Lipid Accumulation in Blastocystis Increases Cell Damage in Co-Cultured Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Blastocystis hominis and Caco-2 Cell Culture
2.2. Assay of Cytopathic Effects (CPE)
2.3. Lipid Staining of Blastocystis hominis
2.4. Total RNA Isolation and cDNA Synthesis of Blastocystis ST7-B
2.5. Library Preparation for RNA-seq
2.6. Quantitative Reverse Transcription PCR (RT-qPCR) Assay
2.7. Protein Extraction for 2DE and Identification via MALDI-TOF-MS
2.8. Fatty Acids Analysis with Gas Chromatography Mass Spectrometry (GC-MS)
2.9. Azocasein Assay for Cysteine Protease Activity
3. Results
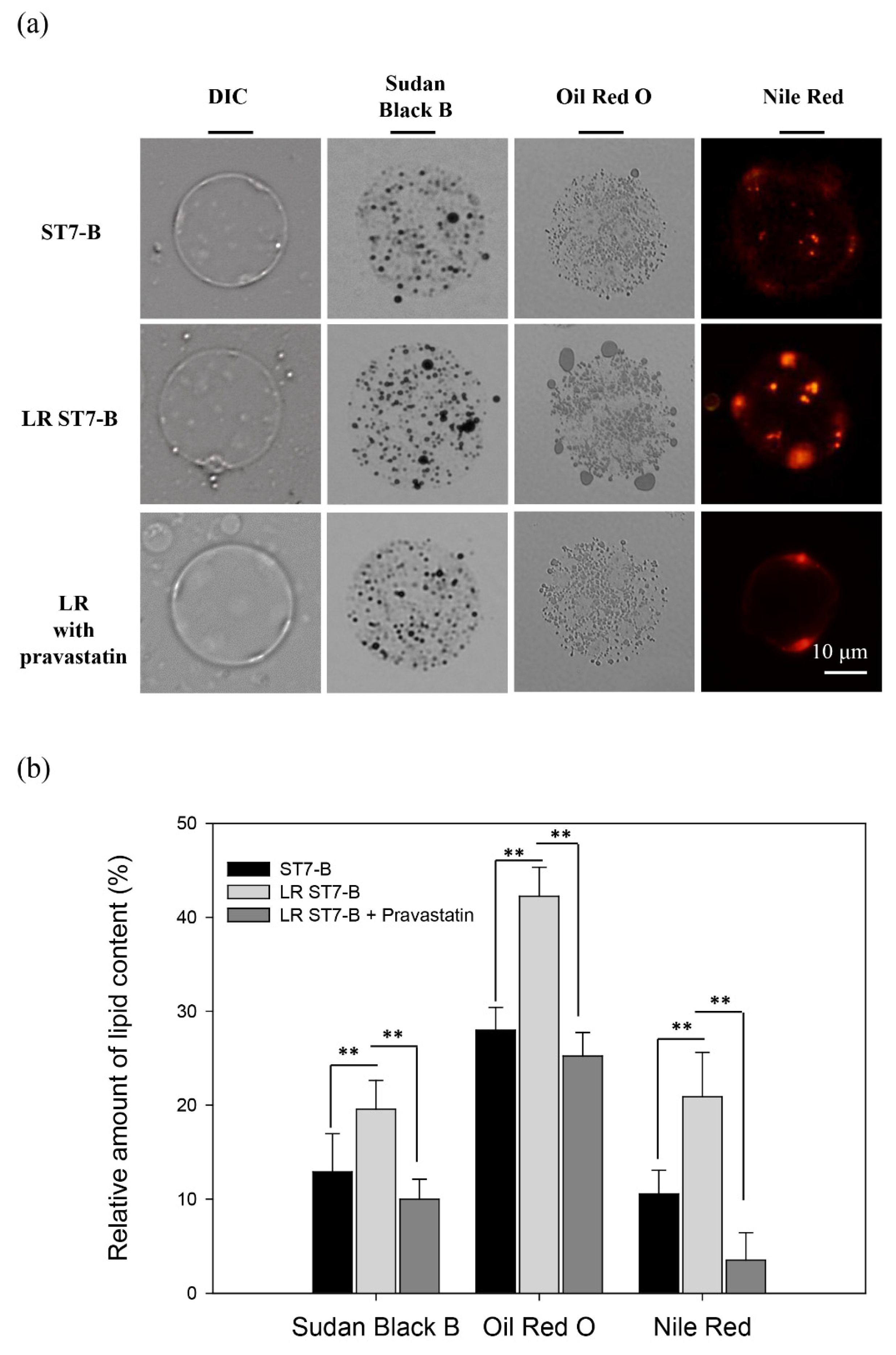
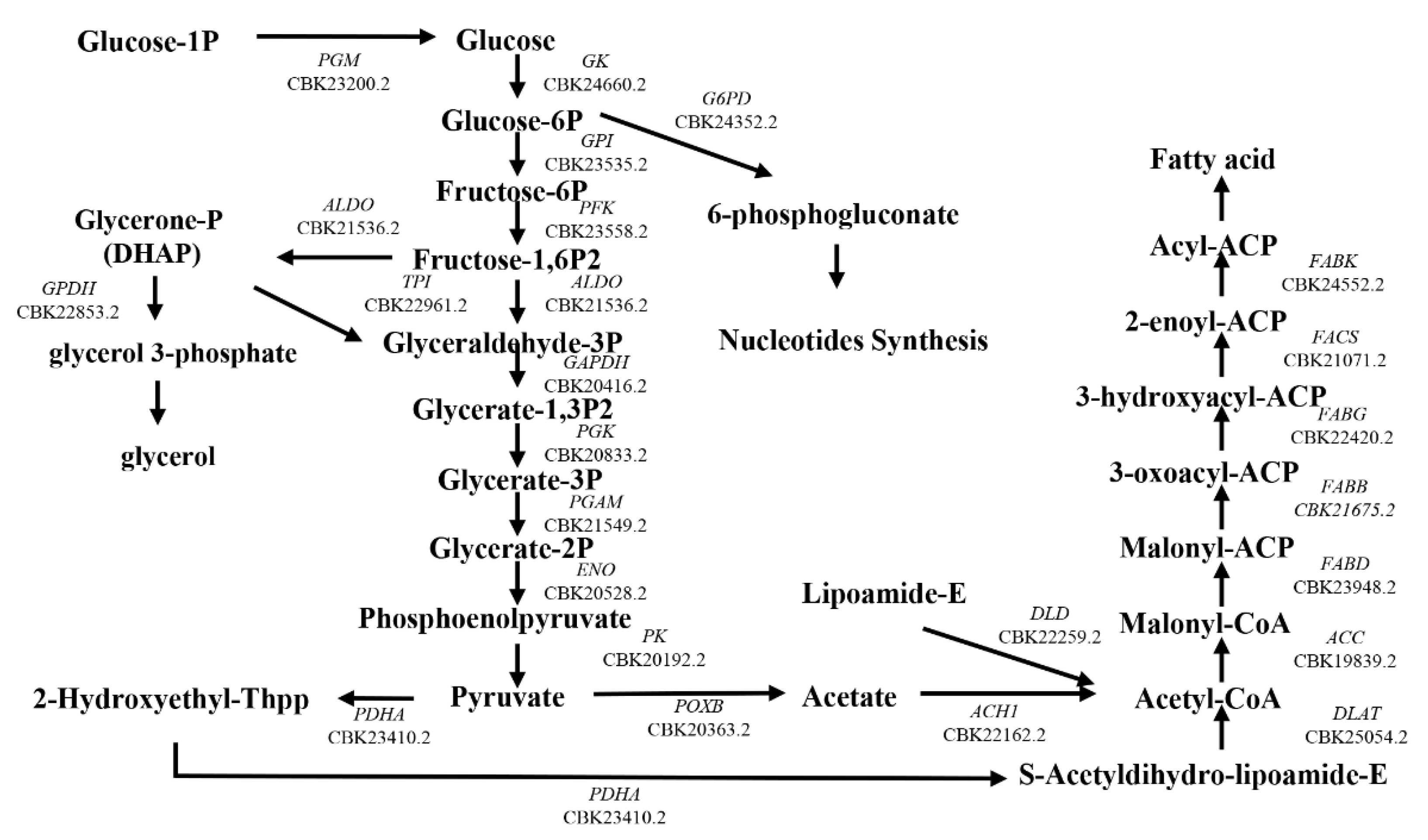
3.1. Lipid Accumulation in ST7-B
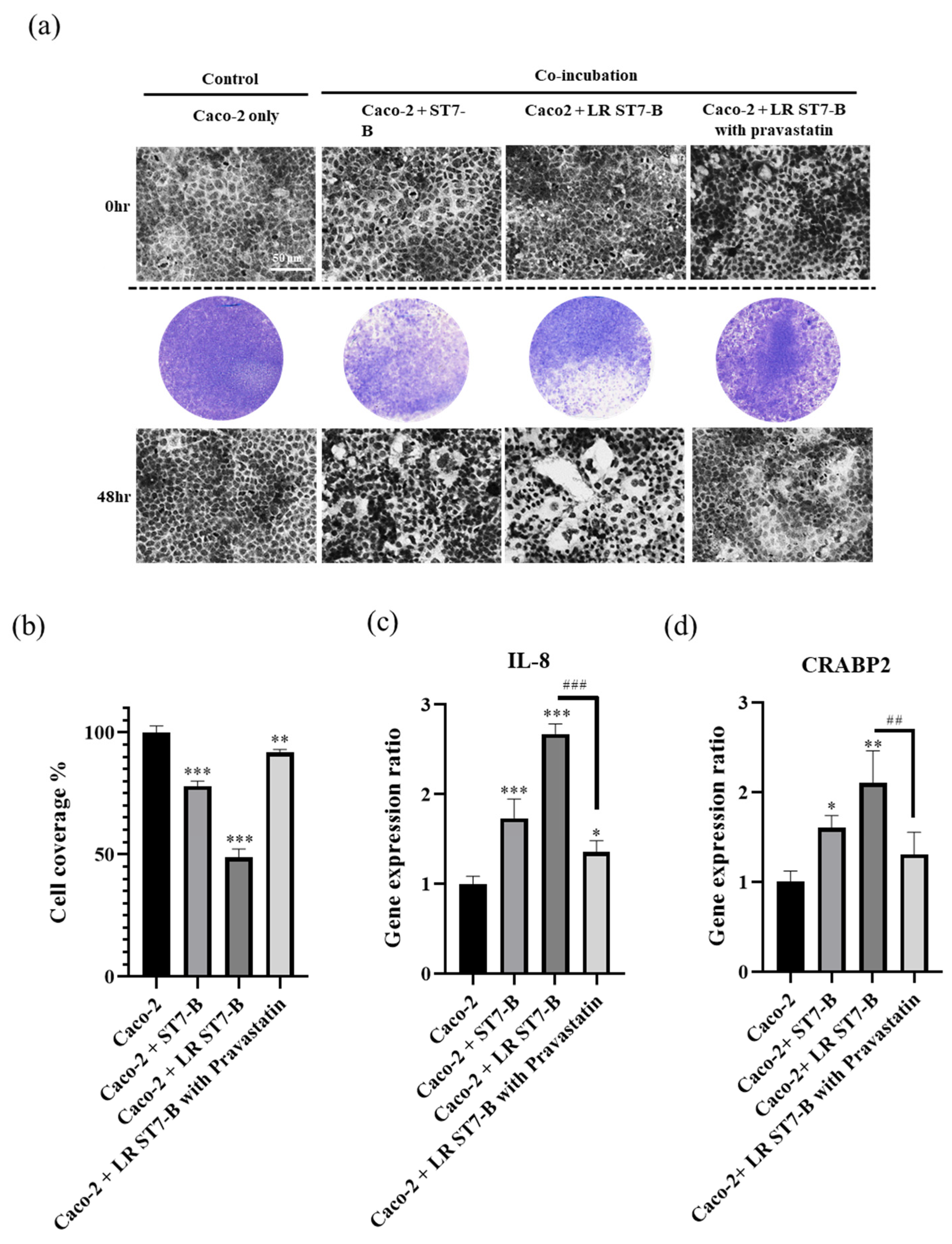
3.2. LR ST7-B Induced Significantly Higher Cell Inflammation and Cell Disruption in Caco-2 Cells
3.3. Cysteine Protease Are Upregulated in LR ST7-B
3.4. Pravastatin Lowered LR ST7-B-Induced Cell Inflammation and Reduced the Disruption of Caco-2 Cells
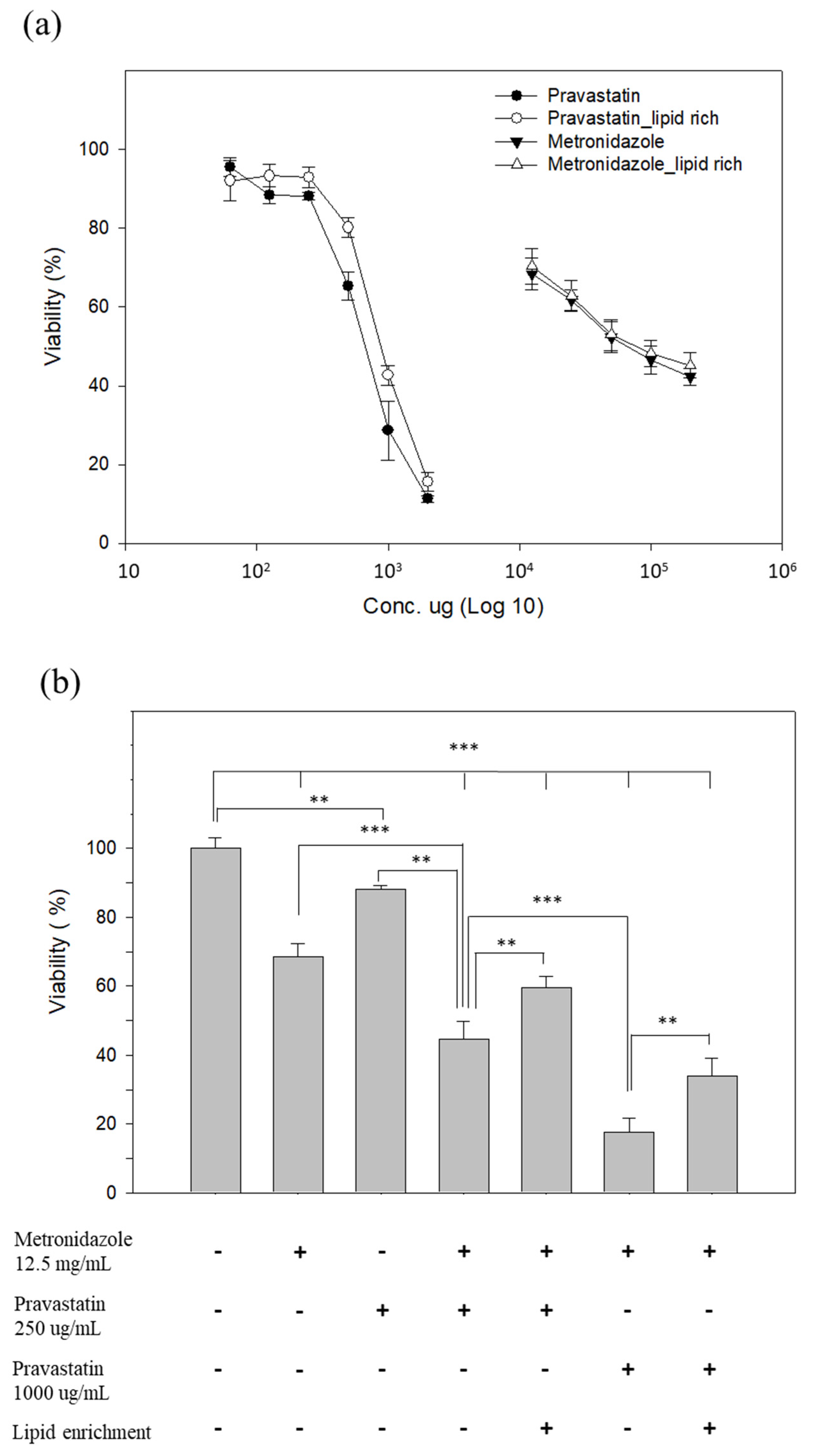
3.5. Pravastatin Reduced the Viability of LR ST7-B
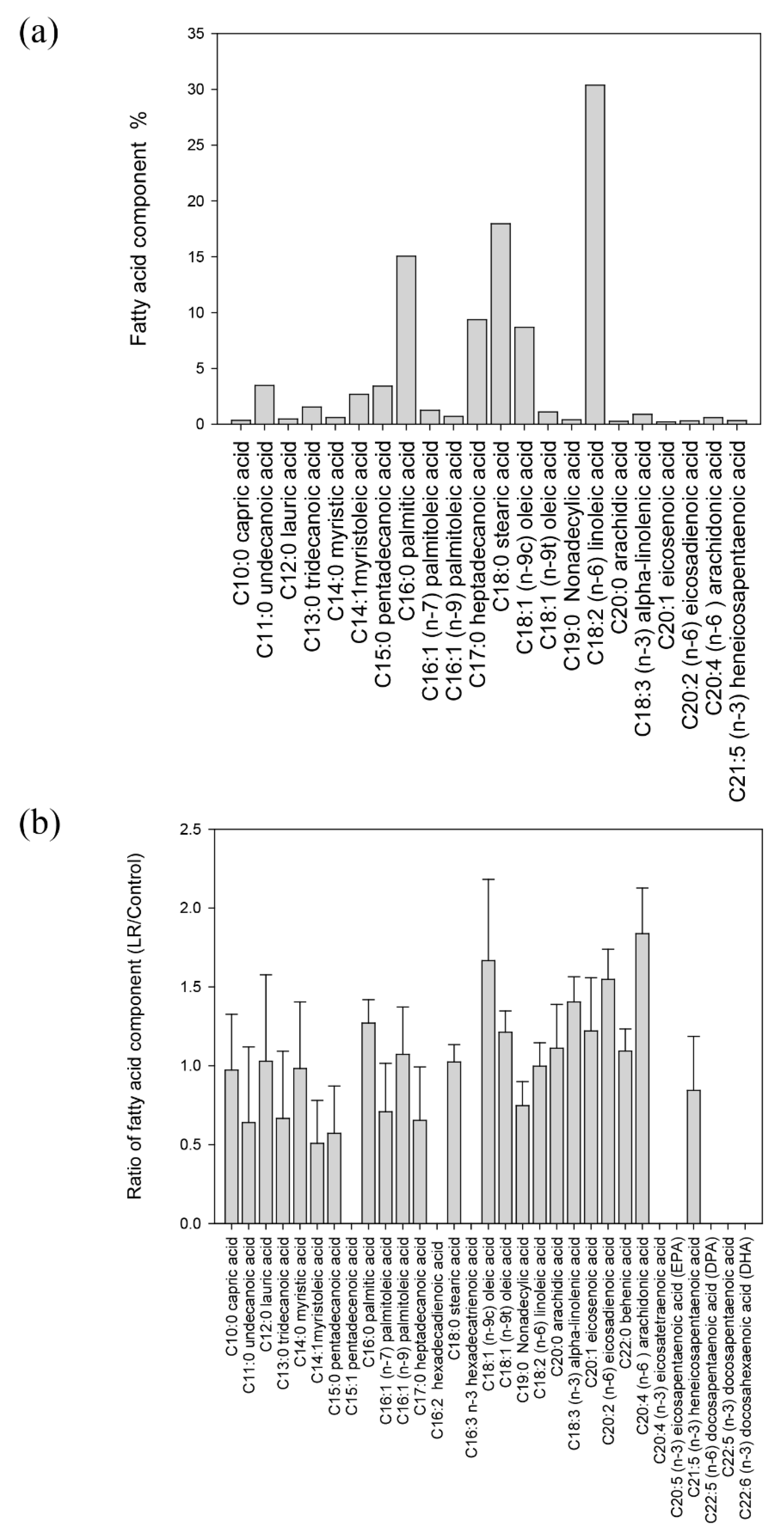
3.6. The Composition of Fatty Acid in Blastocystis ST7-B
3.7. GPDH and G6PD Were Upregulated in LR ST7-B by 2D Electrophoresis and Reverse Transcription PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, K.S. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef]
- Wong, K.H.; Ng, G.C.; Lin, R.T.; Yoshikawa, H.; Taylor, M.B.; Tan, K.S. Predominance of subtype 3 among Blastocystis isolates from a major hospital in Singapore. Parasitol. Res. 2008, 102, 663–670. [Google Scholar] [CrossRef]
- Hirata, T.; Nakamura, H.; Kinjo, N.; Hokama, A.; Kinjo, F.; Yamane, N.; Fujita, J. Prevalence of Blastocystis hominis and Strongyloides stercoralis infection in Okinawa, Japan. Parasitol. Res. 2007, 101, 1717–1719. [Google Scholar] [CrossRef] [PubMed]
- Su, F.H.; Chu, F.Y.; Li, C.Y.; Tang, H.F.; Lin, Y.S.; Peng, Y.J.; Su, Y.M.; Lee, S.D. Blastocystis hominis infection in long-term care facilities in Taiwan: Prevalence and associated clinical factors. Parasitol. Res. 2009, 105, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- El Safadi, D.; Gaayeb, L.; Meloni, D.; Cian, A.; Poirier, P.; Wawrzyniak, I.; Delbac, F.; Dabboussi, F.; Delhaes, L.; Seck, M.; et al. Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect. Dis. 2014, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, A.A.; Canete, R.; Nunez, F.A. Intestinal protozoan and helminth infections in the Municipality San Juan y Martinez, Pinar del Rio, Cuba. Trop. Dr. 2007, 37, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.I.; Goncalves, A.Q.; Sodre, F.C.; Pereira Sdos, R.; Boia, M.N.; de Lemos, E.R.; Daher, R.R. Intestinal protozoa and helminths among Terena Indians in the State of Mato Grosso do Sul: High prevalence of Blastocystis hominis. Rev. Soc. Bras. Med. Trop. 2007, 40, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Basualdo, J.A.; Cordoba, M.A.; de Luca, M.M.; Ciarmela, M.L.; Pezzani, B.C.; Grenovero, M.S.; Minvielle, M.C. Intestinal parasitoses and environmental factors in a rural population of Argentina, 2002–2003. Rev. Inst. Med. Trop. Sao Paulo 2007, 49, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Mirza, H.; Teo, J.D.; Wu, B.; Macary, P.A. Current Views on the Clinical Relevance of Blastocystis spp. Curr. Infect. Dis. Rep. 2010, 12, 28–35. [Google Scholar] [CrossRef]
- Poirier, P.; Wawrzyniak, I.; Vivares, C.P.; Delbac, F.; El Alaoui, H. New insights into Blastocystis spp.: A potential link with irritable bowel syndrome. PLoS Pathog. 2012, 8, e1002545. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Lewis, H.C.; Hammerum, A.M.; Porsbo, L.J.; Nielsen, S.S.; Olsen, K.E.; Arendrup, M.C.; Nielsen, H.V.; Molbak, K. Blastocystis: Unravelling potential risk factors and clinical significance of a common but neglected parasite. Epidemiol. Infect. 2009, 137, 1655–1663. [Google Scholar] [CrossRef]
- Dogruman-Al, F.; Simsek, Z.; Boorom, K.; Ekici, E.; Sahin, M.; Tuncer, C.; Kustimur, S.; Altinbas, A. Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples, and also in IBS/IBD Patients in Ankara, Turkey. PLoS ONE 2010, 5, e15484. [Google Scholar] [CrossRef]
- Olivo-Diaz, A.; Romero-Valdovinos, M.; Gudino-Ramirez, A.; Reyes-Gordillo, J.; Jimenez-Gonzalez, D.E.; Ramirez-Miranda, M.E.; Martinez-Flores, W.A.; Martinez-Hernandez, F.; Flisser, A.; Maravilla, P. Findings related to IL-8 and IL-10 gene polymorphisms in a Mexican patient population with irritable bowel syndrome infected with Blastocystis. Parasitol. Res. 2012, 111, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Guttman, J.A.; Finlay, B.B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 2009, 1788, 832–841. [Google Scholar] [CrossRef]
- Gavard, J.; Gutkind, J.S. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J. Biol. Chem. 2008, 283, 29888–29896. [Google Scholar] [CrossRef]
- Puthia, M.K.; Lu, J.; Tan, K.S. Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human intestinal epithelial cells in an NF-kappaB-dependent manner. Eukaryot. Cell 2008, 7, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Dagci, H.; Ustun, S.; Taner, M.S.; Ersoz, G.; Karacasu, F.; Budak, S. Protozoon infections and intestinal permeability. Acta Trop. 2002, 81, 1–5. [Google Scholar] [CrossRef]
- Mirza, H.; Wu, Z.; Teo, J.D.; Tan, K.S. Statin pleiotropy prevents rho kinase-mediated intestinal epithelial barrier compromise induced by Blastocystis cysteine proteases. Cell. Microbiol. 2012, 14, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Nourrisson, C.; Wawrzyniak, I.; Cian, A.; Livrelli, V.; Viscogliosi, E.; Delbac, F.; Poirier, P. On Blastocystis secreted cysteine proteases: A legumain-activated cathepsin B increases paracellular permeability of intestinal Caco-2 cell monolayers. Parasitology 2016, 143, 1713–1722. [Google Scholar] [CrossRef]
- Vallochi, A.L.; Teixeira, L.; Oliveira, K.D.S.; Maya-Monteiro, C.M.; Bozza, P.T. Lipid Droplet, a Key Player in Host-Parasite Interactions. Front. Immunol. 2018, 9, 1022. [Google Scholar] [CrossRef]
- Araujo-Santos, T.; Rodriguez, N.E.; Moura-Pontes, S.; Dixt, U.G.; Abanades, D.R.; Bozza, P.T.; Wilson, M.E.; Borges, V.M. Role of prostaglandin F2alpha production in lipid bodies from Leishmania infantum chagasi: Insights on virulence. J. Infect. Dis. 2014, 210, 1951–1961. [Google Scholar] [CrossRef]
- Toledo, D.A.; D’Avila, H.; Melo, R.C. Host Lipid Bodies as Platforms for Intracellular Survival of Protozoan Parasites. Front. Immunol. 2016, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Ben Mamoun, C.; Prigge, S.T.; Vial, H. Targeting the Lipid Metabolic Pathways for the Treatment of Malaria. Drug Dev. Res. 2010, 71, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Ekland, E.H.; Ruggles, K.V.; Chan, R.B.; Jayabalasingham, B.; Zhou, B.; Mantel, P.Y.; Lee, M.C.; Spottiswoode, N.; Coburn-Flynn, O.; et al. Profiling the Essential Nature of Lipid Metabolism in Asexual Blood and Gametocyte Stages of Plasmodium falciparum. Cell Host Microbe 2015, 18, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Parton, R.G. Lipid droplets: A unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 2006, 7, 373–378. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, L.; Zhang, S.; Wang, Y.; Du, Y.; Pu, J.; Peng, G.; Chen, Y.; Zhang, H.; Yu, J.; et al. Identification of the major functional proteins of prokaryotic lipid droplets. J. Lipid Res. 2012, 53, 399–411. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Satoh, J.; Enose, Y. Light and electron microscopic localization of lipids in Blastocystis hominis. J. Electron Microsc. 1995, 44, 100–103. [Google Scholar]
- Keenan, T.W.; Zierdt, C.H. Lipid biosynthesis by axenic strains of Blastocystis hominis. Comp. Biochem. Physiol. Biochem. Mol. Biol. 1994, 107, 525–531. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, P.Y.; Liao, J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef]
- Couto, A.S.; Kimura, E.A.; Peres, V.J.; Uhrig, M.L.; Katzin, A.M. Active isoprenoid pathway in the intra-erythrocytic stages of Plasmodium falciparum: Presence of dolichols of 11 and 12 isoprene units. Biochem. J. 1999, 341 Pt 3, 629–637. [Google Scholar] [CrossRef]
- Kapur, N.K.; Musunuru, K. Clinical efficacy and safety of statins in managing cardiovascular risk. Vasc. Health Risk Manag. 2008, 4, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Suresh, G.K.; Tan, K.S.; Thompson, R.C.; Traub, R.J.; Viscogliosi, E.; Yoshikawa, H.; Clark, C.G. Terminology for Blastocystis subtypes—A consensus. Trends Parasitol. 2007, 23, 93–96. [Google Scholar] [CrossRef]
- Poirier, P.; Wawrzyniak, I.; Albert, A.; El Alaoui, H.; Delbac, F.; Livrelli, V. Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: Prospective study of patients with hematological malignancies. J. Clin. Microbiol. 2011, 49, 975–983. [Google Scholar] [CrossRef]
- Huang, K.Y.; Huang, P.J.; Ku, F.M.; Lin, R.; Alderete, J.F.; Tang, P. Comparative transcriptomic and proteomic analyses of Trichomonas vaginalis following adherence to fibronectin. Infect. Immun. 2012, 80, 3900–3911. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Lu, C.K.; Chen, S.F.; Chen, Y.M.; Chen, Y.M. Isolation and characterization of Taiwanese heterotrophic microalgae: Screening of strains for docosahexaenoic acid (DHA) production. Mar. Biotechnol. 2010, 12, 173–185. [Google Scholar] [CrossRef]
- Mirza, H.; Tan, K.S. Blastocystis exhibits inter- and intra-subtype variation in cysteine protease activity. Parasitol. Res. 2009, 104, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Sio, S.W.; Puthia, M.K.; Lee, A.S.; Lu, J.; Tan, K.S. Protease activity of Blastocystis hominis. Parasitol. Res. 2006, 99, 126–130. [Google Scholar] [CrossRef]
- Liao, C.C.; Song, E.J.; Chang, T.Y.; Lin, W.C.; Liu, H.S.; Chen, L.R.; Huang, L.L.; Shin, J.W. Evaluation of cellular retinoic acid binding protein 2 gene expression through the retinoic acid pathway by co-incubation of Blastocystis ST-1 with HT29 cells in vitro. Parasitol. Res. 2016, 115, 1965–1975. [Google Scholar] [CrossRef]
- Wu, Z.; Mirza, H.; Tan, K.S. Intra-subtype variation in enteroadhesion accounts for differences in epithelial barrier disruption and is associated with metronidazole resistance in Blastocystis subtype-7. PLoS Negl. Trop. Dis. 2014, 8, e2885. [Google Scholar] [CrossRef]
- Haresh, K.; Suresh, K.; Khairul Anus, A.; Saminathan, S. Isolate resistance of Blastocystis hominis to metronidazole. Trop. Med. Int. Health 1999, 4, 274–277. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Smith, H.V.; Nagel, R.; Olsen, K.E.; Traub, R.J. Eradication of Blastocystis carriage with antimicrobials: Reality or delusion? J. Clin. Gastroenterol. 2010, 44, 85–90. [Google Scholar] [CrossRef]
- Keenan, T.W.; Huang, C.M.; Zierdt, C.H. Comparative analysis of lipid composition in axenic strains of Blastocystis hominis. Comp. Biochem. Physiol. B 1992, 102, 611–615. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Serricchio, M.; Striepen, B.; Butikofer, P. Lipid synthesis in protozoan parasites: A comparison between kinetoplastids and apicomplexans. Prog. Lipid Res. 2013, 52, 488–512. [Google Scholar] [CrossRef]
- Murphy, D.J. The dynamic roles of intracellular lipid droplets: From archaea to mammals. Protoplasma 2012, 249, 541–585. [Google Scholar] [CrossRef] [PubMed]
- Mbanefo, E.C.; Ahmed, A.M.; Titouna, A.; Elmaraezy, A.; Trang, N.T.; Phuoc Long, N.; Hoang Anh, N.; Diem Nghi, T.; The Hung, B.; Van Hieu, M.; et al. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 45963. [Google Scholar] [CrossRef] [PubMed]
- Lalle, M.; Camerini, S.; Cecchetti, S.; Finelli, R.; Sferra, G.; Muller, J.; Ricci, G.; Pozio, E. The FAD-dependent glycerol-3-phosphate dehydrogenase of Giardia duodenalis: An unconventional enzyme that interacts with the g14-3-3 and it is a target of the antitumoral compound NBDHEX. Front. Microbiol. 2015, 6, 544. [Google Scholar] [CrossRef] [PubMed]
- Toledo, D.A.; Roque, N.R.; Teixeira, L.; Milan-Garces, E.A.; Carneiro, A.B.; Almeida, M.R.; Andrade, G.F.; Martins, J.S.; Pinho, R.R.; Freire-de-Lima, C.G.; et al. Lipid Body Organelles within the Parasite Trypanosoma cruzi: A Role for Intracellular Arachidonic Acid Metabolism. PLoS ONE 2016, 11, e0160433. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.; Dvorak, A.M. Lipid body-phagosome interaction in macrophages during infectious diseases: Host defense or pathogen survival strategy? PLoS Pathog. 2012, 8, e1002729. [Google Scholar] [CrossRef]
- Samuelsson, B. Arachidonic acid metabolism: Role in inflammation. Z. Rheumatol. 1991, 50 (Suppl. S1), 3–6. [Google Scholar]
- Serra, J.; Salvioli, B.; Azpiroz, F.; Malagelada, J.R. Lipid-induced intestinal gas retention in irritable bowel syndrome. Gastroenterology 2002, 123, 700–706. [Google Scholar] [CrossRef]
- Ragavan, N.D.; Kumar, S.; Chye, T.T.; Mahadeva, S.; Shiaw-Hooi, H. Blastocystis sp. in Irritable Bowel Syndrome (IBS)—Detection in Stool Aspirates during Colonoscopy. PLoS ONE 2015, 10, e0121173. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; McKerrow, J.H. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 2002, 120, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Puthia, M.K.; Vaithilingam, A.; Lu, J.; Tan, K.S. Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol. Res. 2005, 97, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Bansal, D.; Bhatti, H.S.; Sehgal, R. Role of cholesterol in parasitic infections. Lipids Health Dis. 2005, 4, 10. [Google Scholar] [CrossRef]
- Kobashigawa, J.A.; Katznelson, S.; Laks, H.; Johnson, J.A.; Yeatman, L.; Wang, X.M.; Chia, D.; Terasaki, P.I.; Sabad, A.; Cogert, G.A.; et al. Effect of pravastatin on outcomes after cardiac transplantation. N. Engl. J. Med. 1995, 333, 621–627. [Google Scholar] [CrossRef]
- Mirza, H.; Teo, J.D.; Upcroft, J.; Tan, K.S. A rapid, high-throughput viability assay for Blastocystis spp. reveals metronidazole resistance and extensive subtype-dependent variations in drug susceptibilities. Antimicrob. Agents Chemother. 2011, 55, 637–648. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-C.; Chen, C.-H.; Shin, J.-W.; Lin, W.-C.; Chen, C.-C.; Chu, C.-T. Lipid Accumulation in Blastocystis Increases Cell Damage in Co-Cultured Cells. Microorganisms 2023, 11, 1582. https://doi.org/10.3390/microorganisms11061582
Liao C-C, Chen C-H, Shin J-W, Lin W-C, Chen C-C, Chu C-T. Lipid Accumulation in Blastocystis Increases Cell Damage in Co-Cultured Cells. Microorganisms. 2023; 11(6):1582. https://doi.org/10.3390/microorganisms11061582
Chicago/Turabian StyleLiao, Chen-Chieh, Chun-Hsien Chen, Jyh-Wei Shin, Wei-Chen Lin, Chien-Chin Chen, and Chun-Ting Chu. 2023. "Lipid Accumulation in Blastocystis Increases Cell Damage in Co-Cultured Cells" Microorganisms 11, no. 6: 1582. https://doi.org/10.3390/microorganisms11061582
APA StyleLiao, C.-C., Chen, C.-H., Shin, J.-W., Lin, W.-C., Chen, C.-C., & Chu, C.-T. (2023). Lipid Accumulation in Blastocystis Increases Cell Damage in Co-Cultured Cells. Microorganisms, 11(6), 1582. https://doi.org/10.3390/microorganisms11061582






