jorgensen, J.M.; Prado, E.L.; Chaima, D.; Malamba, C.; Ashorn, U.; et al. The association of gut microbiota characteristics in Malawian infants with growth and inflammation. Sci. Rep. 2019, 9, 12893. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.K.; Edens, T.J.; Geum, H.M.; Baharmand, I.; Gill, S.K.; Robertson, R.C.; Mutasa, K.; Ntozini, R.; Smith, L.E.; Chasekwa, B.; et al. Maternal fecal microbiome predicts gestational age, birth weight and neonatal growth in rural Zimbabwe. Ebiomedicine 2021, 68, 103421. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.L.; Gootenberg, D.B.; Zhao, G.; Handley, S.A.; Ghebremichael, M.S.; Lim, E.S.; Lankowski, A.; Baldridge, M.T.; Wilen, C.B.; Flagg, M.; et al. Altered Virome and Bacterial Microbiome in Human Immunodeficiency Virus-Associated Acquired Immunodeficiency Syndrome. Cell Host Microbe 2016, 19, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, O.T.; Shaw, S.; Martin, J.C.; Ayeni, F.A.; Scott, K.P. Changes in the gut microbiota of Nigerian infants within the first year of life. PLoS ONE 2022, 17, e0265123. [Google Scholar] [CrossRef] [PubMed]
- Atukunda, P.; Muhoozi, G.K.M.; van den Broek, T.J.; Kort, R.; Diep, L.M.; Kaaya, A.N.; O Iversen, P.; Westerberg, A.C. Child development, growth and microbiota: Follow-up of a randomized education trial in Uganda. J. Glob. Health 2019, 9, 010431. [Google Scholar] [CrossRef]
- Kuhn, L.; Li, F.; Strehlau, R.; Tobin, N.; Patel, F.; Shiau, S.; Wang, S.; Abrams, E.J.; Tiemessen, C.T.; Aldrovandi, G.M. Early antiretroviral therapy in neonates and maturation of the gut microbiome. In Proceedings of the 29th Conference on Retroviruses and Opportunistic Infections, Virtual Conference, 13–16 and 22–24 February 2022. [Google Scholar]
- Chen, H.; Mozzicafreddo, M.; Pierella, E.; Carletti, V.; Piersanti, A.; Ali, S.M.; Ame, S.M.; Wang, C.; Miceli, C. Dissection of the gut microbiota in mothers and children with chronic Trichuris trichiura infection in Pemba Island, Tanzania. Parasites Vectors 2021, 14, 62. [Google Scholar] [CrossRef]
- Nabwera, H.M.; Espinoza, J.L.; Worwui, A.; Betts, M.; Okoi, C.; Sesay, A.K.; Bancroft, R.; Agbla, S.C.; Jarju, S.; Bradbury, R.S.; et al. Interactions between fecal gut microbiome, enteric pathogens, and energy regulating hormones among acutely malnourished rural Gambian children. Ebiomedicine 2021, 73, 103644. [Google Scholar] [CrossRef]
- Obuya, S.; Elkholy, A.; Avuthu, N.; Behring, M.; Bajpai, P.; Agarwal, S.; Kim, H.-G.; El-Nikhely, N.; Akinyi, P.; Orwa, J.; et al. A signature of Prevotella copri and Faecalibacterium prausnitzii depletion, and a link with bacterial glutamate degradation in the Kenyan colorectal cancer patients. J. Gastrointest. Oncol. 2022, 13, 2282–2292. [Google Scholar] [CrossRef]
- de Goffau, M.C.; Jallow, A.T.; Sanyang, C.; Prentice, A.M.; Meagher, N.; Price, D.J.; Revill, P.A.; Parkhill, J.; Pereira, D.I.A.; Wagner, J. Gut microbiomes from Gambian infants reveal the development of a non-industrialized Prevotella-based trophic network. Nat. Microbiol. 2022, 7, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Gebrebrhan, H.; Kambaran, C.; Sivro, A.; Adhiambo, W.; Siele, N.; Becker, M.G.; Li, J.; Choi, S.; Mwatelah, R.S.; Reyes, N.V.; et al. Rectal microbiota diversity in Kenyan MSM is inversely associated with frequency of receptive anal sex, independent of HIV status. Aids 2021, 35, 1091–1101. [Google Scholar] [CrossRef]
- Vonaesch, P.; Morien, E.; Andrianonimiadana, L.; Sanke, H.; Mbecko, J.-R.; Huus, K.E.; Naharimanananirina, T.; Gondje, B.P.; Nigatoloum, S.N.; Vondo, S.S.; et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc. Natl. Acad. Sci. USA 2018, 115, E8489–E8498. [Google Scholar] [CrossRef]
- Yooseph, S.; Kirkness, E.F.; Tran, T.M.; Harkins, D.M.; Jones, M.B.; Torralba, M.G.; O’connell, E.; Nutman, T.B.; Doumbo, S.; Doumbo, O.K.; et al. Stool microbiota composition is associated with the prospective risk of Plasmodium falciparum infection. BMC Genom. 2015, 16, 631. [Google Scholar] [CrossRef] [PubMed]
- Hanachi, M.; Maghrebi, O.; Bichiou, H.; Trabelsi, F.; Bouyahia, N.M.; Zhioua, F.; Belghith, M.; Harigua-Souiai, E.; Baouendi, M.; Guizani-Tabbane, L.; et al. Longitudinal and Comparative Analysis of Gut Microbiota of Tunisian Newborns According to Delivery Mode. Front. Microbiol. 2022, 13, 780568. [Google Scholar] [CrossRef]
- Delgadinho, M.; Ginete, C.; Santos, B.; Mendes, J.; Miranda, A.; Vasconcelos, J.; Brito, M. Microbial gut evaluation in an angolan paediatric population with sickle cell disease. J. Cell. Mol. Med. 2022, 26, 5360–5368. [Google Scholar] [CrossRef] [PubMed]
- Delgadinho, M.; Ginete, C.; Santos, B.; Fernandes, C.; Silva, C.; Miranda, A.; de Vasconcelos, J.N.; Brito, M. How Hydroxyurea Alters the Gut Microbiome: A Longitudinal Study Involving Angolan Children with Sickle Cell Anemia. Int. J. Mol. Sci. 2022, 23, 9061. [Google Scholar] [CrossRef]
- Paganini, D.; Jaeggi, T.; Cercamondi, C.; Kujinga, P.; Moretti, D.; Zimmermann, M. Anemia and Iron Status Are Predictors of Gut Microbiome Composition and Metabolites in Infants and Children in Rural Kenya. FASEB J. 2016, 30, 296.2. [Google Scholar]
- Jaeggi, T.; Kortman, G.A.M.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef]
- Mahdavinia, M.; Rasmussen, H.E.; Engen, P.; Van den Berg, J.P.; Davis, E.; Engen, K.; Green, S.J.; Naqib, A.; Botha, M.; Gray, C.; et al. Atopic dermatitis and food sensitization in South African toddlers: Role of Fiber and Gut Microbiota. Ann. Allergy Asthma Immunol. 2017, 118, 742–743.e3. [Google Scholar] [CrossRef]
- Osakunor, D.N.M.; Munk, P.; Mduluza, T.; Petersen, T.N.; Brinch, C.; Ivens, A.; Chimponda, T.; Amanfo, S.A.; Murray, J.; Woolhouse, M.E.J.; et al. The gut microbiome but not the resistome is associated with urogenital schistosomiasis in preschool-aged children. Commun. Biol. 2020, 3, 155. [Google Scholar] [CrossRef]
- Goosen, C.; Proost, S.; Tito, R.Y.; Baumgartner, J.; Barnabas, S.L.; Cotton, M.F.; Zimmermann, M.B.; Raes, J.; Blaauw, R. The effect of oral iron supplementation on the gut microbiota, gut inflammation, and iron status in iron-depleted South African school-age children with virally suppressed HIV and without HIV. Eur. J. Nutr. 2022, 61, 2067–2078. [Google Scholar] [CrossRef]
- Souai, N.; Zidi, O.; Mosbah, A.; Kosai, I.; El Manaa, J.; Mokhtar, N.B.; Asimakis, E.; Stathopoulou, P.; Cherif, A.; Tsiamis, G.; et al. Impact of the Post-Transplant Period and Lifestyle Diseases on Human Gut Microbiota in Kidney Graft Recipients. Microorganisms 2020, 8, 1724. [Google Scholar] [CrossRef]
- Siraj, Y.A.; Biadgelign, M.G.; Yassin, M.O.; Chekol, Y.Z. Mucosa-associated cultivable aerobic gut bacterial microbiota among colorectal cancer patients attending at the referral hospitals of Amhara Regional State, Ethiopia. Gut Pathog. 2021, 13, 19. [Google Scholar] [CrossRef]
- Tang, M.; Frank, D.N.; Hendricks, A.E.; Ir, D.; Esamai, F.; Liechty, E.; Hambidge, K.M.; Krebs, N.F. Iron in Micronutrient Powder Promotes an Unfavorable Gut Microbiota in Kenyan Infants. Nutrients 2017, 9, 776. [Google Scholar] [CrossRef]
- Afolayan, A.; Ayeni, F.A.; Moissl-Eichinger, C.; Gorkiewicz, G.; Halwachs, B.; Högenauer, C. Impact of a Nomadic Pastoral Lifestyle on the Gut Microbiome in the Fulani Living in Nigeria. Front. Microbiol. 2019, 10, 2138. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Ramadan, M.; Abu Faddan, N.H.; Hassan, E.A.; Ali, M.E.; El-Rehim, A.S.E.D.A.; Abbas, W.A.; Abozaid, M.A.A.; Hassanin, E.; Mohamed, G.A.; et al. Impact of Geographical Location on the Gut Microbiota Profile in Egyptian Children with Type 1 Diabetes Mellitus: A Pilot Study. Int. J. Gen. Med. 2022, 15, 6173–6187. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, S.G.; Abd-Elhameed, R.; Daef, E.; Mohammed, S.M.; Hassan, H.M.; El-Mokhtar, M.A.; Nasreldein, A.; Khedr, E.M. Gut microbiota in forty cases of Egyptian relapsing remitting multiple sclerosis. Iran. J. Microbiol. 2021, 13, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, C.; Sørensen, N.; Ashenafi, S.; Amogne, W.; Kassa, E.; Bekele, A.; Getachew, M.; Gebreselassie, N.; Aseffa, A.; Aderaye, G.; et al. Vitamin D and Phenylbutyrate Supplementation Does Not Modulate Gut Derived Immune Activation in HIV-1. Nutrients 2019, 11, 1675. [Google Scholar] [CrossRef]
- Huus, K.E.; Rodriguez-Pozo, A.; Kapel, N.; Nestoret, A.; Habib, A.; Dede, M.; Manges, A.; Collard, J.-M.; Sansonetti, P.J.; Vonaesch, P.; et al. Immunoglobulin recognition of fecal bacteria in stunted and non-stunted children: Findings from the Afribiota study. Microbiome 2020, 8, 113. [Google Scholar] [CrossRef]
- Malan-Muller, S.; Valles-Colomer, M.; Foxx, C.L.; Vieira-Silva, S.; van den Heuvel, L.L.; Raes, J.; Seedat, S.; Lowry, C.A.; Hemmings, S.M. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur. Neuropsychopharmacol. 2022, 56, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, Z.; Proost, S.; Tito, R.Y.; Raes, J.; Glorieux, G.; Moosa, M.R.; Blaauw, R. The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805. [Google Scholar] [CrossRef]
- Calder, N.; Walsh, K.; Olupot-Olupot, P.; Ssenyondo, T.; Muhindo, R.; Mpoya, A.; Brignardello, J.; Wang, X.; McKay, E.; Morrison, D.; et al. Modifying gut integrity and microbiome in children with severe acute malnutrition using legume-based feeds (MIMBLE): A pilot trial. Cell Rep. Med. 2021, 2, 100280. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Mikulic, N.; A Uyoga, M.; Chenoll, E.; Climent, E.; Howard-Varona, A.; Nyilima, S.; Stoffel, N.U.; Karanja, S.; Kottler, R.; et al. Gut microbiome function and composition in infants from rural Kenya and association with human milk oligosaccharides. Gut Microbes 2023, 15, 2178793. [Google Scholar] [CrossRef]
- Owolabi, A.J.; Senbanjo, I.O.; Oshikoya, K.A.; Boekhorst, J.; Eijlander, R.T.; Kortman, G.A.M.; Hageman, J.H.J.; Samuel, F.; Melse-Boonstra, A.; Schaafsma, A. Multi-Nutrient Fortified Dairy-Based Drink Reduces Anaemia without Observed Adverse Effects on Gut Microbiota in Anaemic Malnourished Nigerian Toddlers: A Randomised Dose–Response Study. Nutrients 2021, 13, 1566. [Google Scholar] [CrossRef] [PubMed]
- Chandiwana, P.; Munjoma, P.T.; Mazhandu, A.J.; Li, J.; Baertschi, I.; Wyss, J.; Jordi, S.B.U.; Mazengera, L.R.; Yilmaz, B.; Misselwitz, B.; et al. Antenatal gut microbiome profiles and effect on pregnancy outcome in HIV infected and HIV uninfected women in a resource limited setting. BMC Microbiol. 2023, 23, 4. [Google Scholar] [CrossRef]
- Jackson, C.L.; Frank, D.N.; Robertson, C.E.; Ir, D.; Kofonow, J.M.; Montlha, M.P.; Mutsaerts, E.A.M.L.; Nunes, M.C.; Madhi, S.A.; Ghosh, D.; et al. Evolution of the Gut Microbiome in HIV-Exposed Uninfected and Unexposed Infants during the First Year of Life. mBio 2022, 13, e0122922. [Google Scholar] [CrossRef]
- Wallenborn, J.T.; Gunier, R.B.; Pappas, D.J.; Chevrier, J.; Eskenazi, B. Breastmilk, Stool, and Meconium: Bacterial Communities in South Africa. Microb. Ecol. 2021, 83, 246–251. [Google Scholar] [CrossRef]
- Olm, M.R.; Dahan, D.; Carter, M.M.; Merrill, B.D.; Yu, F.B.; Jain, S.; Meng, X.; Tripathi, S.; Wastyk, H.; Neff, N.; et al. Robust variation in infant gut microbiome assembly across a spectrum of lifestyles. Science 2022, 376, 1220–1223. [Google Scholar] [CrossRef]
- von Huth, S.; Thingholm, L.B.; Kofoed, P.-E.; Bang, C.; Rühlemann, M.C.; Franke, A.; Holmskov, U. Intestinal protozoan infections shape fecal bacterial microbiota in children from Guinea-Bissau. PLoS Negl. Trop. Dis. 2021, 15, e0009232. [Google Scholar] [CrossRef]
- Rocafort, M.; Noguera-Julian, M.; Rivera, J.; Pastor, L.; Guillén, Y.; Langhorst, J.; Parera, M.; Mandomando, I.; Carrillo, J.; Urrea, V.; et al. Evolution of the gut microbiome following acute HIV-1 infection. Microbiome 2019, 7, 73. [Google Scholar] [CrossRef]
- Desai, C.; Handley, S.A.; Rodgers, R.; Rodriguez, C.; Ordiz, M.I.; Manary, M.J.; Holtz, L.R. Growth velocity in children with Environmental Enteric Dysfunction is associated with specific bacterial and viral taxa of the gastrointestinal tract in Malawian children. PLoS Negl. Trop. Dis. 2020, 14, e0008387. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Aboagye, S.Y.; Ishizaka, A.; Afum, T.; Mensah, G.I.; Asante-Poku, A.; Asandem, D.A.; Parbie, P.K.; Abana, C.Z.-Y.; Kushitor, D.; et al. Gut microbiota signature of pathogen-dependent dysbiosis in viral gastroenteritis. Sci. Rep. 2021, 11, 13945. [Google Scholar] [CrossRef] [PubMed]
- Paganini, D.; A Uyoga, M.; Kortman, G.A.M.; Cercamondi, C.I.; Moretti, D.; Barth-Jaeggi, T.; Schwab, C.; Boekhorst, J.; Timmerman, H.M.; Lacroix, C.; et al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in Kenyan infants. Gut 2017, 66, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Kodio, A.; Coulibaly, D.; Koné, A.K.; Konaté, S.; Doumbo, S.; Guindo, A.; Bittar, F.; Gouriet, F.; Raoult, D.; Thera, M.A.; et al. Blastocystis Colonization Is Associated with Increased Diversity and Altered Gut Bacterial Communities in Healthy Malian Children. Microorganisms 2019, 7, 649. [Google Scholar] [CrossRef]
- Van Zyl, K.N.; Whitelaw, A.C.; Hesseling, A.C.; Seddon, J.A.; Demers, A.-M.; Newton-Foot, M. Association between clinical and environmental factors and the gut microbiota profiles in young South African children. Sci. Rep. 2021, 11, 15895. [Google Scholar] [CrossRef]
- Parbie, P.K.; Mizutani, T.; Ishizaka, A.; Kawana-Tachikawa, A.; Runtuwene, L.R.; Seki, S.; Abana, C.Z.-Y.; Kushitor, D.; Bonney, E.Y.; Ofori, S.B.; et al. Fecal Microbiome Composition in Healthy Adults in Ghana. Jpn. J. Infect. Dis. 2021, 74, 42–47. [Google Scholar] [CrossRef]
- Parbie, P.K.; Mizutani, T.; Ishizaka, A.; Kawana-Tachikawa, A.; Runtuwene, L.R.; Seki, S.; Abana, C.Z.-Y.; Kushitor, D.; Bonney, E.Y.; Ofori, S.B.; et al. Dysbiotic Fecal Microbiome in HIV-1 Infected Individuals in Ghana. Front. Cell. Infect. Microbiol. 2021, 11, 646467. [Google Scholar] [CrossRef]
- Doan, T.; Hinterwirth, A.; Arzika, A.M.; Cotter, S.Y.; Ray, K.J.; O’brien, K.S.; Zhong, L.; Chow, E.D.; Zhou, Z.; Cummings, S.L.; et al. Mass Azithromycin Distribution and Community Microbiome: A Cluster-Randomized Trial. Open Forum Infect. Dis. 2018, 5, ofy182. [Google Scholar] [CrossRef]
- Doan, T.; Arzika, A.M.; Ray, K.J.; Cotter, S.Y.; Kim, J.; Maliki, R.; Zhong, L.; Zhou, Z.; Porco, T.C.; Vanderschelden, B.; et al. Gut Microbial Diversity in Antibiotic-Naive Children After Systemic Antibiotic Exposure: A Randomized Controlled Trial. Clin. Infect. Dis. 2017, 64, 1147–1153. [Google Scholar] [CrossRef]
- Ray, K.J.; Cotter, S.Y.; Arzika, A.M.; Kim, J.; Boubacar, N.; Zhou, Z.; Zhong, L.; Porco, T.C.; Keenan, J.D.; Lietman, T.M.; et al. High-throughput sequencing of pooled samples to determine community-level microbiome diversity. Ann. Epidemiol. 2019, 39, 63–68. [Google Scholar] [CrossRef]
- Ordiz, M.I.; Stephenson, K.; Agapova, S.; Wylie, K.M.; Maleta, K.; Martin, J.; Trehan, I.; Tarr, P.I.; Manary, M.J. Environmental Enteric Dysfunction and the Fecal Microbiota in Malawian Children. Am. J. Trop. Med. Hyg. 2017, 96, 473–476. [Google Scholar] [CrossRef]
- Ordiz, M.I.; Janssen, S.; Humphrey, G.; Ackermann, G.; Stephenson, K.; Agapova, S.; Divala, O.; Kaimila, Y.; Maleta, K.; Zhong, C.; et al. The effect of legume supplementation on the gut microbiota in rural Malawian infants aged 6 to 12 months. Am. J. Clin. Nutr. 2020, 111, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Lungu, E.; Auger, J.; Piano, A.; Dahl, W.J. Higher fiber complementary food alters fecal microbiota composition and normalizes stool form in malawian children: A randomized trial. Afr. J. Food Agric. Nutr. Dev. 2021, 21, 17854–17875. [Google Scholar] [CrossRef]
- Bourke, C.D.; Gough, E.K.; Pimundu, G.; Shonhai, A.; Berejena, C.; Terry, L.; Baumard, L.; Choudhry, N.; Karmali, Y.; Bwakura-Dangarembizi, M.; et al. Cotrimoxazole reduces systemic inflammation in HIV infection by altering the gut microbiome and immune activation. Sci. Transl. Med. 2019, 11, eaav0537. [Google Scholar] [CrossRef] [PubMed]
- Sahly, N.; Moustafa, A.; Zaghloul, M.; Salem, T.Z. Effect of radiotherapy on the gut microbiome in pediatric cancer patients: A pilot study. PeerJ 2019, 7, e7683. [Google Scholar] [CrossRef]
- Kortekangas, E.; Kamng’Ona, A.W.; Fan, Y.; Cheung, Y.B.; Ashorn, U.; Matchado, A.; Poelman, B.; Maleta, K.; Dewey, K.G.; Ashorn, P. Environmental exposures and child and maternal gut microbiota in rural Malawi. Paediatr. Perinat. Epidemiol. 2020, 34, 161–170. [Google Scholar] [CrossRef]
- Hendrixson, D.T.; Naskidashvili, N.; Stephenson, K.B.; Laury, M.L.; Koroma, A.S.; Manary, M.J. An Alternative Oat–Containing, Ready-To-Use, Therapeutic Food Does Not Alter Intestinal Permeability or the 16S Ribosomal RNA Fecal Microbiome Configuration Among Children With Severe Malnutrition in Sierra Leone: A Randomized Controlled Trial. J. Nutr. 2023, 152, 2744–2753. [Google Scholar] [CrossRef]
- Mahdavinia, M.; Rasmussen, H.E.; Botha, M.; Tran, T.D.B.; van den Berg, J.P.; Sodergren, E.; Davis, E.; Engen, K.; Gray, C.; Lunjani, N.; et al. Effects of diet on the childhood gut microbiome and its implications for atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 1636–1637.e5. [Google Scholar] [CrossRef]
- D’souza, A.W.; Moodley-Govender, E.; Berla, B.; Kelkar, T.; Wang, B.; Sun, X.; Daniels, B.; Coutsoudis, A.; Trehan, I.; Dantas, G. Cotrimoxazole Prophylaxis Increases Resistance Gene Prevalence and α-Diversity but Decreases β-Diversity in the Gut Microbiome of Human Immunodeficiency Virus–Exposed, Uninfected Infants. Clin. Infect. Dis. 2020, 71, 2858–2868. [Google Scholar] [CrossRef]
- Almugadam, B.S.; Liu, Y.; Chen, S.-M.; Wang, C.-H.; Shao, C.-Y.; Ren, B.-W.; Tang, L.; Hatziagelaki, E. Alterations of Gut Microbiota in Type 2 Diabetes Individuals and the Confounding Effect of Antidiabetic Agents. J. Diabetes Res. 2020, 2020, 7253978. [Google Scholar] [CrossRef] [PubMed]
- Pickering, H.; Hart, J.D.; Burr, S.; Stabler, R.; Maleta, K.; Kalua, K.; Bailey, R.L.; Holland, M.J. Impact of azithromycin mass drug administration on the antibiotic-resistant gut microbiome in children: A randomized, controlled trial. Gut Pathog. 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Morton, E.R.; Lynch, J.; Froment, A.; Lafosse, S.; Heyer, E.; Przeworski, M.; Blekhman, R.; Ségurel, L. Variation in Rural African Gut Microbiota Is Strongly Correlated with Colonization by Entamoeba and Subsistence. PLoS Genet. 2015, 11, e1005658. [Google Scholar] [CrossRef] [PubMed]
- Alou, M.T.; Million, M.; Traore, S.I.; Mouelhi, D.; Khelaifia, S.; Bachar, D.; Caputo, A.; Delerce, J.; Brah, S.; Alhousseini, D.; et al. Gut Bacteria Missing in Severe Acute Malnutrition, Can We Identify Potential Probiotics by Culturomics? Front. Microbiol. 2017, 8, 899. [Google Scholar] [CrossRef]
- Kristensen, K.H.S.; Wiese, M.; Rytter, M.J.H.; Özçam, M.; Hansen, L.H.; Namusoke, H.; Friis, H.; Nielsen, D.S. Gut Microbiota in Children Hospitalized with Oedematous and Non-Oedematous Severe Acute Malnutrition in Uganda. PLoS Negl. Trop. Dis. 2016, 10, e0004369. [Google Scholar] [CrossRef]
- Davis, J.C.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and Morbidity of Gambian Infants are Influenced by Maternal Milk Oligosaccharides and Infant Gut Microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qin, J.; Tan, C.; Ning, K. The seasonal changes of the gut microbiome of the population living in traditional lifestyles are represented by characteristic species-level and functional-level SNP enrichment patterns. BMC Genom. 2021, 22, 83. [Google Scholar] [CrossRef]
- González, R.; Mandomando, I.; Fumadó, V.; Sacoor, C.; Macete, E.; Alonso, P.L.; Menendez, C. Breast Milk and Gut Microbiota in African Mothers and Infants from an Area of High HIV Prevalence. PLoS ONE 2013, 8, e80299. [Google Scholar] [CrossRef]
- Allali, I.; Boukhatem, N.; Bouguenouch, L.; Hardi, H.; Boudouaya, H.A.; Cadenas, M.B.; Ouldim, K.; Amzazi, S.; Azcarate-Peril, M.A.; Ghazal, H. Gut microbiome of Moroccan colorectal cancer patients. Med. Microbiol. Immunol. 2018, 207, 211–225. [Google Scholar] [CrossRef]
- Karampatsas, K.M.; Faal, A.M.; Jaiteh, M.M.; Garcia-Perez, I.; Aller, S.; Shaw, A.G.; Kopytek, A.M.; Witney, A.A.; Le Doare, K. Gastrointestinal, vaginal, nasopharyngeal, and breast milk microbiota profiles and breast milk metabolomic changes in Gambian infants over the first two months of lactation: A prospective cohort study. Medicine 2022, 101, e31419. [Google Scholar] [CrossRef]
- Lokmer, A.; Aflalo, S.; Amougou, N.; Lafosse, S.; Froment, A.; Tabe, F.E.; Poyet, M.; Groussin, M.; Said-Mohamed, R.; Ségurel, L. Response of the human gut and saliva microbiome to urbanization in Cameroon. Sci. Rep. 2020, 10, 2856. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Petrzelkova, K.J.; Burns, M.B.; Yeoman, C.J.; Amato, K.R.; Vlckova, K.; Modry, D.; Todd, A.; Robinson, C.A.J.; Remis, M.J.; et al. Gut Microbiome of Coexisting BaAka Pygmies and Bantu Reflects Gradients of Traditional Subsistence Patterns. Cell Rep. 2016, 14, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.; Delgadinho, M.; Ginete, C.; Mendes, J.; Vasconcelos, J.; Santos, B. P101: Gut Microbiota Impact on Angolan Children with Sickle Cell Disease. HemaSphere 2022, 6, 18. [Google Scholar] [CrossRef]
- Ordiz, M.I.; May, T.D.; Mihindukulasuriya, K.; Martin, J.; Crowley, J.; Tarr, P.I.; Ryan, K.; Mortimer, E.; Gopalsamy, G.; Maleta, K.; et al. The effect of dietary resistant starch type 2 on the microbiota and markers of gut inflammation in rural Malawi children. Microbiome 2015, 3, 37. [Google Scholar] [CrossRef]
- Shenoy, M.K.; Fadrosh, D.W.; Lin, D.L.; Worodria, W.; Byanyima, P.; Musisi, E.; Kaswabuli, S.; Zawedde, J.; Sanyu, I.; Chang, E.; et al. Gut microbiota in HIV–pneumonia patients is related to peripheral CD4 counts, lung microbiota, and in vitro macrophage dysfunction. Microbiome 2019, 7, 37. [Google Scholar] [CrossRef]
- Katsidzira, L.; Ocvirk, S.; Wilson, A.; Li, J.; Mahachi, C.B.; Soni, D.; DeLany, J.; Nicholson, J.K.; Zoetendal, E.G.; O’keefe, S.J.D. Differences in Fecal Gut Microbiota, Short-Chain Fatty Acids and Bile Acids Link Colorectal Cancer Risk to Dietary Changes Associated with Urbanization Among Zimbabweans. Nutr. Cancer 2019, 71, 1313–1324. [Google Scholar] [CrossRef]
- Even, G.; Lokmer, A.; Rodrigues, J.; Audebert, C.; Viscogliosi, E.; Ségurel, L.; Chabé, M. Changes in the Human Gut Microbiota Associated With Colonization by Blastocystis sp. and Entamoeba spp. in Non-Industrialized Populations. Front. Cell. Infect. Microbiol. 2021, 11, 533528. [Google Scholar] [CrossRef]
- Doan, T.; Hinterwirth, A.; Worden, L.; Arzika, A.M.; Maliki, R.; Abdou, A.; Kane, S.; Zhong, L.; Cummings, S.L.; Sakar, S.; et al. Gut microbiome alteration in MORDOR I: A community-randomized trial of mass azithromycin distribution. Nat. Med. 2019, 25, 1370–1376. [Google Scholar] [CrossRef]
- Pham, T.-P.-T.; Alou, M.T.; Bachar, D.; Levasseur, A.; Brah, S.; Alhousseini, D.; Sokhna, C.; Diallo, A.; Wieringa, F.; Million, M.; et al. Gut Microbiota Alteration is Characterized by a Proteobacteria and Fusobacteria Bloom in Kwashiorkor and a Bacteroidetes Paucity in Marasmus. Sci. Rep. 2019, 9, 9084. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, S.; Diarra, B.; Diabate, S.; Sarro, Y.D.S.; Kone, A.; Kone, B.; Tolofoudie, M.; Baya, B.; Diakite, M.T.; Kodio, O.; et al. Patients infected with Mycobacterium africanum versus Mycobacterium tuberculosis possess distinct intestinal microbiota. PLoS Negl. Trop. Dis. 2020, 14, e0008230. [Google Scholar] [CrossRef]
- Samb-Ba, B.; Mazenot, C.; Gassama-Sow, A.; Dubourg, G.; Richet, H.; Hugon, P.; Lagier, J.-C.; Raoult, D.; Fenollar, F. MALDI-TOF Identification of the Human Gut Microbiome in People with and without Diarrhea in Senegal. PLoS ONE 2014, 9, e87419. [Google Scholar] [CrossRef] [PubMed]
- Byrd, D.A.; Vogtmann, E.; Wu, Z.; Han, Y.; Wan, Y.; Clegg-Lamptey, J.; Yarney, J.; Wiafe-Addai, B.; Wiafe, S.; Awuah, B.; et al. Associations of fecal microbial profiles with breast cancer and nonmalignant breast disease in the Ghana Breast Health Study. Int. J. Cancer 2021, 148, 2712–2723. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Byrd, D.A.; Wan, Y.; Ansong, D.; Clegg-Lamptey, J.; Wiafe-Addai, B.; Edusei, L.; Adjei, E.; Titiloye, N.; Dedey, F.; et al. The oral microbiome and breast cancer and nonmalignant breast disease, and its relationship with the fecal microbiome in the Ghana Breast Health Study. Int. J. Cancer 2022, 151, 1248–1260. [Google Scholar] [CrossRef]
- Wood, L.F.; Brown, B.P.; Lennard, K.; Karaoz, U.; Havyarimana, E.; Passmore, J.-A.S.; Hesseling, A.C.; Edlefsen, P.T.; Kuhn, L.; Mulder, N.; et al. Feeding-Related Gut Microbial Composition Associates With Peripheral T-Cell Activation and Mucosal Gene Expression in African Infants. Clin. Infect. Dis. 2018, 67, 1237–1246. [Google Scholar] [CrossRef]
- Ndungo, E.; Holm, J.B.; Gama, S.; Buchwald, A.G.; Tennant, S.M.; Laufer, M.K.; Pasetti, M.F.; Rasko, D.A. Dynamics of the Gut Microbiome in Shigella -Infected Children during the First Two Years of Life. Msystems 2022, 7, e0044222. [Google Scholar] [CrossRef]
- Toe, L.C.; Kerckhof, F.-M.; De Bodt, J.; Morel, F.B.; Ouedraogo, J.-B.; Kolsteren, P.; Van de Wiele, T. A prebiotic-enhanced lipid-based nutrient supplement (LNSp) increases Bifidobacterium relative abundance and enhances short-chain fatty acid production in simulated colonic microbiota from undernourished infants. FEMS Microbiol. Ecol. 2020, 96, fiaa105. [Google Scholar] [CrossRef]
- Claassen-Weitz, S.; Gardner-Lubbe, S.; Nicol, P.; Botha, G.; Mounaud, S.; Shankar, J.; Nierman, W.C.; Mulder, N.; Budree, S.; Zar, H.J.; et al. HIV-exposure, early life feeding practices and delivery mode impacts on faecal bacterial profiles in a South African birth cohort. Sci. Rep. 2018, 8, 5078. [Google Scholar] [CrossRef]
- Robertson, R.C.; Church, J.A.; Edens, T.J.; Mutasa, K.; Geum, H.M.; Baharmand, I.; Gill, S.K.; Ntozini, R.; Chasekwa, B.; Carr, L.; et al. The fecal microbiome and rotavirus vaccine immunogenicity in rural Zimbabwean infants. Vaccine 2021, 39, 5391–5400. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, L.V.; Moses, A.; Adriko, M.; Faust, C.L.; Tukahebwa, E.M.; Hall, L.J.; Ranford-Cartwright, L.C.; Lamberton, P.H. The impact of storage conditions on human stool 16S rRNA microbiome composition and diversity. PeerJ 2019, 7, e8133. [Google Scholar] [CrossRef]
- Tamburini, F.B.; Maghini, D.; Oduaran, O.H.; Brewster, R.; Hulley, M.R.; Sahibdeen, V.; Norris, S.A.; Tollman, S.; Kahn, K.; Wagner, R.G.; et al. Short- and long-read metagenomics of urban and rural South African gut microbiomes reveal a transitional composition and undescribed taxa. Nat. Commun. 2022, 13, 926. [Google Scholar] [CrossRef]
- Kortekangas, E.; Fan, Y.-M.; Chaima, D.; Lehto, K.-M.; Malamba-Banda, C.; Matchado, A.; Chingwanda, C.; Liu, Z.; Ashorn, U.; Cheung, Y.B.; et al. Associations between Gut Microbiota and Intestinal Inflammation, Permeability and Damage in Young Malawian Children. J. Trop. Pediatr. 2022, 68, fmac012. [Google Scholar] [CrossRef]
- Fassatoui, M.; Lopez-Siles, M.; Díaz-Rizzolo, D.A.; Jmel, H.; Naouali, C.; Abdessalem, G.; Chikhaoui, A.; Nadal, B.; Jamoussi, H.; Abid, A.; et al. Gut microbiota imbalances in Tunisian participants with type 1 and type 2 diabetes mellitus. Biosci. Rep. 2019, 39, bsr20182348. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, C.E.; Sié, A.; Coulibaly, B.; Ouermi, L.; Dah, C.; Tapsoba, C.; Bärnighausen, T.; Ray, K.J.; Zhong, L.; Cummings, S.; et al. Effect of Commonly Used Pediatric Antibiotics on Gut Microbial Diversity in Preschool Children in Burkina Faso: A Randomized Clinical Trial. Open Forum Infect. Dis. 2018, 5, ofy289. [Google Scholar] [CrossRef] [PubMed]
- Goosen, C.; Proost, S.; Baumgartner, J.; Mallick, K.; Tito, R.Y.; Barnabas, S.L.; Cotton, M.F.; Zimmermann, M.B.; Raes, J.; Blaauw, R. Associations of HIV and iron status with gut microbiota composition, gut inflammation and gut integrity in South African school-age children: A two-way factorial case–control study. J. Hum. Nutr. Diet. 2023, 36, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Guengant, J.-P.; May, J.F. African Demography. Glob. J. Emerg. Mark. Econ. 2013, 5, 215–267. [Google Scholar] [CrossRef]
- Laiteerapong, N.; Huang, E.S. Diabetes in Older Adults. Diabetes Am. 2018, 3, 22–26. [Google Scholar]
- Boot, E.; Ekker, M.S.; Putaala, J.; Kittner, S.; De Leeuw, F.-E.; Tuladhar, A.M. Ischaemic stroke in young adults: A global perspective. J. Neurol. Neurosurg. Psychiatry 2020, 91, 411–417. [Google Scholar] [CrossRef]
- Dwiyanto, J.; Hussain, M.H.; Reidpath, D.; Ong, K.S.; Qasim, A.; Lee, S.W.H.; Lee, S.M.; Foo, S.C.; Chong, C.W.; Rahman, S. Ethnicity influences the gut microbiota of individuals sharing a geographical location: A cross-sectional study from a middle-income country. Sci. Rep. 2021, 11, 2618. [Google Scholar] [CrossRef]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocr. 2021, 61, 100912. [Google Scholar] [CrossRef]
- Aljassim, N.; Ostini, R. Health literacy in rural and urban populations: A systematic review. Patient Educ. Couns. 2020, 103, 2142–2154. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B.; Galla, S.; Chakraborty, S.; Cheng, X.; Yeo, J.; Mell, B.; Zhang, H.; et al. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Hajjo, R.; Sabbah, D.A.; Al Bawab, A.Q. Unlocking the Potential of the Human Microbiome for Identifying Disease Diagnostic Biomarkers. Diagnostics 2022, 12, 1742. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Brewster, R.; Tamburini, F.B.; Asiimwe, E.; Oduaran, O.; Hazelhurst, S.; Bhatt, A.S. Surveying Gut Microbiome Research in Africans: Toward Improved Diversity and Representation. Trends Microbiol. 2019, 27, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Pulford, J.; Crossman, S.; Begg, S.; Quach, J.A.; Abomo, P.; El Hajj, T.; Bates, I. Strengthening research management and support services in sub-Saharan African universities and research institutions. AAS Open Res. 2020, 3, 31. [Google Scholar] [CrossRef]
- Aguiar-Pulido, V.; Huang, W.; Suarez-Ulloa, V.; Cickovski, T.; Mathee, K.; Narasimhan, G. Metagenomics, Metatranscriptomics, and Metabolomics Approaches for Microbiome Analysis. Evol. Bioinform. 2016, 12s1, EBO-S36436. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front. Cell. Infect. Microbiol. 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhu, X.; Huang, X.; Murff, H.J.; Ness, R.M.; Seidner, D.L.; Sorgen, A.A.; Blakley, I.C.; Yu, C.; Dai, Q.; et al. On the robustness of inference of association with the gut microbiota in stool, rectal swab and mucosal tissue samples. Sci. Rep. 2021, 11, 14828. [Google Scholar] [CrossRef]
- Claesson, M.J.; Clooney, A.G.; O’Toole, P.W. A clinician’s guide to microbiome analysis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Li, Z.; Wang, Y.; Zhang, Y.; Zhao, M.; Luo, J.; Du, S.; Deng, Z.; Chen, J.; Wang, Y.; et al. Gut microbiome interventions in human health and diseases. Med. Res. Rev. 2019, 39, 2286–2313. [Google Scholar] [CrossRef]
- Li, D.; Gao, C.; Zhang, F.; Yang, R.; Lan, C.; Ma, Y.; Wang, J. Seven facts and five initiatives for gut microbiome research. Protein Cell 2020, 11, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P. Understanding Research Study Designs. Indian J. Crit. Care Med. 2019, 23, S305–S307. [Google Scholar] [CrossRef] [PubMed]
- Lau, F.; Holbrook, A. Chapter 10 Methods for Comparative Studies. In Handbook of eHealth Evaluation: An Evidence-based Approach; Lau, F., Kuziemsky, C., Eds.; University of Victoria: Victoria, BC, USA, 2017; pp. 181–197. Available online: https://www.ncbi.nlm.nih.gov/books/NBK481584/ (accessed on 10 July 2023).
- Musa, A.; PM, E. Statistical Issues in Research I: Overview of Epidemiological Study Design. Niger. J. Clin. Biomed. Res. 2015, 7, 27–33. [Google Scholar]


| Population Group | Percentage (%) |
|---|---|
| Adults | 27.9 |
| Children | 37.1 |
| Infants | 10.4 |
| Not indicated | 2.0 |
| Other | 22.7 |
| Ethnicity/Tribe | Percentage (%) |
| Bambara | 0.7 |
| Black | 2.6 |
| Hadza | 0.7 |
| Mixed ancestry | 1.3 |
| Not indicated | 90.3 |
| Other | 4.6 |
| Gender Ratio (Female/Male) Groups | n |
| Ratios between 0.31–0.99 | 35 |
| Ratios equal to 1 | 9 |
| Ratios between 1.05–5.83 | 52 |
| Females only | 7 |
| Males only | 3 |
| Not indicated | 48 |
| Age range: | 0 months–84 years |
| Study Title | Author’s Name and Year of Publication | General/Key Findings of the Studies | Type of Study |
|---|---|---|---|
| Provision of lipid-based nutrient supplements to mothers during pregnancy and 6 months postpartum and to their infants from 6 to 18 months promotes infant gut microbiota diversity at 18 months of age but not microbiota maturation in a rural Malawian setting: secondary outcomes of a randomised trial | Kamng’Ona et al., 2020 [27] | The findings “did not support the hypothesis that LNS supplementation will promote gut microbiota maturity in Malawian infants”. | Clinical trial |
| Infant gut microbiota characteristics generally do not modify effects of lipid-based nutrient supplementation on growth or inflammation: Secondary analysis of a randomised controlled trial in Malawi | Hughes et al., 2020 [28] | “No conclusive evidence of effect modification was observed in this analysis, the relationships observed before correction for multiple hypothesis testing may be worth additional investigation”. | Clinical trial |
| The gut microbiome and early-life growth in a population with high prevalence of stunting | Robertson et al., 2023 [29] | “HIV exposure shapes maturation of the infant gut microbiota, and the functional composition of the infant gut microbiome is moderately predictive of infant growth in a population at high risk of stunting”. | Sub-study of a clinical trial |
| The impact of anthelmintic treatment on human gut microbiota based on cross-sectional and pre- and post-deworming comparisons in western Kenya | Easton et al., 2019 [30] | The authors were able to “identify changes in the microbiota associated with clearance of N. americanus infection, which were not seen posttreatment in individuals who were uninfected pretreatment”. | Longitudinal and cross-sectional study |
| Alteration of the gut fecal microbiome in children living with HIV on antiretroviral therapy in Yaounde, Cameroon | Abange et al., 2021 [31] | “HIV-infected, antiretroviral therapy (ART) -treated children were characterized by decreased alpha diversity and shifts in community structure. ART regimen was associated with varying degrees of dysbiosis with ritonavir-boosted protease inhibitor (PI/r) based regimens”. | Case-control |
| Evolution in fecal bacterial/viral composition in infants of two central African countries (Gabon and Republic of the Congo) during their first month of life | Brazier et al., 2017 [32] | “The bacterial microbiota communities displayed a similar diversification and expansion in newborns within and between countries during the first four weeks of life”. | Longitudinal study |
| Anaerobe-enriched gut microbiota predicts pro-inflammatory responses in pulmonary tuberculosis | Naidoo et al., 2021 [33] | “Specific anaerobes in cases’ stool predict upregulation of pro-inflammatory immunological pathways, supporting the gut microbiota’s role in TB”. | Cross-sectional study |
| A comparative study of the gut microbiome in Egyptian patients with type I and type II diabetes | Radwan et al., 2020 [34] | The results highlighted “a significant increase in abundance of Gram negative, potentially opportunistic pathogenic taxa (Pseudomonas, Prevotella) in all diabetic groups. The gram-positive Gemella, also had a significant increase in abundance in all diabetic groups. Turicibacter, Terrisporobacter and Clostridium were found to be more abundant in the control group than in type I diabetes (TID)”. | Comparative study |
| Investigations on the interplays between Schistosoma mansoni, praziquantel and the gut microbiome | Schneeberger et al., 2018 [35] | “Overall taxonomic profiling and diversity indicators were found to be close to a “healthy” gut structure in all children. Slight overall compositional changes were observed between S. mansoni-infected and non-infected children. Praziquantel treatment was not linked to a major shift in the gut taxonomic profiles”. | Observational study |
| Maternal human milk oligosaccharide profile modulates the impact of an intervention with iron and Galacto-oligosaccharides in Kenyan infants | Paganini et al., 2019 [36] | “Infants of non-secretor mothers may be more vulnerable to the adverse effect of fortificant iron on the gut microbiota, resulting in decreased abundances of Bifidobacterium and increased abundances of enteropathogens, but also benefit more from the co-provision of Galacto-oligosaccharides in terms of beneficial effects on the gut microbiota and improving iron status”. | Sub-study of a clinical trial |
| Iron-containing micronutrient powders modify the effect of oral antibiotics on the infant gut microbiome and increase post-antibiotic diarrhoea risk: a controlled study in Kenya | Paganini et al., 2019 [37] | “In African infants, iron fortification modifies the response to broad-spectrum antibiotics: iron may reduce their efficacy against potential enteropathogens, particularly pathogenic E. coli, and may increase risk for diarrhoea”. | Clinical trial |
| Design and application of a novel two-amplicon approach for defining eukaryotic microbiota | Popovic et al., 2018 [38] | “The combined sequence information allowed the authors to uncover protozoa, microsporidia, helminths, and fungi, and putative relationships between the eukaryote Blastocystis and bacteria”. | Observational study |
| Brief overview of dietary intake, some types of gut microbiota, metabolic markers and research opportunities in sample of Egyptian women | Hassan et al., 2022 [39] | “Dietary factors, dysbiosis, and the metabolic product short chain fatty acids have been implicated in causing metabolic defects”. | Cross-sectional study |
| Microbiota at multiple body sites during pregnancy in a rural Tanzanian population and effects of moringa-supplemented probiotic yogurt | Bisanz et al., 2015 [40] | “Microbiota analysis by weighted UniFrac distances comparing samples to enrolment showed that moringa-supplemented probiotic yogurt does not affect the microbiota structure and that the faecal microbiotas remained stable over pregnancy”. | Clinical trial |
| Randomised open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children | Bisanz et al., 2014 [41] | “Administration of the probiotic was not observed to have an effect on the gut bacterial community composition. Elevated blood lead was associated with increases in Succinivibrionaceae and Gammaproteobacteria relative abundance levels in stool”. | Pilot clinical trial |
| The microbiome in posttraumatic stress disorder and trauma-exposed controls: An exploratory study | Hemmings et al., 2017 [42] | “Measures of overall microbial diversity were similar among individuals with post-traumatic stress disorder (PTSD) and Trauma-exposed (TE) controls; however, decreased total abundance of Actinobacteria, Lentisphaerae, and Verrucomicrobia was associated with PTSD status”. | Cross-sectional |
| Early benefits of a starter formula enriched in prebiotics and probiotics on the gut microbiota of healthy infants born to HIV+ mothers: A randomised double-blind controlled trial | Cooper et al., 2016 [43] | “The bovine milk-derived oligosaccharides (BMOS) prebiotic in combination with B. lactis probiotic stimulated the growth of bifidobacteria in infants born by cesarean delivery at early life (within the first 10 days) when the gut colonization with bifidobacteria is delayed compared to vaginally born infants”. | Clinical trial |
| The gut microbiome in konzo | Bramble et al., 2021 [44] | “Gut microbiome structure is highly variable depending on region of sampling, but most interestingly, the authors identify unique enrichments of bacterial species and functional pathways that potentially modulate the susceptibility of konzo in prone regions of the Congo”. | Observational study |
| Study of gut microbiome in Egyptian patients with autoimmune thyroid diseases | El-Zawawy et al., 2021 [45] | “Egyptian patients with autoimmune thyroid disorders (ATD) (Graves’ disease (GD) and Hashimoto’s thyroiditis (HT)) show dysbiosis of the gut microbiome”. | Observational study |
| Gut microbiome alterations in patients with stage 4 hepatitis C | Aly et al., 2016 [46] | “The alpha diversity of the healthy persons’ gut microbiomes was higher than those of the hepatitis C virus (HCV) patients. Patients with HCV had a few significant fecal microbiome changes”. | Case-control study |
| Restitution of gut microbiota in Ugandan children administered with probiotics (Lactobacillusrhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12) during treatment for severe acute malnutrition | Castro-Mejía et al., 2020 [47] | “Children with severe acute malnutrition (SAM) have significantly reduced number of observed species and major compositional differences (β-diversity) compared to healthy subjects. Moreover, gut microbiota (GM) diversity and composition change over the course of rehabilitation from SAM and approach the GM of apparently healthy subjects as treatment progresses”. | Sub-study of a clinical trial |
| Characterization and profiling of gut bacterial microbiome and pathobionts among HIV-negative and HIV-infected individuals in Cameroon | Eyongabane Ako et al., 2022 [48] | “Gut pathobionts are circulating among HIV-infected and HIV-negative individuals in Cameroon. Unique gut microbiome OTU (operational taxonomic unit) sequences are significantly high among HIV-infected. Emerging strains of new microorganisms are on the rise”. | Case-control and comparative study |
| The role of probiotics in children with autism spectrum disorder: A prospective, open-label study | Shaaban et al., 2018 [49] | “Probiotics have beneficial effects on both behavioral and gastrointestinal (GI) manifestations of autism spectrum disorder (ASD)”. | Clinical trial |
| Metagenomic analysis of gut microbiota of patients with colorectal cancer at the Federal Medical Centre (FMC), Abeokuta, Ogun State, Nigeria | Sulaimon et al., 2023 [50] | “The taxonomic composition and functional genes of intestinal bacteria were significantly altered in colorectal cancer (CRC). Also, E. coli and P. aeruginosa are at least partially involved in the pathogenesis of CRC”. | Observational study |
| Alteration of gut microbiota in Alzheimer’s disease and their relation to the cognitive impairment | Khedr et al., 2022 [51] | “The current work highlighted a significant relationship between Alzheimer’s disease (AD) and gut microbiota dysbiosis”. | Case-control study |
| Study of the gut microbiome profile in children with autism spectrum disorder: A single tertiary hospital experience | Ahmed et al., 2020 [52] | “The current study showed evidence of changes in the gut microbiome of autism spectrum disorder (ASD) children compared to the unrelated controls. However, the microbiome profile of siblings was more like that of autistic children than that of unrelated controls”. | Observational study |
| Molecular characterization of the gut microbiome in Egyptian patients with remitting relapsing multiple sclerosis | Mekky et al., 2022 [53] | “Egyptian patients with multiple sclerosis exhibit microbial dysbiosis. Multiple sclerosis patients have significantly higher B. fragilis. The level of Prevotella, Lactobacilli and C. perfringes appear much less in MS patients than the control”. | Observational study |
| Dysbiosis signatures of fecal microbiota in South African infants with respiratory, gastrointestinal, and other diseases | Krishnamoorthy et al., 2020 [54] | “The authors showed potential links between the fecal microbiota and clinical parameters, disease-based signature microbiota, and the marker pathogens”. | Case-control study |
| The effects of iron fortification on the gut microbiota in African children: a randomised controlled trial in Cote d’Ivoire | Zimmermann et al., 2010 [55] | “Anaemic African children carry an unfavourable ratio of fecal enterobacteria to bifidobacteria and lactobacilli, which is increased by iron fortification. Thus, iron fortification in this population produces a potentially more pathogenic gut microbiota profile, and this profile is associated with increased gut inflammation”. | Clinical trial |
| Lipid-based nutrient supplements do not affect gut Bifidobacterium microbiota in Malawian infants: A randomised trial | Aakko et al., 2017 [56] | “The dietary supplementation did not have an effect on the Bifidobacterium and S. aureus microbiota composition of the study infants. The fecal bifidobacterial diversity of the infants, however, changed toward a more adult-like microbiota profile within the observed time”. | Clinical trial |
| Associations between HIV status and the gut microbiota in South African children with low iron stores | Goosen et al., 2021 [57] | “Prevotella-enrichment, evident in both groups, was likely influenced by the plant-based diet. The significantly lower relative abundance of beneficial Bifidobacterium among the HIV+ children may be cause for concern as reductions in Bifidobacterium following oral iron supplementation have been reported”. | Comparative cross-sectional study |
| Gut microbiome 16S rRNA gene amplicon taxonomic profiling of hospitalized Moroccan COVID-19 patients | Sehli et al., 2022 [58] | “The 16S rRNA gene meta-taxonomic profiling method revealed differences in microbiome composition and richness changes between hospitalized/treated COVID-19 patients and healthy controls”. | Comparative study |
| Metabolic derangements identified through untargeted metabolomics in a cross-sectional study of Nigerian children with severe acute malnutrition | McMillan et al., 2017 [59] | “The plasma metabolome discriminated children with SAM from controls, while no significant differences were observed in the microbial or small molecule composition of stool”. | Cross-sectional study |
| Parasites and diet as main drivers of the Malagasy gut microbiome richness and function | Mondot et al., 2021 [60] | “High protozoan carriage was associated with higher diversity, richness, and microbial functionalities. Asymptomatic protozoan carriage and dietary habits are the external factors with the deepest impact on gut microbiome”. | Observational study |
| Metagenomic profiling of gut microbiota of diarrhoeic children in Southwest Nigeria | Ugboko et al., 2020 [61] | In diarrheal samples, Firmicutes, Proteobacteria, Actinobacteria, Bacteroidetes, and Fusobacteria were prominent, except Verrucomicrobia. Proteobacteria were notably reduced in controls, with heightened species richness (Escherichia coli, Shigella, etc.) in the diarrhoeic samples, and increased Bifidobacterium, Faeacalibacterium, etc., in controls. | Observational study |
| Evaluating the gut microbiome in children with stunting: Findings from a South African birth cohort | Budree et al., 2019 [62] | “The findings demonstrate a microbial signature associated with stunting in African children”. | Observational study |
| Microbiota richness and diversity in a cohort of underweight HIV positive children aged 24–72 months in Cape Town, South Africa | Van Niekerk et al., 2019 [63] | “Diminished growth of Clostridium Perfringes and increased growth of Enterobacteria was shown, the cohort had low diversity of microbiota. Firmicutes phyla was reasonably well represented in the cohort”. | Observational study |
| The gut microbiota’s influence in the development of foetal alcohol spectrum disorders | Kitchin et al., 2021 [64] | “There were no significant differences in alpha- or beta-diversity, however Bristol Stool Scale and delivery mode was shown to influence beta diversity. Bifidobacteria and Prevotella were found to be higher in infants diagnosed with foetal alcohol spectrum disorder (FASD)”. | Observational study |
| Gut microbiota related to Giardia duodenalis, Entamoeba spp. and Blastocystis hominis infections in humans from Côte d’Ivoire | Iebba et al., 2016 [65] | “This preliminary investigation demonstrates a differential fecal microbiota structure in subjects infected with G. duodenalis or Entamoeba spp./B. hominis”. | Pre-liminary study |
| Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults | Ismail et al., 2011 [66] | “Obesity in Egyptian children and adults is associated with compositional changes in faecal microbiota with increase in the phyla Firmicutes and Bacteroidetes”. | Observational study |
| Gut microbiome community composition is significantly influenced by shared living- space in rural agriculturalists from Burkina Faso | Jacobson et al., 2019 [67] | “Intra-village gut microbiome variation is driven primarily by sharing or lack thereof of quartiers, providing a link between microbial ecological dynamics and living space”. | Observational study |
| Molecular characterization of gut microbial structure and diversity associated with colorectal cancer patients in Egypt | El-Sokkary, 2022 [68] | “The results demonstrated increased abundance of Fusobacterium or Bifidobacterium, and that they can be considered as a sign for impairment or a diseased condition”. | Observational study |
| A prospective study on child morbidity and gut microbiota in rural Malawi | Kortekangas et al., 2019 [69] | “Specific morbidity symptoms might be associated with changes in the relative abundances of several bacterial taxa and overall microbial community composition. There was no clear consistent pattern in the associations between microbiota and morbidity”. | Prospective study |
| Gut bacterial diversity and growth among preschool children in Burkina Faso | Digitale et al., 2020 [70] | The authors “did not find evidence that gut microbial diversity was associated with growth”. | Sub-study of a clinical trial |
| Longitudinal analysis of infant stool bacteria communities before and after acute febrile malaria and artemether-lumefantrine treatment | Mandal et al., 2019 [71] | “In-depth bioinformatics analysis of stool bacteria has revealed for the first time that human malaria episode/artemether-lumefantrine (AL) treatment have minimal effects on gut microbiota in Kenyan infants”. | Longitudinal study |
| Infant and adult gut microbiome and metabolome in rural Bassa and urban settlers from Nigeria | Ayeni et al., 2018 [72] | “The data highlight specific microbiome traits that are progressively lost with urbanization, such as the dominance of pristine fibre degraders and the low inter-individual variation”. | Cross-sectional study |
| New insights on obesity and diabetes from gut microbiome alterations in Egyptian adults | Salah et al., 2019 [73] | “Both obesity and diabetes greatly affect and are affected by gut microbiota. The relative abundance of Firmicutes and Bacteroidetes phyla in obese and diabetic individuals and the F/B ratio are still the most discriminative factors between the patient cases and healthy individuals”. | Comparative study |
| Composition of gut microbiota of children and adolescents with perinatal Human immunodeficiency virus infection taking antiretroviral therapy in Zimbabwe | Flygel et al., 2020 [74] | “Gut microbiota is altered in HIV-infected children, although diversity improves with increasing duration of ART”. | Cross-sectional study |
| Association of maternal prenatal psychological stressors and distress with maternal and early infant faecal bacterial profile | Naudé et al., 2020 [75] | “Maternal prenatal exposure to intimate partner violence is associated with differences in faecal bacterial profiles of mothers at delivery (Lactobacillaceae and Peptostreptococcaceae) and of their infants at birth (family Enterobacteriaceae) and 4–12 weeks of age (genus Weissella). Higher psychological distress during pregnancy is associated with lower infant faecal bacterial profiles of the family Veillonellaceae at 20–28 weeks”. | Longitudinal study |
| Differences in the faecal microbiome in Schistosoma haematobium Infected children vs. uninfected children | Kay et al., 2015 [76] | “There are significant differences in the gut microbiome structure of infected vs. uninfected children and the differences were refractory to antihelminthic praziquantel (PZQ) treatment”. | Cross-sectional study |
| Urogenital schistosomiasis is associated with signatures of microbiome dysbiosis in Nigerian adolescents | Ajibola et al., 2019 [77] | “The adolescent gut microbiome may be shifted towards a dysbiotic state by infection with S. haematobium”. | Observational study |
| Gut microbiome profiles are associated with type 2 diabetes in urban Africans | Doumatey et al., 2020 [78] | “Non-type 2 diabetes (T2D) adults living in a Nigerian city have a characteristic microbial composition that is mainly composed of Firmicutes (Clostridiales) and Actinobacteria (90%). The GM of cases have a bacterial signature consisting of increased sulfate-reducing spp. Desulfovibrio piger, Prevotella, Peptostreptococcus, and Eubacterium and is characterized by a moderate dysbiosis which features a decrease in Firmicutes”. | Case-control study |
| Analysis of human gut microbiota composition associated to the presence of commensal and pathogen microorganisms in Côte d’Ivoire | Di Cristanziano et al., 2021 [79] | “The co-occurrence of Blastocystis and commensal Entamoeba spp., despite the presence of other enteric pathogens including G. duodenalis, seems to preserve a high diversity, favour different bacterial consortia, and does not compromise the intrinsic ability of intestinal microbiota to restore and/or maintain homeostasis”. | Observational study |
| Effects of iron supplementation on dominant bacterial groups in the gut, faecal SCFA and gut inflammation: A randomised, placebo-controlled intervention trial in South African children | Dostal et al., 2014 [80] | “The present study suggests that in African children with a low enteropathogen burden, iron (Fe) status and dietary Fe supplementation did not significantly affect the dominant bacterial groups in the gut, faecal short chain fatty acid (SCFA) concentration or gut inflammation”. | Clinical trial |
| Rectal microbiota among HIV-uninfected, untreated HIV, and treated HIV-infected in Nigeria | Nowak et al., 2017 [81] | “There were subtle shifts in the rectal microbiota among HIV-positive individuals receiving treatment, including a decrease in diversity of the Bacteroidetes phylum driven primarily by a loss in Prevotella. There was a shift towards more pathogenic bacteria. Untreated HIV infection does not significantly alter the rectal microbiota, whereas prior treatment is associated with a shift toward a more pathogenic pattern of microbiota”. | Cross-sectional study |
| Mycobacterium avium subsp. paratuberculosis and microbiome profile of patients in a referral gastrointestinal diseases centre in the Sudan | Elmagzoub et al., 2022 [82] | “A unique microbiome profile of Mycobacterium avium subsp. paratuberculosis (MAP) -positive patients in comparison to MAP-negative was found”. | Cross-sectional study |
| Absence of Mitsuokella multacida is associated with early onset of colorectal cancer | Elkholy et al., 2020 [83] | “These findings suggest that the oncoprotective effect of Mitsuokella multacida should be further investigated”. | Retrospective convenience sample design |
| Profile of microbiota is associated with early onset of colorectal cancer in Egyptian and Kenyan patients | Arafat, 2020 [84] | “The findings suggest that the oncoprotective effect of Mitsuokella multacida should be further investigated”. | Retrospective convenience sample de-sign |
| Gut microbiota in Malawian infants in a nutritional supplementation trial | Cheung et al., 2016 [85] | “Infants who received iron-containing LNS or corn–soya blend (CSB) for 12 months did not differ from non-recipients in the gut microbiota profiles”. | Sub-study of a clinical trial |
| Altered faecal microbiota composition and structure of Ghanaian children with acute gastroenteritis | Quaye et al., 2023 [86] | “The faecal microbiota of acute gastroenteritis (AGE) cases was dominated by disease-associated bacterial genera. Finally, whole microbial community network characteristics differed between AGE cases and controls”. | Cross-sectional case control study |
| The association of gut microbiota characteristics in Malawian infants with growth and inflammation | Kamng’ona et al., 2019 [87] | “Microbiota diversity and maturity were related to growth in weight from 6 to 12 months, but not to growth in length or head circumference or to growth from 12 to 18 months. Microbiota diversity and maturity may also be linked to inflammation, but findings were inconsistent”. | Longitudinal study |
| Maternal fecal microbiome predicts gestational age, birth weight and neonatal growth in rural Zimbabwe | Gough et al., 2021 [88] | “The pregnancy faecal microbiome, primarily the abundance of resistant-starch degraders, is an important contributor to birth weight and neonatal growth, and to a lesser extent gestational age, in infants of rural Zimbabwean mothers who consume a diet high in maize”. | Sub-study of a clinical trial |
| Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome | Monaco et al., 2016 [89] | “Severe immunodeficiency is likely the mechanism leading to changes in the fecal microbiome, including bacteria and viruses. Immune reconstitution, such as through early ART, may restore the healthy enteric microbiome”. | Observational study |
| Changes in the gut microbiota of Nigerian infants within the first year of life | Oyedemi et al., 2022 [90] | “The observed taxonomic differences in the gut microbiota between pre-weaning and weaning samples in Nigerian infants, as well as butyrate production, were influenced by diet. Introduction of solid foods encouraged an increase in microbial diversity, helpful for a healthy life”. | Longitudinal cohort study |
| Child development, growth and microbiota: follow-up of a randomised education trial in Uganda | Atukunda et al., 2019 [91] | “The maternal education intervention had positive effects on child development and growth at three years but did not alter gut microbiota composition”. | Follow-up study of a clinical trial |
| Early antiretroviral therapy in neonates and maturation of the gut microbiome | Kuhn et al., 2022 [92] | “There are detectable benefits associated with breastfeeding in conjunction with early ART in maintaining a more bifidobacteria-rich microbiota profile in infants with HIV”. | Observational study |
| Dissection of the gut microbiota in mothers and children with chronic Trichuris trichiura infection in Pemba Island, Tanzania | Chen et al., 2021 [93] | “Changes in gut microbial composition and structure occur in T. trichiura-infected individuals compared with uninfected individuals”. | Comparative study |
| Interactions between fecal gut microbiome, enteric pathogens, and energy regulating hormones among acutely malnourished rural Gambian children | Nabwera et al., 2021 [94] | “Marasmus SAM is characterized by the collapse of a complex system with nested interactions and key associations between the gut microbiome, enteric pathogens, and energy regulating hormones”. | Cross-sectional observational sub-study of a quasi-experimental study |
| A signature of Prevotella copri and Faecalibacterium prausnitzii depletion, and a link with bacterial glutamate degradation in the Kenyan colorectal cancer patients | Obuya et al., 2022 [95] | “Microbiome and microbial metabolic profiles of CRC patients are different from those of healthy individuals. CRC microbiome dysbiosis, particularly P. copri and F. prausnitzii depletion and glutamate metabolic alterations, are evident in Kenyan CRC patients”. | Cross-sectional observational study |
| Gut microbiomes from Gambian infants reveal the development of a non-industrialized Prevotella-based trophic network | de Goffau et al., 2022 [96] | “Distinct bacterial trophic network clusters were identified, centred around either P. stercorea or F. prausnitzii and were found to develop steadily with age, whereas P. copri, independently of other species, rapidly became dominant after weaning”. | Sub-study of a clinical trial |
| Rectal microbiota diversity in Kenyan MSM is inversely associated with frequency of receptive anal sex, independent of HIV status | Gebrebrhan et al., 2021 [97] | “In addition to decreased alpha diversity in men with HIV infection, the authors also established a dose-dependent relationship between receptive anal sex with multiple partner types, controlling for HIV, and reduced rectal microbiome alpha diversity”. | Cross-sectional study |
| Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa | Vonaesch et al., 2018 [98] | “Stunting is associated with a microbiome “decompartmentalisation” of the gastrointestinal tract characterized by an increased presence of oropharyngeal bacteria from the stomach to the colon”. | Transversal study |
| Gut microbiome profiling of a rural and urban South African cohort reveals biomarkers of a population in lifestyle transition | Oduaran et al., 2020 [17] | “The overall gut microbiome of the cohorts is reflective of their ongoing epidemiological transition. Geographical location was more important for sample clustering than lean/obese status and observed a relatively higher abundance of the Melainabacteria, Vampirovibrio, a predatory bacterium, in Bushbuckridge”. | Pilot study |
| Stool microbiota composition is associated with the prospective risk of Plasmodium falciparum infection | Yooseph et al., 2015 [99] | “The preliminary finding of an association between gut microbiota composition and P. falciparum infection risk suggests that strategic modulation of gut microbiota composition could decrease P. falciparum infection risk in malaria-endemic areas”. | Prospective cohort study |
| Longitudinal and comparative analysis of gut microbiota of Tunisian newborns according to delivery mode | Hanachi et al., 2022 [100] | “Both elective cesarean section (ECS) and vaginally delivered (VD) showed a profile dominated by Proteobacteria, Actinobacteria, and Firmicutes. However, ECS showed an underrepresentation of Bacteroides and an enrichment of opportunistic pathogenic species of the ESKAPE group, starting from the second week”. | Longitudinal study |
| Microbial gut evaluation in an Angolan paediatric population with sickle cell disease | Delgadinho et al., 2022 [101] | “Children with sickle cell disease (SCD) have a higher number of the phylum Actinobacteria. Clostridium cluster XI bacteria was more prevalent in the SCD children, whereas the siblings had a higher abundance of Blautia, Aestuariispira, Campylobacter, Helicobacter, Polaribacter and Anaerorhabdus”. | Cross-sectional study |
| How hydroxyurea alters the gut microbiome: A longitudinal study involving Angolan children with sickle cell anaemia | Delgadinho et al., 2022 [102] | “Hydroxyurea (HU) can influence the diversity and shape of the gut microbiome. However, it is not yet clear if the higher abundance of beneficial bacteria is a direct or indirect effect of HU treatment, being a consequence rather than a cause”. | Longitudinal study |
| Anaemia and iron status are predictors of gut microbiome composition and metabolites in infants and children in rural Kenya | Paganini et al., 2016 [103] | “In infancy, higher haemoglobin (Hb) and better iron status predict higher amounts of pathogenic E. coli. Among older children, higher Hb and better iron status predict lower amounts of pathogenic E. coli. Anaemic infants and children do not have greater gut inflammation but have lower faecal butyrate concentrations. Thus, relationships between anaemia, iron status and pathogenic gut microbiota differ by age in rural Kenya”. | Cross-sectional study |
| Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants | Jaeggi et al., 2015 [104] | “Provision of iron-containing micronutrient powder (MNPs) to weaning infants adversely affects the gut microbiome, increasing pathogen abundance and causing intestinal inflammation”. | Clinical trial |
| Atopic dermatitis and food sensitization in South African toddlers: Role of fibre and gut microbiota | Mahdavinia et al., 2017 [105] | “This pilot study found no major differences in the composition of the gut microbiota of 12- to 36-month-old children with and without AD”. | Pilot study |
| The gut microbiome but not the resistome is associated with urogenital schistosomiasis in preschool-aged children | Osakunor et al., 2020 [106] | “The authors identified differences in the gut microbiome between schistosome infected and uninfected children, showing largely an increase in abundance of specific bacteria”. | Cross-sectional study |
| The effect of oral iron supplementation on the gut microbiota, gut inflammation, and iron status in iron-depleted South African school-age children with virally suppressed HIV and without HIV | Goosen et al., 2022 [107] | “Oral iron supplementation can significantly improve haemoglobin and iron status without increasing pathogenic gut microbial taxa or gut inflammation in iron-depleted virally suppressed HIV+ and HIV−ve school-age children”. | Before-after intervention study with case–control comparisons |
| Impact of the post-transplant period and lifestyle diseases on human gut microbiota in kidney graft recipients | Souai et al., 2020 [108] | “The study shows specific kidney transplant-related effects of the fecal microbiome on graft stability and patient’s health status when compared to healthy subjects. The overall microbial community structure of the kidney transplanted group was clearly different from control subjects”. | Observational study |
| Mucosa-associated cultivable aerobic gut bacterial microbiota among colorectal cancer patients attending at the referral hospitals of Amhara regional state, Ethiopia | Siraj et al., 2021 [109] | “A relative abundance and distributions of cultivable aerobic bacterial microbiota of malignant tissues were significantly different from its adjacent normal tissue biopsies. Families of Enterobacteriaceae and Enterococcaceae were the most frequently recovered bacterial family from malignant tissues”. | Observational study |
| Iron in micronutrient powder promotes an unfavourable gut microbiota in Kenyan infants | Tang et al., 2017 [110] | “MNP fortification over three months in non- or mildly anaemic Kenyan infants can potentially alter the gut microbiome. Addition of iron to the MNP may adversely affect the colonization of potential beneficial microbes and attenuate the decrease of potential pathogens”. | Clinical trial |
| Impact of a nomadic pastoral lifestyle on the gut microbiome in the Fulani living in Nigeria | Afolayan et al., 2019 [111] | “Large differences in the taxonomic and functional composition in the intestinal microbiota between the Fulani and the urban Jarawa population were observed”. | Comparative study |
| Impact of geographical location on the gut microbiota profile in Egyptian children with type 1 diabetes mellitus: A pilot study | Elsherbiny et al., 2022 [112] | “The diabetic groups irrespective of the geographical location showed significantly lower alpha diversity, mean Firmicutes/Bacteroidetes (F/B) ratio, and reduced proportions of Prevotella and Ruminococcus. There were also significantly enriched representations of Actinobacteria, Bacteroidetes, and Proteobacteria and genera Lactobacilli, Bacteroides, and Faecalibacterium pointing to the greater driving power of the disease”. | Case-control study |
| Gut microbiota in forty cases of Egyptian relapsing remitting multiple sclerosis | Elgendy et al., 2021 [113] | “Changes in gut microbiota are associated with exacerbation of multiple sclerosis (MS) disease”. | Prospective study |
| Vitamin D and phenylbutyrate supplementation does not modulate gut derived immune activation in HIV-1 | Missailidis et al., 2019 [114] | “Nutritional supplementation with vitamin D + phenylbutyrate did not modulate gut-derived inflammatory markers or microbial composition in treatment-naïve HIV-1 individuals with active viral replication”. | Clinical trial |
| Immunoglobulin recognition of fecal bacteria in stunted and non-stunted children: Findings from the Afribiota study | Huus et al., 2020 [115] | “Stunted children have a greater proportion of IgA-recognized fecal bacteria. Moreover, there is identification of two putative pathobionts, Haemophilus and Campylobacter, that are broadly targeted by intestinal IgA”. | Observational study |
| Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls | Malan-Muller et al., 2022 [116] | “Mitsuokella, Odoribacter, Catenibacterium and Olsenella, possess a moderate ability to discriminate between PTSD and TEC participants. The summed relative abundance of these genera was higher in individuals with PTSD compared to TEC and was positively correlated with PTSD severity and childhood trauma severity”. | Case-control study |
| The Effect of ss-glucan prebiotic on kidney function, uremic toxins, and gut microbiome in stage 3 to 5 chronic kidney disease (CKD) pre-dialysis participants: A randomised controlled trial | Ebrahim et al., 2022 [117] | “The supplementation of ß-glucan fibre resulted in favourable change in the composition of the gut microbiome. The authors therefore reject the hypothesis that kidney function would change with the prebiotic and accept the hypothesis that the gut microbiome improved with the prebiotic intervention”. | Clinical trial |
| Modifying gut integrity and microbiome in children with severe acute malnutrition using legume-based feeds (MIMBLE): A pilot trial | Calder et al., 2021 [118] | “Cowpea [Cp]-enriched feeds [F] [CpF]) has a positive effect on the fecal microbiota, particularly between days 1 and 7”. | Pilot study |
| Gut microbiome function and composition in infants from rural Kenya and association with human milk oligosaccharides | Derrien et al., 2023 [119] | “Gut microbiome of partially breastfed Kenyan infants over the age of six months is enriched in bacteria from the Bifidobacterium community, including B. infantis, and the high prevalence of a specific HM group may indicate a specific HMO-gut microbiome association”. | Observational study |
| Multi-nutrient fortified dairy-based drink reduces anaemia without observed adverse effects on gut microbiota in anaemic malnourished Nigerian toddlers: A randomised dose–response study | Owolabi et al., 2021 [120] | “Daily consumption of 200–600 mL of iron-fortified multi-nutrient fortified dairy-based drink reduces anaemia without stimulating potential pathogenic gut bacteria in Nigerian toddlers”. | Clinical trial |
| Antenatal gut microbiome profiles and effect on pregnancy outcome in HIV infected and HIV uninfected women in a resource limited setting | Chandiwana et al., 2023 [121] | “Species richness and taxonomy composition of the gut microbiota is altered in HIV-infected pregnant women, possibly reflecting intestinal dysbiosis. Some of the taxa were also associated with low infant birth weight”. | Cross-sectional sub-study |
| Evolution of the gut microbiome in HIV-exposed uninfected and unexposed infants during the first year of life | Jackson et al., 2022 [122] | “Gut microbiomes of HIV-exposed uninfected (HEU) and HIV-unexposed uninfected (HUU) are heavily influenced by the maternal gut microbiome. The microbiotas of HEU and HUU converged over time, mirroring the decrease in the excess of infectious morbidity and mortality in HEU”. | Longitudinal cohort |
| Breastmilk, stool, and meconium: bacterial communities in South Africa | Wallenborn et al., 2022 [123] | “Despite the importance of breastmilk in seeding the infant gut microbiome, the authors found evidence of distinct bacterial communities between breastmilk and stool samples from South African mother-infant dyads”. | Pilot study |
| Robust variation in infant gut microbiome assembly across a spectrum of lifestyles | Olm et al., 2022 [124] | “Infants from all lifestyles begin life with similar bifidobacteria-dominated gut microbiota compositions, but subtle differences detected in early life compound over time”. | Observational study |
| Intestinal protozoan infections shape fecal bacterial microbiota in children from Guinea-Bissau | von Huth et al., 2021 [125] | “Infections with multiple parasite species induces more pronounced changes in the bacterial composition, and certain parasite species significantly changes the diversity of bacteria”. | Prospective no-intervention two-cohort study |
| Evolution of the gut microbiome following acute HIV-1 infection | Rocafort et al., 2019 [126] | “Recent HIV-1 infection is associated with increased fecal shedding of eukaryotic viruses, transient loss of bacterial taxonomic richness, and long-term reductions in microbial gene richness. An HIV-1-associated microbiome signature only becomes evident in chronically HIV-1-infected subjects”. | Sub-study of a prospective observational cohort study |
| Growth velocity in children with environmental enteric dysfunction is associated with specific bacterial and viral taxa of the gastrointestinal tract in Malawian children | Desai et al., 2020 [127] | “The data of the study demonstrate associations between specific bacterial and viral taxa and growth velocity, though the authors cannot yet prove a causal relationship exists between these measures”. | Longitudinal cohort study |
| Gut microbiota signature of pathogen-dependent dysbiosis in viral gastroenteritis | Mizutani et al., 2021 [128] | “This study revealed specific trends in the gut microbiota signature associated with diarrhoea and that pathogen-dependent dysbiosis occurred in viral gastroenteritis. It also revealed that several bacterial taxa with potential pathogenesis, such as Escherichia-Shigella and Klebsiella, are part of healthy commensal microbiota in Ghanaian individuals”. | Comparative study |
| Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in Kenyan infants | Paganini et al., 2017 [129] | “A MNP containing a 5 mg daily dose of highly bioavailable iron is likely safer in that it induces less adverse changes in the gut microbiome and no increase in faecal calprotectin compared with a 12.5 mg iron dose”. | Clinical trial |
| Blastocystis colonization is associated with increased diversity and altered gut bacterial communities in healthy Malian children | Kodio et al., 2019 [130] | “Blastocystis colonization was significantly associated with a higher diversity and richness of the gut bacterial communities in healthy children. Also, Blastocystis colonization was associated with a higher proportion of “beneficial bacteria” (Firmicutes and Bacteroides) than “probable pathogenic bacteria” (Proteobacteria) in the human gut”. | Cross-sectional study |
| Association between clinical and environmental factors and the gut microbiota profiles in young South African children | Nel Van Zyl et al., 2021 [131] | “Prevotella was the most common genus identified in the participants, and after infancy, the gut bacteria were dominated by Firmicutes and Bacteroidetes. In this setting, children exposed to antibiotics and indoor cooking fires were at the most risk for dysbiosis, showing significant losses in gut bacterial diversity”. | Sub-study of a clinical trial |
| Fecal microbiome composition in healthy adults in Ghana | Parbie et al., 2021 [132] | “The fecal microbiome of the Ghanaian adults was dominated by Firmicutes (Faecalibacterium, Subdoligranulum, and Ruminococcaceae UCG-014), Proteobacteria (Escherichia-Shigella and Klebsiella), and Bacteroidetes (Prevotella 9 and Bacteroides), consistent with previous observations in African cohorts”. | Cross-sectional study |
| Dysbiotic fecal microbiome in HIV-1 infected individuals in Ghana | Parbie et al., 2021 [133] | “Analysis revealed significant difference in fecal microbiome diversity and compositions between HIV-1 infected and uninfected individuals. Particularly, the study revealed the characteristics of dysbiotic fecal microbiome in HIV-1 infected adults in Ghana”. | Cross-sectional case-control study |
| Mass azithromycin distribution and community microbiome: A cluster-randomised trial | Doan et al., 2018 [134] | “Two mass azithromycin administrations, 6 months apart, in preschool children led to long-term alterations of the gut microbiome structure and community diversity. Long-term microbial alterations in the community did not imply disease but were associated with an improvement in childhood mortality”. | Clinical trial |
| Gut microbial diversity in antibiotic-naive children after systemic antibiotic exposure: A randomised controlled trial | Doan et al., 2017 [135] | “The paediatric gut microbiome is sensitive to antibiotic exposure. A single dose of antibiotic can result in a significant reduction in bacterial diversity”. | Clinical trial |
| High-throughput sequencing of pooled samples to determine community-level microbiome diversity | Ray et al., 2019 [136] | “Pooling microbiome samples before DNA amplification to estimate community level diversity is a viable and valuable measure to consider in population-level association research studies”. | Observational study |
| Environmental enteric dysfunction and the fecal microbiota in Malawian children | Ordiz et al., 2017 [137] | “Environmental enteric dysfunction (EED) is not associated with a profound fecal dysbiosis, but six genera were identified as having significantly different abundances in EED.” | Clinical trial |
| The effect of legume supplementation on the gut microbiota in rural Malawian infants aged 6 to 12 months | Ordiz et al., 2020 [138] | “Extensive 16S sequencing of fecal samples from a trial of cowpea supplementation in rural African infants did not reveal a microbiota signature for stunting. The association of the Veillonella genus in the feces with health benefits in several populations warrants further investigation”. | Clinical trial |
| Higher fibre complementary food alters fecal microbiota composition and normalises stool form in Malawian children: A randomised trial | Lungu et al., 2021 [139] | “In young Malawian children, feeding a blend of soybean, soy hulls, and maize reduced diarrhoea-type stools and increased the abundance of Akkermansia muciniphila, a bacterial species involved in maintaining intestinal health”. | Clinical trial |
| Co-trimoxazole reduces systemic inflammation in HIV infection by altering the gut microbiome and immune activation | Bourke et al., 2019 [140] | “Co-trimoxazole reduces systemic and intestinal inflammation both indirectly via antibiotic effects on the microbiome, and directly by blunting immune and epithelial cell activation”. | Experimental and longitudinal study |
| Effect of radiotherapy on the gut microbiome in pediatric cancer patients: A pilot study | Sahly et al., 2019 [141] | “A correlation between microbial composition and response to treatment was reported, in which the responders had generally a lower microbial diversity compared to non-responders. In addition, nucleotide changes and deletions in the tested 16S rRNA sequences post radiotherapy were detected”. | Pilot study |
| Environmental exposures and child and maternal gut microbiota in rural Malawi | Kortekangas et al., 2020 [142] | “In this study in a rural Malawian setting, environmental exposures were associated with several subtle aspects of microbiota composition, but authors did not find consistent associations with microbiota maturity or diversity”. | Sub-study of a clinical trial |
| An alternative oat-containing, ready-to-use, therapeutic food does not alter intestinal permeability or the 16S ribosomal RNA fecal microbiome configuration among children with severe malnutrition in Sierra Leone: A randomised controlled trial | Hendrixson et al., 2023 [143] | “Despite remarkably different compositions of oat ready-to-use therapeutic food (o-RUTF) and standard RUTF (s-RUTF), no differences were identified in lactulose permeability or the fecal 16S rRNA configuration among children with SAM receiving these foods”. | Clinical trial |
| Effects of diet on the childhood gut microbiome and its implications for atopic dermatitis | Mahdavinia et al., 2019 [144] | “The authors have identified 2 species of bacteria that seem to be linked to AD and diet and can explain some of the unsolved chain of events in the pathogenesis of this condition”. | Cross-sectional study |
| Co-trimoxazole prophylaxis increases resistance gene prevalence and α-diversity but decreases β-diversity in the gut microbiome of human immunodeficiency virus–exposed, uninfected infants | D’Souza et al., 2020 [145] | “Co-trimoxazole prophylaxis in HEU infants decreased gut microbiome β-diversity and increased antibiotic resistance gene α-diversity and prevalence”. | Experimental study |
| Alterations of gut microbiota in type 2 diabetes individuals and the confounding effect of antidiabetic agents | Almugadam et al., 2020 [146] | “The study revealed a significantly lowered abundance of Faecalibacterium, Fusobacterium, Dialister, and Elusimicrobium in the nontherapeutic T2DM subgroup. Correlation analysis showed a substantial decline in gut microbiota richness and diversity with the duration of illness. Antidiabetic agents restored to some extent the richness and diversity of gut microbiota and improved the abundance of many beneficial bacteria”. | Case-control study |
| Impact of azithromycin mass drug administration on the antibiotic-resistant gut microbiome in children: A randomised, controlled trial | Pickering et al., 2022 [147] | “This study found significant changes in the antimicrobial resistance profile and gut microbiome after four biannual rounds of azithromycin. Abundance of enteropathogen E. albertii was increased after treatment, as well as several opportunistic Acinetobacter pathogens”. | Clinical trial |
| Variation in rural African gut microbiota is strongly correlated with colonization by Entamoeba and subsistence | Morton et al., 2015 [148] | “This study suggests an important role for eukaryotic gut inhabitants and the potential for feedback between helminths, protozoa, microbes, and the host immune response, one that has been largely overlooked in studies of the microbiome”. | Observational study |
| Gut bacteria missing in severe acute malnutrition, can we identify potential probiotics by culturomics? | Alou et al., 2017 [149] | “The authors found a globally decreased diversity, a decrease in the hitherto unknown diversity, a depletion in oxygen-sensitive prokaryotes including Methanobrevibacter smithii and an enrichment in potentially pathogenic Proteobacteria, Fusobacteria and Streptococcus gallolyticus. A complex of 12 species identified only in healthy children using culturomics and metagenomics were identified as probiotics candidates”. | Case-control |
| Gut microbiota in children hospitalized with oedematous and non-oedematous severe acute malnutrition in Uganda | Kristensen et al., 2016 [150] | “The authors found that non-oedematous SAM children have lower GM diversity compared to oedematous SAM children, but no clear compositional differences were found between the two groups of children”. | Observational study |
| Growth and morbidity of Gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota | Davis et al., 2017 [151] | “While bifidobacteria were the dominant genus in the infant gut overall, Dialister and Prevotella were negatively correlated with morbidity, and Bacteroides was increased in infants with abnormal calprotectin”. | Experimental study |
| The seasonal changes of the gut microbiome of the population living in traditional lifestyles are represented by characteristic species-level and functional-level SNP enrichment patterns | Zhu et al., 2021 [152] | “Eight prevalent species have significant single nucleotide polymorphism (SNP) enrichments with the increasing number of SNP, among which only Eubacterium biforme, Eubacterium hallii and Ruminococcus obeum have relatively high species abundances. Many genes in the microbiomes also presented characteristic SNP distributions between the wet season and the dry season”. | Observational study |
| Breast milk and gut microbiota in African mothers and infants from an area of high HIV prevalence | González et al., 2013 [153] | “Both breast milk and faecal microbiota composition varied with lactation period, which might be related to changes in the type of feeding over time and/or in the milk’s biochemical characteristics”. | Cross-sectional study |
| Gut microbiome of Moroccan colorectal cancer patients | Allali et al., 2018 [154] | “CRC stools were markedly different from controls, showing an overrepresentation of 33 genera”. | Observational study |
| Gastrointestinal, vaginal, nasopharyngeal, and breast milk microbiota profiles and breast milk metabolomic changes in Gambian infants over the first two months of lactation: A prospective cohort study | Karampatsas et al., 2022 [155] | “This study’s results indicate that infant gut microbiota are unique bacterial communities, distinct from maternal gut and breast milk, respectively”. | Prospective cohort study |
| Response of the human gut and saliva microbiome to urbanization in Cameroon | Lokmer et al., 2020 [156] | “Urbanization was associated with minor shifts in diversity of the gut and saliva microbiome, but also with changes in the gut microbiome composition that were reminiscent of those associated with industrialization”. | Observational study |
| Gut microbiome of coexisting BaAka pygmies and Bantu reflects gradients of traditional subsistence patterns | Gomez et al., 2016 [157] | “Although the microbiome of both groups is compositionally similar, hunter-gatherers harbor increased abundance of Prevotellaceae, Treponema, and Clostridiaceae, while the Bantu gut microbiome is dominated by Firmicutes”. | Observational study |
| Gut microbiota impact on Angolan children with sickle cell disease | Brito et al., 2022 [158] | “The SCD and control samples exhibited some notable differences in microbiota relative abundance, at different levels of classification”. | Observation-al study |
| The effect of dietary resistant starch type 2 on the microbiota and markers of gut inflammation in rural Malawi children | Ordiz et al., 2015 [159] | “Resistant starch (RS) does not confer physiologically meaningful changes on gut biology, or gut microbial content, after short course treatment. The study data do not preclude the potential use of other prebiotics but provide no data in support of using RS to improve gut health in the studied sub-Saharan childhood population”. | Pilot study |
| Gut microbiota in HIV–pneumonia patients is related to peripheral CD4 counts, lung microbiota, and in vitro macrophage dysfunction | Shenoy et al., 2019 [160] | “This study is the first to reveal a relationship between gut microbiota composition and CD4 status. Moreover, it provides the first evidence that the products of the gut microbiome of HIV-infected patients with low CD4 counts alter monocyte effector phenotypes, reducing their capacity for repair and promoting a program of pro-inflammatory activity”. | Cross-sectional study |
| Differences in fecal gut microbiota, short-chain fatty acids and bile acids link colorectal cancer risk to dietary changes associated with urbanization among Zimbabweans | Katsidzira et al., 2019 [161] | “The gut microbiota composition and activity among rural and urban Zimbabweans retain significant homogeneity (possibly due to retention of dietary fibre), but urban residents have subtle changes, which may indicate a higher CRC risk”. | Exploratory study |
| Changes in the human gut microbiota associated with colonization by Blastocystis sp. and Entamoeba spp. in non-industrialized populations | Even et al., 2021 [162] | “The authors strongly suggest that Blastocystis and Entamoeba likely act through different mechanisms to interact with the gut bacterial microbiota”. | Observational study |
| Gut microbiome alteration in MORDOR I: A community-randomised trial of mass azithromycin distribution | Doan et al., 2019 [163] | “The same mass drug administration (MDA) that caused a reduction in childhood mortality also reduced certain gut bacteria, including known pathogens. Specifically, azithromycin distribution altered microbial metabolic function and decreased organisms known to cause human disease”. | Clinical trial |
| Gut microbiota alteration is characterized by a Proteobacteria and Fusobacteria bloom in Kwashiorkor and a Bacteroidetes paucity in Marasmus | Pham et al., 2019 [164] | “The kwashiorkor gut microbiota was characterized by an increased proportion of Proteobacteria, but there was a decreased proportion of Bacteroidetes in marasmus. Fusobacterium was more frequently cultured from kwashiorkor. All detected potential pathogenic species were enriched in the kwashiorkor gut microbiota”. | Observational study |
| Patients infected with Mycobacterium africanum versus Mycobacterium tuberculosis possess distinct intestinal microbiota | Namasivayam et al., 2020 [165] | “Mycobacterium africanum (MAF) participants have distinct microbiomes compared with Mycobacterium tuberculosis (MTB) patients, displaying decreased diversity and increases in Enterobacteriaceae with respect to healthy participants not observed in the latter patient group”. | Longitudinal study |
| MALDI-TOF identification of the human gut microbiome in people with and without diarrhoea in Senegal | Samb-Ba et al., 2014 [166] | “In individuals with diarrhoea, major commensal bacterial species such as E. coli were significantly decreased (85% versus 64%), as were several Enterococcus spp. (E. faecium and E. casseliflavus) and anaerobes, such as Bacteroides spp. (B. uniformis and B. vulgatus) and Clostridium spp. (C. bifermentans, C. orbiscindens, C. perfringens, and C. symbosium). Conversely, several Bacillus spp. (B. licheniformis, B. mojavensis, and B. pumilus) were significantly more frequent among patients with diarrhoea”. | Comparative study |
| Associations of fecal microbial profiles with breast cancer and non-malignant breast disease in the Ghana breast health study | Byrd et al., 2021 [167] | “Alpha diversity, overall microbiota composition, and taxa with hypothesized estrogen-conjugation and immune-related functions may be associated with breast diseases”. | Case-control study |
| The oral microbiome and breast cancer and non-malignant breast disease, and its relationship with the fecal microbiome in the Ghana breast health study | Wu et al., 2022 [168] | “Some periodontal pathogens are inversely associated with breast cancer and nonmalignant breast disease. Additionally, the oral and fecal microbiome appear to be more correlated among women with breast cancer or nonmalignant breast disease compared to controls”. | Case-control study |
| Feeding-related gut microbial composition associates with peripheral T-cell activation and mucosal gene expression in African infants | Wood et al., 2018 [169] | “Non-exclusive breastfeeding alters the gut microbiota, increasing T-cell activation and, potentially, mucosal recruitment of HIV target cells”. | Prospective, longitudinal study |
| Dynamics of the gut microbiome in Shigella-infected children during the first two years of life. | Ndungo et al., 2022 [170] | “Shigella infection did not profoundly impact overall species diversity but led to the expansion of species known to improve gastrointestinal health and drive the microbiota back to homeostasis”. | Longitudinal study |
| A prebiotic-enhanced lipid-based nutrient supplement (LNSp) increases Bifidobacterium relative abundance and enhances short-chain fatty acid production in simulated colonic microbiota from undernourished infants | Toe et al., 2020 [171] | “Provision of prebiotic enhanced LNS to undernourished children could be a possible strategy to steer the microbiota toward a more beneficial composition and metabolic activity”. | Observational study |
| HIV-exposure, early life feeding practices and delivery mode impacts on faecal bacterial profiles in a South African birth cohort | Claassen-Weitz et al., 2018 [172] | “Major determinants of infant meconium bacterial profiles were mode of feeding and maternal body mass index (BMI). HIV-exposure, on the other hand, was an important contributor to the composition of infant faecal bacterial profiles at 4–12 weeks of life, with HIV-exposed infants having higher bacterial diversity and reduced proportions of bifidobacteria”. | Observational pilot study |
| The fecal microbiome and rotavirus vaccine immunogenicity in rural Zimbabwean infants | Robertson et al., 2021 [173] | “The authors found no clear stool microbiome signature associated with oral rotavirus vaccine (RVV) immunogenicity in rural Zimbabwean infants”. | Observational study |
| The impact of storage conditions on human stool 16S rRNA microbiome composition and diversity | Carruthers et al., 2019 [174] | “Stool storage preservation method significantly influenced the bacterial profiles obtained, however, all samples remained identifiable to their child of origin. Stool stored at ambient temperature for up to 32 h did not significantly influence diversity and had minimal changes upon microbiota composition, which remained relatively stable across time-to-freezing regardless of preservation method used”. | Observational study |
| Short- and long-read metagenomics of urban and rural South African gut microbiomes reveal a transitional composition and undescribed taxa | Tamburini et al., 2022 [175] | “Gut microbiome of South Africans does not conform to a simple “western-nonwestern” axis and contains undescribed microbial diversity”. | Observational study |
| Associations between gut microbiota and intestinal inflammation, permeability, and damage in young Malawian children | Kortekangas et al., 2022 [176] | “The study findings support the hypothesis of an association between the gut microbiota composition and EED assessed by the biomarkers calprotectin, alpha-1 antitrypsin and REG1B in rural Malawian children”. | Observational study |
| Gut microbiota imbalances in Tunisian participants with type 1 and type 2 diabetes mellitus | Fassatoui et al., 2019 [177] | “There was a reduction in the amount of A. muciniphila, Firmicutes, and F. prausnitzii in Tunisian participants with diabetes. The abundance of A. muciniphila was also affected by glycemic level”. | Comparative study |
| Effect of commonly used pediatric antibiotics on gut microbial diversity in preschool children in Burkina Faso: A randomised clinical trial | Oldenburg et al., 2018 [178] | “A short course of azithromycin led to a significant decrease in bacterial diversity of the intestinal microbiome in preschool children. Although the clinical implications of a single course of antibiotics are unclear, the intestinal microbiome in young children is sensitive to antibiotics”. | Clinical trial |
| Associations of HIV and iron status with gut microbiota composition, gut inflammation and gut integrity in South African school-age children: A two-way factorial case–control study | Goosen et al., 2023 [179] | “In 8-to 13-year-old virally suppressed HIV+ and HIV− children with or without iron deficiency (ID), ID was associated with increased gut inflammation and changes in the relative abundance of specific microbiota. In HIV+ children, ID had a cumulative effect that further shifted the gut microbiota to an unfavourable composition”. | Two-way factorial case-control study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pheeha, S.M.; Tamuzi, J.L.; Chale-Matsau, B.; Manda, S.; Nyasulu, P.S. A Scoping Review Evaluating the Current State of Gut Microbiota Research in Africa. Microorganisms 2023, 11, 2118. https://doi.org/10.3390/microorganisms11082118
Pheeha SM, Tamuzi JL, Chale-Matsau B, Manda S, Nyasulu PS. A Scoping Review Evaluating the Current State of Gut Microbiota Research in Africa. Microorganisms. 2023; 11(8):2118. https://doi.org/10.3390/microorganisms11082118
Chicago/Turabian StylePheeha, Sara M., Jacques L. Tamuzi, Bettina Chale-Matsau, Samuel Manda, and Peter S. Nyasulu. 2023. "A Scoping Review Evaluating the Current State of Gut Microbiota Research in Africa" Microorganisms 11, no. 8: 2118. https://doi.org/10.3390/microorganisms11082118
APA StylePheeha, S. M., Tamuzi, J. L., Chale-Matsau, B., Manda, S., & Nyasulu, P. S. (2023). A Scoping Review Evaluating the Current State of Gut Microbiota Research in Africa. Microorganisms, 11(8), 2118. https://doi.org/10.3390/microorganisms11082118







