Abstract
Critically ill COVID-19 patients requiring mechanical ventilation in the intensive care unit are at risk of developing invasive candidiasis. In this study we aimed to (1) characterize oral cultivable mycobiota of mechanically ventilated adult COVID-19 patients in an ICU setting by sampling four distinct oral niches in two fixed time points with regards to oral health status, (2) investigate Candida spp. infections in this population, and (3) compare oral mycobiota with selected bacteriobiota strains during the observation in the ICU. We recruited 56 adult COVID-19 patients who qualified for mechanical ventilation. Patients received either standard or extended oral care procedures with tooth brushing. Oral samples were taken first within 36 h and after 7 days of intubation. Yeast-like fungi were identified by MALDI/TOF mass spectrometry. Yeast infection cases were retrospectively analyzed. Candida spp. in oral sampling was identified in 80.4% and 75.7%, C. albicans in 57.1% and 61.1%, and non-albicans Candida species in 48.2% and 47.2% patients at baseline and follow-up, respectively. There were no differences in the overall CFU counts of Candida spp. species and individual Candida species in oral samples, both at baseline and follow-up. At baseline, a higher prevalence of Candida spp. was associated with a higher identification rate of Lactobacillus spp. (64.4% vs. 27.3%, p = 0.041). At follow-up, there was a borderline lower prevalence of Candida spp. in patients with Lactobacillus spp. identified (57.1% vs. 87.0%, p = 0.057). The incidence rate of candidiasis was 5.4% and the incidence density was 3.1/1000 pds. In conclusion, non-albicans Candida species in oral samples were identified in nearly half of patients. Oral health was moderately impaired. A high incidence of yeast infections, including invasive cases, in patients hospitalized in the ICU due to COVID-19 and requiring mechanical ventilation was noted. Severe COVID-19 and disease-specific interventions within the ICU possibly played a major role promoting Candida spp. infections.
1. Introduction
Candida spp. is one of the most important components of human microbiota [1]. Of various fungi, mainly the yeast-like Candida genus, especially Candida albicans, plays an import role in oral cavity colonization [1]. The prevalence of C. albicans colonization has been previously reported to range between 15 and 30% [1]. In critically ill patients, the isolation rate of Candida spp. can be as high as 50% [2]. In immunocompromised patients, these commensal species can colonize the lower respiratory system and form pathological biofilms on the mucosal surfaces, which has previously been associated with longer duration of mechanical ventilation, increased risk of ventilator associated pneumonia, increased length of intensive care unit (ICU) stay, and higher mortality [3,4]. In this population, Candida spp. can cause opportunistic yeast infections [4]. Previous studies suggested multiple bidirectional interactions between Candida spp. and oral bacteriota [4,5,6]. Oral bacteria can facilitate yeast infections by promoting the expression of virulence genes in C. albicans. Reversely, C. albicans may change the antibiotic resistance patterns of pathogenic bacteria when coexisting in biofilms [7,8]. Recent studies showed that patients with periodontitis have an increased risk of complications in the course of COVID-19 infection and that COVID-19 can exacerbate periodontal disease [9]. Treatment of periodontal disease, including photodynamic therapy adjunctive to standard antimicrobial treatment and mechanical methods of scaling and root planing, may be treated as a preventive measure against potential exacerbation of COVID-19 [9,10].
COVID-19 is caused by the SARS-CoV-2 and causes mild to moderate respiratory illness. In individuals with comorbidities or with compromised immune systems, the risk of severe forms of COVID-19, including acute respiratory distress syndrome (ARDS), is higher [11]. Such critically ill COVID-19 patients, when admitted to the intensive care unit (ICU) and requiring mechanical ventilation, are more likely to develop healthcare-associated infections [12,13]. Cases of both bacterial and fungal infections were reported, with studies focusing on multidrug resistant bacterial strains, mucormycosis, and aspergillosis [12,14,15,16]. As Candida spp. infections in ventilated COVID-19 patients are still under-researched [3], in this study, we intended to provide new evidence in this matter. By implementing individual sampling niches twice—immediately after the initiation of mechanical ventilation and 7 days afterwards—we tried to provide a thorough characterization of oral microbiota and their changes during the hospitalization in the ICU.
In this study, we aimed to (1) characterize oral cultivable mycobiota of mechanically ventilated adult COVID-19 patients in an ICU setting by sampling four distinsct oral niches in two fixed time points with regards to oral health status, (2) investigate Candida spp. infections in this population, and (3) compare oral mycobiota with selected bacteriobiota strains during the observation in the ICU.
2. Materials and Methods
Adult patients admitted to the University Hospital in Krakow, Poland between 1 September 2021 and 31 January 2022,were offered the opportunity to participate in the study and asked for the signed consent form on admission to the hospital. During hospitalization, 56 of them qualified for intubation and were hospitalized in the temporary intensive care unit (ICU) for COVID-19 patients.
The inclusion criteria were as follows:
- SARS-CoV-2 infection confirmed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swabs upon hospital admission;
- Signed consent to participate in the study;
- Patients were admitted to the ICU;
- ntubation due to COVID-19-related pneumonia and acute respiratory distress syndrome (ARDS) within 36 h preceding study procedures.
Demographic and clinical data were gathered from the hospital electronic medical records, including but not limited to age, sex, date of COVID-19 diagnosis, admission to the hospital and ICU, date of intubation, selected comorbidities, and pre- and postintubation treatment, including systemic steroids and antibiotics. SOFA (Sequential Organ Failure Assessment) [17] was calculated at baseline and follow-up. Selected baseline and maximal laboratory results were also extracted. Information on bacterial and yeast infection during the hospitalization was recorded.
2.1. Oral Cavity Sampling Methods
During the observation, patients received two types of oral care—the standard procedure that included cleaning, moisturizing of oral cavity and suction of excess fluid or the extended procedure with additional tooth brushing. The detailed description of the intervention and its effects on oral bacteriobiota has been published elsewhere [13].
The oral cavity health status was inspected and the presence of symptoms of oral candidiasis was recorded. Oral health was assessed using a modified Beck Oral Assessment Scale (BOAS) consisting of 5 subscales, namely assessment of lips, mucosa and gingiva, tongue, teeth, and saliva. Oral samples from four oral habitats (buccal mucosa, tongue, buccal dental surface and gingival pocket) were taken two times, first within 36 h of intubation (baseline) and again after 7 days of intubation (follow-up). Every sample was taken by a trained dentist.
ESwabs™ (COPAN-invented flocked swab with 1 mL of liquid amine in a plastic, screw cap tube, COPAN Diagnostics, Muriera, CA, USA) were used for sampling mucosal surfaces of the posterior part of the dorsum of the tongue and buccae. Tooth Cleanic KerrHawe—KWX-OP-SZ-011 (KerrHawe SA, Biggio, Switzerland) was used to collect the dental plaque from the buccal dental surface side. After the collection, the brush was placed in 1 mL of Liquid Amies in a plastic screw cap tube. Gingival crevicular fluid (GCF) samples were collected with three pieces of PerioPaper Strips (Oraflow, Smithtown, NY, USA), designed to absorb 0–1.2 microliters of fluid. The strips were placed in the gingival pocket for 30–45 s, and then in ESwab tubes (COPAN Diagnostics, Muriera, CA, USA). To prevent a contamination of GCF by saliva, sterile gauze was used to dry the tooth surfaces and remove excess saliva from the mucosae.
The collected samples were immediately delivered to the Chair of Microbiology of Jagiellonian University Medical College. The samples were inoculated for variety of microbiological media to identification bacteria and fungi. The samples were inoculated by the dilution method (dilutions −1 to −6) or qualitative culture method (swabs only). For assessment of fungi growth, Chromagar Candida (Graso, Starogard Gdański, Poland), Sabouraud Agar (Biomaxima, Lublin, Poland) were used. Sabouraud agar and Chromagar Candida were aerobically incubated at 28 °C for 48 h.
The bacterial cultures and results were described in detail earlier [13,16]. After incubation, colonies were counted, reported, and assessed phenotypically. Results being presented as colony forming units (CFU) per mL (CFU/mL). The microorganisms were identified by MALDI TOF MS mass spectrometry (Vitek MS Home bioMérieux, with the V3 version of the database).
In this study, the data concerning yeast species identification and CFU counts from all four sampled oral sites were merged as we aimed to characterize the general status of oral mycobiota.
2.2. Diagnosis of Yeast Infections
Associated healthcare-associated infections (HAI) were identified according to definitions of the Healthcare-Associated Infections Surveillance Network (HAI-Net), European Centre for Disease Prevention and Control (protocol version 4.3), which concerns the ICU, taking into account the Guideline for the Management of Candidiasis of the Infectious Diseases Society of America concerning the diagnosis of candidiasis [18,19].
For the microbiological diagnosing of HAIs, clinical samples including blood, blood obtained from the catheter, tracheal or bronchial secretions, and urine obtained via freshly inserted bladder catheter were collected. Only the first isolate from each patient was selected for microbiological analysis, excluding subsequent cultures from the same patient and infection case. Mycological and bacteriological cultures were conducted in parallel for each material. Blood (arterial, venous, collected by central venous cathether [CVC]) was incubated in a BACT/ALERT® VIRTUO® system (Biomerieux, Marcy-l’Étoile, France). The clinical simples (blood from the positive media) were seeded on Sabouraud glucose agar + gentamicin + chloramphenicol (Thermo Scientific, Waltham, MA, USA) media and incubated at 37 °C (blood), at 35 °C (urine) or at 25 °C and 35 °C simultaneously (lower respiratory tract materials). Interpretation of growth and the titer of non-bronchoscopic lavage and urine cultures was based on ECDC and ASM methodology [19,20]. No additional tests were performed in patients enrolled in the study with positive culture of Candida spp., e.g., detection of Candida antigen and/or anti-Candida antibody in serum or in broncho-alveolar lavage (BAL). Due to severe acute respiratory distress, the BAL was not feasible.
2.3. Ethics Statement
The study and its protocol were approved by the Jagiellonian University Bioethics Committee, decision numbers 1072.6120.333.2020, 7 December 2020 and 1072.6120.353.2020, 16 December 2020. Written informed consent was obtained from each subject prior to participation. Trial registration number: 1072.6120.333.2020.
2.4. Statistical Analysis
The PS Imago Pro ver. 7.0 was used for all statistical analyses. The normality of the continuous variable distribution was assessed using the Shapiro‒Wilk test. Differences between groups were analyzed with Student’s t test or nonparametric test (Mann–Whitney U test) when appropriate. Paired data were analyzed using the Wilcoxon test. Continuous variables are presented as the arithmetic mean () ± standard deviation (SD) or as the median with interquartile range (IQR) when the data were not normally distributed. The distribution of categorical variables was described as counts and percentages. Statistical testing was completed to compare categorical variables using an independent sample chi-squared test or Fisher’s exact test when appropriate and dependent samples with McNemar’s test. We measured the strength association by odds ratio (OR) and 95% confidence intervals (CI). Statistical inference was set at p < 0.05.
3. Results
3.1. Demographic and Clinical Characteristics
The study population included 56 adult patients admitted to an ICU ward who required mechanical ventilation due to COVID-19-related pneumonia. The mean age of the participants was 66.5 ± 12.7 years, and there were 24 (42.9%) females. The population was obese with a mean BMI of 31.9 ± 5.8. Sixteen (28.6%) were admitted directly from the emergency ward, and forty (71.4%) were transferred from another hospital ward. The mean time from COVID-19 diagnosis to intubation was 6.95 ± 6.62 days. On admission to ICU, the median BOAS was 12 (IQR 10–14), and on the follow-up was 11 (IQR 9–14), showing moderate dysfunction of oral health.
Pre-ICU, systemic steroid therapy was used in 76.9%, antibiotics in 63.5%, and antifungal agents in 14.3% of patients. There were no differences in the SOFA score at baseline and follow-up. The full pre-ICU characteristics of the study population have been previously described elsewhere [21]. In the ICU, steroids, antibiotics and antifungal agents were used in 85.7%, 55.4%, and 10.6% of patients, respectively. The overall mortality in this cohort was 76.8% throughout the whole hospitalization period, with 7-day mortality of 33.9%. The clinical characteristics of the study participants are presented in Table 1.

Table 1.
Clinical characteristics of study participants.
3.2. Oral Cultivable Mycobiota Composition
There were no signs of oral thrush either at baseline or follow-up in the studied population. No white plaques or reddish atrophic areas were observed. At baseline, Candida spp. was identified in 80.4% patients. The most common was C. albicans, identified in 57.1%. Non-albicans Candida species were present in 48.2% of patients with C. dubliniensis in 17.9%, followed by C. glabrata in 16.1% and C. kefyr in 14.3% of patients, with the remaining species present in singular cases.
Candida spp. were identified in 75.7% of patients at follow-up. C. albicans was present in 61.1%, and non-albicans Candida species was present in 47.2%. C. dubliniensis were identified in 13.5%, C. kefyr in 13.5%, and C. glabrata in 18.9% of patients. The remaining species were identified rarely. There were no significant differences in the frequency of identification of Candida spp. between the baseline and follow-up sampling. There were no differences in the mycobiota composition between the four prespecified sampling sites, either at baseline or follow-up. The oral mycobiota composition is presented in Table 2.

Table 2.
List of all identified Candia species in oral samples &.
Pre-ICU and in-ICU antifungal agent use, history of diabetes, oral health status, type of oral care procedure, and SOFA score did not significantly affect the frequency of identification and CFU counts of Candida spp. in oral samples. However, the use of steroids and antibiotics, both pre-ICU and in ICU, showed a trend towards higher CFU counts of Candida spp. in baseline and follow-up samples (Figure 1A–D, Tables S2–S8).
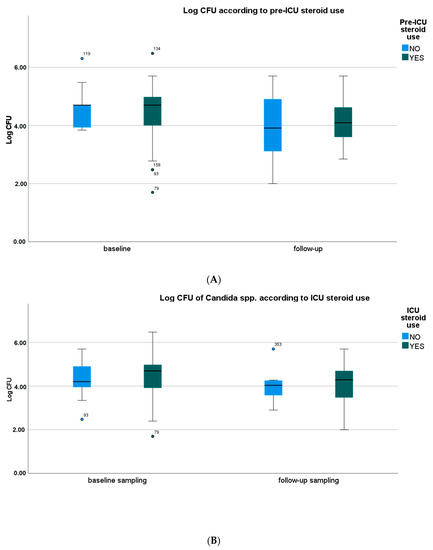
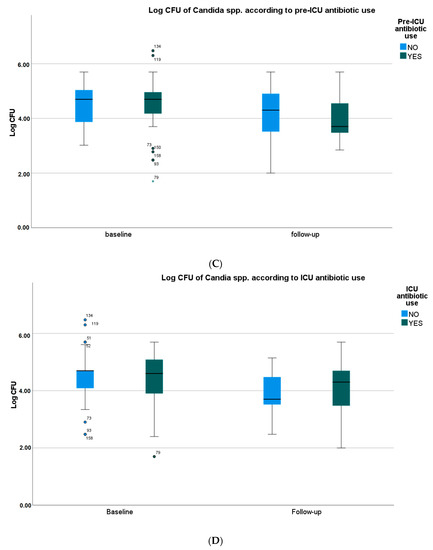
Figure 1.
Yeast species density according to the use of selected drugs. (A) Yeast species density according to the pre-ICU use of systemic steroids. (B) Yeast species density according to the ICU use of systemic steroids. (C) Yeast species density according to the pre-ICU use of antibiotics. (D) Yeast species density according to the ICU use of antibiotics. CFU—colony-forming unit. Medians, IQRs, errors, outliers (dot), and extreme values (asterisk) are presented.
Numerically, CFU counts of Candida spp. strains decreased between the baseline and follow-up sampling. However, there were no significant differences in the overall CFU counts of all Candida spp. species, C. albicans and non-albicans Candida species, and individual Candida species, both at baseline and follow-up (Table 3).

Table 3.
Oral mycobiota composition—quantitative analysis.
3.3. Comparison of Mycobiota with Selected Bacteriobiota Strains
Between the baseline and follow-up oral sampling, a decrease in the overall number of bacteria and fungi species from all sites was observed (median 6 vs. 4, p = 0.005). There were no significant differences in the overall CFU counts of bacterial and Candida spp. strains (Figure 2, Table S1).
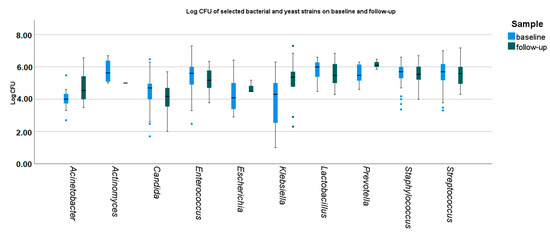
Figure 2.
Baseline and follow-up density of selected bacterial and yeast strains. Medians, IQRs, errors, and outliers (dots) are presented. CFU—colony-forming unit.
At baseline, the CFU counts of identified microbial strains differed significantly, namely Streptococcus spp., Staphyloccus spp., and Lactobacillus were higher, and Candida spp., Escherichia coli, A. baumanii, and K. pneumoniae were lower than the remaining strains (Figure 2 and Figure 3).
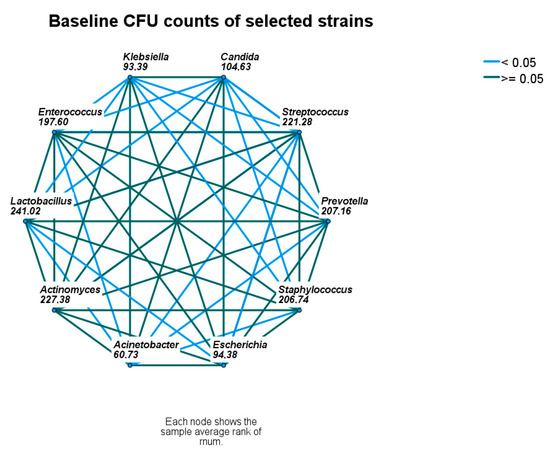
Figure 3.
Baseline comparison of microbial densities. Nodes connected with blue lines differ significantly. CFU—colony-forming unit.
At follow-up, significant differences between the CFU counts of identified microbial strains were less common. CFU counts of Candida spp. were still lower than Enterecoccus spp., Lactobacillus spp., Prevotella spp., and Streptococcus spp. and Staphylococcus spp. The CFU counts of Prevotella spp. remained higher than Candida spp. and A. baumanii. (Figure 2 and Figure 4).
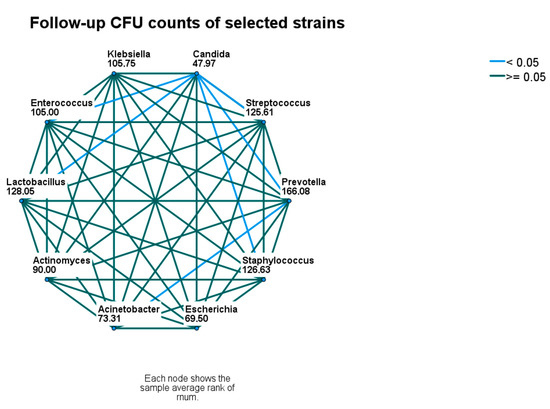
Figure 4.
Follow-up comparison of microbial densities. Nodes connected with blue lines differ significantly. CFU—colony-forming unit.
Considering differences between identification rates of Candida spp. and selected bacterial strains in oral samples from all sampled oral niches, at baseline, higher prevalence of Candida spp. was associated with a higher identification rate of Lactobacillus spp. (64.4% vs. 27.3%, p = 0.041). Conversely, at follow-up, there was a lower prevalence of Candida spp. in patients with Lactobacillus spp. identified, but it was borderline insignificant (57.1% vs. 87.0%, p = 0.057) [11]. There were no associations with the identification rate of A. baumannii, K. pneumoniae, E. faecalis, and P. aeruginosa and the presence of Candida spp strains.
3.4. Yeast Infections
Candida spp. infections were recognized in 7 patients:
- 2 cases of microbiologically confirmed CVC-related bloodstream infection (C. albicans and C. tropicalis) without quantitative CVC blood sample or quantitative or semi-quantitative CVC culture;
- 1 case of primary bloodstream infection, C. glabrata;
- 2 cases of mixed bacterial-fungal symptomatic urinary tract infection:
- ○
- C. glabrata and A. baumannii
- ○
- C. albicans and Enterobacter cloeacae;
- 2 putative cases of mixed bacterial–fungal symptomatic urinary tract infection, in both cases the urine specimens were taken from a Foley catheter:
- ○
- C. albicans and Enterobacter cloeacae
- ○
- C. parapsilosis and Enterococcus faecium;
- 1 putative case of pneumonia—the mixed culture of C. lusitaniae, C. inconspicua, C. tropicalis, and C. albicans with 104 CFU/mL for each were obtained with non-bronchoscopic lavage.
The incidence rate of invasive yeast infections was 5.4%, with the incidence density rate of 3.1/1000 patient days (pds). The incidence rate of all true and putative yeast infections was 12.5%, with the incidence density rate of 7.3/1000 pds.
In the two cases of CVC-related bloodstream infection caused by C. albicans and C. tropicalis, the same species were identified in both the samples from the oral cavity. In the case of primary bloodstream infection caused by C. glabrata, the strain of C. glabrata was also identified in the oral samples.
4. Discussion
The presence of Candida spp. in oral samples, with no signs of thrush or inflammatory lesions on the oral mucosa, indicates that only the oral colonization with Candida spp. was observed in the studied population. Previous studies revealed that Candida spp. comprise oral mycobiota, being present in 30–80% of healthy individuals [22]. Only a small portion of patients with oral Candida spp. colonization present signs of candidiasis [23]. Immunocompromised patients and those hospitalized in the ICU have a higher risk of developing oral candidiasis due to its adhesion ability, antigenic variation, increased production of hydrolytic enzymes, and immunomodulatory activity [24]. In our study, in patients with severe COVID-19 admitted to the ICU and requiring mechanical ventilation, there were no cases of oral candidiasis, but the oral colonization with Candida spp. was common. C. albicans was identified most frequently, but a remarkably high prevalence of non-albicans Candida species in nearly half of the patients was also observed. Of non-albicans Candida species, C. dublinensis dominated, followed by C. glabrata and C. kefyr. To explore any potential shifts during the initial period after ventilation, the oral cavity was sampled once more. The colonization rate remained high in the second sampling 7 days postintubation regardless of antimicrobial drugs and steroid treatment, oral health status, and oral care procedures. Similar to our findings, C. albicans was reported to be the most common species, with marked presence of C. dublinensis, Candida glabrata, Candida parapsilosis, Candida krusei, Candida tropicalis, and Candida pseudotropicalis [22,25]. The colonization of the oral cavity with Candida species is not per se pathological, but as an opportunistic microorganism, it may predispose selected individuals, especially the immune incompetent, to invasive infections [24]. Other factors leading to higher risk invasive candidiasis development include altered local oral mucosal environment, antibiotic or steroid administration, coinfections, age, diabetes, smoking, history of surgical procedures within the oral cavity, or improper oral hygiene [26,27,28,29,30].
Patients in critical condition and hospitalized in the ICU are at even higher risk of invasive candidiasis. It was reported that approx. 30% of the patients in the ICU setting show fungal colonization (defined as Candida spp. growth in at least one sample other than blood, such as urinary culture, are tracheal aspirates) without clinical findings of infection. C. albicans was the most common species [31,32]. Severe COVID-19 alone is a life-threatening infection, with nearly 50% mortality reported [33,34]. As our data showed, the total mortality in this cohort reached 76.8% throughout the whole hospitalization period, with 7-day mortality of 33.9%. Candidemia in an ICU setting has been proven to show high mortality rates exceeding 50% [35,36,37]. Severe COVID-19 associated with candidemia in patients hospitalized in the ICU has the potential to form an extremely dangerous combination.
Many factors have been proposed to predispose for the development of invasive candidiasis in COVID-19 patients. First, the severity of COVID-19 infection possibly played a major role affecting the immune response to infection, enhancing gut microbiota translocation, promoting Candida spp. colonization and qualitative shifts towards higher identification rate of non-albicans Candida species [38]. Previous studies showed significant alarming dysbiosis of oral bacteriota in such populations [18], with high rates of healthcare-acquired infections of Acinetobacter baumannii, Enterococcus faecalis, Escherichia coli, and Klebsiella pneumoniae etiology [13]. Poor oral health status, high frequency of oral colonization by potentially pathogenic bacteria and early postintubation dysbiosis may also play a significant role in the development of candidiasis [13]. The use of corticosteroids was previously reported as the common risk factor for invasive candidiasis due to a complex dysregulation of host immune response to infections [39]. This was also confirmed for COVID-19 patients, in whom, as compared to historical cohorts, the consumption of corticosteroids was even higher, increasing this risk even further [38,40,41]. Numerous studies reported on the widespread use of broad-spectrum antibiotics in COVID-19 patients [33,39]. This led to the dysregulation of normal microbiome in multiple environmental niches. As a result, Candida spp. colonization in the oral cavity and gut was promoted [30,42]. One study also addressed this to high frequency of the use of instrumentation in COVID-19 patients in ICUs, including central venous catheters, arterial lines, and urine bladder catheters [40]. In this study, we identified two cases of Candida spp. bloodstream infections related to central venous catheter (CVC). CVC-related infections are common in the ICU setting, comprising ca. 75% of all ICU bloodstream healthcare-associated infections [43,44]. The risk of candidemia and eventually invasive candidemia is increased in patients with CVC [45]. Additionally, factors, such as age, severe hepatic failure, severity of disease, SOFA score, and septic shock, are unfavorably influencing the prognosis of candidiasis acquired in an ICU [35,42]. Finally, organizational factors may influence the incidence of Candida spp. CVC-related bloodstream infections, with various reported rates for medical vs. surgical ICUs or differing antibiotic or antifungal prophylaxis policies [46].
The reported incidence of putative yeast infections and invasive yeast infections in our study was higher than in data from previously reported historical cohorts. In a large pre-COVID-19-pandemic report from multiple sites in Europe, the cumulative incidence of invasive candidiasis was 7.07 episodes per 1000 ICU admissions [46]. Studies covering the COVID-19 pandemic consistently reported an increase in the rate of invasive candidiasis, with its rates ranging from 0.7 to 23.5% [40]. An increase in the candidemia rate (up to 10-fold) during the COVID-19 pandemic was also reported [32,38,47,48,49,50,51]. In our cohort, we did not find any statistically significant predisposing factors leading to development of candidiasis, probably due to small number of included patients. Similar observations were made by other study groups [47,49].
The etiologies of yeast infections in our cohort encompassed C. albicans and non-albicans Candida species. C. albicans has been reported to be the most frequent cause of invasive candidiasis and candidemia [32,38,49,51], which, however, was not confirmed in our study. Similar results are reported by Papadimitriou-Olivgeris et al., who showed that non-albicans Candida species predominated both before and during the pandemic period, with C. parapsilosis being the most common [52].
In patients that developed candidemia of C. albicans, C tropicalis, and C. glabrata etiology, the strains of C. albicans, C tropicalis, and C. glabrata were identified in samples from the oral cavity. However, we did not perform a molecular genotype analysis of oral and blood Candida spp. strains.
In one putative case of pneumonia, the suspicion of the case of infection was based on the non-BAL material and as such should be treated with caution. According to the literature, pneumonia caused by Candida spp. is exceptional in non-neutropenic patients. The collection of BAL samples is a reference method in the diagnostic process with the parallel use of cultivate and serological methods, supplemented by information from chest imaging and serological tests [53]. UTIs are rarely caused by fungi. The risk factors of candiduria include diabetes, neoplasm, bladder catheterization, the use of wide spectrum antibiotics, immune suppressive therapy, and surgical procedures. Candiduria may be the only sign of candidemia, and when observed during invasive candidiasis, it may be associated with higher mortality [54].
We noted a shift from the high prevalence of both Candida spp. and Lactobacillus spp. at baseline to divergent proportions at follow-up. We suspect that the presence of Lactobacillus spp. at baseline was due to the routine administration of probiotics pre-ICU, according to the local infection prevention standards. In the ICU, however, the probiotics were discontinued. As the hospitalization progressed, the rate of Lactobacillus spp. dropped, and the rate of Candida spp. identification remained stable. There are limited reports on Lactobacillus spp. in displaying antifungal activities. It is assumed that it can produce substances with anticandidal action, contributing to lower prevalence of Candida spp. Still, the specifics of such antifungal mechanisms remain unexplored [5]. Future studies should investigate the impact of postbiotics and parabiotics as regulators of oral cavity homeostasis as they have shown promising results considering oral health and better outcomes of periodontal therapy [55,56,57,58].
Ensuring the safety of medical staff performing procedures in such a high-risk environment is crucial. As a previous review reported, minimally invasive oral treatments can reduce bacteremia and selected periodontal indices, advantaging both the staff and patients [55]. In the case of any pandemic situation, this is even more important, as the reduction of aerosol bacterial load is a major step towards ensuring safer work environment and less patients complications [59].
Our study has some strengths. As Candida spp. infections in ventilated COVID-19 patients are still under-researched, in this study we intended to provide new evidence in this matter. There have been no attempts to investigate the gingival pocket as the source of clinically significant samples of oral microbiota in mechanically ventilated COVID-19 patients. In most cases, oral swabs or saliva samples were tested. By sampling microbiologically diverse individual oral niches during the observation period, we were able to acquire a thorough characterization of oral bacterio- and mycobiota. Moreover, by sampling the oral cavity twice, immediately after the initiation of mechanical ventilation and 7 days afterwards, to our knowledge, for the first time, we were able explore the relationship between the oral mycobiota and selected oral bacterial species in regard to dynamic changes of oral health status in this cohort of patients.
Our study has several limitations. It was relatively small, single-centered, and of retrospective design. Since only patients with COVID-19 were treated in the investigated ICU, there was no control group with non-COVID-19 patients. In this study we used traditional methods for identification of microorganisms with additional MALDI-TOF for species classification. Moreover, the interpretation of yeast cultures obtained via Foley catheter or non-bronchoalveolar lavage should be performed with caution. Detection of Candida antigen and/or anti-Candida antibody in serum or in BAL were not utilized in this study. The pathogenicity of mixed bacterio-fungal biofilms may not be clear. Similarly, the differentiation between the colonization vs. clinically significant Candida spp. infection of the respiratory tract is problematic at times. Finally, we did not genotype the strains of Candida spp. isolated from oral samples and from infection cases.
5. Conclusions
In conclusion, we observed shifts towards a higher identification rate of non-albicans Candida species and divergent proportions of Candida spp. and Lactobacillus spp. in oral samples between the baseline and follow-up in mechanically ventilated adult COVID-19 patients in an ICU setting. Oral health was moderately impaired in this population. A high incidence of yeast infections, including invasive cases, was noted. Severe COVID-19 and disease-specific interventions within the ICU possibly played a major role promoting Candida spp. infections in our study population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11061442/s1.
Author Contributions
Conceptualization, I.G.-M. and J.W.-M.; Methodology, I.G.-M., J.W.-M., D.R., M.N. and A.S.; Formal Analysis, J.W.-M., E.J.-M. and M.K.; Investigation, I.G.-M., J.W.-M., D.R., E.J.-M., A.S. and M.N.; Resources, I.G.-M.; Data Curation, J.W.-M., E.J.-M., D.R., A.S., M.N. and M.K.; Writing—Original Draft Preparation: M.K., I.G.-M. and J.W.-M.; Writing—Review and Editing: M.K., I.G.-M., J.W.-M., E.J.-M. and B.S.-T.; Visualization, M.K. and B.S.-T.; Supervision, I.G.-M. and J.W.-M.; Project administration, I.G.-M.; Funding acquisition, I.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was supported by the National Center for Research and Development CRACoV-HHS project (model of multi-specialist hospital and non-hospital care for patients with SARSCoV-2 infection) through the initiative “Support for specialist hospitals in fighting the spread of SARSCoV-2 infection and in treating COVID-19” (contract number SZPITALE-JEDNOIMIENNE/18/2020). The research described was implemented by consortium of the University Hospital in Krakow and the Jagiellonian University Medical College.
Institutional Review Board Statement
The study and its protocol were approved by the Jagiellonian University Bioethics Committee, decision number 1072.6120.333.2020, 7 December 2020 and 1072.6120.353.2020, 16 December 2020. Written informed consent was obtained from each subject prior to participation. Trial registration number: 1072.6120.333.2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank all the patients who participated in this study. We would like to acknowledge the effort of medical staff in our hospital during the COVID-19 pandemic.
Conflicts of Interest
The authors declare no conflict of interest.
Disclosure
Two articles investigating the same cohort of patients have recently been published [13,21]. In the first article, we included a cross-sectional description of the cohort and focused on bacteriota dysbiosis. In the second article, we analyzed the healthcare-associated bacterial infections and the impact of tooth brushing intervention on microbiological and clinical outcomes. In the current article, we focused on mycobiota, mainly Candida spp. and infections of such etiology.
References
- Szymańska, J.; Wójtowicz, A.; Malm, A. Assessment of Candida spp. frequency in the oral cavity ontocenosis of healthy individuals in different age groups. J. Pre.-Clin. Clin. Res. 2016, 10, 91–94. [Google Scholar] [CrossRef]
- Souza, L.C.D.; da Mota, V.B.R.; de Carvalho, A.V.D.S.Z.; Corrêa, R.D.G.C.F.; Libério, S.A.; Lopes, F.F. Association between pathogens from tracheal aspirate and oral biofilm of patients on mechanical ventilation. Braz. Oral Res. 2017, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Mahmood, M.S.; Ullah, A.; Araf, Y.; Rahaman, T.I.; Moin, A.T.; Hosen, M.J. COVID-19-Associated Candidiasis: Possible Patho-Mechanism, Predisposing Factors, and Prevention Strategies. Curr Microbiol 2022, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, K.M.; Dickson, R.P.; Newton, D.W.; Hoffman, T.C.; Yanik, G.A.; Huffnagle, G.B. Respiratory Tract Colonization by Candida species Portends Worse Outcomes in Immunocompromised Patients. Clin. Pulm. Med. 2018, 25, 197. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Dongari-Bagtzoglou, A. Anticandidal Activities by Lactobacillus Species: An Update on Mechanisms of Action. Front. Oral Health 2021, 2, 47. [Google Scholar] [CrossRef]
- Bertolini, M.; Dongari-Bagtzoglou, A. The Relationship of Candida albicans with the Oral Bacterial Microbiome in Health and Disease. Adv. Exp. Med. Biol. 2019, 1197, 69–78. [Google Scholar] [CrossRef]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; William Costerton, J.; Shirtliff, M.E. Polymicrobial Interactions: Impact on Pathogenesis and Human Disease. Clin. Microbiol. Rev. 2012, 25, 193. [Google Scholar] [CrossRef]
- Allison, D.L.; Willems, H.M.E.; Jayatilake, J.A.M.S.; Bruno, V.M.; Peters, B.M.; Shirtliff, M.E. Candida–Bacteria Interactions: Their Impact on Human Disease. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Martu, M.A.; Maftei, G.A.; Sufaru, I.G.; Jelihovschi, I.; Luchian, I.; Hurjui, L.; Martu, I.; Pasarin, L. COVID-19 and periodontal disease: Etiopathogenic and clinical immplications. Rom. J. Oral Rehabil. 2020, 12, 116–124. [Google Scholar]
- Mocanu, R.C.; Martu, M.-A.; Luchian, I.; Sufaru, I.G.; Maftei, G.A.; Ioanid, N.; Martu, S.; Tatarciuc, M. Microbiologic Profiles of Patients with Dental Prosthetic Treatment and Periodontitis before and after Photoactivation Therapy—Randomized Clinical Trial. Microorganisms 2021, 9, 713. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Gregorczyk-Maga, I.; Anna, P.; Fiema, M.; Kania, M.; Anna, K.; Maga, P.; Jachowicz-Matczak, E.; Romaniszyn, D.; Chmielarczyk, A.; Barbara, Ż.; et al. Impact of tooth brushing on oral bacteriota and health care-associated infections among ventilated COVID-19 patients: An intervention study. Antimicrob. Resist. Infect. Control 2023, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Fiema, M.; Wlodarczyk, A.; Wojkowska-Mach, J.; Garlicki, J.; Gregorczyk-Maga, I. Atypical Presentation of Aspergillus niger Infection in the Oral Cavity as a Prediction of Invasive Pulmonary Aspergillosis in a Patient with COVID-19: Case Report and Literature Review. Microorganisms 2022, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Bonazzetti, C.; Morena, V.; Giacomelli, A.; Oreni, L.; Casalini, G.; Galimberti, L.R.; Bolis, M.; Rimoldi, M.; Ballone, E.; Colombo, R.; et al. Unexpectedly High Frequency of Enterococcal Bloodstream Infections in Coronavirus Disease 2019 Patients Admitted to an Italian ICU: An Observational Study. Crit. Care Med. 2021, 49, e31. [Google Scholar] [CrossRef]
- Russo, A.; Gavaruzzi, F.; Ceccarelli, G.; Borrazzo, C.; Oliva, A.; Alessandri, F.; Magnanimi, E.; Pugliese, F.; Venditti, M. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalized in intensive care unit. Infection 2022, 50, 83. [Google Scholar] [CrossRef]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score—Development, utility and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of Healthcare-Associated Infections and Prevention Indicators in European Intensive Care Units HAI-Net ICU Protocol, Version 2.2; ECDC: Stockholm, Sweden, 2017. [Google Scholar] [CrossRef]
- Leber, A.L. (Ed.) Clinical Microbiology Procedures Handbook, 4th ed.; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
- Gregorczyk-Maga, I.; Fiema, M.; Kania, M.; Kędzierska, J.; Jachowicz, E.; Romaniszyn, D.; Wójkowska-Mach, J. Cultivable oral bacteriota dysbiosis in mechanically ventilated COVID-19 patients. Front. Microbiol. 2022, 13, 4333. [Google Scholar] [CrossRef]
- Arendorf, T.M.; Walker, D.M. The prevalence and intra-oral distribution of Candida albicans in man. Arch. Oral Biol. 1980, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; He, C.; Zhao, C.; Chen, X.; Hua, H.; Yan, Z. Characterization of oral candidiasis and the Candida species profile in patients with oral mucosal diseases. Microb. Pathog. 2019, 134, 103575. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Chaffin, W.L. Oral colonization by Candida albicans. Crit. Rev. Oral Biol. Med. 1999, 10, 359–383. [Google Scholar] [CrossRef]
- Bojang, E.; Ghuman, H.; Kumwenda, P.; Hall, R.A. Immune Sensing of Candida albicans. J. Fungi 2021, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.M.; Bianchi, H.A.; Tadano, T.; Depaula, C.R.; Hoffmann-Santos, H.D.; Leite, D.P.; Hahn, R.C. Factors Related to oral candidiasis in elderly users and non-users of removable dental prostheses. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 17. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A. Diabetes and oral health: An overview. J. Am. Dent. Assoc. 2003, 134, 4S–10S. [Google Scholar] [CrossRef]
- Popa, C.; Filioreanu, A.M.; Stelea, C.; Maftei, G.A.; Popescu, E. Prevalence of oral lesions modulated by patient’s age: The young versus the elderly. Rom. J. Oral Rehabil. 2018, 10, 50–56. [Google Scholar]
- Maftei, G.A.; Martu, C.M.; Popa, C.; Geletu, G.; Danila, V.; Jelihovschi, I.; Foia, L. The biomechanical properties of suture materials and their relationship to bacterial adherence. Mater. Plast. 2019, 56, 980–985. [Google Scholar] [CrossRef]
- Avkan-Oğuz, V.; Çelİk, M.; Eren-Kutsoylu, O.Ö.; Nazli, A.; Uğur, Y.L.; Taylan, A.; Ergan, B.; Irmak, Ç.; Duğral, E.; Özkütük, A.A. Fungal colonization and infections in patients with COVID-19 in intensive care units: A real-life experience at a tertiary-care hospital. Respir. Med. Res. 2022, 82, 100937. [Google Scholar] [CrossRef]
- Niyas, V.K.M.; Rahulan, S.D.; Arjun, R.; Sasidharan, A. ICU-acquired Candidemia in COVID-19 Patients: An Experience from a Tertiary Care Hospital in Kerala, South India. Indian J. Crit. Care Med. 2021, 25, 1207. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Adjei, S.; Hong, K.; Molinari, N.-A.M.; Bull-Otterson, L.; Ajani, U.A.; Gundlapalli, A.V.; Harris, A.M.; Hsu, J.; Kadri, S.S.; Starnes, J.; et al. Mortality Risk Among Patients Hospitalized Primarily for COVID-19 During the Omicron and Delta Variant Pandemic Periods—United States, April 2020–June 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1182–1189. [Google Scholar] [CrossRef]
- Bassetti, M.; Peghin, M.; Carnelutti, A.; Righi, E.; Merelli, M.; Ansaldi, F.; Trucchi, C.; Alicino, C.; Sartor, A.; Toniutto, P.; et al. Clinical characteristics and predictors of mortality in cirrhotic patients with candidemia and intra-abdominal candidiasis: A multicenter study. Intensive Care Med. 2017, 43, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Meltzer, M.I.; Plikaytis, B.D.; Sofair, A.N.; Huie-White, S.; Wilcox, S.; Harrison, L.H.; Seaberg, E.C.; Hajjeh, R.A.; Teutsch, S.M. Excess Mortality, Hospital Stay, and Cost Due to Candidemia: A Case-Control Study Using Data From Population-Based Candidemia Surveillance. Infect. Control. Hosp. Epidemiol. 2005, 26, 540–547. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Madec, Y.; Denoeud-Ndam, L.; Wolff, M.; Fontanet, A.; Bretagne, S.; Dromer, F. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014, 40, 1303–1312. [Google Scholar] [CrossRef]
- Kayaaslan, B.; Eser, F.; Kaya Kalem, A.; Bilgic, Z.; Asilturk, D.; Hasanoglu, I.; Ayhan, M.; Tekce, Y.T.; Erdem, D.; Turan, S.; et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses 2021, 64, 1083. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Kontoyiannis, D.P. Glucocorticoids and invasive fungal infections. Lancet 2003, 362, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; Hong Nguyen, M.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef]
- Riche, C.V.W.; Cassol, R.; Pasqualotto, A.C. Is the Frequency of Candidemia Increasing in COVID-19 Patients Receiving Corticosteroids? J. Fungi 2020, 6, 286. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Ansaldi, F.; Merelli, M.; Cecilia, T.; de Pascale, G.; Diaz-Martin, A.; Luzzati, R.; Rosin, C.; Lagunes, L.; et al. A multicenter study of septic shock due to candidemia: Outcomes and predictors of mortality. Intensive Care Med. 2014, 40, 839–845. [Google Scholar] [CrossRef]
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Yamin, D.H.; Husin, A.; Harun, A. Risk Factors of Candida parapsilosis Catheter-Related Bloodstream Infection. Front. Public Health 2021, 9, 631865. [Google Scholar] [CrossRef]
- Blumberg, H.M.; Jarvis, W.R.; Soucie, J.M.; Edwards, J.E.; Patterson, J.E.; Pfaller, M.A.; Rangel-Frausto, M.S.; Rinaldi, M.G.; Saiman, L.; Wiblin, R.; et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: The NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin. Infect Dis. 2001, 33, 177–186. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Trucchi, C.; Ansaldi, F.; Antonelli, M.; Adamkova, V.; Alicino, C.; Almyroudi, M.-P.; Atchade, E.; et al. Incidence and outcome of invasive candidiasis in intensive care units (icus) in europe: Results of the eucandicu project. Crit. Care 2019, 23, 1–7. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Germinario, B.N.; Ferrante, M.; Frangi, C.; Li Voti, R.; Muccini, C.; Ripa, M.; Canetti, D.; Castiglioni, B.; Oltolini, C.; et al. Candidemia in Coronavirus Disease 2019 (COVID-19) Patients: Incidence and Characteristics in a Prospective Cohort Compared With Historical Non–COVID-19 Controls. Clin. Infect. Dis. 2021, 73, e2838–9. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef]
- Machado, M.; Estévez, A.; Sánchez-Carrillo, C.; Guinea, J.; Escribano, P.; Alonso, R.; Valerio, M.; Padilla, B.; Bouza, E.; Muñoz, P. Incidence of Candidemia Is Higher in COVID-19 versus Non-COVID-19 Patients, but Not Driven by Intrahospital Transmission. J. Fungi 2022, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Gangneux, J.P.; Dannaoui, E.; Fekkar, A.; Luyt, C.E.; Botterel, F.; de Prost, N.; Tadié, J.-M.; Reizine, F.; Houzé, S.; Timsit, J.-F.; et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study. Lancet Respir. Med. 2022, 10, 180–190. [Google Scholar] [CrossRef]
- Macauley, P.; Epelbaum, O. Epidemiology and Mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses 2021, 64, 634. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Kolonitsiou, F.; Kefala, S.; Spiliopoulou, A.; Aretha, D.; Bartzavali, C.; Siapika, A.; Marangos, M.; Fligou, F. Increased incidence of candidemia in critically ill patients during the Coronavirus Disease 2019 (COVID-19) pandemic. Braz. J. Infect. Dis. 2022, 26, 102353. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Olaechea, P.; Alvarez-Lerma, F.; Alvarez-Rocha, L.; Blanquer, J.; Galván, B.; Rodriguez, A.; Zaragoza, R.; Aguado, J.-M.; Mensa, J.; et al. Epidemiology, diagnosis and treatment of fungal respiratory infections in the critically ill patient. Rev. Esp. Quimioter 2013, 26, 173–188. [Google Scholar] [PubMed]
- Sobel, J.D.; Fisher, J.F.; Kauffman, C.A.; Newman, C.A. Candida Urinary Tract Infections—Epidemiology. Clin. Infect. Dis. 2011, 52, S433–S436. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms. 2020, 9, 69. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms. 2022, 10, 337. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Cuggia, G.; Scribante, A. Domiciliary Use of Chlorhexidine vs. Postbiotic Gels in Patients with Peri-Implant Mucositis: A Split-Mouth Randomized Clinical Trial. Appl. Sci. 2022, 12, 2800. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Gallo, S.; Pérez-Albacete Martínez, C.; Maté Sánchez de Val, J.E.; Parisi, L.; Gariboldi, A.; Scribante, A. Ozonized Hydrogels vs. 1% Chlorhexidine Gel for the Clinical and Domiciliary Management of Peri-Implant Mucositis: A Randomized Clinical Trial. J. Clin. Med. 2023, 12, 1464. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Natoli, V.; Bruni, A.; Coscione, C.; Magliano, G.; Giacobbo, G.; Morelli, A.; Moressa, S.; Scribante, A. Bio-Inspired Systems in Nonsurgical Periodontal Therapy to Reduce Contaminated Aerosol during COVID-19: A Comprehensive and Bibliometric Review. J. Clin. Med. 2020, 9, 3914. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).