Microbiota in Irritable Bowel Syndrome and Endometriosis: Birds of a Feather Flock Together—A Review
Abstract
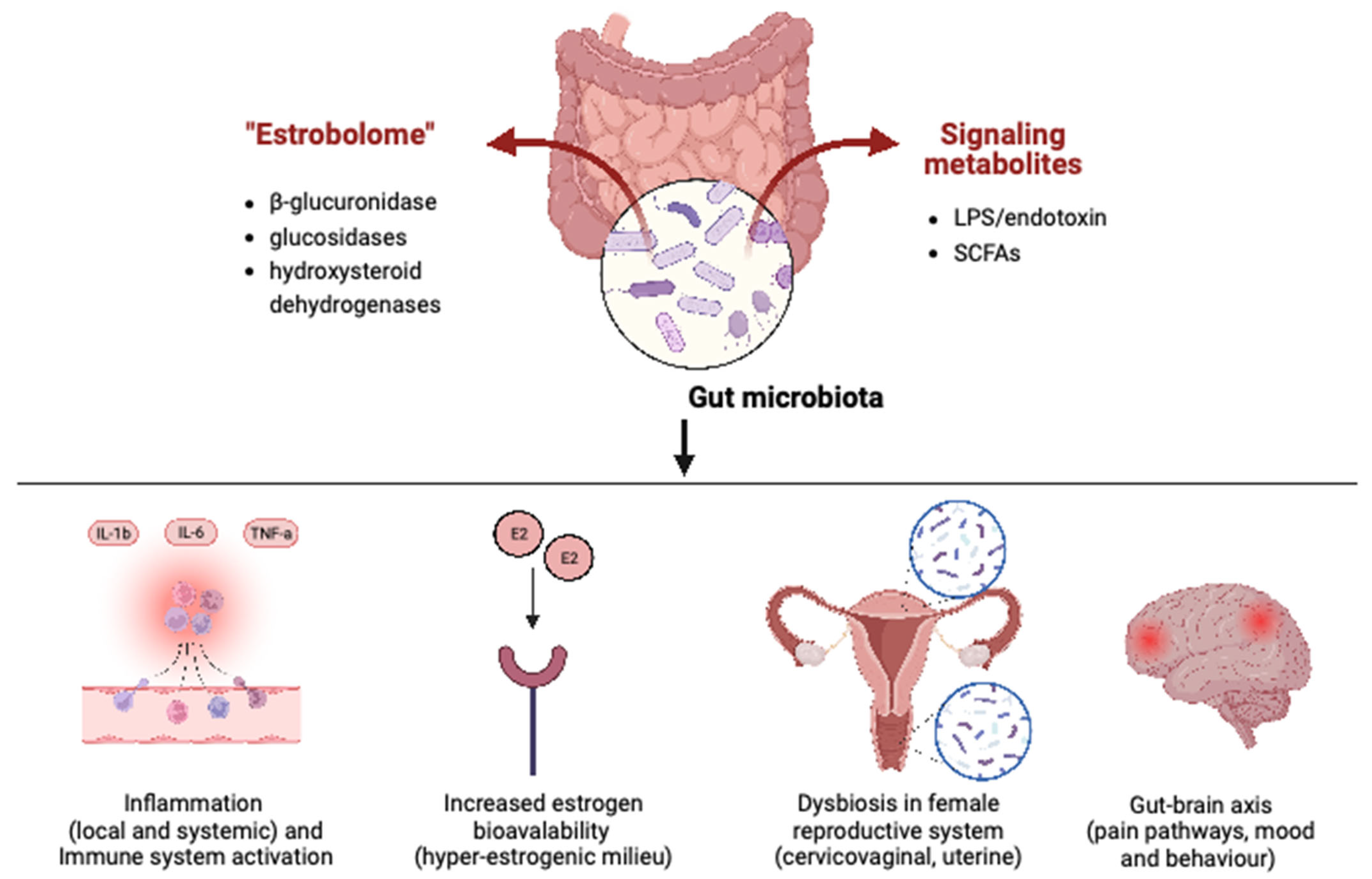
:1. Introduction
2. Microbiota in Irritable Bowel Syndrome
2.1. Insights on Irritable Bowel Syndrome
2.2. Dysbiosis in IBS
| Bacteria | Dysregulation | References | |
|---|---|---|---|
| Phyla | Firmicutes to Bacteroidetes ratio | ↑ | [63] |
| Bacteroidetes | ↑↓ | [59,60,61,63] | |
| Species | Barnesiella intestinihominis | ↓ | [58] |
| Coprococcus | ↓ | [57,58] | |
| Clostridium | ↑ | [63] | |
| Ruminococcus | ↑ | [58] | |
| Lactobacillus | ↓ | [64] | |
| Bifidobacterium | ↓ | [64] | |
| Escherichia coli | ↓ | [64] | |
| Prevotella | ↓ | [61] |
IBS and Small Intestinal Bacterial Overgrowth
2.3. Therapeutical Implications
2.3.1. Low FODMAP Diet
2.3.2. Prebiotics and Probiotics
2.3.3. Antibiotics
2.3.4. Fecal Microbiota Transplantation
3. Microbiota in Endometriosis
3.1. Insights on Endometriosis
3.2. Dysbiosis in Endometriosis
| Bacteria | Dysregulation | References | |
|---|---|---|---|
| Phyla | Firmicutes to Bacteroidetes ratio | ↑ | [154,155] |
| Bacteroidetes | ↑ | [160,161] | |
| Proteobacteria | ↑ | [160,161] | |
| Species | Escherichia coli | ↑ | [160,161] |
| Streptococcus | ↑ | [28] | |
| Gardnerella | ↑↓ | [28,160] | |
| Clostridium | ↓ | [162] | |
| Ruminococcus | ↓ | [162] | |
| Prevotella | ↑ | [160,161] |
Female Genital Tract Microbiome
3.3. Therapeutical Implications
3.3.1. Low FODMAP Diet
3.3.2. Prebiotics and Probiotics
3.3.3. Antibiotics
3.3.4. Fecal Microbiota Transplantation
4. Current Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103, Erratum in Microbiome 2020, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [Green Version]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.; Aleya, L.; Kamel, M. The link among microbiota, epigenetics, and disease development. Environ. Sci. Pollut. Res. Int. 2021, 28, 28926–28964. [Google Scholar] [CrossRef]
- Senchukova, M.A. Microbiota of the gastrointestinal tract: Friend or foe? World J. Gastroenterol. 2023, 29, 19–42. [Google Scholar] [CrossRef]
- Haran, J.P.; McCormick, B.A. Aging, Frailty, and the Microbiome-How Dysbiosis Influences Human Aging and Disease. Gastroenterology 2021, 160, 507–523. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s role in health and diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 36967–36983. [Google Scholar] [CrossRef]
- Karkman, A.; Lehtimäki, J.; Ruokolainen, L. The ecology of human microbiota: Dynamics and diversity in health and disease. Ann. N. Y. Acad. Sci. 2017, 1399, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhang, J.; Deng, J.; Hu, J.; Zhong, Q.; Su, M.; Lin, D.; Xu, T.; Bai, X.; Li, J.; et al. Analysis of the relationship between the gut microbiota enterotypes and colorectal adenoma. Front. Microbiol. 2023, 14, 1097892. [Google Scholar] [CrossRef] [PubMed]
- Novello, M.; Mandarino, F.V.; Di Saverio, S.; Gori, D.; Lugaresi, M.; Duchi, A.; Argento, F.; Cavallari, G.; Wheeler, J.; Nardo, B. Post-operative outcomes and predictors of mortality after colorectal cancer surgery in the very elderly patients. Heliyon 2019, 5, e02363. [Google Scholar] [CrossRef] [Green Version]
- Wiredu Ocansey, D.K.; Hang, S.; Yuan, X.; Qian, H.; Zhou, M.; Valerie Olovo, C.; Zhang, X.; Mao, F. The diagnostic and prognostic potential of gut bacteria in inflammatory bowel disease. Gut Microbes 2023, 15, 2176118. [Google Scholar] [CrossRef]
- Massimino, L.; Barchi, A.; Mandarino, F.V.; Spanò, S.; Lamparelli, L.A.; Vespa, E.; Passaretti, S.; Peyrin-Biroulet, L.; Savarino, E.V.; Jairath, V.; et al. A multi-omic analysis reveals the esophageal dysbiosis as the predominant trait of eosinophilic esophagitis. J. Transl. Med. 2023, 21, 46. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Sinagra, E.; Barchi, A.; Verga, M.C.; Brinch, D.; Raimondo, D.; Danese, S. Gastroparesis: The Complex Interplay with Microbiota and the Role of Exogenous Infections in the Pathogenesis of the Disease. Microorganisms 2023, 11, 1122. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Testoni, S.G.G.; Barchi, A.; Pepe, G.; Esposito, D.; Fanti, L.; Viale, E.; Biamonte, P.; Azzolini, F.; Danese, S. Gastric emptying study before gastric peroral endoscopic myotomy (G-POEM): Can intragastric meal distribution be a predictor of success? Gut 2023, 72, 1019–1020. [Google Scholar] [CrossRef]
- Zamorano, D.; Ivulic, D.; Viver, T.; Morales, F.; López-Kostner, F.; Vidal, R.M. Microbiota Phenotype Promotes Anastomotic Leakage in a Model of Rats with Ischemic Colon Resection. Microorganisms 2023, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Boatman, S.; Kohn, J.; Jahansouz, C. The Influence of the Microbiome on Anastomotic Leak. Clin. Colon Rectal Surg. 2023, 36, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Barchi, A.; Fanti, L.; D’Amico, F.; Azzolini, F.; Esposito, D.; Biamonte, P.; Lauri, G.; Danese, S. Endoscopic vacuum therapy for post-esophagectomy anastomotic dehiscence as rescue treatment: A single center case series. Esophagus 2022, 19, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Esposito, D.; Spelta, G.N.E.; Cavestro, G.M.; Rosati, R.; Parise, P.; Gemma, M.F.; Fanti, L. Double layer stent for the treatment of leaks and fistula after upper gastrointestinal oncologic surgery: A retrospective study. Updates Surg. 2022, 74, 1055–1062. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Barchi, A.; Biamonte, P.; Esposito, D.; Azzolini, F.; Fanti, L.; Danese, S. The prophylactic use of endoscopic vacuum therapy for anastomotic dehiscence after rectal anterior resection: Is it feasible for redo surgery? Tech. Coloproctol. 2022, 26, 319–320. [Google Scholar] [CrossRef]
- Mandarino, F.V.; Barchi, A.; D’Amico, F.; Fanti, L.; Azzolini, F.; Viale, E.; Esposito, D.; Rosati, R.; Fiorino, G.; Bemelman, W.A.; et al. Endoscopic Vacuum Therapy (EVT) versus Self-Expandable Metal Stent (SEMS) for Anastomotic Leaks after Upper Gastrointestinal Surgery: Systematic Review and Meta-Analysis. Life 2023, 13, 287. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain-Gut-Microbiome Axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Leonardi, M.; Hicks, C.; El-Assaad, F.; El-Omar, E.; Condous, G. Endometriosis and the microbiome: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 239–249. [Google Scholar] [CrossRef]
- Qin, R.; Tian, G.; Liu, J.; Cao, L. The gut microbiota and endometriosis: From pathogenesis to diagnosis and treatment. Front. Cell. Infect. Microbiol. 2022, 12, 1069557. [Google Scholar] [CrossRef]
- Salliss, M.E.; Farland, L.V.; Mahnert, N.D.; Herbst-Kralovetz, M.M. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum. Reprod. Update 2021, 28, 92–131. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 2016, 7, 365–383. [Google Scholar] [CrossRef] [Green Version]
- Canakis, A.; Haroon, M.; Weber, H.C. Irritable bowel syndrome and gut microbiota. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host-microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491, Erratum in Gastroenterology 2006, 131, 688. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.D.; Sun, N.; Canakis, A.; Park, W.Y.; Weber, H.C. Irritable Bowel Syndrome and the Gut Microbiome: A Comprehensive Review. J. Clin. Med. 2023, 12, 2558. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [Green Version]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Lovell, R.M.; Ford, A.C. Effect of gender on prevalence of irritable bowel syndrome in the community: Systematic review and meta-analysis. Am. J. Gastroenterol. 2012, 107, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Gralnek, I.M.; Hays, R.D.; Kilbourne, A.; Naliboff, B.; Mayer, E.A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000, 119, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Alizadeh-Tabari, S.; Zamani, V. Systematic review with meta-analysis: The prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Frändemark, Å.; Törnblom, H.; Jakobsson, S.; Simrén, M. Work Productivity and Activity Impairment in Irritable Bowel Syndrome (IBS): A Multifaceted Problem. Am. J. Gastroenterol. 2018, 113, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Wilson, A.; Knight, K.; Wong, J.; Chiou, C.F.; Barghout, V.; Frech, F.; Ofman, J.J. Irritable bowel syndrome, health care use, and costs: A U.S. managed care perspective. Am. J. Gastroenterol. 2003, 98, 600–607. [Google Scholar] [CrossRef]
- Everhart, J.E.; Ruhl, C.E. Burden of digestive diseases in the United States part I: Overall and upper gastrointestinal diseases. Gastroenterology 2009, 136, 376–386. [Google Scholar] [CrossRef]
- Drossman, D.A.; Camilleri, M.; Mayer, E.A.; Whitehead, W.E. AGA technical review on irritable bowel syndrome. Gastroenterology 2002, 123, 2108–2131. [Google Scholar] [CrossRef]
- Saito, Y.A.; Petersen, G.M.; Larson, J.J.; Atkinson, E.J.; Fridley, B.L.; de Andrade, M.; Locke, G.R., 3rd; Zimmerman, J.M.; Almazar-Elder, A.E.; Talley, N.J. Familial aggregation of irritable bowel syndrome: A family case-control study. Am. J. Gastroenterol. 2010, 105, 833–841. [Google Scholar] [CrossRef] [Green Version]
- Lembo, A.; Zaman, M.; Jones, M.; Talley, N.J. Influence of genetics on irritable bowel syndrome, gastro-oesophageal reflux and dyspepsia: A twin study. Aliment Pharmacol. Ther. 2007, 25, 1343–1350. [Google Scholar] [CrossRef]
- Locke, G.R., 3rd; Ackerman, M.J.; Zinsmeister, A.R.; Thapa, P.; Farrugia, G. Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: Evidence of an intestinal channelopathy. Am. J. Gastroenterol. 2006, 101, 1299–1304. [Google Scholar] [CrossRef]
- Ford, A.C.; Thabane, M.; Collins, S.M.; Moayyedi, P.; Garg, A.X.; Clark, W.F.; Marshall, J.K. Prevalence of uninvestigated dyspepsia 8 years after a large waterborne outbreak of bacterial dysentery: A cohort study. Gastroenterology 2010, 138, 1727–1736; quiz e12. [Google Scholar] [CrossRef]
- Marshall, J.K.; Thabane, M.; Garg, A.X.; Clark, W.F.; Moayyedi, P.; Collins, S.M.; Walkerton Health Study Investigators. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut 2010, 59, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Talley, N.J. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: A systematic review. J. Gastroenterol. 2011, 46, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M. Is the irritable gut an inflamed gut? Scand. J. Gastroenterol. Suppl. 1992, 192, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Liebregts, T.; Adam, B.; Bredack, C.; Röth, A.; Heinzel, S.; Lester, S.; Downie-Doyle, S.; Smith, E.; Drew, P.; Talley, N.J.; et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [CrossRef]
- Turcotte, J.F.; Kao, D.; Mah, S.J.; Claggett, B.; Saltzman, J.R.; Fedorak, R.N.; Liu, J.J. Breaks in the wall: Increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest. Endosc. 2013, 77, 624–630. [Google Scholar] [CrossRef]
- Kassinen, A.; Krogius-Kurikka, L.; Mäkivuokko, H.; Rinttilä, T.; Paulin, L.; Corander, J.; Malinen, E.; Apajalahti, J.; Palva, A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007, 133, 24–33. [Google Scholar] [CrossRef]
- Jeffery, I.B.; Das, A.; O’Herlihy, E.; Coughlan, S.; Cisek, K.; Moore, M.; Bradley, F.; Carty, T.; Pradhan, M.; Dwibedi, C.; et al. Differences in Fecal Microbiomes and Metabolomes of People With vs Without Irritable Bowel Syndrome and Bile Acid Malabsorption. Gastroenterology 2020, 158, 1016–1028.e8. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Biagi, E.; Heilig, H.G.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Öhman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.; Simrén, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Tap, J.; Derrien, M.; Törnblom, H.; Brazeilles, R.; Cools-Portier, S.; Doré, J.; Störsrud, S.; Le Nevé, B.; Öhman, L.; Simrén, M. Identification of an Intestinal Microbiota Signature Associated with Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.N.; Wu, H.; Chen, Y.Z.; Chen, Y.J.; Shen, X.Z.; Liu, T.T. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhu, S.; Wang, B.; Duan, L. Alterations of Gut Microbiota in Patients with Irritable Bowel Syndrome Based on 16S rRNA-Targeted Sequencing: A Systematic Review. Clin. Transl. Gastroenterol. 2019, 10, e00012. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Song, Y.; Chaudhary, R.; Mullin, G.E. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet. 2020, 120, 565–586. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome—A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136, Erratum in Front. Microbiol. 2019, 10, 1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choghakhori, R.; Abbasnezhad, A.; Hasanvand, A.; Amani, R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: Association with digestive symptoms and quality of life. Cytokine 2017, 93, 34–43. [Google Scholar] [CrossRef]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef] [Green Version]
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional gastrointestinal disorders: What’s new for Rome IV? Lancet Gastroenterol. Hepatol. 2016, 1, 6–8. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [Green Version]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276, Erratum in Cell 2015, 163, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labus, J.S.; Osadchiy, V.; Hsiao, E.Y.; Tap, J.; Derrien, M.; Gupta, A.; Tillisch, K.; Le Nevé, B.; Grinsvall, C.; Ljungberg, M.; et al. Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, D.; Law, G.R.; Major, G.; Andreyev, H.J.N. A systematic review and meta-analysis on the prevalence of non-malignant, organic gastrointestinal disorders misdiagnosed as irritable bowel syndrome. Sci. Rep. 2022, 12, 1949. [Google Scholar] [CrossRef]
- Kunkel, D.; Basseri, R.J.; Makhani, M.D.; Chong, K.; Chang, C.; Pimentel, M. Methane on breath testing is associated with constipation: A systematic review and meta-analysis. Dig. Dis. Sci. 2011, 56, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lin, H.C.; Enayati, P.; van den Burg, B.; Lee, H.R.; Chen, J.H.; Park, S.; Kong, Y.; Conklin, J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1089–G1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, D.; Klose, P.; Lauche, R.; Dobos, G.; Langhorst, J.; Cramer, H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Schmidt, T.S.B.; Raes, J.; Bork, P. The Human Gut Microbiome: From Association to Modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.M. A role for the gut microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef] [Green Version]
- Krieger-Grübel, C.; Hutter, S.; Hiestand, M.; Brenner, I.; Güsewell, S.; Borovicka, J. Treatment efficacy of a low FODMAP diet compared to a low lactose diet in IBS patients: A randomized, cross-over designed study. Clin. Nutr. ESPEN 2020, 40, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Cope, J.L.; Hollister, E.B.; Tsai, C.M.; McMeans, A.R.; Luna, R.A.; Versalovic, J.; Shulman, R.J. Randomised clinical trial: Gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 42, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Valeur, J.; Småstuen, M.C.; Knudsen, T.; Lied, G.A.; Røseth, A.G. Exploring Gut Microbiota Composition as an Indicator of Clinical Response to Dietary FODMAP Restriction in Patients with Irritable Bowel Syndrome. Dig. Dis. Sci. 2018, 63, 429–436. [Google Scholar] [CrossRef]
- Bennet, S.M.P.; Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Öhman, L.; Simrén, M. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut 2018, 67, 872–881. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef] [Green Version]
- Silk, D.B.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.O.; Tuffnell, Q.; Lee, A.J. Controlled trial of oligofructose in the management of irritable bowel syndrome. J. Nutr. 1999, 129, 1451S–1453S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, M.; Gudmand-Hoyer, E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am. J. Clin. Nutr. 2000, 72, 1570–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, H.L.; Xiao, J.Y. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int. J. Surg. 2020, 75, 116–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Guo, C.; Mu, D.; Feng, B.; Zuo, X.; Li, Y. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: A meta-analysis. BMC Gastroenterol. 2016, 16, 62. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561; quiz 1546, 1562. [Google Scholar] [CrossRef]
- Asha, M.Z.; Khalil, S.F.H. Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A systematic review and meta-analysis. Sultan Qaboos Univ. Med. J. 2020, 20, e13–e24. [Google Scholar] [CrossRef] [Green Version]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J. Gastroenterol. 2015, 21, 3072–3084. [Google Scholar] [CrossRef]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714™ Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am. J. Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- Kogawa, A.C.; Salgado, H.R.N. Status of Rifaximin: A Review of Characteristics, Uses and Analytical Methods. Crit. Rev. Anal. Chem. 2018, 48, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, M.; Lembo, A.; Chey, W.D.; Zakko, S.; Ringel, Y.; Yu, J.; Mareya, S.M.; Shaw, A.L.; Bortey, E.; Forbes, W.P.; et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N. Engl. J. Med. 2011, 364, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fodor, A.A.; Pimentel, M.; Chey, W.D.; Lembo, A.; Golden, P.L.; Israel, R.J.; Carroll, I.M. Rifaximin is associated with modest, transient decreases in multiple taxa in the gut microbiota of patients with diarrhoea-predominant irritable bowel syndrome. Gut Microbes 2019, 10, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zeber-Lubecka, N.; Kulecka, M.; Ambrozkiewicz, F.; Paziewska, A.; Goryca, K.; Karczmarski, J.; Rubel, T.; Wojtowicz, W.; Mlynarz, P.; Marczak, L.; et al. Limited prolonged effects of rifaximin treatment on irritable bowel syndrome-related differences in the fecal microbiome and metabolome. Gut Microbes 2016, 7, 397–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herndon, C.C.; Wang, Y.P.; Lu, C.L. Targeting the gut microbiota for the treatment of irritable bowel syndrome. Kaohsiung J. Med. Sci. 2020, 36, 160–170. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, P.H.; Hilpüsch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef]
- Holvoet, T.; Joossens, M.; Vázquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients with Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results from a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157.e8. [Google Scholar] [CrossRef]
- Madsen, A.M.A.; Halkjær, S.I.; Christensen, A.H.; Günther, S.; Browne, P.D.; Kallemose, T.; Hansen, L.H.; Petersen, A.M. The effect of faecal microbiota transplantation on abdominal pain, stool frequency, and stool form in patients with moderate-to-severe irritable bowel syndrome: Results from a randomised, double-blind, placebo-controlled study. Scand. J. Gastroenterol. 2021, 56, 761–769. [Google Scholar] [CrossRef]
- Ianiro, G.; Eusebi, L.H.; Black, C.J.; Gasbarrini, A.; Cammarota, G.; Ford, A.C. Systematic review with meta-analysis: Efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.D.; Günther, S.; Hansen, L.H.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S.; Dunselman, G.; Dirksen, C.; Hummelshoj, L.; Bokor, A.; Brandes, I.; Brodszky, V.; Canis, M.; Colombo, G.L.; DeLeire, T.; et al. The burden of endometriosis: Costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 2012, 27, 1292–1299, Erratum in Hum. Reprod. 2014, 29, 2073. [Google Scholar] [CrossRef] [Green Version]
- Greene, R.; Stratton, P.; Cleary, S.D.; Ballweg, M.L.; Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 2009, 91, 32–39. [Google Scholar] [CrossRef]
- Tomassetti, C.; Johnson, N.P.; Petrozza, J.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Lee, T.T.M.; Missmer, S.; Vermeulen, N.; International Working Group of AAGL, ESGE, ESHRE and WES; et al. An international terminology for endometriosis, 2021. Hum. Reprod. Open 2021, 2021, hoab029. [Google Scholar] [CrossRef]
- Andres, M.P.; Arcoverde, F.V.L.; Souza, C.C.C.; Fernandes, L.F.C.; Abrão, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef] [Green Version]
- Surrey, E.S.; Soliman, A.M.; Johnson, S.J.; Davis, M.; Castelli-Haley, J.; Snabes, M.C. Risk of Developing Comorbidities Among Women with Endometriosis: A Retrospective Matched Cohort Study. J. Womens Health 2018, 27, 1114–1123. [Google Scholar] [CrossRef]
- Prescott, J.; Farland, L.V.; Tobias, D.K.; Gaskins, A.J.; Spiegelman, D.; Chavarro, J.E.; Rich-Edwards, J.W.; Barbieri, R.L.; Missmer, S.A. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum. Reprod. 2016, 31, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, L.; Dworzynski, K.; Davies, M.; Overton, C.; Guideline Committee. Diagnosis and management of endometriosis: Summary of NICE guidance. BMJ 2017, 358, j3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bafort, C.; Beebeejaun, Y.; Tomassetti, C.; Bosteels, J.; Duffy, J.M. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 2020, 10, CD011031. [Google Scholar] [PubMed]
- Brown, J.; Kives, S.; Akhtar, M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst. Rev. 2012, 2012, CD002122. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 2010, CD008475. [Google Scholar]
- Ottolina, J.; Ferrari, S.; Bartiromo, L.; Bonavina, G.; Salmeri, N.; Schimberni, M.; Makieva, S.; Tandoi, I.; Papaleo, E.; ViganÒ, P.; et al. Ovarian responsiveness in assisted reproductive technology after CO2 fiber laser vaporization for endometrioma treatment: Preliminary data. Minerva Endocrinol. 2020, 45, 288–294. [Google Scholar] [CrossRef]
- Candiani, M.; Ottolina, J.; Salmeri, N.; D’Alessandro, S.; Tandoi, I.; Bartiromo, L.; Schimberni, M.; Ferrari, S.; Villanacci, R. Minimally invasive surgery for ovarian endometriosis as a mean of improving fertility: Cystectomy vs. CO2 fiber laser ablation what do we know so far? Front. Surg. 2023, 10, 1147877. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Mortlock, S.; Ghiasi, M.; Møller, P.L.; Stefansdottir, L.; Galarneau, G.; Turman, C.; Danning, R.; Law, M.H.; Sapkota, Y.; et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat. Genet. 2023, 55, 423–436. [Google Scholar] [CrossRef]
- Salmeri, N.; Ottolina, J.; Bartiromo, L.; Schimberni, M.; Dolci, C.; Ferrari, S.; Villanacci, R.; Arena, S.; Berlanda, N.; Buggio, L.; et al. ’Guess who’? An Italian multicentric study on pigmentation traits prevalence in endometriosis localizations. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 274, 5–12. [Google Scholar] [CrossRef]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef] [Green Version]
- Shafrir, A.L.; Palmor, M.C.; Fourquet, J.; DiVasta, A.D.; Farland, L.V.; Vitonis, A.F.; Harris, H.R.; Laufer, M.R.; Cramer, D.W.; Terry, K.L.; et al. Co-occurrence of immune-mediated conditions and endometriosis among adolescents and adult women. Am. J. Reprod. Immunol. 2021, 86, e13404. [Google Scholar] [CrossRef]
- Coloma, J.L.; Martínez-Zamora, M.A.; Collado, A.; Gràcia, M.; Rius, M.; Quintas, L.; Carmona, F. Prevalence of fibromyalgia among women with deep infiltrating endometriosis. Int. J. Gynaecol. Obstet. 2019, 146, 157–163. [Google Scholar] [CrossRef]
- Miller, J.A.; Missmer, S.A.; Vitonis, A.F.; Sarda, V.; Laufer, M.R.; DiVasta, A.D. Prevalence of migraines in adolescents with endometriosis. Fertil. Steril. 2018, 109, 685–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalani, S.; Choudhry, A.J.; Firth, B.; Bacal, V.; Walker, M.; Wen, S.W.; Singh, S.; Amath, A.; Hodge, M.; Chen, I. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Hum. Reprod. 2018, 33, 1854–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmeri, N.; Li Piani, L.; Cavoretto, P.I.; Somigliana, E.; Viganò, P.; Candiani, M. Endometriosis increases the risk of gestational diabetes: A meta-analysis stratified by mode of conception, disease localization and severity. Sci. Rep. 2023, 13, 8099. [Google Scholar] [CrossRef]
- Salmeri, N.; Farina, A.; Candiani, M.; Dolci, C.; Bonavina, G.; Poziello, C.; Viganò, P.; Cavoretto, P.I. Endometriosis and Impaired Placentation: A Prospective Cohort Study Comparing Uterine Arteries Doppler Pulsatility Index in Pregnancies of Patients with and without Moderate-Severe Disease. Diagnostics 2022, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Missmer, S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef]
- Vanni, V.S.; Villanacci, R.; Salmeri, N.; Papaleo, E.; Delprato, D.; Ottolina, J.; Rovere-Querini, P.; Ferrari, S.; Viganò, P.; Candiani, M. Concomitant autoimmunity may be a predictor of more severe stages of endometriosis. Sci. Rep. 2021, 11, 15372, Erratum in Sci. Rep. 2021, 11, 17715. [Google Scholar] [CrossRef]
- Leuenberger, J.; Kohl Schwartz, A.S.; Geraedts, K.; Haeberlin, F.; Eberhard, M.; von Orellie, S.; Imesch, P.; Leeners, B. Living with endometriosis: Comorbid pain disorders, characteristics of pain and relevance for daily life. Eur. J. Pain. 2022, 26, 1021–1038. [Google Scholar] [CrossRef] [PubMed]
- Salmeri, N.; Gennarelli, G.; Vanni, V.S.; Ferrari, S.; Ruffa, A.; Rovere-Querini, P.; Pagliardini, L.; Candiani, M.; Papaleo, E. Concomitant Autoimmunity in Endometriosis Impairs Endometrium-Embryo Crosstalk at the Implantation Site: A Multicenter Case-Control Study. J. Clin. Med. 2023, 12, 3557. [Google Scholar] [CrossRef]
- Nabi, M.Y.; Nauhria, S.; Reel, M.; Londono, S.; Vasireddi, A.; Elmiry, M.; Ramdass, P.V.A.K. Endometriosis and irritable bowel syndrome: A systematic review and meta-analyses. Front. Med. 2022, 9, 914356. [Google Scholar] [CrossRef]
- Junkka, S.S.; Ohlsson, B. Associations and gastrointestinal symptoms in women with endometriosis in comparison to women with irritable bowel syndrome: A study based on a population cohort. BMC Gastroenterol. 2023, 23, 228. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Angioni, S.; D’Alterio, M.N.; Pirarba, S.; Noto, A.; Santoru, M.L.; Tronci, L.; Fanos, V.; Atzori, L.; Congiu, F. Metabolic Profile of Patients with Severe Endometriosis: A Prospective Experimental Study. Reprod. Sci. 2021, 28, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartiromo, L.; Schimberni, M.; Villanacci, R.; Ottolina, J.; Dolci, C.; Salmeri, N.; Viganò, P.; Candiani, M. Endometriosis and Phytoestrogens: Friends or Foes? A Systematic Review. Nutrients 2021, 13, 2532. [Google Scholar] [CrossRef]
- Saidi, K.; Sharma, S.; Ohlsson, B. A systematic review and meta-analysis of the associations between endometriosis and irritable bowel syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 99–105. [Google Scholar] [CrossRef]
- Chiaffarino, F.; Cipriani, S.; Ricci, E.; Mauri, P.A.; Esposito, G.; Barretta, M.; Vercellini, P.; Parazzini, F. Endometriosis and irritable bowel syndrome: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021, 303, 17–25. [Google Scholar] [CrossRef]
- Jiang, I.; Yong, P.J.; Allaire, C.; Bedaiwy, M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021, 22, 5644. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Tahlak, M.; Keckstein, J.; Wattiez, A.; Martin, D.C. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best. Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 14–26. [Google Scholar] [CrossRef]
- Zizolfi, B.; Foreste, V.; Gallo, A.; Martone, S.; Giampaolino, P.; Di Spiezio Sardo, A. Endometriosis and dysbiosis: State of art. Front. Endocrinol. 2023, 14, 1140774. [Google Scholar] [CrossRef]
- Uzuner, C.; Mak, J.; El-Assaad, F.; Condous, G. The bidirectional relationship between endometriosis and microbiome. Front. Endocrinol. 2023, 14, 1110824. [Google Scholar] [CrossRef] [PubMed]
- Sobstyl, A.; Chałupnik, A.; Mertowska, P.; Grywalska, E. How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 10920. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, M.; Li, D.; Zhang, Z.; Sun, H.; An, M.; Wang, G. Endometriosis induces gut microbiota alterations in mice. Hum. Reprod. 2018, 33, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.; Sun, S.; Bi, Y.; Ding, J.; Cheng, W.; Yu, J.; Zhou, L.; Li, M.; Yu, C. Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am. J. Reprod. Immunol. 2020, 84, e13307. [Google Scholar] [CrossRef] [PubMed]
- Chadchan, S.B.; Cheng, M.; Parnell, L.A.; Yin, Y.; Schriefer, A.; Mysorekar, I.U.; Kommagani, R. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum. Reprod. 2019, 34, 1106–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, J.; Ni, Z.; Cheng, W.; Zhou, L.; Zhai, D.; Sun, S.; Yu, C. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch. Gynecol. Obstet. 2021, 304, 1363–1373. [Google Scholar] [CrossRef]
- Svensson, A.; Brunkwall, L.; Roth, B.; Orho-Melander, M.; Ohlsson, B. Associations Between Endometriosis and Gut Microbiota. Reprod. Sci. 2021, 28, 2367–2377. [Google Scholar] [CrossRef]
- Bailey, M.T.; Coe, C.L. Endometriosis is associated with an altered profile of intestinal microflora in female rhesus monkeys. Hum. Reprod. 2002, 17, 1704–1708. [Google Scholar] [CrossRef] [Green Version]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The Endobiota Study: Comparison of Vaginal, Cervical and Gut Microbiota Between Women with Stage 3/4 Endometriosis and Healthy Controls. Sci. Rep. 2019, 9, 2204. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Yamaguchi, N.; Katamine, S.; Matsuyama, T.; Nakashima, M.; Fujishita, A.; Ishimaru, T.; Masuzaki, H. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil. Steril. 2010, 94, 2860–2863.e1–e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Liu, B.; Liu, Z.; Feng, W.; Liu, M.; Wang, Y.; Peng, D.; Fu, X.; Zhu, H.; Cui, Z.; et al. Gut Microbiota Exceeds Cervical Microbiota for Early Diagnosis of Endometriosis. Front. Cell. Infect. Microbiol. 2021, 11, 788836. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Hiraki, K.; Kitajima, M.; Nakashima, M.; Fushiki, S.; Kitawaki, J. Bacterial contamination hypothesis: A new concept in endometriosis. Reprod. Med. Biol. 2018, 17, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [Green Version]
- Keyama, K.; Kato, T.; Kadota, Y.; Erdenebayar, O.; Kasai, K.; Kawakita, T.; Tani, A.; Matsui, S.; Iwasa, T.; Yoshida, K.; et al. Lipopolysaccharide promotes early endometrial-peritoneal interactions in a mouse model of endometriosis. J. Med. Investig. 2019, 66, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Emani, R.; Alam, C.; Pekkala, S.; Zafar, S.; Emani, M.R.; Hänninen, A. Peritoneal cavity is a route for gut-derived microbial signals to promote autoimmunity in non-obese diabetic mice. Scand. J. Immunol. 2015, 81, 102–109. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Lin, J.; Qian, Y.; Deng, L. Peritoneal fluid concentrations of interleukin-17 correlate with the severity of endometriosis and infertility of this disorder. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 1153–1155. [Google Scholar] [CrossRef]
- Kitawaki, J.; Kado, N.; Ishihara, H.; Koshiba, H.; Kitaoka, Y.; Honjo, H. Endometriosis: The pathophysiology as an estrogen-dependent disease. J. Steroid Biochem. Mol. Biol. 2002, 83, 149–155. [Google Scholar] [CrossRef]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef] [PubMed]
- Pollet, R.M.; D’Agostino, E.H.; Walton, W.G.; Xu, Y.; Little, M.S.; Biernat, K.A.; Pellock, S.J.; Patterson, L.M.; Creekmore, B.C.; Isenberg, H.N.; et al. An Atlas of β-Glucuronidases in the Human Intestinal Microbiome. Structure 2017, 25, 967–977.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Tan, H.; Yang, R.; Yang, F.; Liu, D.; Huang, B.; OuYang, L.; Lei, S.; Wang, Z.; Jiang, S.; et al. Gut dysbiosis-derived β-glucuronidase promotes the development of endometriosis. Fertil. Steril. 2023; S0015-0282(23)00241-8, Epub ahead of print. [Google Scholar] [CrossRef]
- Ustianowska, K.; Ustianowski, Ł.; Machaj, F.; Gorący, A.; Rosik, J.; Szostak, B.; Szostak, J.; Pawlik, A. The Role of the Human Microbiome in the Pathogenesis of Pain. Int. J. Mol. Sci. 2022, 23, 13267. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Bajaj, P.; Bajaj, P.; Madsen, H.; Arendt-Nielsen, L. Endometriosis is associated with central sensitization: A psychophysical controlled study. J. Pain 2003, 4, 372–380. [Google Scholar] [CrossRef]
- Aguilera, M.; Rossini, V.; Hickey, A.; Simnica, D.; Grady, F.; Felice, V.D.; Moloney, A.; Pawley, L.; Fanning, A.; McCarthy, L.; et al. Inflammasome Signaling Regulates the Microbial-Neuroimmune Axis and Visceral Pain in Mice. Int. J. Mol. Sci. 2021, 22, 8336. [Google Scholar] [CrossRef]
- García-Peñarrubia, P.; Ruiz-Alcaraz, A.J.; Martínez-Esparza, M.; Marín, P.; Machado-Linde, F. Hypothetical roadmap towards endometriosis: Prenatal endocrine-disrupting chemical pollutant exposure, anogenital distance, gut-genital microbiota and subclinical infections. Hum. Reprod. Update 2020, 26, 214–246. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Perrotta, A.R.; Borrelli, G.M.; Martins, C.O.; Kallas, E.G.; Sanabani, S.S.; Griffith, L.G.; Alm, E.J.; Abrao, M.S. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: A pilot study. Reprod. Sci. 2020, 27, 1064–1073. [Google Scholar] [CrossRef]
- Hernandes, C.; Silveira, P.; Rodrigues Sereia, A.F.; Christoff, A.P.; Mendes, H.; Valter de Oliveira, L.F.; Podgaec, S. Microbiome Profile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion. Diagnostics 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Zhang, X.; Tang, H.; Zeng, L.; Wu, R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 15. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, R.B.; Mysorekar, I.U. Group therapy on in utero colonization: Seeking common truths and a way forward. Microbiome 2021, 9, 7. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.D.; et al. Does the endometrial cavity have a molecular microbial signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Franasiak, J.M. Endometrial microbiota-new player in town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, K.; Nishioka, K.; Khan, K.N.; Tanaka, Y.; Mori, T.; Nakaya, T.; Kitawaki, J. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am. J. Reprod. Immunol. 2019, 82, e13147. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Kitajima, M.; Hiraki, K.; Nakashima, M.; Masuzaki, H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosis†. Hum. Reprod. 2014, 29, 2446–2456. [Google Scholar] [CrossRef] [Green Version]
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184. [Google Scholar] [CrossRef]
- Xu, M.Q.; Cao, H.L.; Wang, W.Q.; Wang, S.; Cao, X.C.; Yan, F.; Wang, B.M. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J. Gastroenterol. 2015, 21, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Chadchan, S.B.; Naik, S.K.; Popli, P.; Talwar, C.; Putluri, S.; Ambati, C.R.; Lint, M.A.; Kau, A.L.; Stallings, C.L.; Kommagani, R. Gut microbiota and microbiota-derived metabolites promotes endometriosis. Cell Death Discov. 2023, 9, 28. [Google Scholar] [CrossRef]
- Piecuch, M.; Garbicz, J.; Waliczek, M.; Malinowska-Borowska, J.; Rozentryt, P. I Am the 1 in 10-What Should I Eat? A Research Review of Nutrition in Endometriosis. Nutrients 2022, 14, 5283. [Google Scholar] [CrossRef]
- Moore, J.S.; Gibson, P.R.; Perry, R.E.; Burgell, R.E. Endometriosis in patients with irritable bowel syndrome: Specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 201–205. [Google Scholar] [CrossRef]
- Ciebiera, M.; Esfandyari, S.; Siblini, H.; Prince, L.; Elkafas, H.; Wojtyła, C.; Al-Hendy, A.; Ali, M. Nutrition in Gynecological Diseases: Current Perspectives. Nutrients 2021, 13, 1178. [Google Scholar] [CrossRef]
- Itoh, H.; Sashihara, T.; Hosono, A.; Kaminogawa, S.; Uchida, M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 2011, 63, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Uchida, M.; Kobayashi, O. Effects of Lactobacillus gasseri OLL2809 on the induced endometriosis in rats. Biosci. Biotechnol. Biochem. 2013, 77, 1879–1881. [Google Scholar] [CrossRef]
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Uchida, M.; Sashihara, T.; Ji, Z.S.; Li, J.; Tang, Q.; Ni, S.; Song, L.; Kaminogawa, S. Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: Randomized, double-blind, placebo-controlled study. Cytotechnology 2011, 63, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.N.; Fujishita, A.; Muto, H.; Masumoto, H.; Ogawa, K.; Koshiba, A.; Mori, T.; Itoh, K.; Teramukai, S.; Matsuda, K.; et al. Levofloxacin or gonadotropin releasing hormone agonist treatment decreases intrauterine microbial colonization in human endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 103–116. [Google Scholar] [CrossRef]
- Takebayashi, A.; Kimura, F.; Kishi, Y.; Ishida, M.; Takahashi, A.; Yamanaka, A.; Takahashi, K.; Suginami, H.; Murakami, T. The association between endometriosis and chronic endometritis. PloS one 2014, 9, e88354. [Google Scholar] [CrossRef] [Green Version]
- Kitaya, K.; Yasuo, T. Commonalities and Disparities between Endometriosis and Chronic Endometritis: Therapeutic Potential of Novel Antibiotic Treatment Strategy against Ectopic Endometrium. Int. J. Mol. Sci. 2023, 24, 2059. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, A.; Suzuki, M.; Hamaguchi, T.; Watanabe, S.; Iijima, K.; Murofushi, Y.; Shinjo, K.; Osuka, S.; Hariyama, Y.; Ito, M.; et al. Fusobacterium infection facilitates the development of endometriosis through the phenotypic transition of endometrial fibroblasts. Sci. Transl. Med. 2023, 15, eadd1531. [Google Scholar] [CrossRef]
- Gottschick, C.; Deng, Z.L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Wagner-Döbler, I. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome 2017, 5, 99. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Wei, J.; Zhong, Y.; Feng, Y.; Ma, B.; Xiong, Y.; Wei, K.; Tan, B.; Chen, T. Antibiotic Therapy and Vaginal Microbiota Transplantation Reduce Endometriosis Disease Progression in Female Mice via NF-κB Signaling Pathway. Front. Med. 2022, 9, 831115. [Google Scholar] [CrossRef]
- Bowman, K.A.; Broussard, E.K.; Surawicz, C.M. Fecal microbiota transplantation: Current clinical efficacy and future prospects. Clin. Exp. Gastroenterol. 2015, 8, 285–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaranta, G.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation: A Potential Tool for Treatment of Human Female Reproductive Tract Diseases. Front. Immunol. 2019, 10, 2653. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whon, T.W.; Shin, N.R.; Kim, J.Y.; Roh, S.W. Omics in gut microbiome analysis. J. Microbiol. 2021, 59, 292–297. [Google Scholar] [CrossRef]
- Corander, J.; Hanage, W.P.; Pensar, J. Causal discovery for the microbiome. Lancet Microbe 2022, 3, e881–e887. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmeri, N.; Sinagra, E.; Dolci, C.; Buzzaccarini, G.; Sozzi, G.; Sutera, M.; Candiani, M.; Ungaro, F.; Massimino, L.; Danese, S.; et al. Microbiota in Irritable Bowel Syndrome and Endometriosis: Birds of a Feather Flock Together—A Review. Microorganisms 2023, 11, 2089. https://doi.org/10.3390/microorganisms11082089
Salmeri N, Sinagra E, Dolci C, Buzzaccarini G, Sozzi G, Sutera M, Candiani M, Ungaro F, Massimino L, Danese S, et al. Microbiota in Irritable Bowel Syndrome and Endometriosis: Birds of a Feather Flock Together—A Review. Microorganisms. 2023; 11(8):2089. https://doi.org/10.3390/microorganisms11082089
Chicago/Turabian StyleSalmeri, Noemi, Emanuele Sinagra, Carolina Dolci, Giovanni Buzzaccarini, Giulio Sozzi, Miriam Sutera, Massimo Candiani, Federica Ungaro, Luca Massimino, Silvio Danese, and et al. 2023. "Microbiota in Irritable Bowel Syndrome and Endometriosis: Birds of a Feather Flock Together—A Review" Microorganisms 11, no. 8: 2089. https://doi.org/10.3390/microorganisms11082089
APA StyleSalmeri, N., Sinagra, E., Dolci, C., Buzzaccarini, G., Sozzi, G., Sutera, M., Candiani, M., Ungaro, F., Massimino, L., Danese, S., & Mandarino, F. V. (2023). Microbiota in Irritable Bowel Syndrome and Endometriosis: Birds of a Feather Flock Together—A Review. Microorganisms, 11(8), 2089. https://doi.org/10.3390/microorganisms11082089









