Characterization of Growth-Promoting Activities of Consortia of Chlorpyrifos Mineralizing Endophytic Bacteria Naturally Harboring in Rice Plants—A Potential Bio-Stimulant to Develop a Safe and Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Screening for Pesticide-Degrading Bacteria
2.3. Molecular Characterization and Phylogeny of Endophytic Bacteria
2.4. FT-IR for Pesticide-Degrading Activity Confirmation
2.5. Biochemical Analysis
2.6. Indole-3-Acetic Acid (IAA) and ACC Deaminase Production
2.7. Phosphate and Nitrogen Solubilization
2.8. Anti-Bacterial Activity against Multidrug-Resistant Bacteria
2.9. Rice Plant Growth-Promoting Effects of Endophytic Bacteria
2.10. Seed Germination Performance
2.11. Effect of Endophytic Consortia on Growth of Rice Plant and Yield of Grains
2.12. Chlorophyll Content of Fresh Leaf
2.13. Root Length, Shoot Length, and Plant Height
2.14. Plant Dry Matter Production
2.15. Yield Parameters (Grain Yield per Plant)
2.16. Harvesting and Observations
2.17. GC–MS/MS Analysis of Chlorpyrifos Degradation by Each of the Four Consortia
2.18. Statistical Analysis
3. Results
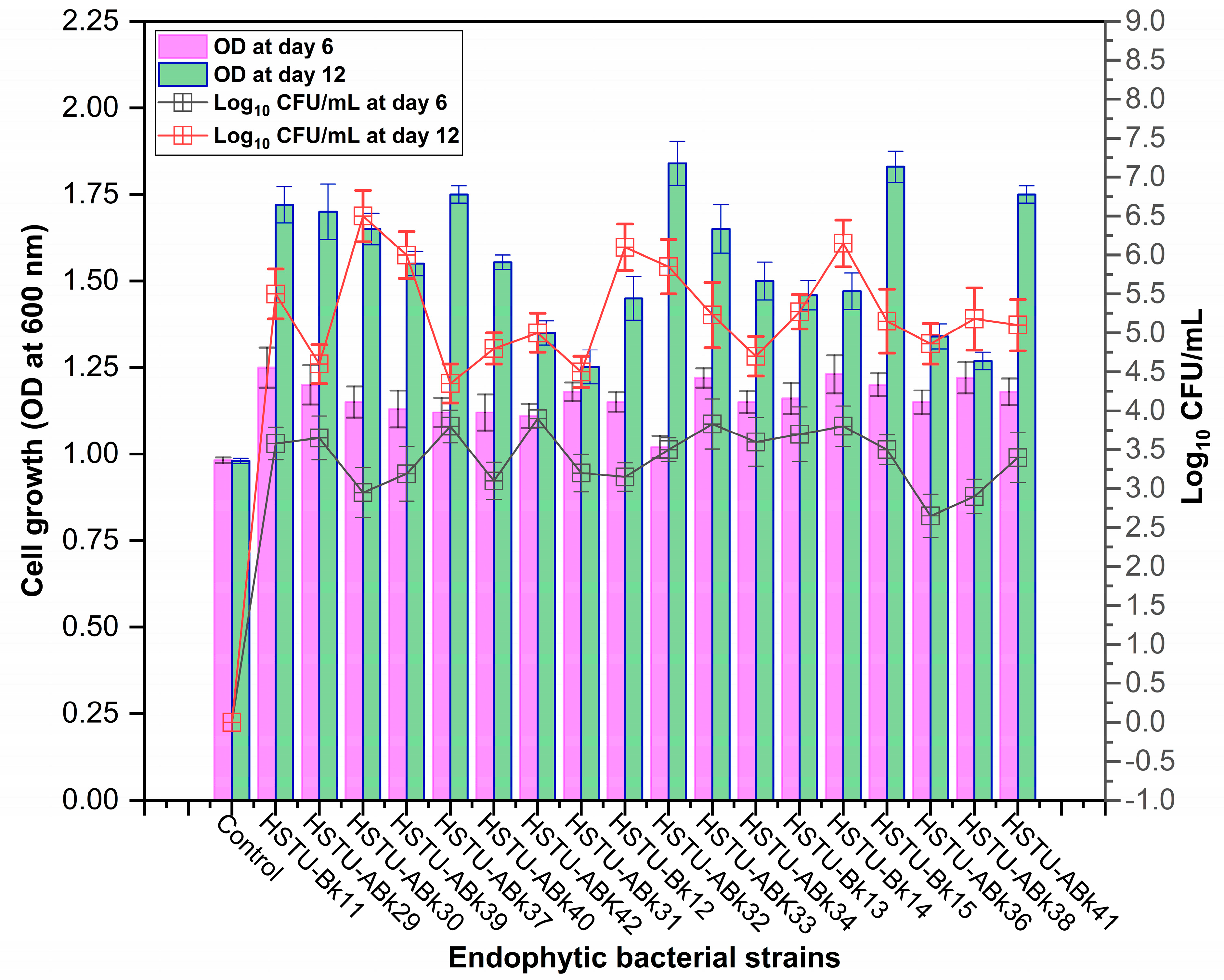
3.1. Isolation and Selection of Chlorpyrifos-Degrading Endophytic Bacteria
3.2. Biochemical Characterization of the Pesticide-Degrading Endophytic Bacteria
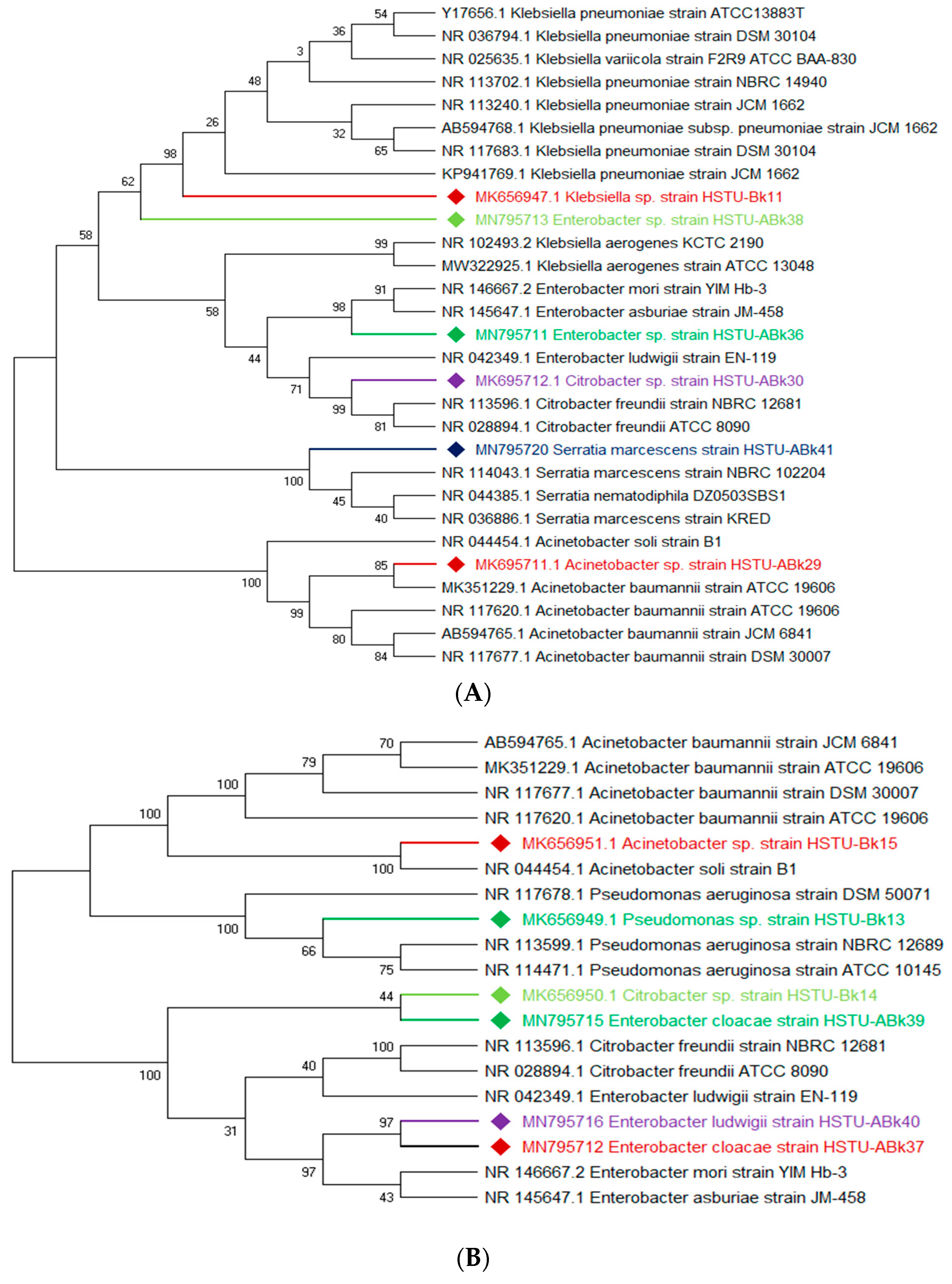
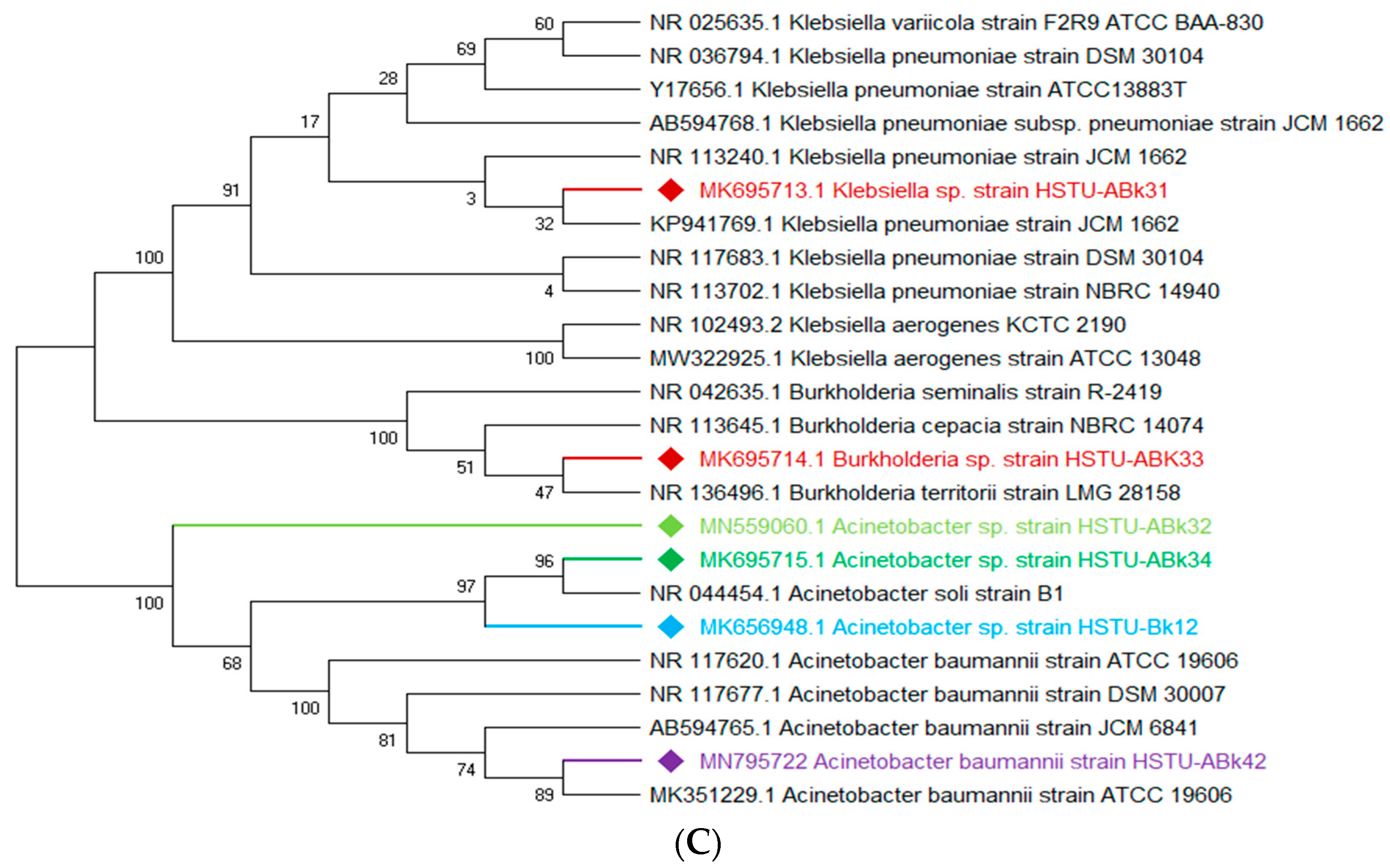
3.3. Molecular Characterization of the Pesticide-Degrading Endophytic Bacteria
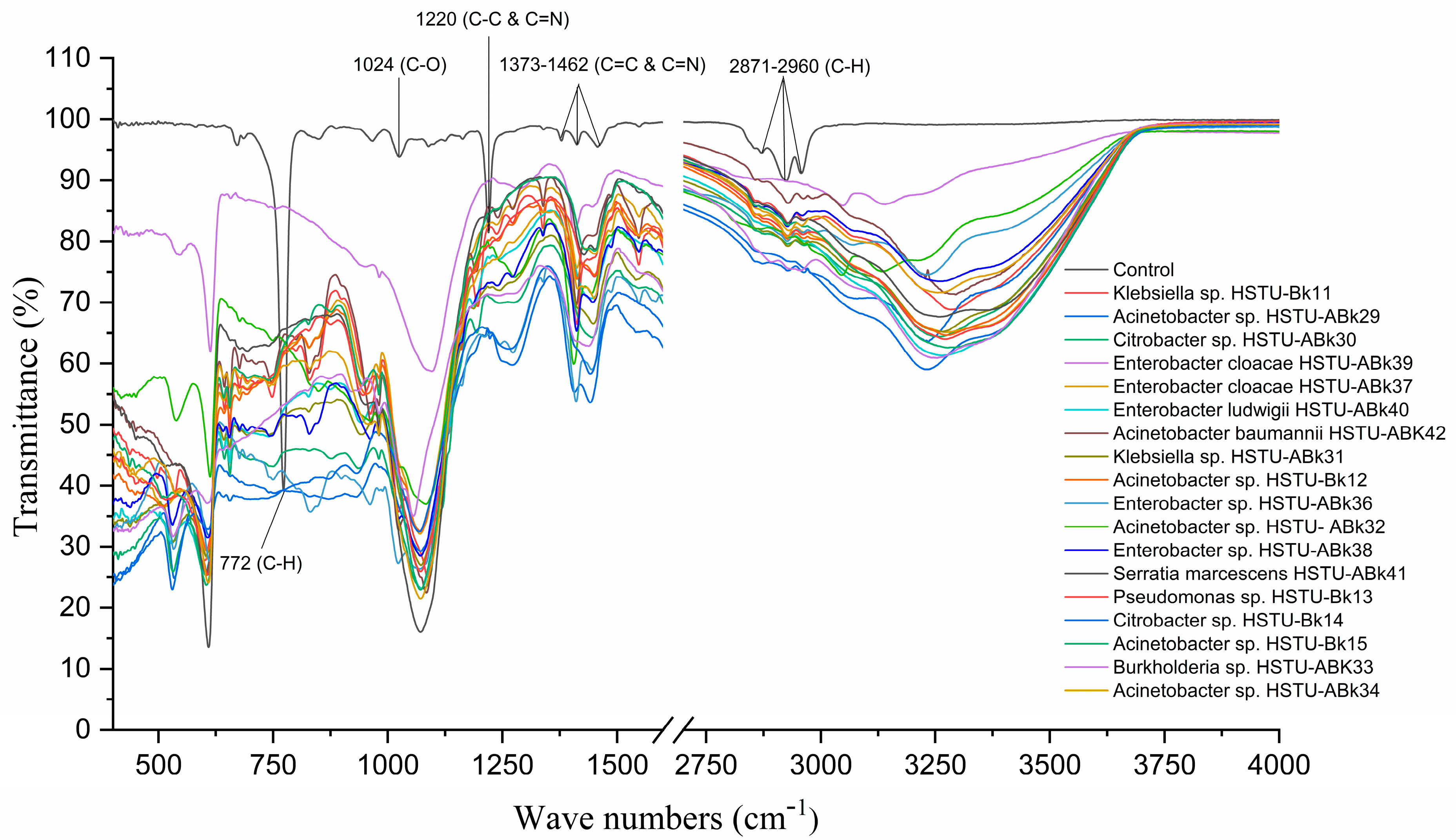
3.4. Chlorpyrifos Biodegradation Confirmation Using FT-IR Analysis
3.5. Plant-Growth-Promoting Traits of the Pesticide-Degrading Endophytic Bacteria
3.5.1. N-Fixation and PO4- Solubilization Activity
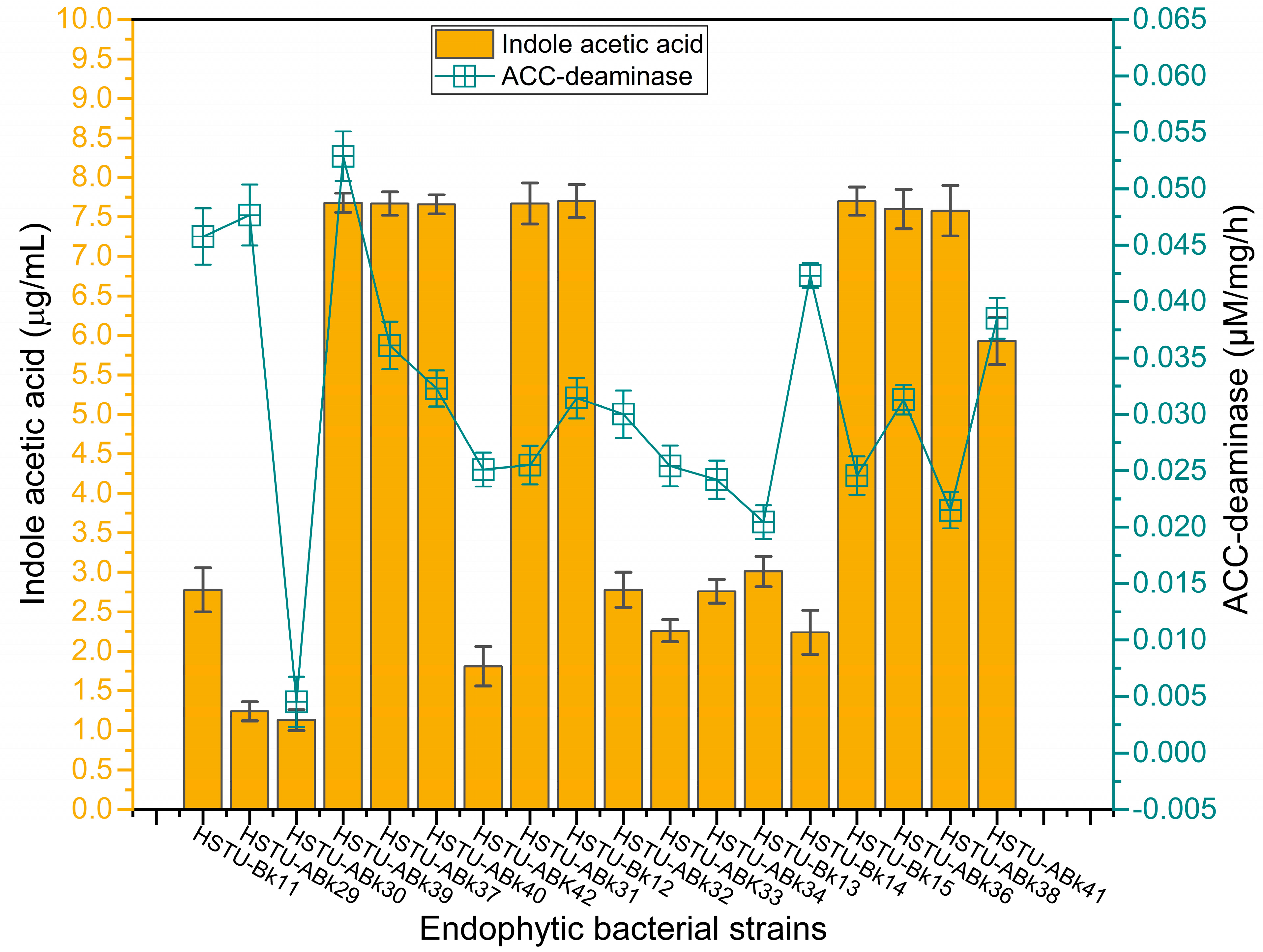
3.5.2. IAA and ACC-Deaminase Activity
3.6. Anti-Bacterial Activity against Multidrug-Resistant Human Pathogenic Bacteria
3.7. Rice Plant Growth-Promoting Effect
3.7.1. Effects of Individual and Consortia of Endophytes on Germination and Seedling Growth
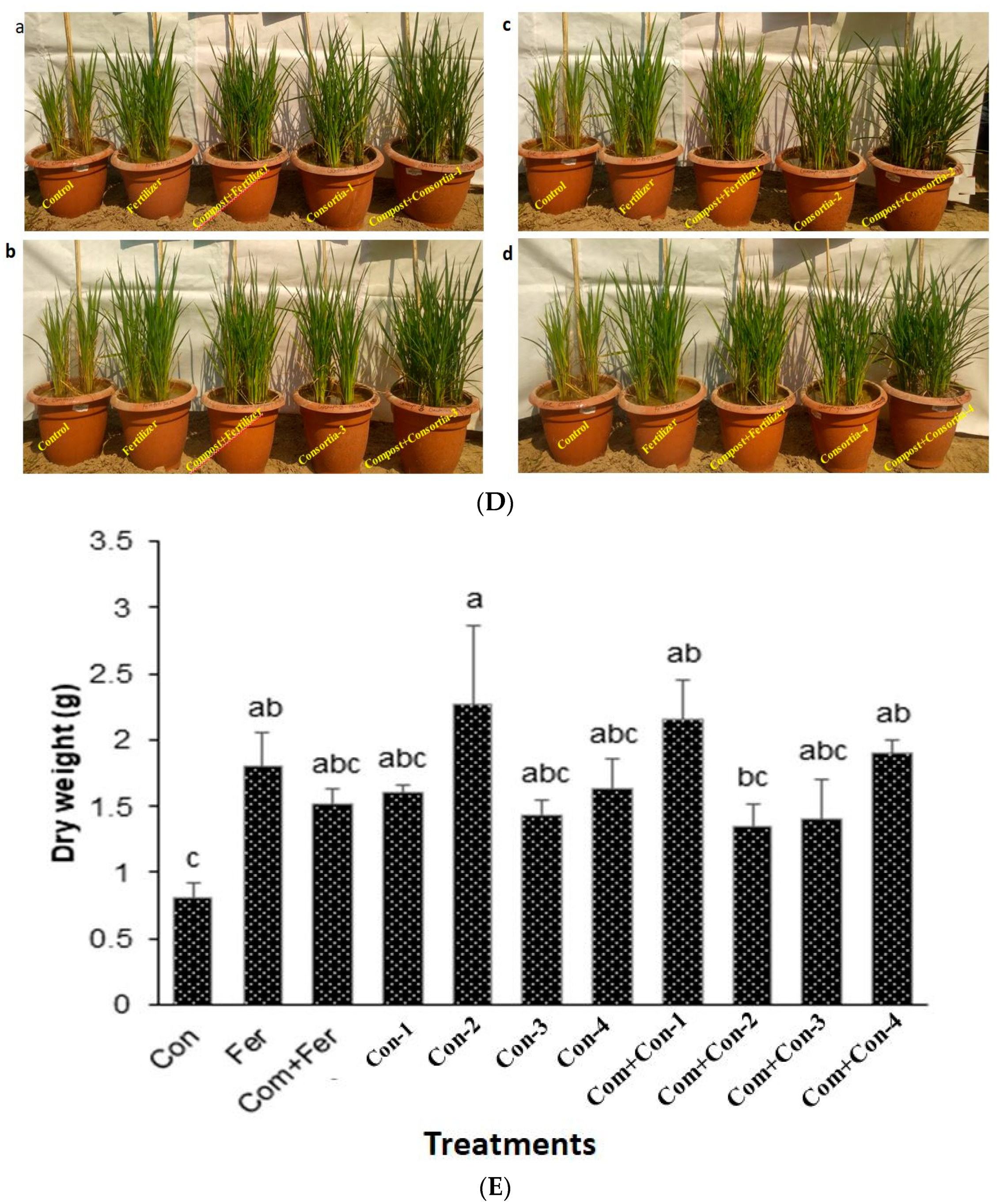
3.7.2. Effects of Consortia on Vegetative and Reproductive Stages and Yield
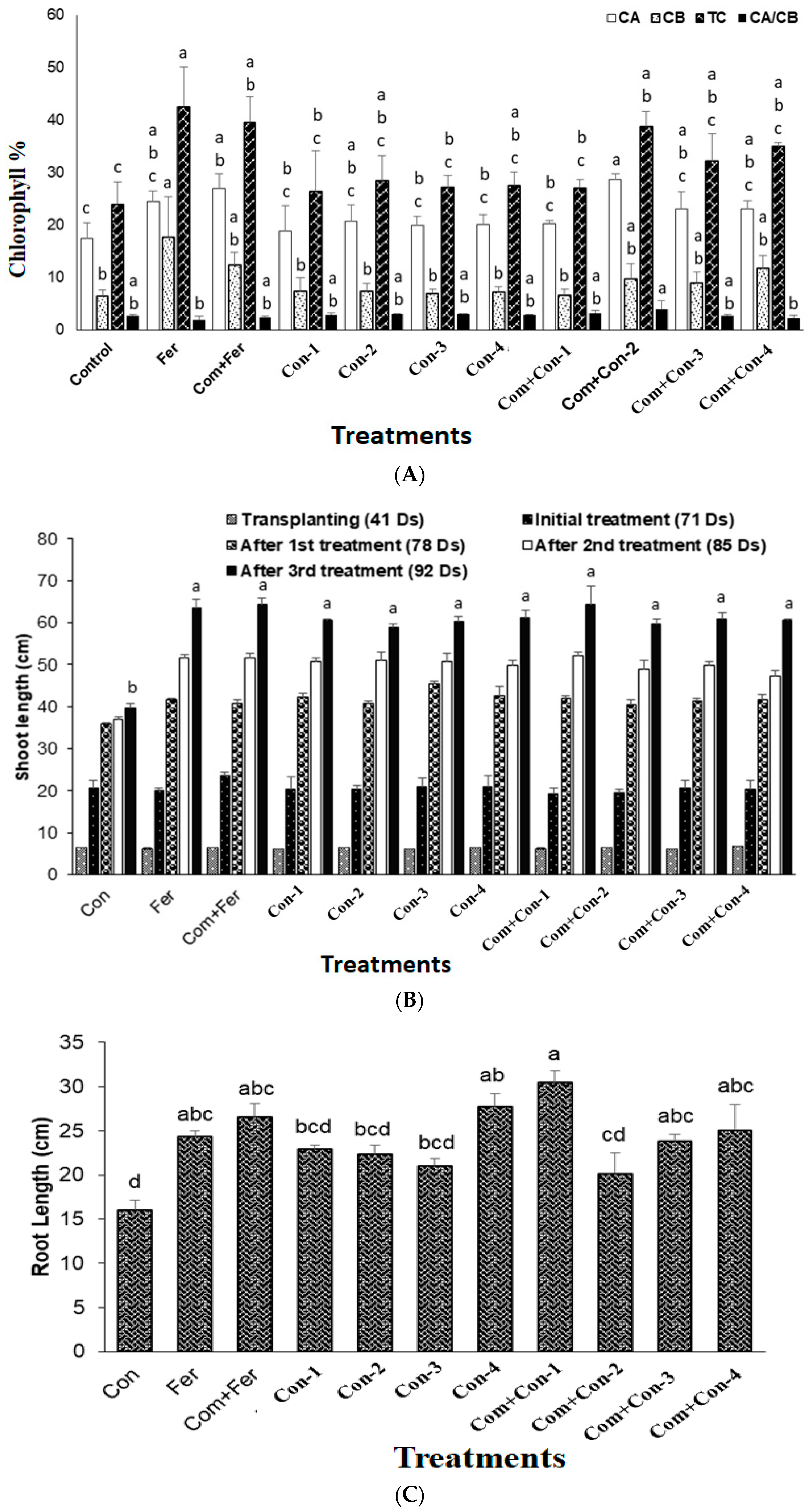
3.7.3. Chlorophyll Content
3.7.4. Root and Shoot Lengths
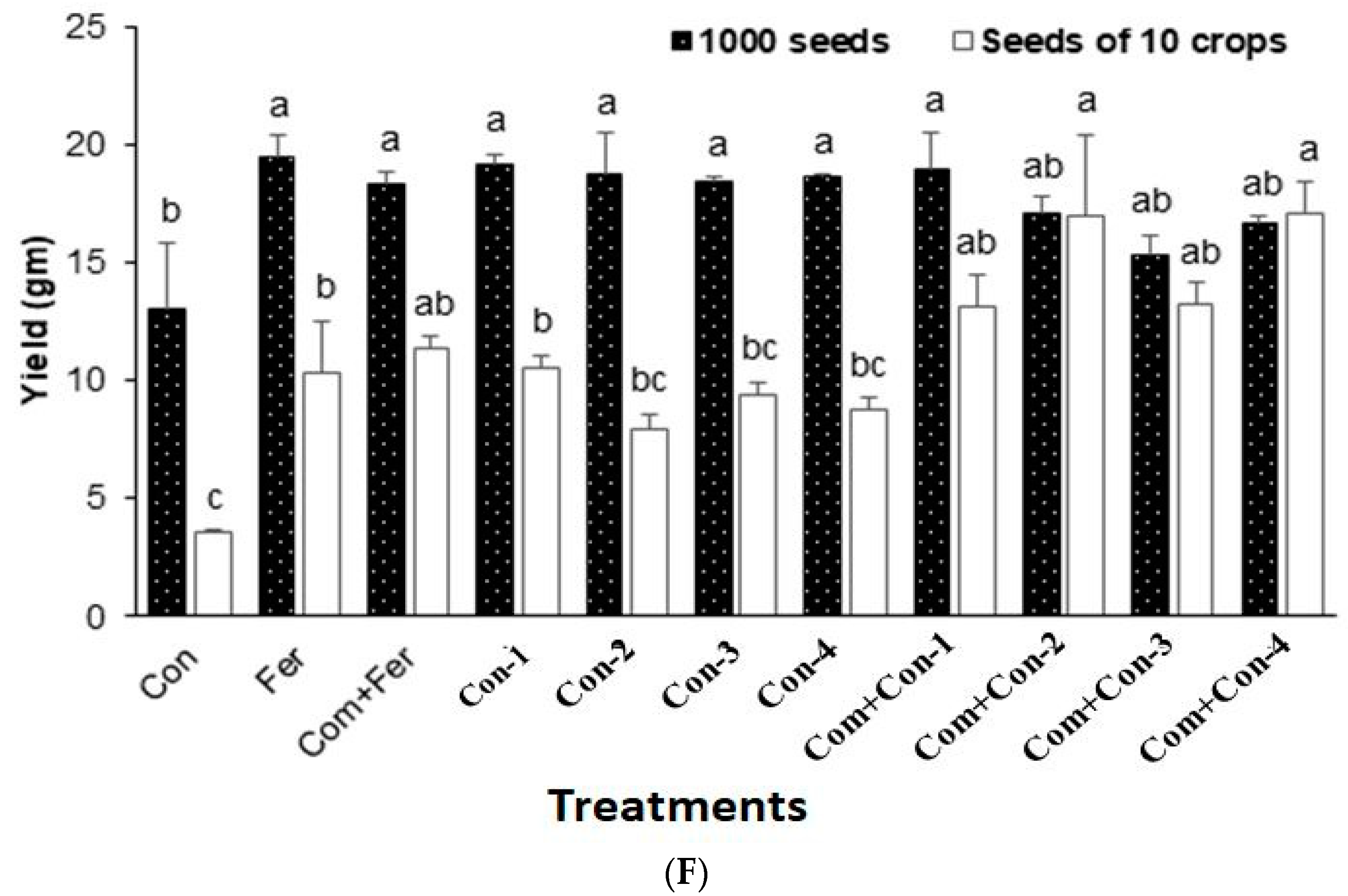
3.7.5. Yield
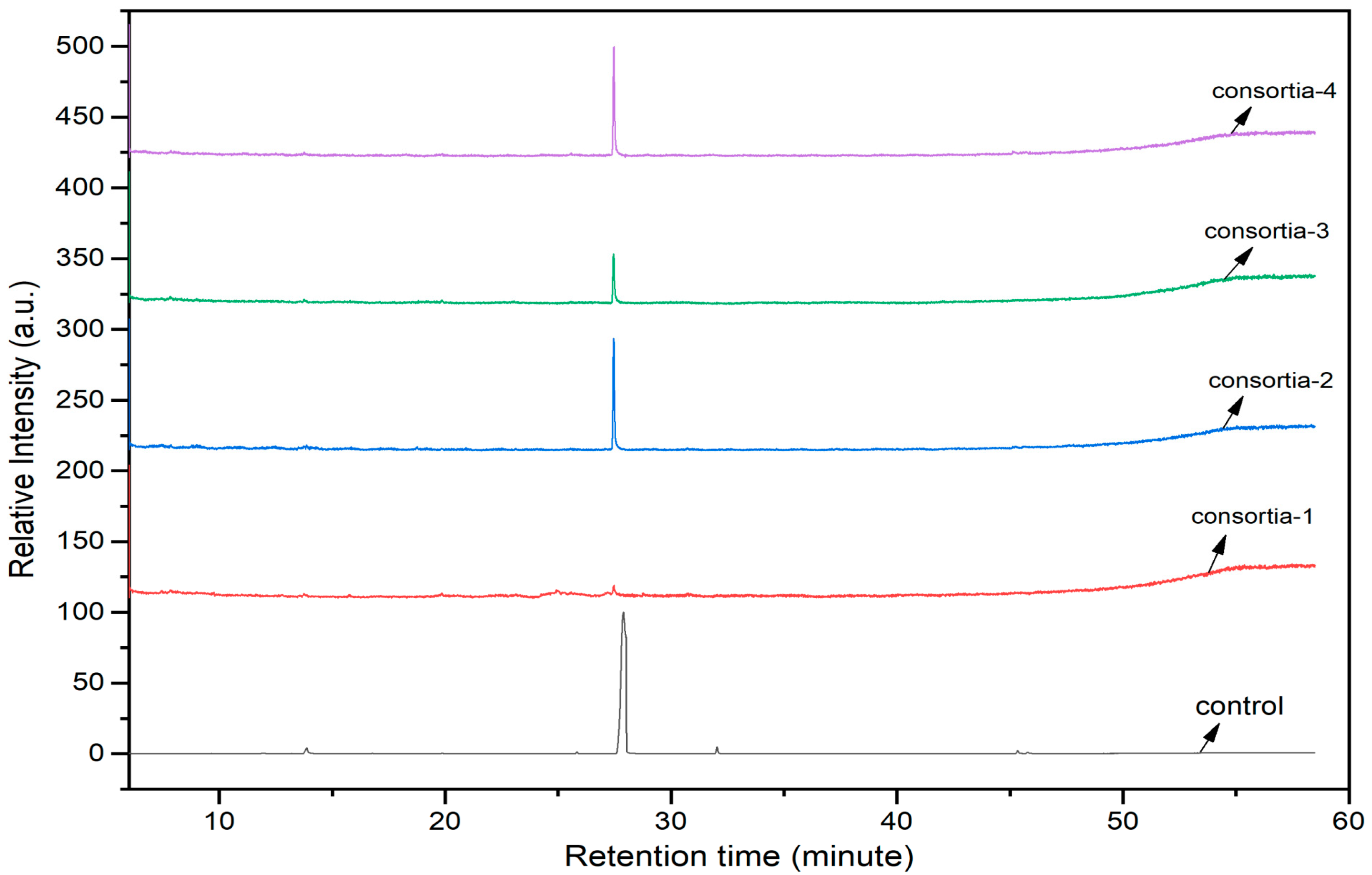
3.8. Roles of Consortia of Endophytic Bacteria in Chlorpyrifos Biodegradation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowdhury, A.Z.; Jahan, S.A.; Islam, M.N.; Moniruzzaman, M.; Alam, M.K.; Zaman, M.A.; Karim, N.; Gan, S.H. Occurrence of organophosphorus and carbamate pesticide residues in surface water samples from the Rangpur district of Bangladesh. Bull. Environ. Contam. Toxicol. 2012, 89, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Abraham, J. Ecofriendly method for bioremediation of chlorpyrifos from agricultural soil by novel fungus Aspergillus terreus JAS1. Water Air Soil Pollut. 2013, 224, 1369. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Lai, X.H.; Shan, C.; Deng, Z.; Ji, Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Brazil. J. Microbiol. 2015, 46, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.B.; Singh, A.; Mukhopadhyay, S. Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: Prospects and challenges. Appl. Microbiol. Biotechnol. 2008, 80, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Aislabie, J.; Lloyd-Jones, G. A review of bacterial-degradation of pesticides. Soil Res. 1995, 33, 925–942. [Google Scholar] [CrossRef]
- Das, S.R.; Haque, M.A.; Akbor, M.A.; AbdullahAlMamun, M.; Debnath, G.C.; Hossain, M.S.; Hasan, Z.; Rahman, A.; Islam, M.A.; Hossain, M.A.; et al. Organophosphorus insecticides mineralizing endophytic and rhizospheric soil bacterial consortium infuence eggplant growth promotion. Arch. Microbiol. 2022, 204, 199. [Google Scholar] [CrossRef]
- Haque, M.A.; Hong, S.Y.; Hwang, C.E.; Kim, S.C.; Cho, K.M. Cloning of an organophosphorus hydrolase (opdD) gene of Lactobacillus sakei WCP904 isolated from chlorpyrifos impregnated kimchi and hydrolysis activities of its gene product for organophosphorus pesticides. Appl. Biol. Chem. 2018, 61, 643–651. [Google Scholar] [CrossRef]
- Zhongli, C.; Shunpeng, L.; Guoping, F. Isolation of methyl parathion-degrading strain M6 and cloning of the methyl parathion hydrolase gene. Appl. Environ. Microbiol. 2001, 67, 4922–4925. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; Lopez-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Narayanan, Z.; Glick, B.R. Secondary metabolites produced by plant growth promoting bacterial endophytes. Microorganisms 2022, 10, 2008. [Google Scholar] [CrossRef]
- Rojas-Sánchez, B.; GuzmánGuzmán, P.; Morales-Cedeño, L.R.; Orozco-Mosqueda, M.C.; Saucedo Martínez, B.C.; Sánchez-Yáñez, J.M.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Bioencapsulation of microbial inoculants: Mechanisms, formulation types and application techniques. Appl. Biosci. 2022, 1, 198–220. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets; FAO: Rome, Italy, 2020. [Google Scholar]
- Gyaneshwar, P.; James, E.K.; Mathan, N.; Reddy, P.M.; Reinhold-Hurek, B.; Ladha, J.K. Endophytic colonization of rice by a diazotrophic strain of Serratia marcescens. J. Bacteriol. 2001, 183, 2634–2645. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Cho, K.M.; Barman, D.N.; Kim, M.K.; Yun, H.D. A potential cellulose microfibril swelling enzyme isolated from Bacillus sp. AY8 enhances cellulose hydrolysis. Process Biochem. 2015, 50, 807–815. [Google Scholar] [CrossRef]
- Haque, M.A.; Hwang, C.E.; Kim, S.C.; Cho, D.Y.; Lee, H.Y.; Cho, K.M.; Lee, J.H. Biodegradation of organophosphate insecticides by two organophosphorus hydrolase genes (opdA and opdE) from isolated Leuconostoc mesenteriodes WCP307 of kimchi origin. Process Biochem. 2020, 94, 340–348. [Google Scholar] [CrossRef]
- Pourbabaee, A.; Soleymani, S.; Farahbakhsh, M.; Torabi, E. Biodegradation of diazinon by the Stenotrophomonas maltophilia PS: Pesticide dissipation kinetics and breakdown characterization using FTIR. Int. J. Environ. Sci. Technol. 2018, 15, 1073–1084. [Google Scholar] [CrossRef]
- Aneja, K.R. Experiments in Microbiology, Plant Pathology and Biotechnology, 4th ed.; New Age International Publishers: New Delhi, India, 2007. [Google Scholar]
- Seeley, H.W.; Vandemark, P.J.; Lee, J.J. Microbes in Action: A laboratory Manual of Microbiology, 4th ed.; W. H. Freeman: New York, NY, USA, 1990. [Google Scholar]
- Abdullah-Al-Mamun, M.; Hossain, M.S.; Debnath, G.C.; Sultana, S.; Rahman, A.; Hasan, Z.; Das, S.R.; Ashik, M.A.; Prodhan, M.Y.; Aktar, S.; et al. Unveiling lignocellulytic traits of a goat omasum inhabitant Klebsiella variicola strain HSTU-AAM51 in light of biochemical and genome analyses. Braz. J. Microbiol. 2022, 53, 99–130. [Google Scholar] [CrossRef]
- Haque, M.A.; Hossain, M.S.; Ahmad, I.; Akbor, M.A.; Rahman, A.; Manir, M.S.; Patel, H.M.; Cho, K.M. Unveiling chlorpyrifos mineralizing and tomato plant-growth activities of Enterobacter sp. strain HSTU-ASh6 using biochemical tests, field experiments, genomics, and in silico analyses. Front. Microbiol. 2022, 13, 1060554. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, A.R.; Park, G.S.; Lim, J.H.; Waqas, M.; Lee, I.J.; Shin, J.H. Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Sci. Biotechnol. 2013, 22, 25–31. [Google Scholar] [CrossRef]
- Rahman, A.; Sitepu, I.R.; Tang, S.Y.; Hashidoko, Y. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci. Biotechnol. Biochem. 2010, 74, 2202–2208. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, N.M.; Davies, W.J.; Tikhonovich, I.A. Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1- carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum). Ann. Appl. Biol. 2015, 167, 11–25. [Google Scholar] [CrossRef]
- Shahzad, S.; Khalid, A.; Arshad, M. Screening rhizobacteria containing ACC-deaminase for growth promotion of chickpea seedlings under axenic conditions. Soil Environ. 2010, 29, 38–46. [Google Scholar]
- Shaharoona, B.; Riffat, B.; Muhammad, A.; Zahir, Z.; Ul-Hassan, Z. 1-Aminocylopropane-1- carboxylate (ACC)-deaminase rhizobacteria extenuates acc-induced classical triple response in etiolated pea seedlings. Pak. J. Bot. 2006, 38, 1491–1499. [Google Scholar]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Fukui, R.; Schroth, M.; Hendson, M.; Hancock, J. Interaction between strains of pseudomonads in sugar beet spermospheres and their relationship to pericarp colonization by Pythium ultimum in soil. Phytopathology 1994, 84, 1322–1330. [Google Scholar] [CrossRef]
- Krishnasamy, V.; Seshu, D. Germination after accelerated ageing and associated characters in rice varieties. Seed Sci. Technol. 1990, 18, 147–156. [Google Scholar]
- Thompson, D.; Clarke, B.; Kobayashi, D. Evaluation of bacterial antagonists for reduction of summer patch symptoms in Kentucky bluegrass. Plant Dis. 1996, 80, 856–862. [Google Scholar] [CrossRef]
- Zhang, J.; Han, C.; Liu, Z. Absorption spectrum estimating rice chlorophyll concentration: Preliminary investigations. J. Plant Breed Crop Sci. 2009, 1, 223–229. [Google Scholar]
- Chadha, A.; Florentine, S.K.; Chauhan, B.S.; Long, B.; Jayasundera, M. Influence of soil moisture regimes on growth, photosynthetic capacity, leaf biochemistry and reproductive capabilities of the invasive agronomic weed; Lactuca serriola. PLoS ONE 2019, 14, e0218191. [Google Scholar] [CrossRef]
- Zaman, N.R.; Chowdhury, U.F.; Reza, R.N.; Chowdhury, F.T.; Sarker, M.; Hossain, M.M.; Akbor, M.A.; Amin, A.; Islam, M.R.; Khan, H. Plant growth promoting endophyte Burkholderia contaminans NZ antagonizes phytopathogen Macrophomina phaseolina through melanin synthesis and pyrrolnitrin inhibition. PLoS ONE 2021, 16, e0257863. [Google Scholar] [CrossRef]
- Dasgupta, S.; Meisner, C.; Huq, M. A pinch or a pint? Evidence of pesticide overuse in Bangladesh. J. Agric. Econ. 2007, 58, 91–114. [Google Scholar] [CrossRef]
- Matin, M.; Malek, M.; Amin, M.; Rahman, S.; Khatoon, J.; Rahman, M.; Aminuddin, M.; Mian, A. Organochlorine insecticide residues in surface and underground water from different regions of Bangladesh. Agric. Ecosyst. Environ. 1998, 69, 11–15. [Google Scholar] [CrossRef]
- Chowdhury, M.A.Z.; Banik, S.; Uddin, B.; Moniruzzaman, M.; Karim, N.; Gan, S.H. Organophosphorus and carbamate pesticide residues detected in water samples collected from paddy and vegetable fields of the Savar and Dhamrai Upazilas in Bangladesh. Int. J. Environ. Res. Public Health 2012, 9, 3318–3329. [Google Scholar] [CrossRef]
- Haque, M.A.; Simo, T.; Prodhan, M.Y.; Ghosh, S.; Hossain, M.S.; Rahman, A.; Sharker, U.K.; Haque, M.A. Enhanced rice plant (BRRI-28) growth at lower doses of urea caused by diazinon mineralizing endophytic bacterial consortia and explorations of relevant regulatory genes in a Klebsiella sp. strain HSTU-F2D4R. Arch. Microbiol. 2023, 205, 231. [Google Scholar] [CrossRef]
- Bargabus, R.L.; Zidack, N.K.; Sherwood, J.E.; Jacobsen, B.J. Characterisation of systemic resistance in sugar beet elicited by a non-pathogenic, phyllosphere-colonizing Bacillus mycoides, biological control agent. Physiol. Mol. Plant Pathol. 2002, 61, 289–298. [Google Scholar] [CrossRef]
- Coombs, J.T.; Michelsen, P.P.; Franco, C.M. Evaluation of endophytic Actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol. Control 2004, 29, 359–366. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Govindasamy, V.; Annapurna, K. Role of antibiosis in suppression of charcoal rot disease by soybean endophyte Paenibacillus sp. HKA-15. Curr. Microbiol. 2007, 55, 25–29. [Google Scholar] [CrossRef]
- Bakker, P.A.; Pieterse, C.M.; Van Loon, L.C. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 2007, 97, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Walker, A.J.; Morgan, A.W.; Wright, D.J. Biodegradation of chlorpyrifos by Enterobacter strain B-14 and its use in bioremediation of contaminated soils. Appl. Environ. Microbiol. 2004, 7, 4855–4863. [Google Scholar] [CrossRef]
- Ghanem, I.; Orfi, M.; Shamma, M. Biodegradation of chlorpyrifos by Klebsiella sp. isolated from an activated sludge sample of waste water treatment plant in Damascus. Folia Microbiol. 2007, 52, 423–427. [Google Scholar] [CrossRef]
- Walitang, D.I.; Kim, K.; Madhaiyan, M.; Kim, Y.K.; Kang, Y.; Sa, T. Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of rice. BMC Microbiol. 2017, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Ge, J.; Li, Y.; He, S.; Zhong, J.; Liu, X.; Yu, X. Enhanced degradation of chlorpyrifos in rice (Oryza sativa L.) by five strains of endophytic bacteria and their plant growth promotional ability. Chemosphere 2017, 184, 505–513. [Google Scholar] [PubMed]
- Taghavi, S.; Van Der Lelie, D.; Hofman, A.; Zhang, Y.B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet 2010, 6, e1000943. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M.; Khan, M.S. Toxicological effects of selective herbicides on plant growth promoting activities of phosphate solubilizing Klebsiella sp. strain PS19. Curr. Microbiol. 2011, 62, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Carrim, A.J.I.; Barbosa, E.C.; Vieira, J.D.G. Enzymatic activity of endophytic bacterial isolates of Jacaranda decurrens Cham.(Carobinha-do-campo). Braz. Arch. Biol. Technol. 2006, 49, 353–359. [Google Scholar] [CrossRef]
- Chimwamurombe, P.M.; Grönemeyer, J.L.; Reinhold-Hurek, B. Isolation and characterization of culturable seed-associated bacterial endophytes from gnotobiotically grown Marama bean seedlings. FEMS Microbiol. 2016, 92, fiw083. [Google Scholar] [CrossRef]
- Ali, N.; Hameed, A.; Ahmed, S. Physicochemical characterization and bioremediation perspective of textile effluent, dyes and metals by indigenous bacteria. J. Hazard. Mater. 2009, 164, 322–328. [Google Scholar] [CrossRef]
- Tony, B.D.; Goyal, D.; Khanna, S. Decolorization of Direct Red 28 by mixed bacterial culture in an up flow immobilized bioreactor. J. Ind. Microbiol. Biotechnol. 2009, 36, 955–960. [Google Scholar] [CrossRef]
- Mattoo, A.J.; Nonzom, S. Endophytes in lignin valorization: A novel approach. Front. Bioeng. Biotechnol. 2022, 10, 895414. [Google Scholar] [CrossRef]
- Li, X.; Geng, X.; Xie, R.; Fu, L.; Jiang, J.; Gao, L.; Sun, J. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum. Biotechnol. Biofuels 2016, 9, 190. [Google Scholar] [CrossRef]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Van, V.T.; Berge, O.; Ke, S.N.; Balandreau, J.; Heulin, T. Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensis on early and late yield component in low fertility sulphate acid soils of Vietnam. Plant Soil 2000, 218, 273–284. [Google Scholar] [CrossRef]
- Shabanamol, S.; Varghese, E.M.; Thampi, M.; Karthika, S.; Sreekumar, J.; Jisha, M.S. Enhancement of growth and yield of rice (Oryza Sativa) by plant probiotic endophyte, Lysinibacillus sphaericus under greenhouse conditions. Commun. Soil Sci. Plant Anal. 2020, 51, 1268–1282. [Google Scholar]
- Tripathi, A.; Pandey, P.; Tripathi, S.N.; Kalra, A. Perspectives and potential applications of endophytic microorganisms in cultivation of medicinal and aromatic plants. Front. Plant Sci. 2022, 13, 985429. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, A.; Abdalla, O.; Li, L.; Ma, J.; Hatab, S.R.; Xu, L.; Guo, J.W.; Rasulov, B.A.; Liu, Y.H.; Hedlund, B.P.; et al. Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahliae. Front. Microbiol. 2018, 9, 924. [Google Scholar] [CrossRef]
- El-Deeb, B.; Fayez, K.; Gherbawy, Y. Isolation and characterization of endophytic bacteria from Plectranthus tenuiflorus medicinal plant in Saudi Arabia desert and their antimicrobial activities. J. Plant Interact. 2013, 8, 56–64. [Google Scholar] [CrossRef]
- Chandrakar, S.; Gupta, A. Antibiotic potential of endophytic actinomycetes of medicinal herbs against human pathogenic bacteria. Proc. Natl. Acad. Sci. India-B Biol. Sci. 2017, 87, 905–915. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, C.; Ye, Y.; Wen, J.; Wu, Y.; Wang, H.; Li, H.; Cai, S.; Cai, W.; Cheng, Z.; et al. Beneficial traits of bacterial endophytes belonging to the core communities of the tomato root microbiome. Agric. Ecosyst. Environ. 2017, 247, 149–156. [Google Scholar] [CrossRef]
- Diale, M.O.; Ubomba-Jaswa, E.; Serepa-Dlamini, M.H. The antibacterial activity of bacterial endophytes isolated from Combretum molle. Afr. J. Biotechnol. 2018, 17, 255–262. [Google Scholar]
- Ek-Ramos, M.J.; Gomez-Flores, R.; Orozco-Flores, A.A.; Rodríguez-Padilla, C.; González-Ochoa, G.; Tamez-Guerra, P. Bioactive products from plant-endophytic Gram-positive bacteria. Front. Microbiol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Fitri, S.N.A.; Gofar, N. Increasing of rice yield by using growth promoting endophytic bacteria from Swamp land. J. Trop. Soils 2010, 15, 271–276. [Google Scholar] [CrossRef]
- Peng, G.; Yuan, Q.; Li, H.; Zhang, W.; Tan, Z. Rhizobium oryzae sp. nov., isolated from the wild rice Oryza alta. Int. J. Syst. Evol. Microbiol. 2008, 58, 2158–5163. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, R.O.; Bellone, C.H.; de Bellone, C.; Boa Sorte, S.; Teixeira, P.M.F.; Dos, S.K.R. Azospirillum inoculation and nitrogen fertilization effect on grain yield and on the diversity of endophytic bacteria in the phyllosphere of rice rainfed crop. Eur. J. Soil Biol. 2009, 45, 36–43. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Dazzo, F.B. Enhancement of rice production using endophytic strains of Rhizobium leguminosarum bv. trifolii in extensive field inoculation trials within the Egypt Nile delta. Plant Soil 2010, 336, 129–142. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.L.; Bergen, M.S.; Kowalski, K.P.; White, J.F. Fungal disease prevention in seedlings of rice (Oryza Sativa) and other grasses by growth-promoting seed-associated endophytic bacteria from invasive Phragmites australis. Microorganisms 2018, 6, 21. [Google Scholar] [CrossRef]
- Doni, F.; Suhaimi, N.S.M.; Mispan, M.S.; Fathurrahman, F.; Marzuki, B.M.; Kusmoro, J.; Uphoff, N. Microbial contributions for rice Production: From conventional crop management to the use of ‘Omics’ Technologies. Int. J. Mol. Sci. 2022, 23, 737. [Google Scholar] [CrossRef]
- Tie, J.; Qiao, Y.; Jin, N.; Gao, X.; Liu, Y.; Lyu, J.; Zhang, G.; Hu, L.; Yu, J. Yield and rhizosphere soil environment of greenhouse Zucchini in response to different planting and breeding waste composts. Microorganisms 2023, 11, 1026. [Google Scholar] [CrossRef]
- Sullins, K.N.; Dillard, S.L.; Held, D.W.; Carroll, E.P. Utility of plant growth-promoting Rhizobacteria for sustainable production of Bermudagrass forage. Microorganisms 2023, 11, 863. [Google Scholar] [CrossRef]
- Adachi, S.; Yamamoto, T.; Nakae, T.; Yamashita, M.; Uchida, M.; Karimata, R.; Ichihara, N.; Soda, K.; Ochiai, T.; Ao, R.; et al. Genetic architecture of leaf photosynthesis in rice revealed by different types of reciprocal mapping populations. J. Exp. Bot. 2019, 70, 5131–5144. [Google Scholar] [CrossRef] [PubMed]
| Isolates | Oxi | Cit | Cat | MIU | Mot | Ure | VP | MR | TSI | Lac | Suc | Dex | Mal | CMC | Xy | Amy | Pro | CR | TB | BTB | AB | TB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Klebsiella sp. HSTU-Bk11 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Acinetobacter sp. HSTU-Abk29 | + | + | + | - | - | + | + | - | + | + | + | + | + | + | + | + | + | + | + | - | + | + |
| Citrobacter sp. HSTU-Abk30 | + | + | + | + | + | - | - | + | + (Fe) | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Enterobacter cloacae HSTU-Abk39 | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Enterobacter cloacae HSTU-Abk37 | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Enterobacter ludwigii HSTU-Abk40 | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Acinetobacter baumannii HSTU-ABK42 | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Klebsiella sp. Strain HSTU-Abk31 | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Acinetobacter sp. Strain HSTU-Bk12 | + | + | + | + | + | - | + | - | + | + | + | + | + | + | - | + | + | + | + | + | + | + |
| Acinetobacter sp. HSTU-Abk32 | + | + | + | - | - | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | - |
| Burkholderia sp. HSTU-ABK33 | + | + | + | - | - | - | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Acinetobacter sp. HSTU-Abk34 | + | + | + | + | + | + | + | - | + | + | + | + | + | - | + | + | + | + | + | + | + | + |
| Pseudomonas sp. HSTU-Bk13 | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + |
| Citrobacter sp. HSTU-Bk14 | + | + | + | - | - | + | + | - | + (Fe) | + | + | + | + | - | + | + | + | + | + | + | + | + |
| Acinetobacter sp. HSTU-Bk15 | + | + | + | + | + | - | - | + | + | + | + | + | - | + | + | + | + | + | + | + | + | |
| Enterobacter sp. HSTU-Abk36 | + | + | + | + | + | - | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Enterobacter sp. HSTU-Abk38 | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Serratia marcescens HSTU-Abk41 | + | + | + | + | + | + | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Isolates | Multidrug-Resistant Human Pathogenic Bacteria | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus (mm ± SE) | E. coli (mm ± SE) | Klebshilla (mm ± SE) | S. epidermidis (mm ± SE) | |||||||||
| 16 h | 32 h | 48 h | 16 h | 32 h | 48 h | 16 h | 32 h | 48 h | 16 h | 32 h | 48 h | |
| Klebsiella sp. HSTU-Bk11 | 12.75 ± 0.25 | 15 ± 0.2 | 20.5 ± 0.5 | - | 8.5 ± 0.5 | 7.5 ± 0.5 | - | - | - | 13.5 ± 3.5 | 17 ± 5.0 | 18.5 ± 1.5 |
| Acinetobacter sp. HSTU-ABk29 | - | 6.5 ± 1.5 | 6.5 ± 1.5 | - | - | - | - | - | - | 9.5 ± 1.5 | 13 ± 2.0 | 12 ± 0.0 |
| Citrobacter sp. HSTU-ABk30 | - - | 11 ± 0.0 | 10.5 ± 0.5 | - - | - - | - - | - - | - - | - - | - - | - - | - - |
| Enterobacter cloacae HSTU-ABk39 | 40 ± 2 | 41.5 ± 1.5 ** | 40 ± 0.0 | - | - | - | - | - | 9 ± 1.0 | - | - | 9.5 ± 1.5 |
| Enterobacter cloacae HSTU-ABk37 | - | - | 10 ± 0.0 | 6 ± 0.0 | 6 ± 0.0 | 6 ± 0.0 | - | - | - | - | 9.5 ± 1.5 | 10 ± 0.0 |
| Enterobacter ludwigii HSTU-ABk40 | - | - | - | 6 ± 0.0 | 6 ± 0.0 | 6 ± 0.0 | - | - | 8 ± 0.0 | - | - | - |
| Acinetobacter baumannii HSTU-ABK42 | - | - | - | - | - | - | 8.5 ± 1.5 | - | - | - | - | - |
| Klebsiella sp. HSTU-ABk31 | - | - | - | - | 7 ± 0.0 | 7 ± 0.0 | 8.5 ± 0.5 | - | - | - | - | - |
| Acinetobacter sp. HSTU-Bk12 | - | - | - | - | 5.5 ± 0.5 | 6 ± 0.0 | 6.5 ± 0.5 | - | - | - | - | - |
| Acinetobacter sp. HSTU-ABk32 | 16.5 ± 0.5 | 16.5 ± 0.5 | 17 ± 0.0 | 8.5 ± 0.5 | 8.5 ± 0.5 | 10 ± 0.0 | - | 7 ± 0.0 | 15 ± 0.0 | 9 ± 0.0 | 10.5 ± 0.5 | 9.5 ± 0.5 |
| Burkholderia sp. HSTU-ABK33 | - | - | - | - | - | - | - | 5.5 ± 0.5 | 5.5 ± 0.5 | - | - | - |
| Acinetobacter sp. HSTU-ABk34 | 18 ± 2.0 | 26 ± 0.6 ** | 24.5 ± 4.5 | 12.5 ± 0.5 | 12.5 ± 0.5 | 12.5 ± 0.5 | 9.5 ± 1.5 | 9.5 ± 1.5 | 10.5 ± 0.5 | 10 ± 0.0 | 11 ± 0.0 | 10.5 ± 0.5 |
| Pseudomonas sp. HSTU-Bk13 | 15 ± 1.0 | 16 ± 2.0 | 17.5 ± 0.5 | 8 ± 1.0 | 7.5 ± 0.5 | 7.5 ± 0.5 | 6.5 ± 0.5 | 18.5 ± 1.5 | 18.5 ± 1.5 | 19 ± 1.0 | ||
| Citrobacter sp. HSTU-Bk14 | 10 ± 2.0 | 11.5 ± 1.5 | 11.5 ± 1.5 | - | - | - | - | - | - | - | - | - |
| Acinetobacter sp. HSTU-Bk15 | 11.5 ± 1.5 | 16.5 ± 0.5 | 23.5 ± 1.5 | - | - | - | 17 ± 1.0 | 16 ± 2.0 | 16 ± 3.0 | 16 ± 1.0 | 16 ± 1.0 | 17.5 ± 2.5 |
| Enterobacter HSTU-ABk36 | - | - | - | 6 ± 0.0 | 11.5 ± 2.5 | 14.5 ± 0.5 | 10 ± 1.0 | 10.5 ± 0.5 | 9 ± 1.0 | 25 ± 10.0 | 28.5 ± 10.5 | 29 ± 9.0 ** |
| Enterobacter sp. HSTU-ABk38 | - | 11.5 ± 0.5 | 11.5 ± 0.5 | - | - | - | - | 6 ± 0.0 | 7 ± 0.0 | - | - | - |
| Serratia marcescens HSTU-ABk41 | - | - | - | - | - | - | - | 6 ± 0.0 | 6.5 ± 0.5 | - | - | - |
| Treatment | Germination % (Mean ± SE) | Shoot Length after 8 Days (Mean ± SE) | Shoot Length after 12 Days (Mean ± SE) | Root Length after 8 Days (Mean ± SE) | Root Length After 12 Days (Mean ± SE) | Vigor Index (Mean ± SE) |
|---|---|---|---|---|---|---|
| Klebsiella sp. HSTU-Bk11 | 95.56 ± 2.22 a | 5.00 ± 0.50 abcd | 6.77 ± 1.01 abc | 6.73 ± 1.92 ab | 7.43 ± 0.46 abc | 1116.67 ± 119.46 abc |
| Acinetobacter sp. HSTU-ABk29 | 91.11 ± 5.88 ab | 5.40 ± 0.31 abcd | 7.37 ± 0.33 abc | 6.43 ± 1.32 abcd | 7.50 ± 0.76 abc | 1068.89 ± 79.08 abc |
| Citrobacter sp. HSTU-ABk30 | 95.56 ± 2.22 a | 4.77 ± 0.12 bcd | 5.57 ± 0.58 bcd | 6.33 ± 1.20 abcd | 6.77 ± 1.65 abc | 1056.00 ± 83.44 abc |
| Enterobacter cloacae HSTU-ABk39 | 88.89 ± 2.22 ab | 4.73 ± 0.39 bcd | 7.30 ± 0.80 abc | 3.90 ± 0.56 dc | 4.97 ± 0.93 c | 771.56 ± 105.30 cd |
| Enterobacter cloacae HSTU-ABk37 | 93.33 ± 0.0 a | 5.33 ± 0.22 abcd | 5.50 ± 0.53 cd | 7.67 ± 0.33 ab | 6.27 ± 1.50 bc | 1213.33 ± 51.40 ab |
| Enterobacter ludwigii HSTU-ABk40 | 93.33 ± 3.85 a | 5.33 ± 0.38 abcd | 7.33 ± 0.45 abc | 6.83 ± 0.69 ab | 7.83 ± 0.44 ab | 1137.11 ± 105.13 ab |
| Acinetobacter baumannii HSTU-ABK42 | 88.89 ± 2.22 ab | 5.17 ± 0.17 abcd | 6.50 ± 0.29 abcd | 7.17 ± 0.88 ab | 7.50 ± 1.04 abc | 1098.89 ± 101.99 abc |
| Klebsiella sp. HSTU-ABk31 | 95.56 ± 2.22 a | 5.90 ± 0.31 abc | 6.53 ± 0.30 abcd | 7.27 ± 0.67 ab | 7.07 ± 1.38 abc | 1256.67 ± 16.51 ab |
| Acinetobacter sp. HSTU-Bk12 | 93.33 ± 0.00 a | 5.10 ± 0.10 abcd | 7.27 ± 0.59 abc | 6.07 ± 0.70 bcd | 6.67 ± 0.17 abc | 1042.22 ± 71.76 abc |
| Acinetobacter sp. HSTU-ABk32 | 93.33 ± 0.00 a | 5.27 ± 0.15 abcd | 6.63 ± 0.93 abc | 6.10 ± 0.31 abcd | 8.03 ± 0.27 ab | 1060.89 ± 41.86 abc |
| Burkholderia sp. HSTU-ABK33 | 95.56 ± 2.22 a | 6.27 ± 0.46 a | 7.00 ± 0.50 abc | 6.67 ± 0.41 abc | 9.07 ± 0.64 a | 1237.56 ± 87.10 ab |
| Acinetobacter sp. HSTU-ABk34 | 88.89 ± 2.22 ab | 6.00 ± 0.40 ab | 7.57 ± 0.35 ab | 7.83 ± 0.60 ab | 8.10 ± 0.47 ab | 1228.89 ± 86.47 ab |
| Pseudomonas sp. HSTU-Bk13 | 93.33 ± 3.85 a | 5.43 ± 0.64 abcd | 6.37 ± 1.10 abcd | 7.33 ± 0.67 ab | 6.70 ± 1.82 abc | 1196.22 ± 138.18 ab |
| Citrobacter sp. HSTU-Bk14 | 91.11 ± 5.8 ab | 4.83 ± 0.33 bcd | 8.17 ± 0.64 a | 7.50 ± 0.29 ab | 7.33 ± 0.44 abc | 1128.89 ± 111.31 |
| Acinetobacter sp. HSTU-Bk15 | 97.78 ± 2.22 a | 4.73 ± 0.26 bcd | 6.97 ± 0.75 abc | 5.20 ± 0.51 bcd | 7.20 ± 0.85 abc | 969.56 ± 62.34 |
| Enterobacter sp. HSTU-ABk36 | 95.56 ± 2.22 a | 5.77 ± 0.64 abcd | 6.10 ± 0.32 bcd | 8.80 ± 0.76 a | 7.27 ± 0.93 abc | 1386.00 ± 100.34 a |
| Enterobacter sp. HSTU-ABk38 | 97.78 ± 2.22 a | 4.40 ± 0.20 de | 6.53 ± 0.62 abcd | 6.73 ± 0.23 ab | 9.17 ± 0.17 a | 1087.11 ± 18.72 abc |
| Serratia marcescens HSTU-ABk41 | 93.33 ± 3.85 a | 4.77 ± 0.37 bcd | 7.33 ± 0.73 abc | 6.00 ± 1.53 bcd | 7.83 ± 1.20 ab | 1009.78 ± 188.26 bc |
| Control | 82.22 ± 5.88 b | 3.27 ± 0.43 e | 4.60 ± 0.46 d | 4.00 ± 0.29 cd | 6.13 ± 0.52 bc | 600.44 ± 80.22 d |
| LSD of individual bacteria | 9.55 | 1.40 | 2.02 | 2.73 | 2.78 | 354.61 |
| Consortia/Group-1 | 96.67 ± 3.3 a | 6.45 ± 0.05 a | 8.5 ± 1 a | 6.65 ± 0.35 a | 8.75 ± 0.75 a | 1267.33 ± 72 a |
| Consortia/Group-2 | 93.33 ± 6.7 a | 6.4 ± 0.2 a | 8.7 ± 0.1 a | 5.7 ± 0.3 a | 8.15 ± 1.85 a | 1128.67 ± 71 ab |
| Consortia/Group-3 | 93.33 ± 0.0 a | 5.5 ± 0.5 b | 8.8 ± 0.5 a | 6.5 ± 0.5 a | 7.15 ± 0.55 ab | 1050.00 ± 116 ab |
| Consortia/Group-4 | 93.33 ± 00 a | 9.75 ± 0.25 ab | 7.5 ± 1 ab | 7.1 ± 0.1 a | 7.25 ± 0.25 ab | 1176.00 ± 37 ab |
| Control | 93.33 ± 0.0 a | 4.55 ± 0.05 b | 5.85 ± 0.35 b | 6 ± 1.0 a | 5.5 ± 0.0 b | 984.67 ± 98 b |
| LSD of group treatment | 8.93 | 1.55 | 2.39 | 1.88 | 2.56 | 273.50 |
| Similarity of Hit | Search Spectrum | Soft Ionization (SI) | Spectrum | Molecular Weight (Da) | Molecular Form | Molecular Structure |
|---|---|---|---|---|---|---|
| 1,2,3,4,5,8 | 76,69,67,66,65,57 | 2921 | 88 | 2 | Chlorpyrifos | 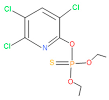 |
| 6 | 59 | 6515 | 38 | 4 | 2-Hydroxy-3,5,6 trichloropyridine | 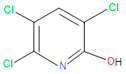 |
| 7 | 58 | 6515 | 38 | 4 | 2-Hydroxy-3,5,6-trichloropyridine | 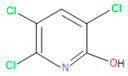 |
| 9 | 56 | 2588 | 3 | 6 | Phorate sulfoxide | 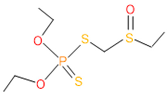 |
| 10 | 56 | 2588 | 3 | 6 | Phorate sulfoxide | 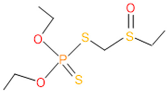 |
| 11 | 55 | 5598 | 13 | 0 | Chloropyriphos-methyl | 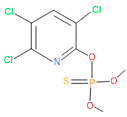 |
| 12 | 55 | 2588 | 3 | 6 | Phorate sulfoxide | 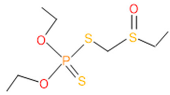 |
| 13 | 55 | 2588 | 4 | 7 | Phorate sulfoxide | 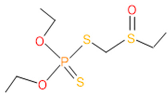 |
| 14 | 54 | 2921 | 88 | 2 | Chlorpyrifos |  |
| 15 | 53 | 2588 | 4 | 7 | Phorate sulfone | 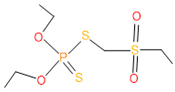 |
| 16 | 53 | 16,947 | 69 | 6 | Carbonochloridic | 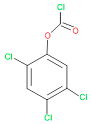 |
| 17 | 52 | 5598 | 15 | 2 | Phosphoric acid |  |
| 18 | 51 | 2021 | 58 | 1 | dl-2-.beta.-Thienyl-.alpha.-alanine | 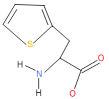 |
| 19 | 50 | 2497 | 7 | 6 | Oxydisulfoton | 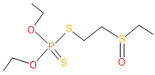 |
| 20 | 50 | 683 | 8 | 9 | Diethyl methanephosphonate |  |
| 21 | 50 | 683 | 8 | 9 | Diethyl methanephosphonate |  |
| 22 | 50 | 5598 | 13 | 0 | Chloropyriphos-methyl | 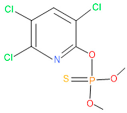 |
| 23 | 50 | 17,297 | 40 | 4 | Carbofenothion sulfoxide | 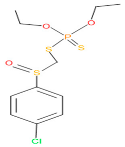 |
| 24 | 49 | 0 | 0 | 0 | Acetamide, |  |
| 25 | 49 | 1970 | 40 | 7 | 4-Pyridinol, |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodhan, M.Y.; Rahman, M.B.; Rahman, A.; Akbor, M.A.; Ghosh, S.; Nahar, M.N.-E.-N.; Simo; Shamsuzzoha, M.; Cho, K.M.; Haque, M.A. Characterization of Growth-Promoting Activities of Consortia of Chlorpyrifos Mineralizing Endophytic Bacteria Naturally Harboring in Rice Plants—A Potential Bio-Stimulant to Develop a Safe and Sustainable Agriculture. Microorganisms 2023, 11, 1821. https://doi.org/10.3390/microorganisms11071821
Prodhan MY, Rahman MB, Rahman A, Akbor MA, Ghosh S, Nahar MN-E-N, Simo, Shamsuzzoha M, Cho KM, Haque MA. Characterization of Growth-Promoting Activities of Consortia of Chlorpyrifos Mineralizing Endophytic Bacteria Naturally Harboring in Rice Plants—A Potential Bio-Stimulant to Develop a Safe and Sustainable Agriculture. Microorganisms. 2023; 11(7):1821. https://doi.org/10.3390/microorganisms11071821
Chicago/Turabian StyleProdhan, Md. Yeasin, Md. Bokhtiar Rahman, Aminur Rahman, Md. Ahedul Akbor, Sibdas Ghosh, Mst. Nur-E-Nazmun Nahar, Simo, Md. Shamsuzzoha, Kye Man Cho, and Md. Azizul Haque. 2023. "Characterization of Growth-Promoting Activities of Consortia of Chlorpyrifos Mineralizing Endophytic Bacteria Naturally Harboring in Rice Plants—A Potential Bio-Stimulant to Develop a Safe and Sustainable Agriculture" Microorganisms 11, no. 7: 1821. https://doi.org/10.3390/microorganisms11071821
APA StyleProdhan, M. Y., Rahman, M. B., Rahman, A., Akbor, M. A., Ghosh, S., Nahar, M. N.-E.-N., Simo, Shamsuzzoha, M., Cho, K. M., & Haque, M. A. (2023). Characterization of Growth-Promoting Activities of Consortia of Chlorpyrifos Mineralizing Endophytic Bacteria Naturally Harboring in Rice Plants—A Potential Bio-Stimulant to Develop a Safe and Sustainable Agriculture. Microorganisms, 11(7), 1821. https://doi.org/10.3390/microorganisms11071821








