Is There Any Difference in the In Situ Immune Response in Active Localized Cutaneous Leishmaniasis That Respond Well or Poorly to Meglumine Antimoniate Treatment or Spontaneously Heal?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry
2.3. Immunofluorescence
2.4. Statistical Analysis
3. Results
3.1. PRL Patients Had More Severe Skin Lesions and Showed Greater Positivity in the Parasite Detection Tests
3.2. Different In Situ Cellular Profiles Are Correlated with Patient Characteristics
3.3. Distribution of CD4+ and CD8+ T Lymphocytes and CD22+ B Lymphocytes
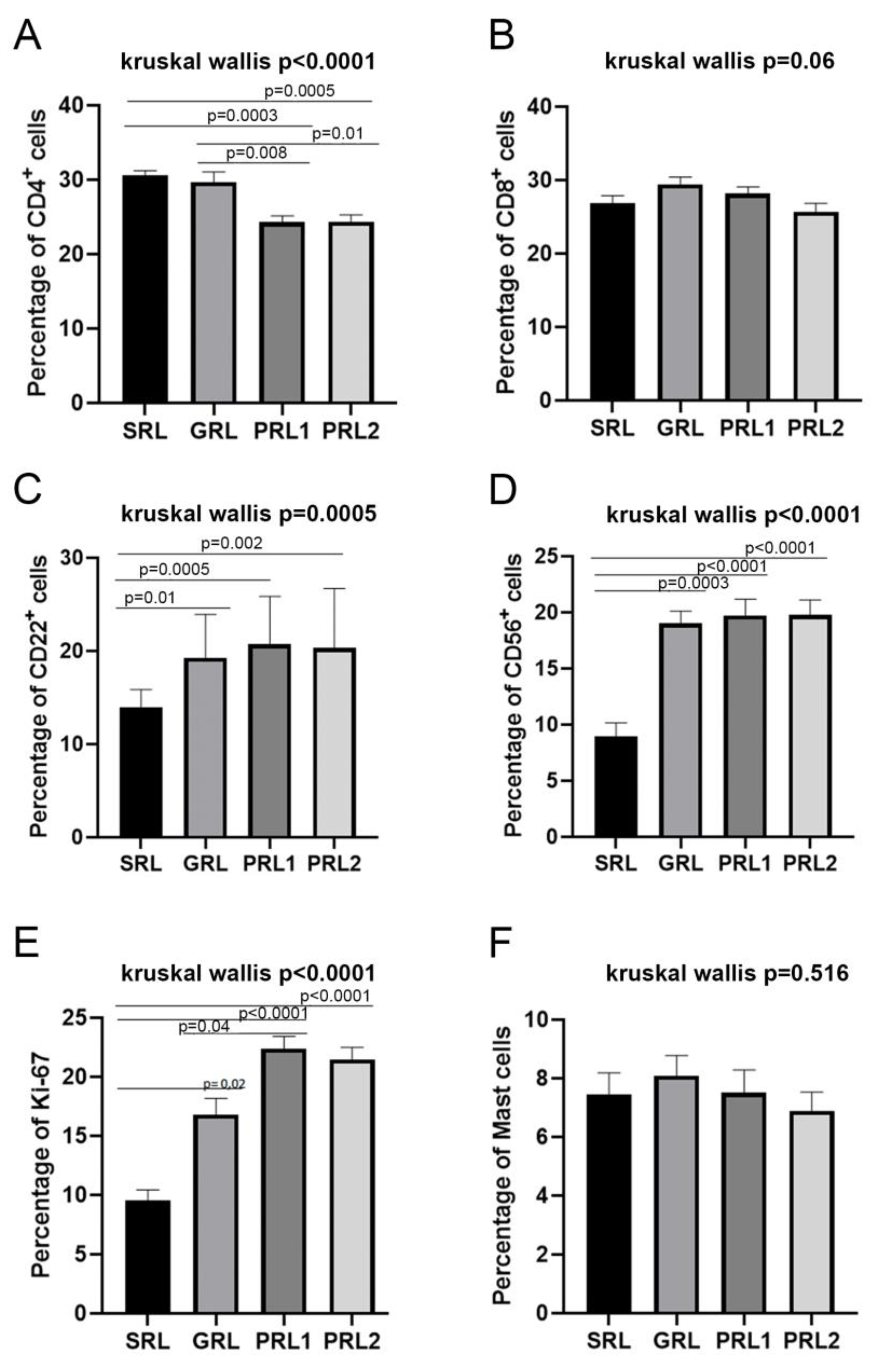
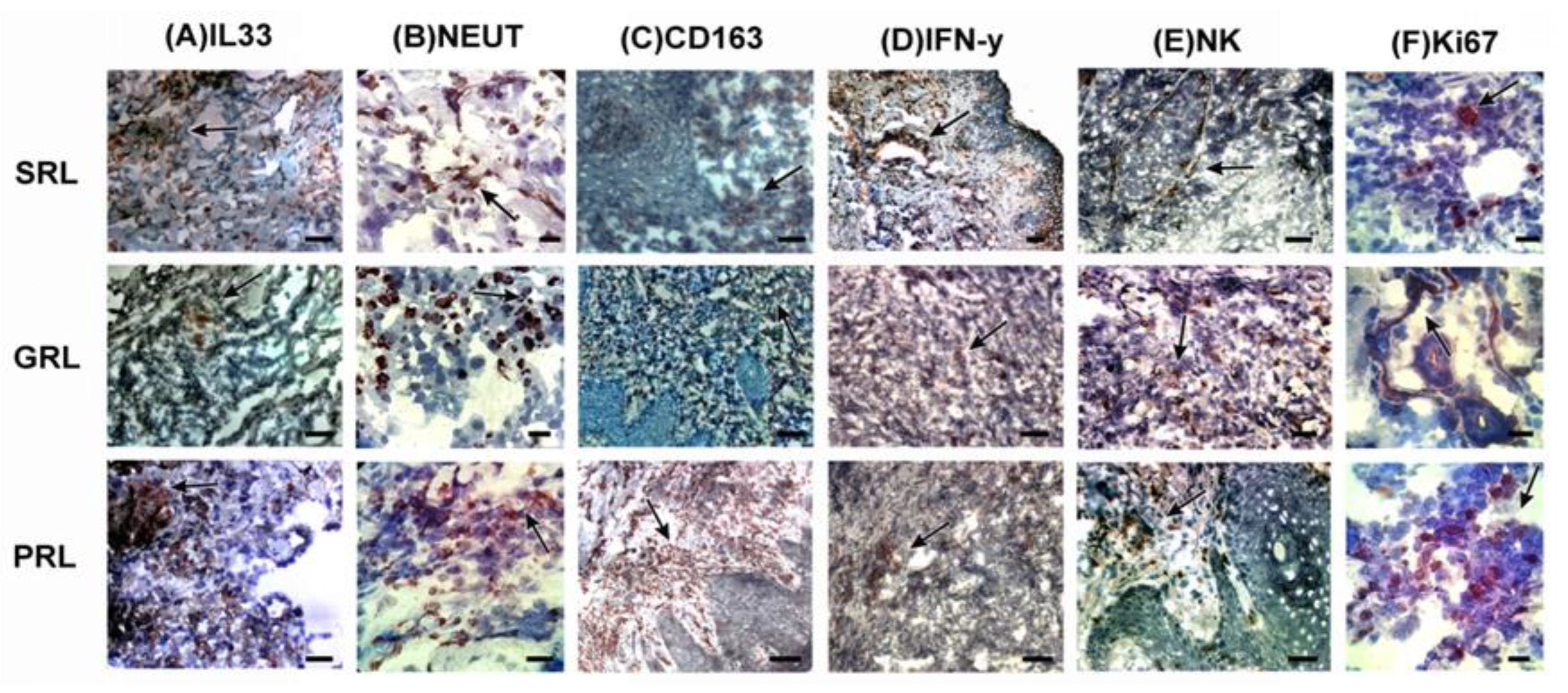
3.4. M2 Macrophages Were Associated with Poor Treatment Response While IFN-γ and NOS2 Expression Were Related to a Better Response and Spontaneous Healing

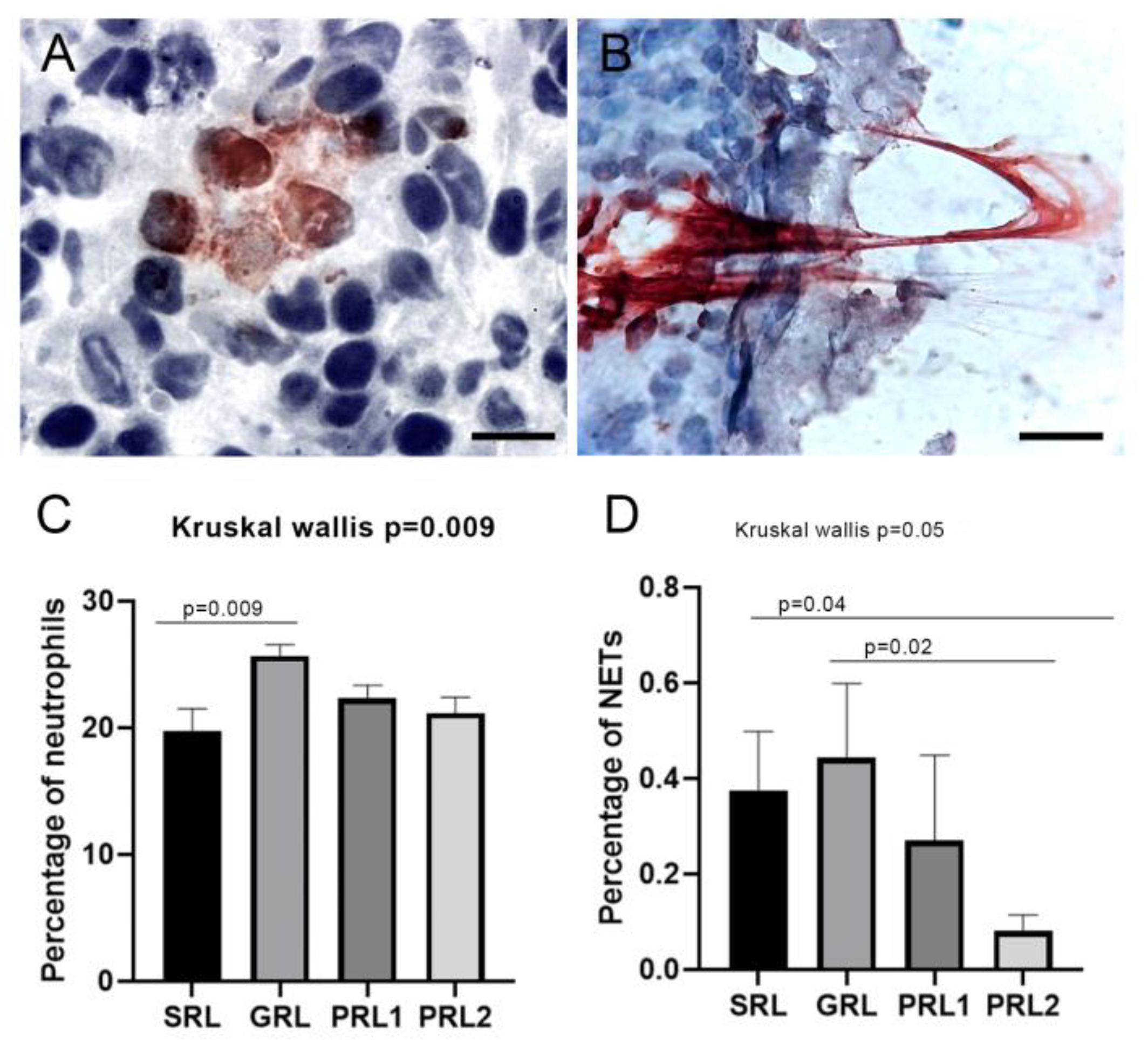
3.5. Neutrophil Extracellular Traps (NETs) Were Associated with Spontaneous Healing and Good Treatment Response
3.6. Evaluation of the Distribution of Mast Cells, CD56+ Natural Killer (NK) Cells, and Cellular Proliferation Marker (Ki-67)
3.7. Distribution of FoxP3, CD25, IL-33, and ST2L in LCL Lesions

3.8. Leishmania spp. were Detected in Lesions from all Groups with a Tendency to Be More Concentrated in the Lesions from PRL Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PAHO. Organización Panamericana de la Salud Manual de Procedimientos Para Vigilancia y Control de Las Leishmaniasis En Las Américas; PAHO: Washington, DC, USA, 2019; ISBN 978-92-75-32063-1. [Google Scholar]
- Mattos, M.d.S. Aspectos clínicos, laboratoriais e epidemiológicos da leishmaniose tegumentar americana—Casuística do Hospital Evandro Chagas/FIOC-RUZ/RJ no período de janeiro de 1987 a dezembro de 1991. Rev. Soc. Bras. Med. Trop. 1993, 26, 261–262. [Google Scholar] [CrossRef]
- Marzochi, M.C.d.A.; Marzochi, K.B.F. Tegumentary and Visceral Leishmaniases in Brazil: Emerging Anthropozoonosis and Possibilities for Their Control. Cad. Saúde Pública 1994, 10, S359–S375. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Guerra, J.A.; Talhari, S.; Paes, M.G.; Garrido, M.; Talhari, J.M. Aspectos clínicos e diagnósticos da leishmaniose tegumentar americana em militares simultaneamente expostos à infecção na Amazônia. Rev. Soc. Bras. Med. Trop. 2003, 36, 587–590. [Google Scholar] [CrossRef]
- Silveira, F.T.; Lainson, R.; Corbett, C.E. Clinical and Immunopathological Spectrum of American Cutaneous Leishmaniasis with Special Reference to the Disease in Amazonian Brazil: A Review. Memórias Inst. Oswaldo Cruz 2004, 99, 239–251. [Google Scholar] [CrossRef]
- Oliveira-Ribeiro, C.; Pimentel, M.I.F.; Oliveira, R.d.V.C.; Fagundes, A.; Madeira, M.d.F.; Mello, C.X.; Mouta-Confort, E.; Valete-Rosalino, C.M.; Vasconcellos, E.d.C.F.; Lyra, M.R.; et al. Clinical and Laboratory Profiles of Patients with Early Spontaneous Healing in Cutaneous Localized Leishmaniasis: A Historical Cohort Study. BMC Infect. Dis. 2017, 17, 559. [Google Scholar] [CrossRef] [PubMed]
- Locksley, R.M.; Heinzel, F.P.; Sadick, M.D.; Holaday, B.J.; Gardner, K.D. Murine Cutaneous Leishmaniasis: Susceptibility Correlates with Differential Expansion of Helper T-Cell Subsets. Ann. De L’institut Pasteur/Immunol. 1987, 138, 744–749. [Google Scholar] [CrossRef]
- Heinzel, F.P.; Sadick, M.D.; Holaday, B.J.; Coffmanj, R.L.; Locksley, R.M. Reciprocal Expression of Interferon y or Interleukin 4 during the Resolution or Progression of Murine Leishmaniasis: Evidence for Expansion of Distinct Helper T Cell Subsets. J. Exp. Med. 1989, 169, 59–72. [Google Scholar] [CrossRef]
- Awasthi, A.; Mathur, R.K.; Saha, B. Immune Response to Leishmania Infection. Indian J. Med. Res. 2004, 119, 238–258. [Google Scholar]
- Machado, P.; Kanitakis, J.; Almeida, R.; Chalon, A.; Araújo, C.; Caravalho, M.E. Evidence of in Situ Cytotoxicity in American Cutaneous Leishmaniasis. Eur. J. Dermatol 2002, 12, 449–451. [Google Scholar]
- Rojas, R.; Valderama, L.; Valderama, M.; Varona, X.M.; Ouellette, M.; Saravia, G.M. Resistance to Antimony and Treatment Failure in Human Leishmania (Viannia) Infection. J. Inf. Dis. 2006, 193, 1375–1383. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Hueb, M.; Santos, T.A.R.R.d.; Fontes, C.J.F. Fatores associados ao insucesso do tratamento da leishmaniose cutânea com antimoniato de meglumina. Rev. Soc. Bras. Med. Trop. 2006, 39, 139–145. [Google Scholar] [CrossRef]
- Brahim, L.R.; Valete-Rosalino, C.M.; Antônio, L.d.F.; Pimentel, M.I.F.; Lyra, M.R.; Paes, L.E.d.C.; Costa, A.D.d.; Vieira, I.F.; Dias, C.M.G.; Duque, M.C.d.O.; et al. Low Dose Systemic or Intralesional Meglumine Antimoniate Treatment for American Tegumentary Leishmaniasis Results in Low Lethality, Low Incidence of Relapse, and Low Late Mucosal Involvement in a Referral Centre in Rio de Janeiro, Brazil (2001–2013). Memórias Inst. Oswaldo Cruz 2017, 112, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.H.; Frézard, F.; Pereira, N.P.; Moura, A.S.; Ramos, L.M.Q.C.; Carvalho, G.B.; Rocha, M.O.C. American Tegumentary Leishmaniasis in Brazil: A Critical Review of the Current Therapeutic Approach with Systemic Meglumine Antimoniate and Short-Term Possibilities for an Alternative Treatment. Trop. Med. Int. Health 2019, 24, 380–391. [Google Scholar] [CrossRef]
- Rugani, J.N.; Gontijo, C.M.F.; Frézard, F.; Soares, R.P.; Monte-Neto, R.L.d. Antimony Resistance in Leishmania (Viannia) Braziliensis Clinical Isolates from Atypical Lesions Associates with Increased ARM56/ARM58 Transcripts and Reduced Drug Uptake. Memórias Inst. Oswaldo Cruz 2019, 114, e190111. [Google Scholar] [CrossRef]
- Unger, A.; O’Neal, S.; Machado, P.R.L.; Guimarães, L.H.; Morgan, D.J.; Schriefer, A.; Bacellar, O.; Glesby, M.J.; Carvalho, E.M. Association of Treatment of American Cutaneous Leishmaniasis Prior to Ulcer Development with High Rate of Failure in Northeastern Brazil. Am. J. Trop. Med. Hyg. 2009, 80, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Gonçalves, L.; Liarte, D.; Lima, D.; Guimarães, F.; Resende, D.; Santi, A.; Oliveira, L.; Velloso, J.; Delfino, R.; et al. Comparative Transcriptomic Analysis of Antimony Resistant and Susceptible Leishmania Infantum Lines. Parasites Vectors 2020, 13, 600. [Google Scholar] [CrossRef]
- García-Bustos, M.F.; González-Prieto, G.; Paniz-Mondolfi, A.E.; Parodi, C.; Beckar, J.; Monroig, S.; Ramos, F.; Mora, M.C.; Delgado-Noguera, L.A.; Hashiguchi, Y.; et al. Risk Factors for Antimony Treatment Failure in American Cutaneous Leishmaniasis in Northwestern-Argentina. PLoS Negl. Trop. Dis. 2021, 15, e0009003. [Google Scholar] [CrossRef]
- Gagini, T.; de Oliveira Schubach, A.; de Fatima Madeira, M.; Maria Valete-Rosalino, C.; Fernandes Pimentel, M.I.; da Silva Pacheco, R. Genotypic Profiles of Leishmania (Viannia) Braziliensis Strains from Cutaneous Leishmaniasis Patients and Their Relationship with the Response to Meglumine Antimoniate Treatment: A Pilot Study. Parasite 2017, 24, 34. [Google Scholar] [CrossRef] [PubMed]
- Baptista, C.; Miranda, L.d.F.C.; Madeira, M.d.F.; Leon, L.L.P.; Conceição-Silva, F.; Schubach, A.d.O. In Vitro Sensitivity of Paired Leishmania (Viannia) Braziliensis Samples Isolated before Meglumine Antimoniate Treatment and after Treatment Failure or Reactivation of Cutaneous Leishmaniasis. Dis. Markers 2015, 2015, 943236. [Google Scholar] [CrossRef] [PubMed]
- Nylén, S.; Eidsmo, L. Tissue Damage and Immunity in Cutaneous Leishmaniasis. Parasite Immunol. 2012, 34, 551–561. [Google Scholar] [CrossRef]
- Vieira, É.L.M.; Keesen, T.S.L.; Machado, P.R.; Guimarães, L.H.; Carvalho, E.M.; Dutra, W.O.; Gollob, K.J. Immunoregulatory Profile of Monocytes from Cutaneous Leishmaniasis Patients and Association with Lesion Size. Parasite Immunol. 2013, 35, 65–72. [Google Scholar] [CrossRef]
- Conceição-Silva, F.; Leite-Silva, J.; Morgado, F.N. The Binomial Parasite-Host Immunity in the Healing Process and in Reactivation of Human Tegumentary Leishmaniasis. Front. Microbiol. 2018, 9, 1308. [Google Scholar] [CrossRef] [PubMed]
- Conceição-Silva, F.; Morgado, F.N. Leishmania Spp-Host Interaction: There Is Always an Onset, but Is There an End? Front. Cell Infect. Microbiol. 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Mendez, S.; Lira, R.; Kadambi, N.; Milon, G.; Sacks, D. A Natural Model of Leishmania Major Infection Reveals a Prolonged “Silent” Phase of Parasite Amplification in the Skin before the Onset of Lesion Formation and Immunity. J. Immunol. 2000, 165, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Morgado, F.N.; Schubach, A.; Rosalino, C.M.V.; Quintella, L.P.; Santos, G.; Salgueiro, M.; Conceição-Silva, F. Is the in Situ Inflammatory Reaction an Important Tool to Understand the Cellular Immune Response in American Tegumentary Leishmaniasis? Br. J. Dermatol. 2008, 158, 50–58. [Google Scholar] [CrossRef]
- Novais, F.O.; Santiago, R.C.; Báfica, A.; Khouri, R.; Afonso, L.; Borges, V.M.; Brodskyn, C.; Barral-Netto, M.; Barral, A.; de Oliveira, C.I. Neutrophils and Macrophages Cooperate in Host Resistance against Leishmania Braziliensis Infection. J. Immunol. 2009, 183, 8088–8098. [Google Scholar] [CrossRef]
- Morgado, F.N.; Nascimento, M.T.C.; Saraiva, E.M.; de Oliveira-Ribeiro, C.; Madeira, M.d.F.; da Costa-Santos, M.; Vasconcellos, E.C.F.; Pimentel, M.I.F.; Rosandiski Lyra, M.; Schubach, A.d.O.; et al. Are Neutrophil Extracellular Traps Playing a Role in the Parasite Control in Active American Tegumentary Leishmaniasis Lesions? PLoS ONE 2015, 10, e0133063. [Google Scholar] [CrossRef]
- Faria, D.R.; Souza, P.E.A.; Durães, F.V.; Carvalho, E.M.; Gollob, K.J.; Machado, P.R.; Dutra, W.O. Recruitment of CD8+ T Cells Expressing Granzyme A Is Associated with Lesion Progression in Human Cutaneous Leishmaniasis. Parasite Immunol. 2009, 31, 432–439. [Google Scholar] [CrossRef]
- da Silva Santos, C.; Brodskyn, C.I. The Role of CD4 and CD8 T Cells in Human Cutaneous Leishmaniasis. Front. Public Health 2014, 2, 165. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Bacellar, O.; Carvalho, E.M. Protection and Pathology in Leishmania Braziliensis Infection. Pathogens 2022, 11, 466. [Google Scholar] [CrossRef]
- Kima, P.E.; Soong, L. Interferon Gamma in Leishmaniasis. Front. Immunol. 2013, 4, 156. [Google Scholar] [CrossRef] [PubMed]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.d.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef] [PubMed]
- Sandoval Pacheco, C.M.; Araujo Flores, G.V.; Gonzalez, K.; de Castro Gomes, C.M.; Passero, L.F.D.; Tomokane, T.Y.; Sosa-Ochoa, W.; Zúniga, C.; Calzada, J.; Saldaña, A.; et al. Macrophage Polarization in the Skin Lesion Caused by Neotropical Species of Leishmania sp. J. Immunol. Res. 2021, 2021, 5596876. [Google Scholar] [CrossRef]
- Maspi, N.; Abdoli, A.; Ghaffarifar, F. Pro- and Anti-Inflammatory Cytokines in Cutaneous Leishmaniasis: A Review. Pathog. Glob. Health 2016, 110, 247–260. [Google Scholar] [CrossRef]
- Guimarães-Costa, A.B.; Nascimento, M.T.C.; Froment, G.S.; Soares, R.P.P.; Morgado, F.N.; Conceição-Silva, F.; Saraiva, E.M. Leishmania Amazonensis Promastigotes Induce and Are Killed by Neutrophil Extracellular Traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748–6753. [Google Scholar] [CrossRef]
- Abi Abdallah, D.S.; Denkers, E.Y. Neutrophils Cast Extracellular Traps in Response to Protozoan Parasites. Front. Immunol. 2012, 3, 382. [Google Scholar] [CrossRef]
- Conceição-Silva, F.; Reis, C.S.M.; De Luca, P.M.; Leite-Silva, J.; Santiago, M.A.; Morrot, A.; Morgado, F.N. The Immune System Throws Its Traps: Cells and Their Extracellular Traps in Disease and Protection. Cells 2021, 10, 1891. [Google Scholar] [CrossRef]
- Da-Cruz, A.M.; Conceição-Silva, F.; Bertho, A.L.; Coutinho, S.G. Leishmania-Reactive CD4+ and CD8+ T Cells Associated with Cure of Human Cutaneous Leishmaniasis. Infect. Immun. 1994, 62, 2614–2618. [Google Scholar] [CrossRef] [PubMed]
- Maretti-Mira, A.C.; de Oliveira-Neto, M.P.; Da-Cruz, A.M.; de Oliveira, M.P.; Craft, N.; Pirmez, C. Therapeutic Failure in American Cutaneous Leishmaniasis Is Associated with Gelatinase Activity and Cytokine Expression. Clin. Exp. Immunol. 2011, 163, 207–214. [Google Scholar] [CrossRef]
- Bacellar, O.; Faria, D.; Nascimento, M.; Cardoso, T.M.; Gollob, K.J.; Dutra, W.O.; Scott, P.; Carvalho, E.M. Interleukin 17 Production among Patients with American Cutaneous Leishmaniasis. J. Infect. Dis. 2009, 200, 75–78. [Google Scholar] [CrossRef]
- Morgado, F.N.; Schubach, A.; Vasconcellos, E.; Azeredo-Coutinho, R.B.; Valete-Rosalino, C.M.; Quintella, L.P.; Santos, G.; Salgueiro, M.; Palmeiro, M.R.; Conceição-Silva, F. Signs of an in Situ Inflammatory Reaction in Scars of Human American Tegumentary Leishmaniasis. Parasite Immunol. 2010, 32, 285–295. [Google Scholar] [CrossRef]
- Rodrigues, K.M.P.; Oliveira, M.P.; Maretti-Mira, A.C.; Oliveira-Neto, M.P.; Mattos, M.S.; Silva, L.; Soares, D.A.; Dolci, E.L.L.; Perico, R.A.P.N.; Pirmez, C. Influence of the Notch System in the Therapeutic Response of American Tegumentary Leishmaniasis. Br. J. Dermatol. 2011, 164, 1228–1234. [Google Scholar] [CrossRef]
- Amato, V.S.; de Andrade, H.F.; Duarte, M.I.S. Mucosal Leishmaniasis: In Situ Characterization of the Host Inflammatory Response, before and after Treatment. Acta Trop. 2003, 85, 39–49. [Google Scholar] [CrossRef]
- Conceição-Silva, F.; Morgado, F.N.; Costa-Santos, M.d.; Miranda-Nascimento, C.; Schubach, A.O.; Oliveira-Mendes, S. Leishmania Braziliensis and in Situ Host Immune Response: Dispute or Partnership? Rev. Da Soc. Bras. De Med. Trop. 2010, 43, 64–71. [Google Scholar]
- Gomes, A.H.S.; Martines, R.B.; Kanamura, C.T.; Barbo, M.L.P.; Iglezias, S.D.; Lauletta Lindoso, J.A.; Pereira-Chioccola, V.L. American Cutaneous Leishmaniasis: In Situ Immune Response of Patients with Recent and Late Lesions. Parasite Immunol. 2017, 39, e12423. [Google Scholar] [CrossRef] [PubMed]
- Jayasena Kaluarachchi, T.D.; Weerasekera, M.M.; McBain, A.J.; Ranasinghe, S.; Wickremasinghe, R.; Yasawardene, S.; Jayanetti, N.; Wickremasinghe, R. Diagnosing Cutaneous Leishmaniasis Using Fluorescence in Situ Hybridization: The Sri Lankan Perspective. Pathog. Glob. Health 2019, 113, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.; Sacks, D. Immune Privilege in Sites of Chronic Infection: Leishmania and Regulatory T Cells. Immunol. Rev. 2006, 213, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Katara, G.K.; Ansari, N.A.; Verma, S.; Ramesh, V.; Salotra, P. Foxp3 and IL-10 Expression Correlates with Parasite Burden in Lesional Tissues of Post Kala Azar Dermal Leishmaniasis (PKDL) Patients. PLoS Negl. Trop. Dis. 2011, 5, e1171. [Google Scholar] [CrossRef]
- Anderson, C.F.; Oukka, M.; Kuchroo, V.J.; Sacks, D. CD4(+)CD25(-)Foxp3(-) Th1 Cells Are the Source of IL-10-Mediated Immune Suppression in Chronic Cutaneous Leishmaniasis. J. Exp. Med. 2007, 204, 285–297. [Google Scholar] [CrossRef]
- Rodrigues, F.M.D.; Coelho Neto, G.T.; Menezes, J.G.P.B.; Gama, M.E.A.; Gonçalves, E.G.; Silva, A.R.; Laurenti, M.D.; Corbett, C.E.P.; Silveira, F.T.; Gomes, C.M.C. Expression of Foxp3, TGF-β and IL-10 in American Cutaneous Leishmaniasis Lesions. Arch. Dermatol. Res. 2014, 306, 163–171. [Google Scholar] [CrossRef]
- Cataldo, J.I.; Conceição-Silva, F.; Antônio, L.d.F.; Schubach, A.d.O.; Marzochi, M.C.d.A.; Valete-Rosalino, C.M.; Pimentel, M.I.F.; Lyra, M.R.; Oliveira, R.d.V.C.d.; Barros, J.H.d.S.; et al. Favorable Responses to Treatment with 5 Mg Sbv/Kg/Day Meglumine Antimoniate in Patients with American Tegumentary Leishmaniasis Acquired in Different Brazilian Regions. Rev. Soc. Bras. Med. Trop. 2018, 51, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Saheki, M.N.; Lyra, M.R.; Bedoya-Pacheco, S.J.; Antônio, L.d.F.; Pimentel, M.I.F.; Salgueiro, M.d.M.; Vasconcellos, É.d.C.F.E.; Passos, S.R.L.; Santos, G.P.L.d.; Ribeiro, M.N.; et al. Low versus High Dose of Antimony for American Cutaneous Leishmaniasis: A Randomized Controlled Blind Non-Inferiority Trial in Rio de Janeiro, Brazil. PLoS ONE 2017, 12, e0178592. [Google Scholar] [CrossRef] [PubMed]
- Miranda, L.d.F.C.; Pacheco, R.d.S.; Pimentel, M.I.F.; Salgueiro, M.d.M.; Silva, A.F.d.; Mello, C.X.d.; Barros, J.H.d.S.; Valete-Rosalino, C.M.; Madeira, M.d.F.; Xavier, S.C.d.C.; et al. Geospatial Analysis of Tegumentary Leishmaniasis in Rio de Janeiro State, Brazil from 2000 to 2015: Species Typing and Flow of Travelers and Migrants with Leishmaniasis. PLoS Negl. Trop. Dis. 2019, 13, e0007748. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.O.; Coutinho, C.E.R.; Madeira, M.F.; Bottino, C.G.; Vieira, R.T.; Nascimento, S.B.; Bernardino, A.; Bourguignon, S.C.; Corte-Real, S.; Pinho, R.T.; et al. Leishmaniasis Treatment--a Challenge That Remains: A Review. Parasitol. Res. 2008, 103, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Millott, S.; Parkinson, C.; Palmer, R.M.; Moncada, S. Macrophage Killing of Leishmania Parasite in Vivo Is Mediated by Nitric Oxide from L-Arginine. J. Immunol. 1990, 144, 4794–4797. [Google Scholar] [CrossRef]
- Chagas, A.C.; Oliveira, F.; Debrabant, A.; Valenzuela, J.G.; Ribeiro, J.M.C.; Calvo, E. Lundep, a Sand Fly Salivary Endonuclease Increases Leishmania Parasite Survival in Neutrophils and Inhibits XIIa Contact Activation in Human Plasma. PLoS Pathog. 2014, 10, e1003923. [Google Scholar] [CrossRef]
- Laurenti, M.D.; Gidlund, M.; Ura, D.M.; Sinhorini, I.L.; Corbett, C.E.P.; Goto, H. The Role of Natural Killer Cells in the Early Period of Infection in Murine Cutaneous Leishmaniasis. Braz. J. Med. Biol. Res. 1999, 32, 323–325. [Google Scholar] [CrossRef]
- Wei, X.Q.; Charles, I.G.; Smith, A.; Ure, J.; Feng, G.J.; Huang, F.P.; Xu, D.; Muller, W.; Moncada, S.; Liew, F.Y. Altered Immune Responses in Mice Lacking Inducible Nitric Oxide Synthase. Nature 1995, 375, 408–411. [Google Scholar] [CrossRef]
- Murray, H.W.; Nathan, C.F. Macrophage Microbicidal Mechanisms In Vivo: Reactive Nitrogen versus Oxygen Intermediates in the Killing of Intracellular Visceral Leishmania Donovani. J. Exp. Med. 1999, 189, 741–746. [Google Scholar] [CrossRef]
- Bogdan, C.; Röllinghoff, M.; Diefenbach, A. The Role of Nitric Oxide in Innate Immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Qadoumi, M.; Becker, I.; Donhauser, N.; Röllinghoff, M.; Bogdan, C. Expression of Inducible Nitric Oxide Synthase in Skin Lesions of Patients with American Cutaneous Leishmaniasis. Infect. Immun. 2002, 70, 4638–4642. [Google Scholar] [CrossRef] [PubMed]
- Palmeiro, M.R.; Morgado, F.N.; Valete-Rosalino, C.M.; Martins, A.C.; Moreira, J.; Quintella, L.P.; de Oliveira Schubach, A.; Conceição-Silva, F. Comparative Study of the in Situ Immune Response in Oral and Nasal Mucosal Leishmaniasis. Parasite Immunol. 2012, 34, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Farah, F.S.; Samra, S.A.; Nuwayri-Salti, N. The Role of the Macrophage in Cutaneous Leishmaniasis. Immunology 1975, 29, 755–764. [Google Scholar] [PubMed]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the Macrophage: A Multifaceted Interaction. Future Microbiol. 2015, 10, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Barral, A.; Costa, J.M.; Bittencourt, A.L.; Barral-Netto, M.; Carvalho, E.M. Polar and Subpolar Diffuse Cutaneous Leishmaniasis in Brazil: Clinical and Immunopathologic Aspects. Int. J. Dermatol. 1995, 34, 474–479. [Google Scholar] [CrossRef]
- Christensen, S.M.; Belew, A.T.; El-Sayed, N.M.; Tafuri, W.L.; Silveira, F.T.; Mosser, D.M. Host and Parasite Responses in Human Diffuse Cutaneous Leishmaniasis Caused by L. Amazonensis. PLoS Negl. Trop. Dis. 2019, 13, e0007152. [Google Scholar] [CrossRef]
- Gabriel, C.; McMaster, W.R.; Girard, D.; Descoteaux, A. Leishmania Donovani Promastigotes Evade the Antimicrobial Activity of Neutrophil Extracellular Traps. J. Immunol. 2010, 185, 4319–4327. [Google Scholar] [CrossRef]
- Saffarzadeh, M.; Preissner, K.T. Fighting against the Dark Side of Neutrophil Extracellular Traps in Disease: Manoeuvres for Host Protection. Curr. Opin. Hematol. 2013, 20, 3–9. [Google Scholar] [CrossRef]
- Farrera, C.; Fadeel, B. Macrophage Clearance of Neutrophil Extracellular Traps Is a Silent Process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef]
- Mendonça, S.C.; Coutinho, S.G.; Amendoeira, R.R.; Marzochi, M.C.; Pirmez, C. Human American Cutaneous Leishmaniasis (Leishmania b. Braziliensis) in Brazil: Lymphoproliferative Responses and Influence of Therapy. Clin. Exp. Immunol. 1986, 64, 269–276. [Google Scholar] [PubMed]
- Souza, M.A.; Castro, M.C.A.B.; Oliveira, A.P.; Almeida, A.F.; Reis, L.C.; Silva, C.J.; Brito, M.E.F.; Pereira, V.R.A. American Tegumentary Leishmaniasis: Cytokines and Nitric Oxide in Active Disease and after Clinical Cure, with or without Chemotherapy. Scand. J. Immunol. 2012, 76, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.N.; Ribeiro, L.E.; Schrieffer, A.; Machado, P.; Carvalho, E.M.; Bacellar, O. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Human Tegumentary Leishmaniasis. Cytokine 2014, 66, 127–132. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous Leishmaniasis: Immune Responses in Protection and Pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef]
- Rodriguez-Pinto, D.; Navas, A.; Blanco, V.M.; Ramírez, L.; Garcerant, D.; Cruz, A.; Craft, N.; Saravia, N.G. Regulatory T Cells in the Pathogenesis and Healing of Chronic Human Dermal Leishmaniasis Caused by Leishmania (Viannia) Species. PLoS Negl. Trop. Dis. 2012, 6, e1627. [Google Scholar] [CrossRef]
- Bahrami, F.; Darabi, H.; Riazi-Rad, F.; Khaze, V.; Ajdary, S.; Alimohammadian, M.H. FOXP3 Expression and Frequency of Regulatory T Cells in Healed Individuals from Leishmania Major Infection and the Asymptomatic Cases. Hum. Immunol. 2014, 75, 1026–1033. [Google Scholar] [CrossRef]
- Oliveira-Neto, M.P.; Mattos, M.; da Silva, C.; de Souza, F.; Fernandes, O.; Pirmez, C. Leishmaniasis Recidiva Cutis in New World Cutaneous Leishmaniasis. Int. J. Dermatol. 1998, 37, 846–849. [Google Scholar] [CrossRef]
- Azeredo-Coutinho, R.B.G.; Conceição-Silva, F.; Schubach, A.; Cupolillo, E.; Quintella, L.P.; Madeira, M.F.; Pacheco, R.S.; Valete-Rosalino, C.M.; Mendonça, S.C.F. First Report of Diffuse Cutaneous Leishmaniasis and Leishmania Amazonensis Infection in Rio de Janeiro State, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Kropf, P.; Schopf, L.R.; Chung, C.L.; Xu, D.; Liew, F.Y.; Sypek, J.P.; Müller, I. Expression of Th2 Cytokines and the Stable Th2 Marker ST2L in the Absence of IL-4 during Leishmania Major Infection. Eur. J. Immunol. 1999, 29, 3621–3628. [Google Scholar] [CrossRef]
- Hardman, C.; Ogg, G. Interleukin-33, Friend and Foe in Type-2 Immune Responses. Curr. Opin. Immunol. 2016, 42, 16–24. [Google Scholar] [CrossRef]
- Lu, J.; Kang, J.; Zhang, C.; Zhang, X. The Role of IL-33/ST2L Signals in the Immune Cells. Immunol. Lett. 2015, 164, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Oboki, K.; Ohno, T.; Kajiwara, N.; Saito, H.; Nakae, S. IL-33 and IL-33 Receptors in Host Defense and Diseases. Allergol. Int. 2010, 59, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M. Role of IL-33 in Inflammation and Disease. J. Inflamm. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Rostan, O.; Gangneux, J.-P.; Piquet-Pellorce, C.; Manuel, C.; McKenzie, A.N.J.; Guiguen, C.; Samson, M.; Robert-Gangneux, F. The IL-33/ST2 Axis Is Associated with Human Visceral Leishmaniasis and Suppresses Th1 Responses in the Livers of BALB/c Mice Infected with Leishmania Donovani. mBio 2013, 4, e00383-13. [Google Scholar] [CrossRef] [PubMed]
- Schubach, A.; Marzochi, M.C.; Cuzzi-Maya, T.; Oliveira, A.V.; Araujo, M.L.; Oliveira, A.L.; Pacheco, R.S.; Momen, H.; Conceicao-Silva, F.; Coutinho, S.G.; et al. Cutaneous Scars in American Tegumentary Leishmaniasis Patients: A Site of Leishmania (Viannia) Braziliensis Persistence and Viability Eleven Years after Antimonial Therapy and Clinical Cure. Am. J. Trop. Med. Hyg. 1998, 58, 824–827. [Google Scholar] [CrossRef]

| Clinical Characteristics | ||||
|---|---|---|---|---|
| Variable | SRL (n = 14) | GRL (n = 20) | PRL (n = 24) | p-Value |
| Sex | 0.496 | |||
| Female | 7 | 6 | 9 | |
| Male | 7 | 14 | 15 | |
| Age (Mean ± SEM) | 37.00 ± 15.21 | 37.40 ± 16.00 | 37.29 ± 13.73 | 0.995 |
| (Min–Max) | (17–64) | (16–72) | (18–72) | |
| Mean number of lesions | 1.0 | 1.5 | 2.6 | 0.032 * |
| (Min–Max) | (1–1) | (1–3) | (1–9) | |
| Largest diameter of a lesion (mm) | 80 | 80 | 90 | 0.05 |
| (Min–Max) | (10–80) | (5–80) | (5–90) | |
| Mean diameter of a lesion (mm) (Mean ± SEM) | 29.16 ± 18.62 | 34.52 ± 16.15 | 40.09 ± 20.04 | 0.396 |
| Evolution of lesions (months) | 4.4 ± 5.79 | 3.3 ± 2.60 | 4.5 ± 1.60 | 0.413 |
| (Mean ± SEM) (Min–Max) | (1–3) | (1–12) | (1–7) | |
| Number of positive parasitological exams | ||||
| 1 | 12 patients (85.71%) (A or B) | 4 patients (20.00%) (A, B, or C) | 0 | <0.0001 ** |
| 2 | 2 patients (14.29%) (A and B) | 3 patients (15.00%) (A, B, or C) | 2 patients (8.33%) (A, B, or C) | |
| 3 or more | 0 | 13 patients (65.00%) (A, B, and C) | 22 patients (91.67%) (A, B, and C) | |
| Marker | SRL % (Mean + SEM) (Min–Max) | GRL % (Mean + SEM) (Min–Max) | PRL1 % (Mean + SEM) (Min–Max) | PRL2 % (Mean + SEM) (Min–Max) |
|---|---|---|---|---|
| CD4 | 30.59 ± 2.38 | 29.71 ± 6.02 | 24.35 ± 4.07 | 24.39 ± 4.29 |
| (27.18–34.31) | (20.21–40.22) | (18.00–35.63) | (16.95–33.33) | |
| CD8 | 26.81 ± 2.76 | 29.46 ± 4.32 | 28.13 ± 4.69 | 25.68 ± 5.76 |
| (21.34–33.20) | (22.94–36.72) | (19.72–35.59) | (13.95–40.40) | |
| CD22 | 13.46 ± 1.87 | 19.28 ± 4.65 | 20.70 ± 5.16 | 20.30 ± 6.40 |
| (12.10–18.75) | (12.00–27.14) | (13.00–30.88) | (7.02–21.28) | |
| CD68 | 17.00 ± 5.40 | 26.48 ± 5.20 | 30.34 ± 6.73 | 29.90 ± 5.48 |
| (4.89–28.14) | (17.67–37.10) | (17.06–41.45) | (20.79–41.51) | |
| CD163 | 14.31 ± 6.12 | 19.21 ± 4.55 | 23.15 ± 5.08 | 22.37 ± 5.43 |
| (6.47–23.40) | (9.00–28.68) | (12.72–30.88) | (12.21–30.63) | |
| CD206 | 16.52 ± 4.29 | 17.15 ± 4.13 | 17.90 ± 5.11 | 17.00 ± 5.52 |
| (9.85–24.53) | (5.44–23.44) | (8.56–29.82) | (7.44–27.66) | |
| CD56 | 7.76 ± 4.05 | 19.00 ± 4.92 | 19.72 ± 7.11 | 19.77 ± 6.53 |
| (4.31–22.38) | (9.38–27.18) | (7.23–37.08) | (10.00–34.95) | |
| Mast cell Tryptase | 7.44 ± 2.81 | 8.08 ± 3.08 | 7.51 ± 3.77 | 6.88 ± 3.16 |
| (3.33–13.40) | (4.00–13.23) | (3.00–17.16) | (3.00–16.58) | |
| Neutrophil elastase | 19.49 ± 1.13 | 25.67 ± 3.93 | 22.32 ± 5.05 | 21.16 ± 6.16 |
| (10.79–36.19) | (19.98–35.14) | (13.97–31.94) | (10.21–33.95) | |
| Ki-67 | 8.98 ± 0.03 | 16.82 ± 6.07 | 22.38 ± 5.10 | 21.47 ± 5.03 |
| (4.79–17.15) | (8.13–26.29) | (14.02–33.59) | (11.57–32.49) | |
| FoxP3 | 12.92 ± 4.52 | 16.24 ± 4.06 | 12.83 ± 4.62 | 10.18 ± 4.11 |
| (7.69–24.75) | (10.58–25.18) | (3.70–20.06) | (2.71–19.61) | |
| CD25 | 8.03 ± 4.29 | 16.78 ± 4.05 | 19.44 ± 5.71 | 18.16 ± 6.43 |
| (3.41–19.96) | (11.17–23.72) | (10.00–27.27) | (8.79–28.97) | |
| IFN-γ | 24.67 ± 4.74 | 18.86 ± 6.10 | 12.36 ± 4.15 | 14.86 ± 4.04 |
| (13.22–32.61) | (4.64–32.90) | (5.23–16.43) | (10.00–23.29) | |
| ST2L | 7.12 ± 2.22 | 14.27 ± 5.22 | 12.70 ± 4.26 | 11.31 ± 4.61 |
| (4.00–11.89) | (8.00–25.21) | (6.00–21.11) | (5.33–21.28) | |
| IL-33 | 16.33 ± 4.70 | 9.91 ± 4.54 | 13.05 ± 5.11 | 11.98 ± 4.27 |
| (4.80–24.16) | (4.11–20.32) | (4.11–20.32) | (4.23–18.48) |
| Intensity | Group Number of Patients (% within Each Group) | p-Value * | |||
|---|---|---|---|---|---|
| SRL | GRL | PRL1 | PRL2 | ||
| 1 (discrete) | 0 | 3 (15%) | 11 (45.83%) | 14 (58.34%) | |
| 2 (moderate) | 3 (21%) | 4 (20%) | 9 (37.50%) | 6 (25%) | 0.0002 |
| 3 (intense) | 4 (28%) | 8 (40%) | 3 (12.50%) | 2 (8.33%) | |
| 4 to 5 (very intense) | 7 (50%) | 5 (25%) | 1 (4.17%) | 2 (8.33%) | |
| Group | Leishmania spp. (mm2) (Mean) (Min–Max) | p-Value * | NETs (mm2) (Mean) (Min–Max) | p-Value * |
|---|---|---|---|---|
| SRL | 0.08 (0–0.28) | 0.37 (0–1.03) | ||
| GRL | 0.10 (0–0.44) | 0.08 | 0.44 (0–1.92) | 0.05 |
| PRL1 | 0.33 (0–1.18) | 0.27 (0–4.29) | ||
| PRL2 | 0.29 (0–0.76) | 0.08 (0–0.63) |
| Marker | SRL | GRL | PRL |
|---|---|---|---|
| CD4/CD8 | CD4 > CD8 | CD4 ≥ CD8 | CD8 ≥ CD4 |
| CD22 | + | ++ | ++ |
| CD68 | + | ++ | +++ |
| CD163 | + | ++ | +++ |
| CD206 | ++ | ++ | ++ |
| CD56 | + | ++ | ++ |
| Mast cell tryptase | + | + | + |
| Neutrophil elastase | + | ++ | ++ |
| Ki-67 | + | ++ | +++ |
| FoxP3 | + | ++ | + |
| CD25 | + | ++ | ++ |
| IFN-γ | +++ | ++ | + |
| ST2L | + | ++ | ++ |
| IL-33 | +++ | + | ++ |
| NOS2 | +++ | ++ | + |
| Predominance of Th1 response with presence of a balanced immune response | High number of inflammatory cells, but still presenting a controlled Th1-Th2 balance | Predominance of an unbalanced immune response with tendency towards a Th2 response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite-Silva, J.; Oliveira-Ribeiro, C.; Morgado, F.N.; Pimentel, M.I.F.; Lyra, M.R.; Fagundes, A.; Miranda, L.F.C.; Valete-Rosalino, C.M.; Schubach, A.O.; Conceição-Silva, F. Is There Any Difference in the In Situ Immune Response in Active Localized Cutaneous Leishmaniasis That Respond Well or Poorly to Meglumine Antimoniate Treatment or Spontaneously Heal? Microorganisms 2023, 11, 1631. https://doi.org/10.3390/microorganisms11071631
Leite-Silva J, Oliveira-Ribeiro C, Morgado FN, Pimentel MIF, Lyra MR, Fagundes A, Miranda LFC, Valete-Rosalino CM, Schubach AO, Conceição-Silva F. Is There Any Difference in the In Situ Immune Response in Active Localized Cutaneous Leishmaniasis That Respond Well or Poorly to Meglumine Antimoniate Treatment or Spontaneously Heal? Microorganisms. 2023; 11(7):1631. https://doi.org/10.3390/microorganisms11071631
Chicago/Turabian StyleLeite-Silva, Jéssica, Carla Oliveira-Ribeiro, Fernanda Nazaré Morgado, Maria Inês Fernandes Pimentel, Marcelo Rosandiski Lyra, Aline Fagundes, Luciana Freitas Campos Miranda, Claudia Maria Valete-Rosalino, Armando Oliveira Schubach, and Fátima Conceição-Silva. 2023. "Is There Any Difference in the In Situ Immune Response in Active Localized Cutaneous Leishmaniasis That Respond Well or Poorly to Meglumine Antimoniate Treatment or Spontaneously Heal?" Microorganisms 11, no. 7: 1631. https://doi.org/10.3390/microorganisms11071631
APA StyleLeite-Silva, J., Oliveira-Ribeiro, C., Morgado, F. N., Pimentel, M. I. F., Lyra, M. R., Fagundes, A., Miranda, L. F. C., Valete-Rosalino, C. M., Schubach, A. O., & Conceição-Silva, F. (2023). Is There Any Difference in the In Situ Immune Response in Active Localized Cutaneous Leishmaniasis That Respond Well or Poorly to Meglumine Antimoniate Treatment or Spontaneously Heal? Microorganisms, 11(7), 1631. https://doi.org/10.3390/microorganisms11071631








