Synergistic Immunostimulatory Activities of Probiotic Strains, Leuconostoc lactis and Weissella cibaria, and the Prebiotic Oligosaccharides They Produce
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Lactic Acid Bacteria
2.2. Culture of Animal Cells
2.2.1. Cell Lines and Culture Conditions
2.2.2. Preparation of Lactic Acid Bacterial Sample
2.3. Evaluation of Probiotic Characteristics of Lactic Acid Bacteria
2.3.1. Acid Tolerance of Lactic Acid Bacteria
2.3.2. Bile Tolerance
2.3.3. Gut-Adhesion Ability in an In Vitro Model
2.3.4. Antioxidant Activity of Lactic Acid Bacteria
2.4. Preparation of Oligosaccharides
2.5. Preparation of Synbiotics Containing Oligosaccharide-Producing Probiotic Strains and Their Corresponding Oligosaccharides
2.6. Evaluation of Immunostimulatory Activities
2.6.1. Cell-Viability Assay
2.6.2. Nitric Oxide Assay
2.6.3. Total-RNA Extraction and the Synthesis of cDNA
2.6.4. Real-Time Quantitative Polymerase Chain Reaction (PCR)
2.6.5. Protein Extraction
2.6.6. Western Blotting
2.7. Statistical Analyses
3. Results
3.1. Probiotic Characteristics of Oligosaccharide-Producing Lactic Acid Bacteria
3.1.1. Acid and Bile Tolerances and the Ability to Adhere to HT-29 Cells
3.1.2. Antioxidant Activities of Oligosaccharide-Producing Lactic Acid Bacteria
3.2. Immunostimulatory Activities of Prebiotic-Producing Lactic Acid Bacteria
3.2.1. Cell Viability
3.2.2. Production of NO
3.2.3. Quantitative Analysis of Cytokine Expression in Macrophages Treated with Lactic Acid Bacteria
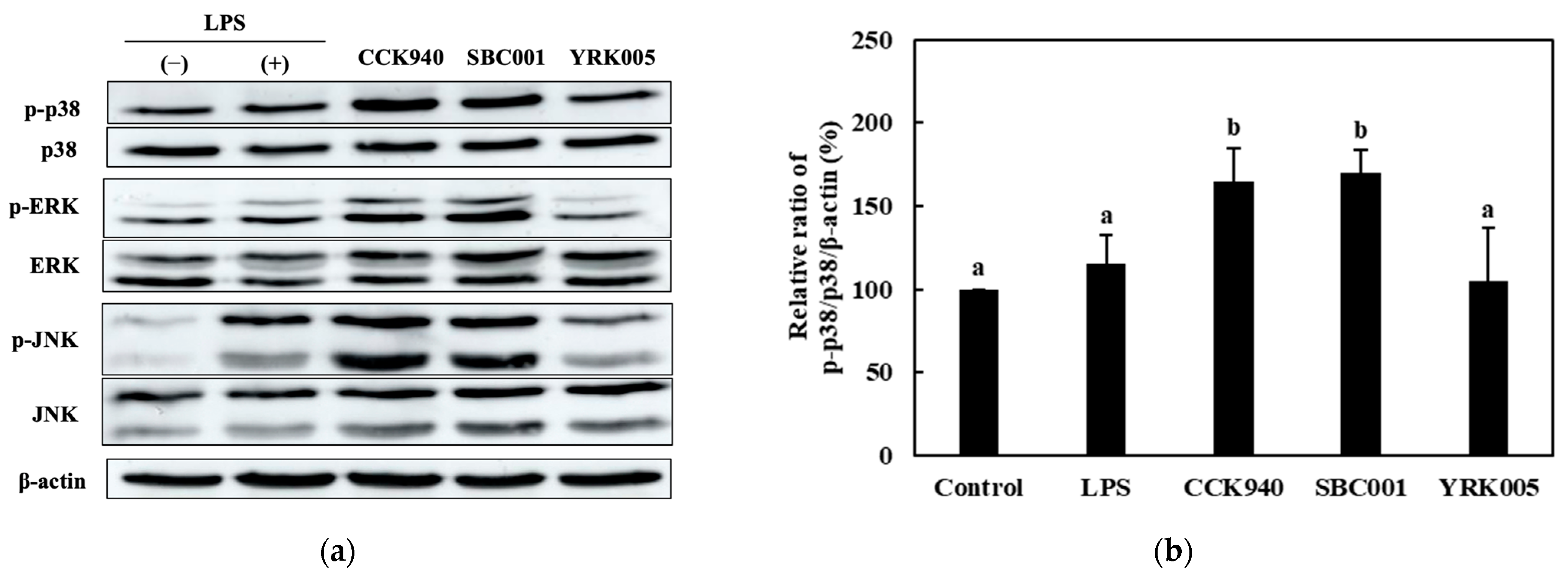
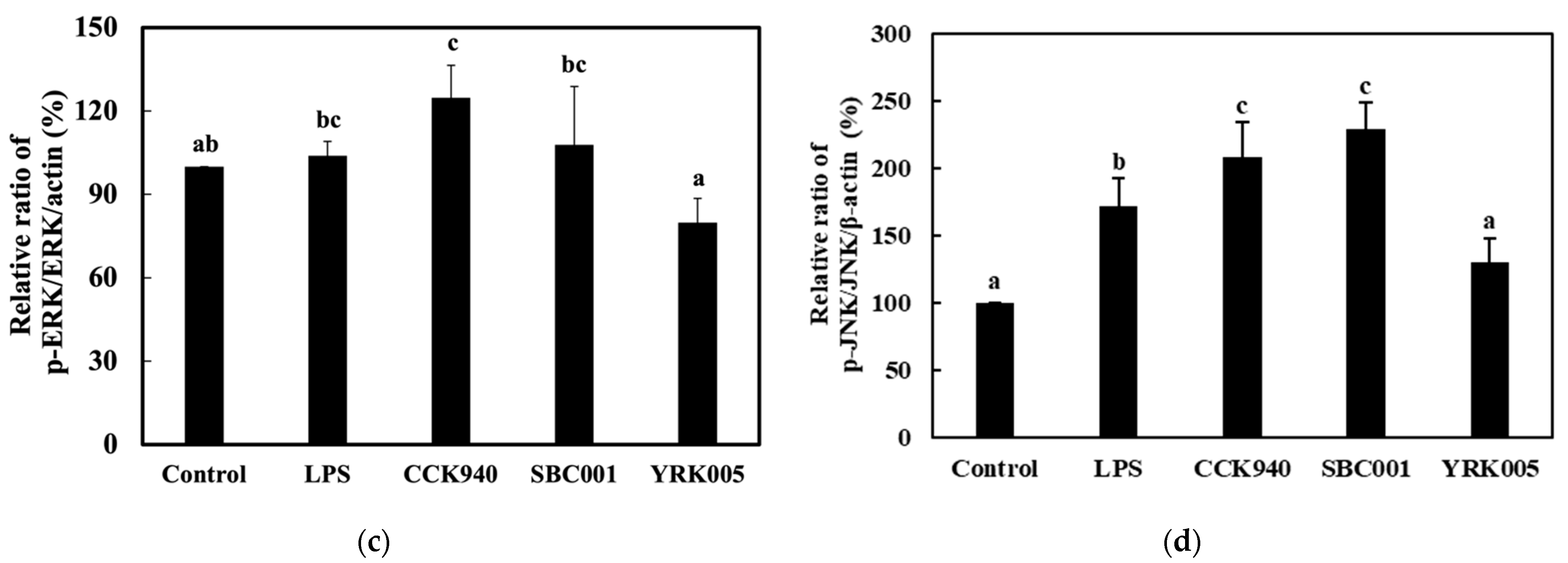
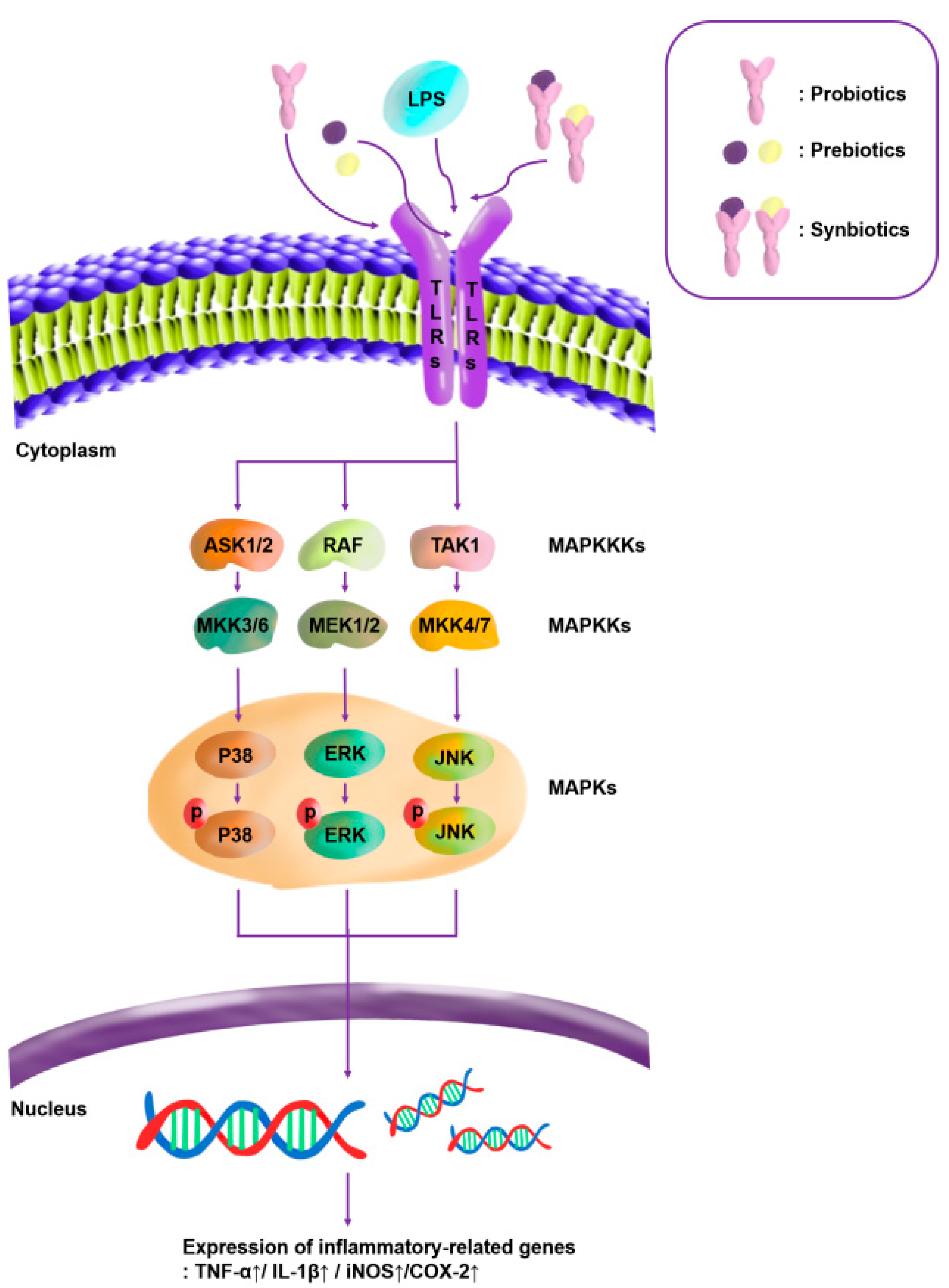
3.2.4. Quantitative Analysis of the Expression of the Mitogen-Activated Protein Kinase (MAPK)-Pathway Proteins in Macrophages Treated with Lactic Acid Bacterial Strains
3.3. Synergistic Immunostimulatory Activities of Probiotics and the Prebiotics They Produced
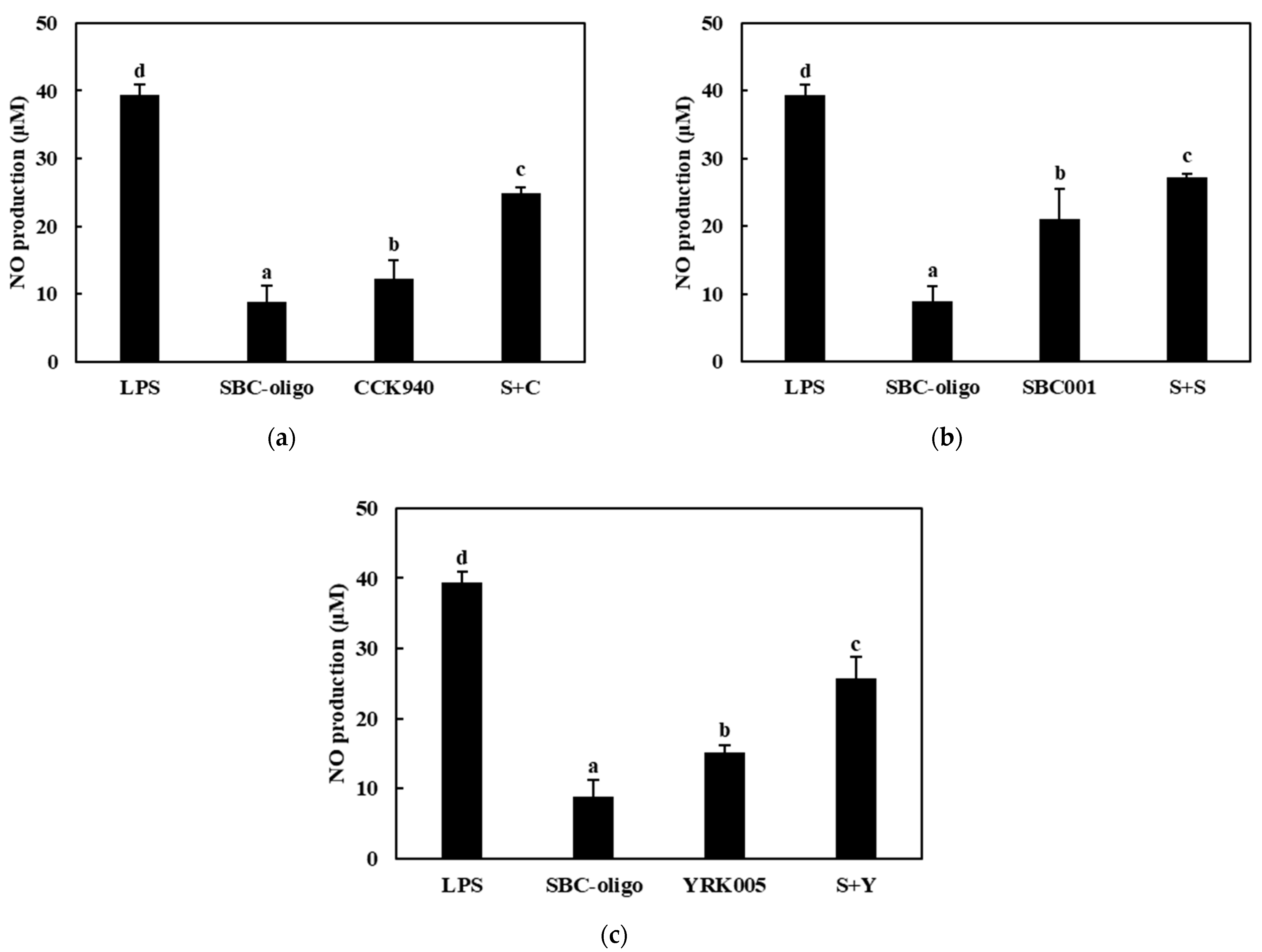
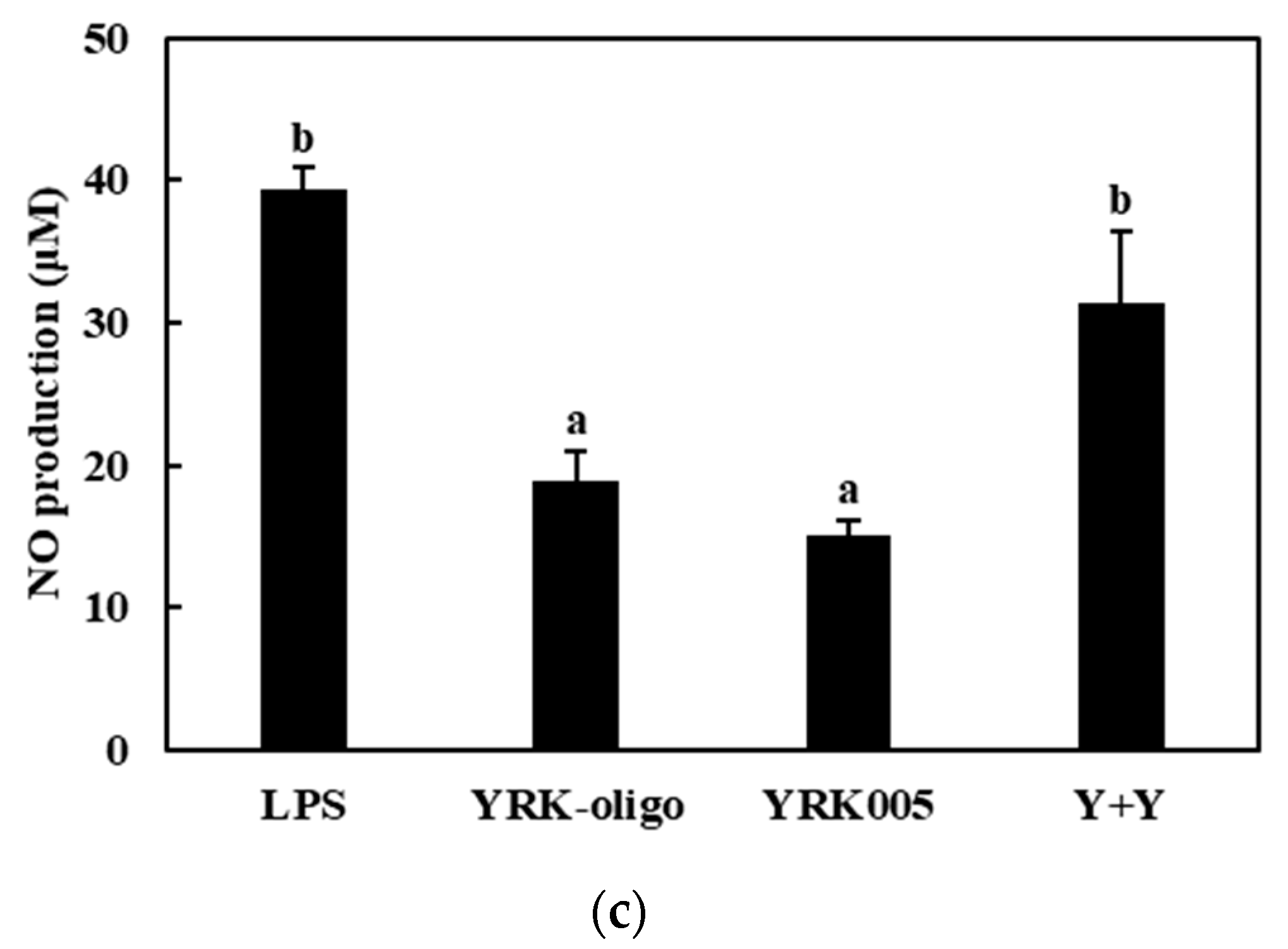
3.3.1. Production of NO
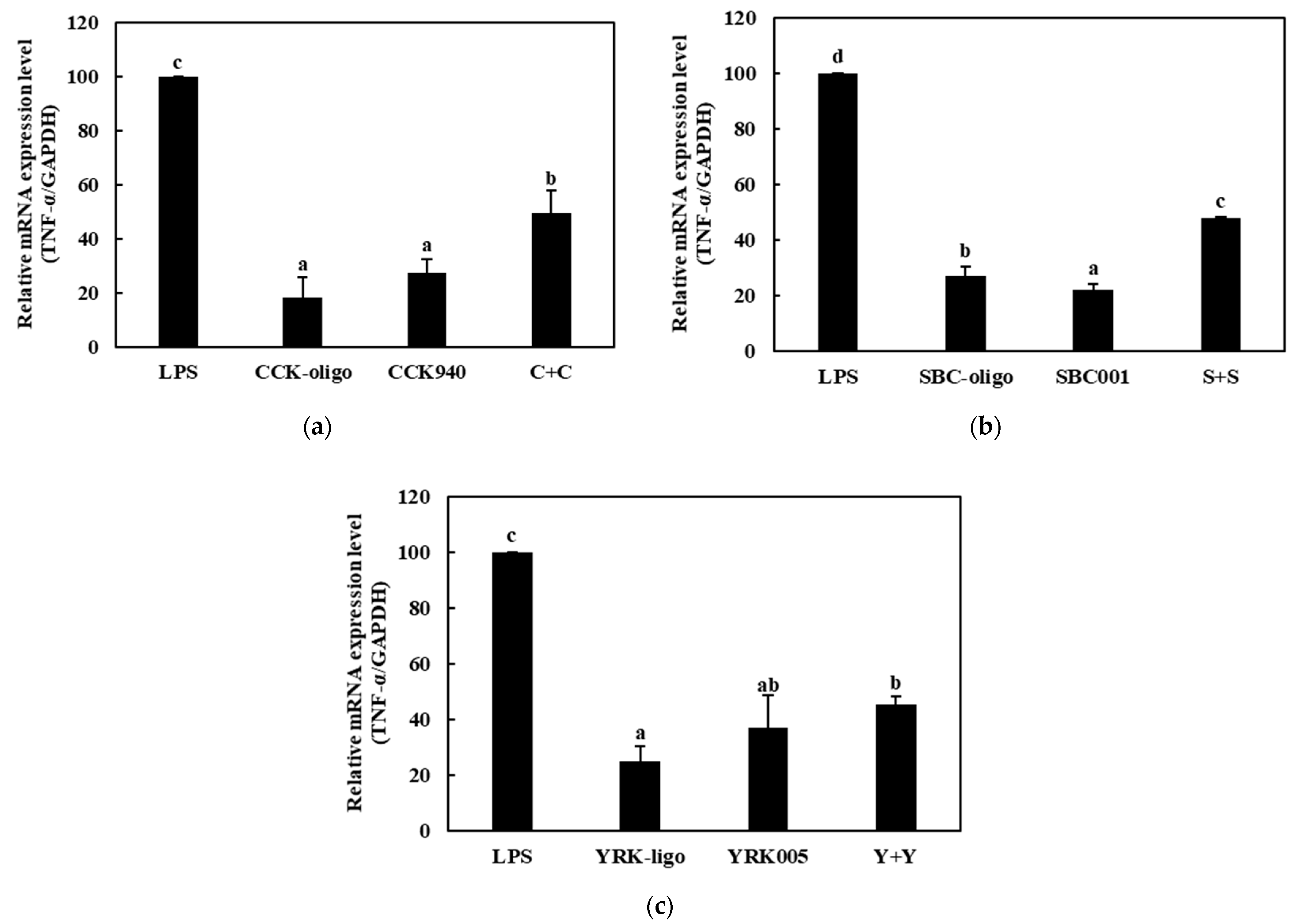
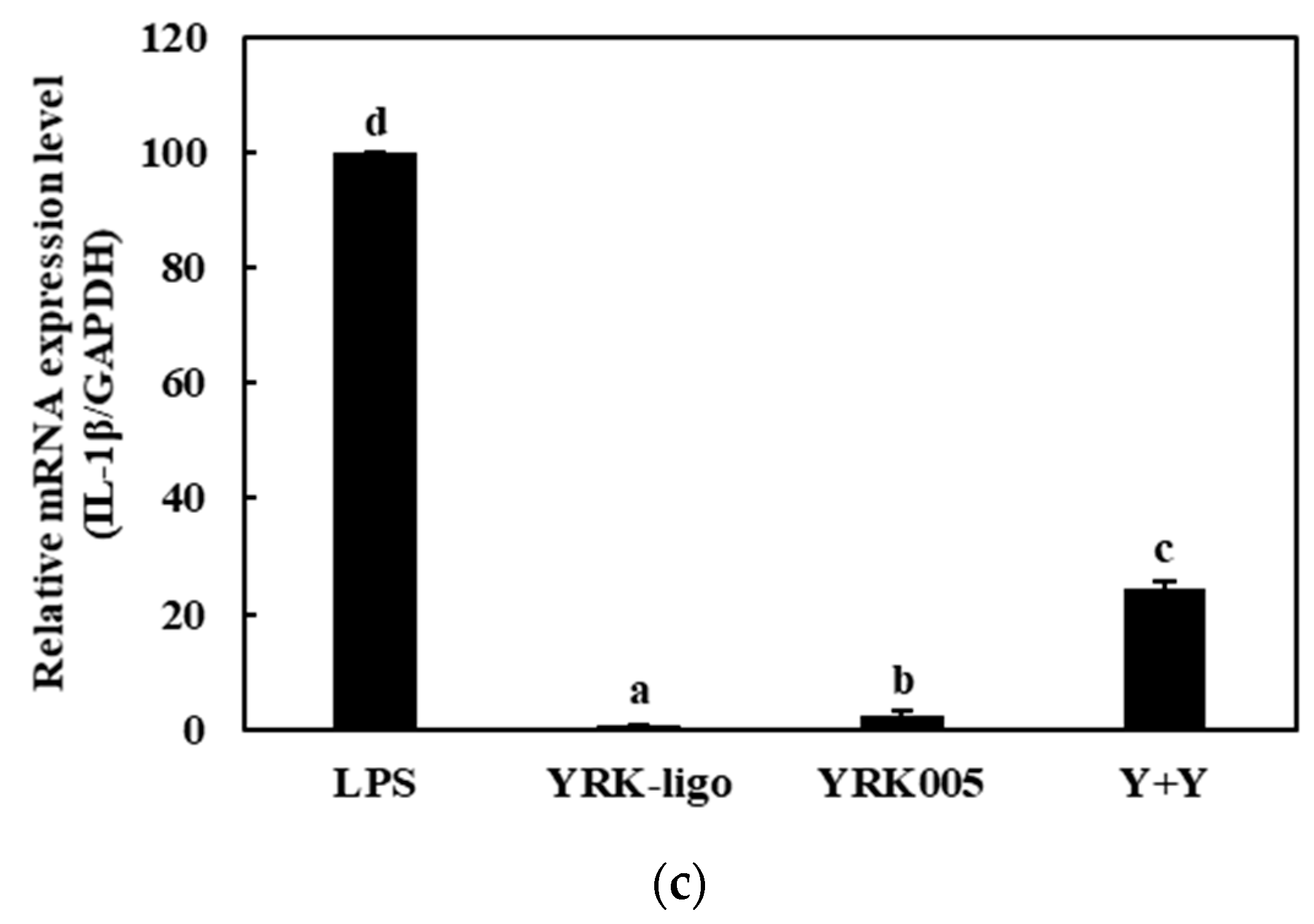
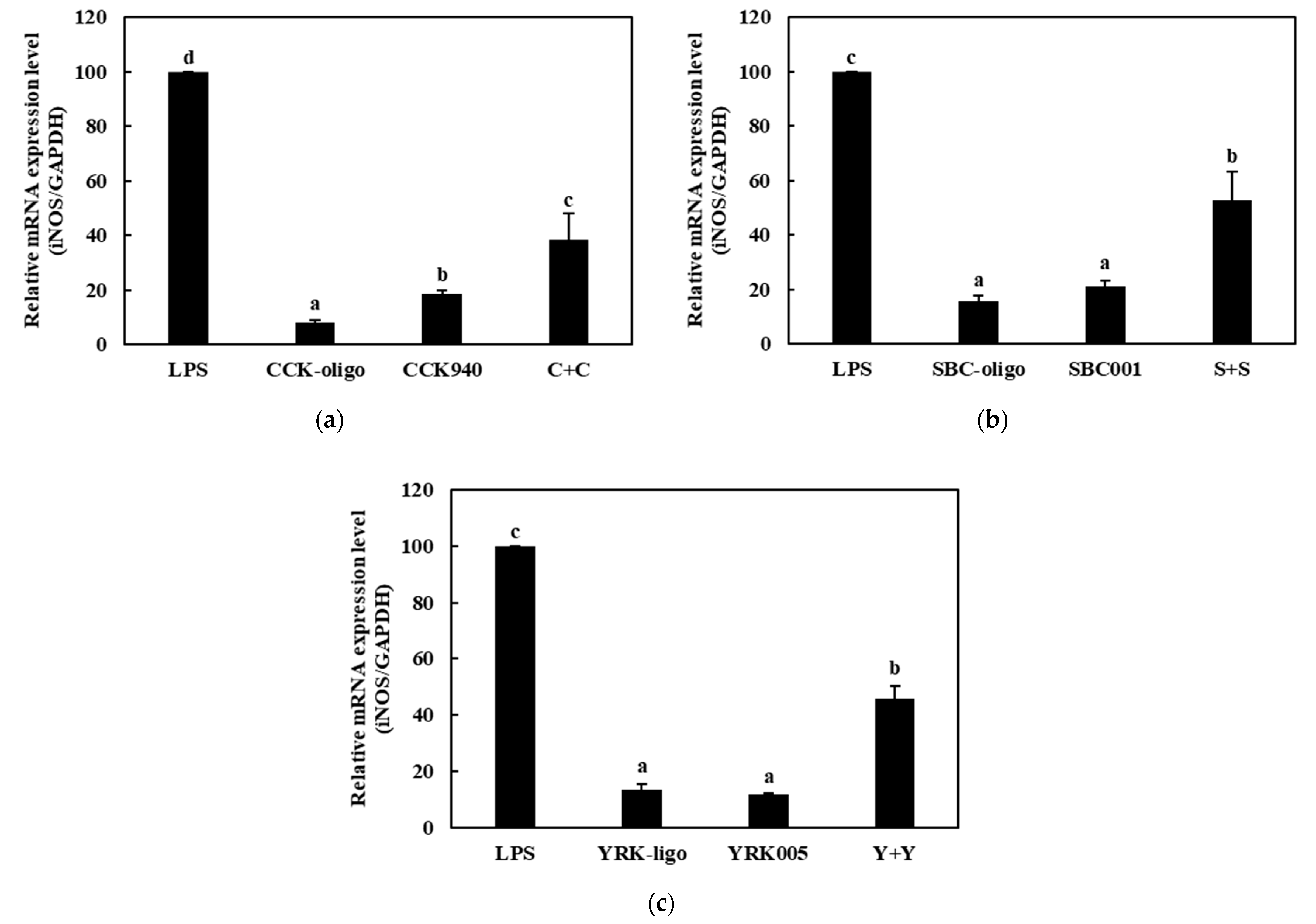
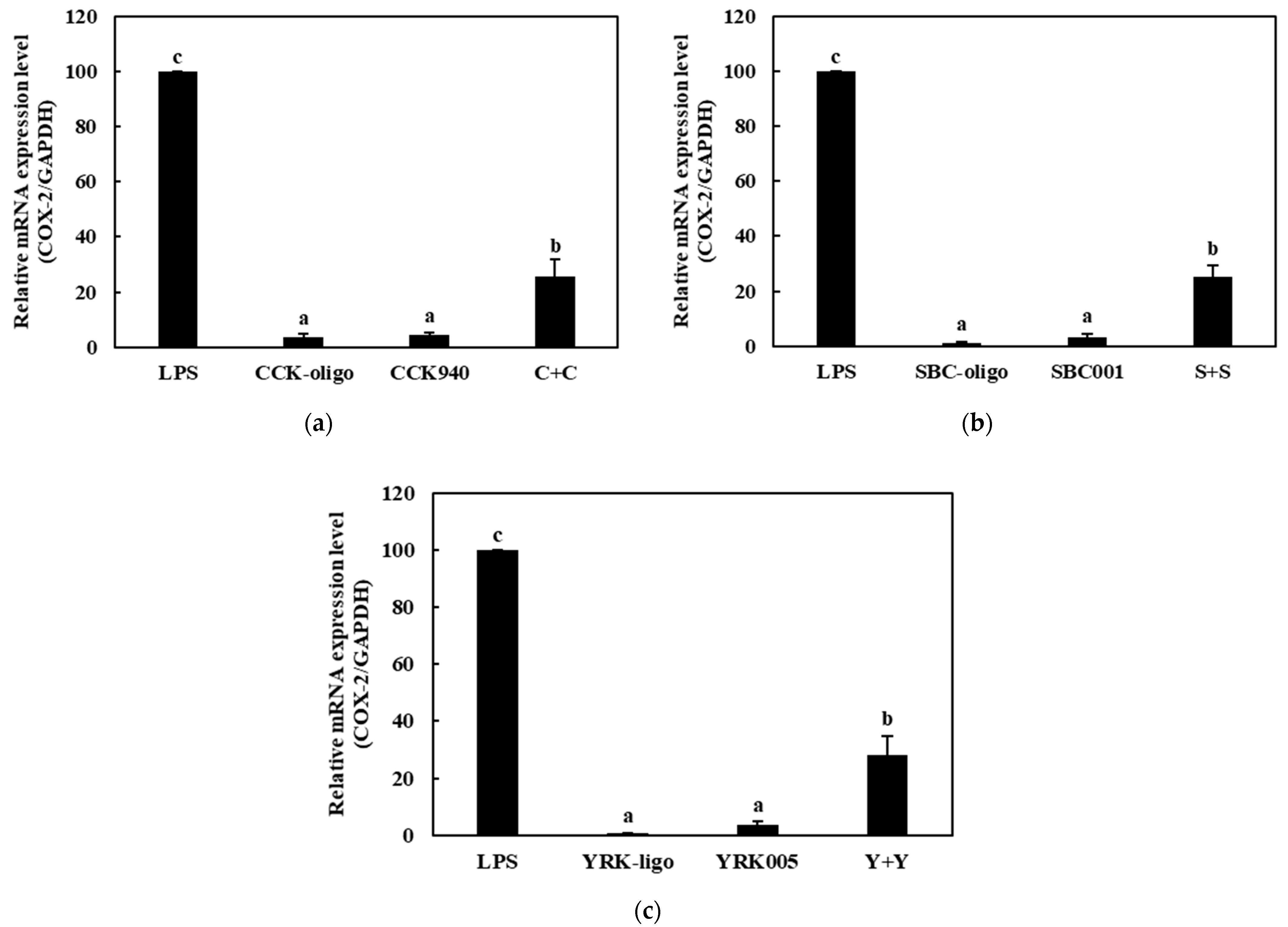
3.3.2. Quantitative Analysis of the Expressions of Cytokine Genes in Macrophages Treated with Synbiotics
3.3.3. Quantitative Analysis of the Expression of the MAPK-Pathway Proteins in Macrophages Treated with Synbiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seo, J.-H.; Lee, H. Characteristics and Immunomodulating Activity of Lactic Acid Bacteria for the Potential Probiotics. Korean J. Food Sci. Technol. 2007, 39, 681–687. [Google Scholar]
- Food Agriculture Organization/World Health Organization Working Group. Guidelines for the Evaluation of Probiotics in Food; Report of a Joint FAO/WHO; FAO/WHO: London, ON, Canada, 2002.
- Soccol, C.R.; de Vandenberghe, L.P.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; De Dea Lindner, J.; Pandey, A.; Thomaz-Soccol, V. The Potential of Probiotics: A Review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Foligné, B.; Daniel, C.; Pot, B. Probiotics from Research to Market: The Possibilities, Risks and Challenges. Curr. Opin. Microbiol. 2013, 16, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Manning, T.S.; Gibson, G.R. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G.; Follador, R. Metabolism of Oligosaccharides and Starch in Lactobacilli: A Review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef]
- Sarbini, S.R.; Rastall, R.A. Prebiotics: Metabolism, Structure, and Function. Funct. Food Rev. 2011, 3, 93–106. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Pandey, K.; Naik, S.; Vakil, B. Probiotics, Prebiotics and Synbiotics—A Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Chung, C.H.; Day, D.F. Glucooligosaccharides from Leuconostoc Mesenteroides B-742 (ATCC 13146): A Potential Prebiotic. J. Ind. Microbiol. Biotechnol. 2002, 29, 196–199. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Jang, J.-K.; Lee, B.-H.; Park, Y.-S. Structural Analysis of Gluco-Oligosaccharides Produced by Leuconostoc Lactis and Their Prebiotic Effect. Molecules 2019, 24, 3998. [Google Scholar] [CrossRef]
- Kothari, D.; Goyal, A. Gentio-Oligosaccharides from Leuconostoc Mesenteroides NRRL B-1426 Dextransucrase as Prebiotics and as a Supplement for Functional Foods with Anti-Cancer Properties. Food Funct. 2015, 6, 604–611. [Google Scholar] [CrossRef]
- Schwab, C.; Mastrangelo, M.; Corsetti, A.; Gänzle, M. Formation of Oligosaccharides and Polysaccharides by Lactobacillus Reuteri LTH5448 and Weissella Cibaria 10M in Sorghum Sourdoughs. Cereal Chem. 2008, 85, 679–684. [Google Scholar] [CrossRef]
- Lee, S.; Song, I.H.; Park, Y.-S. In Vivo and in Vitro Study of Immunostimulation by Leuconostoc lactis-Produced Gluco-Oligosaccharides. Molecules 2019, 24, 3994. [Google Scholar] [CrossRef]
- Kelly, G. Inulin-Type Prebiotics—A Review: Part 1. Altern. Med. Rev. 2008, 13, 315–329. [Google Scholar]
- Bosscher, D.; Van Loo, J.; Franck, A. Inulin and Oligofructose as Prebiotics in the Prevention of Intestinal Infections and Diseases. Nutr. Res. Rev. 2006, 19, 216–226. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Collins, M.D.; Gibson, G.R. Probiotics, Prebiotics, and Synbiotics: Approaches for Modulating the Microbial Ecology of the Gut. Am. J. Clin. Nutr. 1999, 69, 1052S–1057S. [Google Scholar] [CrossRef]
- Deepak, M.; Barak, S. Functional Foods: Sources and Health Benefits; Scientific Publishers: Jodhpur, India, 2017; pp. 173–204. [Google Scholar]
- Parkin, J.; Cohen, B. An Overview of the Immune System. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Everett, H.; McFadden, G. Apoptosis: An Innate Immune Response to Virus Infection. Trends. Microbiol. 1999, 7, 160–165. [Google Scholar] [CrossRef]
- Delves, P.; Roitt, I. L14 The Immune System (Part II). N. Engl. J. Med. 2000, 343, 108–117. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–555. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage Activation and Polarization. Front. Biosci. 2008, 13, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and Prebiotics Associated with Aquaculture: A Review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.R.; Frøkiær, H.; Pestka, J.J. Lactobacilli Differentially Modulate Expression of Cytokines and Maturation Surface Markers in Murine Dendritic Cells. J. Immunol. 2002, 168, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Niers, L.E.M.; Timmerman, H.M.; Rijkers, G.T.; Van Bleek, G.M.; Van Uden, N.O.P.; Knol, E.F.; Kapsenberg, M.L.; Kimpen, J.L.L.; Hoekstra, M.O. Identification of Strong Interleukin-10 Inducing Lactic Acid Bacteria Which down-Regulate T Helper Type 2 Cytokines. Clin. Exp. Allergy 2005, 35, 1481–1489. [Google Scholar] [CrossRef]
- Von der Weid, T.; Bulliard, C.; Schiffrin, E.J. Induction by a Lactic Acid Bacterium of a Population of CD4+ T Cells with Low Proliferative Capacity That Produce Transforming Growth Factor β and Interleukin-10. Clin. Diagn. Lab. Immunol. 2001, 8, 695–701. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Cytokines and Macrophages. Biomed. Pharmacother. 1994, 48, 445–453. [Google Scholar] [CrossRef]
- Coppack, S.W. Pro-Inflammatory Cytokines and Adipose Tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.-S. Oligosaccharide Production by Leuconostoc lactis CCK940 Which Has Glucansucrase Activity. Food Eng. Prog. 2017, 21, 383–390. [Google Scholar] [CrossRef]
- Kim, M.; Jang, J.K.; Park, Y.S. Production Optimization, Structural Analysis, and Prebiotic-and Anti-Inflammatory Effects of Gluco-Oligosaccharides Produced by Leuconostoc lactis SBC001. Microorganisms 2021, 9, 200. [Google Scholar] [CrossRef]
- Park, J.; Park, Y.S. Gluco-Oligosaccharides from Weissella cibaria as Potential Prebiotics. Food Eng. Prog. 2021, 25, 305–312. [Google Scholar] [CrossRef]
- Lee, S.; Park, G.G.; Jang, J.K.; Park, Y.S. Optimization of Oligosaccharide Production from Leuconostoc lactis Using a Response Surface Methodology and the Immunostimulating Effects of These Oligosaccharides on Macrophage Cells. Molecules 2018, 23, 2118. [Google Scholar] [CrossRef]
- Kwon, A.; Park, Y.S. Immunostimulatory Activity of Synbiotics Using Lactococcus lactis SG-030 and Glucooligosaccharides from Weissella cibaria YRK005. Microorganisms 2021, 9, 2437. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Du, G.; Chen, J. Enhanced Acid Tolerance in Lactobacillus Casei by Adaptive Evolution and Compared Stress Response during Acid Stress. Biotechnol. Bioprocess Eng. 2012, 17, 283–289. [Google Scholar] [CrossRef]
- Rehaiem, A.; Belgacem, Z.B.; Edalatian, M.R.; Martínez, B.; Rodríguez, A.; Manai, M.; Guerra, N.P. Assessment of Potential Probiotic Properties and Multiple Bacteriocin Encoding-Genes of the Technological Performing Strain Enterococcus Faecium MMRA. Food Control 2014, 37, 343–350. [Google Scholar] [CrossRef]
- Song, M.; Yun, B.; Moon, J.-H.; Park, D.-J.; Lim, K.; Oh, S. Characterization of Selected Lactobacillus Strains for Use as Probiotics. Food Sci. Anim. Resour. 2015, 35, 551–556. [Google Scholar] [CrossRef]
- Jeon, E.B.; Son, S.-H.; Jeewanthi, R.K.C.; Lee, N.-K.; Paik, H.-D. Characterization of Lactobacillus plantarum Lb41, an Isolate from Kimchi and Its Application as a Probiotic in Cottage Cheese. Food Sci. Biotechnol. 2016, 25, 1129–1133. [Google Scholar] [CrossRef]
- Shuwen, Z.; Lu, L.; Yanling, S.; Hongjuan, L.; Qi, S.; Xiao, L.; Jiaping, L. Antioxidative Activity of Lactic Acid Bacteria in Yogurt. Afr. J. Microbiol. Res. 2011, 5, 5194–5201. [Google Scholar]
- Son, S.-H.; Yang, S.-J.; Jeon, H.-L.; Yu, H.-S.; Lee, N.-K.; Park, Y.-S.; Paik, H.-D. Antioxidant and Immunostimulatory Effect of Potential Probiotic Lactobacillus paraplantarum SC61 Isolated from Korean Traditional Fermented Food, Jangajji. Microb. Pathog. 2018, 125, 486–492. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Schillinger, U. Introduction to Pre-and Probiotics. Food Res. Int. 2002, 35, 109–116. [Google Scholar] [CrossRef]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A Water-Soluble Tetrazolium Salt Useful for Colorimetric Cell Viability Assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Ghadimi, D.; de Vrese, M.; Heller, K.J.; Schrezenmeir, J. Lactic acid bacteria enhance autophagic ability of mononuclear phagocytes by increasing Th1 autophagy-promoting cytokine (IFN-gamma) and nitric oxide (NO) levels and reducing Th2 autophagy-restraining cytokines (IL-4 and IL-13) in response to Mycobacterium tuberculosis antigen. Int. Immunopharmacol. 2010, 10, 694–706. [Google Scholar] [PubMed]
- Kang, M.J.; Jeong, H.; Kim, S.; Shin, J.; Song, Y.; Lee, B.-H.; Park, H.-G.; Lee, T.-H.; Jiang, H.-H.; Han, Y.-S.; et al. Structural analysis and prebiotic activity of exopolysaccharide produced by probiotic strain Bifidobacterium bifidum EPS DA-LAIM. Food Sci. Biotechnol. 2023, 32, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lin, C.; Chen, Y.; Kao, S. Anti-inflammatory Effects of Perilla Frutescens Leaf Extract on Lipopolysaccharide-stimulated RAW264. 7 Cells. Mol. Med. Rep. 2014, 10, 1077–1083. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory Effects of Chitooligosaccharides on RAW 264.7 Mouse Macrophages via Regulation of the MAPK and PI3K/Akt Signaling Pathways. Mar. Drugs 2019, 17, 36. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Wang, Z.; Wang, Y.; Xie, M.; Xie, J. Sulfated Polysaccharide from Cyclocarya paliurus Enhances the Immunomodulatory Activity of Macrophages. Carbohydr. Polym. 2017, 174, 669–676. [Google Scholar] [CrossRef]
- Wynn, T.A.; Freund, Y.R.; Paulnock, D.M. TNF-α Differentially Regulates Ia Antigen Expression and Macrophage Tumoricidal Activity in Two Murine Macrophage Cell Lines. Cell. Immunol. 1992, 140, 184–196. [Google Scholar] [CrossRef]
- Nakao, S.; Kuwano, T.; Tsutsumi-miyahara, C.; Ueda, S. Infiltration of COX-2—Expressing Macrophages Is a Prerequisite for IL-1 β—Induced Neovascularization and Tumor Growth. J. Clin. Investig. 2005, 115, 2979–2991. [Google Scholar] [CrossRef]
- Kim, K.-T.; Yang, S.J.; Paik, H.-D. Probiotic Properties of Novel Probiotic Levilactobacillus brevis KU15147 Isolated from Radish Kimchi and Its Antioxidant and Immune-Enhancing Activities. Food Sci. Biotechnol. 2021, 30, 257–265. [Google Scholar] [CrossRef]
- Won, J.H.; Im, H.T.; Kim, Y.H.; Yun, K.J.; Park, H.J.; Choi, J.W.; Lee, K.T. Anti-Inflammatory Effect of Buddlejasaponin IV through the Inhibition of INOS and COX-2 Expression in RAW 264.7 Macrophages via the NF-ΚB Inactivation. Br. J. Pharmacol. 2006, 148, 216–225. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and P38 Protein Kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Huang, G.; Shi, L.Z.; Chi, H. Regulation of JNK and P38 MAPK in the Immune System: Signal Integration, Propagation and Termination. Cytokine 2009, 48, 161–169. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK Signaling Cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, D.; Park, Y.S. Genome analysis revealed a repertoire of oligosaccharide utilizing CAZymes in Weissella confusa CCK931 and Weissella cibaria YRK005. Food Sci. Biotechnol. 2023, 32, 553–564. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, N.; Gupta, D.; Lee, H.J.; Park, Y.S. Comparative genome analysis of four Leuconostoc strains with a focus on carbohydrate-active enzymes and oligosaccharide utilization pathways. Comput. Struct. Biotechnol. J. 2022, 20, 4771–4785. [Google Scholar] [CrossRef]
- Adesioye, F.A.; Makhalanyane, T.P.; Biely, P.; Cowan, D.A. Phylogeny, Classification and Metagenomic Bioprospecting of Microbial Acetyl Xylan Esterases. Enzyme Microb. Technol. 2016, 93, 79–91. [Google Scholar] [CrossRef]
- Schwab, C.; Sørensen, K.I.; Gänzle, M.G. Heterologous Expression of Glycoside Hydrolase Family 2 and 42 β-Galactosidases of Lactic Acid Bacteria in Lactococcus lactis. Syst. Appl. Microbiol. 2010, 33, 300–307. [Google Scholar] [CrossRef]
- Egloff, M.-P.; Uppenberg, J.; Haalck, L.; van Tilbeurgh, H. Crystal Structure of Maltose Phosphorylase from Lactobacillus brevis: Unexpected Evolutionary Relationship with Glucoamylases. Structure 2001, 9, 689–697. [Google Scholar] [CrossRef]
- Siziya, I.N.; Jung, J.-H.; Seo, M.-J.; Lim, M.-C.; Seo, D.-H. Whole-cell bioconversion using non-Leloir transglycosylation reactions: A review. Food. Sci. Biotechnol. 2023, 32, 749–768. [Google Scholar] [CrossRef]
- Kumar, S.; Bansal, K.; Sethi, S.K. Comparative Genomics Analysis of Genus Leuconostoc Resolves Its Taxonomy and Elucidates Its Biotechnological Importance. Food Microbiol. 2022, 106, 104039. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Stevens, H.; Van Riel, M.; Simons, G.; De Vos, W.M. Leuconostoc lactis Beta-Galactosidase Is Encoded by Two Overlapping Genes. J. Bacteriol. 1992, 174, 4475–4481. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Han, N.S.; Kim, J.H. Purification and Characterization of Beta-Glucosidase from Weissella Cibaria 37. J. Microbiol. Biotechnol. 2012, 22, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Achour, L.; Paineau, D.; Riottot, M.; Attar, A.; Bornet, F. Four-week short chain fructo-oligosaccharides ingestion leads to increasing fecal bifidobacteria and cholesterol excretion in healthy elderly volunteers. Nutr. J. 2007, 6, 42. [Google Scholar] [CrossRef]
- Liong, M.T.; Shah, N.P. Effects of a Lactobacillus casei synbiotic on serum lipoprotein, intestinal microflora, and organic acids in rats. J. Dairy Sci. 2006, 89, 1390–1399. [Google Scholar] [CrossRef]
- Yu, X.; Yin, J.; Li, L.; Luan, C.; Zhang, J.; Zhao, C.; Li, S. Prebiotic Potential of Xylooligosaccharides Derived from Corn Cobs and Their In Vitro Antioxidant Activity When Combined with Lactobacillus. J. Microbiol. Biotechnol. 2015, 25, 1084–1092. [Google Scholar] [CrossRef]
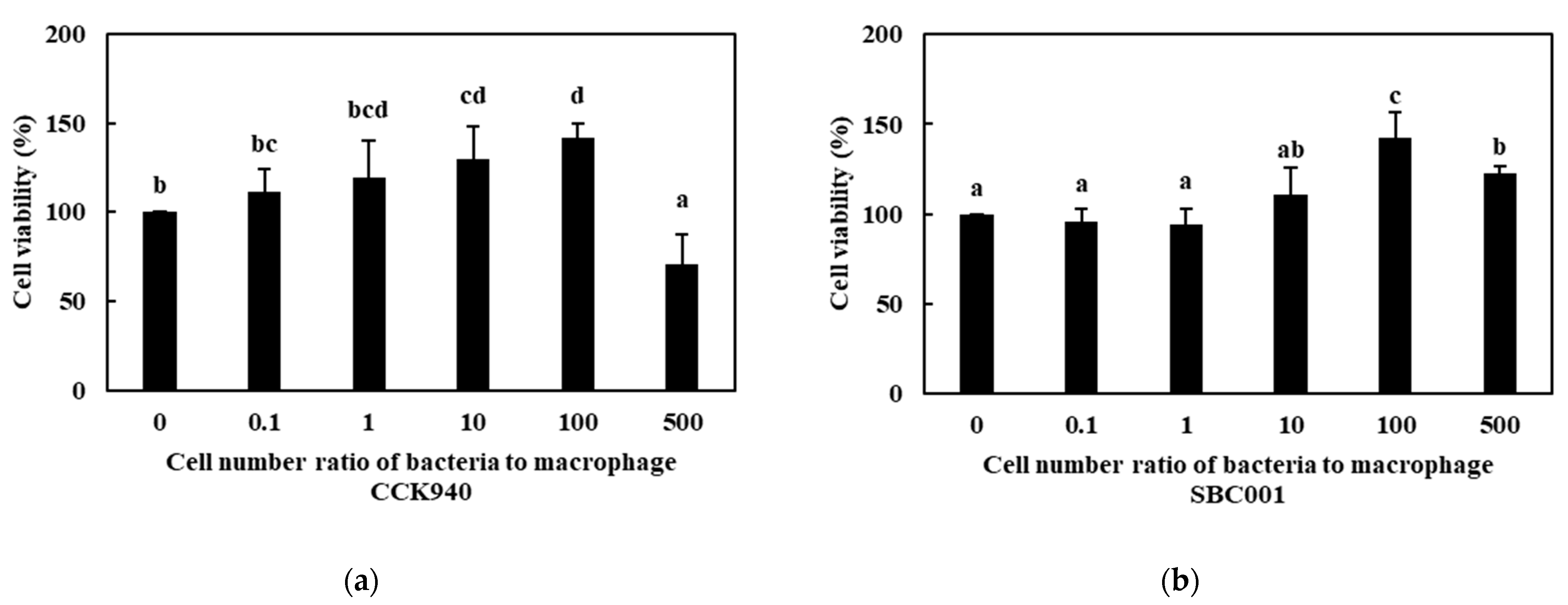
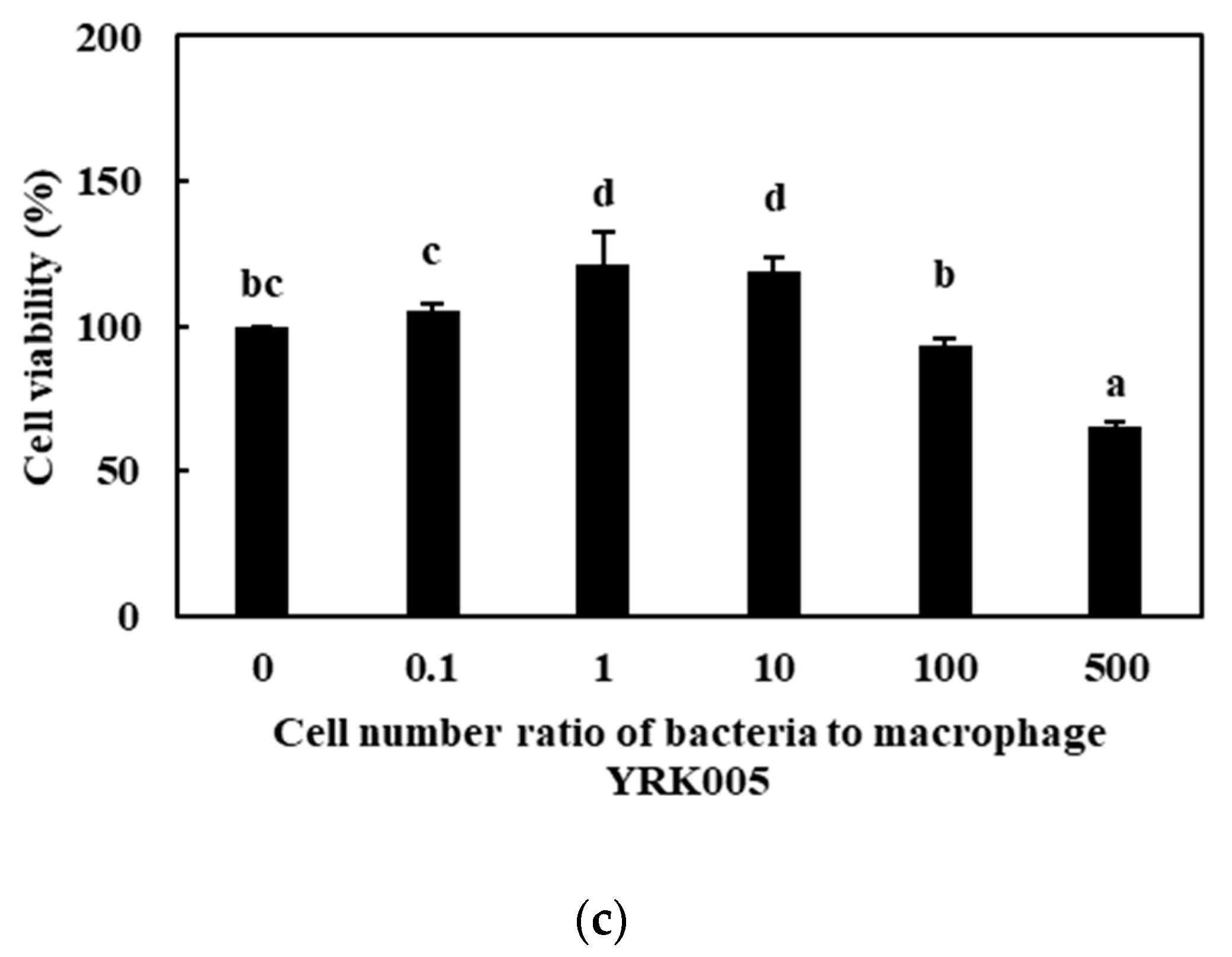
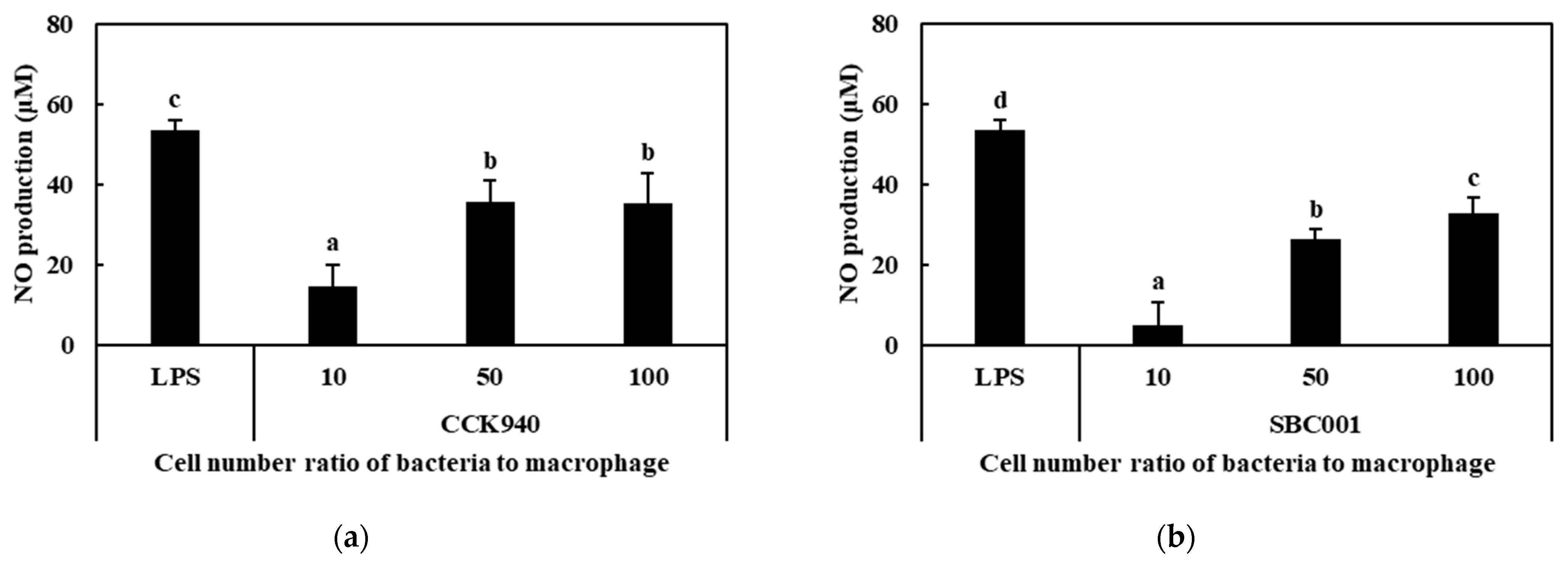


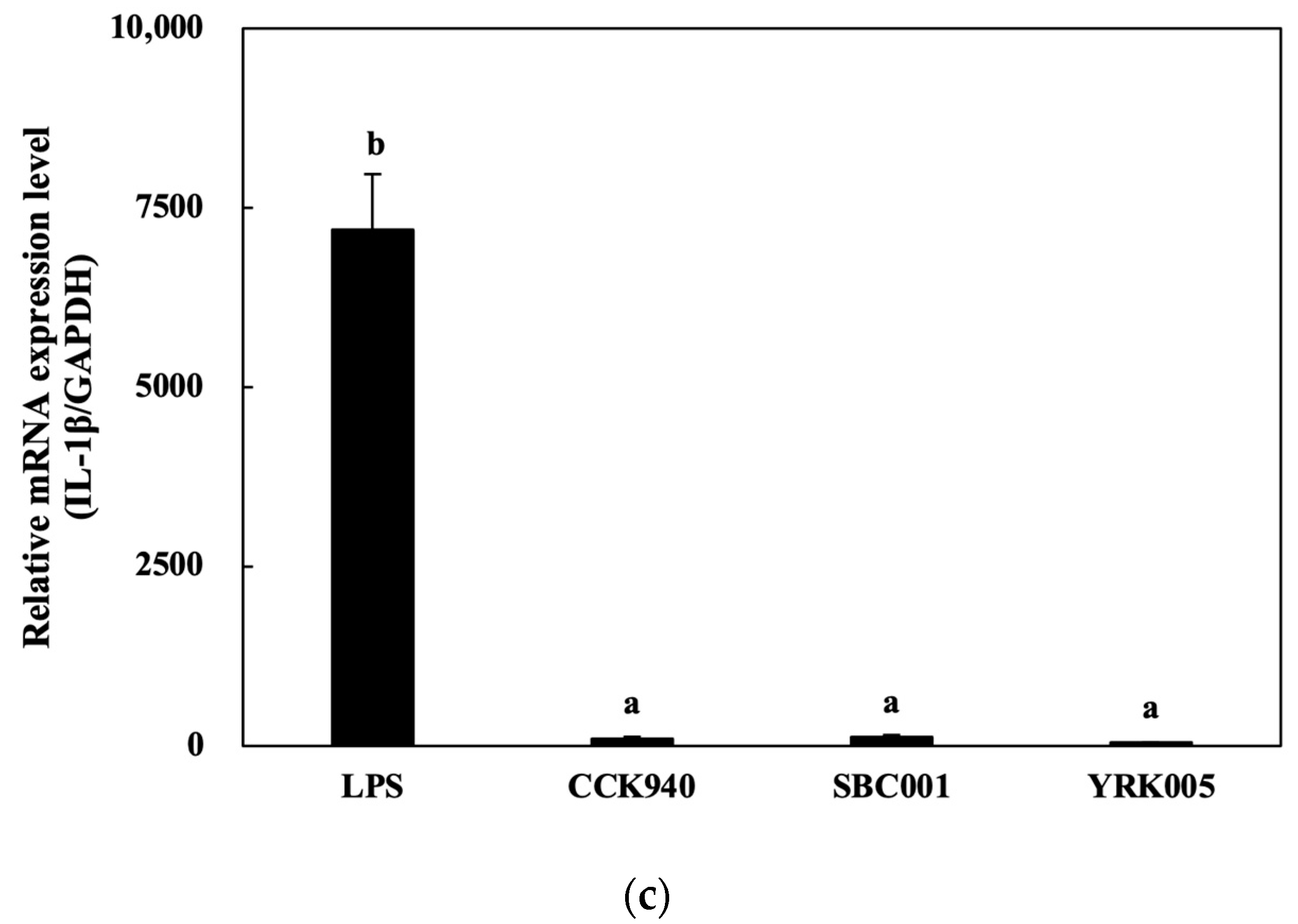



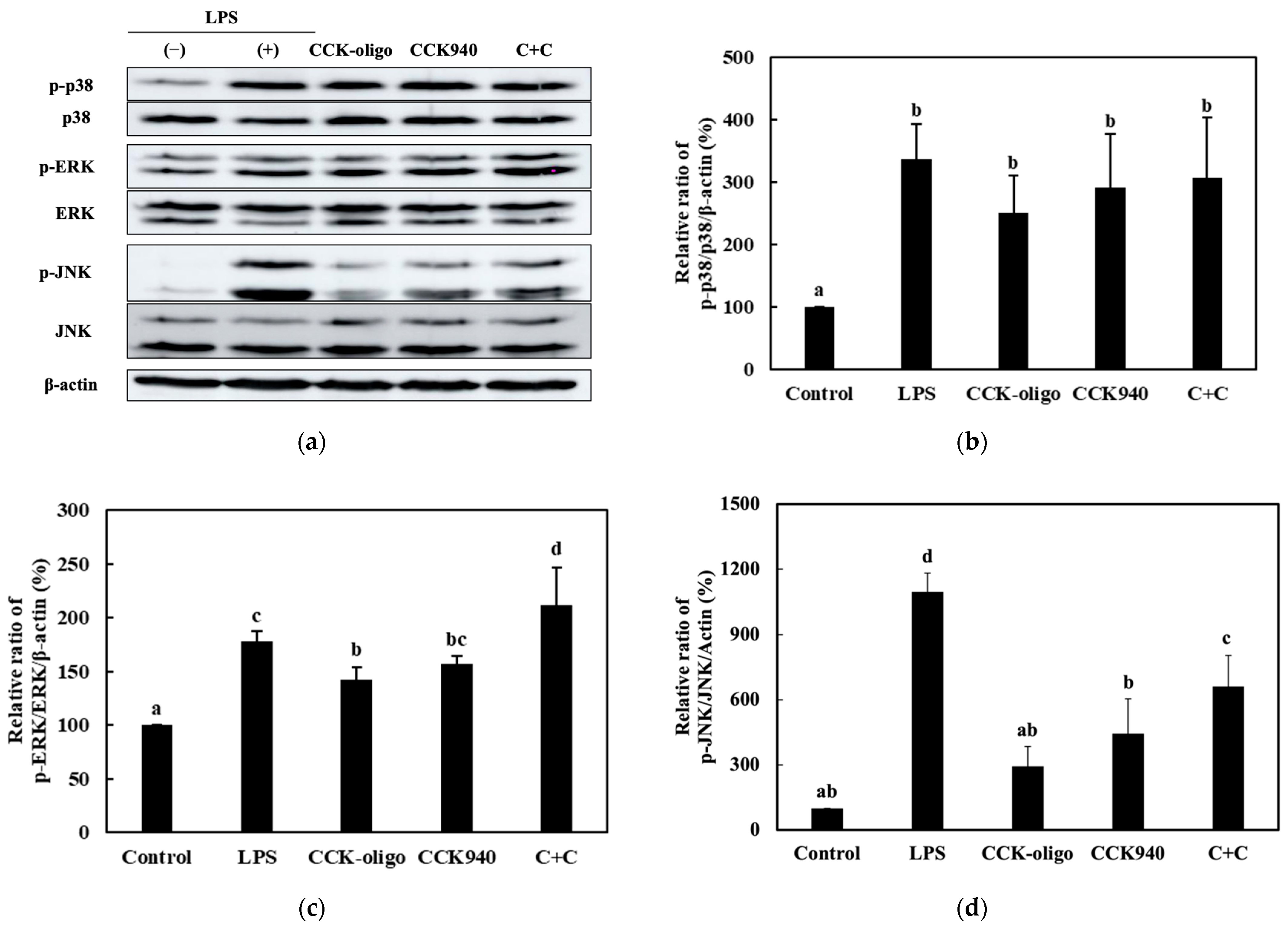
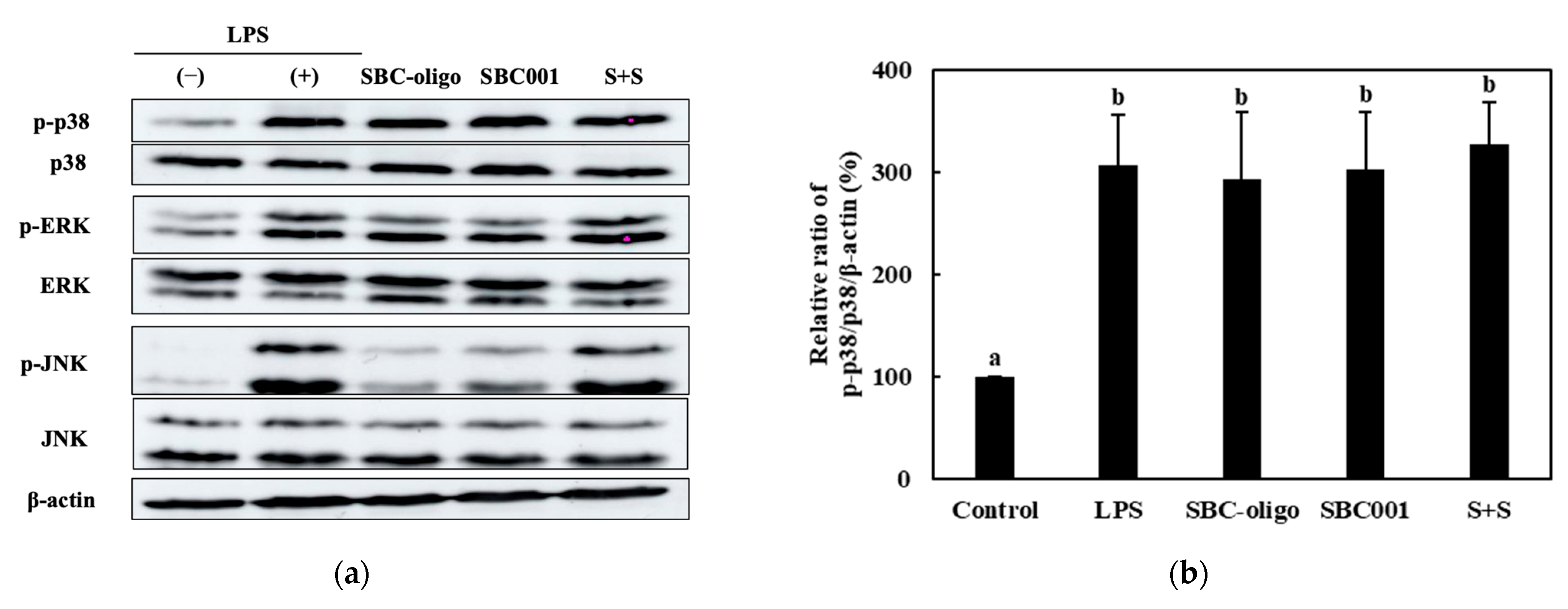
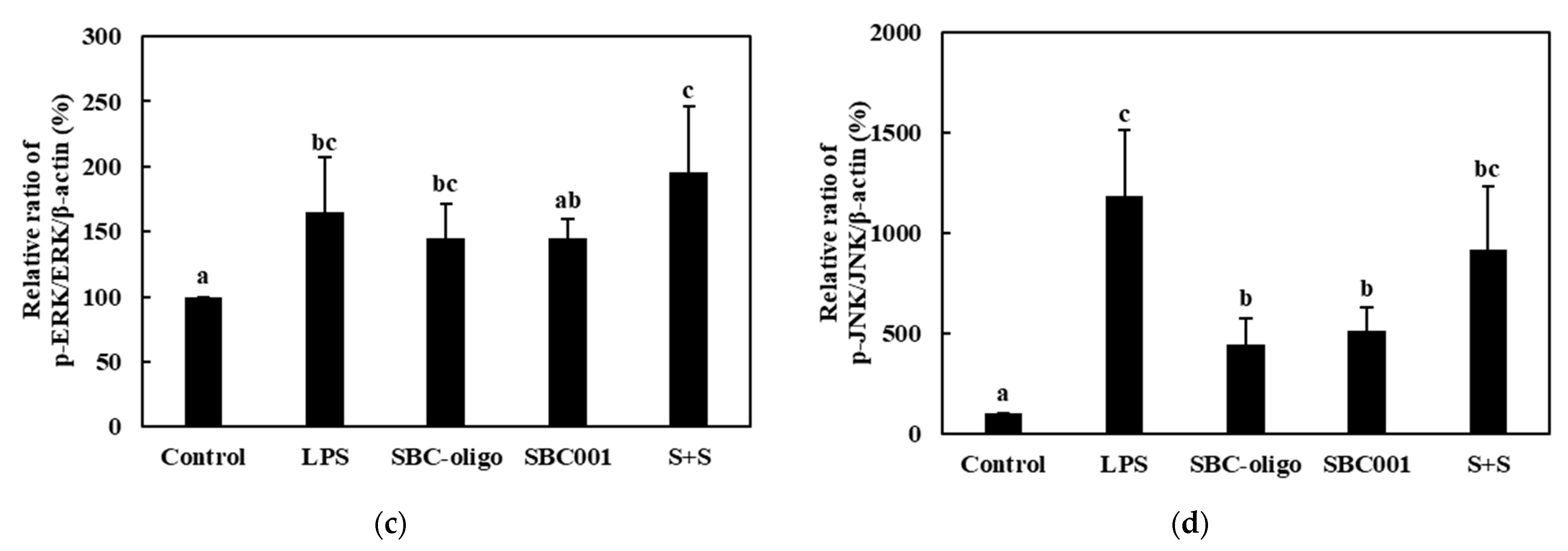
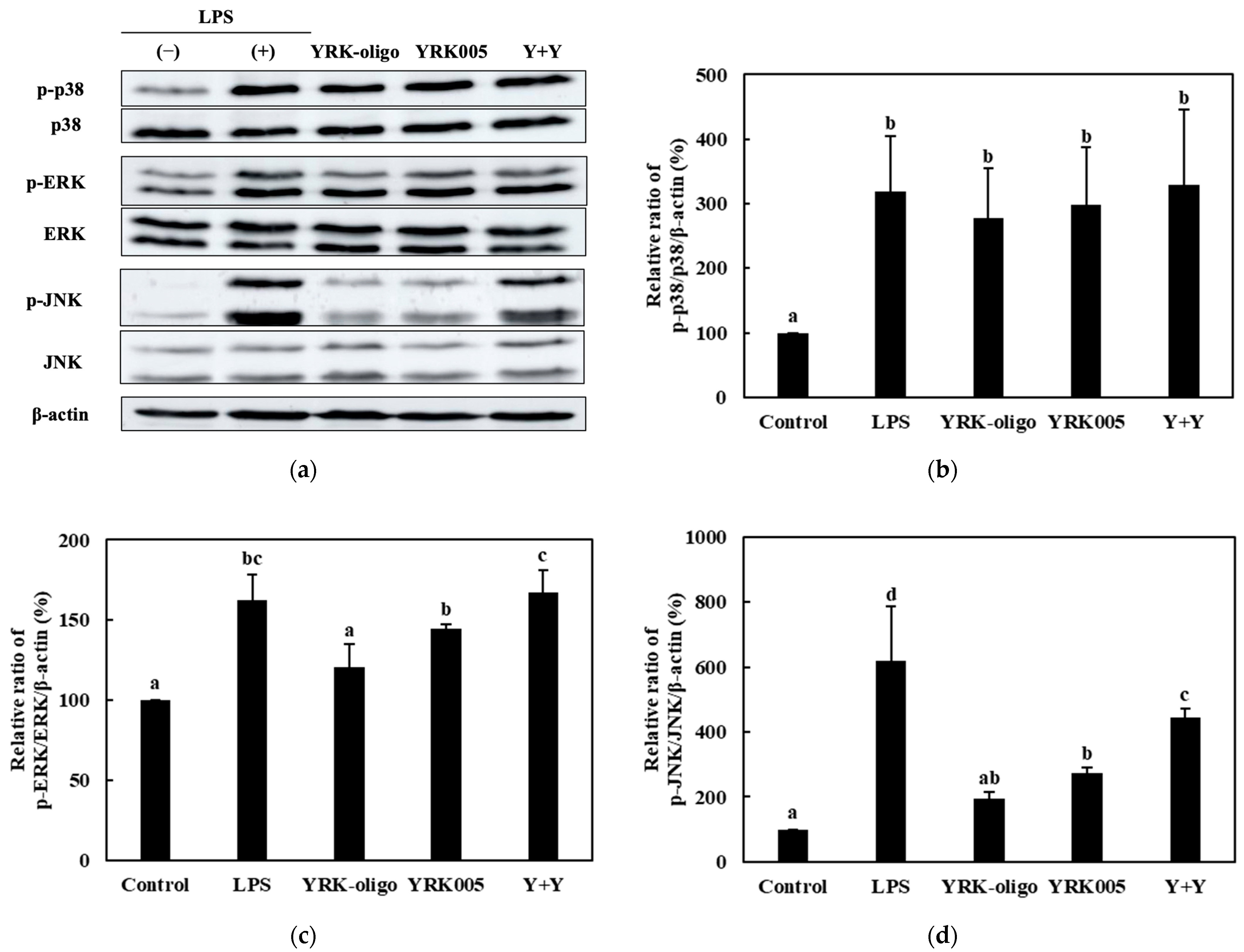
| Sample | Synbiotic Preparation |
|---|---|
| C + C | CCK-oligosaccharides + Leuconostoc lactis CCK940 |
| C + S | CCK-oligosaccharides + L. lactis SBC001 |
| C + Y | CCK-oligosaccharides + Weissella cibaria YRK005 |
| S + C | SBC-oligosaccharides + L. lactis CCK940 |
| S + S | SBC-oligosaccharides + L. lactis SBC001 |
| S + Y | SBC-oligosaccharides + W. cibaria YRK005 |
| Y + C | YRK-oligosaccharides + L. lactis CCK940 |
| Y + S | YRK-oligosaccharides + L. lactis SBC001 |
| Y + Y | YRK-oligosaccharides + W. cibaria YRK005 |
| Gene | Primer Sequence | |
|---|---|---|
| GAPDH | Forward Reverse | 5′-ATC CCA TCA CCA TCT TCC AG-3′ 5′-CCT GCT TCA CCA CCT TCT TG-3′ |
| TNF-α | Forward Reverse | 5′-ATG AGC ACA GAA AGC ATG ATC CG-3′ 5′-CCA AAG TAG ACC TGC CCG GAC TC-3′ |
| IL-1β | Forward Reverse | 5′-ATG GCA ACT GTT CCT GAA CTC AACT-3′ 5′-CAG GAC AGG TAT AGA TTC TTT CCT T-3′ |
| iNOS | Forward Reverse | 5′-AAT GGC AAC ATC AGG TCG GCC ATC ACT-3′ 5′-GCT GTG TGT CAC AGA AGT CTC GAA CTC-3′ |
| Strain | Acid Tolerance (%) | Bile Tolerance (%) | Adhesion Ability (%) |
|---|---|---|---|
| Leuconostoc lactis CCK940 | 87.2 ± 3.9 b | 75.9 ± 1.3 a | 54.2 ± 11.1 a |
| L. lactis SBC001 | 52.3 ± 3.3 a | 81.9 ± 0.3 b | 55.7 ± 10.7 a |
| Weissella cibaria YRK005 | 54.0 ± 3.4 a | 82.4 ± 1.9 b | 67.6 ± 5.4 a |
| Strain | Antioxidant Activity (%) |
|---|---|
| Leuconostoc lactis CCK940 | 56.8 ± 3.1 a |
| L. lactis SBC001 | 67.1 ± 2.2 b |
| Weissella cibaria YRK005 | 64.5 ± 3.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Kwon, A.; Jeong, H.; Park, Y.-S. Synergistic Immunostimulatory Activities of Probiotic Strains, Leuconostoc lactis and Weissella cibaria, and the Prebiotic Oligosaccharides They Produce. Microorganisms 2023, 11, 1354. https://doi.org/10.3390/microorganisms11051354
Jeong S, Kwon A, Jeong H, Park Y-S. Synergistic Immunostimulatory Activities of Probiotic Strains, Leuconostoc lactis and Weissella cibaria, and the Prebiotic Oligosaccharides They Produce. Microorganisms. 2023; 11(5):1354. https://doi.org/10.3390/microorganisms11051354
Chicago/Turabian StyleJeong, Seoyoung, Ayeon Kwon, Huijin Jeong, and Young-Seo Park. 2023. "Synergistic Immunostimulatory Activities of Probiotic Strains, Leuconostoc lactis and Weissella cibaria, and the Prebiotic Oligosaccharides They Produce" Microorganisms 11, no. 5: 1354. https://doi.org/10.3390/microorganisms11051354
APA StyleJeong, S., Kwon, A., Jeong, H., & Park, Y.-S. (2023). Synergistic Immunostimulatory Activities of Probiotic Strains, Leuconostoc lactis and Weissella cibaria, and the Prebiotic Oligosaccharides They Produce. Microorganisms, 11(5), 1354. https://doi.org/10.3390/microorganisms11051354






