Abstract
Consumers’ recent interest in healthier diets has increased the demand for food products with functional properties, such as probiotics. However, most probiotic food types available on the market are of dairy origin, which limits their consumption by individuals with food intolerances and by those who adhere to strict vegan and vegetarian diets. The aim of the current review is to assess both the limitations and impacts of the addition of probiotic microorganisms to fruit, vegetable, and/or mixed juices. Thus, an integrative literature review was herein carried out. A bibliographic survey was carried out in the following databases: Lilacs, Medline, Web of Science, Scopus, and Scielo. In addition, searches for studies published in English from 2010 to 2021 were carried out, based on the following meshes: “fruit”, ‘‘vegetable”, ‘‘juice”, and “probiotics”, which were used both in combination with each other and with Boolean operators such as “AND” and “OR”. Although 254 articles were initially found in the literature search, only 21 of them were selected to compose the final sample. The included studies mainly addressed microorganism viability and physicochemical analyses. Overall, fruit and/or vegetable juices can be suitable matrices used to help the development of probiotic food types. However, the microorganisms added to these products must be capable of adapting to and surviving in them to enable a product’s success. Therefore, factors such as pH, fiber content, amino acids, and phenolic compounds play an essential role in the survival of probiotic microorganisms. Given the wide variety of analyses, a comparison between parameters was the major limitation of the present study. Future studies should focus on filling the gaps persisting in the development of probiotic fruit and/or vegetable juices as well as mixed juices.
1. Introduction
In recent years, there has been an increase in the consumption of fruits and vegetables, mainly due to their health benefits. China is the largest producer of fruits, followed by India and Brazil [1]. The presence of bioactive compounds such as carotenoids, polyphenols, and vitamins, as well as dietary fiber and minerals, makes these foods important for maintaining a healthy lifestyle [2,3]. Including fruits and vegetables in the diet through juices is a practical way to eat healthy foods [3,4]. New trends in the consumption of natural foods and a healthy lifestyle have boosted sales of fruit and vegetable juices.
The possibility of diversifying raw materials in the preparation of juices has added to consumers’ desire for products that are beneficial to health, directing interest in adding compounds with functional properties. Foods claimed to have functional properties are defined as foods that, in addition to their nutritional values, confer benefits on body functions and have become increasingly popular [5].
Consumers’ recent interest in healthier diets has increased the demand for food products with functional properties, i.e., those presenting at least one nutrient or non-nutrient with positive effects, either metabolic or physiological, on human body development and maintenance, among other functions. Fruit juices are potential matrices for the insertion of probiotics since they have several nutritional characteristics favorable for growth while also meeting consumer needs for more natural and healthier foods [6,7].
Probiotic food types fall into this group; moreover, they are associated with several advantages, such as improving intestinal health and treating diseases such as obesity and type 2 diabetes [8,9,10]. According to the scientific literature, when inserted into food, the microbial cultures used must have counts of 108 to 109 cells per gram of product [11,12,13].
The cultures of microorganisms most used for producing probiotic foods may be lactic acid bacteria (LAB) of the genera Lactobacillus, Lacticaseibacillus, Lactiplantibacillus, Limosilactobacillus and Bifidobacterium, such as the species Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus delbrueckii subsp. lactis, Lacticaseibacillus casei, Lacticaseibacillus paracasei, Lacticaseibacillus rhamnosus, Lactiplantibacillus plantarum subsp. plantarum, Limosilactobacillus reuteri, Limosilactobacillus fermentum, Bifidobacterium longum, B. bifidum, B. infantiles, B. breve, B. animalis, B. lactis, and species from other genera, such as Streptococcus thermophilus, Streptococcus spp., Enterococcus faecium and Saccharomyces cerevisiae [14]. It is important to note that there was a reclassification of the genus Lactobacillus, with the definition of 23 new genera based on phenotypic, genotypic, and ecological characteristics, to define in a more delimited way the specificities and characteristics of each group contained in this old genus [15].
Probiotic microorganisms are those that when administered in adequate amounts can have beneficial health effects on hosts [16]. Thus, these microorganisms can be added to food items to help develop products with probiotic properties. However, probiotic microorganisms must survive storage, either in capsules or in food, as well as be consumed on a regular basis to provide the desired benefits. Furthermore, it is worth emphasizing that probiotics must also survive their passage through the gastrointestinal tract (GT) [17].
On the other hand, most probiotic food types traded today are of dairy origin. However, intolerance associated with the intake of milk and dairy products, as well as the increased number of individuals adhering to a vegan lifestyle and strict vegetarian diets in recent years, may limit their consumption. Thus, fruit and vegetable-based products can be an alternative to help overcome this issue [18].
It is essential to point out that the food matrix and the food administration form can also influence the survival and multiplication of probiotics, as well as possibly helping to maintain the viability of microorganisms during the product’s shelf life [17,18,19]. Thus, the digestion of liquid food is faster and reduces their contact time with bile acids and low stomach pH, a fact that enables microorganism cultures to develop resistance to these adverse conditions [20].
The intake of mixed juices, as well as fruit and vegetable juices, has great potential to increase both in Brazil and abroad [21,22]. These drinks hold significant amounts of vitamins, minerals, fiber, antioxidants, and bioactive compounds. Thus, in addition to the increasing consumption of these nutrients by the population, juices also meet the emerging demand for healthier and more natural products [20].
From this perspective, some authors have investigated the use of fruit and/or vegetable juices as potential matrices to help develop probiotic food types [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. However, these products have some stressors capable of hindering the adaptation and survival abilities of microorganisms, namely low pH, oxygen level, antimicrobial components, and storage temperature [24].
Given the increased demand for and benefits resulting from the intake of probiotic food types, as well as the challenges and advantages assumingly associated with the development of beverages derived from non-dairy matrices (Figure 1), the aim of the current study was to assess the viability of probiotic microorganisms and the impacts of their addition to fruit, vegetables, and/or mixed juices based on an integrative literature review.
Figure 1.
The addition of probiotic microorganisms in fruit, vegetable and/or mixed juices.
2. Materials and Methods
An integrative review study was conducted based on the method suggested by Souza et al. [25]. This was split into six stages, namely: guiding question elaboration; inclusion and exclusion criteria establishment and search in the literature; and definition of information to be extracted from the selected studies, including study assessment, interpretation of results, and review presentation.
Firstly, the following guiding question was defined: what are the limitations and impacts of adding probiotic microorganisms to fruit and vegetable juices? Then, data collection was carried out, and databases, search strategies, and inclusion and exclusion criteria were defined.
A bibliographic survey was carried out in July 2021 through an electronic search performed in the following databases available in the Virtual Health Library: Lilacs, Medline, Web of Science, Scopus, and Scielo. Health Sciences Descriptors (DeCS) and Medical Subject Headings (Mesh) were used as strategies to search for articles. Boolean operators “and” and “or” were used with keywords “fruit”, “vegetable”, “juice”, “probiotics”, as well as with combinations of them.
The inclusion criteria comprised original articles published in English between 2010 and 2021 that addressed the limitations and impacts of adding microorganisms to fruit and vegetable juices at the time to develop new products. Exclusion criteria comprised review articles, book chapters, editorials, letters to the editor, and studies that did not address the topic associated with the purpose of the current review. Publications available in more than one database were only considered once.
The initial search in the literature resulted in 254 articles associated with the herein selected keywords and descriptors. Then, articles published in duplicate were excluded in compliance with the adopted criteria. After the exclusion procedure was completed, 121 studies were considered eligible for the review. They were subjected to a pre-selection stage, according to which their title and abstract were assessed. Whether the selected studies were linked to the current research’s guiding question was also assessed at this stage. After the pre-selection process was completed, 29 articles were identified and assessed to check whether they provided information about the development of fruit and vegetable juices added with probiotics, as well as about their physicochemical, microbiological, and sensory features. After conducting a careful and objective assessment, 21 articles were selected and thoroughly evaluated. They were organized based on the categories of collected information (title, author, year, journal, sample, material and methods, and main results). A flowchart of the steps taken from the bibliographic survey to article selection is shown in Figure 2.
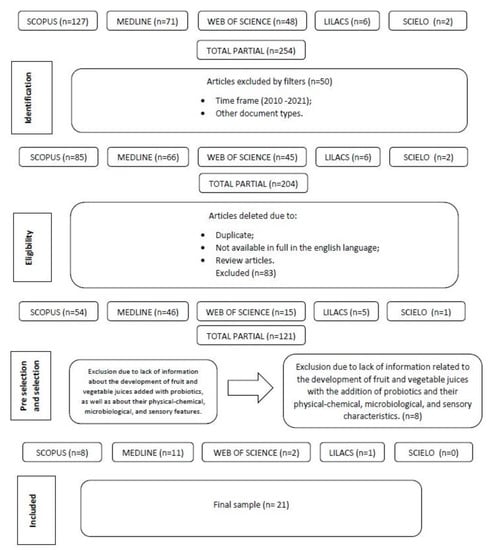
Figure 2.
Flowchart showing article selection steps.
Data were collected from the selected studies, grouped in charts, tables, and thematic approaches, and evaluated and interpreted based on the literature. Finally, the results and discussion were herein presented and organized to help form a better understanding of the topic of this review.
3. Results
Of the 254 initially identified articles, 21 were included in the current review. Six (6; 2.4%) of them were indexed in the Lilacs database; 71 (27.9%) in Medline; 127 (50%) in Scopus; 48 (18.9%) in Web of Science; and two (0.8%) in Scielo.
3.1. Main Juice-Featuring Analyses
The herein selected articles mainly addressed the viability and physical-chemical analyses of microorganisms (Table 1). The most performed analyses comprised viability tests, which were carried out in 21 studies included in the current review. They were followed by pH (n = 17), total titratable acidity (n = 10), total soluble solids (n = 8), organic acids (n = 6), total sugars (n = 6), instrumental color (n = 6), sensory (n = 5), antioxidant capacity (n = 5), phenolic compounds (n = 5), microbiological (n = 4), and reduced sugars/carbohydrates (n = 3) analyses. However, other juice features were analyzed in less than three of the selected publications, namely: quantification of macronutrients (lipids, proteins, and carbohydrates) [26], the formation of volatile compounds [10,27], and amino acids [27], anthocyanin concentrations [26,28], viscosity [29], turbidity [30], and fiber [31] and inulin contents [32].

Table 1.
Studies focused on assessing the viability and impacts of adding probiotic microorganisms to fruit and mixed juices: Main analyses carried out, juice type, inoculated microorganism, study conditions, and main results.
From this perspective, there is no standard in the analyses carried out in studies that investigate the development of fruit and vegetable juices added with probiotic microorganisms. This fact limits likely comparisons between results and the formation of a consensus in the literature about the physicochemical aspects associated with developing fruit and vegetable juices with these microorganisms. Thus, any innovative product should be subjected to an in-depth assessment to help fill the gaps associated with the impacts of adding probiotics to plant-origin products. On the other hand, it is essential to emphasize that specific analyses, such as those focused on measuring the amount of inulin added to juices and anthocyanin concentration, were mentioned in some studies. Still, their inclusion depends on the research type and the assessed food matrix.
Thus, studies need to be planned to consider each raw material used, the conditions of inoculation of probiotic microorganisms, and physical-chemical, microbiological, sensory, and nutritional parameters. With all this information, it is possible to analyze more deeply the impacts of the addition of microorganisms to vegetable matrices such as fruit and vegetable juices.
3.2. Juices Used in Studies
With respect to juice featuring, fruits were the main matrices used to prepare the juices assessed in the analyzed studies. The main fruit used was the orange, being used as a food matrix in six studies, followed by apple, pomegranate, and grape, used in five, four and two studies, respectively. Only two studies used vegetables to prepare juices: pumpkin [39] and Chinese jujube [10]. Orange is an important fruit recognized for its sensory and nutritional characteristics and health benefits, as well as being an important source of vitamins, mainly vitamin C, fiber, and minerals. It has high antioxidant capacity due to its high content of bioactive compounds such as ascorbic acid, flavonoids, and carotenoids [1,22,27,43].
Mixed juices presented different formulations comprising different fruit and vegetable types, which were combined with each other at different proportions during preparation. This result may be associated with the variety of and accessibility to fruits and vegetables since the surveys were conducted in different countries at different times. Mixed juices have been explored as an alternative to replace ultra-processed beverages and are a convenient way to consume products with high nutritional value [31].
3.3. Types and Number of Probiotic Microorganisms Used in Juices
All selected articles used bacteria as probiotic microorganisms for research purposes; Lactobacillus was the main tested genus (Table 1). This bacterial genus encompasses Gram-positive microorganisms naturally found in human GT. Lactobacillus strains are mostly used because they are generally recognized as safe (GRAS), as well as because of their benefits and suitability, not only in terms of origin, safety, and resistance but also in terms of growth properties in vitro and during processing, and because of their functional features [40]. According to Frakolaki et al. [46], the strains must endure the acid gastric juice, the bile, and the pancreatic enzymes in order to reach the small intestine. Then, they must be able to adhere to the intestinal surfaces. Furthermore, the strains need appropriate technological characteristics, as resistance to aerobic conditions and could be product in industrial scale.
In addition to these factors, probiotic strains need to be storage stable, present satisfactory counts and not interfere with the sensory attributes of the products. With so many necessary factors to be met, it is clear that developing probiotic food is a challenge.
Some of the selected articles included other bacterial genera but the authors considered them as probiotics. There is often some confusion in defining a strain as a probiotic. It is important to highlight that there is a difference between commensal and probiotic microorganisms. Commensal microorganisms in the gut are usually sources of probiotic strains but they cannot be called “probiotics” because the strains still need to be isolated and their possible health effects characterized and evaluated. Another problem is considering live cultures present in fermented foods as probiotic [16].
With respect to the microorganisms’ inoculation form applied to juices, five studies assessed the addition of bacteria in the microencapsulated form. These studies aimed at comparing the impacts of adding microorganisms to juices in their microencapsulated and free forms. According to Rengadu et al. [44], microencapsulation is an efficient method to assist in the protection of probiotics. This technique enables it to survive in food and during passage through the GT. There are different materials that can be used in the microencapsulation process such as polysacaharides, which can be used in combination with gelatine, alginate carrageenan, and starch. Another advantage to these methods is the fact that microcapsules provide an appropriate anaerobic condition to the probiotic bacteria and work as a physical obstacle to the acidic conditions associated with fruit juice [44]. In addition, it is important that the use of this method does not interfere with the survival of microorganisms and their action.
Although data from the literature indicate that the addition of microencapsulated probiotics to foods is a good proposal, it is worth noting that depending on the material used, dissolution may occur, altering the quality parameters of the juice, including color, flavor, viscosity, and aroma. Then, if the juice matrix is an adequate environment for probiotics, this enables its possible use in free form.
Concerning to the addition of isolated or combined microorganisms, probiotics in juices were individually assessed in some studies, except for the study by Rengadu et al. [44], who evaluated the impact of microorganisms separately, and analyzed the accumulation of Lacticaseibacillus casei and Bifidobacterium animalis combined in the same sample.
The number of probiotics inoculated in the tested juices ranged from approximately 106 to 1010 CFU/mL. According to the criteria established by FAO/WHO [19], these products must hold at least 106 to 107 CFU/mL of microorganisms during their shelf life to be considered probiotic. However, according to the National Health Surveillance Agency in Brazil, the minimum number of probiotics in the daily recommendation of these products must range from 108 to 109 CFU [8]. It is important to note that, depending on the country, the number of probiotic microorganisms may vary according to what the authors consider an appropriate recommendation. This could explain the diversity in the values found regarding the amount of initial inoculum added to the juices.
It is essential highlighting that juices and vegetables are unconventional matrices for probiotic microorganisms, besides having stressors, with emphasis on as low pH. Therefore, microorganism inoculation in amounts slightly above the limits established by the legislation would be an interesting strategy since cultures must adapt to and survive in juices to enable these products to exert their benefits.
3.4. Probiotic Microorganisms’ Viability in Juices
The viability of probiotics is directly linked to features of the food matrix and added microorganisms, as well as interactions between them. Although there is no consensus in the literature about the exact number of viable cells necessary to trigger probiotic effects, most studies adopt values ranging from 10⁶ to 10⁷ CFU/mL [26,31,38,45].
Many factors can influence the viability of probiotic microorganisms in food products during production, processing, and storage. The characteristics related to the probiotic microorganisms involved, such as strains and the amount of inoculum used, directly influence their viability. In addition, the characteristics of the food are also important, such as pH, titratable acidity, molecular oxygen, water activity, and the presence of salt and sugar. In addition, the addition of chemical substances such as bacteriocins and artificial flavors and colorings should be considered, as should the conditions applied in processing such as heat treatment, incubation temperature, cooling, packaging type, material and storage methods, and production scale [47].
According to Rengadu et al. [44], low pH, nutrient depletion and lactic acid accumulation during storage time can hinder probiotic bacteria survival and affect their effective dose necessary for consumption purposes. Still, it is essential to emphasize that the metabolic specificities of each microorganism, as well as their ability to adapt to breeders’ stressful conditions, are also crucial factors enabling the survival of probiotics [31]. The pH value directly influences the functioning of enzymes, the stability of molecules, and, consequently, cellular metabolism. The different microorganisms have a maximum, optimum, and minimum pH value to enable their growth. Generally, the presence of organic acids (such as lactic, acetic, formic, and benzoic acids) affects the survival of microorganisms, as they are often found in a non-dissociated form and are thus able to more easily penetrate the cell. Organic acids enter the microbial cell and dissociate, releasing H+ ions and causing significant alterations in cellular functioning and the inhibition of microbial growth [48].
De Oliveira et al. [31] assessed three Lactobacillus strains (Lactiplantibacillus plantarum LP 299V, Lacticaseibacillus rhamnosus GG, and Lactobacillus acidophilus LA—14) in mixed mango and carrot juice at different concentrations. According to the aforementioned authors, L. acidophilus appears to be more demanding when it comes to pH conditions, oxygen level and nutrient viability, in addition to presenting viability lower than that of other microorganisms. On the other hand, the tested L. plantarum strain has shown greater viability because this microorganism is acknowledged for its excellent adaptation to adverse conditions, its high capacity to ferment different sugars, and its efficient transport system. Despite this, all strains tested in the current study have shown viability higher than 7 log10 CFU/mL after fermentation and at the end of 35-day storage at 8 °C.
On the other hand, the study by Mokhtari et al. [30] assessed bacteria belonging to different genera (Lactobacillus acidophilus and Bifidobacterium bifidum). The results show that L. acidophilus is more resistant than B. bifidum under acidic conditions. Therefore, it presented better compatibility with the investigated conditions (grape juice with pH 3.8 during 8-week storage). However, this difference was not significant, and both microorganisms recorded viability higher than 7.0 log10 CFU/mL after 60 days at 4 °C.
Thus, in line with other studies, bacteria belonging to the genus Lactobacillus are often resistant and capable of surviving in juices with a pH ranging from 3.7 to 4.3. On the other hand, bifidobacteria are less acid-tolerant and pH values close to 4.6 can be detrimental to their survival. However, as shown in the current review, the aforementioned probiotics, even at lower concentrations, have shown good viability in juices with low pH. In these cases, this parameter alone cannot explain the herein observed trends.
From another perspective, the chemical composition of nutrients, balance, and viability, as well as the presence of inhibitory compounds and intrinsic factors in food, can be decisive features used to select the appropriate matrix to help develop probiotic products. This review has found that six studies assessed the same microorganism in more than one juice. In this way, it is possible to analyze in more detail the impacts of the composition and characteristics of the juices on the survival of probiotic microorganisms.
The study by Bhat et al. [34] assessed the growth of the microorganism species Weissella Kimchi R-3 in orange, pomegranate, and carrot juices under different fermentation (72 h/37 °C) and storage (12 days/25 °C or 5 weeks/4 °C) conditions. The results indicated that the growth of microorganisms during fermentation time, as well as their viability under both storage conditions, were higher in carrot juice. The assessment showed that a considerable number of bacteria remained until the end of the storage period. However, the numbers of bacteria in orange and pomegranate juice were undetectable in the second week of refrigerated storage as well as during 3-to-6–day storage at room temperature. Thus, the primary justification for this finding depends on the low pH of these matrices and the temperature conditions. The metabolism of microorganisms is more active at 25 °C, which increases the production of organic acids and the depletion of nutrients. On the other hand, 4 °C is the temperature outside the optimal microorganism growth range; therefore, it is an adverse condition for bacterial growth. The reduced pH value improves the quantity of undissociated organic acids in fermented products and contributes to the bactericidal effect of these substances [47,49].
However, as previously mentioned, in addition to pH, other features of juices can also hinder the survival of probiotic microorganisms in them. For example, Srisukchayakul et al. [37] tested the addition of bacteria, previously adapted to acidic media, to pomegranate, cranberry and lime juices. The results proved that although all three matrices presented a similar pH, microorganisms were less resistant to cranberry and pomegranate juices due to the high concentration of phenolic compounds with antimicrobial action. According to Dinkçi et al. [49], plant-based materials such as fruits and vegetables impact probiotic viability depending on which phenolic compounds are present, the amount, and the proportion of vegetable added to the product. Thus, it is possible that the choice of which raw material to use can directly influence the viability of the chosen probiotic culture.
On the other hand, according to Olivares et al. [38], maintaining the viability of probiotic cultures in fruit juices is a challenging task since these products have high concentrations of dissolved oxygen. It is worth emphasizing that the most used probiotic microorganisms are of the anaerobic and microaerophilic types; therefore, the presence of oxygen in the product at more significant rates can lead to toxicity and viability loss. Thus, the aforementioned authors have pointed out that vitamin C, as an oxygen scavenger, may have a protective effect during the storage period as well as promote a more favorable anaerobic environment (not observed during fermentation) [38]. However, it is necessary to consider that vitamin C is a very unstable compound and long periods of storage can cause its reduction in food. As a consequence, damage to the survival of anaerobic cells may occur.
With respect to matrix composition, De Oliveira et al. [31] assessed different formulations associating mango juice with carrot, in addition to different Lactobacillus species. The study indicated higher microorganism viability in juice presenting higher carrot pulp concentration due to its high fiber content. The authors explained that dietary fibers can significantly influence the survival of probiotic microorganisms after processing and during storage time. Soluble fibers can be used as a substrate for the growth of probiotic microorganisms, whereas insoluble fibers can protect these bacteria by acting as a physical barrier. Furthermore, the growth of carrot pulp was also high, a factor that can be considered a bias and that may have provided greater stability for microorganisms in this formulation.
However, Valero-cases and Frutos [32] investigated inulin addition to mixed carrot and orange juice by adding Lactobacillus plantarum CECT 220 to it. They observed higher microorganism viability in juices added with inulin after 15-day storage. However, viability began to decrease in juices without fiber addition after this time. This happened due to the lower concentration of monosaccharides in these samples. Therefore, inulin was a carbon source available for the tested strain during storage time, and it may also have protected the used microorganism during refrigerated storage by preventing cell damage, mainly by physically immobilizing inulin-structured cells since this polymer forms aggregates in aqueous media. One of the great doubts regarding probiotic products of plant origin is related to the viability of probiotic cultures in non-dairy matrices. Prebiotics are characterized as non-digestible compounds by the body that remain intact in the colon, serving as a substrate for beneficial microorganisms present in the human microbiota. In this way, they may be able to increase the viability of cultures of microorganisms inserted in food matrices [6,47,49]. As presented in the previous research, prebiotics such as inulin could help in the survival of probiotic microorganisms.
The herein presented results suggest that the viability of the microorganisms resulted from the synergistic and antagonistic actions of different parameters. pH can be detrimental to the viability of microorganisms, for example, but protein and dietary fiber can protect cells from this type of stress. Finally, although acidity is a disadvantage for the survival of probiotics in juices, incorporating probiotic bacteria in fruit juices can help increase their resistance to subsequent stressful acidic conditions, such as those observed in the human gastrointestinal tract [23].
According to Tripathi and Giti [47], food components play significant roles in food, either providing protection, remaining neutral, or causing harm to probiotic viability. In addition, food additives (sugars, sweeteners, salts, aroma compounds, flavoring and coloring agents, and bacteriocins) could significantly influence the development and viability of probiotic bacteria.
3.5. Impact of Probiotic Microorganisms’ Addition on Juices’ Quality Features
3.5.1. pH and Total Titratable Acidity
pH is an indicator of juice quality and possible microbiological activity. Thus, evaluating this parameter becomes important to understand the possible impacts of adding probiotic microorganisms to juices.
Most studies consider pH one of the most critical factors affecting the survival of probiotic bacteria since they must resist the acidity of the juices in order to grow. Thus, both the drop in pH and increase in acidity help to prevent the development of unwanted microorganisms during storage time as well as increase the shelf life of juices. However, if the amount of produced acid is too high, it can affect the product’s sensory features and, consequently, its acceptability [29,40,41,44].
Therefore, the results of the assessed studies have shown that all articles performing pH and acidity analyses during fermentation reported reductions and increases in these parameters, respectively. According to these studies, these findings were associated with the metabolism of microorganisms since they use carbohydrates found in food matrices as an energy source as well as synthesized organic acids.
Moreover, 9 of the 11 articles that carried out pH analyses during storage time also observed a pH decrease in at least one of the tested juices. The explanation for this finding was the same as previously cited. Zhu et al. [42] in turn have also highlighted that an increase in acidity and decrease in pH may have taken place due to juice sugar hydrolysis induced by enzymes released from dead probiotic cells.
However, divergent results were found in the study by Bonaccorso et al. [43], according to whom pH remained constant for 35-day storage at 5 °C. According to the aforementioned authors, it may have happened due to the reduced metabolism of these bacteria at low temperatures. In addition, Majeed et al. [33] evaluated the pH of apple juice added with Bacillus coagulans MTCC 5856 for 6 months under refrigeration (4–6 °C), and they did not observe changes in this parameter; the authors also used the justification associated with refrigeration temperature to explain this finding.
Furthermore, Garcia et al. [29] assessed five Lactobacillus strains in apple, grape or orange juices kept under refrigeration at 4 °C for 21 days (each strain in one sample). The authors did not observe changes in pH and acidity until the 14th day of storage. In addition, pH in apple juice increased on the 21st day of storage, regardless of the strain or microorganisms’ proliferation, as well as in grape juice, depending on the added culture (Table 1).
Changes in pH and acidity values can be caused by different factors, as suggested by the aforementioned authors. Thus, it is necessary to know the initial pH of the matrix used and, after inoculation, monitor possible changes during storage. Additionally, changes in pH and acidity values may also occur depending on the strain used and its concentration added to the product.
3.5.2. Total Soluble Solids, Organic Acids, Reducing Sugars/Carbohydrates
Total soluble solids are an essential quality parameter for the development of new products. This parameter mostly represents the sugar content as well as a small portion of soluble proteins and amino acids, among other organic materials. Thus, the value of soluble solids is likely to affect the product’s taste since it can indicate its sweetness level [50].
Only 3 of the 8 articles analyzing total soluble solids have analyzed this parameter during fermentation time; two of them found a decrease in this quality parameter. The other five studies analyzed total solids during storage time; half of them did not find significant differences in these values. According to De Oliveira et al. [31], the maintenance of total soluble solid values may be associated with the action of microorganisms in the hydrolysis of insoluble sugars, a fact that promoted balance in this parameter during storage time.
However, Garcia et al. [29] observed different results for total soluble solids. Total solids content increased in apple juice added with L. brevis 59, L. fermentum 111, and L. pentosus 129 and decreased in all orange juice samples during the 21-day storage. According to the aforementioned authors, the decrease observed in this parameter was associated with microorganisms’ consumption of matrix sugars, whereas the increase observed in the hydrolysis of sugar was associated with enzymes released from dead Lactobacillus cells.
As previously mentioned, probiotics can metabolize sugars in juice during fermentation and form organic acids. The produced acids can also work as critical secondary carbon sources for microorganisms. Therefore, six articles have evaluated organic acid levels in juices.
The results reported by Wu et al. [28] suggested a decreasing trend in pyruvic, shikimic, citric, and malic acid contents as well as an increasing trend in lactic acid content and blueberry and blackberry juices during fermentation time. These authors advocated that potential probiotics (S. thermophilus and L. plantarum) can biotransform malic or pyruvic acid into lactic acid or into other products. Furthermore, probiotic microorganisms can also degrade citric acid to produce lactic and acetic acids and diacetyl. Li et al. [10] and de Garcia et al. [29] also observed the same trend towards increased lactic acid, decreased citric acid, and malic acid biotransformation.
Wu et al. [28] and Garcia et al. [29] also associated viable microorganism counts with organic acids’ metabolism. Thus, microorganisms with higher viable counts presented the best enzymatic activity and, consequently, the highest consumption of sugar and the most significant lactic acid formation.
All studies focused on analyzing reducing sugars, and total sugars reported a decrease in these parameters after fermentation. This finding is explained by the consumption of these nutrients by probiotic microorganisms, which used them as an energy source to grow in juices. The total soluble solids content reflects the flavor and other sensorial substances that are characteristic of the juice. The presence of sugars can influence consumer preference and may lead to acceptance or rejection and is considered a determining factor for turbidity. Thus, changes in these parameters can directly impact the acceptability of juices added with probiotic microorganisms.
3.5.3. Phenolic Compounds and Antioxidant Capacity
Beneficial effects attributed to phenolic compounds are associated with their antioxidant activity. Fruits, vegetables, and derivatives of them are the primary source of antioxidants in the human diet. However, phenolic compounds can be altered during processing, storage, and fermentation time [51].
Although several studies have already investigated the effect of fermentation on the quality and functionality of fruit and vegetable juices, information in the literature about changes in their phenolic profiles and antioxidant capacity remains scarce [10,23]. Therefore, five studies performed this analysis to help better understand the effect of these compounds on the survival of strains as well as the effect of probiotics on the phenolic profile of the juices [10,26,28,36,51].
It is important to emphasize that phenolic compounds play an essential role in the viability of probiotic microorganisms [23]. Valero-cases et al. [35], for example, stated that probiotic bacteria’s growth in pomegranate juice was associated with the metabolism of most phenolic compounds found in this fruit to a greater or lesser extent, depending on the used strain.
On the other hand, Almeida Bianchini Campos et al. [26] have shown that total phenolic compounds’ content was higher in the control juice than in the fermented one. According to these authors, this finding may be explained by the decrease in pH since the co-pigmentation of these compounds is favored by medium acidification. However, anthocyanin content did not change after fermentation since these flavonoids remain stable in acidic media.
Similarly, Mustafa et al. [36] assessed the behavior of phenolic compounds in pomegranate juice at different fermentation temperatures (30 °C, 35 °C, and 37 °C). The results indicated increased phenolic acid and flavonoid contents after 24-h of fermentation. These changes were associated with the used strains and the temperature conditions the samples were exposed to. The variation in the content of free phenolic compounds in fermentation may be related to the action of microbial enzymes that attach glycosidic bonds to these compounds. Considering that each microorganism has an enzymatic profile, different impacts can be observed, and this generates unique flavors and products after the fermentation process. Thus, fermentation with different species of microorganisms causes different enzymatic reactions, followed by different releases of phenolics from the cell wall of food matrices [36].
Wu et al. [28] observed significant changes in phenolic compounds in black and blueberry juices after 48-h of fermentation. Both juices presented similar variations in some phenolic acids. Chlorogenic acid and procyanidin contents decreased, whereas gallic, caffeic, syringic and ferulic acid contents increased. According to the aforementioned authors, anthocyanins in juices may have been degraded into syringic, ferulic and gallic acids. Furthermore, probiotics may have metabolized chlorogenic acid into caffeic acid during fermentation time.
Based on the study by Wu et al. [28], Li et al. [10] reported that the increase observed in total phenolic compounds in Chinese jujube juices after fermentation may also be linked to the activity of hydrolytic enzymes from microorganisms acting on complex phenolic compounds. Furthermore, the results indicated that the used strains have different phenolic acid metabolization capacities. Probiotic strains added with L. plantarum recorded higher phenolic acid metabolization rates in blueberry and blackberry juices than those added with S. thermophilus and B. bifidum. Overall, microorganisms can produce hydrolytic enzymes that in turn act in phenolic complexes. According to the aforementioned authors, species belonging to the genus Lactobacillus are capable of producing larger numbers of these enzymes than the ones belonging to other genera.
Probiotic strains, such as Bifidobacterium lactis BS 05, Lactobacillus acidophilus LA 06 and Levilactobacillus brevis LBR01, have already been acknowledged and patented for their antioxidant activity. In addition, there is also evidence in the literature of the antioxidant capacity of L. plantarum, Lactobacillus helveticus, L. acidophilus, Limosilactobacillus fermentum, L. casei, Lactobacillus GG and of some bifidobacterial strains [52]. However, as reported in the herein reviewed articles, antioxidant property is linked to specific strains [10,28].
Microorganisms have increased the antioxidant capacity in 3 of the 5 studies carried out in this analysis, as shown in Table 1. Values may change depending on the used strain. According to Li et al. [10] and Wu et al. [28], probiotics can play a key role in changing the phenolic profile of matrices. The aforementioned authors have also associated this fact with increased antioxidant capacity. However, based on the study conducted by Almeida Bianchini Campos et al. [26] and Valero-cases et al. [35], microorganisms only helped in maintaining high antioxidant capacity. From another perspective, Mustafa et al. [36] have tested the antioxidant capacity in pomegranate juice added with four lactobacillus strains (Lacticaseibacillus casei NRRL B-1922; Lacticaseibacillus casei NRRL B-227; Lacticaseibacillus Bulgaria CFFC B0043; Ligilactobacillus salivarius NRRL B-1949) and adjusted the pH to 2.5; 4.0 and 5.5. According to these authors, this parameter was also affected by pH change. The addition of microorganisms has considerably increased antioxidant capacity at pH 4, but this parameter decreased at pH 2.5 and 5.5. According to Mustafa et al. [36], this fact indicates that pH is an important factor to modify the metabolite profiles of fermented juices. The appropriate pH value favors the action of enzymes on the cell wall of the food matrix during fermentation. Thus, it contributes to the release of cell wall components during fermentation and induces the release of phenolic compounds from food matrices, thus contributing to the antioxidant capacity.
Finally, based on the results of the herein reviewed studies, most lactic acid bacteria have oxygen-free radical scavenging systems. Therefore, another possible mechanism to be taken into consideration lies in the synthesis of bioactive peptides as effective antioxidant activity modes in food products added with probiotic bacteria [52].
3.5.4. Instrumental Color Analysis
Color is an important aspect used in food products to attract consumers since it is linked to food taste, nutrition, and quality. Overall, fruit and vegetable juices have pigments that can be altered due to chemical reactions and the microbial growth taking place during fermentation [53].
Studies by De Oliveira et al. [31] and Mokhtari et al. [30] did not report perceptual changes in juice color during storage time, regardless of formulation or the addition of microorganisms. Li et al. [3] have emphasized that Chinese jujube juice fermentation made the samples lighter and less red. On the other hand, Almeida Bianchini Campos et al. [26] observed that fermented pineapple and “juçara” juices showed a higher trend to turn red and yellow, respectively.
Anthocyanins are pigments accounting for the color of several juices; they are easily degraded by chemical reactions under different oxygen, enzyme, pH and temperature conditions [54]. However, these red pigments are more stable in acidic media. Therefore, fermentation and, consequently, the decrease in pH may explain the results reported by Almeida Bianchini Campos et al. [26].
Rengadu et al. [44] assessed color changes in apple juice added with free microorganisms and resistant starch microcapsules. They observed that microcapsules significantly affected juice color. Visual inspection has shown that the control sample acquired a golden yellow color, although the juice turned slightly darker and cloudy after the microcapsules were added to it. According to the aforementioned authors, the observed color variation could result from microcapsules’ dispersion in the juice at the time the samples were analyzed.
Finally, according to Mokhtari et al. [30], although the presence of alginate microcapsules (clear) in contrast to grape juice initially reduced the sample’s color over the storage period, no significant additional change in color was observed. However, changes observed in the product hindered the color sensory analysis. Thus, grape juices added with microcapsules recorded the lowest acceptability rate for this parameter.
3.5.5. Turbidity and Viscosity
Turbidity is an indicator of particle stability and is a decisive visual quality attribute for consumer acceptance of juices. Usually, consumers associate loss of turbidity with deterioration and degradation of quality.
Mokhtari et al. [30] have analyzed turbidity in grape juice added with microorganisms (Lactobacillus acidophilus—PTCC 1643 and Bifidobacterium bifidum—PTCC 1644), either in their encapsulated or free form, during storage at 4 °C for 60 days. At the beginning of the storage period, turbidity in treatments added with free bacteria was significantly higher than that observed for the control and for samples added with encapsulated bacteria. At the end of the storage period, turbidity in treatments added with free bacteria significantly increased and was slightly higher in juices added with L. acidophilus. According to the aforementioned authors, this happened due to the microorganism’s greater resistance and metabolic activity in the medium. Furthermore, a slight increase in turbidity was observed in juices added with microencapsulated microorganisms compared to the control. According to these authors, this happened due to the release of materials from microcapsules, along with the release of metabolites during bacterial growth in the juice. In addition, the contact of calcium alginate in the capsules with minerals in grape juice, mainly with sodium and phosphorus, during the longer storage period, led to gradual capsule structure degradation and, consequently, to increased turbidity.
Garcia et al. [29] assessed the viscosity of apple, orange and grape juices added with five Lactobacillus strains (Lactiplantibacillus plantarum 49; Levilactobacillus brevis 59; Lactobacillus paracasei 108; Limosilactobacillus fermentum 111; Lactiplantibacillus pentosus 129). The aforementioned authors observed increased viscosity in apple juice added with L. plantarum 49, L. brevis 59, or L. fermentum 111, as well as in the control, for the first time at 21-day storage. However, this parameter decreased in samples with L. paracasei 108 or L. pentosus 129. Viscosity in orange and grape juices has increased in samples added with microorganisms at 21-day storage, regardless of strain, as well as in control samples. According to these authors, the increased viscosity observed in fruit juices may be associated with the ability of some Lactobacillus species to produce exopolysaccharides capable of acting as texturizing agents, as well as to increase the viscosity of the final product and to interact with other juice constituents, such as proteins.
However, since juice samples without lyophilized Lactobacillus cells’ addition have also shown increased viscosity, this finding was explained based on the argument that the interaction among compounds found in fruit juices (sugars, pectin, and proteins) can help strengthen hydrogen bonds between solutes and that this process results in a decrease in intermolecular space as well as an increase in the product’s viscosity.
3.5.6. Amino Acids
Only one article assessed the presence of amino acids in probiotic juices. According to the study by Xu et al. [27], 18 amino acids were identified in mixed juice comprising Chinese jujube, apple, orange, and carrot; aspartic acid was the most abundant among them, followed by glutamic acid and proline. The first two acids recorded a post-fermentation decrease, whereas proline recorded a slight increase. The metabolic activity of the investigated three probiotics has changed amino acids’ type and content in the juice; these changes have the potential to change the juice’s flavor during the fermentation process.
3.5.7. Formation of Volatile Compounds
The composition and concentration of volatile compounds are aspects of great interest for the development of fruit and vegetable juices added with probiotics since they have a direct influence on sensory properties as well as product acceptance. Furthermore, only studies conducted by Li et al. [10] and Xu et al. [27] focused on investigating fermentation effects on the profile and content of volatile compounds.
According to Li et al. [10], fermentation significantly improved the formation of volatile compounds, mainly for L. plantarum in Muzao Chinese jujube juice and for L. casei in Hetian Chinese jujube juice. With respect to the profile of the formed volatile compounds, alcohols were important aromatic compounds found in both fermented juices. They contributed to their light aroma and acted as solvents for other aromatic substances. Still, fermentation had a positive influence on acetaldehyde production to a lesser extent; in other words, it may have given positive aromatic attributes to the investigated juices. Furthermore, acid production increased, mainly in Muzao juice fermented by L. helveticus and in Hetian juice fermented by L. casei, and it gave a sour taste to the juice. According to the aforementioned authors, ketones and esters always give a pleasant odor to food products. Fermentation increased ketone formation, mainly in Muzao juice fermented by L. plantarum and in Hetian juice fermented by L. casei. Samples fermented by L. plantarum also showed an increase in esters content. Based on these results, these authors have emphasized that aroma development in fermented juices is a complex and dynamic process since different strains have different metabolic patterns in different environments.
On the other hand, Xu et al. [27] identified 36 compounds in mixed juices comprising Chinese jujube with orange, carrot, and control apple, whereas 34 compounds were found in the fermented juice. Alcohols and alkenes were the total volatile compound classes prevailing in the analyzed samples.
The aforementioned authors also identified a few aldehydes in the analyzed samples, which can be attributed to their instability in food matrices. Therefore, they were likely reduced to alcohol or oxidized into acids due to microbial activity. This is a positive result, since, according to these authors, high aldehyde concentrations lead to a reduction in product acceptability. Finally, total ester content in the fermented juices significantly increased. Esters are widely studied and appear to contribute to fruity notes. Yet, the aforementioned authors declared that the fermented juices presented intense floral and fruity notes that in turn may be associated with their high alcohol, ketone, and terpene contents.
In short, both previously mentioned studies included an analysis of volatile compounds in their investigation process. Despite being a complex process, the addition of probiotic microorganisms and, consequently, the fermentation process have a positive impact on the formation of volatile compounds. It is essential to point out that this finding may be associated with the adopted conditions since both studies used Chinese jujube juice as the matrix, even at different ratios and combinations, as well as the addition of L. plantarum strains for fermentation purposes.
3.5.8. Microbiological Analysis
Microbiological analysis is essential to help collect information about contaminating microorganisms and, consequently, to guarantee the safety of the product to be developed [55]. However, only 4 of the 21 articles included in the current review performed this analysis.
Molds and yeasts are the most common contaminating microorganisms found in fruit and vegetable juices since they can multiply under these products’ acidic conditions. On the other hand, bacterial growth in fruit juice depends on pH, humidity, temperature, and storage time, as well as on water activity, preservative concentration, treatment application, sugar content and the amount of raw material [55].
Accordingly, all herein assessed studies performed a mold and yeast analysis, except for the study by Almeida Bianchini Campos et al. [26]. Valero-cases and Frutos [32] subjected carrot and orange juices to pasteurization (90 °C/5 min), as well as to microbiological analysis of molds and yeasts, during 30-day storage at 4 °C. The fermented juice did not show colony-forming units, whereas non-fermented juices presented numbers of molds and yeasts lower than 3 log10 CFU/mL. Based on this result, the aforementioned authors justified that, in addition to thermal treatment, the fermentation of juices by probiotic microorganisms can help maintain their microbiological safety and prolong their shelf life since this process can inhibit contaminating flora growth by increasing lactic acid levels and decreasing pH in juices.
From another perspective, Olivares et al. [38] and Bonaccorso et al. [43] compared the microbiological features of juices added with free or microencapsulated microorganisms. However, the results in their study were divergent.
Olivares et al. [38] analyzed the growth of aerobic microorganisms, molds, and yeasts, as well as E. coli in pasteurized pineapple, raspberry, and orange juices (88 °C/90 s) stored under refrigeration (4 °C) for 28 days. All juices added with free bacteria, except for the pineapple juice, presented numbers of molds, yeasts and aerobic bacteria lower than those observed for juices added with microencapsulated microorganisms. E. coli results were negative in all samples. Probiotic microorganisms in free forms may have directly affected the survival of contaminating microorganisms. These results may be related to the composition, pH value, presence of antimicrobial compounds and competition for space and nutrients in the juices.
On the other hand, Bonaccorso et al. [43] observed the multiplication of microorganisms such as Leuconostoc spp., mesophiles, psychotrophics, molds and yeasts in orange juice free from prior treatment or the addition of preservatives. Furthermore, the authors observed that the number of microorganisms was higher in samples added with free probiotic bacteria than in those added with microencapsulated microorganisms.
Although these authors did not present a justification for these results, they mentioned that microencapsulation could be a promising approach to help preserve juices since it improves their stability. Although fruit juice is a suitable matrix used to grow spoilage microorganisms, such as molds and yeasts, and Leuconostoc spp., this strategy was more effective in reducing the excessive growth of microorganisms in comparison to the addition of free probiotics.
It is worth mentioning that there were studies carried out in different countries among the herein selected articles; thus, each article has followed a specific legislation to check the safety of the developed juices. Almeida Bianchini Campos [26], for example, investigated E. coli and Salmonella sp. in mixed pasteurized pineapple and jussara juice (88 °C/2 min). The aforementioned authors reported E. coli values lower than 1 log10 CFU/mL and a lack of Salmonella sp. in 25 mL of sample. These findings met the requirements of the current Brazilian legislation. Bonaccorso et al. [43] stated that values recorded for contaminating microorganisms in all samples were in compliance with the safety limits set by the European Union legislation. Finally, Olivares et al. [38] used the Chilean legislation based on the Codex Alimentarius as a reference to classify juice samples as safe.
3.5.9. Sensory Analysis
The effects of probiotics on the sensory features of juices depend on fruit type, probiotic organism, storage temperature, and supplementation with prebiotics and protectants. However, there is an increase in acidity levels as well as a decrease in sweetness levels during the fermentation or storage period of probiotic fruit juices. This happens due to sugar consumption for the growth of microbial cells or maintenance purposes; this process can lead to undesirable changes in the flavor of the juices as well as decrease their acceptability [35,52,55].
Accordingly, all studies performing this analysis recorded sensory changes as well as lower scores on attributes and acceptability rates after fermentation, except for the study conducted by Wu et al. [28], who conducted a sensory analysis of blackberry and blueberry juices fermented by three probiotic bacteria strains (Lactiplantibacillus plantarum BNCC 337796; Streptococcus thermophilus CGMCC1.8748; Bifidobacterium bifidum CGMCC1.5090). The aforementioned authors observed that blueberry juice added with L. plantarum recorded the highest scores in the overall sensory assessment in comparison to all other samples. However, the opposite was observed for blackberry juice. Thus, a likely solution for juices whose overall acceptance may be negatively affected by probiotics lies in using strategies to mitigate this impact from a sensory perspective, i.e., the addition of ingredients such as volatile compounds or even other fruit juices.
Thus, studies such as the ones conducted by Dimitrovski et al. [39] and Güney and Güngörmüşler [40] have added juices made of other fruits to help mitigate the impacts of probiotic microorganisms on the investigated products’ sensory features. These authors observed that the initial juice acceptance increased when this approach was adopted to prepare juices [23,52,56]. Therefore, probiotic microorganisms can negatively affect the sensory quality of juices depending on the used matrix. Thus, the proper selection of fruits and vegetables can be a decisive factor in the acceptance of the prepared probiotic product.
3.6. Microorganisms’ (Microencapsulated x Free) Inoculation Method and Changes in Juice Features and in Microorganisms’ Viability
Studies focusing on comparing physical-chemical parameters between products inoculated with free and microencapsulated probiotics observed the most intense changes in juices added with free bacteria. The main argument used to explain this finding was that free bacteria would have unrestricted access to nutrients in the juice and that it would consume more sugars. Consequently, it would lead to higher acidity as well as decreased pH and total soluble solids [30,38,43,44,45].
On the other hand, all articles focused on comparing viability have observed a larger number of viable cells in juices added with microencapsulated probiotics. The explanation given by most authors for this outcome was that these microcapsules could work as a physical barrier to protect microorganisms from adverse environmental conditions, such as low pH [30,38,43,44].
Mokhtari et al. [30] had the only study that focused on assessing sensory features among the herein selected ones. According to the authors, encapsulation can change the perception about the color and appearance of fruit juices. Moreover, microcapsules in juices likely change the product’s mouthfeel, mainly because they are liquid products. On the other hand, a higher overall acceptance level was observed for products added with microencapsulated bacteria. Thus, encapsulation can enable a more controlled environment and therefore lead to fewer changes in the product’s taste [30].
4. Final Considerations
Based on the herein analyzed studies, it was found that, overall, fruit and/or vegetable juices can be propitious matrices used to develop probiotic foods. However, above all, the added microorganisms must be able to adapt to and survive in the environment to guarantee the product’s success.
The results of the current study have shown that pH is one of the most critical factors enabling the survival of microorganisms. In addition, the synergy between the food matrix and added strain, as well as fiber content, phenolic compounds, and amino acids, also plays an essential role in the maintenance of probiotic microorganisms inoculated in juices. Furthermore, microencapsulation is a promising technology to help improve the viability of microorganisms by creating a more controlled environment with fewer physicochemical and, likely, sensory changes.
On the other hand, fermentation can lead to undesirable sensory changes, which can hinder the process of developing juices with probiotic fruits and vegetables. However, fermentation can help improve the product’s microbiological safety since it appears to inhibit the growth of contaminating microorganisms.
It is also important to emphasize that probiotic microorganisms play an essential role in improving the antioxidant activity of the matrices they are inoculated in. However, this effect depends on the added strain.
Finally, given the wide variety of analyses conducted in the herein assessed studies, the main limitation of the present study refers to the comparison between parameters. Therefore, future studies should focus on filling the remaining gaps observed in the development of probiotic fruit and/or vegetable juices and mixed juices to develop a consensus about this process.
Author Contributions
Conceptualization, M.S.M. and J.F.B.d.S.J.; methodology, M.S.M. and J.F.B.d.S.J.; formal analysis M.S.M. and J.F.B.d.S.J.; writing—original draft preparation M.S.M., M.M.D. and J.F.B.d.S.J.; writing—review and editing, M.S.M., M.M.D. and J.F.B.d.S.J.; supervision, J.F.B.d.S.J.; project administration, J.F.B.d.S.J.; funding acquisition, J.F.B.d.S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any external funding. Espírito Santo Research and Innovation Support Foundation (FAPES) supported the APC.
Data Availability Statement
Data supporting this study’s findings will be made available upon request to the corresponding author.
Acknowledgments
We are grateful to the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/CAPES) for supporting the Post-Graduate Program in Nutrition and Health. We thank Espírito Santo Research and Innovation Support Foundation (FAPES) for supporting the payment of publication fees by Process Edital Fapes N. 05/2023-Publicação de artigos técnico-científicos. We thank the Research and Graduate Studies of the Federal University of Espírito Santo (Pró Reitoria de Pesquisa e Pós Graduação—PRPPG, UFES) for all the support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Statista. Leading Producers of Fresh Fruit Worldwide in 2021. Available online: https://www.statista.com/statistics/279164/global-top-producers-of-selected-fresh-fruit-worldwide/ (accessed on 20 January 2023).
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A Review of Recent Trends in the Development of the Microbial Safety of Fruits and Vegetables. Trends Food Sci. Technol. 2020, 103, 321–332. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.; Bohn, T. Bioaccessibility of Phytoene and Phytofluene Is Superior to Other Carotenoids from Selected Fruit and Vegetable Juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.K.; Meireles, M.A.A.; Saldaña, M.D.A. Supercritical Carbon Dioxide Technology: A Promising Technique for the Non-Thermal Processing of Freshly Fruit and Vegetable Juices. Trends Food Sci. Technol. 2020, 97, 381–390. [Google Scholar] [CrossRef]
- Farag, M.A.; Abdelwareth, A.; Sallam, I.E.; el Shorbagi, M.; Jehmlich, N.; Fritz-Wallace, K.; Serena Schäpe, S.; Rolle-Kampczyk, U.; Ehrlich, A.; Wessjohann, L.A.; et al. Metabolomics Reveals Impact of Seven Functional Foods on Metabolic Pathways in a Gut Microbiota Model. J. Adv. Res. 2020, 23, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Fonteles, T.V.; Rodrigues, S. Prebiotic in Fruit Juice: Processing Challenges, Advances, and Perspectives. Curr. Opin. Food Sci. 2018, 22, 55–61. [Google Scholar] [CrossRef]
- Lillo-Pérez, S.; Guerra-Valle, M.; Orellana-Palma, P.; Petzold, G. Probiotics in Fruit and Vegetable Matrices: Opportunities for Nondairy Consumers. LWT 2021, 151, 112106. [Google Scholar] [CrossRef]
- Brazil Resolução No 19, de 30 de Abril de 1999. Regulamento Técnico Para Procedimento de Registro de Alimentos Com Alegações de Propriedades Funcionais e Ou de Saúde Em Sua Rotulagem. Diário Oficial Da União; Poder Executivo. 1999. Available online: http://antigo.anvisa.gov.br/documents/10181/2718376/RES_19_1999_COMP.pdf/311b03f5-c2f5-4b97-89a8-30331f8145f3 (accessed on 20 January 2023).
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef]
- Li, T.; Jiang, T.; Liu, N.; Wu, C.; Xu, H.; Lei, H. Biotransformation of Phenolic Profiles and Improvement of Antioxidant Capacities in Jujube Juice by Select Lactic Acid Bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef]
- WHO; FAO. Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Porto, M.R.A.; Okina, V.S.; Pimentel, T.C.; Garcia, S.; Prudencio, S.H. Beet and Orange Mixed Juices Added with Lactobacillus Acidophilus. Nutr. Food Sci. 2018, 48, 76–87. [Google Scholar] [CrossRef]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan Probiotic Products: A Modern Tendency or the Newest Challenge in Functional Foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef]
- James, A.; Wang, Y. Characterization, Health Benefits and Applications of Fruits and Vegetable Probiotics. CyTA—J. Food 2019, 17, 770–780. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Gomes da Cruz, A.; Daga, M. Strategies to Improve the Functionality of Probiotics in Supplements and Foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Otles, S.; Ozyurt, V.H. Probiotic, and Prebiotic Beverages. In Functional and Medicinal Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–458. [Google Scholar]
- FAO/WHO Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. 2002. Available online: https://www.foodinprogress.com/wp-content/uploads/2019/04/Guidelines-for-the-Evaluation-of-Probiotics-in-Food.pdf (accessed on 20 January 2023).
- Priyadarshini, A.; Priyadarshini, A. Market Dimensions of the Fruit Juice Industry. In Fruit Juices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 15–32. [Google Scholar]
- Statista Other Juice, Juice Mixtures & Smoothies—Brazil|Forecast. Available online: https://www.statista.com/outlook/cmo/non-alcoholic-drinks/juices/other-juice-juice-mixtures-smoothies/brazil (accessed on 24 February 2023).
- Grand View Research Inc. Fruit and Vegetable Juice Market Report, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/fruit-vegetable-juice-market (accessed on 24 February 2023).
- Perricone, M.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M. Challenges for the Production of Probiotic Fruit Juices. Beverages 2015, 1, 95–103. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.; Kachrimanidou, V.; Bosnea, L.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- de Souza, M.T.; da Silva, M.D.; de Carvalho, R. Integrative Review: What Is It? How to Do It? Einstein 2010, 8, 102–106. [Google Scholar] [CrossRef]
- de Almeida Bianchini Campos, R.C.; Martins, E.M.F.; de Andrade Pires, B.; do Carmo Gouveia Peluzio, M.; da Rocha Campos, A.N.; Ramos, A.M.; de Castro Leite Júnior, B.R.; de Oliveira Martins, A.D.; da Silva, R.R.; Martins, M.L. In Vitro and in Vivo Resistance of Lactobacillus rhamnosus GG Carried by a Mixed Pineapple (Ananas comosus L. Merril) and Jussara (Euterpe edulis Martius) juice to the gastrointestinal Tract. Food Res. Int. 2019, 116, 1247–1257. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical Analysis and Flavor Properties of Blended Orange, Carrot, Apple and Chinese Jujube Juice Fermented by Selenium-Enriched Probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of Blueberry and Blackberry Juices Using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of Probiotics, Metabolism of Phenolics, Antioxidant Capacity in Vitro and Sensory Evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Garcia, E.F.; de Oliveira Araújo, A.; Luciano, W.A.; de Albuquerque, T.M.R.; de Oliveira Arcanjo, N.M.; Madruga, M.S.; dos Santos Lima, M.; Magnani, M.; Saarela, M.; de Souza, E.L. The Performance of Five Fruit-Derived and Freeze-Dried Potentially Probiotic Lactobacillus Strains in Apple, Orange, and Grape Juices. J. Sci. Food Agric. 2018, 98, 5000–5010. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M. Survival of encapsulated probiotics in pasteurized grape juice and evaluation of their properties during storage. Food Sci. Technol. Int. 2019, 25, 120–129. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.M.; de Castro Leite Júnior, B.R.; Martins, E.M.F.; Martins, M.L.; Vieira, É.N.R.; de Barros, F.A.R.; Cristianini, M.; de Almeida Costa, N.; Ramos, A.M. Mango and carrot mixed juice: A new matrix for the vehicle of probiotic Lactobacilli. J. Food Sci. Technol. 2021, 58, 98–109. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Frutos, M.J. Effect of Inulin on the Viability of L. plantarum during Storage and In Vitro Digestion and on Composition Parameters of Vegetable Fermented Juices. Plant Foods Hum. Nutr. 2017, 72, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F. Evaluation of the Stability of Bacillus Coagulans MTCC 5856 during Processing and Storage of Functional Foods. Int. J. Food Sci. Technol. 2016, 51, 894–901. [Google Scholar] [CrossRef]
- Bhat, B.; Gupta, M.; Tabia, S.; Bijender, A.; Bajaj, K. Growth and Viability of Probiotic Weissella Kimchi R-3 in Fruit and Vegetable Beverages. Indian J. Biochem. Biophys. 2017, 54, 191–199. [Google Scholar]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; Frutos, M.J. Influence of Fermentation with Different Lactic Acid Bacteria and in Vitro Digestion on the Biotransformation of Phenolic Compounds in Fermented Pomegranate Juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef]
- Mustafa, S.M.; Chua, L.S.; El-Enshasy, H.A.; Abd Majid, F.A.; Hanapi, S.Z.; Abdul Malik, R. Effect of Temperature and pH on the Probiotication of Punica Granatum Juice Using Lactobacillus Species. J. Food Biochem. 2019, 43, e12805. [Google Scholar] [CrossRef]
- Srisukchayakul, P.; Charalampopoulos, D.; Karatzas, K.A. Study on the Effect of Citric Acid Adaptation toward the Subsequent Survival of Lactobacillus plantarum NCIMB 8826 in Low PH Fruit Juices during Refrigerated Storage. Food Res. Int. 2018, 111, 198–204. [Google Scholar] [CrossRef]
- Olivares, A.; Soto, C.; Caballero, E.; Altamirano, C. Survival of Microencapsulated Lactobacillus casei (Prepared by Vibration Technology) in Fruit Juice during Cold Storage. Electron. J. Biotechnol. 2019, 42, 42–48. [Google Scholar] [CrossRef]
- Dimitrovski, D.; Dimitrovska-Vetadjoka, M.; Hristov, H.; Doneva-Shapceska, D. Developing probiotic pumpkin juice by fermentation with commercial probiotic strain Lactobacillus Casei 431. J. Food Process. Preserv. 2021, 45, e15245. [Google Scholar] [CrossRef]
- Güney, D.; Güngörmüşler, M. Development and Comparative Evaluation of a Novel Fermented Juice Mixture with Probiotic Strains of Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2021, 13, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebian, M.; Mahmoudi, R.; Shakouri, M.J. Probiotic Viability, Physicochemical Characterization and Sensory Properties of Cornelian Cherry (Cornus mas L.) Juice Supplemented with Lactobacillus acidophilus and Lactobacillus delbrueckii. J. Chem. Health Risks 2020, 10, 253–260. [Google Scholar] [CrossRef]
- Zhu, W.; Lyu, F.; Naumovski, N.; Ajlouni, S.; Ranadheera, C.S. Functional efficacy of probiotic Lactobacillus sanfranciscensis in apple, orange and tomato juices with special reference to storage stability and in vitro gastrointestinal survival. Beverages 2020, 6, 13. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Russo, N.; Romeo, A.; Carbone, C.; Grimaudo, M.A.; Alvarez-Lorenzo, C.; Randazzo, C.; Musumeci, T.; Caggia, C. Coating Lacticaseibacillus rhamnosus GG in Alginate Systems: An Emerging Strategy Towards Improved Viability in Orange Juice. AAPS PharmSciTech 2021, 22, 123. [Google Scholar] [CrossRef]
- Rengadu, D.; Gerrano, A.S.; Mellem, J.J. Microencapsulation of Lactobacillus casei and Bifidobacterium animalis enriched with resistant starch from vigna unguiculata. Starch—Stärke 2021, 73, 2000247. [Google Scholar] [CrossRef]
- Marques da Silva, T.; Sonza Pinto, V.; Ramires Fonseca Soares, V.; Marotz, D.; Cichoski, A.J.; Queiroz Zepka, L.; Jacob Lopes, E.; de Bona da Silva, C.; de Menezes, C.R. Viability of Microencapsulated Lactobacillus acidophilus by complex coacervation associated with enzymatic crosslinking under application in different fruit juices. Food Res. Int. 2021, 141, 110190. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A Review of the Microencapsulation Techniques for the Incorporation of Probiotic Bacteria in Functional Foods. Crit. Rev. Food Sci. Nutr. 2021, 61, 1515–1536. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic Functional Foods: Survival of Probiotics during Processing and Storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Erkmen, O.; Bozoglu, T.F. (Eds.) Microbial Sources and Factors Affecting Microorganisms. In Food Microbiology: Principles into Practice; Wiley: Hoboken, NJ, USA, 2016; pp. 81–82. ISBN 9781119237761. [Google Scholar]
- Dinkçi, N.; Akdeniz, V.; Akalin, A.S. Survival of Probiotics in Functional Foods during Shelf Life. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Elsevier: London, UK, 2019; pp. 201–233. [Google Scholar]
- Hadiwijaya, Y.; Putri, I.E.; Mubarok, S.; Hamdani, J.S. Rapid and non-destructive prediction of total soluble solids of guava fruits at various storage periods using handheld near-infrared instrument. IOP Conf. Ser. Earth Environ. Sci. 2020, 458, 012022. [Google Scholar] [CrossRef]
- Guiné, R.; Barroca, M. Influence of processing and storage on fruit juices phenolic compounds. Int. J. Med. Biol. Front. 2014, 20, 45–58. [Google Scholar]
- Fernandes Pereira, A.L.; Rodrigues, S. Turning Fruit Juice into Probiotic Beverages. In Fruit Juices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 279–287. [Google Scholar]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Das, K.K.; Uddin, M.A. The Microbiological Quality of Commercial Fruit Juices-Current Perspectives. Bangladesh J. Microbiol. 2019, 35, 128–133. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.J.; Narala, V.R.; Joshi, V.K. Development of New Probiotic Foods—A Case Study on Probiotic Juices. In Therapeutic, Probiotic, and Unconventional Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 55–78. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).