Tomato Plant Microbiota under Conventional and Organic Fertilization Regimes in a Soilless Culture System
Abstract
1. Introduction
2. Materials and Methods
2.1. Tomato Plants Grown in Soilless Culture Systems
2.2. Sample Collection and DNA Extraction
2.3. High-Throughput Sequencing of 16S rRNA and Data Processing
2.4. Analyses of Core Microbiota, Bacterial Abundance, and Diversity
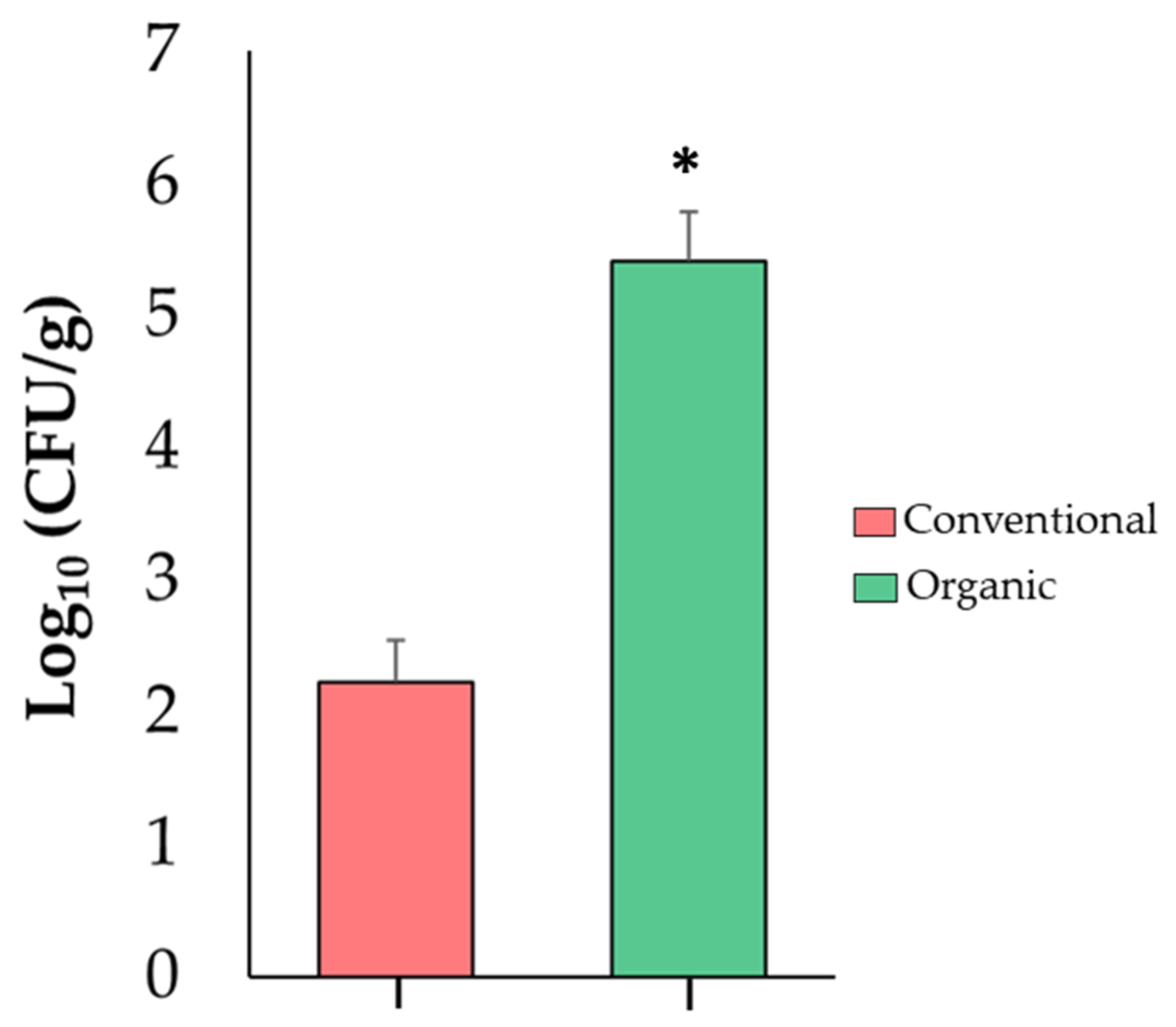
2.5. Absolute Quantification of Enterobacteriaceae in Tomato Fruits
2.6. Statistical Analyses
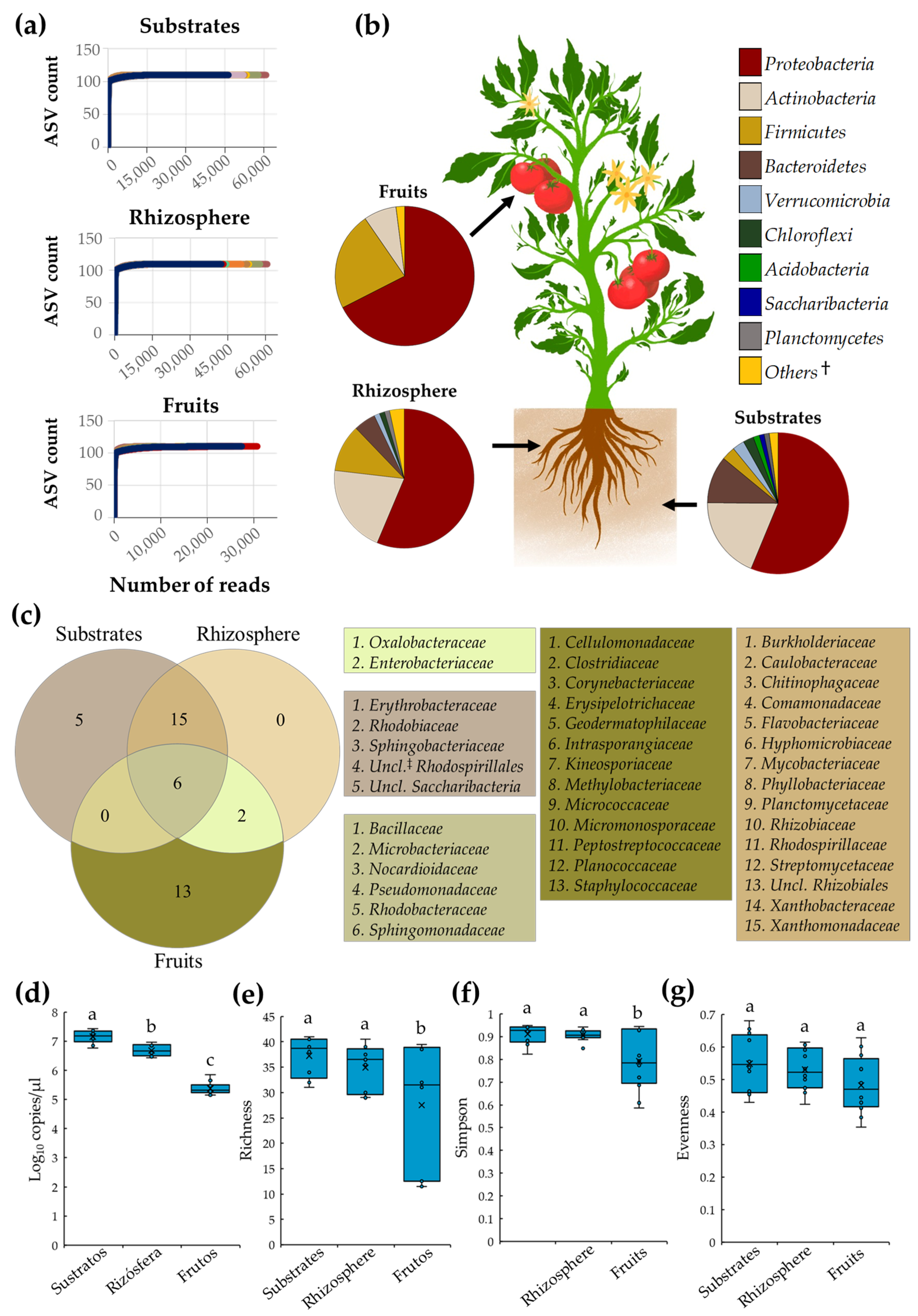
3. Results
3.1. Tomato Core Microbiota
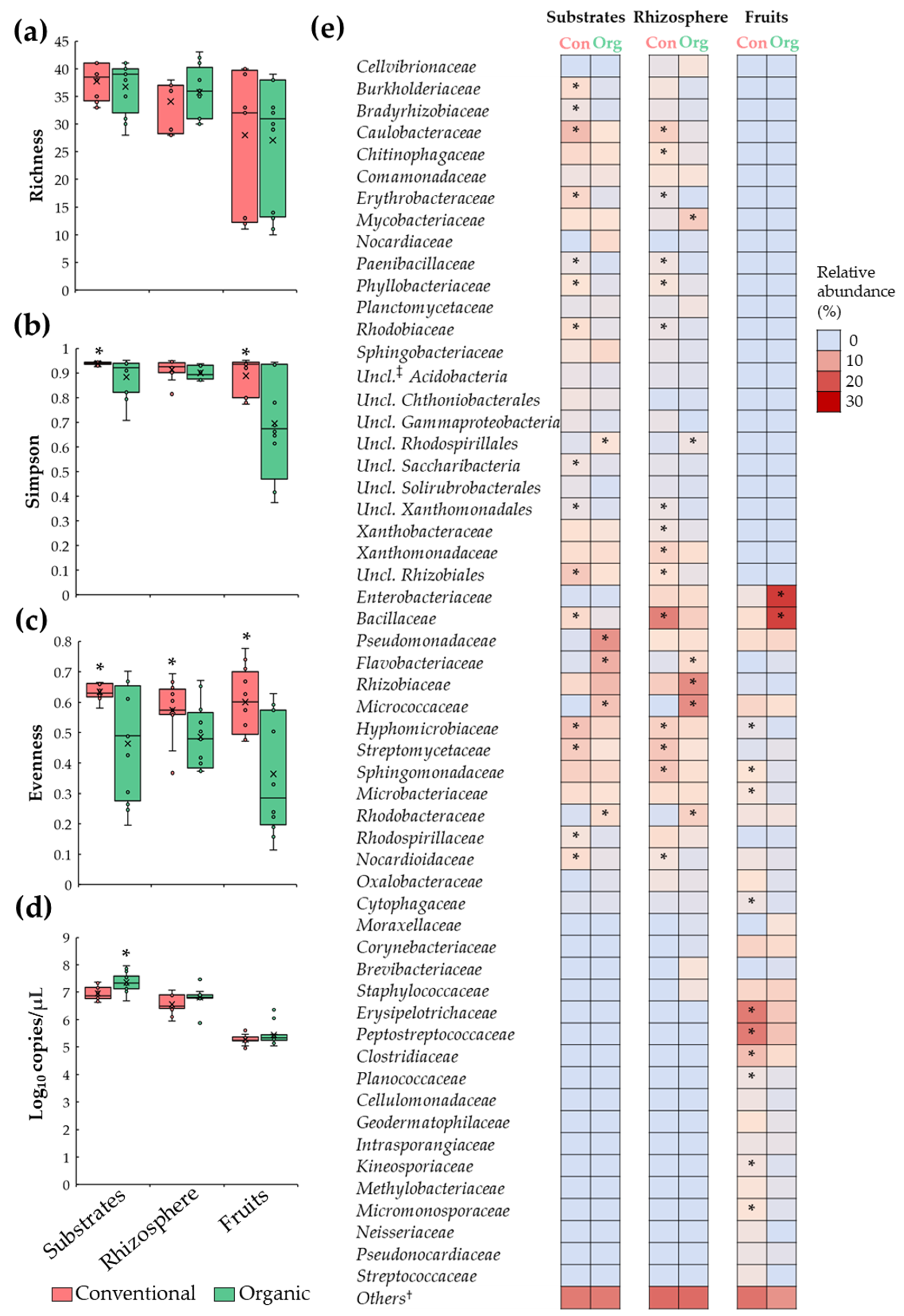
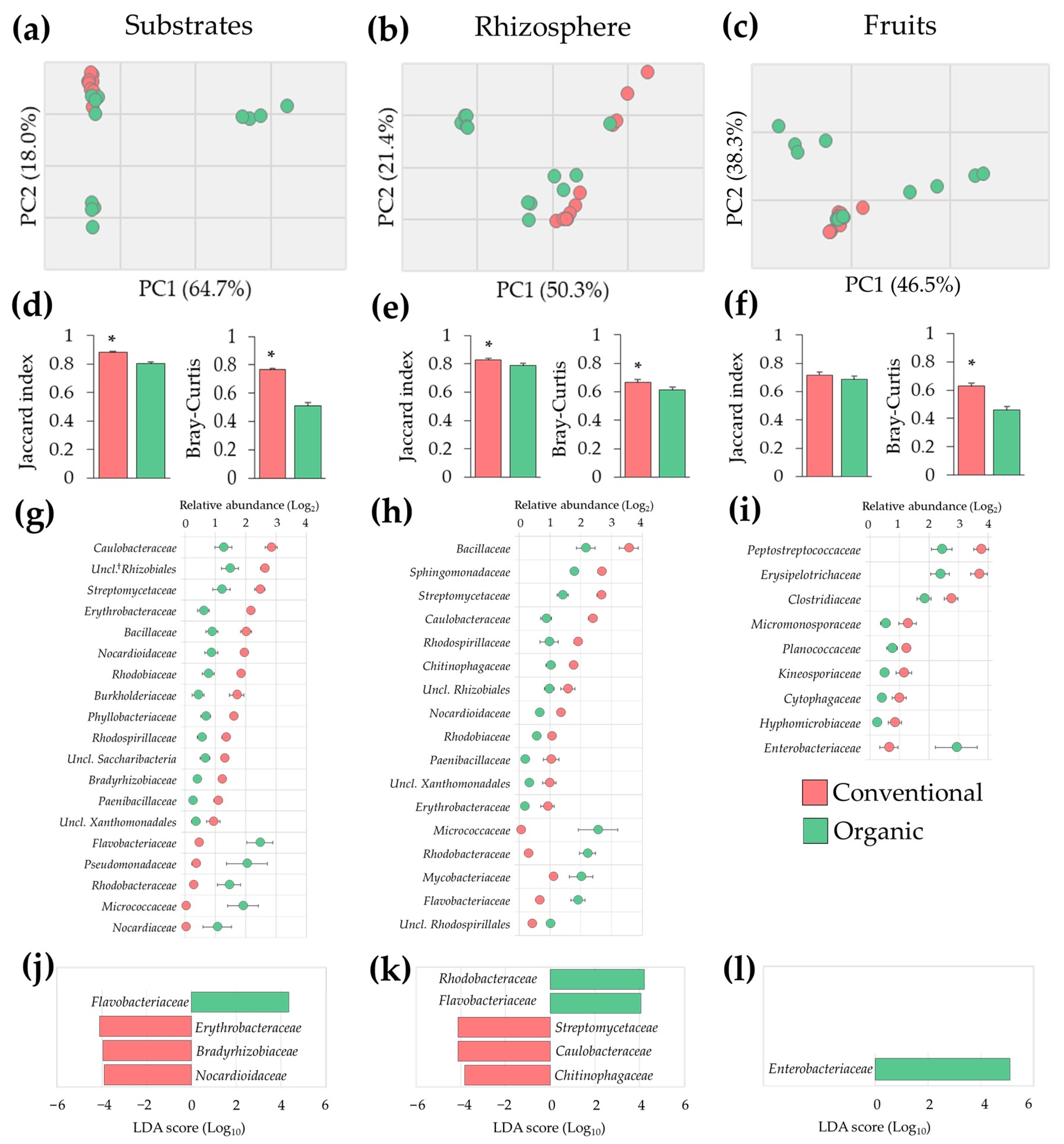
3.2. Differences in Tomato Microbiota between Conventional and Organic Soilless Culture Systems
3.3. Microbial Dysbiosis Biomarkers
3.4. Absolute Quantification of Enterobacteriaceae in Tomato Fruits
4. Discussion
4.1. Tomato Core Microbiota in Soilless Culture Systems
4.2. Bacterial Relative Abundance Is Influenced by Conventional and Organic Soilless Culture Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Ortega, W.M.; Martínez, V.; Nieves, M.; Simón, I.; Lidón, V.; Fernandez-Zapata, J.C.; Martinez-Nicolas, J.J.; Cámara-Zapata, J.M.; García-Sánchez, F. Agricultural and Physiological Responses of Tomato Plants Grown in Different Soilless Culture Systems with Saline Water under Greenhouse Conditions. Sci. Rep. 2019, 9, 6733. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.C.; Telhado, S.F.P. Organic Food: A Comparative Study of the Effect of Tomato Cultivars and Cultivation Conditions on the Physico-Chemical Properties. Foods 2015, 4, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Floare-Avram, C.V.; Covaciu, F.; Voica, C.; Puscas, R.; Feher, I.; Marincas, O.; Magdas, D.A. Differentiation of Tomatoes Based on Isotopic, Elemental and Organic Markers. J. Food Sci. Technol. 2020, 57, 2222–2232. [Google Scholar] [CrossRef]
- Putra, P.A.; Yuliando, H. Soilless Culture System to Support Water Use Efficiency and Product Quality: A Review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing Sustainability of Growing Media Constituents and Stand-Alone Substrates in Soilless Culture Systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M. The Evolution of Soilless Systems towards Ecological Sustainability in the Perspective of a Circular Economy. Is It Really the Opposite of Organic Agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Nik Ibrahim, N.N.L.; Wayayok, A.; Hashim, N. Some Emerging Opportunities of Nanotechnology Development for Soilless and Microgreen Farming. Agronomy 2021, 11, 1213. [Google Scholar] [CrossRef]
- Vinha, A.F.; Barreira, S.V.P.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Organic versus Conventional Tomatoes: Influence on Physicochemical Parameters, Bioactive Compounds and Sensorial Attributes. Food Chem. Toxicol. 2014, 67, 139–144. [Google Scholar] [CrossRef]
- Azarbad, H. Conventional vs. Organic Agriculture—Which One Promotes Better Yields and Microbial Resilience in Rapidly Changing Climates? Front. Microbiol. 2022, 13, 903500. [Google Scholar] [CrossRef]
- Vigar, V.; Myers, S.; Oliver, C.; Arellano, J.; Robinson, S.; Leifert, C. A Systematic Review of Organic Versus Conventional Food Consumption: Is There a Measurable Benefit on Human Health? Nutrients 2019, 12, 7. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Moura, C.F.H.; Gomes-Filho, E.; Marco, C.A.; Urban, L.; Miranda, M.R.A. The Impact of Organic Farming on Quality of Tomatoes Is Associated to Increased Oxidative Stress during Fruit Development. PLoS ONE 2013, 8, e56354. [Google Scholar] [CrossRef]
- Ronga, D.; Caradonia, F.; Vitti, A.; Francia, E. Agronomic Comparisons of Heirloom and Modern Processing Tomato Genotypes Cultivated in Organic and Conventional Farming Systems. Agronomy 2021, 11, 349. [Google Scholar] [CrossRef]
- Ronga, D.; Lovelli, S.; Zaccardelli, M.; Perrone, D.; Ulrici, A.; Francia, E.; Milc, J.; Pecchioni, N. Physiological Responses of Processing Tomato in Organic and Conventional Mediterranean Cropping Systems. Sci. Hortic. 2015, 190, 161–172. [Google Scholar] [CrossRef]
- Riahi, A.; Hdider, C.; Sanaa, M.; Tarchoun, N.; Kheder, M.B.; Guezal, I. Effect of Conventional and Organic Production Systems on the Yield and Quality of Field Tomato Cultivars Grown in Tunisia. J. Sci. Food Agric. 2009, 89, 2275–2282. [Google Scholar] [CrossRef]
- Mubarok, S.; Farhah, F.F.; Anas; Suwali, N.; Kurnia, D.; Kusumiyati; Suminar, E.; Ezura, H. Data on the Yield and Quality of Organically Hybrids of Tropical Tomato Fruits at Two Stages of Fruit Maturation. Data Brief 2019, 25, 104031. [Google Scholar] [CrossRef] [PubMed]
- Adekiya, A.O.; Dahunsi, S.O.; Ayeni, J.F.; Aremu, C.; Aboyeji, C.M.; Okunlola, F.; Oyelami, A.E. Organic and In-Organic Fertilizers Effects on the Performance of Tomato (Solanum lycopersicum) and Cucumber (Cucumis sativus) Grown on Soilless Medium. Sci. Rep. 2022, 12, 12212. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, W.; Ghini, R.; Galvão, J.A.H.; Siloto, R.C. Organic and Conventional Tomato Cropping Systems. Sci. Agric. 2004, 61, 253–259. [Google Scholar] [CrossRef]
- Grunert, O.; Robles-Aguilar, A.A.; Hernandez-Sanabria, E.; Schrey, S.D.; Reheul, D.; Van Labeke, M.-C.; Vlaeminck, S.E.; Vandekerckhove, T.G.L.; Mysara, M.; Monsieurs, P.; et al. Tomato Plants Rather than Fertilizers Drive Microbial Community Structure in Horticultural Growing Media. Sci. Rep. 2019, 9, 9561. [Google Scholar] [CrossRef] [PubMed]
- Grunert, O.; Hernandez-Sanabria, E.; Buysens, S.; De Neve, S.; Van Labeke, M.-C.; Reheul, D.; Boon, N. In-Depth Observation on the Microbial and Fungal Community Structure of Four Contrasting Tomato Cultivation Systems in Soil Based and Soilless Culture Systems. Front. Plant Sci. 2020, 11, 520834. [Google Scholar] [CrossRef]
- Readyhough, T.; Neher, D.A.; Andrews, T. Organic Amendments Alter Soil Hydrology and Belowground Microbiome of Tomato (Solanum lycopersicum). Microorganisms 2021, 9, 1561. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kong, H.G.; Song, G.C.; Ryu, C.-M. Disruption of Firmicutes and Actinobacteria Abundance in Tomato Rhizosphere Causes the Incidence of Bacterial Wilt Disease. ISME J. 2021, 15, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Elkabetz, D.; Leibman-Markus, M.; Sayas, T.; Schneider, A.; Jami, E.; Kleiman, M.; Bar, M. Cytokinin Drives Assembly of the Phyllosphere Microbiome and Promotes Disease Resistance through Structural and Chemical Cues. ISME J. 2022, 16, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Runge, P.; Ventura, F.; Kemen, E.; Stam, R. Distinct Phyllosphere Microbiome of Wild Tomato Species in Central Peru upon Dysbiosis. Microb. Ecol. 2023, 85, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Mehlferber, E.C.; McCue, K.F.; Ferrel, J.E.; Koskella, B.; Khanna, R. Temporally Selective Modification of the Tomato Rhizosphere and Root Microbiome by Volcanic Ash Fertilizer Containing Micronutrients. Appl. Environ. Microbiol. 2022, 88, e00049-22. [Google Scholar] [CrossRef] [PubMed]
- USDA AMS Guidelines for Organic Crop Certification. Available online: https://www.ams.usda.gov/sites/default/files/media/Crop%20-%20Guidelines.pdf (accessed on 1 April 2023).
- Allard, S.M.; Walsh, C.S.; Wallis, A.E.; Ottesen, A.R.; Brown, E.W.; Micallef, S.A. Solanum lycopersicum (Tomato) Hosts Robust Phyllosphere and Rhizosphere Bacterial Communities When Grown in Soil Amended with Various Organic and Synthetic Fertilizers. Sci. Total Environ. 2016, 573, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Vargas, P.; Bosmans, L.; Van Calenberge, B.; Van Kerckhove, S.; Lievens, B.; Rediers, H. Bacterial Community Dynamics of Tomato Hydroponic Greenhouses Infested with Hairy Root Disease. FEMS Microbiol. Ecol. 2021, 97, fiab153. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.; Evanich, D.J.; Shi, Y.; Xu, Y.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-Resolution Spatiotemporal Transcriptome Mapping of Tomato Fruit Development and Ripening. Nat. Commun. 2018, 9, 364. [Google Scholar] [CrossRef]
- Allard, S.M.; Ottesen, A.R.; Micallef, S.A. Rain Induces Temporary Shifts in Epiphytic Bacterial Communities of Cucumber and Tomato Fruit. Sci. Rep. 2020, 10, 1765. [Google Scholar] [CrossRef]
- Ottesen, A.R.; González Peña, A.; White, J.R.; Pettengill, J.B.; Li, C.; Allard, S.; Rideout, S.; Allard, M.; Hill, T.; Evans, P.; et al. Baseline Survey of the Anatomical Microbial Ecology of an Important Food Plant: Solanum lycopersicum (Tomato). BMC Microbiol. 2013, 13, 114. [Google Scholar] [CrossRef]
- Piraine, R.E.A.; Leite, F.P.L.; Bochman, M.L. Mixed-Culture Metagenomics of the Microbes Making Sour Beer. Fermentation 2021, 7, 174. [Google Scholar] [CrossRef]
- Can-Herrera, L.A.; Gutierrez-Canul, C.D.; Dzul-Cervantes, M.A.A.; Pacheco-Salazar, O.F.; Chi-Cortez, J.D.; Carbonell, L.S. Identification by Molecular Techniques of Halophilic Bacteria Producing Important Enzymes from Pristine Area in Campeche, Mexico. Braz. J. Biol. 2021, 83, e246038. [Google Scholar] [CrossRef] [PubMed]
- Puón-Peláez, X.-H.D.; McEwan, N.R.; Gómez-Soto, J.G.; Álvarez-Martínez, R.C.; Olvera-Ramírez, A.M. Metataxonomic and Histopathological Study of Rabbit Epizootic Enteropathy in Mexico. Animals 2020, 10, 936. [Google Scholar] [CrossRef] [PubMed]
- Çelik, Z.C.; Çakiris, A.; Yanıkoğlu, F.; Abacı, N.; Ekmekçi, S.S.; Ilgın, C.; Çelik, H.; Tağtekin, D. Metagenomic Analysis of Black-Stained Plaques in Permanent Dentition. Arch. Oral Biol. 2021, 128, 105171. [Google Scholar] [CrossRef]
- Gómez-Govea, M.A.; Ramírez-Ahuja, M.d.L.; Contreras-Perera, Y.; Jiménez-Camacho, A.J.; Ruiz-Ayma, G.; Villanueva-Segura, O.K.; Trujillo-Rodríguez, G.d.J.; Delgado-Enciso, I.; Martínez-Fierro, M.L.; Manrique-Saide, P.; et al. Suppression of Midgut Microbiota Impact Pyrethroid Susceptibility in Aedes aegypti. Front. Microbiol. 2022, 13, 2025. [Google Scholar] [CrossRef]
- Ravi, R.K.; Walton, K.; Khosroheidari, M. MiSeq: A Next Generation Sequencing Platform for Genomic Analysis. Methods Mol. Biol. 2018, 1706, 223–232. [Google Scholar] [CrossRef]
- Del Fabbro, C.; Scalabrin, S.; Morgante, M.; Giorgi, F.M. An Extensive Evaluation of Read Trimming Effects on Illumina NGS Data Analysis. PLoS ONE 2013, 8, e85024. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Neu, A.T.; Allen, E.E.; Roy, K. Defining and Quantifying the Core Microbiome: Challenges and Prospects. Proc. Natl. Acad. Sci. USA 2021, 118, e2104429118. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Abellan-Schneyder, I.; Matchado, M.S.; Reitmeier, S.; Sommer, A.; Sewald, Z.; Baumbach, J.; List, M.; Neuhaus, K. Primer, Pipelines, Parameters: Issues in 16S rRNA Gene Sequencing. mSphere 2021, 6, e01202-20. [Google Scholar] [CrossRef] [PubMed]
- Nathani, N.M.; Patel, A.K.; Dhamannapatil, P.S.; Kothari, R.K.; Singh, K.M.; Joshi, C.G. Comparative Evaluation of Rumen Metagenome Community Using qPCR and MG-RAST. AMB Express 2013, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Větrovský, T.; Baldrian, P. The Variability of the 16S rRNA Gene in Bacterial Genomes and Its Consequences for Bacterial Community Analyses. PLoS ONE 2013, 8, e57923. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Bardenhorst, S.; Vital, M.; Karch, A.; Rübsamen, N. Richness Estimation in Microbiome Data Obtained from Denoising Pipelines. Comput. Struct. Biotechnol. J. 2022, 20, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, L.; Gotelli, N.J. Diversity-Disease Relationships and Shared Species Analyses for Human Microbiome-Associated Diseases. ISME J. 2019, 13, 1911–1919. [Google Scholar] [CrossRef]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and Using Diversity Indices: Insights for Ecological Applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef]
- Jaccard, P. The Distribution of the Flora in the Alpine Zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-Induced Assemblage of a Plant-Beneficial Bacterial Consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Liu, F.; Hewezi, T.; Lebeis, S.L.; Pantalone, V.; Grewal, P.S.; Staton, M.E. Soil Indigenous Microbiome and Plant Genotypes Cooperatively Modify Soybean Rhizosphere Microbiome Assembly. BMC Microbiol. 2019, 19, 201. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, J.; Xiao, Z.; Zhu, X.; Wang, J.; Wu, H.; Wu, Y.; Zhang, Z.; Lin, W. Barcoded Pyrosequencing Reveals a Shift in the Bacterial Community in the Rhizosphere and Rhizoplane of Rehmannia Glutinosa under Consecutive Monoculture. Int. J. Mol. Sci. 2018, 19, 850. [Google Scholar] [CrossRef] [PubMed]
- Resendiz-Nava, C.N.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Rivera-Pastrana, D.M.; Mercado-Silva, E.M.; Nava, G.M. A Comprehensive Evaluation of Enterobacteriaceae Primer Sets for Analysis of Host-Associated Microbiota. Pathogens 2022, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Kurina, I.; Popenko, A.; Klimenko, N.; Koshechkin, S.; Chuprikova, L.; Filipenko, M.; Tyakht, A.; Alexeev, D. Development of qPCR Platform with Probes for Quantifying Prevalent and Biomedically Relevant Human Gut Microbial Taxa. Mol. Cell. Probes 2020, 52, 101570. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere Signaling: Insights into Plant–Rhizomicrobiome Interactions for Sustainable Agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef]
- Timm, C.M.; Carter, K.R.; Carrell, A.A.; Jun, S.-R.; Jawdy, S.S.; Vélez, J.M.; Gunter, L.E.; Yang, Z.; Nookaew, I.; Engle, N.L.; et al. Abiotic Stresses Shift Belowground Populus-Associated Bacteria Toward a Core Stress Microbiome. mSystems 2018, 3, e00070-17. [Google Scholar] [CrossRef]
- Qi, M.; Berry, J.C.; Veley, K.W.; O’Connor, L.; Finkel, O.M.; Salas-González, I.; Kuhs, M.; Jupe, J.; Holcomb, E.; Glavina del Rio, T.; et al. Identification of Beneficial and Detrimental Bacteria Impacting Sorghum Responses to Drought Using Multi-Scale and Multi-System Microbiome Comparisons. ISME J. 2022, 16, 1957–1969. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A Review on the Plant Microbiome: Ecology, Functions, and Emerging Trends in Microbial Application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Singh, B.K.; Trivedi, P. Microbiome and the Future for Food and Nutrient Security. Microb. Biotechnol. 2017, 10, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.; Mosca, A.; Dimaria, G.; Nicotra, D.; Tessitori, M.; Privitera, G.F.; Pulvirenti, A.; Leonardi, C.; Catara, V. Soil and Soilless Tomato Cultivation Promote Different Microbial Communities That Provide New Models for Future Crop Interventions. Int. J. Mol. Sci. 2022, 23, 8820. [Google Scholar] [CrossRef] [PubMed]
- Genitsaris, S.; Stefanidou, N.; Leontidou, K.; Matsi, T.; Karamanoli, K.; Mellidou, I. Bacterial Communities in the Rhizosphere and Phyllosphere of Halophytes and Drought-Tolerant Plants in Mediterranean Ecosystems. Microorganisms 2020, 8, 1708. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Du, Y.; Zhu, W.; Pang, X.; Wang, Z. Effects of Organic Materials on Soil Bacterial Community Structure in Long-Term Continuous Cropping of Tomato in Greenhouse. Open Life Sci. 2022, 17, 381–392. [Google Scholar] [CrossRef]
- Escobar-Rodríguez, C.; Novak, J.; Buchholz, F.; Uetz, P.; Bragagna, L.; Gumze, M.; Antonielli, L.; Mitter, B. The Bacterial Microbiome of the Tomato Fruit Is Highly Dependent on the Cultivation Approach and Correlates with Flavor Chemistry. Front. Plant Sci. 2021, 12, 775722. [Google Scholar] [CrossRef]
- Gorrasi, S.; Pasqualetti, M.; Muñoz-Palazon, B.; Novello, G.; Mazzucato, A.; Campiglia, E.; Fenice, M. Comparison of the Peel-Associated Epiphytic Bacteria of Anthocyanin-Rich “Sun Black” and Wild-Type Tomatoes under Organic and Conventional Farming. Microorganisms 2022, 10, 2240. [Google Scholar] [CrossRef]
- Allard, S.M.; Ottesen, A.R.; Brown, E.W.; Micallef, S.A. Insect Exclusion Limits Variation in Bacterial Microbiomes of Tomato Flowers and Fruit. J. Appl. Microbiol. 2018, 125, 1749–1760. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.S.; Zhan, Y.; Zhang, Z.; Liu, Y.; Wei, Y.; Xu, T.; Li, J. Tomato Microbiome under Long-Term Organic and Conventional Farming. iMeta 2022, 1, e48. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhang, Z.; Liu, Y.; Wei, Y. Tomato Endophytic Bacteria Composition and Mechanism of Suppressiveness of Wilt Disease (Fusarium oxysporum). Front. Microbiol. 2021, 12, 731764. [Google Scholar] [CrossRef]
- Ikeda-Ohtsubo, W.; Brugman, S.; Warden, C.H.; Rebel, J.M.J.; Folkerts, G.; Pieterse, C.M.J. How Can We Define “Optimal Microbiota?”: A Comparative Review of Structure and Functions of Microbiota of Animals, Fish, and Plants in Agriculture. Front. Nutr. 2018, 5, 90. [Google Scholar] [CrossRef]
- Bruto, M.; Prigent-Combaret, C.; Muller, D.; Moënne-Loccoz, Y. Analysis of Genes Contributing to Plant-Beneficial Functions in Plant Growth-Promoting Rhizobacteria and Related Proteobacteria. Sci. Rep. 2014, 4, 6261. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Zarandi, M.; Saberi Riseh, R.; Tarkka, M.T. Actinobacteria as Effective Biocontrol Agents against Plant Pathogens, an Overview on Their Role in Eliciting Plant Defense. Microorganisms 2022, 10, 1739. [Google Scholar] [CrossRef] [PubMed]
- Narsing Rao, M.P.; Lohmaneeratana, K.; Bunyoo, C.; Thamchaipenet, A. Actinobacteria–Plant Interactions in Alleviating Abiotic Stress. Plants 2022, 11, 2976. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elyousr, K.A.M.; Khalil Bagy, H.M.M.; Hashem, M.; Alamri, S.A.M.; Mostafa, Y.S. Biological Control of the Tomato Wilt Caused by Clavibacter michiganensis subsp. Michiganensis Using Formulated Plant Growth-Promoting Bacteria. Egypt. J. Biol. Pest Control 2019, 29, 54. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus Subtilis: A Plant-Growth Promoting Rhizobacterium That Also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- López, S.M.Y.; Pastorino, G.N.; Fernández-González, A.J.; Franco, M.E.E.; Fernández-López, M.; Balatti, P.A. The Endosphere Bacteriome of Diseased and Healthy Tomato Plants. Arch. Microbiol. 2020, 202, 2629–2642. [Google Scholar] [CrossRef]
- Samayoa, B.E.; Shen, F.-T.; Lai, W.-A.; Chen, W.-C. Screening and Assessment of Potential Plant Growth-Promoting Bacteria Associated with Allium cepa Linn. Microbes Environ. 2020, 35, ME19147. [Google Scholar] [CrossRef]
- Pearce, S.L.; Oakeshott, J.G.; Pandey, G. Insights into Ongoing Evolution of the Hexachlorocyclohexane Catabolic Pathway from Comparative Genomics of Ten Sphingomonadaceae Strains. G3 2015, 5, 1081–1094. [Google Scholar] [CrossRef]
- Zhao, Q.; Yue, S.; Bilal, M.; Hu, H.; Wang, W.; Zhang, X. Comparative Genomic Analysis of 26 Sphingomonas and Sphingobium Strains: Dissemination of Bioremediation Capabilities, Biodegradation Potential and Horizontal Gene Transfer. Sci. Total Environ. 2017, 609, 1238–1247. [Google Scholar] [CrossRef]
- Wang, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Natarajan, D.; Ma, Y. Plant Growth-Promoting Bacteria in Metal-Contaminated Soil: Current Perspectives on Remediation Mechanisms. Front. Microbiol. 2022, 13, 966226. [Google Scholar] [CrossRef]
- Kang, S.-M.; Imran, M.; Shaffique, S.; Kwon, E.-H.; Park, Y.-S.; Lee, I.-J. Growth and Photosynthetic Characteristics of Sesame Seedlings with Gibberellin-Producing Rhodobacter sphaeroides SIR03 and Biochar. Int. J. Plant Biol. 2022, 13, 257–269. [Google Scholar] [CrossRef]
- Azaroual, S.E.; Kasmi, Y.; Aasfar, A.; El Arroussi, H.; Zeroual, Y.; El Kadiri, Y.; Zrhidri, A.; Elfahime, E.; Sefiani, A.; Meftah Kadmiri, I. Investigation of Bacterial Diversity Using 16S rRNA Sequencing and Prediction of Its Functionalities in Moroccan Phosphate Mine Ecosystem. Sci. Rep. 2022, 12, 3741. [Google Scholar] [CrossRef]
- Antoniou, A.; Tsolakidou, M.-D.; Stringlis, I.A.; Pantelides, I.S. Rhizosphere Microbiome Recruited from a Suppressive Compost Improves Plant Fitness and Increases Protection against Vascular Wilt Pathogens of Tomato. Front. Plant Sci. 2017, 8, 2022. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.-T.; Wang, W.-H.; Tsui, C.K.; Cai, L. Changes in Bacterial and Fungal Microbiomes Associated with Tomatoes of Healthy and Infected by Fusarium oxysporum f. sp. Lycopersici. Microb. Ecol. 2021, 81, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.; Satpute, A.; Albrecht, U.; Strauss, S.L. Impact of Soil Microbial Amendments on Tomato Rhizosphere Microbiome and Plant Growth in Field Soil. Microb. Ecol. 2020, 80, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Choi, J.; Lee, P.A.; Roy, N.; Khan, R.; Lee, H.J.; Weon, H.Y.; Kong, H.G.; Lee, S.-W. Alteration of Bacterial Wilt Resistance in Tomato Plant by Microbiota Transplant. Front. Plant Sci. 2020, 11, 1186. [Google Scholar] [CrossRef]
- Renaut, S.; Masse, J.; Norrie, J.P.; Blal, B.; Hijri, M. A Commercial Seaweed Extract Structured Microbial Communities Associated with Tomato and Pepper Roots and Significantly Increased Crop Yield. Microb. Biotechnol. 2019, 12, 1346–1358. [Google Scholar] [CrossRef]
- Nakayasu, M.; Ohno, K.; Takamatsu, K.; Aoki, Y.; Yamazaki, S.; Takase, H.; Shoji, T.; Yazaki, K.; Sugiyama, A. Tomato Roots Secrete Tomatine to Modulate the Bacterial Assemblage of the Rhizosphere. Plant Physiol. 2021, 186, 270–284. [Google Scholar] [CrossRef]
- Santoyo, G. How Plants Recruit Their Microbiome? New Insights into Beneficial Interactions. J. Adv. Res. 2021, 40, 45–58. [Google Scholar] [CrossRef]
- Bao, L.; Cai, W.; Cao, J.; Zhang, X.; Liu, J.; Chen, H.; Wei, Y.; Zhuang, X.; Zhuang, G.; Bai, Z. Microbial Community Overlap between the Phyllosphere and Rhizosphere of Three Plants from Yongxing Island, South China Sea. Microbiologyopen 2020, 9, e1048. [Google Scholar] [CrossRef]
- Dong, C.-J.; Wang, L.-L.; Li, Q.; Shang, Q.-M. Bacterial Communities in the Rhizosphere, Phyllosphere and Endosphere of Tomato Plants. PLoS ONE 2019, 14, e0223847. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial Life in the Phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The Importance of the Microbiome of the Plant Holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the Yields of Organic and Conventional Agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Paul Chowdhury, S.; Babin, D.; Sandmann, M.; Jacquiod, S.; Sommermann, L.; Sørensen, S.J.; Fliessbach, A.; Mäder, P.; Geistlinger, J.; Smalla, K.; et al. Effect of Long-term Organic and Mineral Fertilization Strategies on Rhizosphere Microbiota Assemblage and Performance of Lettuce. Environ. Microbiol. 2019, 21, 2426–2439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, W.; Zhou, Z.; Huang, G.; Wang, X.; Han, Q.; Liu, G. The Application of Mixed Organic and Inorganic Fertilizers Drives Soil Nutrient and Bacterial Community Changes in Teak Plantations. Microorganisms 2022, 10, 958. [Google Scholar] [CrossRef]
- Grunert, O.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Perneel, M.; Van Labeke, M.-C.; Reheul, D.; Boon, N. Mineral and Organic Growing Media Have Distinct Community Structure, Stability and Functionality in Soilless Culture Systems. Sci. Rep. 2016, 6, 18837. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Vannette, R.L.; Igwe, A.; Blundell, R.; Casteel, C.L.; Gaudin, A.C.M. Effects of Agricultural Management on Rhizosphere Microbial Structure and Function in Processing Tomato Plants. Appl. Environ. Microbiol. 2019, 85, e01064-19. [Google Scholar] [CrossRef]
- Huang, F.; Mo, C.; Li, L.; Shi, J.; Yang, Y.; Liao, X. Organic Fertilizer Application Mediates Tomato Defense Against Pseudomonas syringae Pv. Tomato, Possibly by Reshaping the Soil Microbiome. Front. Microbiol. 2022, 13, 939911. [Google Scholar] [CrossRef]
- Kraut-Cohen, J.; Shapiro, O.H.; Dror, B.; Cytryn, E. Pectin Induced Colony Expansion of Soil-Derived Flavobacterium Strains. Front. Microbiol. 2021, 12, 651891. [Google Scholar] [CrossRef]
- Lidbury, I.D.E.A.; Scanlan, D.J.; Murphy, A.R.J.; Christie-Oleza, J.A.; Aguilo-Ferretjans, M.M.; Hitchcock, A.; Daniell, T.J. A Widely Distributed Phosphate-Insensitive Phosphatase Presents a Route for Rapid Organophosphorus Remineralization in the Biosphere. Proc. Natl. Acad. Sci. USA 2022, 119, e2118122119. [Google Scholar] [CrossRef]
- Rilling, J.I.; Acuña, J.J.; Sadowsky, M.J.; Jorquera, M.A. Putative Nitrogen-Fixing Bacteria Associated with the Rhizosphere and Root Endosphere of Wheat Plants Grown in an Andisol from Southern Chile. Front. Microbiol. 2018, 9, 2710. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Gonçalves, L.S.A.; de Oliveira, A.L.M. Diversity and Plant Growth-Promoting Functions of Diazotrophic/N-Scavenging Bacteria Isolated from the Soils and Rhizospheres of Two Species of Solanum. PLoS ONE 2020, 15, e0227422. [Google Scholar] [CrossRef] [PubMed]
- Nonthakaew, N.; Panbangred, W.; Songnuan, W.; Intra, B. Plant Growth-Promoting Properties of Streptomyces spp. Isolates and Their Impact on Mung Bean Plantlets’ Rhizosphere Microbiome. Front. Microbiol. 2022, 13, 967415. [Google Scholar] [CrossRef]
- Tang, T.; Sun, X.; Dong, Y.; Liu, Q. Erythrobacter aureus sp. Nov., a Plant Growth-Promoting Bacterium Isolated from Sediment in the Yellow Sea, China. 3 Biotech 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Favero, V.O.; Carvalho, R.H.; Motta, V.M.; Leite, A.B.C.; Coelho, M.R.R.; Xavier, G.R.; Rumjanek, N.G.; Urquiaga, S. Bradyrhizobium as the Only Rhizobial Inhabitant of Mung Bean (Vigna radiata) Nodules in Tropical Soils: A Strategy Based on Microbiome for Improving Biological Nitrogen Fixation Using Bio-Products. Front. Plant Sci. 2021, 11, 602645. [Google Scholar] [CrossRef]
- Passari, A.K.; Upadhyaya, K.; Singh, G.; Abdel-Azeem, A.M.; Thankappan, S.; Uthandi, S.; Hashem, A.; Abd_Allah, E.F.; Malik, J.A.; A.S.A.; et al. Enhancement of Disease Resistance, Growth Potential, and Photosynthesis in Tomato (Solanum lycopersicum) by Inoculation with an Endophytic Actinobacterium, Streptomyces thermocarboxydus Strain BPSAC147. PLoS ONE 2019, 14, e0219014. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Goyal, R.K.; Schmidt, M.A.; Hynes, M.F. Molecular Biology in the Improvement of Biological Nitrogen Fixation by Rhizobia and Extending the Scope to Cereals. Microorganisms 2021, 9, 125. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic Nitrogen Fixation and the Challenges to Its Extension to Nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef]
- de Ponti, T.; Rijk, B.; van Ittersum, M.K. The Crop Yield Gap between Organic and Conventional Agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Ottesen, A.R.; Gorham, S.; Pettengill, J.B.; Rideout, S.; Evans, P.; Brown, E. The Impact of Systemic and Copper Pesticide Applications on the Phyllosphere Microflora of Tomatoes. J. Sci. Food Agric. 2015, 95, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Telias, A.; White, J.R.; Pahl, D.M.; Ottesen, A.R.; Walsh, C.S. Bacterial Community Diversity and Variation in Spray Water Sources and the Tomato Fruit Surface. BMC Microbiol. 2011, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, A.; Ramachandran, P.; Reed, E.; Gu, G.; Gorham, S.; Ducharme, D.; Newell, M.; Rideout, S.; Turini, T.; Hill, T.; et al. Metagenome Tracking Biogeographic Agroecology: Phytobiota of Tomatoes from Virginia, Maryland, North Carolina and California. Food Microbiol. 2019, 79, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. The Changing Face of the Family Enterobacteriaceae (Order: “Enterobacterales”): New Members, Taxonomic Issues, Geographic Expansion, and New Diseases and Disease Syndromes. Clin. Microbiol. Rev. 2021, 34, e00174-20. [Google Scholar] [CrossRef]
- Kang, E.; Crouse, A.; Chevallier, L.; Pontier, S.M.; Alzahrani, A.; Silué, N.; Campbell-Valois, F.-X.; Montagutelli, X.; Gruenheid, S.; Malo, D. Enterobacteria and Host Resistance to Infection. Mamm. Genome 2018, 29, 558–576. [Google Scholar] [CrossRef]
- Balali, G.I.; Yar, D.D.; Afua Dela, V.G.; Adjei-Kusi, P. Microbial Contamination, an Increasing Threat to the Consumption of Fresh Fruits and Vegetables in Today’s World. Int. J. Microbiol. 2020, 2020, 3029295. [Google Scholar] [CrossRef]
- Abbott, D.W.; Boraston, A.B. Structural Biology of Pectin Degradation by Enterobacteriaceae. Microbiol. Mol. Biol. Rev. 2008, 72, 301–316. [Google Scholar] [CrossRef]
- Palacio-Bielsa, A.; Roselló, M.; Llop, P.; López, M.M. Erwinia spp. from Pome Fruit Trees: Similarities and Differences among Pathogenic and Non-Pathogenic Species. Trees 2012, 26, 13–29. [Google Scholar] [CrossRef]
- Charkowski, A.; Blanco, C.; Condemine, G.; Expert, D.; Franza, T.; Hayes, C.; Hugouvieux-Cotte-Pattat, N.; Solanilla, E.L.; Low, D.; Moleleki, L.; et al. The Role of Secretion Systems and Small Molecules in Soft-Rot Enterobacteriaceae Pathogenicity. Annu. Rev. Phytopathol. 2012, 50, 425–449. [Google Scholar] [CrossRef]
- Rasch, M.; Andersen, J.B.; Nielsen, K.F.; Flodgaard, L.R.; Christensen, H.; Givskov, M.; Gram, L. Involvement of Bacterial Quorum-Sensing Signals in Spoilage of Bean Sprouts. Appl. Environ. Microbiol. 2005, 71, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharousi, Z.S.; Guizani, N.; Al-Sadi, A.M.; Al-Bulushi, I.M.; Shaharoona, B. Hiding in Fresh Fruits and Vegetables: Opportunistic Pathogens May Cross Geographical Barriers. Int. J. Microbiol. 2016, 2016, 4292417. [Google Scholar] [CrossRef] [PubMed]
- Boehme, S.; Werner, G.; Klare, I.; Reissbrodt, R.; Witte, W. Occurrence of Antibiotic-Resistant Enterobacteria in Agricultural Foodstuffs. Mol. Nutr. Food Res. 2004, 48, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Saksena, R.; Malik, M.; Gaind, R. Bacterial Contamination and Prevalence of Antimicrobial Resistance Phenotypes in Raw Fruits and Vegetables Sold in Delhi, India. J. Food Saf. 2020, 40, e12739. [Google Scholar] [CrossRef]
- Waturangi, D.E.; Hudiono, F.; Aliwarga, E. Prevalence of Pathogenic Escherichia Coli from Salad Vegetable and Fruits Sold in Jakarta. BMC Res. Notes 2019, 12, 247. [Google Scholar] [CrossRef]
- Feng, P.C.H.; Reddy, S. Prevalences of Shiga Toxin Subtypes and Selected Other Virulence Factors among Shiga-Toxigenic Escherichia Coli Strains Isolated from Fresh Produce. Appl. Environ. Microbiol. 2013, 79, 6917–6923. [Google Scholar] [CrossRef]
- Bell, R.L.; Zheng, J.; Burrows, E.; Allard, S.; Wang, C.Y.; Keys, C.E.; Melka, D.C.; Strain, E.; Luo, Y.; Allard, M.W.; et al. Ecological Prevalence, Genetic Diversity, and Epidemiological Aspects of Salmonella Isolated from Tomato Agricultural Regions of the Virginia Eastern Shore. Front. Microbiol. 2015, 6, 415. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Harlee, N.A.; Smelser, A.M.; Schneider, K.R. Salmonella Enterica Contamination of Market Fresh Tomatoes: A Review. J. Food Prot. 2018, 81, 1193–1213. [Google Scholar] [CrossRef]
- Santos, R.F.C.; Nascimento, J.D.S.; Geimba, M.P.; Hessel, C.T.; Tondo, E.C. First Report of Human Gastroenteritis Caused by Escherichia Coli O157:NM in Brazil. Foodborne Pathog. Dis. 2017, 14, 665–666. [Google Scholar] [CrossRef]
- Cummings, K.; Barrett, E.; Mohle-Boetani, J.C.; Brooks, J.T.; Farrar, J.; Hunt, T.; Fiore, A.; Komatsu, K.; Werner, S.B.; Slutsker, L. A Multistate Outbreak of Salmonella enterica Serotype Baildon Associated with Domestic Raw Tomatoes. Emerg. Infect. Dis. 2001, 7, 1046–1048. [Google Scholar] [CrossRef]
- Gupta, S.K.; Nalluswami, K.; Snider, C.; Perch, M.; Balasegaram, M.; Burmeister, D.; Lockett, J.; Sandt, C.; Hoekstra, R.M.; Montgomery, S. Outbreak of Salmonella Braenderup Infections Associated with Roma Tomatoes, Northeastern United States, 2004: A Useful Method for Subtyping Exposures in Field Investigations. Epidemiol. Infect. 2007, 135, 1165–1173. [Google Scholar] [CrossRef]
- Hedberg, C.W.; Angulo, F.J.; White, K.E.; Langkop, C.W.; Schell, W.L.; Stobierski, M.G.; Schuchat, A.; Besser, J.M.; Dietrich, S.; Helsel, L.; et al. Outbreaks of Salmonellosis Associated with Eating Uncooked Tomatoes: Implications for Public Health. The Investigation Team. Epidemiol. Infect. 1999, 122, 385–393. [Google Scholar] [CrossRef]
- Behravesh, C.B.; Blaney, D.; Medus, C.; Bidol, S.A.; Phan, Q.; Soliva, S.; Daly, E.R.; Smith, K.; Miller, B.; Taylor, T.; et al. Multistate Outbreak of Salmonella serotype Typhimurium Infections Associated with Consumption of Restaurant Tomatoes, USA, 2006: Hypothesis Generation through Case Exposures in Multiple Restaurant Clusters. Epidemiol. Infect. 2012, 140, 2053–2061. [Google Scholar] [CrossRef]
- Heaton, J.C.; Jones, K. Microbial Contamination of Fruit and Vegetables and the Behaviour of Enteropathogens in the Phyllosphere: A Review. J. Appl. Microbiol. 2008, 104, 613–626. [Google Scholar] [CrossRef]
- Müller, L.; Kjelsø, C.; Frank, C.; Jensen, T.; Torpdahl, M.; Søborg, B.; Dorleans, F.; Rabsch, W.; Prager, R.; Gossner, C.M.; et al. Outbreak of Salmonella Strathcona Caused by Datterino Tomatoes, Denmark, 2011. Epidemiol. Infect. 2016, 144, 2802–2811. [Google Scholar] [CrossRef]
- Reller, M.E.; Nelson, J.M.; Mølbak, K.; Ackman, D.M.; Schoonmaker-Bopp, D.J.; Root, T.P.; Mintz, E.D. A Large, Multiple-Restaurant Outbreak of Infection with Shigella flexneri Serotype 2a Traced to Tomatoes. Clin. Infect. Dis. 2006, 42, 163–169. [Google Scholar] [CrossRef]
- Holden, N.; Pritchard, L.; Toth, I. Colonization Outwith the Colon: Plants as an Alternative Environmental Reservoir for Human Pathogenic Enterobacteria. FEMS Microbiol. Rev. 2009, 33, 689–703. [Google Scholar] [CrossRef]
- Gu, G.; Hu, J.; Cevallos-Cevallos, J.M.; Richardson, S.M.; Bartz, J.A.; van Bruggen, A.H.C. Internal Colonization of Salmonella enterica Serovar Typhimurium in Tomato Plants. PLoS ONE 2011, 6, e27340. [Google Scholar] [CrossRef]
- de Moraes, M.H.; Desai, P.; Porwollik, S.; Canals, R.; Perez, D.R.; Chu, W.; McClelland, M.; Teplitski, M. Salmonella Persistence in Tomatoes Requires a Distinct Set of Metabolic Functions Identified by Transposon Insertion Sequencing. Appl. Environ. Microbiol. 2017, 83, e03028-16. [Google Scholar] [CrossRef]
- Bonanomi, G.; Alioto, D.; Minutolo, M.; Marra, R.; Cesarano, G.; Vinale, F. Organic Amendments Modulate Soil Microbiota and Reduce Virus Disease Incidence in the TSWV-Tomato Pathosystem. Pathogens 2020, 9, 379. [Google Scholar] [CrossRef]
- Caradonia, F.; Ronga, D.; Catellani, M.; Giaretta Azevedo, C.V.; Terrazas, R.A.; Robertson-Albertyn, S.; Francia, E.; Bulgarelli, D. Nitrogen Fertilizers Shape the Composition and Predicted Functions of the Microbiota of Field-Grown Tomato Plants. Phytobiomes J. 2019, 3, 315–325. [Google Scholar] [CrossRef]
- Jiang, S.-Q.; Yu, Y.-N.; Gao, R.-W.; Wang, H.; Zhang, J.; Li, R.; Long, X.-H.; Shen, Q.-R.; Chen, W.; Cai, F. High-Throughput Absolute Quantification Sequencing Reveals the Effect of Different Fertilizer Applications on Bacterial Community in a Tomato Cultivated Coastal Saline Soil. Sci. Total Environ. 2019, 687, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiong, W.; Zhang, R.; Hang, X.; Wang, D.; Li, R.; Shen, Q. Continuous Application of Different Organic Additives Can Suppress Tomato Disease by Inducing the Healthy Rhizospheric Microbiota through Alterations to the Bulk Soil Microflora. Plant Soil 2018, 423, 229–240. [Google Scholar] [CrossRef]
| Production Week 1 | |||||
|---|---|---|---|---|---|
| 8 | 26 | 44 | |||
| Soilless Culture System | Niche | Composite Samples 2 | Total of Samples | ||
| Conventional | Substrates | 4 | 4 | 4 | n = 12 |
| Rhizosphere | 4 | 4 | 4 | n = 12 | |
| Fruits | 4 | 4 | 4 | n = 12 | |
| Organic | Substrates | 4 | 4 | 4 | n = 12 |
| Rhizosphere | 4 | 4 | 4 | n = 12 | |
| Fruits | 4 | 4 | 4 | n = 12 | |
| Total 3 | n = 72 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resendiz-Nava, C.N.; Alonso-Onofre, F.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Rivera-Pastrana, D.M.; Stasiewicz, M.J.; Nava, G.M.; Mercado-Silva, E.M. Tomato Plant Microbiota under Conventional and Organic Fertilization Regimes in a Soilless Culture System. Microorganisms 2023, 11, 1633. https://doi.org/10.3390/microorganisms11071633
Resendiz-Nava CN, Alonso-Onofre F, Silva-Rojas HV, Rebollar-Alviter A, Rivera-Pastrana DM, Stasiewicz MJ, Nava GM, Mercado-Silva EM. Tomato Plant Microbiota under Conventional and Organic Fertilization Regimes in a Soilless Culture System. Microorganisms. 2023; 11(7):1633. https://doi.org/10.3390/microorganisms11071633
Chicago/Turabian StyleResendiz-Nava, Carolina N., Fernando Alonso-Onofre, Hilda V. Silva-Rojas, Angel Rebollar-Alviter, Dulce M. Rivera-Pastrana, Matthew J. Stasiewicz, Gerardo M. Nava, and Edmundo M. Mercado-Silva. 2023. "Tomato Plant Microbiota under Conventional and Organic Fertilization Regimes in a Soilless Culture System" Microorganisms 11, no. 7: 1633. https://doi.org/10.3390/microorganisms11071633
APA StyleResendiz-Nava, C. N., Alonso-Onofre, F., Silva-Rojas, H. V., Rebollar-Alviter, A., Rivera-Pastrana, D. M., Stasiewicz, M. J., Nava, G. M., & Mercado-Silva, E. M. (2023). Tomato Plant Microbiota under Conventional and Organic Fertilization Regimes in a Soilless Culture System. Microorganisms, 11(7), 1633. https://doi.org/10.3390/microorganisms11071633







