Abstract
The prevalence and genetic character of Wolbachia endosymbionts in field-collected Aedes aegypti mosquitoes were examined for the first time in Taiwan. A total of 665 Ae. aegypti were screened for Wolbachia infection using a PCR assay targeting the Wolbachia surface protein (wsp) gene. In general, the prevalence of Wolbachia infection was detected in 3.3% Ae. aegypti specimens (2.0% female and 5.2% male). Group-specific Wolbachia infection was detected with an infection rate of 1.8%, 0.8% and 0.8% in groups A, B and A&B, respectively. Genetic analysis demonstrated that all Wolbachia strains from Taiwan were phylogenetically affiliated with Wolbachia belonging to the supergroups A and B, with high sequence similarities of 99.4–100% and 99.2–100%, respectively. Phylogenetic relationships can be easily distinguished by maximum likelihood (ML) analysis and were congruent with the unweighted pair group with the arithmetic mean (UPGMA) method. The intra- and inter-group analysis of genetic distance (GD) values revealed a lower level within the Taiwan strains (GD < 0.006 for group A and GD < 0.008 for group B) and a higher level (GD > 0.498 for group A and GD > 0.286 for group B) as compared with other Wolbachia strains. Our results describe the first detection and molecular identification of Wolbachia endosymbiont in field-caught Ae. aegypti mosquitoes collected from Taiwan, and showed a low Wolbachia infection rate belonging to supergroups A and B in Ae. aegypti mosquitoes.
1. Introduction
Wolbachia is a facultative intracellular and naturally occurring endosymbiont found in a wide range of terrestrial arthropods and nematodes [1,2,3,4,5]. This bacterium was first discovered in the reproductive tissues of the Culex pipiens mosquito, and Wolbachia pipientis was firstly described [6]. In the insect host, it is estimated to be naturally present in 60–76% of known species [7,8,9]. Wolbachia endosymbiont is not known to directly infect vertebrates and contains a powerful ability to manipulate the reproductive system in diverse ways, such as parthenogenesis, feminization of males and inducing cytoplasmic incompatibility (CI), which cause deleterious alterations of the reproductive system in invertebrate hosts that will lead to the suppression of vector populations and interference in pathogen transmission [10]. This fascinating aspect of its ability has inspired researchers targeting this endosymbiont for vector control. Indeed, this reducing ability of Wolbachia has been utilized to eradicate the mosquito species of Culex pipiens fatigans [11]. Most recently, Wolbachia strains of wMel and wAlbB have been successfully transfected into Aedes aegypti mosquitoes and shown to inhibit/reduce infections with zika, dengue, chikungunya, yellow fever and Plasmodium [12,13,14,15,16,17,18]. Although Wolbachia have demonstrated the detrimental role of blocking the transmission of mosquito-borne viruses [18,19,20,21,22,23], the existence and genetic identity of Wolbachia endosymbiont in field-caught Aedes aegypti mosquitoes of Taiwan has never been investigated.
The Aedes aegypti is a major mosquito species around the world and is incriminated as the transmission vector for several mosquito-borne viruses that infect humans, especially dengue, zika, yellow fever, and chikungunya viruses [23,24,25]. Although previous studies have claimed that the Ae. aegypti is not naturally infected with Wolbachia [7,26,27], recent investigations have provided solid evidence of natural Wolbachia infection detected in Ae. aegypti, including the detection of Wolbachia endosymbiont in the larvae and adults of field-collected Ae. aegypti from Malaysia, India, USA, and Philippines [28,29,30,31]. Thus, this evidence clearly demonstrates that the natural infection of Wolbachia endosymbiont in Ae. aegypti mosquitoes appears to be more commonly observed than previously described. However, there has been no research focusing on the genetic composition and affiliation of Wolbachia endosymbiont in field-caught Ae. aegypti mosquitoes in Taiwan.
The molecular approach provides the feasibility to differentiate the genetic variance at the individual base-pair level and provides a much more powerful method for discriminating the genetic diversity between and within supergroups of Wolbachia endosymbionts [32,33,34,35,36,37]. Current investigations focused on the molecular markers of Wolbachia surface protein (wsp) and 16S rDNA genes have demonstrated the existence of at least 16 supergroups [8,38,39,40,41]. The supergroups A and B are mainly found in arthropods, and may alter reproduction [42]. Thus, molecular analysis based on the genetic variation of the wsp gene has made it possible to facilitate the genetic discrimination of taxonomically similar Wolbachia endosymbionts within various mosquitoes.
It is postulated that the Wolbachia endosymbionts in field-caught Ae. aegypti mosquitoes of Taiwan may be genetically different from the existing common groups of Wolbachia throughout the world. Thus, the objectives of this study are to examine the presence of Wolbachia in field-caught Ae. aegypti from Taiwan and to determine the genetic identity of Wolbachia endosymbionts detected in Ae. aegypti mosquitoes. In addition, the phylogenetic affiliation of Wolbachia strains detected in Ae. aegypti mosquitoes of Taiwan was further analyzed by comparing their differential nucleotide composition with other Wolbachia strains described from various biological and geographical sources that have been documented in GenBank.
2. Materials and Methods
2.1. Field Collection and Genetic Identification of Mosquito Specimens
All specimens of adult Ae. aegypti mosquitoes investigated in this study were collected from three districts (Fongshan, Cianjhen, and Lingya) of Kaohsiung City, located in southern Taiwan (Figure 1). All these mosquitoes were captured from several places close to human houses by using ovitrap and photocatalyst mosquito traps for a period of four weeks. These traps were placed in or outside the house and were operated continuously overnight, from 16:00 p.m. to 08:00 a.m. the following morning. All mosquito specimens were identified to the species level based on their morphological characteristics, as previously described [43], and collected specimens were stored at −80 °C for further molecular analysis. The genetic identification of the mosquito species of southern Taiwan was compared with the sequences documented in GenBank and performed by targeting the mitochondrial CO1 gene.
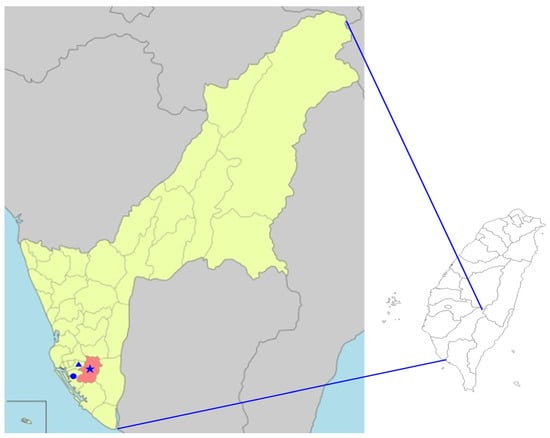
Figure 1.
Map of Kaohsiung City of Taiwan showing the mosquito collection sites from 3 districts of Fongshan (★), Cianjhen (●), and Lingya (▲) in Kaohsiung City.
2.2. DNA Extraction from Mosquito Specimens
Genomic DNA was extracted from individual mosquito specimens collected in this investigation. In general, the individual mosquito specimen was placed in a 1.5 mL microcentrifuge tube that was filled with 180 μL of lysing buffer solution equipped with a DNeasy Blood & Tissue Kit (catalogue no. 69506, Qiagen, Taipei, Taiwan), and then the samples were homogenized with a tissue homogenizer (TissueLyser II, Qiagen, Hilden, Germany), as instructed by the manufacturer. The homogenated fluid was further centrifuged at room temperature and the supernatant fluid was further processed by a DNeasy Blood & Tissue Kit, as instructed by the manufacturer. After filtration, the filtrated solution was collected with a second vial and the DNA concentration in the filtrated solution was measured with a microplate spectrophotometer (Epoch, Biotek, Shoreline, WA, USA), and the extracted DNA was stored at −80 °C for further analysis.
2.3. Wolbachia DNA Amplification via Nested Polymerase Chain Reaction (nPCR)
Extracted DNA samples from the mosquito specimens were used as a template for PCR amplification. Initially, the primer set of 81F (5′-TGGTCCAATAAGTGATGAAGAAAC-3′) and 691R (5′-AAAAATTAAACGCTACTCCA-3′) was used to amplify the universal wsp gene. A nested PCR was then performed using the group-A-specific primer set of 328F (5′-CCAGCAGATACTATTGCG-3′) and 691R, which amplified with a product of approximately 360 bp, and the primer set of 81F and 522R (5′-ACCAGCTTTTGCTTGATA-3′) served as the group-B-specific primers, which amplified with a product of approximately 440 bp, as previously described [37]. All PCR reagents and Taq polymerase enzymes were obtained and used as instructed by the supplier (Takara Shuzo Co., Ltd., Kyoto, Japan). The PCR amplification was performed with a thermocycler (Veriti, Applied Bioosystems, Taipei, Taiwan), and each 25 μL reaction mixture contained a 3 μL DNA template, 1.5 μL forward and reverse primers, 2.5 μL 10× PCR buffer (Mg2+), 2 μL dNTP mixture (10 mM each), 1 unit of Taq DNA polymerase and was filled up with an adequate volume of ddH2O. For comparison, adequate amounts of sterile distilled water were added in the reaction mixture for serving as a negative control. The PCR conditions were started with a pre-cycle of denaturation at 94 °C for 5 min and then amplified for 35 cycles with the conditions of denaturation at 94 °C for 1 min, annealing at 53 °C/55 °C for group A/B for 1 min, extension at 72 °C for 1 min, and a final extension step at 72 °C for 10 min. For visualizing the DNA products, all amplified products were electrophoresed on 1.5% agarose gels in Tris-Borate-EDTA (TBE) buffer and then the gels were stained with ethidium bromide. A 100 bp DNA ladder (GeneRuler, Thermo Scientific & Invitrogen, Taichung, Taiwan) was used as the standard marker for comparison. A negative control of distilled water was included in parallel with each amplification.
2.4. Genetic Identification of Mosquito Species
DNA samples extracted from Wolbachia-infected and uninfected mosquito specimens were used for identifying the genetic identity of tested mosquito by targeting the mitochondrial CO1 gene. The primer sets of CO1-F1/CO1-R1 were used to amplify the CO1 gene of mosquitoes, as described previously [44]. The PCR conditions for performing CO1 gene amplification were started with a pre-cycle of denaturation at 95 °C for 5 min and 5 cycles with the conditions of 94 °C for 40 s, 45 °C for 1 min, and 72 °C for 1 min. Thereafter, 35 cycles took place with the conditions of denaturation at 94 °C for 40 s, annealing at 52 °C for 1 min, extension at 72 °C for 1 min, and then a final extension step at 72 °C for 10 min. PCR amplification was performed with a thermocycler (Veriti, Applied Bioosystems, Taipei, Taiwan), and each 25 μL reaction mixture contained 3 μL DNA template, 1.5 μL forward and reverse primers, 2.5 μL 10× PCR buffer (Mg2+), 2 μL dNTP mixture (10 mM each), and 1 unit of Taq DNA polymerase and was filled up with an adequate volume of ddH2O. A negative control of distilled water was included in parallel with each amplification.
2.5. Phylogenetic Analysis Based on Wolbachia wsp Gene
Selective samples with clear bands on agarose gel were used for gene sequencing. In principle, 10 μL of each selective sample was submitted for DNA sequencing (Mission Biotech Co., Ltd., Taipei, Taiwan). After purification, sequencing reaction was performed with 25 cycles under the same conditions and the same primer set of the initial amplification of mosquito’s DNA with the dye-deoxy terminator reaction method using the Big Dye Terminator Cycle Sequencing Kit in an ABI Prism 377-96 DNA Sequencer (Applied Biosystems, Foster City, CA, USA). The determined sequences were initially edited with BioEdit software (V5.3) and aligned with the CLUSTAL W software [45]. Thereafter, the aligned sequences of Wolbachia wsp gene from Taiwan specimens were analyzed by comparing with other Wolbachia sequences containing 5 group A, 5 group B and 2 outgroup strains identified from the different biological and geographical origins documented in GenBank (Table 1). Phylogenetic analysis was performed with maximum likelihood (ML) compared with the unweighted pair group with arithmetic mean (UPGMA) method to estimate the phylogeny of the entire alignment using the MEGA X software package [46]. The genetic distance values of inter- and intra-species variations were also analyzed with the Kimura two-parameter model [47]. All phylogenetic trees were constructed and performed with 1000 bootstrap replications to evaluate the reliability of the construction, as described previously [48].

Table 1.
Wolbachia strains used for phylogenetic analysis in this study.
2.6. Nucleotide Sequence Accession Numbers
The nucleotide sequences of PCR-amplified wsp genes of 12 group A and 5 group B Wolbachia detected in field-caught Ae. aegypti mosquitoes of Taiwan have been registered and assigned the following GenBank accession numbers: group A of KH-FS-Ae-10409-F1 (OP882272), KH-FS-Ae-10410-F4 (OP882273), KH-FS-Ae-10410-F5 (OP882274), KH-FS-Ae-10410-F7 (OP882275), KH-FS-Ae-10410-F8 (OP882276), KH-FS-Ae-10410-F12 (OP882277), KH-FS-Ae-10411-M5 (OP882278), KH-FS-Ae-10411-M9 (OP882279), KH-FS-Ae-10411-M10 (OP882280), KH-FS-Ae-10411-M11 (OP882281), KH-FS-Ae-10411-M12 (OP882282), and KH-FS-Ae-10411-M13 (OP882283); group B of KH-FS-Ae-10410-F4 (OP896740), KH-FS-Ae-10411-M9 (OP896741), KH-FS-Ae-10411-M10 (OP896742), KH-FS-Ae-10411-M11 (OP896743) and KH-FS-Ae-10411-M12 (OP896744), respectively.
3. Results
3.1. Detection of Wolbachia in Field-Caught Ae. aegypti Mosquitoes
The presence of Wolbachia endosymbiont was detected in Ae. aegypti mosquitoes with a nested PCR assay targeting the group-specific wsp gene. The amplified products were visualized on gels with a molecular size of approximately 360 bp and 440 bp for group A and group B Wolbachia, respectively. The Wolbachia infection was detected in 3.3% (22/665) individual Ae. aegypti mosquitoes collected from Kaohsiung, Taiwan. An infection rate of 5.2% and 2.0% was detected in males and females, respectively (Table 2). In addition, group-specific Wolbachia infection with groups A, B and A&B was detected in 1.8% (12/665), 0.8% (5/665) and 0.8% (5/665) of mosquito specimens, respectively (Table 2).

Table 2.
Detection of Wolbachia infection in wild-caught Aedes aegypti mosquitoes collected from southern Taiwan with nested-PCR assay targeting the Wolbachia surface protein (wsp) gene.
3.2. Genetic Analysis of Wolbachia Detected in Field-Caught Ae. aegypti Mosquitoes
To identify the genetic identity of Wolbachia endosymbionts in Ae. aegypti mosquitoes of Taiwan, the wsp gene sequences of 12 group A and 5 group B Taiwan Wolbachia strains were aligned and analyzed with the downloaded Wolbachia sequences of 5 group A, 5 group B, and 2 outgroup strains from various origins documented in GenBank. The results revealed that all Wolbachia strains detected in Taiwan Ae. aegypti were genetically affiliated with the Wolbachia type strains of supergroups A (GenBank no. KY817476) and B (GenBank no. AF020059), with a high sequence similarity of 99.4–100% and 99.2–100%, respectively (Table 3 and Table 4). Based on the genetic distance (GD) values, the intra- and inter-species analysis revealed a lower level (GD < 0.006) of genetic divergence within the group A Taiwan strains as compared with the group B (GD > 0.498) and outgroup (GD > 0.788) Wolbachia strains (Table 3). In addition, a lower level (GD < 0.008) was observed within the group B Taiwan strains as compared with the group A (GD > 0.302) and outgroup (GD > 0.515) Wolbachia strains (Table 4).

Table 3.
Intra- and inter-group analysis of genetic distance values a based on the wsp gene sequences between the group A Wolbachia strains of Taiwan and other Wolbachia strains belonging to the supergroups A and B and outgroup documented in GenBank.

Table 4.
Intra- and inter-group analysis of genetic distance values a based on the wsp gene sequences between the group B Wolbachia strains of Taiwan and other Wolbachia strains belonging to the supergroups B and A and outgroup documented in GenBank.
3.3. Phylogenetic Analysis of Wolbachia Detected in Field-Caught Ae. aegypti Mosquitoes
Based on the sequence alignment of wsp genes, phylogenetic relationships were analyzed to reveal the genetic affiliation among 29 Wolbachia strains used in this study. The repeatability of the clustering specimens presented in phylogenetic trees was analyzed using bootstrap analysis. The phylogenetic relationships of group A and group B Wolbachia strains were constructed using the ML method, which showed one major clade of supergroup A and two major clades of supergroup B, which could be easily distinguished from other Wolbachia strains (Figure 2) and were congruent with UPGMA analysis (Figure 3). In principle, 12 group A and 5 group B Taiwan Wolbachia strains were analyzed with 2 outgroup strains and 5 other Wolbachia strains belonging to the groups A and B, respectively (Figure 2 and Figure 3). These comparable Wolbachia included Wolbachia strains from Ae. aegypti, Ae. albopictus, and Culex quinquefasciatus mosquitoes documented in GenBank (Table 1). Results revealed that all Taiwan Wolbachia strains constituted a monophyletic clade genetically affiliated to the Wolbachia strains of supergroups A (wAlbA) and B (WalbB), respectively. The discrimination from other Wolbachia strains could be easily demonstrated in the same group A or B with a bootstrap value of 99 and 100 in both ML and UPGMA analysis, respectively (Figure 2 and Figure 3). These results demonstrated a lower genetic divergence within the same group of Wolbachia detected in Ae. aegypti mosquitoes from Taiwan, but a higher genetic divergence from other Wolbachia groups documented from various biological and geographical origins.
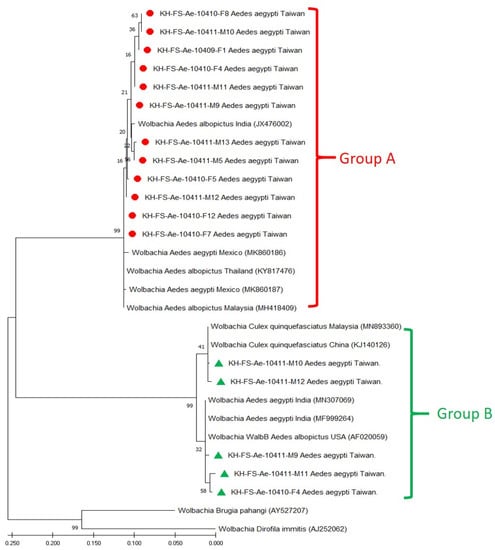
Figure 2.
Phylogenetic relationships based on the Wolbachia surface protein (wsp) gene sequences from 12 specimens of group A (indicated as ●) and 5 specimens of group B (indicated as ▲) of Aedes aegypti collected from Taiwan, compared with 12 other specimens belonging to supergroups A and B and outgroup Wolbachia documented in GenBank. The tree was constructed and analyzed with the Maximum Likelihood method using 1000 bootstraps replicates. Numbers at the nodes indicate the percentages of reliability of each branch of the tree. Branch length is drawn proportional to the estimated sequence divergence.
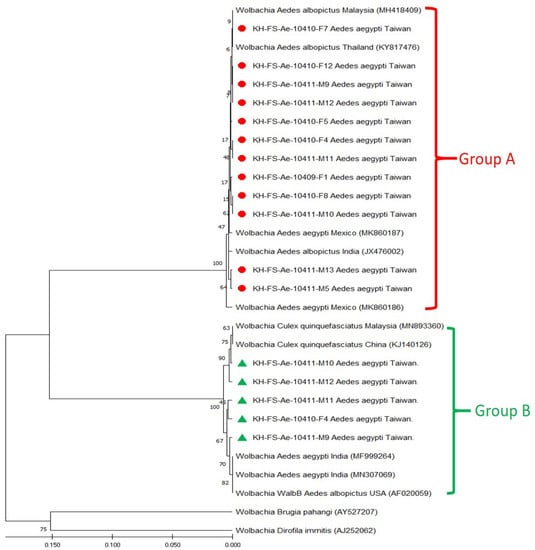
Figure 3.
Phylogenetic relationships based on the Wolbachia surface protein (wsp) gene sequences from 12 specimens of group A (indicated as ●) and 5 specimens of group B (indicated as ▲) of Aedes aegypti collected from Taiwan, compared with 12 other specimens belonging to supergroups A and B and outgroup Wolbachia documented in GenBank. The tree was constructed and analyzed with the UPGMA method using 1000 bootstrap replicates. Numbers at the nodes indicate the percentages of reliability of each branch of the tree. The branch length was drawn proportional to the estimated sequence divergence.
3.4. Molecular Identification of Field-Caught Ae. aegypti Mosquitoes of Taiwan
To further identify the Taiwan strain of Aedes mosquitoes, a PCR assay was performed by targeting the mitochondrial CO1 gene of selected Wolbachia-infected and uninfected Aedes mosquitoes. The CO1 gene sequences of six Aedes mosquitoes (four Wolbachia-infected and two uninfected) from Kaohsiung of Taiwan were genetically analyzed with sixteen other mosquito specimens belonging to three Aedes species (Ae. aegypti, Ae. albopictus and Ae. flavopictus) and four Culex species (Cx. quinquefasciatus, Cx. tritaeniorhynchus, Cx. fuscanus and Cx. gelidus). The results demonstrated that all Taiwan Aedes samples were genetically affiliated to the Ae. aegypti group, with a high sequence similarity (99.71–100% similarity), and can be obviously discriminated from other strains of Aedes and Culex mosquitoes (Figure 4). All these Ae. aegypti collected from Taiwan were registered and assigned GenBank numbers (OP889677, OP889681 and OP895029-032).
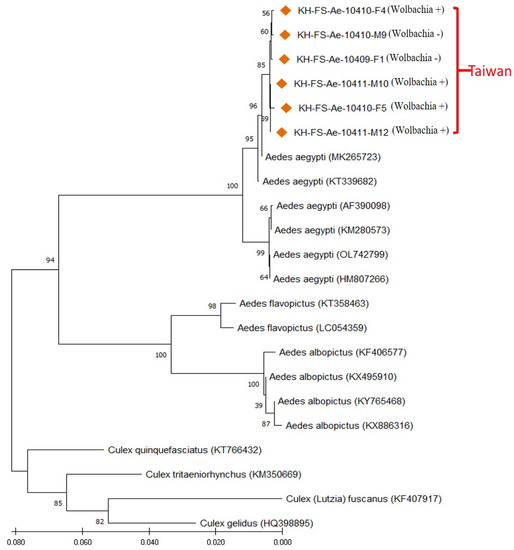
Figure 4.
Phylogenetic relationships based on the mosquito CO1 gene sequences from 6 specimens of Aedes aegypti collected from Taiwan (indicated with ◆) were compared with 16 specimens of various Aedes and Culex species documented in GenBank. The tree was constructed and analyzed with the Neighbor-Joining method using 1000 bootstraps replicates. Numbers at the nodes indicate the percentages of reliability of each branch of the tree. The branch length is drawn proportional to the estimated sequence divergence.
4. Discussion
The present study represents the first description regarding the molecular identification of Wolbachia endosymbiont in field-caught Ae. aegypti mosquitoes collected from southern Taiwan. In this study, the overall Wolbachia infection rate (3.3%) that was detected in Ae. aegypti mosquitoes collected from Taiwan was lower than the reported infection rates in previous studies, which were described as 16.8% in Manila, Philippines, 25% in Kuala Lumpur, Malaysia, and 57.4% in New Mexico, USA, respectively [28,29,30]. In addition, another study, investigating Ae. aegypti mosquitoes from India, also demonstrated the natural occurrence of Wolbachia infection [31]. Our detection of Wolbachia infection was performed with an individual mosquito sample. However, the Wolbachia infection reported by previous studies was identified by testing samples with pooled mosquitoes. Thus, this may partially explain the higher Wolbachia infection reported in previous studies. In any case, the present study reveals the first molecular detection of Wolbachia endosymbiont existing in Ae. aegypti mosquitoes collected from Kaohsiung of Taiwan, and provides the first convincing sequences (GenBank accession numbers: OP882272-83 and OP896740-4) of Wolbachia endosymbionts detected in field-collected Ae. aegypti mosquitoes of southern Taiwan.
The existence of natural Wolbachia infection in Ae. aegypti mosquitoes remains controversial. Although Ae. albopictus mosquitoes have been verified with natural Wolbachia infections [7,26,27], a recent global survey from various countries described the lack of natural Wolbachia infections in Ae. aegypti [27]. However, numerous recent studies have contradicted this claim, and have provided evidence of natural Wolbachia infections in Ae. aegypti mosquitoes [28,29,30,31]. In addition, the persistence of a low presence of Wolbachia sequences was also detected in the midgut of Ae. aegypti mosquitoes [49,50]. Indeed, results from this investigation indicated a low prevalence (3.3%) of natural Wolbachia infections in Ae. aegypti mosquitoes collected from the fields of southern Taiwan, and the Wolbachia infection in male (5.2%) was higher than in the female (2.0%). The reality of this biological characteristic may vary in Aedes mosquitoes distributed in various geographical areas or countries. Thus, the present study clearly demonstrated a low prevalence of natural Wolbachia infections in field-caught Ae. aegypti mosquitoes collected from southern Taiwan and revealed the possibility of the persistence of Wolbachia endosymbiont existing in natural Ae. aegypti populations.
The genetic group of the Wolbachia strain existing in field-collected Ae. aegypti mosquitoes needs to be further identified. Although previous reports described how most of the Wolbachia strains discovered in Ae. aegypti mosquitoes were identified as the supergroup B Wolbachia [29,30,31,32], results from the present observation demonstrated that a single Wolbachia strain (0.8% of group B and 1.8% of group A) was detected in the majority of the Wolbachia-infected Ae. aegypti mosquitoes from Taiwan. Only 0.8% (5/665) were simultaneously infected with supergroups A and B of Wolbachia endosymbiont (Table 2). The possible mechanisms regarding the modification rescue property [51] and the association with the bacteriophage WO infection [52] have been described with regard to the presence of supergroup A and co-infection with supergroups A and B in Ae. aegypti mosquitoes. In addition, the present study also revealed a higher Wolbachia infection in male Ae. aegypti, and whether this observation may explain the high possibility of maternal transmission of Wolbachia in the natural Ae. aegypti mosquito population requires further investigation. Thus, the genetic variation of Wolbachia strains in field-collected Ae. aegypti mosquitoes distributed in different geographical areas or countries needs to be further classified.
The genetic affiliation of Wolbachia strains detected in Ae. aegypti mosquitoes can be classified by comparing the sequence similarity of the wsp gene of the Wolbachia endosymbiont. Indeed, the sequence comparison of the wsp gene of Wolbachia endosymbiont has been shown to be useful for determining the genetic affiliation of Wolbachia strains among various species of arthropod hosts [37,42]. In the present study, the phylogenetic analysis of the wsp gene from Ae. aegypti mosquitoes of Taiwan displayed a high genetic similarity associated with the supergroups A and B (Figure 2 and Figure 3). The Wolbachia strains of group A are mainly affiliated with the wAlbA strain identified from Aedes albopictus (GenBank accession no. KY817476), and the Wolbachia strains of group B are affiliated with the WalbB strain identified from either Ae. albopictus (GenBank accession no. AF020059) or Aedes aegypti (GenBank accession no. MF999264). The phylogenetic trees constructed by either the Maximum Likelihood (ML) method or the unweighted pair group with arithmetic mean analysis (UPGMA) strongly support genetic discrimination, recognizing the separation of different supergroups between the Wolbachia strains detected in Ae. aegypti mosquitoes collected from Taiwan and other supergroups of Wolbachia strains from different biological and geographical origins. Accordingly, results from this study reveal that genetic identities of Wolbachia endosymbionts detected in field-caught Ae. aegypti collected from southern Taiwan were classified as a monophyletic group which was genetically affiliated to the supergroups A and B of Wolbachia endosymbionts. Further study should deepen the molecular analysis of various target genes of Wolbachia to reveal the reality of Wolbachia supergroups.
Due to the detrimental effects of Wolbachia endosymbiont on mosquito reproduction and pathogen replication, it is interested to evaluate the possible application of Wolbachia infections in natural mosquito populations. Indeed, mosquitoes infected with specific Wolbachia strains (wMel and wAlbB) have shown the ability to inhibit/limit a variety of human pathogens in mosquitoes, including dengue, chikungunya, zika and Plasmodium [12,13,14,15,16,17,18,19,20,21], and Wolbachia endosymbionts can be transmitted vertically from infected females to their offspring. These inherited Wolbachia can manipulate the host population through cytoplasmic incompatibility (CI) to regulate the mosquito’s reproduction. In general, when Wolbachia-infected males mate with females which are uninfected or harboring a different Wolbachia type, early embryo death occurs [16,20]. In addition, Wolbachia-induced CI has been used as a proposed strategy for reducing the mosquito population in the field by releasing laboratory-produced Wolbachia-infected males [9,11,17]. Indeed, field releases of Wolbachia-infected males of Aedes mosquitoes have been tested in several countries, including Australia, China, Singapore, the USA and Italy, and have significantly reduced the population densities of wild Aedes mosquito in the field [53,54,55,56]. However, there are still local differences between Ae. aegypti populations and the variation in persistence of Wolbachia infection in the field mosquitoes. Indeed, our study also found a low prevalence of Wolbachia infection in natural Aedes aegypti. Thus, any efforts or attempts to apply this Wolbachia-induced strategy in suppressing wild Aedes mosquito populations requires further testing and geographical analysis for promising adequate applications in different areas or countries.
In recent decades, dengue fever infection has been recognized as the major mosquito-borne human infection in Taiwan, and there were significant outbreaks of human infections with dengue fever during the years 2014–2015 that resulted in hundreds of deaths in southern Taiwan [57]. Although there have been no subsequent outbreaks of dengue fever in that region since then, sporadic human infections of domestic and imported human cases have still been reported in the following years. Although Ae. aegypti mosquitoes are incriminated as the main vector for the transmission of dengue virus, the mass spraying of insecticides for adult mosquitoes and the reduction of breeding sources for larval mosquitoes are routinely used as the traditional control strategies in Taiwan. It is postulated that transinfection of a suitable Wolbachia strain into local Ae. aegypti mosquitoes may cause the suppression of the Ae. aegypti population in a local environment [9,10,11,53,54,55,56]. However, the ability for laboratory mass-reared Wolbachia-infected males to compete with wild males for wild females and the adequate ratio for releasing Wolbachia-infected males are critical for the evaluation of the feasibility of this method in the natural environment. Thus, an open field trial is necessary for analyzing the possibility of applying this strategy by releasing Wolbachia-infected males to mate with females in the natural environment, and to follow up the suppressive impacts on Aedes aegypti populations in Taiwan.
5. Conclusions
This study describes the first molecular detection and genetic classification of the Wolbachia endosymbionts discovered in field-caught Ae. aegypti mosquitoes collected from southern Taiwan. Phylogenetic analysis based on the wsp gene of Ae. aegypti mosquitoes revealed them to be either singly or superinfected with both groups A and B of Wolbachia endosymbionts. In addition, this investigation also describes strong evidence of new findings of group A Wolbachia detected in field-collected Ae. aegypti mosquitoes. Due to the possible application of Wolbachia endosymbionts for the biological control of mosquito populations, the potential role of Wolbachia endosymbionts in vector mosquitoes and their microbiome interactions within mosquitoes need to be further identified.
Author Contributions
Conceptualization, C.-M.S. and L.-L.C.; investigation, C.-M.S. and L.-L.C.; formal analysis, C.-M.S. and L.-L.C.; writing of the manuscript, C.-M.S. and L.-L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by grants from the Ministry of Science and Technology (MOST 111-2314-B-037-031; NSTC 112-2923-B-037-001) and from a cooperative project between the National Pingtung University of Science and Technology and Kaohsiung Medical University, Taiwan (NPUST-KMU-112-P007).
Data Availability Statement
All data described in this paper are available after publication.
Acknowledgments
The authors are grateful to the staff of the Department of Health, Kaohsiung City Government, for helping in the collection of mosquitoes from various districts of Kaohsiung City in southern Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Werren, J.H. Biology of Wolbachia. Ann. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef] [PubMed]
- Tram, U.; Sullivan, W. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 2002, 296, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Bandi, C.; Anderson, T.J.; Genchi, C.; Blaxter, M.L. Phylogeny of Wolbachia in filarial nematodes. Proc. R. Soc. Lond. Biol. Sci. 1998, 265, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.; Schmetz, C.; Bandi, C.; Bonow, I.; Mand, S.; Fischer, K.; Buttner, D.W. Tunga penetrans: Molecular identification of Wolbachia endobacteria and their recognition by antibodies against proteins of endobacteria from filarial parasites. Exp. Parasitol. 2002, 102, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Bazzocchi, C.; Jamnongluk, W.; O’Neill, S.L.; Anderson, T.J.C.; Genchi, C.; Bandi, C. Wsp gene sequences from the Wolbachia of filarial nematodes. Cur. Microbiol. 2000, 41, 96–100. [Google Scholar] [CrossRef]
- Hertig, M.; Wolbach, S.B. Studies on Rickettsia-Like Micro-Organisms in Insects. J. Med. Res. 1924, 44, 329–374.7. [Google Scholar]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Jeyaprakash, A.; Hoy, M.A. Long PCR improves Wolbachia DNA amplification: Wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 2000, 9, 393–405. [Google Scholar] [CrossRef]
- Rasgon, J.L. Wolbachia induces male-specific mortality in the mosquito Culex pipiens (LIN strain). PLoS ONE 2012, 7, e30381. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Laven, H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 1967, 216, 383–384. [Google Scholar] [CrossRef]
- Aliota, M.T.; Peinado, S.A.; Velez, I.D.; Osorio, J.E. The wMel strain of Wolbachia reduces transmission of zika virus by Aedes aegypti. Sci. Rep. 2016, 6, 28792. [Google Scholar] [CrossRef]
- Aliota, M.T.; Walker, E.C.; Yepes, A.; Velez, I.D.; Christensen, B.M.; Osorio, J.E. The wMel Strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004677. [Google Scholar] [CrossRef]
- Nazni, W.A.; Hoffmann, A.A.; NoorAfizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, G.M.; Arif, M.A.; Thohir, H.; NurSyamimi, H.; et al. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr. Biol. 2019, 29, 4241–4248. [Google Scholar] [CrossRef]
- Frentiu, F.D.; Zakir, T.; Walker, T.; Popovici, J.; Pyke, A.T.; van den Hurk, A.; McGraw, E.A.; O’Neill, S.C. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl. Trop. Dis. 2014, 8, e2688. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- McMenimen, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.; Sidhu, M.; Wang, Y.F.; O’Neill, S.L. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The endosymbiotic bacterium Wolbachia induces resistence to dengue virus in Aedes aegypti. PLoS Path. 2010, 6, e1000833. [Google Scholar] [CrossRef]
- Hancock, P.A.; Sinkins, S.P.; Godfray, H.C. Strategies for introducing Wolbachia to reduce transmission of mosquito-borne diseases. PLoS Negl. Trop. Dis. 2011, 5, e1024. [Google Scholar] [CrossRef]
- Walker, T.J.P.H.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.J.; Turelli, M. Wolbachia versus dengue: Evolutionary forecasts. Evol. Med. Public Health 2013, 2013, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Mehlhom, H. Declining malaria, rising of dengue and zika virus: Insights for mosquito vector control. Parasitol. Res. 2016, 115, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Kittayapong, P.; Baisley, K.J.; Baimai, V.; O’Neill, S.L. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2000, 37, 340–345. [Google Scholar] [CrossRef]
- Ricci, I.; Cancrini, G.; Gabrielli, S.; D’amelio, S.; Favia, G. Searching for Wolbachia (Rickettsiales, Rickettsiaceae) in mosquitoes (Diptera: Culicidae): Large polymerase chain reaction survey and new identifications. J. Med. Entomol. 2002, 39, 562–567. [Google Scholar] [CrossRef]
- Gloria-Soria, A.; Chiodo, T.G.; Powell, J.R. Lack of evidence for natural Wolbachia infections in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2018, 55, 1354–1356. [Google Scholar] [CrossRef]
- Teo, C.H.; Lim, P.K.; Voon, K.; Mak, J.W. Detection of dengue viruses and Wolbachia in Aedes aegypti and Aedes albopictus larvae from four urban localities in Kuala Lumpur, Malaysia. Trop. Biomed. 2017, 34, 583–597. [Google Scholar]
- Carvajal, T.M.; Hashimoto, K.; Harnandika, R.K.; Amalin, D.M.; Watanabe, K. Detection of Wolbachia in field-collected Aedes aegypti mosquitoes in metropolitan Manila, Philippines. Parasit. Vectors 2019, 12, 361. [Google Scholar] [CrossRef]
- Kulkarni, A.; Yu, W.; Jiang, J.; Sanchez, C.; Karna, A.K.; Martinezk, K.J.L.; Hanley, K.A.; Buenemann, M.; Hansen, I.A.; Xue, R.D.; et al. Wolbachia pipientis in Aedes aegypti populations in New Mexico and Florida, USA. Ecol. Evol. 2019, 9, 6148–6156. [Google Scholar] [CrossRef]
- Balaji, S.; Jayachandran, S.; Prabagaran, S.R. Evidence for the natural occurrence of Wolbachia in Aedes aegypti mosquitoes. FEMS Microbiol. Lett. 2019, 366, fnz055. [Google Scholar] [CrossRef]
- Benson, M.J.; Gawronski, J.D.; Eveleigh, D.E.; Benson, D.R. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Appl. Environ. Microbiol. 2004, 70, 616–620. [Google Scholar] [CrossRef]
- Bordenstein, S.; Rosengaus, R.B. Discovery of a novel Wolbachia super group in Isoptera. Curr. Microbiol. 2005, 51, 393–398. [Google Scholar] [CrossRef]
- Andreotti, R.; Perez, A.A.; Scoles, G.A. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 2011, 11, 6–9. [Google Scholar] [CrossRef]
- Carpi, G.; Cagnacci, F.; Wittekindt, N.E.; Zhao, F.; Qi, J.; Tomsho, L.P.; Drautz, D.I.; Rizzoli, A.; Schuster, S.C. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS ONE 2011, 6, e25604. [Google Scholar] [CrossRef]
- Bing, X.L.; Xia, W.Q.; Gui, J.D.; Yan, G.H.; Wang, X.W.; Liu, S.S. Diversity and evolution of the Wolbachia endosymbionts of Bemisia (Hemiptera: Aleyrodidae) whiteflies. Ecol. Evol. 2014, 4, 2714–2737. [Google Scholar] [CrossRef]
- Zhou, W.; Rousset, F.; O’Neil, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef]
- Ruang-Areerate, T.; Kittayapong, P.; Baimai, V.; O’Neill, S.L. Molecular phylogeny of Wolbachia endosymbionts in Southeast Asian mosquitoes (Diptera: Culicidae) based on wsp gene sequences. J. Med. Entomol. 2003, 40, 1–5. [Google Scholar] [CrossRef]
- Chai, H.N.; Du, Y.Z.; Qiu, B.L.; Zhai, B.P. Detection and phylogenetic analysis of Wolbachia in the Asiatic rice leafroller, Cnaphalocrocis medinalis, in Chinese populations. J. Insect Sci. 2011, 11, 123. [Google Scholar] [CrossRef]
- Zhang, X.; Norris, D.E.; Rasgon, J.L. Distribution and molecular characterization of Wolbachia endosymbionts and filarial nematodes in Maryland populations of the lone star tick (Amblyomma americanum). FEMS Microbiol. Ecol. 2011, 77, 50–56. [Google Scholar] [CrossRef]
- Wang, G.H.; Jia, L.Y.; Xiao, J.H.; Huang, D.W. Discovery of a new Wolbachia supergroup in cave spider species and the lateral transfer of phage WO among distant hosts. Infect. Genet. Evol. 2016, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Zhang, W.; Guo, L.R. Evolution and phylogeny of Wolbachia: Reproductive parasites of arthropods. Proc. R. Soc. Lond. Biol. Sci. 1995, 261, 55–63. [Google Scholar]
- WHO. Pictorial Identification Key of Important Disease Vectors in the WHO South-East Asia Region; WHO: Geneva, Switzerland, 2020; ISBN 978-92-9022-758-8. [Google Scholar]
- Chan, A.; Chiang, L.P.; Hapuarachchi, H.C.; Tan, C.H.; Pang, S.C.; Lee, R.; Lee, K.S.; Ng, L.C.; Lam-Phua, S.G. DNA barcoding: Complementing morphological identification of mosquito species in Singapore. Parasit. Vectors 2014, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Bio. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 52, 1119–1134. [Google Scholar]
- Coon, K.L.; Brown, M.R.; Strand, M.R. Mosquitoes host communities of bacterial that are essential for development but vary greatly between local habitats. Mol. Ecol. 2016, 25, 5806–5826. [Google Scholar] [CrossRef]
- Thongsripong, P.; Chandler, J.A.; Green, A.B.; Kittayapong, P.; Wilcox, B.A.; Kapan, D.D.; Bennett, S.N. Mosquito vector-associated microbiota: Metabarcoding bacteria and eukaryotic symbionts across habitat types in Thailand endemic for dengue and other arthropod-borne diseases. Ecol. Evol. 2018, 8, 1352–1368. [Google Scholar] [CrossRef]
- Atyame, C.M.; Labbe, P.; Dumas, E.; Milesi, P.; Charlat, S.; Fort, P.; Weill, M. Wolbachia divergence and the evolution of cytoplasmic incompatibility in Culex pipiens. PLoS ONE 2014, 9, e87336. [Google Scholar] [CrossRef]
- Ravikumar, H.; Ramachandraswamy, N.; Sampathkumar, S.; Prakash, B.M.; Huchesh, H.C.; Uday, J.; Puttaraju, H.P. A preliminary survey for Wolbachia and bacteriophage WO infection in Indian mosquitoes (Diptera: Culicidae). Trop. Biomed. 2010, 27, 384–393. [Google Scholar]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Crawford, J.E.; Clarke, D.W.; Criswell, V.; Desnoyer, M.; Cornel, D.; Deegan, B.; Gong, K.; Hopkins, K.C.; Howell, P.; Hyde, J.S.; et al. Efficient production of male Wolbachia infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nat. Biotechnol. 2020, 38, 482–492. [Google Scholar] [CrossRef]
- National Environment Agency, Singapore. Project Wolbachia Singapore. Available online: https://www.nea.gov.sg/corporate-functions/resources/research/wolbachia-aedes-mosquito-suppression-strategy (accessed on 15 June 2023).
- Caputo, B.; Moretti, R.; Manica, M.; Serini, P.; Lampazzi, E.; Bonanni, M.; Fabbri, G.; Pichler, V.; della Torre, A. A bacterium against the tiger: Preliminary evidence of fertility reduction after release of Aedes albopictus males with manipulated Wolbachia infection in an Italian urban area. Pest Manag. Sci. 2020, 76, 1324–1332. [Google Scholar] [CrossRef]
- Dengue Fever in Taiwan. CDC, Taiwan. Available online: https://www.cdc.gov.tw/Disease/SubIndex/WYbKe3aE7LiY5gb-eA8PBw (accessed on 15 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).