Abstract
The respiratory tract of lung transplant recipients (LTR) is likely to be colonized with non-fermentative Gram-negative rods. As a consequence of the improvements in molecular sequencing and taxonomy, an increasing number of bacterial species have been described. We performed a review of the literature of bacterial infections in LTR involving non-fermentative Gram-negative rods with exclusion of Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Achromobacter spp. and Burkholderia spp. Overall, non-fermenting GNR were recovered from 17 LTR involving the following genera: Acetobacter, Bordetella, Chryseobacterium, Elizabethkinga, Inquilinus, and Pandoraea. We then discuss the issues raised by these bacteria, including detection and identification, antimicrobial resistance, pathogenesis, and cross-transmission.
1. Introduction
The respiratory tracts of patients with chronic lung disease and lung transplant recipients (LTR) is likely to be colonized with non-fermentative Gram-negative rods (GNR) such as Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Achromobacter spp. or Burkholderia spp., the latter being mainly recovered from cystic fibrosis (CF) patients. Since the beginning of the 2000s, improvements in molecular sequencing and bacterial taxonomy have led to an increasing number of bacterial genus and species description. Bacterial families of closely related species of well described pathogens were extended: Alcaligenaceae (e.g., Achromobacter spp.), Burkholderiaceae (e.g., Burkholderia spp.), Pseudomonadaceae (e.g., P. aeruginosa), Xanthomonadaceae (e.g., S. maltophilia). Furthermore, bacterial genera were reclassified as members of the families Rhodospirillaceae, Flavobacteriaceae, Acetobacteraceae, and Weeksellaceae. Some species belonging to these families were associated with infections in immunocompromised patients or patients with chronic respiratory diseases such as CF. Most of these bacterial species are environmental saprophytes that can be recovered from soil, plants, or water. They present characteristics that raise questions and challenges: (i) as they are rarely isolated, they could be under-recognized as pathogens; (ii) their detection and identification in clinical laboratories could be problematic; (iii) multidrug-resistance is common and specific guidelines are lacking to assess their susceptibility to antimicrobial drugs; (iv) colonization of the respiratory tract and infection might be difficult to distinguish, (v) finally, hospital cross-transmission and its prevention remains unclear.
The incidence of post-transplant infections is higher in LTR than in other solid-organ recipients [1]. Most infections are due to common respiratory viruses such as influenza. However, bacterial infections are not uncommon. Early post-transplant infections mainly involve the bacteria of the recipient rather than those of the donor [2]. Consequently, LTR previously colonized with non-fermenting Gram-negative rods (GNR) are at risk of infection involving these bacteria. Conversely, late-onset infections could involve a wide range of bacteria including vaccine-preventable pathogens such as Streptococcus pneumoniae or Haemophilus influenzae.
We performed a narrative review of bacterial infections in LTR involving uncommon species of the families Burkholderiaceae, Rhodospirillaceae, Pseudomonadaceae, Weeksellaceae, Flavobacteriaceae, Alcaligenaceae, Acebobacteraceae, and Xanthomonadaceae. We retrieved published cases of uncommon GNR recovered from LTR to identify singularities related to their, (i) detection and identification, (ii) antimicrobial resistance, (iii) role in infections, and (iv) cross-transmission and prevention.
2. Methods of the Narrative Review
Studies were searched from PubMed from inception to 30 September 2022. All bacterial genera belonging to 8 bacterial families Acetobacteraceae, Alcaligenaceae, Burkholderiaceae, Flavobacteriaceae, Pseudomonaceae, Rhodospirillaceae, Xanthomonadaceae, Weeksellaceae as defined by the Taxonomy browser of the National Library of Medicine were included in the general review [3]. 1174 articles were identified from PubMed of which 15 case reports were found eligible (Figure 1).
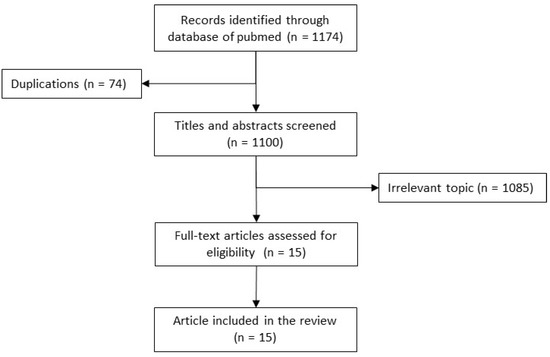
Figure 1.
Flow diagram. Relevant article Detection, Identification, Antimicrobial resistance, cross-transmission and its prevention.
The common species and complex Pseudomonas aeruginosa, Achromobacter xylosoxydans, Burkholderia cepacia complex, and Stenotrophomonas maltophilia were excluded. Case reports and cohorts of LTR from whom non-fermenting GNR were isolated whether the patients were infected or not were eligible for review. Considering case-reports and case-series, the following data were extracted from each eligible article: socio-demographic characteristics, the reason for lung transplantation, other predisposing conditions, past medical history including antimicrobial treatment prior to bacterial isolation, clinical findings at the time of isolation and associated micro-organisms, and outcome. Data relative to detection and identification, antimicrobial resistance, role in infection, cross-transmission and its prevention were extracted. Overall, non-fermenting GNR were recovered from 17 LTR involving the following genera: Acetobacter, Bordetella, Chryseobacterium, Elizabethkinga, Inquilinus, and Pandoraea (Table 1). Except for the genera Bordetella, all could be classified as opportunistic environmental saprophytes.

Table 1.
Bacterial genera involved in infections or colonization in lung transplant recipients.
3. Review of Cases (Table 2)
3.1. Acetobacter indonesiensis
Two cases of A. indonesiensis infections have been reported in LTR over the past years [4,5]. One of them was a 31-year-old man with CF and the second was a 51-year-old woman with hypersensitivity pneumonitis, extrinsic allergic alveolitis, and short telomere syndrome. Neither of them was previously known to be colonized with A. indonesiensis. In both patients, A. indonesiensis was recovered from at least three respiratory samples with the first positive one within the month following the lung transplantation. A. indonesiensis was not recovered from other samples such as blood cultures. Despite both patients being considered infected, they recovered with antibiotic drugs inactive against A. indonesiensis. A. indonesiensis was not recovered in any subsequent respiratory tract sample after seven months of follow-up for one of them.
3.2. Chryseobacterium spp.
Chryseobacterium spp. was mentioned once in a retrospective series of clinically relevant infections following lung transplantation in South Korea [6]. The patient developed pneumonia between 1 and 6 months following the transplantation. No other microbiological or clinical data are available for this case.
3.3. Elizabethkinga spp.
Elizabethkinga spp. was associated with septic shock in a 26 year-old man who had received lung transplantation five years before for end-stage CF [7]. The patient was first admitted for hypoxic respiratory failure attributed to the respiratory syncytial virus. During his hospital stay, he developed septic shock. Elizabethkinga was isolated from blood culture, peritoneal fluid, and bronchoalveolar lavage (BAL). Microbiological clearance was obtained with intravenous piperacillin-tazobactam and levofloxacin. However, on day 15, he developed a cardiac arrest secondary to acidosis and severe hypercapnic respiratory failure. He died on day 19 of his hospital stay.
3.4. Inquilinus limosus
I. limosus was isolated from 3 LTR with early post-operative infections [8,9,10]. All of them were transplanted for CF and were previously colonized with I. limosus. The first one was a 22-year-old woman who presented with pulmonary infiltrate one week after lung transplantation [8]. I. limosus was isolated with Enterococcus spp. and coagulase-negative Staphylococcus from a BAL. The patient recovered and was discharged 6 weeks after the transplantation. I. limosus was not isolated from her respiratory tract after one year of follow-up. The second patient, a 31-year-old man, successively developed a bacteraemic lung empyema on post-operative day 38, and a contralateral lung empyema 8 months later [9]. I. limosus was recovered from blood culture and the empyema during the first infection and the empyema only during the second one. He completely recovered from both episodes with surgical and antimicrobial treatment. The authors did not report whether I. limosus was isolated from the patient between the two infections and after the second one. The last patient, a 45-year-old LTR woman eight years ago, presented an I. limosus bacteraemia after a SARS-CoV-2 infection [10]. I. limosus was isolated from three consecutive respiratory samples and from an aerobic blood culture vial. She recovered with an antimicrobial regimen. She was colonized before the lung transplantation, but I. limosus has not been isolated since then.
3.5. Pandoraea spp.
Pandoraea spp. was the most frequent bacterial genera isolated from LTR with six patients infected. Five patients were lung transplanted for CF, while the remaining one was a 30-year-old man with end-stage pulmonary sarcoidosis complicated by nocardiosis and mycetomas [11]. P. pnomenusa was never isolated from this latter patient before the lung transplantation. Immediately post-operatively he presented with sepsis and pulmonary effusions. P. pnomenusa was isolated from multiple sets of blood cultures and respiratory samples. No other pathogens were found. The patient developed progressive acute respiratory distress syndrome and died on post-operative day 17 from refractive septic shock and multiple organ failure.
All five lung transplanted patients for CF were previously colonized with Pandoraea spp. Post-operatively, Pandoraea spp. was associated with colonization in three patients and infection in two patients. One patient was a 30-year-old female who died 3 weeks after lung transplantation from Pseudomonas aeruginosa septic shock [12]. The two other patients were doing well after lung transplants [13,14]. Of the two infected patients, a 21-year-old woman died from P. noserga septic shock on post-operative day 25 [15]. The second patient, a 30-year-old woman, developed a pleural effusion positive for P. apista and P. aeruginosa on post-operative day 45 [14]. She recovered with appropriate treatment.
Of note, one CF patient colonized with an isolate of P. sputorum was recused for lung transplantation due to concern about the isolate [16]. However, P. apista clearance was reported following lung transplantation in a 21 year-old woman with CF [17].
3.6. Bordetella spp.
In contrast to the other genus for which an environmental source is suspected or proved, Bordetella species are mainly transmitted through contact with infected humans or pets. Furthermore, while B. pertussis and B. parapertussis are fastidious micro-organisms that cultivate in vitro on Bordet-Jengou selective agar, other Bordetella species such as B. bronchiseptica, B. petrii, B. trematum grow on standard media such as blood Columbia or chocolate agar.
A single case of B. pertussis infection was reported in an LTR for CF [18]. The patient presented with acute respiratory distress syndrome five years after the transplantation. He recovered from whooping cough with antibiotics, but he required a prolonged ICU stay complicated by hypoxic-ischaemic optic neuropathy leading to blindness. His sister tested positive for B. pertussis during his hospital stay. Nevertheless, the author did not report if the patient had been vaccinated against whooping cough or not.
Of the three patients with B. bronchiseptica infection, two were transplanted for CF and one for anti-MDA-5-associated clinically amyopathic dermatomyositis [19,20]. They were all in contact with dogs and two contracted a Kennel cough within six months following the transplantation, while the third contracted it one year later. Vaccination status of the contact pet is reported in a single case, a puppy was not vaccinated against B. bronchiseptica [19]. All three patients required hospital management of B. bronchiseptica infection. Two of them recovered with an appropriate antibiotic regimen, while a 10-year-old girl died from respiratory failure.

Table 2.
Cases involving opportunistic Gram-negative rods.
Table 2.
Cases involving opportunistic Gram-negative rods.
| No. | Age | Sex | Reason for Lung Transplantation | Infection | Isolation before Transplant | Species | Other Associated Pathogens | Past History of Antimicrobial Treatment | Medical History | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | M | Cystic fibrosis | Early post-operative pneumonia/Colonization | No | A. indonesiensis | P. aeruginosa S. aureus | Peri-operative prophylaxis with colistin, tobramycin, ceftazidime, and linezolid |
| Recovery | [21] |
| 2 | 51 | F |
| Early post-operative Pneumonia/Colonization | No | A. indonesiensis | n.a. * | Post-operative prophylaxis cotrimoxazole, vancomycin, piperacillin/tazobactam |
| Recovery | [4] |
| 3 | n.a. | n.a. | n.a. | Pneumonia 1 to 6 months after transplant | n.a. | Chryseobacterium spp. | n.a. | n.a. | n.a. | n.a. | [6] |
| 4 | 26 | M | Cystic fibrosis | Septic shock 5 years after transplant | No | Elizabethkinga spp. | P. aeruginosa | n.a. |
| Death | [7] |
| 5 | 22 | F | Cystic fibrosis | Early post-operative pulmonary infiltrate (week 1) | Yes | I. limosus | n.a. | Peri-operative prophylaxis with imipenem, tobramycin, ceftazidime, and aerosolized colistin |
| Recovery | [8] |
| 6 | 31 | M | Cystic fibrosis | Early bacteraemic lung empyema on postoperative day 38 and month 8 | Yes | I. limosus | no | Peri-operative prophylaxis with piperacillin-tazobactam, tobramycin, cotrimoxazole, nebulized amphotericin, azithromycin |
| Recovery | [9] |
| 7 | 45 | F | Cystic fibrosis | Bacteremia 8 years after lung transplantation | Yes | I. limosus | No | n.a. |
| Recovery | [10] |
| 8 | 30 | F | Cystic fibrosis | Early post-operative pleural effusion | Yes | P. apista | P. aeruginosa | n.a. |
| Recovery | [14] |
| 9 | 36 | M | Cystic fibrosis | Colonization | Yes | P. apista | n.a. | n.a. |
| Recovery | [14] |
| 10 | 21 | F | Csytic fibrosis | Early post-operative septic choc | Yes | P. nosoerga | No | Peri-operative prophylaxis piperacillin/tazobactam, tigecyclin |
| Death | [15] |
| 11 | 30 | M | End-stage pulmonary sarcoidosis complicated by nocardiosis and mycetomas. Prednisone 50 mg daily | Septic shock | No | P. pnomenusa | n.a. | Peri-operative prophylaxis Ceftazidime, vancomycin. |
| Death | [11] |
| 12 | n.a. | n.a. | Cystic fibrosis | Colonization | n.a. | P. pulmonicola | n.a. | n.a. | n.a. | Recovery | [13] |
| 13 | 30 | F | Cystic fibrosis | Colonization | Yes | P. pulmonicola | P. aeruginosa | n.a. |
| Death | [12] |
| 14 | 42 | M | Cystic fibrosis | ARDS 5 years post-transplant | no | B. pertussis | No | n.a. |
| Recovery | [18] |
| 15 | 10 | F | Cystic fibrosis | Late post-transplant pneumonia (1-year) | no | B. bronchiseptica | P. aeruginosa, A. fumigatus, K. pneumoniae, S. maltophilia. | n.a. |
| Death | [19] |
| 16 | 15 | M | Cystic fibrosis | Bronchitis 3 months post-transplant | no | B. bronchiseptica | B. cepacia | n.a. |
| Recovery | [19] |
| 17 | 51 | M | MDA-5 associated clinically amyopathic dermatomyositis. | Bronchitis 4 months post-transplant | no | B. bronchiseptica | No | n.a. |
| Recovery | [20] |
* n.a.: not available. ** BAL: Broncho-alveolar fluid.
4. Issues Raised by Opportunistic GNR
4.1. Detection and Identification
These fastidious non-fermenting Gram-negative rods present several microbiological characteristics that make their culture, identification, and antimicrobial susceptibility testing (AST) challenging in clinical laboratories. The results of external quality control trials of diagnostic microbiology of CF isolates highlight these difficulties [22]. A total of 31 and 37 laboratories that handled CF patient samples participated in this program in 2008 and 2009, respectively [22]. While P. aeruginosa, Staphylococcus aureus, Burkholderia spp. strains were correctly recovered and identified by most participants, 19.4% and 45.2% did not detect or misidentify a strain of P. pnomenusa, respectively. These rates were 27.0% and 10.8%, respectively for a strain of I. limosus. However, since these programs, methods of identification have been improved and the diffusion of the MALDI-TOF mass-spectrometry should have led to a decrease in the rate of misidentification.
Respiratory samples are frequently polymicrobial. Consequently, slow-growing micro-organisms such as I. limosus or Pandoraea spp. could be overgrown by bacteria of the commensal flora, such as staphylococci, streptococci, Capnocytophaga spp., or fast-growing pathogens such as P. aeruginosa. However, they could be cultivated on a Burkholderia cepacia selective agar plate that could therefore enhance their recovery and their isolation. However, Burkholderia selective agar is mainly used for respiratory samples of CF patients but not those from lung transplant recipients or patients with other respiratory diseases [23]. The respiratory samples of CF patients are incubated for 5 days to allow the growth of slow-growing micro-organisms. Indeed, among the 17 patients included in the present review, 14 were for CF with sputa and were incubated for 5 days. While bronchoscopy samples are incubated for 4 or 5 days, sputa from LTR are incubated only for 2 days, and consequently do not allow the culture of fastidious GNR. The benefit of the use of Burkholderia selective agar media and the prolonged incubation of cultures from LTR, as for CF samples, remains to be assessed. Furthermore, the morphotype of the colonies is not specific and it is sometimes confusing. For instance, I. limosus colonies display a mucoid morphotype similar to mucous strains or P. aeruginosa. Consequently, they are likely misidentified as this pathogen using morphologic characteristics only [24].
Phenotypic and biochemical methods provide frequently inconsistent identification of these species, i.e., either no identification or misidentification [16,25,26,27,28,29]. Indeed, Ralstonia spp. were previously misidentified as Burkholderia spp., I. limosus as Roseomonas gilardii, Sphingomonas paucimobilis or Agrobacterium radiobacter [30,31,32]. Conversely, some strains were misidentified as belonging to these species. For instance, a Burkholderia gladioli strain has been misidentified as Empedobacter spp, a genus of the Weeksellaceae family [33], and B. trematum could be misidentified as B. bronchiseptica [34]. These lower performances could be related to inherent limitations of biochemical methods [35]: (i) their databases are limited and could lack several species; (ii) not all of the strains of the same species have the same characteristics; (iii) the same strain may produce different results in repeated tests. MALDI-TOF mass spectrometry has greatly improved the identification of cultivable micro-organisms in clinical laboratories including fastidious bacteria [36,37]. In comparison to 16S rDNA gene sequencing, most of the clinical isolates are correctly identified at the species level [38,39]. Furthermore, MALDI-TOF mass spectrometer databases are regularly updated to include new species and improve the identification of closely related species that remain difficult to distinguish. However, closely related species, for instance, Elizabethkingia species, could not be distinguished using a MALDI-TOF mass spectrometer equipped with commercial reference databases [40]. In-house expanded spectrum databases could be implemented for their identification [41]. The 16S rDNA gene sequencing displays high performance for the identification of all these bacteria. However, it is not able to distinguish some closely related species that display a high level of sequence homology [31,42]. Alternative molecular methods such as gyrB gene sequencing or specific PCR could resolve ambiguous identifications but they required specific reagents and skills. Consequently, they are performed by a low number of laboratories [21,43,44,45,46,47].
4.2. Antimicrobial Resistance
Assessing the antimicrobial susceptibility of opportunistic GNR is also challenging. Species-related methods (inoculum, temperature, and delay of incubation) and breakpoints lack in both CLSI and EUCAST guidelines [48,49,50]. Antimicrobial susceptibility testing was mainly performed and interpreted using Burkholderia spp., Pseudomonas aeruginosa, or non-related species guidelines. As they are slow-growing the incubation could require a prolonged delay of up to 48 h.
Species’ intrinsic antimicrobial resistance remains unclear. Most data are from case reports or case series that included a few strains. Furthermore, a high rate of strains was recovered from CF patients that could have been colonized for several years or treated with several courses of antimicrobials. Consequently, the pattern of wild-type strains is little assessed. Considering these findings, performing AST is therefore mandatory when an antibiotic regimen is required. In colonized patients, antimicrobial susceptibility of previously recovered strains should be considered when empiric therapy is required.
In vitro, most β-lactams including β-lactams/β-lactamases inhibitor combinations are inactive except for the carbapenems [4,5,25,26,51,52,53,54,55]. However, the emergence of meropenem resistance in an I. limosus strain was reported during a meropenem course in a 16 year-old girl with CF [24]. Fluoroquinolones, aminoglycosides, and cyclins exhibit a variable activity [4,5,25,26,51,52,53,54,55,56]. Colistin is inactive in vitro against Inquilinus spp., Pandoraea spp., Ralstonia spp., and A. indonesensis [4,5,25,51,52,54,55]. It has been suggested that the clinical use of nebulized colistin to treat P. aeruginosa infection in CF patients enhances the selection and colonization of colistin-resistant species [21]. Fosfomycin and trimethoprime-sulfametoxazole combination were almost always found inactive [25,51,52,54], suggesting in a comparable manner to other non-fermenting Gram-negative rods, the drugs are inactive in vivo against these species.
Genetic determinants of resistance have been identified mainly for β-lactams. All these species intrinsically express broad-spectrum β-lactamases such as OXA-62 in Pandoraea spp. [57,58], OXA-22 and OXA-60 in Ralstonia spp. [59], Inq1 in I. limosus [56], BlaB and GOB in Elizabethkingia spp. [60,61], or IND-16 and OXA-209 Chryseobacterium spp. [62,63,64]. Some strains were found to harbour several genes coding β-lactamases [63,65]. However, broad-spectrum β-lactamases genes could be either chromosomally or plasmid-encoded, Elizabethkingia spp. has the singularity of harbouring a high diversity of chromosomally encoded Metallo-β-lactamases [66]. Moreover, other mechanisms including efflux-pump, genomic mutation, or methylation could take part in antimicrobial resistance to β-lactams and other classes of drugs such as fluoroquinolones [42,67,68,69].
4.3. Pathogenesis
All these opportunistic GNR could colonize the respiratory tract of patients with underlying diseases. I. limosus and Pandoraea spp. were almost exclusively recovered from the respiratory tract of CF patients [14,21,24]. Conversely, Chryseobacterium spp. and Elizabethkinga spp. have been isolated from various clinical contexts including meningitis in newborns or bacteremia in immunocompromised patients [40,70,71,72,73,74].
Interestingly, the two patients with I. limosus early post-operative infections were previously colonized and both recovered from their infection. I. limosus colonization in CF patients is associated with specific serum antibody response [24]. It can be speculated that the antibodies against I. limosus could have contributed to favorable outcomes in these patients. Of the four patients previously colonized with Pandoraea spp. prior to lung transplantation, two were found to have been colonized after the surgery, one recovered from P. apista septic shock, and one died from P. pnomenusa septic shock. The potential role of immune response consecutive to chronic colonization remains to be assessed for this genus.
Considering A. indonesiensis, despite the bacteria being recovered from multiple respiratory samples in both patients [4,5], colonization rather than infection could not be ruled out as both recovered without effective antimicrobials.
Indeed, as they could be associated with chronic colonization of the respiratory tract, the significance of their recovery remains unclear. However, clinical signs, spirometry, and declining radiographic parameters were reported in some patients following the isolation of I. limosus and Pandoraea spp. [24,75,76,77]. The outcome of CF patients colonized with Inquilinus spp was assessed in a retrospective case-control study that included 17 patients with at least one respiratory culture positive for Inquilinus spp compared with age-matched CF controls with chronic P. aeruginosa colonization [53]. After five years of follow-up, the patients colonized with I. limosus showed a similar decrease in spirometry value, BMI percentiles, and rate of pulmonary exacerbation, to that of those colonized with P. aeruginosa.
In silico analyses have suggested their genomes encoded several virulence factors and determinants associated with environmental survival and encompassed genes associated with flagella, adhesion, capsule polysaccharide synthesis and antiphagocytosis, secretion systems, cytokine production and cytotoxicity, and biofilm formation for instance [63,67,78,79]. The genome of Elizabethkingia is predicted to encode 270 putative virulence factors [80]. While different species of Elizabethkingia shared the same virulence factors, 162 predicted genes for virulence factors have been reported as unique in Elizabethkingia anophelis and 6 in Elizabethkingia meningoseptica.
Inquilinus spp. strain LMG 20,952 produces two exopolysaccharides (EPS) that exhibit the same charge per sugar residue as alginate, an exopolysaccharide produced by P. aeruginosa [81,82]. Alginate is involved in biofilm formation and also enhances adhesion to solid surfaces. However, Inquilinus spp. EPS displays a different confirmation suggesting it could have a specific role [83]. Pandoraea spp. strains were able in vitro to invade lung epithelial cells and kill Galleria mellonella larvae [13,84]. Virulence was inconsistent regarding bacterial strain and species. However, P. pulmonicola strains were generally the most virulent of the species tested in comparison to other species, P. apista and P. pnomenusa, or to Burkholderia cenocepacia. Chryseobacterium spp. and Elizabethkingia spp. are able in vitro to produce biofilm, the latter could invade respiratory tract epithelial cells in a murine model [85,86,87,88].
The airways are polymicrobial and bacteria have mutual interactions [89,90]. The kinetics of biofilm formation by I. limosus is disturbed in the presence of P. aeruginosa [91]. However, bacterial growth in a polymicrobial environment protects the target microorganism from the effect of the antimicrobial agent [91,92].
4.4. Cross-Transmission and Prevention
While there are no specific guidelines for Pandoraea spp., I. limosus, and Ralstonia spp., CF patients have been previously placed on isolation procedures during hospitalization when colonized or infected with these micro-organisms [12,14,93,94]. Furthermore, in a CF centre, an epidemic spread of P. pulmonicola was controlled by implementing additional cross-transmission prevention measures [95]. Of note, preventative measures were not strictly followed in the CF department before additional control measures were implemented.
It was suggested that arrangements must be set up for CF patients infected with Burkholderia cepacia complex and methicillin-resistant Staphylococcus aureus, for example, separate clinics and appropriate inpatient segregation [96,97]. However, the American guidelines consider the lack of definitive support for cohort segregation. Nevertheless, people with CF should be separated from other patients regardless of their respiratory tract culture results [98]. In CF patients, additional infection prevention and control (IPC) measures are controversial as they are mainly based on theoretical benefit more than proven efficacy [99].
Regarding the low frequency of isolation of these fastidious GNRs and the absence of evidence in LTR, specific infection prevention and control measures are probably not required. The compliance with standard precautions, which is inconsistent in several wards including CF centers [100,101], should be enhanced in order to prevent the cross-transmission of all microorganisms from asymptomatic and symptomatic carriers. Indeed, to be successful, infection control measures need to be simple, universally applied, and acceptable [99]. In addition, as for CF, LTR should probably be separated from other patients regardless of their respiratory tract culture results.
5. Issues Raised by Bordetella spp.
In contrast to the other genus for which an environmental source is suspected or proved, Bordetella species are mainly transmitted through contact with infected humans or pets. While they belong to the same genus, Bordetella species differ in several characteristics. B. pertussis and B. parapertussis are fastidious micro-organisms that cultivate in vitro on selective agar such as Bordet-Jengou media. Due to the lower prevalence of whooping cough and the development of PCR few laboratories now perform B. pertussis and B. parapertussis culture. Commercial or in-house PCR assays are also available for these pathogens. Consequently, clinical laboratories should be aware when B. pertussis and B. parapertussis are suspected. Conversely, other Bordetella species such as B. bronchiseptica, B. petrii, and B. trematum grow on standard media such as Columbia supplemented with blood or chocolate agar.
B. bronchiseptica is a zoonotic respiratory infectious agent of Kennel cough. The bacteria is transmitted by companion animals, mainly dogs and cats. The prevalence of B. bronchiseptica was assessed to be up to 4.9% in symptomatic cats and up to 19.5% in rescue catteries [102,103,104]. Bacterial isolation from healthy dogs ranged from 0.0% to 45.6% while it ranged from 3.3% to 78.7% in dogs with respiratory disease [105]. To date, no human transmission of B. bronchiseptica has been reported. B. bronchiseptica is mainly associated with bronchitis and pneumonia in immunocompromised patients or those with chronic lung disease.
Whooping cough and Kennel cough are preventable infectious diseases. For whooping cough, the vaccine status of the only case included in the present review was not available. While the vaccine response of LTR could differ from immunocompetent patients, there are no specific guidelines for LTR. The Pertussis vaccine is recommended or mandatory for newborns and infants in several European countries [106]. However, vaccine schedules differ among countries [106]. When possible, vaccination should be started already during the pre-transplantation period when the patient is on the waiting list. Booster vaccinations should be given post-transplantation, but only when immunosuppression has been tapered. Parental vaccine cocooning prevents pertussis infection in infants [107,108]. A similar strategy is also probably adapted for close contact with LTR. Consequently, we suggest recommendations for Pertussis vaccination should be encouraged in LTR as well as persons of close environment. Assessing immune response to pertussis vaccine in LTR is required.
We found little data regarding the vaccination of contact pets of LTR. Of the three LTR infected with B. bronchiseptica, the contact pet was vaccinated in a single case. Nevertheless, as an effective vaccine is available for dogs [109], LTR should be encouraged to vaccinate companion animals. Furthermore, the pertussis vaccine offers some cross-protection against B. bronchiseptica and therefore helps further mitigate the risk of zoonotic infection of this organism from pets to their owners [110]. While CF patients are aware of the risk of Whooping cough and the benefit of vaccination [109], a similar campaign should be in place for patients with other chronic lung diseases.
6. Conclusions
There are few reports of bacterial infections involving uncommon GNR in LTR, and their incidence remains to be assessed. Several methods could enhance the recovery of these bacteria, especially from polymicrobial samples such as respiratory tract samples. The use of selective media such a Burkholderia cepacia selective agar and prolonged incubation for the recovery of fastidious microorganisms should probably be assessed. Antimicrobial susceptibility testing and interpretation should also be standardized as well as assessing the intrinsic species pattern of resistance. Whooping cough and kennel cough are preventable infectious diseases. LTR and their relations should be encouraged to be vaccinated as well as companion animals for B. bronchiseptica.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Polly Gobin for the proofreading and English correction.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Waller, K.M.J.; De La Mata, N.L.; Wyburn, K.R.; Hedley, J.A.; Rosales, B.M.; Kelly, P.J.; Ramachandran, V.; Shah, K.K.; Morton, R.L.; Rawlinson, W.D.; et al. Notifiable Infectious Diseases Among Organ Transplant Recipients: A Data-Linked Cohort Study, 2000–2015. Open Forum Infect. Dis. 2022, 9, ofac337. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Miyoshi, K.; Kurosaki, T.; Otani, S.; Sugimoto, S.; Yamane, M.; Oto, T.; Toyooka, S. Airway bacteria of the recipient but not the donor are relevant to post-lung transplant pneumonia. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 833–840. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Taxonomy Browser n.d. Available online: https://www.ncbi.nlm.nih.gov/taxonomy (accessed on 8 October 2022).
- Basu, S.S.; Delaney, M.L.; Li, N.; Onderdonk, A.B.; Bry, L. Acetobacter indonesiensis Pneumonia after Lung Transplantation. Emerg. Infect. Dis. 2018, 24, 598–599. [Google Scholar] [CrossRef]
- Bittar, F.; Reynaud-Gaubert, M.; Thomas, P.; Boniface, S.; Raoult, D.; Rolain, J.-M. Acetobacter indonesiensis pneumonia after lung transplant. Emerg. Infect. Dis. 2008, 14, 997–998. [Google Scholar] [CrossRef]
- Bae, M.; Lee, S.-O.; Jo, K.-W.; Choi, S.; Lee, J.; Chae, E.J.; Do, K.-H.; Choi, D.-K.; Choi, I.-C.; Hong, S.-B.; et al. Infections in Lung Transplant Recipients during and after Prophylaxis. Infect. Chemother. 2020, 52, 600–610. [Google Scholar] [CrossRef]
- Ramanan, P.; Razonable, R. Elizabethkingia species sepsis after lung transplantation: Case report and literature review. Transpl. Infect. Dis. 2013, 15, E229–E234. [Google Scholar] [CrossRef] [PubMed]
- Pitulle, C.; Citron, D.M.; Bochner, B.; Barbers, R.; Appleman, M.D. Novel Bacterium Isolated from a Lung Transplant Patient with Cystic Fibrosis. J. Clin. Microbiol. 1999, 37, 3851–3855. [Google Scholar] [CrossRef] [PubMed]
- Goeman, E.; Shivam, A.; Downton, T.; Glanville, A.R. Bacteremic Inquilinus limosus empyema in an Australian lung transplant patient with cystic fibrosis. J. Hearth Lung Transplant. 2015, 34, 1220–1223. [Google Scholar] [CrossRef]
- Farfour, E.; Zrounba, M.; Roux, A.; Revillet, H.; Vallée, A.; Vasse, M. Inquilinus limosus Bacteremia in Lung Transplant Recipient after SARS-CoV-2 Infection. Emerg. Infect. Dis. 2023, 29, 642–644. [Google Scholar] [CrossRef]
- Stryjewski, M.E.; LiPuma, J.J.; Messier, J.R.H.; Reller, L.B.; Alexander, B.D. Sepsis, multiple organ failure, and death due to Pandoraea pnomenusa infection after lung transplantation. J. Clin. Microbiol. 2003, 41, 2255–2257. [Google Scholar] [CrossRef]
- Kokcha, S.; Bittar, F.; Reynaud-Gaubert, M.; Mely, L.; Gomez, C.; Gaubert, J.-Y.; Thomas, P.; Rolain, J.-M. Pandoraea pulmonicola chronic colonization in a cystic fibrosis patient, France. New Microbes New Infect. 2013, 1, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Caraher, E.; Collins, J.; Herbert, G.; Murphy, P.G.; Gallagher, C.G.; Crowe, M.J.; Callaghan, M.; McClean, S. Evaluation of in vitro virulence characteristics of the genus Pandoraea in lung epithelial cells. J. Med. Microbiol. 2008, 57, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.M.; LiPuma, J.J.; Rosenbluth, D.B.; Dunne, W.M. Chronic colonization with Pandoraea apista in cystic fibrosis patients determined by repetitive-element-sequence PCR. J. Clin. Microbiol. 2006, 44, 833–836. [Google Scholar] [CrossRef]
- Peyclit, L.; Baron, S.A.; Reynaud-Gaubert, M.; Cassir, N.; Rolain, J.M. Fatal Pandoraea nosoerga infection after combined liver-lung transplantation for cystic fibrosis: A recontamination by the pre-transplantation strain n.d. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2403–2406. [Google Scholar] [CrossRef]
- Pimentel, J.D.; MacLeod, C. Misidentification of Pandoraea sputorum isolated from sputum of a patient with cystic fibrosis and review of Pandoraea species infections in transplant patients. J. Clin. Microbiol. 2008, 46, 3165–3168. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, I.M.; Johansen, H.K.; Frederiksen, B.; Pressler, T.; Hansen, A.; Vandamme, P.; Høiby, N.; Koch, C. Epidemic Spread of Pandoraea apista, a New Pathogen Causing Severe Lung Disease in Cystic Fibrosis Patients. Pediatr. Pulmonol. 2003, 36, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Panchabhai, T.S.; Bandyopadhyay, D.; Kapoor, A.; Akindipe, O.; Lane, C.; Krishnan, S. Acute ischemic optic neuropathy with extended prone position ventilation in a lung transplant recipient. Int. J. Crit. Illn. Inj. Sci. 2016, 6, 45–47. [Google Scholar] [CrossRef]
- Ner, Z.; Ross, L.A.; Horn, M.V.; Keens, T.G.; MacLaughlin, E.F.; Starnes, V.A.; Woo, M.S. Bordetella bronchiseptica infection in pediatric lung transplant recipients. Pediatr. Transplant. 2003, 7, 413–417. [Google Scholar] [CrossRef]
- Deitchman, A.R.; Kalchiem-Dekel, O.; Todd, N.; Reed, R.M. Rapidly progressive interstitial lung disease due to anti-melanoma differentiation associated protein-5 requiring a bilateral lung transplant, and complicated by kennel cough. Respir. Med. Case Rep. 2019, 28, 100886. [Google Scholar] [CrossRef]
- Bittar, F.; Leydier, A.; Bosdure, E.; Toro, A.; Reynaud-Gaubert, M.; Boniface, S.; Stremler, N.; Dubus, J.-C.; Sarles, J.; Raoult, D.; et al. Inquilinus limosus and cystic fibrosis. Emerg. Infect. Dis. 2008, 14, 993–995. [Google Scholar] [CrossRef]
- Hogardt, M.; Ulrich, J.; Riehn-Kopp, H.; Tümmler, B. EuroCareCF quality assessment of diagnostic microbiology of cystic fibrosis isolates. J. Clin. Microbiol. 2009, 47, 3435–3438. [Google Scholar] [CrossRef] [PubMed]
- Farfour, E.; Botterel, F.; Pozzetto, B. Infections Broncho-Pulmonaires (Hors Tuberculose et Mucoviscidose); REMIC 7; Société Française de Microbiologie: Paris, France, 2022; pp. 1–16. [Google Scholar]
- Schmoldt, S.; Latzin, P.; Heesemann, J.; Griese, M.; Imhof, A.; Hogardt, M. Clonal analysis of Inquilinus limosus isolates from six cystic fibrosis patients and specific serum antibody response. J. Med. Microbiol. 2006, 55, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Chiron, R.; Marchandin, H.; Counil, F.; Jumas-Bilak, E.; Freydière, A.-M.; Bellon, G.; Husson, M.-O.; Turck, D.; Brémont, F.; Chabanon, G.; et al. Clinical and microbiological features of Inquilinus sp. isolates from five patients with cystic fibrosis. J. Clin. Microbiol. 2005, 43, 3938–3943. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.E.; Rhoads, D.D.; Wilson, D.A.; Highland, K.B.; Richter, S.S.; Procop, G.W. Inquilinus limosus in pulmonary disease: Case report and review of the literature. Diagn. Microbiol. Infect. Dis. 2016, 86, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Adley, C.; Saieb, F. Comparison of bioMérieux API 20NE and Remel RapID NF Plus, identification systems of type strains of Ralstonia pickettii. Lett. Appl. Microbiol. 2005, 41, 136–140. [Google Scholar] [CrossRef]
- Ryan, M.P.; Pembroke, J.T.; Adley, C.C. Genotypic and phenotypic diversity of Ralstonia pickettii and Ralstonia insidiosa isolates from clinical and environmental sources including High-purity Water. Diversity in Ralstonia pickettii. BMC Microbiol. 2011, 11, 194. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Köthe, J.; Wirths, B.; Sigge, A.; Poppert, S.; Johnson, J.R.; Scheutz, F.; Ulleryd, P.; Kuskowski, M.A.; O’Bryan, T.T.; et al. Superiority of molecular techniques for identification of gram-negative, oxidase-positive rods, including morphologically nontypical Pseudomonas aeruginosa, from patients with cystic fibrosis. J. Clin. Microbiol. 2005, 43, 3895–3900. [Google Scholar] [CrossRef]
- Salvador-García, C.; Yagüe-Guirao, G.; Pastor-Vivero, M.D.; Sáez-Nieto, J.A. Chronic colonization of Inquilinus limosus in a patient with cystic fibrosis: First report in Spain. Enferm. Infecc. Microbiol. Clin. 2013, 31, 414–415. [Google Scholar] [CrossRef]
- Bittar, F.; Richet, H.; Dubus, J.-C.; Reynaud-Gaubert, M.; Stremler, N.; Sarles, J.; Raoult, D.; Rolain, J.-M. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS ONE 2008, 3, e2908. [Google Scholar] [CrossRef]
- Kidd, T.J.; Ramsay, K.A.; Hu, H.; Bye, P.T.P.; Elkins, M.R.; Grimwood, K.; Harbour, C.; Marks, G.B.; Nissen, M.D.; Robinson, P.J.; et al. Low rates of Pseudomonas aeruginosa misidentification in isolates from cystic fibrosis patients. J. Clin. Microbiol. 2009, 47, 1503–1509. [Google Scholar] [CrossRef]
- Brizendine, K.D.; Baddley, J.W.; Pappas, P.G.; Leon, K.J.; Rodriguez, J.M. Fatal Burkholderia gladioli infection misidentified as Empedobacter brevis in a lung transplant recipient with cystic fibrosis. Transpl. Infect. Dis. 2012, 14, E13–E18. [Google Scholar] [CrossRef]
- Buechler, C.; Neidhöfer, C.; Hornung, T.; Neuenhoff, M.; Parčina, M. Detection and characterization of clinical bordetella trematum isolates from chronic wounds. Pathogens 2021, 10, 966. [Google Scholar] [CrossRef] [PubMed]
- Bosshard, P.P.; Zbinden, R.; Abels, S.; Böddinghaus, B.; Altwegg, M.; Böttger, E.C. 16S rRNA gene sequencing versus the API 20 NE system and the VITEK 2 ID-GNB card for identification of nonfermenting Gram-negative bacteria in the clinical laboratory. J. Clin. Microbiol. 2006, 44, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Souza, H.A.P.H.D.M.D.; Dalla-Costa, L.M.; Vicenzi, F.J.; De Souza, D.C.; Riedi, C.A.; Filho, N.A.R.; Pillonetto, M. MALDI-TOF: A useful tool for laboratory identification of uncommon glucose non-fermenting gram-negative bacteria associated with cystic fibrosis. J. Med. Microbiol. 2014, 63, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Farfour, E.; Leto, J.; Barritault, M.; Barberis, C.; Meyer, J.; Dauphin, B.; Le Guern, A.-S.; Leflèche, A.; Badell, E.; Guiso, N.; et al. Evaluation of the andromas matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of aerobically growing gram-positive bacilli. J. Clin. Microbiol. 2012, 50, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.F.; Barrios, R.; Zintgraff, J.; Martínez, C.; Irazu, L.; Vay, C.; Prieto, M. Utility of platforms Viteks MS and Microflex LT for the identification of complex clinical isolates that require molecular methods for their taxonomic classification. PLoS ONE 2019, 14, e0218077. [Google Scholar] [CrossRef]
- Bittar, F.; Rolain, J.-M. Detection and accurate identification of new or emerging bacteria in cystic fibrosis patients. Clin. Microbiol. Infect. 2010, 16, 809–820. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Chow, W.-N.; Foo, C.-H.; Curreem, S.O.T.; Lo, G.C.-S.; Teng, J.L.L.; Chen, J.H.K.; Ng, R.H.Y.; Wu, A.K.L.; Cheung, I.Y.Y.; et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci. Rep. 2016, 6, 26045. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Perng, C.-L.; Jian, M.-J.; Lee, S.-Y.; Sun, J.-R.; Shang, H.-S. Multicentre study evaluating matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of clinically isolated Elizabethkingia species and analysis of antimicrobial susceptibility. Clin. Microbiol. Infect. 2019, 25, 340–345. [Google Scholar] [CrossRef]
- Liang, C.-Y.; Yang, C.-H.; Lai, C.-H.; Huang, Y.-H.; Lin, J.-N. Genomic Features, Comparative Genomic Analysis, and Antimicrobial Susceptibility Patterns of Chryseobacterium arthrosphaerae Strain ED882-96 Isolated in Taiwan. Genes 2019, 10, 309. [Google Scholar] [CrossRef]
- Coward, A.; Kenna, D.T.; Woodford, N.; Turton, J.F.; Armstrong, M.; Auckland, C.; Bowler, I.; Burns, P.; Cargill, J.; Carroll, M.; et al. Structured surveillance of Achromobacter, Pandoraea and Ralstonia species from patients in England with cystic fibrosis. J. Cyst. Fibros. 2020, 19, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; LiPuma, J.J. Use of the gyrB gene for the identification of Pandoraea species. FEMS Microbiol. Lett. 2002, 208, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Liu, L.; Vandamme, P.; LiPuma, J.J. Identification of Pandoraea species by 16S ribosomal DNA-based PCR assays. J. Clin. Microbiol. 2001, 39, 4452–4455. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Vandamme, P.; LiPuma, J.J. Infection by Ralstonia species in cystic fibrosis patients: Identification of R. pickettii and R. mannitolilytica by polymerase chain reaction. Emerg. Infect. Dis. 2002, 8, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Maroye, P.; Doermann, H.; Rogues, A.; Gachie, J.; Mégraud, F. Investigation of an outbreak of Ralstonia pickettii in a paediatric hospital by RAPD. J. Hosp. Infect. 2000, 44, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, M.M. Antimicrobial susceptibility testing for glucose-nonfermenting gram-negative bacteria: The tip of the iceberg. Antimicrob. Agents Chemother. 2020, 64, e00011-20. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 12.0, Valid from 2022-01-01 2022. Available online: http://www.eucast.org (accessed on 8 October 2022).
- CLSI M100-ED30:2020; Performance Standards for Antimicrobial Susceptibility Testing, 30th ed. Clinical and laboratory Standard Institute: Wayne, PA, USA, 2020.
- Kiratisin, P.; Koomanachai, P.; Kowwigkai, P.; Pattanachaiwit, S.; Aswapokee, N.; Leelaporn, A. Early-onset prosthetic valve endocarditis caused by Inquilinus sp. Diagn. Microbiol. Infect. Dis. 2006, 56, 317–320. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Essig, A.; Sommerburg, O. Inquilinus limosus in patients with cystic fibrosis, Germany. Emerg. Infect. Dis. 2005, 11, 457–459. [Google Scholar] [CrossRef]
- Lenhart-Pendergrass, P.M.; Caverly, L.J.; Wagner, B.D.; Sagel, S.D.; Nick, J.A.; LiPuma, J.J.; Martiniano, S.L. Clinical characteristics and outcomes associated with Inquilinus infection in cystic fibrosis. J. Cyst. Fibros. 2021, 20, 310–315. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Iula, D.V.; Pagliuca, C.; Pastore, G.; Pagliarulo, C.; Catania, M.R.; Colicchio, R.; Picardi, M.; Raia, V.; Salvatore, P. Identification of Inquilinus limosus in cystic fibrosis: A first report in Italy. New Microbiol. 2014, 37, 567–571. [Google Scholar]
- Kohlmann, R.; Barenberg, K.; Anders, A.; Gatermann, S.G. Acetobacter indonesiensis Bacteremia in Child with Metachromatic Leukodystrophy. Emerg. Infect. Dis. 2016, 22, 1681–1683. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.; Power, P.; Gutkind, G.; Di Conza, J.A. INQ-1, a chromosome-encoded AmpC β-lactamase from Inquilinus limosus. J. Antimicrob. Chemother. 2014, 69, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Queenan, A.M.; Bauernfeind, A. Novel carbapenem-hydrolyzing oxacillinase OXA-62 from Pandoraea pnomenusa. Antimicrob. Agents Chemother. 2006, 50, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Bauernfeind, A. Intrinsic carbapenem-hydrolyzing oxacillinases from members of the genus pandoraea. Antimicrob. Agents Chemother. 2015, 59, 7136–7141. [Google Scholar] [CrossRef] [PubMed]
- Fluit, A.C.; Bayjanov, J.R.; Aguilar, M.D.; Cantón, R.; Tunney, M.M.; Elborn, J.S.; van Westreenen, M.; Ekkelenkamp, M.B. Characterization of clinical Ralstonia strains and their taxonomic position. Antonie van Leeuwenhoek 2021, 114, 1721–1733. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, D.; Niu, S.; Chen, Q.; Lin, Q.; Zhang, X. Mbls, rather than efflux pumps, led to carbapenem resistance in fosfomycin and aztreonam/avibactam resistant elizabethkingia anophelis. Infect. Drug Resist. 2021, 14, 315–327. [Google Scholar] [CrossRef]
- González, L.J.; Vila, A.J. Carbapenem resistance in Elizabethkingia meningoseptica is mediated by metallo-β-lactamase BlaB. Antimicrob. Agents Chemother. 2012, 56, 1686–1692. [Google Scholar] [CrossRef]
- Woodford, N.; Palepou, M.-F.I.; Babini, G.S.; Holmes, B.; Livermore, D.M. Carbapenemases of Chryseobacterium (Flavobacterium) meningosepticum: Distribution of blaB and characterization of a novel metallo-β-lactamase gene, blaB3, in the type strain, NCTC 10016. Antimicrob. Agents Chemother. 2000, 44, 1448–1452. [Google Scholar] [CrossRef]
- Damas, M.S.F.; Ferreira, R.L.; Campanini, E.B.; Soares, G.G.; Campos, L.C.; Laprega, P.M.; da Costa, A.S.; Freire, C.C.D.M.; Pitondo-Silva, A.; Cerdeira, L.T.; et al. Whole genome sequencing of the multidrug-resistant Chryseobacterium indologenes isolated from a patient in Brazil. Front. Med. 2022, 9, 2143. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, X.; Feng, C.; Li, A.; Dong, H.; Wu, S.; Zheng, B. Whole genome sequencing uncovers a novel IND-16 metallo-β-lactamase from an extensively drug-resistant Chryseobacterium indologenes strain J31. Gut Pathog. 2016, 8, 47. [Google Scholar] [CrossRef]
- Cimmino, T.; Rolain, J.-M. Whole genome sequencing for deciphering the resistome of Chryseobacterium indologenes, an emerging multidrug-resistant bacterium isolated from a cystic fibrosis patient in Marseille, France. New Microbes New Infect. 2016, 12, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhang, Q.; Gu, Z. Molecular diversity of chromosomal metallo-β-lactamase genes in Elizabethkingia genus. Int. J. Antimicrob. Agents 2020, 56, 105978. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.; Di Conza, J.; Gutkind, G. Draft genome sequence of Inquilinus limosus strain MP06, a multidrug-resistant clinical isolate. Braz. J. Microbiol. 2015, 46, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-L.; Ee, R.; Yong, D.; Yu, C.-Y.; Ang, G.-Y.; Tee, K.-K.; Yin, W.-F.; Chan, K.-G. Complete Genome Sequence Analysis of Pandoraea pnomenusa Type Strain DSM 16536(T) Isolated from a Cystic Fibrosis Patient. Front. Microbiol. 2016, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Yang, Y.; Su, J.; Xu, X.; Wang, M.; Chen, Y.; Li, Y. Clinical and molecular characteristics of Chryseobacterium indologenes isolates at a teaching hospital in Shanghai, China. Ann. Transl. Med. 2021, 9, 668. [Google Scholar] [CrossRef]
- Shinha, T.; Ahuja, R. Bacteremia due to Elizabethkingia meningoseptica. IDCases 2015, 2, 13–15. [Google Scholar] [CrossRef]
- Zdziarski, P.; Paściak, M.; Rogala, K.; Korzeniowska-Kowal, A.; Gamian, A. Elizabethkingia miricola as an opportunistic oral pathogen associated with superinfectious complications in humoral immunodeficiency: A case report. BMC Infect. Dis. 2017, 17, 763. [Google Scholar] [CrossRef]
- Arbune, M.; Fotea, S.; Nechita, A.; Stefanescu, V. Emerging Infection with Elizabethkingia meningoseptica in Neonate. A Case Report. J. Crit. Care Med. 2018, 4, 96–100. [Google Scholar] [CrossRef]
- Ceyhan, M.; Yıldırım, I.; Tekelı, A.; Yurdakok, M.; Us, E.; Altun, B.; Kutluk, T.; Cengiz, A.B.; Gurbuz, V.; Barın, C.; et al. A Chryseobacterium meningosepticum outbreak observed in 3 clusters involving both neonatal and non-neonatal pediatric patients. Am. J. Infect. Control. 2008, 36, 453–457. [Google Scholar] [CrossRef]
- Olbrich, P.; Rivero-Garvía, M.; Falcón-Neyra, M.D.; Lepe, J.A.; Cisneros, J.M.; Márquez-Rivas, J.; Neth, O. Chryseobacterium indologenes central nervous system infection in infancy: An emergent pathogen? Infection 2014, 42, 179–183. [Google Scholar] [CrossRef]
- Hayes, D.; Murphy, B.S.; Kuhn, R.J.; Anstead, M.I.; Feola, D.J. Mucoid Inquilinus limosus in a young adult with cystic fibrosis. Pediatr. Pulmonol. 2009, 44, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.; Andersson, M.I.; Ives, A.; Hull, J.; Chapman, S.J.; Flight, W.G. Familial cluster of Inquilinus limosus infection among three brothers with cystic fibrosis. Respir. Med. Case Rep. 2021, 34, 101487. [Google Scholar] [CrossRef]
- Poore, T.S.; Virella-Lowell, I.; Guimbellot, J.S. Potential pathogenicity of Inquilinus limosus in a pediatric patient with cystic fibrosis. Pediatr. Pulmonol. 2018, 53, E21–E23. [Google Scholar] [CrossRef] [PubMed]
- Millard, A.D.; Westblade, L.F.; LiPuma, J.J.; Vavikolanu, K.; Read, T.D.; Pallen, M.; Burd, E.M.; Constantinidou, C.I. Draft Genome Sequence of the Pandoraea apista LMG 16407 Type Strain. Genome Announc. 2015, 3, e01300-15. [Google Scholar] [CrossRef]
- Mwanza, E.P.; Hugo, A.; Charimba, G.; Hugo, C.J. Pathogenic Potential and Control of Chryseobacterium Species from Clinical, Fish, Food and Environmental Sources. Microorganisms 2022, 10, 895. [Google Scholar] [CrossRef]
- Zajmi, A.; Teo, J.; Yeo, C.C. Epidemiology and Characteristics of Elizabethkingia spp. Infections in Southeast Asia. Microorganisms 2022, 10, 882. [Google Scholar] [CrossRef]
- Herasimenka, Y.; Cescutti, P.; Impallomeni, G.; Rizzo, R. Exopolysaccharides produced by Inquilinus limosus, a new pathogen of cystic fibrosis patients: Novel structures with usual components. Carbohydr. Res. 2007, 342, 2404–2415. [Google Scholar] [CrossRef]
- Maunders, E.; Welch, M. Matrix exopolysaccharides; the sticky side of biofilm formation. FEMS Microbiol. Lett. 2017, 364, 120. [Google Scholar] [CrossRef]
- Kuttel, M.; Ravenscroft, N.; Foschiatti, M.; Cescutti, P.; Rizzo, R. Conformational properties of two exopolysaccharides produced by Inquilinus limosus, a cystic fibrosis lung pathogen. Carbohydr. Res. 2012, 350, 40–48. [Google Scholar] [CrossRef]
- Costello, A.; Herbert, G.; Fabunmi, L.; Schaffer, K.; Kavanagh, K.; Caraher, E.M.; Callaghan, M.; McClean, S. Virulence of an emerging respiratory pathogen, genus Pandoraea, in vivo and its interactions with lung epithelial cells. J. Med. Microbiol. 2011, 60, 289–299. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Chiu, C.-H.; Chu, C.; Tang, P.; Su, L.-H. Invasion of murine respiratory tract epithelial cells by Chryseobacterium meningosepticum and identification of genes present specifically in an invasive strain. New Microbiol. 2006, 29, 55–62. [Google Scholar] [PubMed]
- Lin, P.-Y.; Chen, H.-L.; Huang, C.-T.; Su, L.-H.; Chiu, C.-H. Biofilm production, use of intravascular indwelling catheters and inappropriate antimicrobial therapy as predictors of fatality in Chryseobacterium meningosepticum bacteraemia. Int. J. Antimicrob. Agents 2010, 36, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Puah, S.M.; Fong, S.P.; Kee, B.P.; Puthucheary, S.; Chua, K.H. Molecular identification and biofilm-forming ability of Elizabethkingia species. Microb. Pathog. 2022, 162, 105345. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Lo, H.-H.; Hsieh, H.-Y.; Chang, S.-M. Identification, epidemiological relatedness, and biofilm formation of clinical Chryseobacterium indologenes isolates from central Taiwan. J. Microbiol. Immunol. Infect. 2015, 48, 559–564. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2009, 8, 15–25. [Google Scholar] [CrossRef]
- Rogers, G.B.; Hoffman, L.R.; Whiteley, M.; Daniels, T.W.; Carroll, M.P.; Bruce, K.D. Revealing the dynamics of polymicrobial infections: Implications for antibiotic therapy. Trends Microbiol. 2010, 18, 357–364. [Google Scholar] [CrossRef]
- Magalhães, A.P.; Lopes, S.P.; Pereira, M.O. Insights into Cystic Fibrosis Polymicrobial Consortia: The Role of Species Interactions in Biofilm Development, Phenotype, and Response to In-Use Antibiotics. Front. Microbiol. 2017, 7, 2146. [Google Scholar] [CrossRef]
- O’brien, T.J.; Figueroa, W.; Welch, M. Decreased efficacy of antimicrobial agents in a polymicrobial environment. ISME J. 2022, 16, 1694–1704. [Google Scholar] [CrossRef]
- Coman, I.; Bilodeau, L.; Lavoie, A.; Carricart, M.; Tremblay, F.; Zlosnik, J.; Berthiaume, Y. Ralstonia mannitolilytica in cystic fibrosis: A new predictor of worse outcomes. Respir. Med. Case Rep. 2017, 20, 48–50. [Google Scholar] [CrossRef]
- Cooke, R.; O’neill, W.; Xu, J.; Moore, J.; Elborn, J. Inquilinus limosus isolated from a cystic fibrosis patient: First UK report. Br. J. Biomed. Sci. 2007, 64, 127–129. [Google Scholar] [CrossRef]
- Degand, N.; Lotte, R.; Le Butor, C.D.; Segonds, C.; Thouverez, M.; Ferroni, A.; Vallier, C.; Mély, L.; Carrère, J. Epidemic spread of Pandoraea pulmonicola in a cystic fibrosis center. BMC Infect. Dis. 2015, 15, 583. [Google Scholar] [CrossRef] [PubMed]
- The UK Cystic Fibrosis Trust Infection Control Group. Standards for the Clinical Care of Children and Adults with Cystic Fibrosis in the UK; The UK Cystic Fibrosis Trust Infection Control Group: Bromley, UK, 2011. [Google Scholar]
- The UK Cystic Fibrosis Trust Infection Control Group. The Burkholderia cepacia Complex—Suggestions for Prevention and Infection Control; The UK Cystic Fibrosis Trust Infection Control Group: Bromley, UK, 2004. [Google Scholar]
- Saiman, L.; Siegel, J.D.; Lipuma, J.J.; Brown, R.; Bryson, E.A.; Chambers, M.J.; Downer, V.S.; Fliege, J.; Hazle, L.A.; Jain, M.; et al. Infection Prevention and Control Guideline for Cystic Fibrosis: 2013 Update. Infect. Control. Hosp. Epidemiology 2014, 35, s1–s67. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.R.; Smith, S.J.; Rowbotham, N.J. Infection prevention and control in cystic fibrosis: One size fits all The argument against. Paediatr. Respir. Rev. 2019, 36, 94–96. [Google Scholar] [CrossRef]
- Stockwell, R.E.; Wood, M.E.; Ballard, E.; Moore, V.; Wainwright, C.E.; Bell, S.C. Current infection control practices used in Australian and New Zealand cystic fibrosis centers. BMC Pulm. Med. 2020, 20, 16–18. [Google Scholar] [CrossRef]
- Saiman, L.; Zhou, J.J.; Jiang, X.; Kosorok, M.R.; Muhlebach, M.S. Surveying Cystic Fibrosis Care Centers to Assess Adoption of Infection Prevention and Control Recommendations. Infect. Control. Hosp. Epidemiol. 2018, 39, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Foley, P.; Yason, C.; Vanderstichel, R.; Muckle, A. Prevalence of feline herpesvirus-1, feline calicivirus, chlamydia felis, and bordetella bronchiseptica in a population of shelter cats on Prince Edward island. Can. J. Vet. Res. 2020, 84, 181–188. [Google Scholar]
- Berger, A.; Willi, B.; Meli, M.L.; Boretti, F.S.; Hartnack, S.; Dreyfus, A.; Lutz, H.; Hofmann-Lehmann, R. Feline calicivirus and other respiratory pathogens in cats with Feline calicivirusrelated symptoms and in clinically healthy cats in Switzerland. BMC Veter. Res. 2015, 11, 282. [Google Scholar] [CrossRef]
- Binns, S.H.; Dawson, S.; Speakman, A.J.; Cuevas, L.E.; Gaskell, C.J.; Hart, C.A.; Morgan, K.L.; Gaskell, R.M. Prevalence and risk factors for feline Bordetella bronchiseptica infection. Veter. Rec. 1999, 144, 575–580. [Google Scholar] [CrossRef]
- Day, M.; Carey, S.; Clercx, C.; Kohn, B.; MarsilIo, F.; Thiry, E.; Freyburger, L.; Schulz, B.; Walker, D. Aetiology of Canine Infectious Respiratory Disease Complex and Prevalence of its Pathogens in Europe. J. Comp. Pathol. 2020, 176, 86–108. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Vaccine Scheduler—Pertussis: Recommended Vaccinations n.d. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=3&SelectedCountryIdByDisease=-1 (accessed on 8 October 2022).
- Saul, N.; Wang, K.; Bag, S.; Baldwin, H.; Alexander, K.; Chandra, M.; Thomas, J.; Quinn, H.; Sheppeard, V.; Conaty, S. Effectiveness of maternal pertussis vaccination in preventing infection and disease in infants: The NSW Public Health Network case-control study. Vaccine 2018, 36, 1887–1892. [Google Scholar] [CrossRef]
- Rowe, S.L.; Tay, E.L.; Franklin, L.J.; Stephens, N.; Ware, R.; Kaczmarek, M.C.; Lester, R.A.; Lambert, S.B. Effectiveness of parental cocooning as a vaccination strategy to prevent pertussis infection in infants: A case-control study. Vaccine 2018, 36, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Rendall, J.C.; Millar, B.C. A doggy tale: Risk of zoonotic infection with Bordetella bronchiseptica for cystic fibrosis (CF) patients from live licenced bacterial veterinary vaccines for cats and dogs. J. Clin. Pharm. Ther. 2022, 47, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Rendall, J.C.; Millar, B.C. Does Bordetella pertussis vaccine offer any cross-protection against Bordetella bronchiseptica? Implications for pet owners with cystic fibrosis. J. Clin. Pharm. Ther. 2021, 46, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).