Sex- and Age-Dependent Associations between Parabacteroides and Obesity: Evidence from Two Population Cohort
Abstract
1. Introduction
2. Method
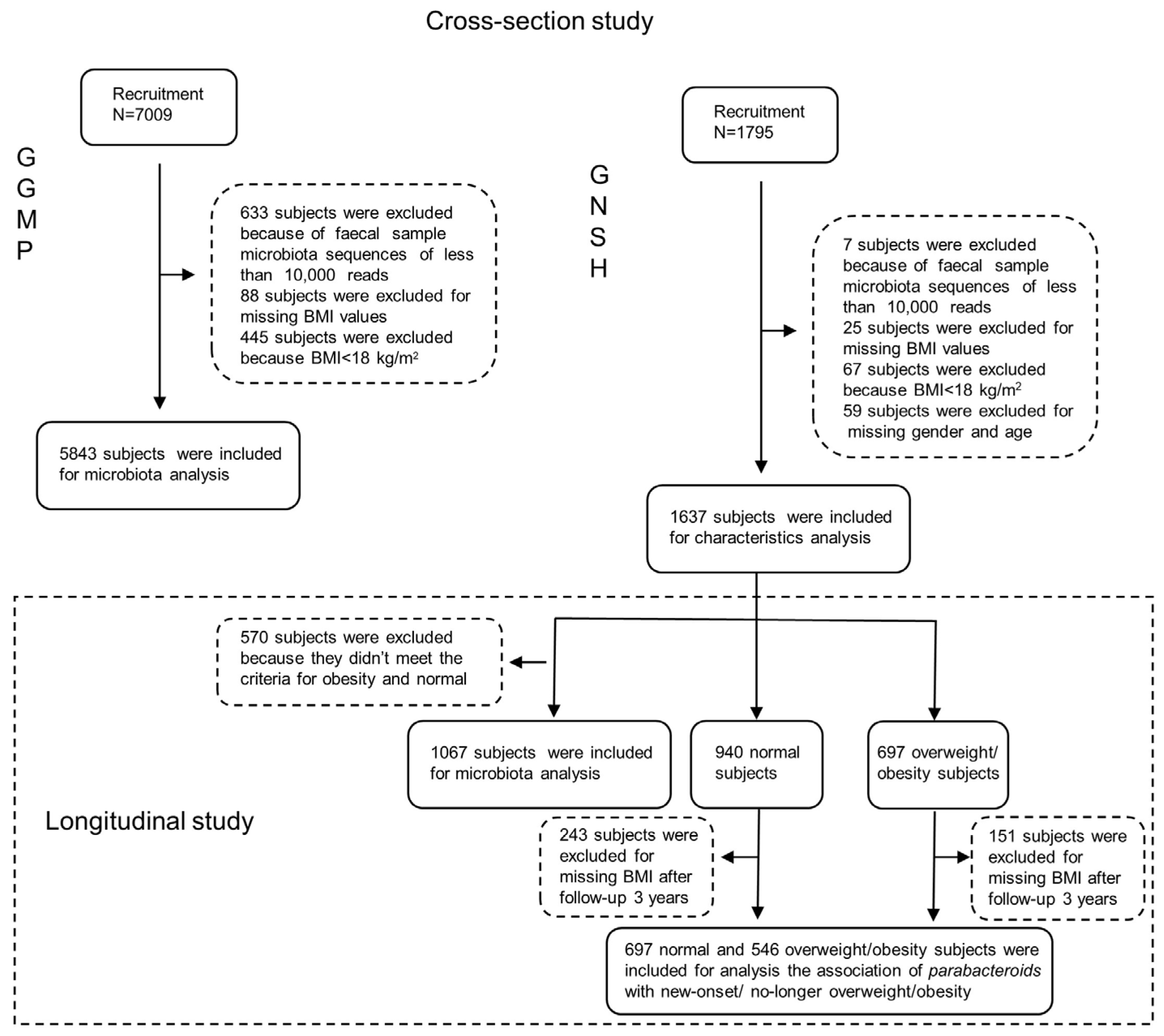
2.1. Study Design and Participants
2.2. The Assessment of Obesity/Overweight, BMI Change, and Other Covariates
2.3. Stool Samples and Bioinformatics Analysis
2.4. Statistical Analysis
3. Results
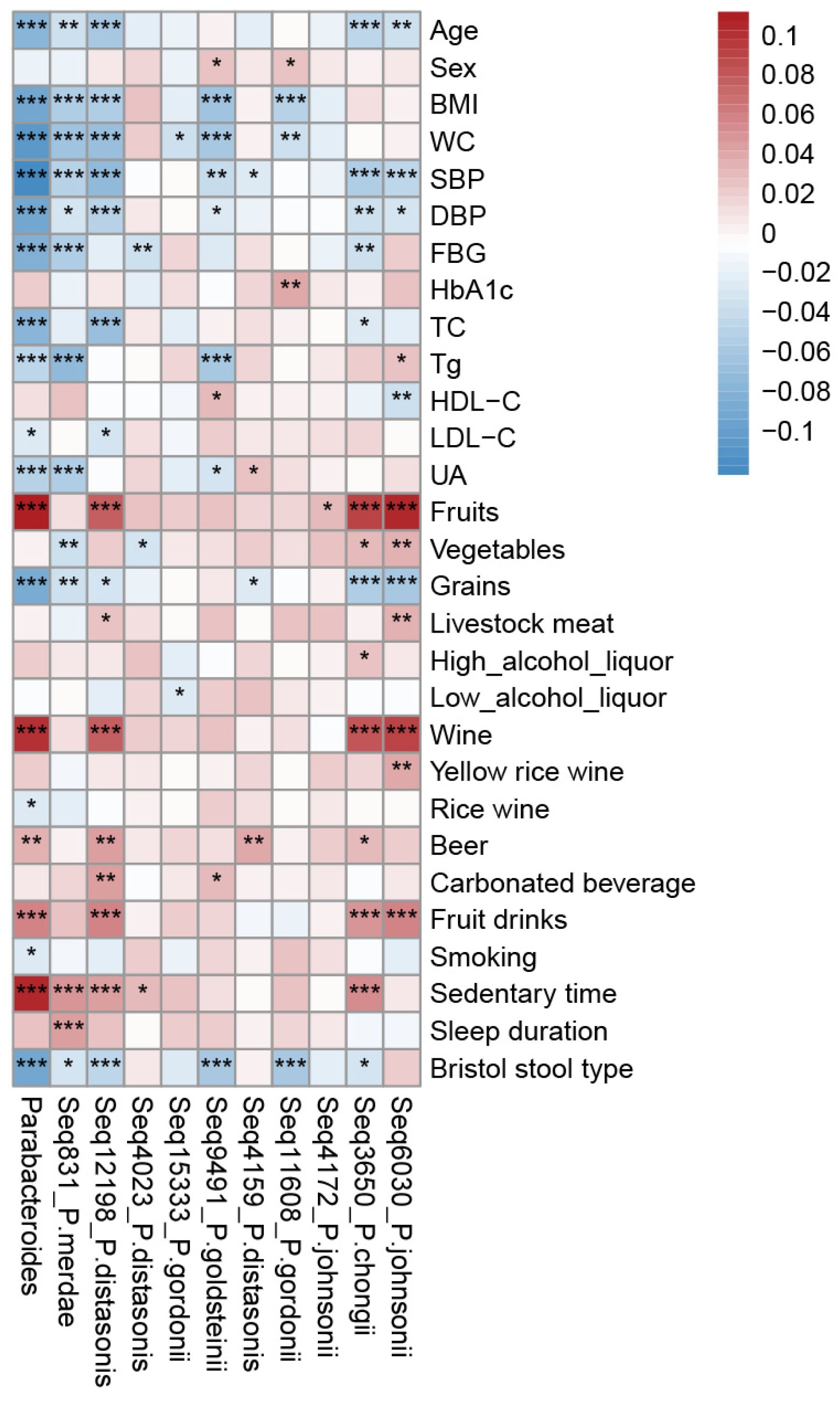
3.1. Parabacteroides Distribution and Covariates in the Studied Population
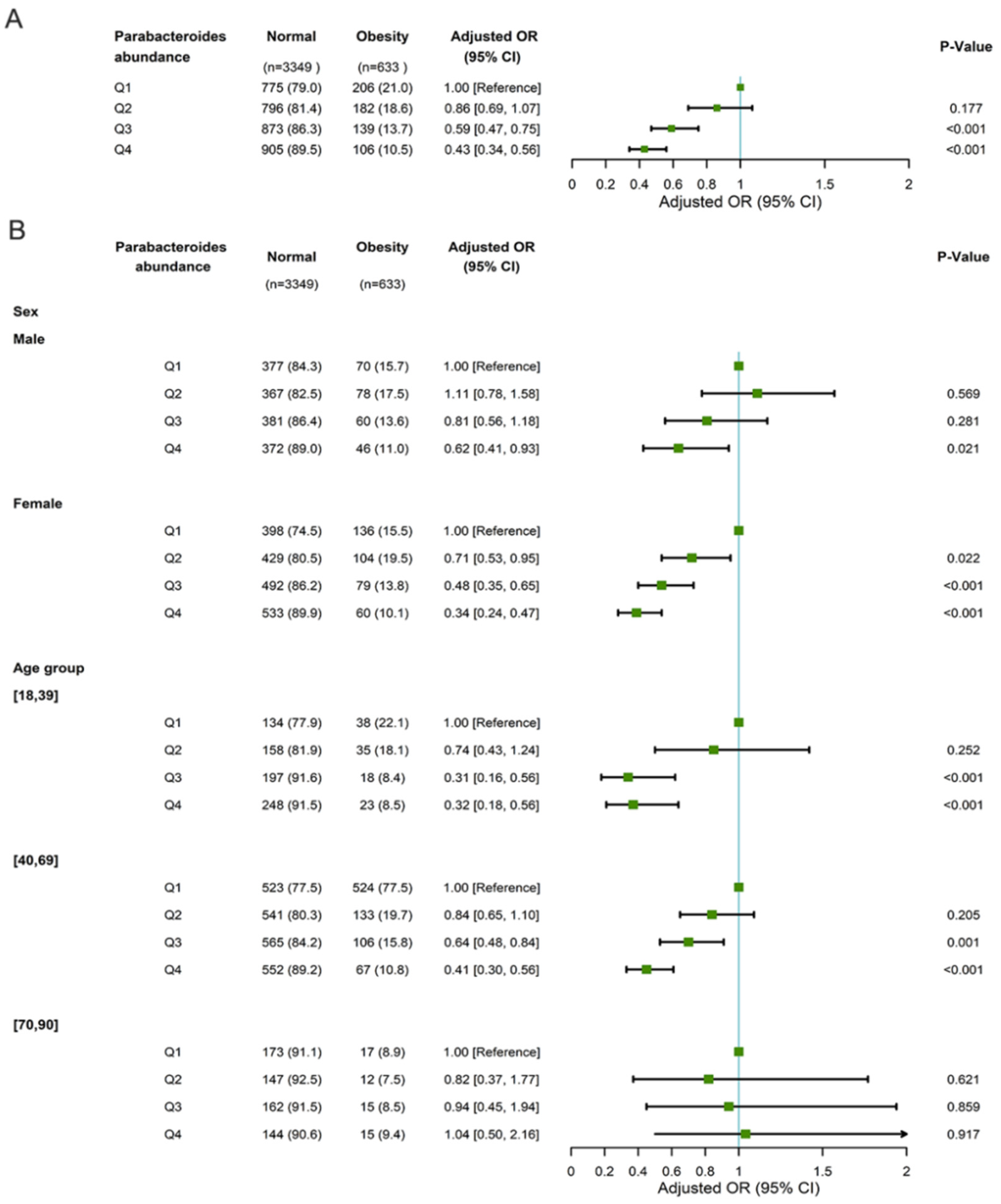
3.2. The Association between Parabacteroides Abundance and Obesity
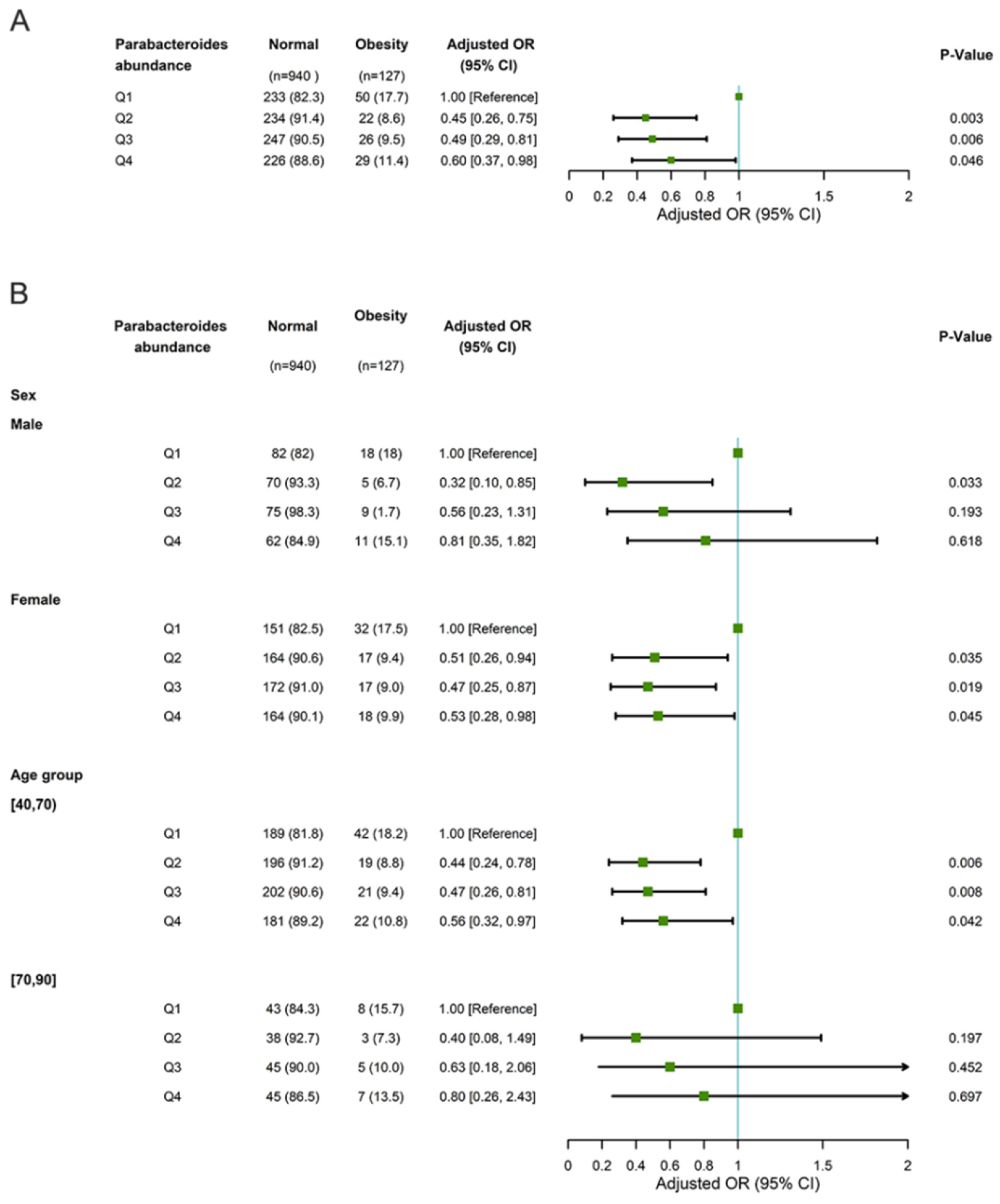
3.3. The Association of Parabacteroides Abundance with New-Onset Overweight/Obesity and No Longer Being Overweight/Obese
3.4. Prospective Association between Long-Term Weight Change Pattern and Parabacteroides in GNHS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017, 288, 1–8. [Google Scholar]
- Aminian, A.; Wilson, R.; Al-Kurd, A.; Tu, C.; Milinovich, A.; Kroh, M.; Rosenthal, R.J.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; et al. Association of Bariatric Surgery with Cancer Risk and Mortality in Adults with Obesity. JAMA 2022, 327, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Pugliese, G.; Liccardi, A.; Graziadio, C.; Barrea, L.; Muscogiuri, G.; Colao, A. Obesity and infectious diseases: Pathophysiology and epidemiology of a double pandemic condition. Int. J. Obes. 2022, 46, 449–465. [Google Scholar] [CrossRef]
- Colleluori, G.; Aguirre, L.; Phadnis, U.; Fowler, K.; Armamento-Villareal, R.; Sun, Z.; Brunetti, L.; Park, J.H.; Kaipparettu, B.A.; Putluri, N.; et al. Aerobic Plus Resistance Exercise in Obese Older Adults Improves Muscle Protein Synthesis and Preserves Myocellular Quality Despite Weight Loss. Cell Metab. 2019, 30, 261–273.e6. [Google Scholar] [CrossRef]
- Bouchard, C. Genetics of Obesity: What We Have Learned over Decades of Research. Obesity 2021, 29, 802–820. [Google Scholar] [CrossRef]
- Pan, X.-F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated with Obesity, Lipid Metabolism, and Metabolic Health—Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Wu, T.-R.; Lin, C.-S.; Chang, C.-J.; Lin, T.-L.; Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Lu, C.-C.; Young, J.D.; Lai, H.-C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- D’oplinter, A.d.W.; Rastelli, M.; Van Hul, M.; Delzenne, N.M.; Cani, P.D.; Everard, A. Gut microbes participate in food preference alterations during obesity. Gut Microbes 2021, 13, 1959242. [Google Scholar] [CrossRef] [PubMed]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Haange, S.-B.; Rolle-Kampczyk, U.; Engelmann, B.; Dietrich, A.; Thieleking, R.; Wiegank, C.; Fries, C.; Horstmann, A.; Villringer, A.; et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl. Psychiatry 2021, 11, 500. [Google Scholar] [CrossRef]
- Alderete, T.L.; Jones, R.B.; Shaffer, J.P.; Holzhausen, E.A.; Patterson, W.B.; Kazemian, E.; Chatzi, L.; Knight, R.; Plows, J.F.; Berger, P.K.; et al. Early life gut microbiota is associated with rapid infant growth in Hispanics from Southern California. Gut Microbes 2021, 13, 1961203. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Wu, S.; Zheng, H.-M.; Li, P.; Sheng, H.-F.; Chen, M.-X.; Chen, Z.-H.; Ji, G.-Y.; Zheng, Z.-D.; et al. Linking gut microbiota, metabolic syndrome and economic status based on a population-level analysis. Microbiome 2018, 6, 172. [Google Scholar] [CrossRef]
- He, Y.; Wu, W.; Zheng, H.-M.; Li, P.; McDonald, D.; Sheng, H.-F.; Chen, M.-X.; Chen, Z.-H.; Ji, G.-Y.; Zheng, Z.-D.; et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef]
- Gou, W.; Ling, C.-W.; He, Y.; Jiang, Z.; Fu, Y.; Xu, F.; Miao, Z.; Sun, T.-Y.; Lin, J.-S.; Zhu, H.-L.; et al. Interpretable Machine Learning Framework Reveals Robust Gut Microbiome Features Associated with Type 2 Diabetes. Diabetes Care 2021, 44, 358–366. [Google Scholar] [CrossRef]
- Zhou, B. Prospective study for cut-off points of body mass index in Chinese adults. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi 2002, 23, 431–434. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta-Zuluaga, J.; Kelley, S.T.; Chen, Y.; Escobar, J.S.; Mueller, N.T.; Ley, R.E.; McDonald, D.; Huang, S.; Swafford, A.D.; Knight, R.; et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. Msystems 2019, 4, e00261-19. [Google Scholar] [CrossRef] [PubMed]
- Santos-Marcos, J.A.; Haro, C.; Vega-Rojas, A.; Alcala-Diaz, J.F.; Molina-Abril, H.; Leon-Acuña, A.; Lopez-Moreno, J.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; et al. Sex Differences in the Gut Microbiota as Potential Determinants of Gender Predisposition to Disease. Mol. Nutr. Food Res. 2019, 63, e1800870. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Das, M.; Jeffery, I.B.; O’Toole, P.W. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. Elife 2020, 9, e50240. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Qiu, W.; Zheng, H.; Qi, C.; Hu, S.; Wu, W.; Wang, H.; Wu, G.; Cao, P.; Ma, Z.; et al. Ageing trajectory of the gut microbiota is associated with metabolic diseases in a chronological age-dependent manner. Gut 2022, 72, 1431–1433. [Google Scholar] [CrossRef]
- Yan, H.; Qin, Q.; Yan, S.; Chen, J.; Yang, Y.; Li, T.; Gao, X.; Ding, S. Comparison of the Gut Microbiota in Different Age Groups in China. Front. Cell. Infect. Microbiol. 2022, 12, 877914. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Liu, L.; Zhao, S.-S.; You, J.-Q.; Zhao, X.-J.; Liu, H.-X.; Xu, G.-W.; Wen, D.-L. Interplay between dietary intake, gut microbiota, and metabolic profile in obese adolescents: Sex-dependent differential patterns. Clin. Nutr. 2022, 41, 2706–2719. [Google Scholar] [CrossRef]
- Wang, J.-J.; Pang, X.-Y.; Zhao, L.-P.; Tian, L.; Wang, X.-P. Sex differences in colonization of gut microbiota from a man with short-term vegetarian and inulin-supplemented diet in germ-free mice. Sci. Rep. 2016, 6, 36137. [Google Scholar] [CrossRef]
- Li, R.; Andreu-Sánchez, S.; Kuipers, F.; Fu, J. Gut microbiome and bile acids in obesity-related diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101493. [Google Scholar] [CrossRef]
- Zeibich, L.; Koebele, S.V.; Bernaud, V.E.; Ilhan, Z.E.; Dirks, B.; Northup-Smith, S.N.; Neeley, R.; Maldonado, J.; Nirmalkar, K.; Files, J.A.; et al. Surgical Menopause and Estrogen Therapy Modulate the Gut Microbiota, Obesity Markers, and Spatial Memory in Rats. Front. Cell. Infect. Microbiol. 2021, 11, 702628. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall | Q1 | Q2 | Q3 | Q4 | p-Value |
|---|---|---|---|---|---|---|
| No. of participants | 5843 | 1416 | 1408 | 1440 | 1433 | |
| Age, year a | 52.98 (14.34) | 54.37 (13.74) | 53.51 (13.85) | 52.54 (14.57) | 51.39 (15.04) | <0.001 |
| Male (n, %) | 2598 (44.5) | 645 (45.6) | 638 (45.3) | 647 (44.9) | 612 (42.7) | 0.4 |
| BMI, kg/m2 a | 23.78 (3.23) | 24.22 (3.36) | 23.97 (3.33) | 23.52 (3.14) | 23.41 (3.02) | <0.001 |
| WC, cm a | 81.27 (9.36) | 82.82 (9.39) | 81.52 (9.68) | 80.62 (9.21) | 80.12 (8.99) | <0.001 |
| SBP, mmHg a | 132.24 (21.78) | 135.59 (22.13) | 133.16 (21.99) | 131.24 (21.59) | 128.76 (20.91) | <0.001 |
| DBP, mmHg a | 77.98 (11.49) | 79.34 (11.28) | 78.49 (11.79) | 77.70 (11.33) | 76.47 (11.34) | <0.001 |
| FBG, mmol/L b | 5.32 [4.93, 5.83] | 5.42 [4.98, 5.93] | 5.36 [4.98, 5.84] | 5.30 [4.90, 5.81] | 5.26 [4.87, 5.72] | <0.001 |
| HbA1c b | 4.90 [4.40, 5.30] | 4.90 [4.40, 5.30] | 4.90 [4.50, 5.30] | 4.90 [4.50, 5.30] | 4.90 [4.50, 5.30] | 0.62 |
| TC, mmol/L a | 5.26 (0.85) | 5.34 (0.87) | 5.29 (0.84) | 5.24 (0.87) | 5.16 (0.84) | <0.001 |
| Tg, mmol/L b | 1.10 [0.76, 1.64] | 1.16 [0.80, 1.71] | 1.10 [0.76, 1.70] | 1.05 [0.75, 1.56] | 1.09 [0.74, 1.63] | <0.001 |
| HDL-C, mmol/L b | 1.22 [1.00, 1.47] | 1.21 [0.98, 1.46] | 1.22 [1.00, 1.46] | 1.22 [1.01, 1.48] | 1.21 [1.00, 1.47] | 0.343 |
| LDL-C, mmol/L a | 3.28 (0.95) | 3.31 (0.97) | 3.28 (0.92) | 3.29 (0.95) | 3.24 (0.95) | 0.188 |
| UA, μmol/L a | 338.18 (91.98) | 345.15 (93.77) | 341.33 (93.40) | 332.25 (91.69) | 333.73 (88.46) | <0.001 |
| Variable | Risk Ratio * (95% CI) | p Value |
|---|---|---|
| Parabacteroides | 0.51 (0.23, 1.32) | 0.134 |
| Seq4172_ P. johnsonii | 0.89 (0.36, 1.91) | 0.786 |
| Seq831_ P. merdae | 1.20 (0.76, 1.91) | 0.440 |
| Seq12198_ P. distasonis | 0.91 (0.53, 1.50) | 0.716 |
| Seq4023_ P. distasonis | 1.20 (0.61, 2.21) | 0.571 |
| Seq6030_ P. johnsonii | 0.94 (0.51, 1.62) | 0.831 |
| Seq15333_ P. gordonii | 0.58 (0.24, 1.22) | 0.185 |
| Variable | Risk Ratio * (95% CI) | p Value |
|---|---|---|
| Parabacteroides | 0.73 (0.17, 2.16) | 0.618 |
| Seq4172_ P. johnsonii | 0.76 (0. 38, 1.66) | 0.459 |
| Seq831_ P. merdae | 0.72 (0.43, 1.18) | 0.196 |
| Seq12198_ P. distasonis | 1.10 (0.64, 1.95) | 0.725 |
| Seq4023_ P. distasonis | 0.96 (0.51, 1.96) | 0.911 |
| Seq6030_ P. johnsonii | 1.46 (0.75, 3.13) | 0.289 |
| Seq15333_ P. gordonii | 0.82 (0.40, 1.88) | 0.87 |
| The Main OTUs of Parabacteroides | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | Parabacteroides | Seq831 | Seq12198 | Seq4023 | Seq6030 | Seq4172 | ||||||
| Overall (n = 1243) | β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value |
| Model 1 | −2.40 (−6.79, 1.98) | 0.283 | −0.01 (−5.12, 5.09) | 0.996 | −2.86 (−14.543, 8.822) | 0.631 | −5.307 (−18.511, 7.897) | 0.431 | 22.54 (−11.31, 56.39) | 0.192 | −22.94 (−44.73, −1.14) | 0.039 |
| Model 2 | −2.46 (−6.84, 1.92) | 0.271 | −0.27 (−5.45, 4.91) | 0.919 | −2.083 (−13.896, 9.730) | 0.729 | −5.176 (−18.450, 8.099) | 0.444 | 22.34 (−11.34, 56.12) | 0.195 | −24.052 (−43.90, −0.38) | 0.046 |
| Female (n = 808) | ||||||||||||
| Model 1 | −0.38 (−5.91, 5.14) | 0.891 | 2.77 (−4.01, 9.57) | 0.422 | −4.75 (−18.89, 9.38) | 0.509 | −4.35 (−23.50, 14.79) | 0.655 | 23.92 (−12.74, 60.59) | 0.201 | −31.30 (−57.27, −5.32) | 0.018 |
| Model 2 | −0.63 (−6.15, 4.88) | 0.821 | 2.61 (−4.16, 9.40) | 0.449 | −5.54 (−19.66, 8.58) | 0.441 | −2.73 (−21.88, 16.42) | 0.780 | 22.98 (−13.62, 59.59) | 0.218 | −30.67 (−56.59, −4.74) | 0.021 |
| Male (n = 435) | ||||||||||||
| Model 1 | −4.42 (−10.69, 1.84) | 0.166 | −6.42 (−13.93, 1.09) | 0.094 | 8.81 (−13.95, 31.59) | 0.447 | −6.97 (−23.44, 9.49) | 0.406 | 45.96 (−5.70, 97.64) | 0.081 | 2.93 (−35.47, −41.35) | 0.881 |
| Model 2 | −4.20 (−10.45, 2.03) | 0.186 | −6.17 (−13.64, 1.30) | 0.106 | 11.26 (−11.44, 33.96) | 0.330 | −5.63 (−22.05, 10.75) | 0.50 | 39.01 (−12.80, 90.81) | 0.140 | 4.35 (−33.86, 42.57) | 0.823 |
| 40–69 years old (n = 1032) | ||||||||||||
| Model 1 | −1.21 (−5.57, 3.13) | 0.583 | 1.25 (−4.02, 6.53) | 0.640 | −4.90 (−16.97, 7.16) | 0.425 | −7.15 (−20.83, 6.52) | 0.305 | 19.11 (−9.42, 47.64) | 0.189 | −28.7 (−51.37, 6.07) | 0.013 |
| Model 2 | −1.27 (−5.63, 3.07) | 0.565 | 1.18 (−4.09, 6.45) | 0.660 | −5.02 (−17.08, 7.03) | 0.424 | −6.24 (−19.94, 7.44) | 0.370 | 17.85 (−10.69, 46.40) | 0.22 | −27.74 (−50.41, 5.08) | 0.016 |
| 70–90 years old (n = 211) | ||||||||||||
| Model 1 | −2.71 (−15.42, 10.03) | 0.676 | −5.99 (−21.98, 10.00) | 0.461 | 9.47 (−26.09, 45.04) | 0.60 | −1.85 (−40.94, 37.24) | 0.926 | 130.44 (−11.96, 272.85) | 0.072 | −6.94 (−63.87, 49.98) | 0.81 |
| Model 2 | −3.03 (−15.53, 9.46) | 0.539 | −4.91 (−20.62, 10.81) | 0.539 | 8.23 (−26.69, 43.14) | 0.643 | 3.59 (−34.93, 42.12) | 0.854 | 122.64 (−18.14, 263.41) | 0.087 | −11.17 (−67.09, 44.74) | 0.694 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Zhang, X.; Fu, J.; Duan, Z.; Qiu, W.; Cai, Y.; Ma, W.; Zhou, H.; Chen, Y.; Zheng, J.; et al. Sex- and Age-Dependent Associations between Parabacteroides and Obesity: Evidence from Two Population Cohort. Microorganisms 2023, 11, 2087. https://doi.org/10.3390/microorganisms11082087
Zhang F, Zhang X, Fu J, Duan Z, Qiu W, Cai Y, Ma W, Zhou H, Chen Y, Zheng J, et al. Sex- and Age-Dependent Associations between Parabacteroides and Obesity: Evidence from Two Population Cohort. Microorganisms. 2023; 11(8):2087. https://doi.org/10.3390/microorganisms11082087
Chicago/Turabian StyleZhang, Feng, Xiru Zhang, Jingxiang Fu, Zhuo Duan, Wen Qiu, Yijia Cai, Wenjun Ma, Hongwei Zhou, Yuming Chen, Jusheng Zheng, and et al. 2023. "Sex- and Age-Dependent Associations between Parabacteroides and Obesity: Evidence from Two Population Cohort" Microorganisms 11, no. 8: 2087. https://doi.org/10.3390/microorganisms11082087
APA StyleZhang, F., Zhang, X., Fu, J., Duan, Z., Qiu, W., Cai, Y., Ma, W., Zhou, H., Chen, Y., Zheng, J., & He, Y. (2023). Sex- and Age-Dependent Associations between Parabacteroides and Obesity: Evidence from Two Population Cohort. Microorganisms, 11(8), 2087. https://doi.org/10.3390/microorganisms11082087










