Muricauda okinawensis sp. Nov. and Muricauda yonaguniensis sp. Nov., Two Marine Bacteria Isolated from the Sediment Core near Hydrothermal Fields of Southern Okinawa Trough
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling, Isolation and Maintenance
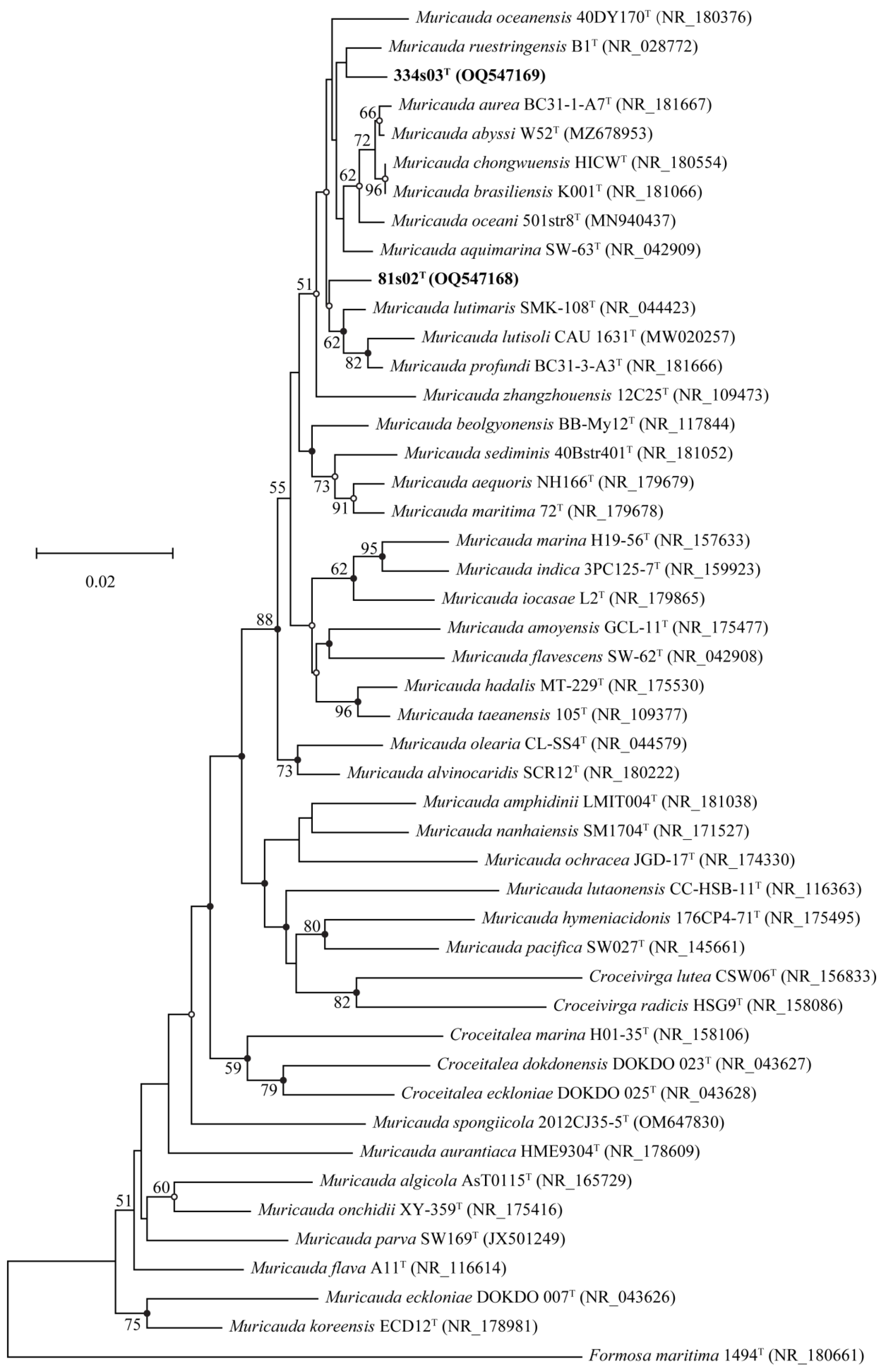
2.2. 16S rRNA Gene Sequence and Phylogeny
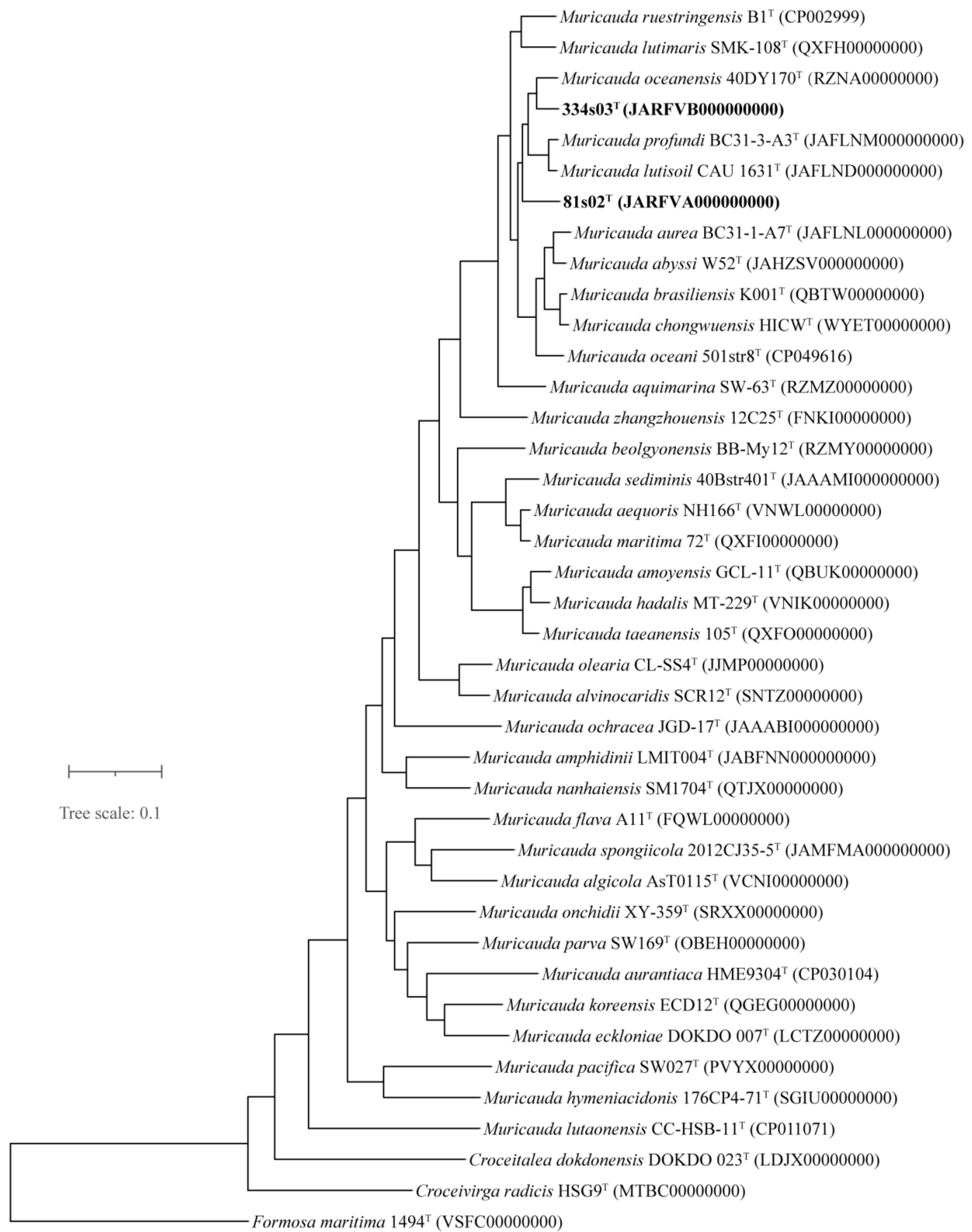
2.3. Genomic Characterization
2.4. Morphological, Physiological and Biochemical Characterization
2.5. Chemotaxonomic Characterization
3. Results and Discussion
3.1. Phylogenetic and Genome Analysis
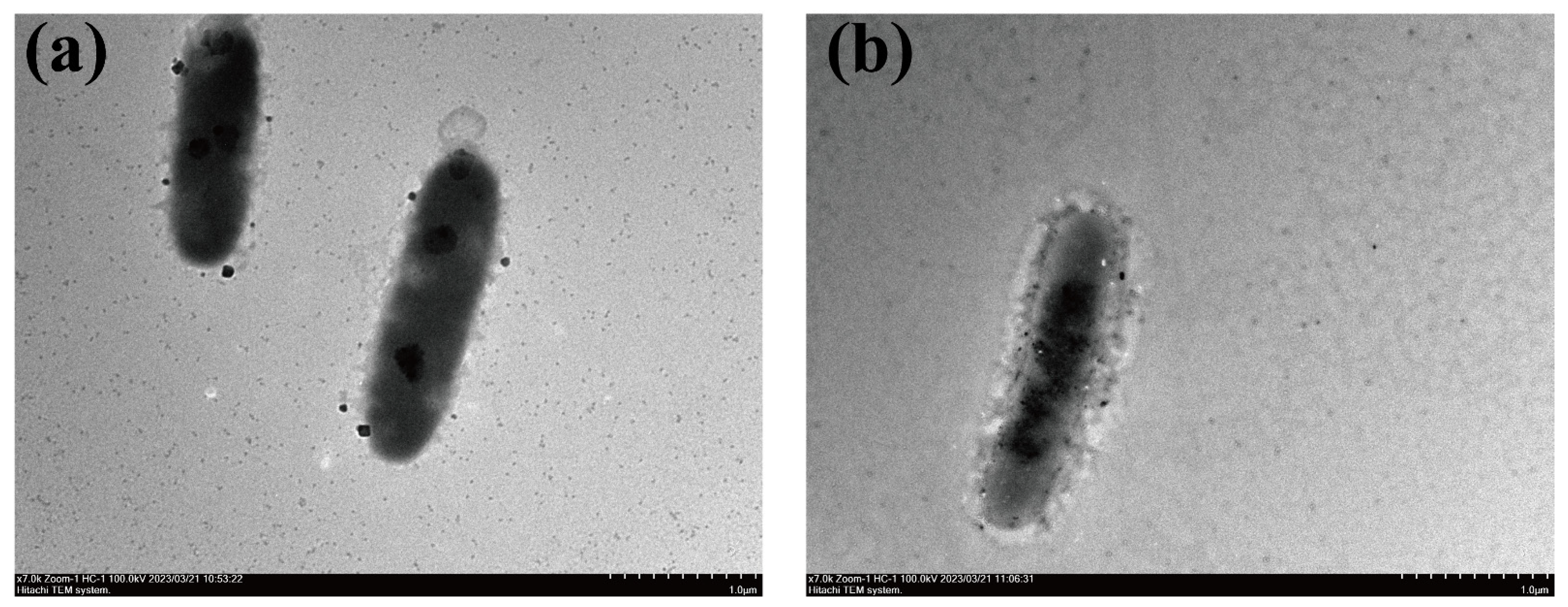
3.2. Morphological, Physiological and Biochemical Characteristics
3.3. Chemotaxonomic Characteristics
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 ♯ | 8 ♯ | 9 ♯ | 10 ♯ |
|---|---|---|---|---|---|---|---|---|---|---|
| Growth at: | ||||||||||
| Temperature Range (°C) | 10–40 | 10–40 | 10–38 * | 10–40 * | 10–44 * | 8–40 * | 15–37 | 15–40 | 15–40 | 8–42 |
| Optimum temperature | 30 | 30–35 | 30 * | 28–32 * | 30–37 * | 20–30 * | 28–33 | 25–30 | 35 | 25–30 |
| pH Range | 6.0–9.5 | 6.0–9.5 | 5.0–8.0 * | 6.0–8.5 * | 6.0–9.0 * | 6.0–9.0 * | 6.0–8.0 | 6.0–8.0 | 5.5–9.0 | 5.5–9.0 |
| Optimum pH | 7.0–7.5 | 7.5 | 7.0–8.0 * | 7.5 * | 7.0 * | 8.0 * | ND | 7 | 6.5–7.0 | 7 |
| NaCl Range (%, w/v) | 0.5–10.0 | 0.5–9.0 | 1.0–10.0 * | 2.0–10.0 * | 0.5–9.0 * | 0.5–9.0 * | 0.5–9.0 | 0.5–8.0 | 0.5–10.0 | 0.5–10.0 |
| Nitrate reduction | − | − | − | + | w | − | − | w | − | − |
| Indole production | − | − | − | + | − | − | − | ND | − | − |
| Hydrolysis of: | ||||||||||
| Arginine | − | − | − | + | − | − | ND | ND | − | + |
| Casein | + | − | + | − | + | + | ND | ND | ND | − |
| Gelatin | − | − | − | + | − | − | − | − | − | − |
| Starch | − | − | − | − | − | − | − | ND | w | − |
| Tween 80 | + | + | − | − | + | + | + | ND | + | − |
| Urease | − | − | − | + | − | − | − | ND | − | + |
| Acid production from: | ||||||||||
| l-Arabinose | − | w | w | + | − | w | ND | ND | + | ND |
| d-Xylose | − | w | w | + | w | + | ND | ND | + | ND |
| d-Galactose | + | w | w | + | w | w | ND | ND | + | ND |
| l-Rhamnose | − | − | − | + | − | − | ND | ND | w | ND |
| N-Acetyl-glucosamine | + | − | w | + | + | w | ND | ND | ND | ND |
| Inulin | − | − | − | − | + | w | ND | ND | ND | ND |
| Glycogen | + | + | w | − | − | − | ND | ND | ND | ND |
| Enzymic activities: | ||||||||||
| Trypsin | + | + | − | + | + | + | + | + | + | + |
| α-Chymotrypsin | − | − | w | + | + | − | + | + | + | − |
| β-Glucuronidase | − | − | + | − | − | − | − | w | + | − |
| N-Acetyl-glucosaminidase | + | − | + | + | + | + | + | + | + | + |
| DNA G+C content (mol%) | 41.6 | 41.9 | 40.1 | 42.1 | 43.4 | 41.4 | 41.6 | 41.4 | 42.4 | 42.8 |
| Fatty Acid | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Straight-chain: | ||||||
| C12:0 | ND | ND | TR | TR | 0.56 | TR |
| C16:0 | 1.95 | 2.07 | 0.74 | 0.93 | 0.54 | 1.25 |
| C18:0 | 0.74 | 0.64 | TR | ND | TR | TR |
| Branched: | ||||||
| iso-C13:0 | TR | TR | 0.79 | 0.84 | 1.07 | 0.81 |
| iso-C14:0 | TR | ND | 2.23 | ND | 4.76 | 5.00 |
| iso-C15:0 | 39.08 | 39.60 | 38.29 | 46.61 | 36.85 | 38.33 |
| iso-C15:1 G | 13.07 | 15.04 | 19.76 | 10.64 | 15.23 | 15.18 |
| iso-C16:0 | 0.77 | 0.53 | 2.43 | 1.35 | 0.68 | 1.00 |
| iso-C17:0 | 0.77 | TR | TR | 1.00 | TR | 0.74 |
| anteiso-C15:0 | 0.87 | 1.97 | 4.16 | 1.37 | 3.66 | 3.55 |
| Unsaturated: | ||||||
| C18:1 ω9c | 0.54 | 0.70 | TR | TR | TR | TR |
| Hydroxy: | ||||||
| C15:0 3-OH | ND | ND | 0.72 | TR | 1.77 | 0.59 |
| C16:0 3-OH | 0.68 | 0.61 | TR | TR | 0.58 | 0.64 |
| C17:0 2-OH | TR | ND | 0.83 | TR | 1.34 | 0.98 |
| C17:0 3-OH | ND | ND | TR | TR | 1.45 | 0.59 |
| iso-C15:0 3-OH | 5.16 | 5.05 | 4.21 | 4.74 | 5.53 | 4.49 |
| iso-C16:0 3-OH | 1.53 | 0.85 | 3.98 | 1.51 | 2.53 | 1.92 |
| iso-C17:0 3-OH | 26.68 | 27.72 | 15.95 | 22.48 | 19.21 | 18.97 |
| Summed features: * | ||||||
| 2 | 0.59 | 0.59 | TR | TR | TR | TR |
| 3 | 2.05 | 2.00 | 1.28 | 1.72 | 0.96 | 1.34 |
| 9 | 1.93 | 0.60 | 0.66 | 1.79 | TR | 0.82 |
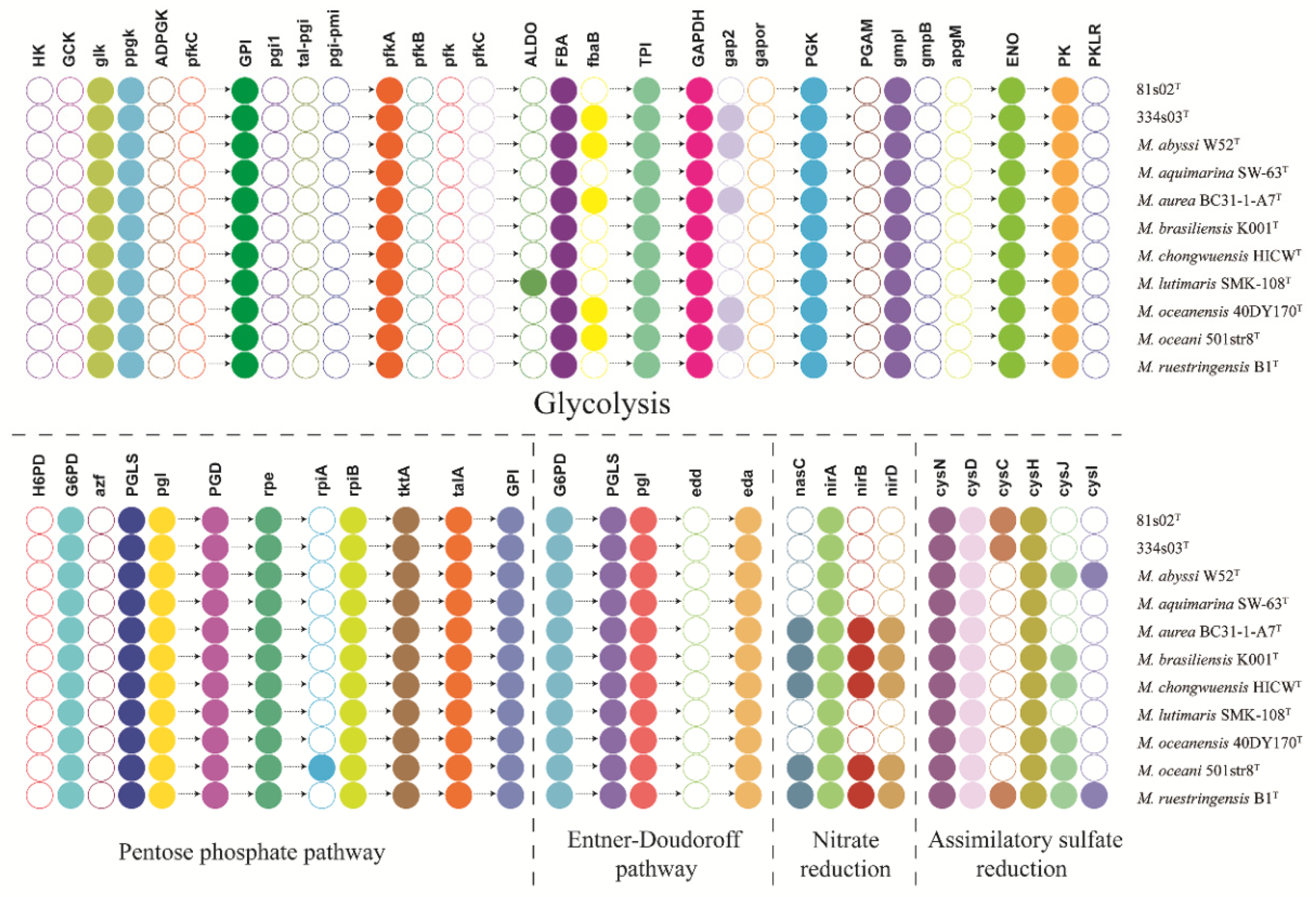
3.4. Genome Attributes and Comparative Genome Analysis
4. Conclusions
4.1. Description of Muricauda okinawensis sp. Nov.
4.2. Description of Muricauda yonaguniensis sp. Nov.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MES | 2-N-Morpholino ethanesulfonic acid hydrate |
| PIPES | Piperazine -1, 4-bis (2-ethanesulfonic acid) |
| HEPES | 2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid |
| CAPSO | Capso sodium salt |
| PNPG | 4-Nitrophenyl β-d-glucopyranoside |
References
- Bruns, A.; Rohde, M.; Berthe-Corti, L. Muricauda ruestringensis gen. nov., sp. nov., a facultatively anaerobic, appendaged bacterium from German North Sea intertidal sediment. Int. J. Syst. Evol. Microbiol. 2001, 51, 1997–2006. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, M.H.; Oh, T.K.; Park, Y.H. Muricauda flavescens sp. nov. and Muricauda aquimarina sp. nov., isolated from a salt lake near Hwajinpo Beach of the East Sea in Korea, and emended description of the genus Muricauda. Int. J. Syst. Evol. Microbiol. 2005, 55, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.Y.; Kim, M.H.; Bae, G.D.; Zhang, G.I.; Kim, Y.H.; Cho, B.C. Muricauda olearia sp. nov., isolated from crude-oil-contaminated seawater, and emended description of the genus Muricauda. Int. J. Syst. Evol. Microbiol. 2009, 59, 1856–1861. [Google Scholar] [CrossRef]
- Liang, J.; Yin, Q.; Zheng, X.; Wang, Y.; Song, Z.M.; Zhang, Y.; Hao, L.; Xu, Y. Muricauda onchidii sp. nov., isolated from a marine invertebrate from South China Sea, and transfers of Flagellimonas algicola, Flagellimonas pacifica and Flagellimonas maritima to Muricauda algicola comb. nov., Muricauda parva nom. nov. and Muricauda aurantiaca nom. nov., respectively, and emended description of the genus Muricauda. Int. J. Syst. Evol. Microbiol. 2021, 71, 004982. [Google Scholar] [CrossRef]
- García-López, M.; Meier-Kolthoff, J.P.; Tindall, B.J.; Gronow, S.; Woyke, T.; Kyrpides, N.C.; Hahnke, R.L.; Göker, M. Analysis of 1,000 type-strain genomes improves taxonomic classification of Bacteroidetes. Front. Microbiol. 2019, 10, 02083. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.H.; Chun, B.H.; Khan, S.A.; Jeon, C.O. Flagellimonas algicola sp. nov., isolated from a marine red alga, Asparagopsis taxiformis. Curr. Microbiol. 2020, 77, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Z.; Dai, X.; Zhang, X.H. Spongiibacterium pacificum sp. nov., isolated from seawater of South Pacific Gyre and emended description of the genus Spongiibacterium. Int. J. Syst. Evol. Microbiol. 2015, 65, 154–158. [Google Scholar] [CrossRef]
- Choi, S.; Lee, J.H.; Kang, J.W.; Choe, H.N.; Seong, C.N. Flagellimonas aquimarina sp. nov., and transfer of Spongiibacterium flavum Yoon and Oh 2012 and S. pacificum Gao et al. 2015 to the genus Flagellimonas Bae et al. 2007 as Flagellimonas flava comb. nov. and F. pacifica comb. nov., respectively. Int. J. Syst. Evol. Microbiol. 2018, 68, 3266–3272. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H.; Cha, I.; Joh, K. Flagellimonas maritima sp. nov., isolated from surface seawater. Int. J. Syst. Evol. Microbiol. 2020, 70, 187–192. [Google Scholar] [CrossRef]
- Bae, S.S.; Kwon, K.K.; Yang, S.H.; Lee, H.S.; Kim, S.J.; Lee, J.H. Flagellimonas eckloniae gen. nov., sp. nov., a mesophilic marine bacterium of the family Flavobacteriaceae, isolated from the rhizosphere of Ecklonia kurome. Int. J. Syst. Evol. Microbiol. 2007, 57, 1050–1054. [Google Scholar] [CrossRef]
- Yoon, B.J.; Oh, D.C. Spongiibacterium flavum gen. nov., sp. nov., a member of the family Flavobacteriaceae isolated from the marine sponge Halichondria oshoro, and emended descriptions of the genera Croceitalea and Flagellimonas. Int. J. Syst. Evol. Microbiol. 2012, 62, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Liu, J.; Wu, Y.; Zhang, X.H. Muricauda lutea sp. nov., isolated from seawater. Int. J. Syst. Evol. Microbiol. 2017, 67, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Y.; Chen, X.; Liu, D.; Song, H.; Liu, J.; Du, Z.J. Croceivirga litoralis sp. nov., isolated from coastal surface water, and reclassification of Muricauda lutea as Croceivirga lutea comb. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 6348–6354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, X.; Qiao, Y.; Wang, Y.; Zhang, X.H. Muricauda pacifica sp. nov., isolated from seawater of the South Pacific Gyre. Int. J. Syst. Evol. Microbiol. 2015, 65, 4087–4092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Lai, Q.; Du, Y.; Sun, F.; Shao, Z. Muricauda indica sp. nov., isolated from deep sea water. Int. J. Syst. Evol. Microbiol. 2018, 68, 881–885. [Google Scholar] [CrossRef]
- Dang, Y.R.; Sun, Y.Y.; Sun, L.L.; Yuan, X.X.; Li, Y.; Qin, Q.L.; Chen, X.L.; Zhang, Y.Z.; Shi, M.; Zhang, X.Y. Muricauda nanhaiensis sp. nov., isolated from seawater of the South China Sea. Int. J. Syst. Evol. Microbiol. 2019, 69, 2089–2094. [Google Scholar] [CrossRef]
- Wang, D.; Wu, Y.; Liu, Y.; Liu, B.; Gao, Y.; Yang, Y.; Zhang, Y.; Liu, C.; Huo, Y.; Tang, A.; et al. Muricauda abyssi sp. nov., a marine bacterium isolated from deep seawater of the Mariana Trench. Int. J. Syst. Evol. Microbiol. 2022, 72, 005615. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kang, S.J.; Jung, Y.T.; Oh, T.K. Muricauda lutimaris sp. nov., isolated from a tidal flat of the Yellow Sea. Int. J. Syst. Evol. Microbiol. 2008, 58, 1603–1607. [Google Scholar] [CrossRef]
- Kim, J.M.; Jin, H.M.; Jeon, C.O. Muricauda taeanensis sp. nov., isolated from a marine tidal flat. Int. J. Syst. Evol. Microbiol. 2013, 63, 2672–2677. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, Y.; Khim, J.S.; Yang, D.; Pathiraja, D.; Choi, I.G.; Kim, J.J. Muricauda ochracea sp. nov., isolated from a tidal flat in the Republic of Korea. Int. J. Syst. Evol. Microbiol. 2020, 70, 4555–4561. [Google Scholar] [CrossRef]
- Li, G.; Lai, Q.; Yan, P.; Shao, Z. Roseovarius amoyensis sp. nov. and Muricauda amoyensis sp. nov., isolated from the Xiamen coast. Int. J. Syst. Evol. Microbiol. 2019, 69, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Y.; Guo, Q.; Lai, Q.; Wei, J.; Zheng, T.; Tian, Y. Muricauda zhangzhouensis sp. nov., isolated from mangrove sediment. Int. J. Syst. Evol. Microbiol. 2013, 63, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Sun, Q.L.; Sun, Y.Y.; Yu, C.; Sun, L. Muricauda iocasae sp. nov., isolated from deep sea sediment of the South China Sea. Int. J. Syst. Evol. Microbiol. 2018, 68, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Zhu, S.; Chen, T.; Ren, N.; Chen, X.; Chen, Y.; Xue, Z.; Shen, X.; Huang, Y.; Yang, J.; et al. Muricauda oceani sp. nov., isolated from the East Pacific Ocean. Int. J. Syst. Evol. Microbiol. 2020, 70, 3839–3844. [Google Scholar] [CrossRef]
- Zhu, S.; Xue, Z.; Huang, Y.; Chen, X.; Ren, N.; Chen, T.; Chen, Y.; Yang, J.; Chen, J. Muricauda sediminis sp. nov., isolated from western Pacific Ocean sediment. Int. J. Syst. Evol. Microbiol. 2019, 71, 004757. [Google Scholar] [CrossRef]
- Park, J.S. Muricauda hymeniacidonis sp. nov., isolated from sponge of Hymeniacidon sinapium. Int. J. Syst. Evol. Microbiol. 2019, 69, 3800–3805. [Google Scholar] [CrossRef]
- Liu, L.; Yu, M.; Zhou, S.; Fu, T.; Sun, W.; Wang, L.; Zhang, X.H. Muricauda alvinocaridis sp. nov., isolated from shrimp gill from the Okinawa Trough. Int. J. Syst. Evol. Microbiol. 2020, 70, 1666–1671. [Google Scholar] [CrossRef]
- Shin, J.Y.; Park, J.S. Muricauda spongiicola sp. nov., isolated from the sponge Callyspongia elongata. Int. J. Syst. Evol. Microbiol. 2023, 73, 005702. [Google Scholar] [CrossRef]
- Takai, K.; Nakagawa, S.; Nunoura, T. Comparative investigation of microbial communities associated with hydrothermal activities in the Okinawa Trough. In Subseafloor Biosphere Linked to Hydrothermal Systems: TAIGA Concept; Ishibashi, J., Okino, K., Sunamura, M., Eds.; Springer: Tokyo, Japan, 2015; pp. 421–435. [Google Scholar]
- Takai, K.; Nakamura, K. Compositional, physiological and metabolic variability in microbial communities associated with geochemically diverse, deep-sea hydrothermal vent fluids. In Geomicrobiology: Molecular and Environmental Perspective; Barton, L.L., Mandl, M., Loy, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 251–283. [Google Scholar]
- Inagaki, F.; Kuypers, M.M.; Tsunogai, U.; Ishibashi, J.; Nakamura, K.; Treude, T.; Ohkubo, S.; Nakaseama, M.; Gena, K.; Chiba, H.; et al. Microbial community in a sediment-hosted CO2 lake of the southern Okinawa Trough hydrothermal system. Proc. Natl. Acad. Sci. USA 2006, 103, 14164–14169. [Google Scholar] [CrossRef]
- Nakagawa, S.; Takai, K.; Inagaki, F.; Hirayama, H.; Nunoura, T.; Horikoshi, K.; Sako, Y. Distribution, phylogenetic diversity and physiological characteristics of epsilon-Proteobacteria in a deep-sea hydrothermal field. Environ. Microbiol. 2005, 7, 1619–1632. [Google Scholar] [CrossRef]
- Yanagawa, K.; Breuker, A.; Schippers, A.; Nishizawa, M.; Ijiri, A.; Hirai, M.; Takaki, Y.; Sunamura, M.; Urabe, T.; Nunoura, T.; et al. Microbial community stratification controlled by the subseafloor fluid flow and geothermal gradient at the Iheya North hydrothermal field in the Mid-Okinawa Trough (Integrated Ocean Drilling Program Expedition 331). Appl. Environ. Microbiol. 2014, 80, 6126–6135. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, K.; Ijiri, A.; Breuker, A.; Sakai, S.; Miyoshi, Y.; Kawagucci, S.; Noguchi, T.; Hirai, M.; Schippers, A.; Ishibashi, J.; et al. Defining boundaries for the distribution of microbial communities beneath the sediment-buried, hydrothermally active seafloor. ISME J. 2017, 11, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, K.; Morono, Y.; Beer, D.; Haeckel, M.; Sunamura, M.; Futagami, T.; Hoshino, T.; Terada, T.; Nakamura, K.; Urabe, T.; et al. Metabolically active microbial communities in marine sediment under high-CO2 and low-pH extremes. ISME J. 2013, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, M.; Liu, Y.; Liu, J.; Wu, Y.; Li, L.; Liu, J.; Wang, M.; Zhang, X.H. Comparative analyses of the bacterial community of hydrothermal deposits and seafloor sediments across Okinawa Trough. J. Mar. Syst. 2018, 180, 162–172. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Q.L.; Zeng, Z.G.; Chen, S.; Sun, L. Microbial diversity in the deep-sea sediments of Iheya North and Iheya Ridge, Okinawa Trough. Microbiol. Res. 2015, 177, 43–52. [Google Scholar] [CrossRef]
- Cao, W.; Wang, L.; Saren, G.; Yu, X.; Li, Y. Variable microbial communities in the non-hydrothermal sediments of the Mid-Okinawa Trough. Geomicrobiol. J. 2020, 37, 774–782. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Cao, W.R.; Lu, D.C.; Sun, X.K.; Sun, Y.Y.; Saren, G.; Yu, X.K.; Du, Z.J. Seonamhaeicola maritimus sp. nov., isolated from coastal sediment. Int. J. Syst. Evol. Microbiol. 2020, 70, 902–908. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Fitch, W.M. On the problem of discovering the most parsimonious tree. Am. Nat. 1977, 111, 223–257. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, R.; Zhu, H.; Ruan, J.; Qian, W.; Fang, X.; Shi, Z.; Li, Y.; Li, S.; Shan, G.; Kristiansen, K.; et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010, 20, 265–272. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid Annotations using Subsystems Technology. BMC Genomics 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef] [PubMed]
- Drula, E.; Garron, M.L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Glöckner, F.O.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef]
- Chun, J.; Rainey, F.A. Integrating genomics into the taxonomy and systematics of the Bacteria and Archaea. Int. J. Syst. Evol. Microbiol. 2014, 64, 316–324. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Waite, D.W.; Rinke, C.; Skarshewski, A.; Chaumeil, P.A.; Hugenholtz, P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 2018, 36, 996–1004. [Google Scholar] [CrossRef]
- Chaumeil, P.A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 2020, 36, 1925–1927. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Bowman, J.P. Description of Cellulophaga algicola sp. nov., isolated from the surfaces of Antarctic algae, and reclassification of Cytophaga uliginosa (ZoBell and Upham 1944) Reichenbach 1989 as Cellulophaga uliginosa comb. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 1861–1868. [Google Scholar] [CrossRef]
- Smibert, R.M.; Krieg, N.R. Phenotypic characteristics. In Methods for General and Molecular Biology; Gerhardt, P., Murray, R.G.E., Wood, W.A., Krieg, N.R., Eds.; American Society for Microbiology: Washington, DC, USA, 1994; pp. 607–654. [Google Scholar]
- Jorgensen, J.H.; Turnidge, J.D.; Washington, J.A. Antibacterial susceptibility tests: Dilution and disk diffusion methods. In Manual of Clinical Microbiology; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; American Society for Microbiology: Washington, DC, USA, 1999; pp. 1526–1543. [Google Scholar]
- Hiraishi, A.; Ueda, Y.; Ishihara, J.; Mori, T. Comparative lipoquinone analysis of influent sewage and activated sludge by high-performance liquid chromatography and photodiode array detection. J. Gen. Appl. Microbiol. 1996, 42, 457–469. [Google Scholar] [CrossRef]
- Minnikin, D.E.; O’Donnell, A.G.; Goodfellow, M.; Alderson, G.; Athalye, M.; Schaal, A.; Parlett, J.H. An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J. Microbiol. Meth. 1984, 2, 233–241. [Google Scholar] [CrossRef]
- Kroppenstedt, R.M. Separation of bacterial menaquinones by HPLC using reverse phase (RP18) and a silver loaded ion exchanger as stationary phases. J. Liq. Chromatogr. 1982, 5, 2359–2367. [Google Scholar] [CrossRef]
- Tindall, B.J.; Sikorski, J.; Smibert, R.A.; Krieg, N.R. Phenotypic characterization and the principles of comparative systematics. In Methods for General and Molecular Microbiology, 3rd ed.; Reddy, C.A., Beveridge, T.J., Breznak, J.A., Marzluf, G.A., Schmidt, T.M., Snyder, L.R., Eds.; American Society for Microbiology: Washington, DC, USA, 2007; pp. 330–393. [Google Scholar]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; MIDI Technical Note #101; MIDI, Inc.: Newark, DE, USA, 2001. [Google Scholar]
- Michael, R.; Rosselló-Móra, R. Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. USA 2009, 106, 19126–19131. [Google Scholar] [CrossRef]
- Chen, M.X.; He, X.Y.; Li, H.Y. Muricauda chongwuensis sp. nov., isolated from coastal seawater of China. Arch. Microbiol. 2021, 203, 6245–6252. [Google Scholar] [CrossRef]
- Vizzotto, C.S.; Peixoto, J.; Green, S.J.; Lopes, F.A.C.; Ramada, M.H.S.; Júnior, O.R.P.; Pinto, O.H.B.; Tótola, M.R.; Thompson, F.L.; Krüger, R.H. Muricauda brasiliensis sp. nov., isolated from a mat-forming cyanobacterial culture. Braz. J. Microbiol. 2021, 52, 325–333. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, R.; Lai, Q.; Shao, Z. Muricauda aurea sp. nov. and Muricauda profundi sp. nov., two marine bacteria isolated from deep sea sediment of Pacific Ocean. Int. J. Syst. Evol. Microbiol. 2022, 72, 005217. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Wu, D.; Sun, C.; Cheng, H.; Xu, X.W.; Wu, M.; Wu, Y.H. Muricauda maritima sp. nov., Muricauda aequoris sp. nov. and Muricauda oceanensis sp. nov., three marine bacteria isolated from seawater. Int. J. Syst. Evol. Microbiol. 2020, 70, 6240–6250. [Google Scholar] [CrossRef]
- Takahashi, M.; Sasaki, Y.; Ida, S.; Morikawa, H. Nitrite reductase gene enrichment improves assimilation of NO2 in Arabidopsis. Plant. Physiol. 2001, 126, 731–741. [Google Scholar] [CrossRef]
- Benini, S. Carbohydrate-active enzymes: Structure, activity, and reaction products. Int. J. Mol. Sci. 2020, 21, 2727. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Deng, X.; Jiang, M.; Zeng, Z.; Chang, F. Muricauda okinawensis sp. Nov. and Muricauda yonaguniensis sp. Nov., Two Marine Bacteria Isolated from the Sediment Core near Hydrothermal Fields of Southern Okinawa Trough. Microorganisms 2023, 11, 1580. https://doi.org/10.3390/microorganisms11061580
Cao W, Deng X, Jiang M, Zeng Z, Chang F. Muricauda okinawensis sp. Nov. and Muricauda yonaguniensis sp. Nov., Two Marine Bacteria Isolated from the Sediment Core near Hydrothermal Fields of Southern Okinawa Trough. Microorganisms. 2023; 11(6):1580. https://doi.org/10.3390/microorganisms11061580
Chicago/Turabian StyleCao, Wenrui, Xingyu Deng, Mingyu Jiang, Zhigang Zeng, and Fengming Chang. 2023. "Muricauda okinawensis sp. Nov. and Muricauda yonaguniensis sp. Nov., Two Marine Bacteria Isolated from the Sediment Core near Hydrothermal Fields of Southern Okinawa Trough" Microorganisms 11, no. 6: 1580. https://doi.org/10.3390/microorganisms11061580
APA StyleCao, W., Deng, X., Jiang, M., Zeng, Z., & Chang, F. (2023). Muricauda okinawensis sp. Nov. and Muricauda yonaguniensis sp. Nov., Two Marine Bacteria Isolated from the Sediment Core near Hydrothermal Fields of Southern Okinawa Trough. Microorganisms, 11(6), 1580. https://doi.org/10.3390/microorganisms11061580







