Nannochloris sp. Microalgae Strain for Treatment of Dairy Wastewaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese Whey Preparation for Inoculum and Characterization
2.2. Microalgae Cultivation and Growth Conditions
2.3. Effect of Stress Factors on Biomass Productivity and Biocompound Accumulation
3. Results
3.1. Cheese Whey Characterization
3.2. Growth Medium Analysis after Microalgae Cultivation
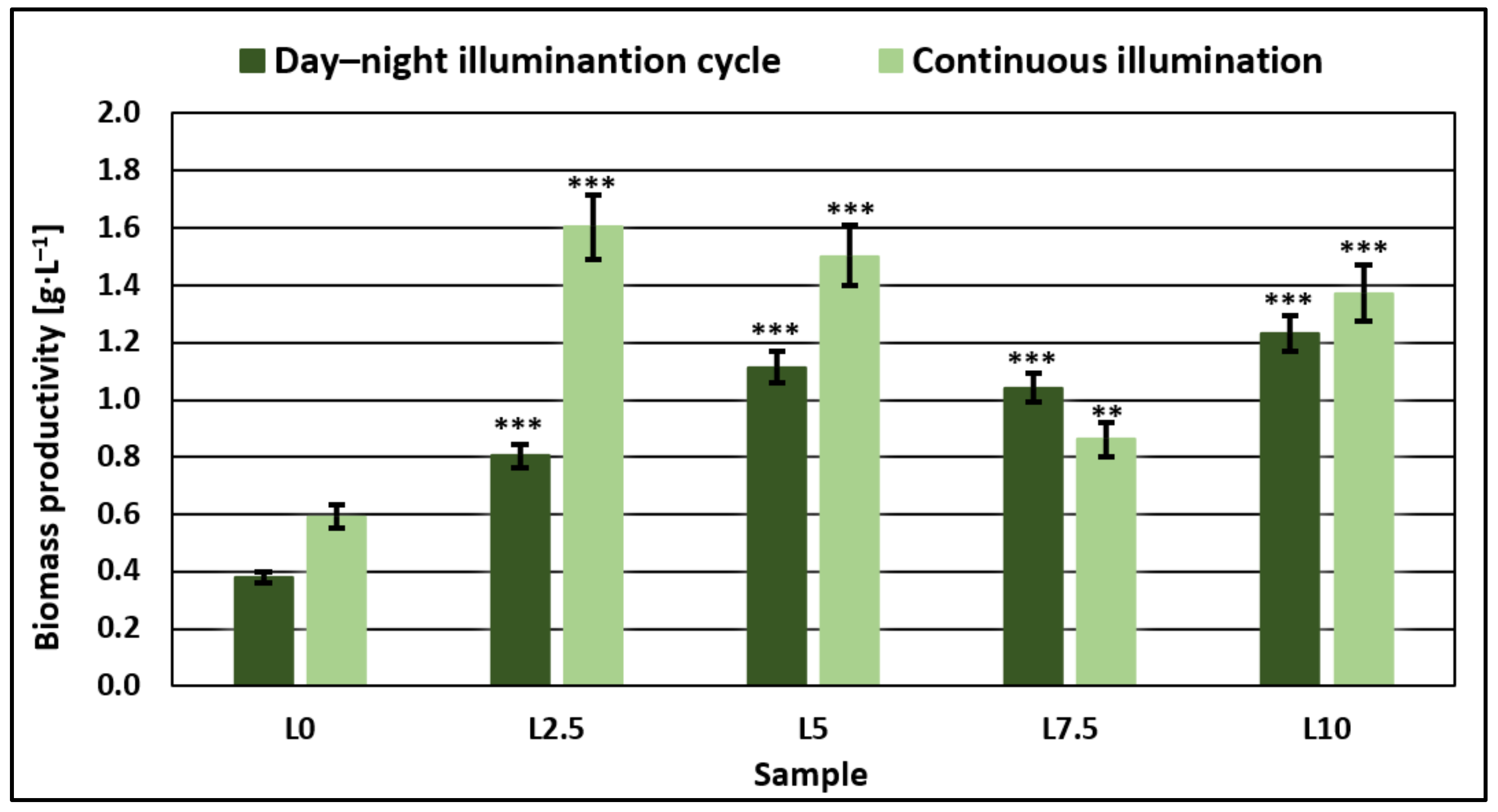
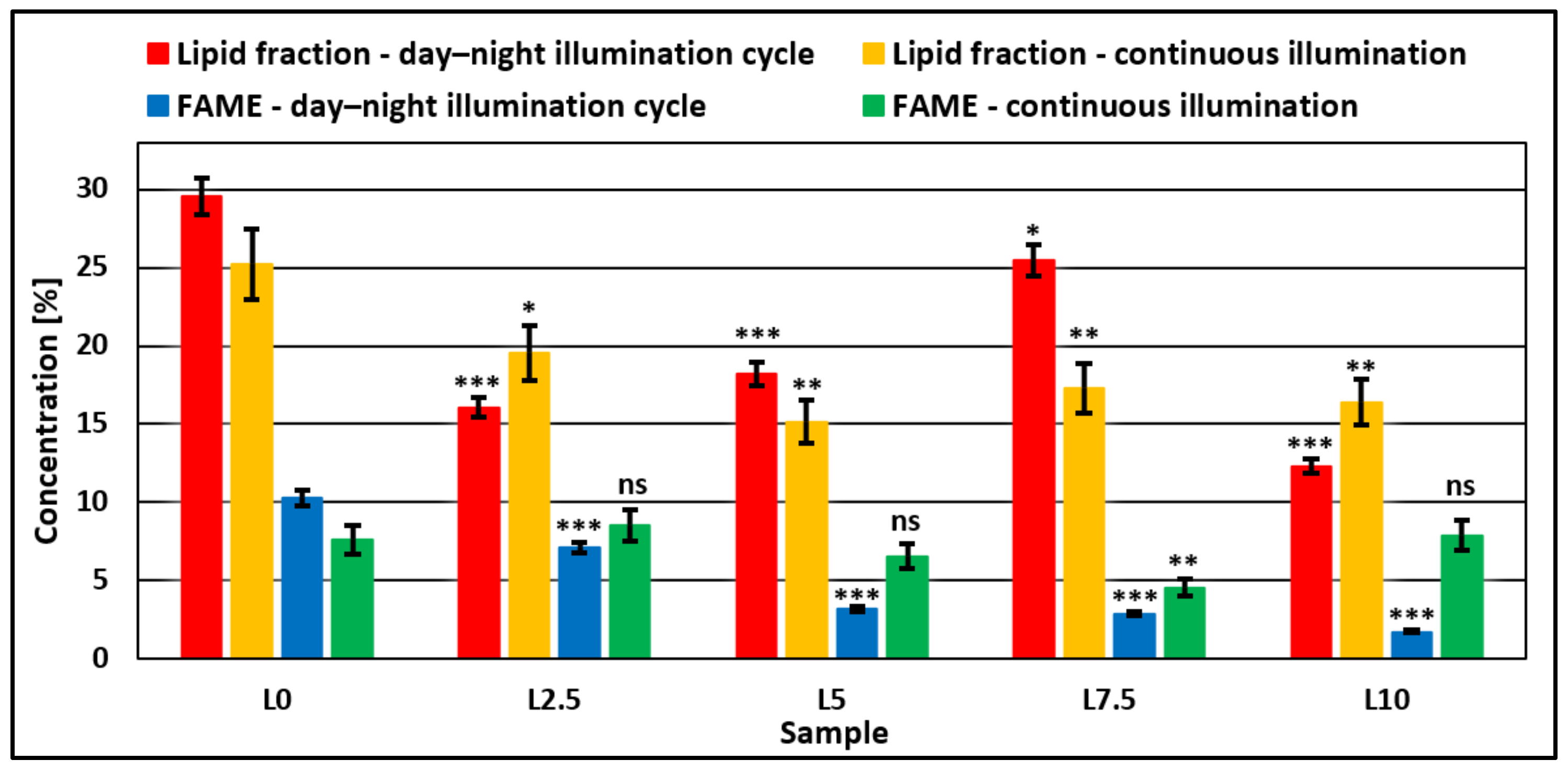
3.3. Microalgae Biomass Productivity and Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- El-Dalatony, M.M.; Salama, E.-S.; Kurade, M.B.; Hassan, S.H.A.; Oh, S.-E.; Kim, S.; Jeon, B.-H. Utilization of Microalgal Biofractions for Bioethanol, Higher Alcohols, and Biodiesel Production: A Review. Energies 2017, 10, 2110. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae—A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Darda, S.; Papalas, T.; Zabaniotou, A. Biofuels journey in Europe: Currently the way to low carbon economy sustainability is still a challenge. J. Clean. Prod. 2019, 208, 575–588. [Google Scholar] [CrossRef]
- Stepan, E.; Enascuta, C.-E.; Oprescu, E.-E.; Radu, E.; Radu, A.; Galan, A.-M.; Vasilievici, G.; Lavric, V.; Velea, S. Intermediates for synthetic paraffinic kerosene from microalgae. Fuel 2016, 172, 29–36. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O.; Imanah, O.E.; Ndibe, H. Biofuels from microalgae biomass: A review of conversion processes and procedures. Arab. J. Chem. 2022, 15, 103591. [Google Scholar] [CrossRef]
- Kothari, R.; Tyagi, V.; Pathak, A. Waste-to-energy: A way from renewable energy sources to sustainable development. Renew. Sustain. Energy Rev. 2010, 14, 3164–3170. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Yunus Khan, T.M.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ejeromedoghene, O.; Okoye, C.O.; Ezeorba, T.P.C.; Nyaruaba, R.; Ikechukwu, C.K.; Oladipo, A.; Orege, J.I. Microalgae biorefinery: An integrated route for the sustainable production of high-value-added products. Energy Convers. Manag. X 2022, 16, 100323. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. Green fuel production: Processes applied to microalgae. Environ. Chem. Lett. 2013, 11, 315–324. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, A.; Zhang, D.; Li, L. Comparison of cell rupturing by ozonation and ultrasonication for algal lipid extraction from Chlorella vulgaris. Environ. Technol. 2014, 35, 931–937. [Google Scholar] [CrossRef]
- Singh, B.; Guldhe, A.; Rawat, I.; Bux, F. Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew. Sustain. Energy Rev. 2014, 29, 216–245. [Google Scholar] [CrossRef]
- Zhu, L. Microalgal culture strategies for biofuel production: A review. Biofuels Bioprod. Biorefining 2015, 9, 801–814. [Google Scholar] [CrossRef]
- Benemann, J.R.; Woertz, I.; Lundquist, T. Autotrophic Microalgae Biomass Production: From Niche Markets to Commodities. Ind. Biotechnol. 2018, 14, 3–10. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnology 2023, 13, 100205. [Google Scholar] [CrossRef]
- Biswas, T.; Chatterjee, D.; Barman, S.; Chakraborty, A.; Halder, N.; Banerjee, S.; Raychaudhuri, S. Cultivable Bacterial Community Analysis of Dairy Activated Sludge for Value Addition to Dairy Wastewater. Microbiol. Biotechnol. Lett. 2019, 47, 585–595. [Google Scholar] [CrossRef]
- Gupta, S.K.; Ansari, F.A.; Nasr, M.; Rawat, I.; Nayunigari, M.K.; Bux, F. Cultivation of Chlorella sorokiniana and Scenedesmus obliquus in wastewater: Fuzzy intelligence for evaluation of growth parameters and metabolites extraction. J. Clean. Prod. 2017, 147, 419–430. [Google Scholar] [CrossRef]
- Hoh, D.; Watson, S.; Kan, E. Algal biofilm reactors for integrated wastewater treatment and biofuel production: A review. Chem. Eng. J. 2016, 287, 466–473. [Google Scholar] [CrossRef]
- Nayak, J.K.; Ghosh, U.K. Post treatment of microalgae treated pharmaceutical wastewater in photosynthetic microbial fuel cell (PMFC) and biodiesel production. Biomass-Bioenergy 2019, 131, 105415. [Google Scholar] [CrossRef]
- Choi, H.-J. Dairy wastewater treatment using microalgae for potential biodiesel application. Environ. Eng. Res. 2016, 21, 393–400. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Z.; Wang, X.; Yuan, Z. Cultivation of Chlorella sp. using raw dairy wastewater for nutrient removal and biodiesel production: Characteristics comparison of indoor bench-scale and outdoor pilot-scale cultures. Bioresour. Technol. 2015, 192, 382–388. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Pareek, N. Comparative Appraisal of Biomass Production, Remediation, and Bioenergy Generation Potential of Microalgae in Dairy Wastewater. Front. Microbiol. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Kiani, H.; Azimi, Y.; Li, Y.; Mousavi, M.; Cara, F.; Mulcahy, S.; McDonnell, H.; Blanco, A.; Halim, R. Nitrogen and phosphate removal from dairy processing side-streams by monocultures or consortium of microalgae. J. Biotechnol. 2023, 361, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mercado, I.; Álvarez, X.; Verduga, M.-E.; Cruz, A. Enhancement of Biomass and Lipid Productivities of Scenedesmus sp. Cultivated in the Wastewater of the Dairy Industry. Processes 2020, 8, 1458. [Google Scholar] [CrossRef]
- Gălan, A.-M.; Vlaicu, A.; Vintilă, A.C.N.; Cîlţea-Udrescu, M.; Cerchezan, G.; Frone, A.N.; Vasilievici, G.; Paulenco, A. Microalgae Strain Porphyridium purpureum for Nutrient Reduction in Dairy Wastewaters. Sustainability 2022, 14, 8545. [Google Scholar] [CrossRef]
- Karimian, A.; Mahdavi, M.A.; Gheshlaghi, R. Algal cultivation strategies for enhancing production of Chlorella sorokiniana IG-W-96 biomass and bioproducts. Algal Res. 2022, 62, 102630. [Google Scholar] [CrossRef]
- Santos, L.F.d.; Gonçalves, C.M.; Ishii, P.L.; Suguimoto, H.H. Deproteinization: An integrated-solution approach to increase efficiency in β-galactosidase production using cheese whey powder (CWP) solution. Rev. Ambiente Água 2017, 12, 643–651. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Oancea, F.; Velea, S.; Stepan, E.; Ilie, L. Nannochloris sp. Strain for Obtainment of Bio-Based Aviation Fuel. WO2015072873A1, 21 May 2015. [Google Scholar]
- Mishra, P.; Prasad, S.M. Evaluation of anticandidal activities of Spirulina metabolite against Candida albicance. Int. J. Pharmacol. Sci. Res. 2015, 6, 1000–1007. [Google Scholar]
- Galan (Popescu), A.M. Natural Oils and Polyphenolic Compounds for Use as Dietary Supplements. Ph.D. Thesis, University Politehnica of Bucharest, Bucharest, Romania, 2017. [Google Scholar]
- Ji, B.; Wang, S.; Silva, M.R.U.; Zhang, M.; Liu, Y. Microalgal-bacterial granular sludge for municipal wastewater treatment under simulated natural diel cycles: Performances-metabolic pathways-microbial community nexus. Algal Res. 2021, 54, 102198. [Google Scholar] [CrossRef]
- Xiong, J.-Q.; Kurade, M.B.; Kim, J.R.; Roh, H.-S.; Jeon, B.-H. Ciprofloxacin toxicity and its co-metabolic removal by a freshwater microalga Chlamydomonas mexicana. J. Hazard. Mater. 2017, 323, 212–219. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Galan, A.-M.; Calinescu, I.; Radu, E.; Oprescu, E.E.; Vasilievici, G.; Velea, S. Development of a New Method for Determination of the Oil Content from Microalgae Lipid Fraction. Rev. de Chim. 2017, 68, 671–674. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M. Influence of Nutrient-Stress Conditions on Chlorella vulgaris Biomass Production and Lipid Content. Catalysts 2021, 11, 573. [Google Scholar] [CrossRef]
- Chang, J.; Le, K.; Song, X.; Jiao, K.; Zeng, X.; Ling, X.; Shi, T.; Tang, X.; Sun, Y.; Lin, L. Scale-up cultivation enhanced arachidonic acid accumulation by red microalgae Porphyridium purpureum. Bioprocess Biosyst. Eng. 2017, 40, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Plöhn, M.; Spain, O.; Sirin, S.; Silva, M.; Escudero-Oñate, C.; Ferrando-Climent, L.; Allahverdiyeva, Y.; Funk, C. Wastewater treatment by microalgae. Physiol. Plant. 2021, 173, 568–578. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process. Eng. 2021, 40, 101747. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Cruz, C.G.; da Rosa, A.P.C. Insights into the technology utilized to cultivate microalgae in dairy effluents. Biocatal. Agric. Biotechnol. 2021, 35, 102106. [Google Scholar] [CrossRef]

| Component | Concentration |
|---|---|
| NaHCO3 | 16.80 g/L |
| K2HPO4 | 0.50 g/L |
| NaNO3 | 2.50 g/L |
| K2SO4 | 1.00 g/L |
| NaCl | 1.00 g/L |
| MgSO4 · 7H2O | 0.20 g/L |
| CaCl2 · 2H2O | 0.04 g/L |
| Microelement solution 1 | 1 mL/L |
| Chelated Fe solution 2 | 5 mL/L |
| Parameter (Unit) | Value |
|---|---|
| COD (mg/L) | 68,300 ± 3730 |
| TN (mg/L) | 280 ± 11 |
| TP (mg/L) | 1228 ± 46 |
| Lactose (g/L) | 61 ± 3.5 |
| pH | 6.08 ± 0.17 |
| NaCl (%) | 2.50 ± 0.1 |
| Sample ID | ||||||
|---|---|---|---|---|---|---|
| L0 | L2.5 | L5 | L7.5 | L10 | ||
| Added DCW (mL) | - | 8.1 ± 0.1 | 16.4 ± 0.2 | 24.6 ± 0.3 | 32.8 ± 0.4 | |
| Before microalgae cultivation | Lactose (g/L) | - | 2.5 | 5 | 7.5 | 10 |
| COD (mg/L) | - | 2800 ± 14 | 5601 ± 37 | 8401 ± 58 | 11201 ± 81 | |
| TN (mg/L) | 412 ± 9 | 406 ± 7 | 401 ± 12 | 396 ± 11 | 390 ± 14 | |
| TP (mg/L) | 89 ± 4 | 136 ± 5 | 182 ± 4 | 229 ± 6 | 276 ± 8 | |
| After microalgae cultivation | Lactose (g/L) | - | 0.0110 ± 0.0012 | 0.0078 ± 0.0018 | 0.0110 ± 0.0035 | 0.0249 ± 0.0059 |
| COD (mg/L) | - | 385 ± 3 | 470 ± 5 | 615 ± 8 | 660 ± 11 | |
| TN (mg/L) | 290 ± 7 | 240 ± 6 | 190 ± 2 | 70 ± 3 | 40 ± 2 | |
| TP (mg/L) | 74 ± 3 | 78 ± 2 | 80 ± 3 | 94 ± 4 | 96 ± 5 | |
| Parameter reduction | Lactose (%) | - | 99.56 ± 0.05 | 99.84 ± 0.036 | 99.85 ± 0.047 | 99.75 ± 0.059 |
| COD (%) | - | 86 ± 0.27 | 92 ± 0.35 | 93 ± 0.33 | 94 ± 0.37 | |
| TN (%) | 30 ± 0.14 | 41 ± 0.12 | 53 ± 0.24 | 82 ± 0.26 | 90 ± 0.32 | |
| TP (%) | 17 ± 0.10 | 43 ± 0.15 | 56 ± 0.25 | 59 ± 0.23 | 65 ± 0.30 | |
| Sample ID | ||||||
|---|---|---|---|---|---|---|
| L0 | L2.5 | L5 | L7.5 | L10 | ||
| Added DCW (mL) | - | 8.5 ± 0.1 | 17.1 ± 0.2 | 25.6 ± 0.3 | 34.1 ± 0.4 | |
| Before microalgae cultivation | Lactose (g/L) | - | 2.5 | 5 | 7.5 | 10 |
| COD (mg/L) | - | 3084 ± 21 | 6171 ± 43 | 9254 ± 67 | 12342 ± 94 | |
| TN (mg/L) | 412 ± 9 | 365 ± 4 | 360 ± 7 | 355 ± 6 | 350 ± 8 | |
| TP (mg/L) | 89 ± 4 | 141 ± 3 | 203 ± 6 | 264 ± 7 | 325 ± 7 | |
| After microalgae cultivation | Lactose (g/L) | - | 0.0570 ± 0.0030 | 0.059 ± 0.0035 | 0.0640 ± 0.0115 | 0.1290 ± 0.0090 |
| COD (mg/L) | - | 415 ± 5 | 400 ± 4 | 880 ± 9 | 900 ± 12 | |
| TN (mg/L) | 330 ± 8 | 180 ± 6 | 170 ± 3 | 140 ± 4 | 20 ± 2 | |
| TP (mg/L) | 78 ± 2 | 76 ± 3 | 80 ± 3 | 86 ± 4 | 60 ± 2 | |
| Parameter reduction | Lactose (%) | - | 97.72 ± 0.054 | 98.82 ± 0.056 | 99.15 ± 0.053 | 98.71 ± 0.120 |
| COD (%) | - | 87 ± 0.37 | 94 ± 0.41 | 90 ± 0.36 | 93 ± 0.39 | |
| TN (%) | 20 ± 0.22 | 51 ± 0.14 | 53 ± 0.28 | 61 ± 0.27 | 94 ± 0.36 | |
| TP (%) | 12 ± 0.15 | 46 ± 0.12 | 61 ± 0.29 | 67 ± 0.31 | 82 ± 0.34 | |
| Fatty Acid | Day–Night Illumination System | Continuous Illumination System | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L0 | L2.5 | L5 | L7.5 | L10 | L0 | L2.5 | L5 | L7.5 | L10 | |
| Palmitic acid (C16:0) | 16.35 | 14.71 | 16.00 | 14.62 | 16.04 | 15.19 | 16.51 | 17.90 | 13.03 | 19.85 |
| Palmitoleic acid (C16:1) | 4.72 | 3.50 | 7.06 | 4.66 | 5.12 | 5.12 | 2.61 | 5.04 | 4.22 | 6.98 |
| 7,10 Hexadecadienoic acid (C16:2) | 7.89 | 14.41 | 8.88 | 7.96 | 12.82 | 12.25 | 15.44 | 12.80 | 11.10 | 5.86 |
| 7,10,13 Hexadecatrienoic acid (C16:3) | 8.21 | 7.32 | 1.23 | 3.40 | 5.72 | 7.80 | 4.00 | 2.65 | 6.72 | 2.82 |
| Stearic acid (C18:0) | 17.22 | 2.00 | 4.85 | 4.02 | 2.38 | 1.77 | 2.89 | 2.21 | 4.11 | 7.21 |
| Oleic acid (C18:1) | 12.74 | 6.69 | 34.35 | 33.25 | 12.85 | 8.87 | 8.53 | 12.97 | 15.70 | 19.45 |
| Linoleic acid (C18:2) | 6.50 | 31.09 | 21.84 | 17.06 | 25.89 | 26.01 | 31.55 | 26.44 | 26.21 | 27.20 |
| Linolenic acid (C18:3) | 26.37 | 20.28 | 5.79 | 15.03 | 19.18 | 22.99 | 18.47 | 19.99 | 18.91 | 10.63 |
| Saturated fatty acids | 33.57 | 16.71 | 20.85 | 18.64 | 18.42 | 16.96 | 19.40 | 20.11 | 17.14 | 27.06 |
| Unsaturated fatty acids | 66.43 | 83.29 | 79.15 | 81.36 | 81.58 | 83.04 | 80.60 | 79.89 | 82.86 | 72.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulenco, A.; Vintila, A.C.N.; Vlaicu, A.; Ciltea-Udrescu, M.; Galan, A.-M. Nannochloris sp. Microalgae Strain for Treatment of Dairy Wastewaters. Microorganisms 2023, 11, 1469. https://doi.org/10.3390/microorganisms11061469
Paulenco A, Vintila ACN, Vlaicu A, Ciltea-Udrescu M, Galan A-M. Nannochloris sp. Microalgae Strain for Treatment of Dairy Wastewaters. Microorganisms. 2023; 11(6):1469. https://doi.org/10.3390/microorganisms11061469
Chicago/Turabian StylePaulenco, Anca, Alin Cristian Nicolae Vintila, Alexandru Vlaicu, Mihaela Ciltea-Udrescu, and Ana-Maria Galan. 2023. "Nannochloris sp. Microalgae Strain for Treatment of Dairy Wastewaters" Microorganisms 11, no. 6: 1469. https://doi.org/10.3390/microorganisms11061469
APA StylePaulenco, A., Vintila, A. C. N., Vlaicu, A., Ciltea-Udrescu, M., & Galan, A.-M. (2023). Nannochloris sp. Microalgae Strain for Treatment of Dairy Wastewaters. Microorganisms, 11(6), 1469. https://doi.org/10.3390/microorganisms11061469






