Sanitizing Hatching Eggs with Essential Oils: Avian and Microbiological Safety
Abstract
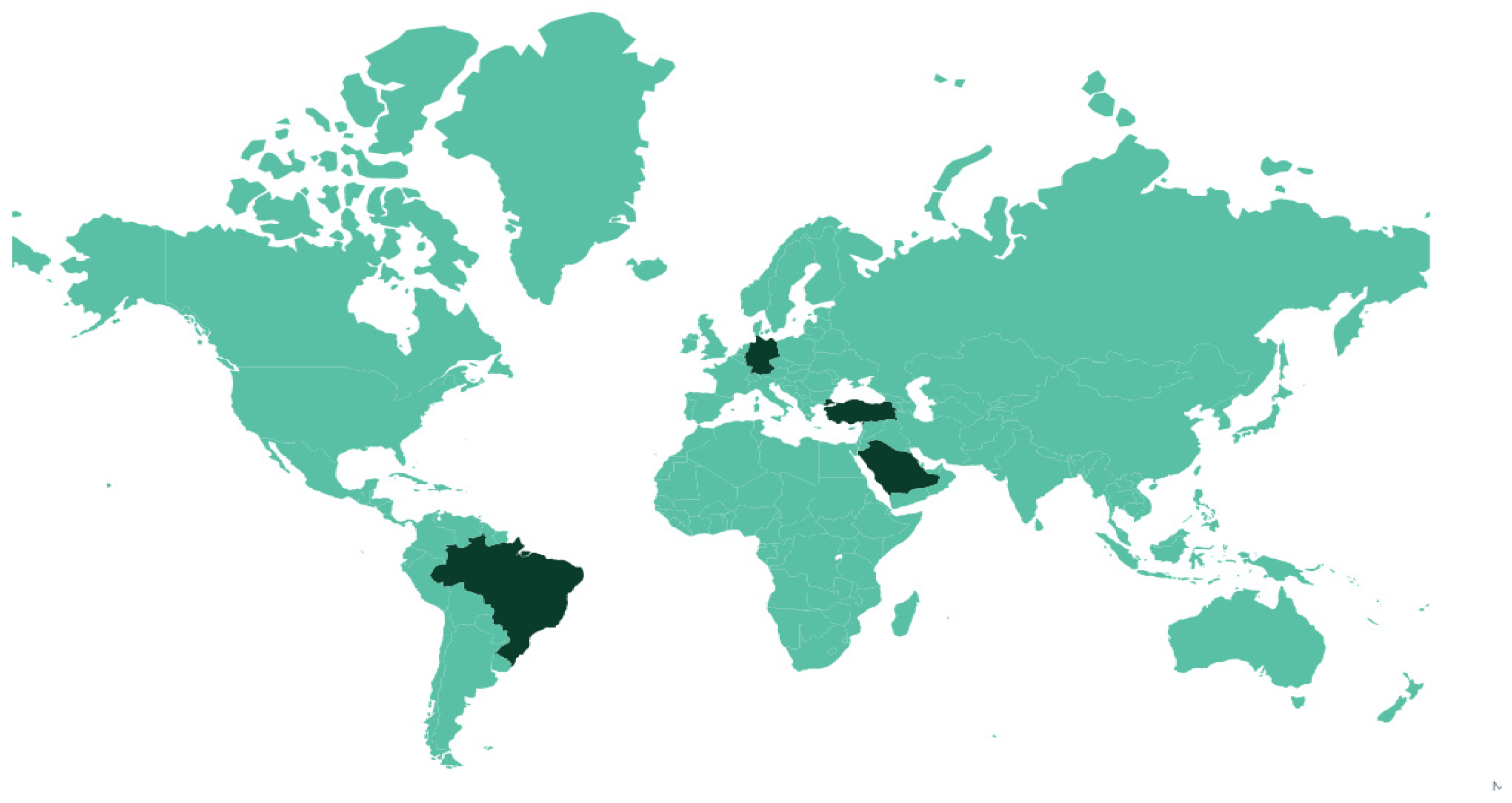
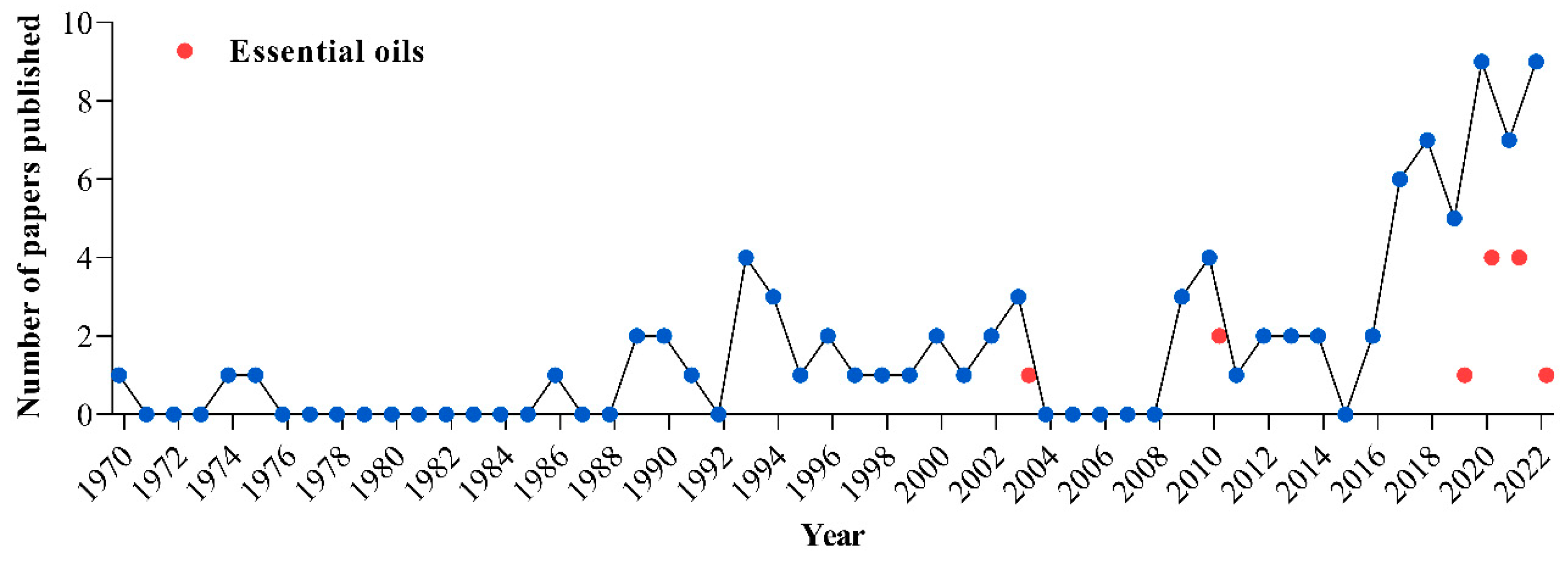
1. Introduction
2. Paper Search Method
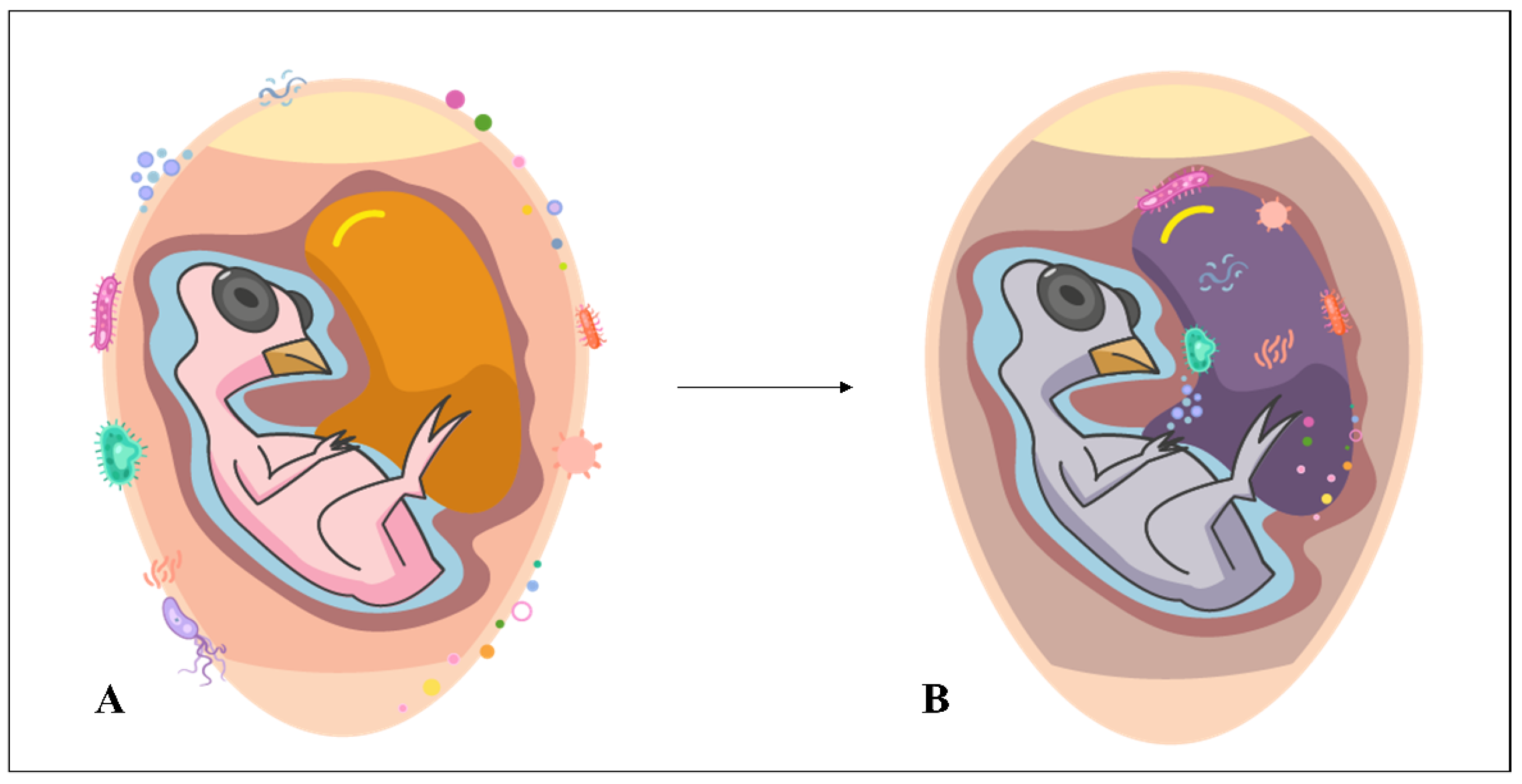
3. Eggshell Microorganisms: Risks for Poultry Embryos and Chicks
4. Essential Oils and Their In Vitro Antimicrobial Activity
- Bacteria:
- Cell membrane alteration and increased permeability.
- Stops energy production.
- Blocks active transport.
- Fungi:
- Cell membrane disruption, alteration, and inhibition of cell wall formation.
- Dysfunction of fungal mitochondria.
- Inhibition of efflux pumps.
| Essential Oil | Majority Element | Analysis | Method | Microorganism | Origin Microorganism | Study |
|---|---|---|---|---|---|---|
| Thymus vulgaris Origanum vulgare Mentha pulegium | - | B* | Disk diffusion |
| ATCC | [34] |
| Allium sativum | Diallyl disulfide (44.6%) | B |
| ATCC and NCTC | [35] | |
| Cinnamomum cassia Syzygium aromaticum | Eugenol (72.13%) Eugenol (83.63%) | B | Agar dilution |
| ATCC and human clinical isolate | [36] |
| Thymus vulgaris Foeniculum vulgare Cuminum cyminum | - | B and F* | Disk diffusion |
| MTCC | [37] |
| Origanum vulgare Origanum majorana | - | B | Disk diffusion and broth microdilution |
| Poultry meat | [38] |
| Thymus vulgaris Origanum vulgare | Thymol (41.60%) Carvacrol (53.4%) | B | Broth microdilution |
| Clinical isolate and poultry meat isolate | [39] |
| Lippia rotundifolia Lippia origanoides | - | B | Disk diffusion and dilution |
| Poultry feces | [40] |
| Thymus schimperi Rosmarinus officinalis Eucalyptus globulus | Carvacrol (71.02%) α-Pinene (50.83%) 1,8-Cineole (63.00%) | B | Well diffusion |
| - | [41] |
| Pimenta pseudocaryophyllus Citrus Terpenes | - Limonene (28.67%) | B | Disk diffusion |
| ATCC | [42] |
| Lavandula × intermedia Lavandula angustifolia | Linalool (57.10%) Linalool (53.97%) | B and F | Well diffusion and broth microdilution |
| ATCC, NCTC, and food and clinical isolates | [43] |
| Kaempferia galanga Cymbopogon flexuosus Pogostemon cablin Curcuma caesia Cymbopogon winterianus Clausena heptaphylla Cinnamomum tamala Ocimum sanctum Cinnamomum camphora | P-Methoxycinnamate (27.84%) Geranial (Citral a) (42.14%) Patchouli alcohol (32.33%) Eucalyptol (15.05%) Citronellal (38.68%) (E)-Anethole (53.49%) Eugenol (72.33%) Eugenol (41.89%) Camphor (49.43%) | B and F | Disk diffusion and broth dilution |
| ATCC | [44] |
| Aloysia triphylla Cinnamomum zeylanicum Cymbopogon citratus Litsea cubeba Mentha piperita | Limonene (E)-Cinamaldeído Neral Geranial Mentol | B and F | Disk diffusion and broth microdilution |
| Poultry | [45] |
| Syzygium aromaticum | - | B | Disk diffusion and dilution |
| Poultry | [46] |
| Satureja kitaibelii | p-Cymene (24.4%) | B | Broth microdilution |
| ATCC | [47] |
| Origanum vulgare | Germacrene D (21.5%) | |||||
| Achillea millefolium | Camphor (9.8%) | |||||
| Achillea clypeolata | 1,8-Cineole (45.1%) | |||||
| Thymus serpyllum | Geraniol (63.4%) | |||||
| Origanum vulgare | Carvacrol (66.98%) | B | Broth microdilution |
| Intensive poultry farms (boot swabs) | [48] |
| Melaleuca alternifolia | Terpinen-4-ol (>30%) | B and F | Modified zone of inhibition test with glass cylinders |
| ATCC | [49] |
| Rosmarinus officinalis | 1,8-Cineole (>30%) | |||||
| Cinnamomum cassia | Trans-cinnamaldehyde (>30%) | |||||
| Cymbopogon flexuosus | Citral (81.84%) | B and F | Disk diffusion and serial dilution |
| NCIM and isolated from blight and blast infected leaves | [50] |
| Cymbopogon martini | Geraniol (63.79%) | |||||
| Eucalyptus citridora | Citronellal (76.80%) | |||||
| Pelargonium spp. | Geraniol (22.38%) | |||||
| Cymbopogon winterianus | Citronellal (34.10%) | |||||
| Satureja hortensis | Thymol (41.28%) | B | Disk diffusion and broth microdilution |
| Poultry infections | [51] |
| Origanum vulgare | Carvacrol (65.80%) | B and F | Disk diffusion |
| ATCC | [52] |
| Melaleuca alternifolia | Terpinen-4-ol (39.60%) | |||||
| Citrus limonum, Cinnamomum cassia, Eugenia caryophyllus, Eucalyptus globulus, and Rosmarinus officinalis | - | |||||
| Ocimum basilicum | Linalool (65.20%) | |||||
| Crithmum maritimum | γ-Terpinene (32.9%) | B and F | Well Diffusion and broth microdilution |
| ATCC and DSMZ | [53] |
| Cuminum cyminum | Cumin aldehyde (30.2%) | |||||
| Cupressus arizonica | α-Pinene (41.0%) | |||||
| Pimpinella anisum | (E)-Anethole (96.7%) | |||||
| Zingiber officinale | - | B | Disk diffusion |
| ATCC | [54] |
| Citrus aurantifolia | ||||||
| Cymbopogon citratus | ||||||
| Origanum vulgare | Carvacrol (68.72%) | B | Broth microdilution |
| ATCC and isolated food | [55] |
| Thymus vulgaris | Thymol (54.60%) | |||||
| Eugenia caryophyllus | Eugenol (86.25%) | |||||
| Cinnamomum cassia | Trans-cinnamaldehyde (86.57%) | |||||
| Ocimum basilicum | Estragole (60.98%) | B | Broth microdilution |
| ATCC | [56] |
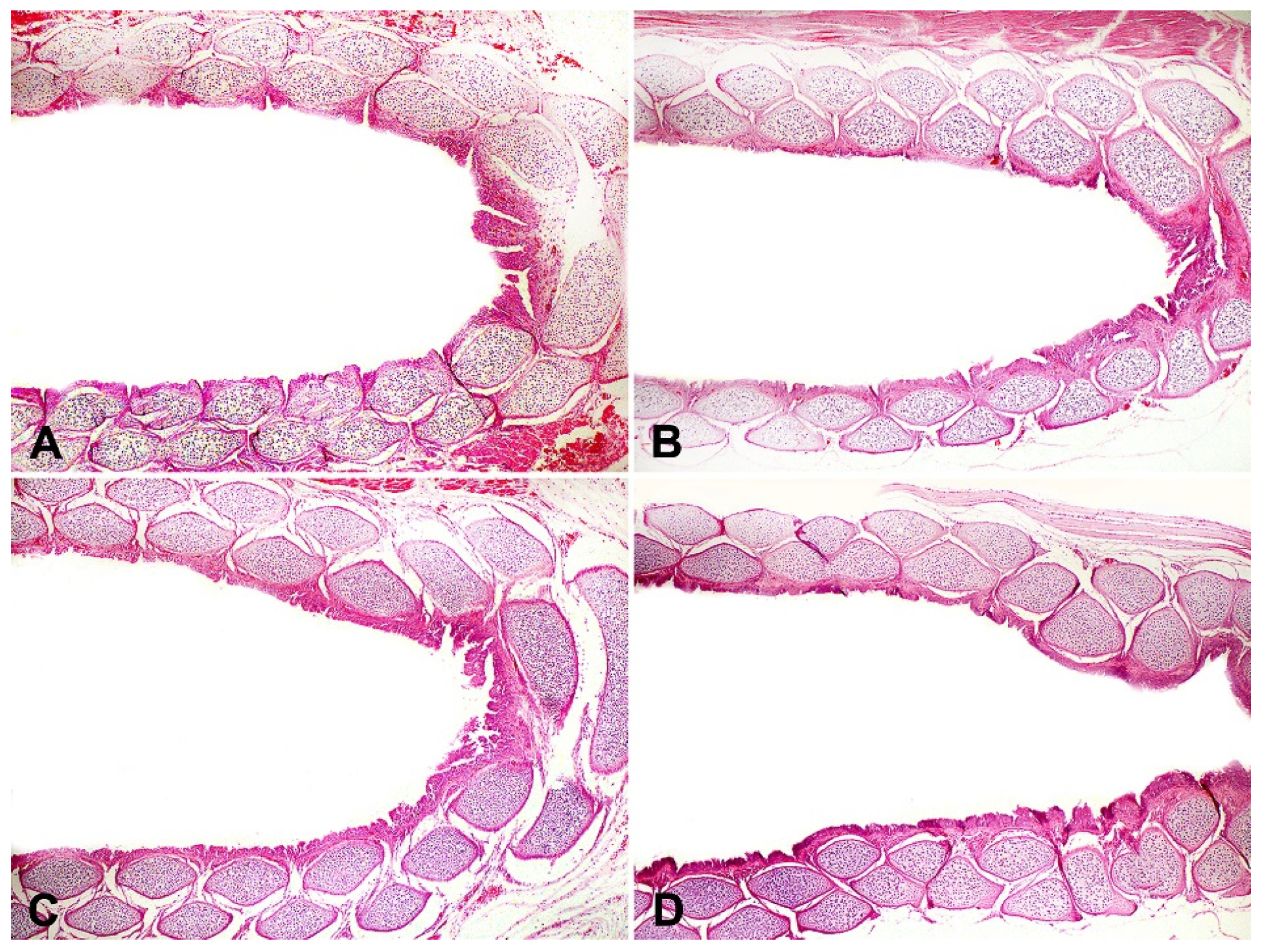
5. Antimicrobial Effect of Essential Oils In Vivo (Eggshells)
6. Toxicity of Essential Oils for Poultry Embryos and Chicks
7. Comparing Essential Oils and Formaldehyde for Sanitizing Hatching Eggs
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rezaee, M.S.; Liebhart, D.; Hess, C.; Hess, M.; Paudel, S. Bacterial Infection in Chicken Embryos and Consequences of Yolk Sac Constitution for Embryo Survival. Vet. Pathol. 2021, 58, 71–79. [Google Scholar] [CrossRef]
- Fardows, J.; Shamsuzzaman, S.M. Detection of Potential Pathogenic Aerobic Bacteria from Egg Shell and Egg Contents of Hen Collected from Poultry. Bangladesh Med. Res. Counc. Bull. 2015, 41, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.d.S.; McManus, C.; Salgado, C.B.; dos Santos, V.M. Effects of Sanitizers on Microbiological Control of Hatching Eggshells and Poultry Health during Embryogenesis and Early Stages after Hatching in the Last Decade. Animals 2022, 12, 2826. [Google Scholar] [CrossRef]
- Araújo, W.A.G.; Albino, L.F.T. Incubação Comercial [Commercial Incubation]; Transworld Research Network: Trivandrum, India, 2011. [Google Scholar]
- Oliveira, G.d.S.; dos Santos, V.M.; Nascimento, S.T. Essential Oils as Sanitisers for Hatching Eggs. Worlds Poult. Sci. J. 2021, 77, 605–617. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; dos Santos, V.M.; McManus, C. Propolis: Effects on the Sanitisation of Hatching Eggs. Worlds Poult. Sci. J. 2022, 78, 261–272. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; McManus, C.; dos Santos, V.M. Garlic as Active Principle of Sanitiser for Hatching Eggs. Worlds Poult. Sci. J. 2022, 78, 1037–1052. [Google Scholar] [CrossRef]
- Jahantigh, M. Bacteriological Study of Dead-in-Shell Embryos of Ostrich. Iran J. Vet. Res. 2010, 11, 88–90. [Google Scholar]
- Fonseca, B.B.; Beletti, M.E.; Melo, R.T.; Mendonça, E.P.; Vieira, C.U.; Levenhagen, M.A.; Rossi, D.A. Transfer, Viability and Colonisation of Campylobacter jejuni in the Chicken Vitellus and in Embryos. Br. Poult. Sci. 2011, 52, 279–286. [Google Scholar] [CrossRef]
- Hincke, M.; Gautron, J.; Rodriguez-Navarro, A.B.; McKee, M.D. The Eggshell: Structure and Protective Function. In Improving the Safety and Quality of Eggs and Egg Products: Egg Chemistry, Production and Consumption; Woodhead Publishing: Sawston, UK, 2011; pp. 151–182. [Google Scholar]
- Weil, A.J.; Volentine, J.A. Infection of the Developing Chick Embryo with Dysentery Bacilli. Proc. Soc. Exp. Biol. Med. 1940, 44, 160–161. [Google Scholar] [CrossRef]
- Kwanashie, C.N.; Kazeem, H.M.; Umoh, J.U.; Abdu, P.A. Aspergillus Species Associated with Dead-in-Shell Chick Embryo in Some Hatcheries in Northwest Nigeria. Eurasian J. Vet. Sci. 2014, 30, 11–13. [Google Scholar] [CrossRef]
- Saleemi, M.K.; Khan, M.Z.; Khan, A.; Hassan, Z.U.; Khan, W.A.; Rafique, S.; Fatima, Z.; Sultan, A. Embryotoxic and Histopathological Investigations of In-Ovo Inoculation of Aflatoxigenic Fungal Extracts in Chicken Embryos. Pak. Vet. J. 2015, 35, 403–408. [Google Scholar]
- Karunarathna, R.; Ahmed, K.A.; Liu, M.; Yu, C.; Popowich, S.; Goonewardene, K.; Gunawardana, T.; Kurukulasuriya, S.; Gupta, A.; Ayalew, L.E.; et al. Non-Viable Chicken Embryos: An Overlooked Niche Harbouring a Significant Source of Multidrug Resistant Bacteria in the Poultry Production. Int. J. Vet. Sci. Med. 2020, 8, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kizerwetter-Świda, M.; Binek, M. Bacterial Microflora of the Chicken Embryos and Newly Hatched Chicken. J. Anim. Feed Sci. 2008, 17, 224–232. [Google Scholar] [CrossRef][Green Version]
- Karunarathna, R.; Popowich, S.; Wawryk, M.; Chow-Lockerbie, B.; Ahmed, K.A.; Yu, C.; Liu, M.; Goonewardene, K.; Gunawardana, T.; Kurukulasuriya, S.; et al. Increased Incidence of Enterococcal Infection in Nonviable Broiler Chicken Embryos in Western Canadian Hatcheries as Detected by Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight Mass Spectrometry. Avian. Dis. 2017, 61, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, S.; Islam, M.; Khatun, M.; Akhter, L.; Sultana, S. Characterization of Bacteria Associated with Omphalitis in Chicks. Bangladesh Vet. 2012, 29, 63–68. [Google Scholar] [CrossRef]
- Amer, M.M.; Elbayoumi, K.M.; Amin Girh, Z.M.S.; Mekky, H.M.; Rabie, N.S. A Study On Bacterial Contamination of Dead in Shell Chicken Embryos and Culled One Day Old Chicks. Int. J. Pharm. Clin. Res. 2017, 7, 5–11. [Google Scholar]
- Far, R.; Peighambari, S.M.; Sadrzadeh, A.; Badouei, A. Bacterial Contamination of Dead-in-Shell Embryos in Ostrich Hatcheries and Antimicrobial Resistance Patterns of Isolated Escherichia coli. Iran. J. Vet. Med. 2013, 7, 169–175. [Google Scholar]
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Recent Updates on the Chemistry, Bioactivities, Mode of Action, and Industrial Applications of Plant Essential Oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Tu, X.F.; Hu, F.; Thakur, K.; Li, X.L.; Zhang, Y.S.; Wei, Z.J. Comparison of Antibacterial Effects and Fumigant Toxicity of Essential Oils Extracted from Different Plants. Ind. Crops Prod. 2018, 124, 192–200. [Google Scholar] [CrossRef]
- Hatami, T.; Johner, J.C.F.; Zabot, G.L.; Meireles, M.A.A. Supercritical Fluid Extraction Assisted by Cold Pressing from Clove Buds: Extraction Performance, Volatile Oil Composition, and Economic Evaluation. J. Supercrit. Fluids 2019, 144, 39–47. [Google Scholar] [CrossRef]
- Shukla, A.; Naik, S.N.; Goud, V.V.; Das, C. Supercritical CO2 Extraction and Online Fractionation of Dry Ginger for Production of High-Quality Volatile Oil and Gingerols Enriched Oleoresin. Ind. Crops Prod. 2019, 130, 352–362. [Google Scholar] [CrossRef]
- Jadhav, N.L.; Garule, P.A.; Pinjari, D.V. Comparative Study of Ultrasound Pretreatment Method with Conventional Hydrodistillation Method for Extraction of Essential Oil from Piper betle L. (Paan). Indian Chem. Eng. 2022, 64, 132–140. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial Activity of Cinnamon Essential Oils and Their Synergistic Potential with Antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Selles, S.M.A.; Kouidri, M.; Belhamiti, B.T.; Ait Amrane, A. Chemical Composition, In Vitro Antibacterial and Antioxidant Activities of Syzygium aromaticum Essential Oil. J. Food Meas. Charact. 2020, 14, 2352–2358. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Soares, G.A.B.E.; Bhattacharya, T.; Chakrabarti, T.; Tagde, P.; Cavalu, S. Exploring Pharmacological Mechanisms of Essential Oils on the Central Nervous System. Plants 2022, 11, 21. [Google Scholar] [CrossRef]
- Ezeorba, T.P.C.; Chukwudozie, K.I.; Ezema, C.A.; Anaduaka, E.G.; Nweze, E.J.; Okeke, E.S. Potentials for Health and Therapeutic Benefits of Garlic Essential Oils: Recent Findings and Future Prospects. Pharmacol. Res. Mod. Chin. Med. 2022, 3, 100075. [Google Scholar] [CrossRef]
- Code of Federal Regulations (CFR). Part 182.20 Essential Oils, Oleoresins (Solvent-Free), and Natural Extractives (including Distillates). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.20. (accessed on 16 May 2023).
- Silva, N.; Alves, S.; Gonçalves, A.; Amaral, J.S.; Poeta, P. Antimicrobial Activity of Essential Oils from Mediterranean Aromatic Plants against Several Foodborne and Spoilage Bacteria. Food Sci. Technol. Int. 2013, 19, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Chekki, R.Z.; Snoussi, A.; Hamrouni, I.; Bouzouita, N. Chemical composition, antibacterial and antioxidant activities of Tunisian garlic (Allium sativum) essential oil and ethanol extract. Med. J. Chem. 2014, 3, 947–956. [Google Scholar] [CrossRef]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; da Silva Probst, I.; Fernandes Júnior, A. Antimicrobial Activity of Essential Oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Bhaisare, D.B.; Thyagarajan, D.; Churchil, R.R.; Punniamurthy, N. In Vitro Antimicrobial Efficacy of Certian Herbal Seeds Essential Oils against Important Poultry Microbes. Indian J. Anim. Res. 2016, 50, 561–564. [Google Scholar] [CrossRef]
- de Marques, J.L.; Volcão, L.M.; Funck, G.D.; Kroning, I.S.; da Silva, W.P.; Fiorentini, Â.M.; Ribeiro, G.A. Antimicrobial Activity of Essential Oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus Isolated from Poultry Meat. Ind. Crops Prod. 2015, 77, 444–450. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil-Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Souza, D.S.; Almeida, A.C.; Andrade, V.A.; Marcelo, N.A.; Azevedo, I.L.; Martins, E.R.; Figueiredo, L.S. Antimicrobial Activity of Lippia origanoides and Lippia rotundifolia Oils against Enterobacteria Isolated from Poultry. Arq. Bras. Med. Vet. Zootec. 2015, 67, 940–944. [Google Scholar] [CrossRef][Green Version]
- Mekonnen, A.; Yitayew, B.; Tesema, A.; Taddese, S. In vitro Antimicrobial Activity of Essential Oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. Int. J. Microbiol. 2016, 2016, 9545693. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; de Alencar, S.M.; de Sousa, R.L.M.; Moreno, A.M.; Da Gloria, E.M. Antimicrobial Activity of Several Essential Oils on Pathogenic and Beneficial Bacteria. Ind. Crops Prod. 2017, 97, 128–136. [Google Scholar] [CrossRef]
- Blažeković, B.; Yang, W.; Wang, Y.; Li, C.; Kindl, M.; Pepeljnjak, S.; Vladimir-Knežević, S. Chemical Composition, Antimicrobial and Antioxidant Activities of Essential Oils of Lavandula × intermedia ‘Budrovka’ and L. angustifolia Cultivated in Croatia. Ind. Crops Prod. 2018, 123, 173–182. [Google Scholar] [CrossRef]
- Munda, S.; Dutta, S.; Pandey, S.K.; Sarma, N.; Lal, M. Antimicrobial Activity of Essential Oils of Medicinal and Aromatic Plants of the North East India: A Biodiversity Hot Spot. J. Essent. Oil-Bear. Plants 2019, 22, 105–119. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Tosi, G.; Massi, P.; Pistelli, L.; Mancianti, F. In Vitro Antimicrobial Activity of Essential Oils against Salmonella Enterica Serotypes Enteritidis and Typhimurium Strains Isolated from Poultry. Molecules 2019, 24, 900. [Google Scholar] [CrossRef] [PubMed]
- Kammon, A. In Vitro Antimicrobial Activity of Clove Oil against Gram Negative Bacteria Isolated from Chickens. Approaches Poult. Dairy Vet. Sci. 2019, 6, 542–546. [Google Scholar] [CrossRef]
- Aćimović, M.; Zorić, M.; Zheljazkov, V.D.; Pezo, L.; Čabarkapa, I.; Jeremić, J.S.; Cvetković, M. Chemical Characterization and Antibacterial Activity of Essential Oil of Medicinal Plants from Eastern Serbia. Molecules 2020, 25, 5482. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, M.; Cacaci, M.; Barbanti, L.; Martini, C.; Sanguinetti, M.; Benvenuti, S.; Tosi, G.; Fiorentini, L.; Scozzoli, M.; Bugli, F.; et al. Origanum vulgare Essential Oil vs. A Commercial Mixture of Essential Oils: In vitro Effectiveness on Salmonella spp. from Poultry and Swine Intensive Livestock. Antibiotics 2020, 9, 763. [Google Scholar] [CrossRef]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St. Rose, T.; Puchalski, K.; Langland, J. Antimicrobial Activity of the Volatile Substances from Essential Oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef]
- Mangalagiri, N.P.; Panditi, S.K.; Jeevigunta, N.L.L. Antimicrobial Activity of Essential Plant Oils and Their Major Components. Heliyon 2021, 7, e06835. [Google Scholar] [CrossRef]
- Seyedtaghiya, M.H.; Fasaei, B.N.; Peighambari, S.M. Antimicrobial and Antibiofilm Effects of Satureja hortensis Essential Oil against Escherichia coli and Salmonella Isolated from Poultry. Iran. J. Microbiol. 2021, 13, 74–80. [Google Scholar] [CrossRef]
- Blejan, E.I.; Popa, D.E.; Costea, T.; Cioacă, A.; Olariu, L.; Ghica, M.; Georgescu, M.; Stancov, G.; Arsene, A.L. The in vitro Antimicrobial Activity of Some Essential Oils from Aromatic Plants. Farmacia 2021, 69, 290–298. [Google Scholar] [CrossRef]
- Campana, R.; Tiboni, M.; Maggi, F.; Cappellacci, L.; Cianfaglione, K.; Morshedloo, M.R.; Frangipani, E.; Casettari, L. Comparative Analysis of the Antimicrobial Activity of Essential Oils and Their Formulated Microemulsions against Foodborne Pathogens and Spoilage Bacteria. Antibiotics 2022, 11, 447. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; Pires, P.G.D.S.; dos Santos, V.M. Combination of Cassava Starch Biopolymer and Essential Oils for Coating Table Eggs. Front. Sustain. Food Syst. 2022, 6, 957229. [Google Scholar] [CrossRef]
- Milagres de Almeida, J.; Crippa, B.L.; Martins Alencar de Souza, V.V.; Perez Alonso, V.P.; da Motta Santos Júnior, E.; Siqueira Franco Picone, C.; Prata, A.S.; Cirone Silva, N.C. Antimicrobial Action of Oregano, Thyme, Clove, Cinnamon and Black Pepper Essential Oils Free and Encapsulated against Foodborne Pathogens. Food Control 2023, 144, 109356. [Google Scholar] [CrossRef]
- de Araújo, M.V.; da Oliveira, G.S.; McManus, C.; Vale, I.R.R.; Salgado, C.B.; da Pires, P.G.S.; de Campos, T.A.; Gonçalves, L.F.; Almeida, A.P.C.; dos Martins, G.S.; et al. Preserving the Internal Quality of Quail Eggs Using a Corn Starch-Based Coating Combined with Basil Essential Oil. Processes 2023, 11, 1612. [Google Scholar] [CrossRef]
- Upadhyaya, I.; Upadhyay, A.; Kollanoor-Johny, A.; Baskaran, S.A.; Mooyottu, S.; Darre, M.J.; Venkitanarayanan, K. Rapid Inactivation of Salmonella Enteritidis on Shell Eggs by Plant-Derived Antimicrobials. Poult. Sci. 2013, 92, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Balasubramanian, B.; Rankin, K.; Shah, T.; Donoghue, A.M.; Upadhyaya, I.; Sartini, B.; Luo, Y.; Upadhyay, A. Trans-Cinnamaldehyde Nanoemulsion Wash Inactivates Salmonella Enteritidis on Shelled Eggs without Affecting Egg Color. Poult. Sci. 2023, 102, 102523. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, I.; Yin, H.B.; Nair, M.S.; Chen, C.H.; Upadhyay, A.; Darre, M.J.; Venkitanarayanan, K. Efficacy of Fumigation with Trans-Cinnamaldehyde and Eugenol in Reducing Salmonella Enterica Serovar Enteritidis on Embryonated Egg Shells. Poult. Sci. 2015, 94, 1685–1690. [Google Scholar] [CrossRef]
- Oliveira, G.S.; Nascimento, S.T.; Dos Santos, V.M.; Dallago, B.S.L. Spraying Hatching Eggs with Clove Essential Oil Does Not Compromise the Quality of Embryos and One-Day-Old Chicks or Broiler Performance. Animals 2021, 11, 2045. [Google Scholar] [CrossRef]
- Cui, H.; Ma, C.; Li, C.; Lin, L. Enhancing the Antibacterial Activity of Thyme Oil against Salmonella on Eggshell by Plasma-Assisted Process. Food Control 2016, 70, 183–190. [Google Scholar] [CrossRef]
- de Andrade, M. Avaliação do óleo Essencial de Citronela (Cymbopogon winterianus) Como Agente Alternativo na Desinfecção de ovos Incubáveis [Evaluation of Citronella Essential Oil (Cymbopogon winterianus) as an Alternative Agent in the Disinfection of Hatching Eggs]; Graduation Completion Work; Federal Technological University of Paraná: Curitiba, Paraná, Brazil, 28 June 2018. [Google Scholar]
- Nogueira, W.C.L.; Pena, A.C.S.; de Souza, C.N.; Azevedo, I.L.; Fariafilho, D.E.; Almeida, A.C. Disinfection of Fertile Eggs of Free-Range Poultry with Essential Oils. Rev. Bras. Saude Prod. Anim. 2019, 20, e0822019. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; Nascimento, S.T.; dos Santos, V.M.; Silva, M.G. Clove Essential Oil in the Sanitation of Fertile Eggs. Poult. Sci. 2020, 99, 5509–5516. [Google Scholar] [CrossRef]
- Phothisuwan, S.; Preechatiwong, W.; Matan, N. Enhancement of Antibacterial Activity of Essential Oil Vapor Released from a Paper Egg Tray in Combination with UV-C Radiation against Pathogenic Bacteria on Chicken Eggs. J. Food Process. Preserv. 2020, 44, e14794. [Google Scholar] [CrossRef]
- Bekhet, G.; Khalifa, A.Y.Z. Essential Oil Sanitizers to Sanitize Hatching Eggs. J. Appl. Anim. Res. 2022, 50, 695–701. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; McManus, C.C.; Salgado, C.B.; Pires, P.G.d.S.; dos Santos, V.M. Rice Flour Coating Supplemented with Rosemary Essential Oil to Preserve the Internal, Microbiological and Sensory Quality of Quail Eggs. Acta Aliment. 2023, 52, 294–304. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; Dos Santos, V.M. Essential Oils and Propolis as Additives in Egg Coatings. Worlds Poult. Sci. J. 2022, 78, 1053–1066. [Google Scholar] [CrossRef]
- Gatea, S.M.; Altaie, S.M.S.; Khafaji, S.S.; Aljanabi, T.K.; Shatti, D.H.; Hussain, M.A. Influence of Spraying Different Solutions at Different Incubation Periods on Hatchability Parameters of Local Iraqi’s Eggs. IOP Conf. Ser. Earth Environ. Sci. 2019, 388, 012034. [Google Scholar] [CrossRef]
- Harry, E.G.; Binstead, J.A. Studies on Disinfection of Eggs and Incubators. V: The Toxicity of Formaldehyde to the Developing Embryo. Br. Vet. J. 1961, 117, 532–539. [Google Scholar] [CrossRef]
- Zeweil, H.S.; Rizk, R.E.; Bekhet, G.M.; Ahmed, M.R. Comparing the Effectiveness of Egg Disinfectants against Bacte-ria and Mitotic Indices of Developing Chick Embryos. J. Basic Appl. Zool. 2015, 70, 1–15. [Google Scholar] [CrossRef]
- de Oliveira, S.Z. Toxicidade do óleo Essencial de Melaleuca a Organismos não-alvo [Toxicity of Melaleuca Essential Oil to Non-target Organism]. Master’s Dissertation, Federal Technological University of Paraná, Curitiba, Paraná, Brazil, 28 February 2023. [Google Scholar]
- Tebrün, W.; Motola, G.; Hafez, M.H.; Bachmeier, J.; Schmidt, V.; Renfert, K.; Reichelt, C.; Brüggemann-Schwarze, S.; Pees, M. Preliminary Study: Health and Performance Assessment in Broiler Chicks Following Application of Six Different Hatching Egg Disinfection Protocols. PLoS ONE 2020, 15, e0232825. [Google Scholar] [CrossRef]
- Demirci, F.; Paper, D.H.; Franz, G.; Hüsnü Can Başer, K. Investigation of the Origanum onites L. Essential Oil Using the Chorioallantoic Membrane (CAM) Assay. J. Agric. Food Chem. 2004, 52, 251–254. [Google Scholar] [CrossRef]
- Ali, R.; Elzahraa Mostafa, D.F.; Aboulqasem, H.; Ali, R.A.; Aboulqasem, H.E. The Effect of Co-Treatment with Retinoic Acid on Rescuing Citral Induced Morphological Anomalies during Chick Embryo Development. Assiut. Univ. J. Zool. 2019, 1, 42–63. [Google Scholar]
- Zhang, X.; Peng, Y.; Wu, C. Chicken Embryonic Toxicity and Potential in vitro Estrogenic and Mutagenic Activity of Carvacrol and Thymol in Low Dose/Concentration. Food Chem. Toxicol. 2021, 150, 112038. [Google Scholar] [CrossRef]
- Ulucay, I.O.; Yildirim, I. Hatching Traits of Quail (Coturnix Coturnix Japonica) Eggs Disinfected with Carvacrol, Cinnamaldehyde or Thymol. J. Appl. Anim. Res. 2010, 38, 139–142. [Google Scholar] [CrossRef]
- Zeweil, H.; Rizk, R.; Bekhet, G.; Ahmed, R. Effect of Egg Disinfection on Hatching Performance for Bandarah Chicken Strain. Egypt. Poult. Sci. J. 2013, 33, 289–307. [Google Scholar]
- Goze, A.; Gose, I.; Alim, A.; Saygin, H.; Alim, B.A. Investigation of Effects of Essential Oil from Berries of Juniperus excelsa Bieb. Subsp Excelsa (Cupressaceae) on Angiogenesis in Shell-Less Chick Embryo (CAM) Culture. J. Essent. Oil-Bear. Plants 2015, 18, 1100–1107. [Google Scholar] [CrossRef]
- Bekhet, G.M.; Sayed, A.A. Oregano-Oil Antagonist Lipopolysaccharide (LPS) Induced Toxicity in Pre- and Post-Hatch Chick Embryo. J. Appl. Anim. Res. 2021, 49, 211–220. [Google Scholar] [CrossRef]
- Oladokun, S.; Macisaac, J.; Rathgeber, B.; Adewole, D. Essential Oil Delivery Route: Effect on Broiler Chicken’s Growth Performance, Blood Biochemistry, Intestinal Morphology, Immune, and Antioxidant Status. Animals 2021, 11, 3386. [Google Scholar] [CrossRef]
- Yaseen, A.; Gaafar, K.; Abou-elkhaire, R. Response of Broiler Chicken to in Ovo Administration of Nano Encapsulated Thyme Oil. J. Curr. Vet. Res. 2022, 4, 166–172. [Google Scholar] [CrossRef]
- Aberbour, A.; Touazi, L.; Benberkane, A.; Aissanou, S.; Sherasiya, A.; Iguer-Ouada, M.; Hornick, J.L.; Moula, N. The Effect of In Ovo Administration of Rosemary Essential Oil on Hatchability, Relative Hatching Weight, and Embryo Mortality Rate in Japanese Quail (Coturnix Coturnix Japonica). Animals 2023, 13, 1217. [Google Scholar] [CrossRef]
- Allen, J.; Balasubramanian, B.; Donoghue, A.M.; Upadhyaya, I.; Luo, Y.; Upadhyay, A. Effect of Trans-Cinnamaldehyde Nanoemulsion Wash on Chicken Embryo Development in Fertilized Eggs. Poult. Sci. 2023, 102, 102812. [Google Scholar] [CrossRef] [PubMed]
- Cadirci, S. Disinfection of Hatching Eggs by Formaldehyde Fumigation—A Review. Eur. Poult. Sci. 2009, 73, 116–123. [Google Scholar]
- Melo, E.F.; Clímaco, W.L.S.; Triginelli, M.V.; Vaz, D.P.; de Souza, M.R.; Baião, N.C.; Pompeu, M.A.; Lara, L.J.C. An Evaluation of Alternative Methods for Sanitizing Hatching Eggs. Poult. Sci. 2019, 98, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Hayretdaǧ, S.; Kolankaya, D. Investigation of the Effects of Pre-Incubation Formaldehyde Fumigation on the Tracheal Epithelium of Chicken Embryos and Chicks. Turk. J. Vet. Anim. Sci. 2008, 32, 263–267. [Google Scholar]
- Qu, M.; Lu, J.; He, R. Formaldehyde from Environment. In Formaldehyde and Cognition; He, H., Ed.; Springer Science + Business Media B.V.: Berlin, Germany, 2017; Volume 1, pp. 1–19. [Google Scholar]
- Dan, S.; Pant, M.; Kaur, T.; Pant, S. Toxic effect of formaldehyde: A systematic review. Int. Res. J. Mod. Eng. Technol. Sci. 2020, 2, 179–189. [Google Scholar]
- Bernardini, L.; Barbosa, E.; Charão, M.F.; Brucker, N. Formaldehyde Toxicity Reports from In Vitro and in vivo Studies: A Review and Updated Data. Drug Chem. Toxicol. 2020, 45, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Fouad, W.; Abdel-Hafez, M.S. Effect of Spraying Hatching Eggs of Japanese Quails by Live Yeast on Physiological Changes in the Embryonic Development, Hatchability and Total Bacterial Count. Egypt. Poult. Sci. J. 2017, 37, 1303–1321. [Google Scholar]
- Toghyani, P.; Shahzamani, S.; Gholami Ahangaran, M.; Ali Mousavi Firouzabadi, S. Comparison of Eucalyptus Extract and Formaldehyde on Hatchability and Survival Rate of Chicks in Disinfection of Fertile Eggs. Int. J. Pharm. Res. Allied Sci. 2020, 9, 105–109. [Google Scholar]
- Copur, G.; Arslan, M.; Duru, M.; Baylan, M.; Canogullari, S.; Aksan, E. Use of Oregano (Origanum onites L.) Essential Oil as Hatching Egg Disinfectant. Afr. J. Biotechnol. 2010, 8, 2531–2538. [Google Scholar]
- Shahein, E.H.A.; Sedeek, E.K. Role of Spraying Hatching Eggs with Natural Disinfectants on Hatching Characteristics and Eggshell Bacterial Counts. Egypt. Poult. Sci. J. 2014, 34, 213–230. [Google Scholar] [CrossRef]
| Essential Oil or Its Component | Essential Oil Concentration | Eggshell Application | Egg Type | Eggshell Contamination | Eggshell Microbial Load | Study |
|---|---|---|---|---|---|---|
| Carvacrol | 0.25, 0.5 and 0.75% | Immersing | Chick | Inoculation |
| [57] |
| Eugenol | ||||||
| Trans-cinnamaldehyde | ||||||
| Thymus vulgaris | 0.25, 0.5 and 1 mg/mL | Immersing | Chick | Inoculation |
| [61] |
| Cymbopogon winterianus | 0.1, 0.15 and 0.2% | Spraying | Chick | Natural |
| [62] |
| Cymbopogon flexuosus | 1% | Immersing | Chick | Natural |
| [63] |
| Lippia rotundifolia | ||||||
| Syzygium aromaticum | 0.39% | Spraying | Chick | Natural |
| [64] |
| Syzygium aromaticum | 10–80 µg/g | Vaporizing | Chick | Inoculation |
| [65] |
| Natural |
| |||||
| Origanum vulgare | 0.5% | Immersing | Chick | Natural |
| [66] |
| Cuminum cyminum | ||||||
| Trans-cinnamaldehyde | 0.48% | Immersing | Chick | Inoculation |
| [58] |
| Essential Oil or Its Component | Essential Oil Concentration | Egg Application Method | Application Target | Egg Type | Authors’ Findings for Embryos and Chicks | Study |
|---|---|---|---|---|---|---|
| Origanum vulgare | 0.2 and 0.4% or 0.5% | Sanitizing | Eggshell | Chick |
| [66,71,78] |
| Cuminum cyminum | ||||||
| Juniperus excelsa | 10% ratio of 9 (oil):1 (ethyl alcohol) | Micropipetting | Blastodisc | Chick |
| [79] |
| Cymbopogon winterianus | 0.1, 0.15 and 0.2% | Sanitizing | Eggshell | Chick |
| [62] |
| Syzygium aromaticum | 0.39% | Sanitizing | Eggshell | Chick |
| [60] |
| Origanum vulgare | 0.5% | Sanitizing | Embryo | Chick |
| [80] |
| Commercial blend | 0.2 mL ratio of 2 (saline): 1 (Commercial blend) | Injecting | Amnion | Chick |
| [81] |
| Thymus vulgaris | 0.03 mL/egg | Injecting | Embryo | Chick |
| [82] |
| Rosmarinus officinalis | 1 µL or 3 µL/egg | Injecting | Air chamber | Quail |
| [83] |
| Trans-cinnamaldehyde | 0.48% | Washing | Eggshell | Chick |
| [84] |
| Compounds | Bacterial Count (log) a | Hatchability (%) a | Significance b | Most Efficient | Study |
|---|---|---|---|---|---|
| Origanum onites | <0.47 | >1.98 | * TBC ns Hatchability | Essential oil | [93] |
| Formaldehyde | <0.06 | >1.89 | |||
| Thymus vulgaris | <1.68 | >6.95 | * | Formaldehyde | [94] |
| Formaldehyde | <1.81 | >9.70 | |||
| Syzygium aromaticum | <1.19 | >10.66 | ns | Similar | [64] |
| Paraformaldehyde | <1.26 | >7.84 | |||
| Origanum vulgare | <6.33 | >12.05 | * | Essential oils | [66] |
| Cuminum cyminum | <6.13 | >11.70 | |||
| Formaldehyde | <3.03 | <2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.d.S.; McManus, C.; de Araújo, M.V.; de Sousa, D.E.R.; de Macêdo, I.L.; Castro, M.B.d.; Santos, V.M.d. Sanitizing Hatching Eggs with Essential Oils: Avian and Microbiological Safety. Microorganisms 2023, 11, 1890. https://doi.org/10.3390/microorganisms11081890
Oliveira GdS, McManus C, de Araújo MV, de Sousa DER, de Macêdo IL, Castro MBd, Santos VMd. Sanitizing Hatching Eggs with Essential Oils: Avian and Microbiological Safety. Microorganisms. 2023; 11(8):1890. https://doi.org/10.3390/microorganisms11081890
Chicago/Turabian StyleOliveira, Gabriel da Silva, Concepta McManus, Maria Viviane de Araújo, Davi Emanuel Ribeiro de Sousa, Isabel Luana de Macêdo, Marcio Botelho de Castro, and Vinícius Machado dos Santos. 2023. "Sanitizing Hatching Eggs with Essential Oils: Avian and Microbiological Safety" Microorganisms 11, no. 8: 1890. https://doi.org/10.3390/microorganisms11081890
APA StyleOliveira, G. d. S., McManus, C., de Araújo, M. V., de Sousa, D. E. R., de Macêdo, I. L., Castro, M. B. d., & Santos, V. M. d. (2023). Sanitizing Hatching Eggs with Essential Oils: Avian and Microbiological Safety. Microorganisms, 11(8), 1890. https://doi.org/10.3390/microorganisms11081890






