The Fight against Poliovirus Is Not Over
Abstract
1. Introduction
2. Poliovirus
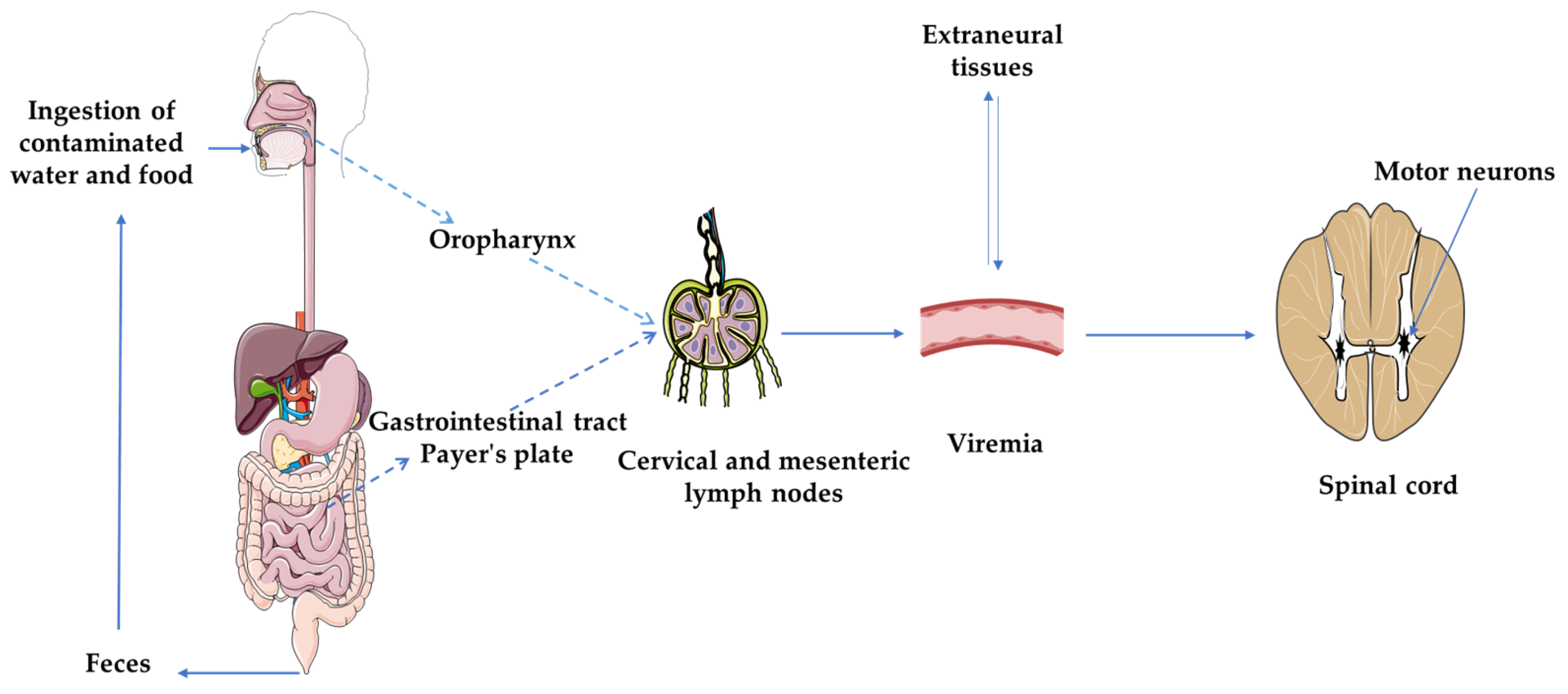
2.1. Structure and Pathogenesis
2.2. Diagnosis
3. Paralytic Polio Vaccines and Eradication of WPV
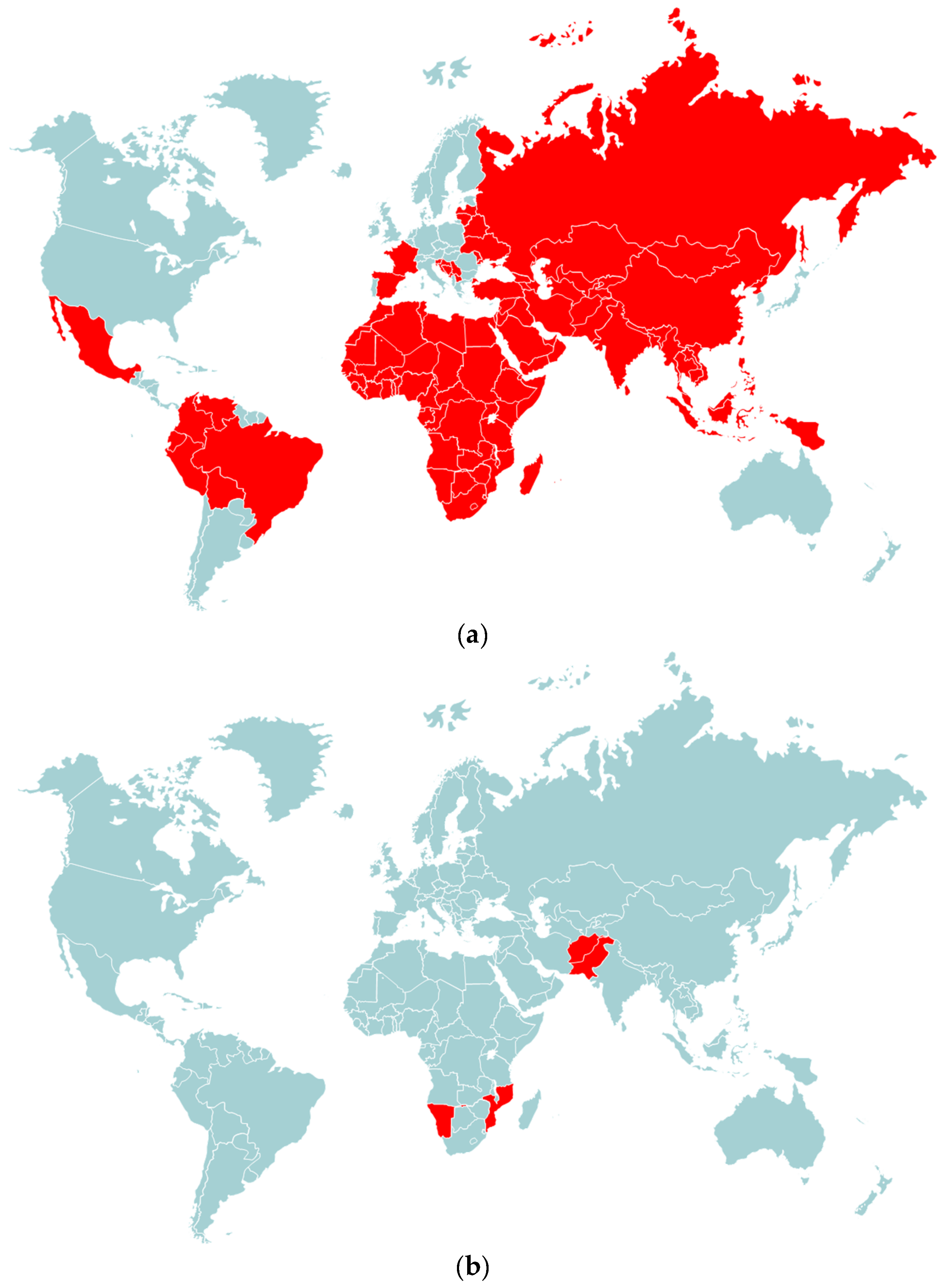
4. Emergence of Vaccine-Derived Poliovirus Strains
5. New Strategies to Fight against PV
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, B.; Oberste, M.S.; Maher, K.; Pallansch, M.A. Complete genomic sequencing shows that polioviruses and members of human enterovirus species C are closely related in the noncapsid coding region. J. Virol. 2003, 77, 8973–8984. [Google Scholar] [CrossRef]
- Baggen, J.; Thibaut, H.J.; Strating, J.R.P.M.; van Kuppeveld, F.J.M. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018, 16, 368–381. [Google Scholar] [CrossRef]
- Pallansch, M.A.; Roos, R. Enteroviruses: Polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Nathanson, N. The pathogenesis of poliomyelitis: What we don’t know. Adv. Virus. Res. 2008, 71, 1–50. [Google Scholar] [PubMed]
- World Health Organization. Weekly Epidemiological Record 2022, 97, 277–300. Available online: https://apps.who.int/iris/handle/10665/357167 (accessed on 15 April 2023).
- Davlantes, E.; Greene, S.A.; Tobolowsky, F.A.; Biya, O.; Wiesen, E.; Abebe, F.; Weldetsadik, M.B.; Eboh, V.A.; Chisema, M.N.; da Conceição Mário, B.; et al. Update on Wild Poliovirus Type 1 Outbreak-Southeastern Africa, 2021–2022. MMWR Morb. Mortal. Wkly Rep. 2023, 72, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Datta, S.D.; Quddus, A.; Vertefeuille, J.F.; Burns, C.C.; Jorba, J.; Wassilak, S.G.F. Progress Toward Polio Eradication-Worldwide, January 2016–March 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 524–528. [Google Scholar] [CrossRef]
- Agol, V.I. Molecular mechanisms of poliovirus variation and evolution. Curr. Top. Microbiol. Immunol. 2006, 299, 211–259. [Google Scholar] [PubMed]
- Jorba, J.; Diop, O.M.; Iber, J.; Henderson, E.; Zhao, K.; Quddus, A.; Sutter, R.; Vertefeuille, J.F.; Wenger, J.; Wassilak, S.G.F.; et al. Update on Vaccine-Derived Poliovirus Outbreaks-Worldwide, January 2018–June 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1024–1028. [Google Scholar] [CrossRef]
- Blondel, B.; Autret, A.; Brisac, C.; Pelletier, I.; Martin-Latil, S.; Jegouic, S.; Bessaud, M.; Joffret, M.L.; Balanant, J.; Colbère-Garapin, F.; et al. Evolution génétique du poliovirus: Succès et difficultés de l’éradication de la poliomyélite paralytique [Genetic evolution of poliovirus: Success and difficulties in the eradication of paralytic poliomyelitis]. Med. Trop. 2008, 68, 189–202. [Google Scholar]
- Yeh, M.T.; Bujaki, E.; Dolan, P.T.; Smith, M.; Wahid, R.; Konz, J.; Weiner, A.J.; Bandyopadhyay, A.S.; Van Damme, P.; De Coster, I.; et al. Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence. Cell Host Microbe. 2020, 27, 736–751.e8. [Google Scholar] [CrossRef]
- Contreras, G.; Dimock, K.; Furesz, J.; Gardell, C.; Hazlett, D.; Karpinski, K.; McCorkle, G.; Wu, L. Genetic characterization of Sabin types 1 and 3 poliovaccine virus following serial passage in the human intestinal tract. Biologicals 1992, 20, 15–26. [Google Scholar] [CrossRef]
- Minor, P.D.; Dunn, G.; Ramsay, M.E.; Brown, D. Effect of different immunisation schedules on the excretion and reversion of oral poliovaccine strains. J. Med. Virol. 2005, 75, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Macadam, A.J.; Ferguson, G.; Stone, D.M.; Meredith, J.; Knowlson, S.; Auda, G.; Almond, J.W.; Minor, P.D. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: Relevance to poliomyelitis eradication. J. Virol. 2006, 80, 8653–8663. [Google Scholar] [CrossRef] [PubMed]
- Kew, O.M.; Sutter, R.W.; de Gourville, E.M.; Dowdle, W.R.; Pallansch, M.A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 2005, 59, 587–635. [Google Scholar] [CrossRef]
- Rakoto-Andrianarivelo, M.; Jegouic, S.; Bessaud, M.; Delpeyroux, F. Poliovirus et entérovirus C, même espèce, même “tribu” virale [Polioviruses and species C enteroviruses, viruses of the same species and “tribe”]. Med. Sci. 2008, 24, 452–453. [Google Scholar]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. Ictv Report Consortium: ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Guo, H.; Li, Y.; Liu, G.; Jiang, Y.; Shen, S.; Bi, R.; Huang, H.; Cheng, T.; Wang, C.; Wei, W. A second open reading frame in human enterovirus determines viral replication in intestinal epithelial cells. Nat. Commun. 2019, 10, 4066. [Google Scholar] [CrossRef]
- Lulla, V.; Dinan, A.M.; Hosmillo, M.; Chaudhry, Y.; Sherry, L.; Irigoyen, N.; Nayak, K.M.; Stonehouse, N.J.; Zilbauer, M.; Goodfellow, I.; et al. An upstream protein-coding region in enteroviruses modulates virus infection in gut epithelial cells. Nat. Microbiol. 2019, 4, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Racaniello, V.R. One hundred years of poliovirus pathogenesis. Virology 2006, 344, 9–16. [Google Scholar] [CrossRef]
- Mueller, S.; Wimmer, E.; Cello, J. Poliovirus and poliomyelitis: A tale of guts, brains, and an accidental event. Virus Res. 2005, 11, 175–193. [Google Scholar] [CrossRef]
- Nathanson, N.; Kew, O.M. From emergence to eradication: The epidemiology of poliomyelitis deconstructed. Am. J. Epidemiol. 2010, 172, 1213–1229. [Google Scholar] [CrossRef]
- He, Y.; Mueller, S.; Chipman, P.R.; Bator, C.M.; Peng, X.; Bowman, V.D.; Mukhopadhyay, S.; Wimmer, E.; Kuhn, R.J.; Rossmann, M.G. Complexes of poliovirus serotypes with their common cellular receptor, CD155. J. Virol. 2003, 77, 4827–4835. [Google Scholar] [CrossRef]
- Kaplan, G.; Freistadt, M.S.; Racaniello, V.R. Neutralization of poliovirus by cell receptors expressed in insect cells. J. Virol. 1990, 64, 4697–4702. [Google Scholar] [CrossRef]
- Gómez Yafal, A.; Kaplan, G.; Racaniello, V.R.; Hogle, J.M. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology 1993, 197, 501–505. [Google Scholar] [CrossRef]
- Bodian, D. Emerging concept of poliomyelitis infection. Science 1955, 122, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B. Pathogenesis of poliomyelitis; reappraisal in the light of new data. Science 1956, 123, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Freistadt, M.S.; Fleit, H.B.; Wimmer, E. Poliovirus receptor on human blood cells: A possible extraneural site of poliovirus replication. Virology 1993, 195, 798–803. [Google Scholar] [CrossRef]
- Wyatt, H.V. Incubation of poliomyelitis as calculated from the time of entry into the central nervous system via the peripheral nerve pathways. Rev. Infect. Dis. 1990, 12, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Sartwell, P.E. La période d’incubation de la poliomyélite. Am. J. St. Publique Nations Health 1952, 42, 1403–1408. [Google Scholar] [CrossRef]
- Horstmann, D.M.; Paul, J.R. The incubation period in human poliomyelitis and its implications. J. Am. Med. Assoc. 1947, 135, 11–14. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.S.; Harper, J.; Murray, A.; Lexau, C.; Bahta, L.; Christensen, J.; Cebelinski, E.; Fuller, S.; Kline, S.; Wallace, G.S.; et al. Vaccine-derived poliomyelitis 12 years after infection in Minnesota. N. Engl. J. Med. 2011, 364, 2316–2323. [Google Scholar] [CrossRef]
- Sutter, R.W.; Patriarca, P.A.; Suleiman, A.J.M.; Brogan, S.; Malankar, P.G.; Cochi, S.L.; Al-Ghassani, A.A.K.; El-Bualy, M.S. Attributable risk of DTP (diphtheria and tetanus toxoids and pertussis vaccine) injection in provoking paralytic poliomyelitis during a large outbreak in Oman. J. Infect. Dis. 1992, 165, 444–449. [Google Scholar] [CrossRef]
- Strebel, P.M.; Ion-Nedelcu, N.; Baughman, A.L.; Sutter, R.W.; Cochi, S.L. Intramuscular injections within 30 days of immunization with oral poliovirus vaccine--a risk factor for vaccine-associated paralytic poliomyelitis. N. Engl. J. Med. 1995, 332, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Lim, Y.W. Post-poliomyelitis syndrome: Case report and review of the literature. Ann. Acad. Med. Singap. 2005, 34, 447–449. [Google Scholar] [PubMed]
- Julien, J.; Leparc-Goffart, I.; Lina, B.; Fuchs, F.; Foray, S.; Janatova, I.; Aymard, M.; Kopecka, H. Postpolio syndrome: Poliovirus persistence is involved in the pathogenesis. J. Neurol. 1999, 246, 472–476. [Google Scholar] [CrossRef]
- Li Hi Shing, S.; Chipika, R.H.; Finegan, E.; Murray, D.; Hardiman, O.; Bede, P. Post-polio Syndrome: More Than Just a Lower Motor Neuron Disease. Front. Neurol. 2019, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, H.J.; Tresser, N.; Hogan, R.E.; Martin, E. Pathological analysis of spinal cords from survivors of poliomyelitis. Ann. N. Y. Acad. Sci. 1995, 753, 390–393. [Google Scholar] [CrossRef]
- Sharief, M.K.; Hentges, R.; Ciardi, M. Intrathecal immune response in patients with the post-polio syndrome. N. Engl. J. Med. 1991, 325, 749–755. [Google Scholar] [CrossRef]
- Bickerstaffe, A.; Beelen, A.; Lutter, R.; Nollet, F. Elevated plasma inflammatory mediators in post-polio syndrome: No association with long-term functional decline. J. Neuroimmunol. 2015, 289, 162–167. [Google Scholar] [CrossRef]
- Gonzalez, H.; Khademi, M.; Andersson, M.; Wallström, E.; Borg, K.; Olsson, T. Prior poliomyelitis-evidence of cytokine production in the central nervous system. J. Neurol. Sci. 2002, 205, 9–13. [Google Scholar] [CrossRef]
- Wood, D.J.; Hull, B. L20B cells simplify culture of polioviruses from clinical samples. J. Med. Virol. 1999, 58, 188–192. [Google Scholar] [CrossRef]
- Gerloff, N.; Sun, H.; Mandelbaum, M.; Maher, C.; Nix, W.A.; Zaidi, S.; Shaukat, S.; Seakamela, L.; Nalavade, U.P.; Sharma, D.K.; et al. Diagnostic Assay Development for Poliovirus Eradication. J. Clin. Microbiol. 2018, 56, e01624-17. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Ling, H.; Yan, D.; Nishimura, Y.; Yoshida, H.; Wakita, T.; Shimizu, H. Development of a reverse transcription-loop-mediated isothermal amplification (RT-LAMP) system for a highly sensitive detection of enterovirus in the stool samples of acute flaccid paralysis cases. BMC Infect. Dis. 2009, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.; Sun, H.; Jeffries-Miles, S.; Gerloff, N.; Mandelbaum, M.; Pang, H.; Collins, N.; Burns, C.C.; Vega, E. Culture-Independent Detection of Poliovirus in Stool Samples by Direct RNA Extraction. Microbiol. Spectr. 2021, 9, e0066821. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y. Isolation and Characterization of Vaccine-Derived Polioviruses, Relevance for the Global Polio Eradication Initiative. Methods Mol. Biol. 2016, 1387, 213–226. [Google Scholar]
- Kilpatrick, D.R.; Ching, K.; Iber, J.; Chen, Q.; Yang, S.J.; De, L.; Williams, A.J.; Mandelbaum, M.; Sun, H.; Oberste, M.S.; et al. Identification of vaccine-derived polioviruses using dual-stage real-time RT-PCR. J. Virol. Methods. 2014, 197, 25–28. [Google Scholar] [CrossRef]
- Kilpatrick, D.R.; Yang, C.F.; Ching, K.; Vincent, A.; Iber, J.; Campagnoli, R.; Mandelbaum, M.; De, L.; Yang, S.J.; Nix, A.; et al. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J. Clin. Microbiol. 2009, 47, 1939–1941. [Google Scholar] [CrossRef]
- Van der Avoort, H.G.; Hull, B.P.; Hovi, T.; Pallansch, M.A.; Kew, O.M.; Crainic, R.; Wood, D.J.; Mulders, M.N.; van Loon, A.M. Comparative study of five methods for intratypic differentiation of polioviruses. J. Clin. Microbiol. 1995, 33, 2562–2566. [Google Scholar] [CrossRef]
- Balanant, J.; Guillot, S.; Candrea, A.; Delpeyroux, F.; Crainic, R. The natural genomic variability of poliovirus analyzed by a restriction fragment length polymorphism assay. Virology 1991, 184, 645–654. [Google Scholar] [CrossRef]
- World Health Organization. Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004. Available online: https://apps.who.int/iris/handle/10665/68762 (accessed on 8 April 2023).
- Hammon, W.M.; Coriell, L.L.; Ludwig, E.H.; Mcallister, R.M.; Greene, A.E.; Sather, G.E.; Wehrle, P.F. Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. 5. Reanalysis of results based on laboratory-confirmed cases. J. Am. Med. Assoc. 1954, 156, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.P.; Molodecky, N.A.; Pons-Salort, M.; O’Reilly, K.M.; Grassly, N.C. Impact of inactivated poliovirus vaccine on mucosal immunity: Implications for the polio eradication endgame. Expert. Rev. Vaccines 2015, 14, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccine Supply and Quality Unit. In Manual of Laboratory Methods for Testing of Vaccines Used in the WHO Expanded Programme on Immunization; World Health Organization: Geneva, Switzerland, 1994. Available online: https://apps.who.int/iris/handle/10665/63576 (accessed on 10 April 2023).
- Zhaori, G.; Sun, M.; Faden, H.S.; Ogra, P.L. Nasopharyngeal secretory antibody response to poliovirus type 3 virion proteins exhibit different specificities after immunization with live or inactivated poliovirus vaccines. J. Infect. Dis. 1989, 159, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Saletti, G.; Çuburu, N.; Yang, J.S.; Dey, A.; Czerkinsky, C. Enzyme-linked immunospot assays for direct ex vivo measurement of vaccine-induced human humoral immune responses in blood. Nat. Protoc. 2013, 8, 1073–1087. [Google Scholar] [CrossRef]
- Herremans, T.M.; Reimerink, J.H.; Buisman, A.M.; Kimman, T.G.; Koopmans, M.P. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J. Immunol. 1999, 162, 5011–5018. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Molodecky, N.A.; Verma, H.; Sharma, P.; Yang, J.S.; Saletti, G.; Ahmad, M.; Bahl, S.K.; Wierzba, T.F.; Nandy, R.K.; et al. Human Circulating Antibody-Producing B Cell as a Predictive Measure of Mucosal Immunity to Poliovirus. PLoS ONE 2016, 11, e0146010. [Google Scholar] [CrossRef]
- Rachlin, A.; Patel, J.C.; Burns, C.C.; Jorba, J.; Tallis, G.; O’Leary, A.; Wassilak, S.G.F.; Vertefeuille, J.F. Progress Toward Polio Eradication-Worldwide, January 2020–April 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 650–655. [Google Scholar] [CrossRef]
- Hull, H.F.; Ward, N.A.; Hull, B.P.; Milstien, J.B.; de Quadros, C. Paralytic poliomyelitis: Seasoned strategies, disappearing disease. Lancet 1994, 343, 1331–1337. [Google Scholar] [CrossRef]
- Hinman, A.R.; Foege, W.H.; de Quadros, C.A.; Patriarca, P.A.; Orenstein, W.A.; Brink, E.W. The case for global eradication of poliomyelitis. Bull. World Health Organ. 1987, 65, 835–840. [Google Scholar]
- World Health Organization. Weekly Polio Analyses WPV. 2023. Available online: https://https://polioeradication.org/wp-content/uploads/2023/05/weekly-polio-analyses-WPV-20230509.pdf (accessed on 9 May 2023).
- Hird, T.R.; Grassly, N.C. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012, 8, e1002599. [Google Scholar] [CrossRef]
- Quarleri, J. Poliomyelitis is a current challenge: Long-term sequelae and circulating vaccine-derived poliovirus. Geroscience 2023, 45, 707–717. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.S.; Garon, J.; Seib, K.; Orenstein, W.A. Polio vaccination: Past, present and future. Future Microbiol. 2015, 10, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.W.; John, T.J.; Jain, H.; Agarkhedkar, S.; Ramanan, P.V.; Verma, H.; Deshpande, J.; Singh, A.P.; Sreevatsava, M.; Malankar, P.; et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: A randomised, double-blind, controlled trial. Lancet 2010, 376, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Mehndiratta, M.M.; Mehndiratta, P.; Pande, R. Poliomyelitis: Historical facts, epidemiology, and current challenges in eradication. Neurohospitalist 2014, 4, 223–229. [Google Scholar] [CrossRef]
- Estívariz, C.F.; Pallansch, M.A.; Anand, A.; Wassilak, S.G.; Sutter, R.W.; Wenger, J.D.; Orenstein, W.A. Poliovirus vaccination options for achieving eradication and securing the endgame. Curr. Opin. Virol. 2013, 3, 309–315. [Google Scholar] [CrossRef]
- Cuba IPV Study Collaborative Group. Randomized, placebo-controlled trial of inactivated poliovirus vaccine in Cuba. N. Engl. J. Med. 2007, 356, 1536–1544. [Google Scholar] [CrossRef]
- Laassri, M.; Lottenbach, K.; Belshe, R.; Wolff, M.; Rennels, M.; Plotkin, S.; Chumakov, K. Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains. J. Infect. Dis. 2005, 192, 2092–2098. [Google Scholar] [CrossRef]
- Onorato, I.M.; Modlin, J.F.; McBean, A.M.; Thoms, M.L.; Losonsky, G.A.; Bernier, R.H. Mucosal immunity induced by enhance-potency inactivated and oral polio vaccines. J. Infect. Dis. 1991, 163, 1–6. [Google Scholar] [CrossRef]
- Henry, J.L.; Jaikaran, E.S.; Davies, J.R.; Tomlinson, A.J.; Mason, P.J.; Barnes, J.M.; Beale, A.J. A study of poliovaccination in infancy: Excretion following challenge with live virus by children given killed or living poliovaccine. J. Hyg. 1966, 64, 105–120. [Google Scholar] [CrossRef]
- Murdin, A.D.; Barreto, L.; Plotkin, S. Inactivated poliovirus vaccine: Past and present experience. Vaccine 1996, 14, 735–746. [Google Scholar] [CrossRef]
- Anis, E.; Kopel, E.; Singer, S.R.; Kaliner, E.; Moerman, L.; Moran-Gilad, J.; Sofer, D.; Manor, Y.; Shulman, L.M.; Mendelson, E.; et al. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro. Surveill. 2013, 18, 20586. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Giri, S.; Karthikeyan, A.S.; Iturriza-Gomara, M.; Muliyil, J.; Abraham, A.; Grassly, N.C.; Kang, G. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: An open-label, randomised controlled trial. Lancet 2014, 384, 1505–1512. [Google Scholar] [CrossRef]
- Jafari, H.; Deshpande, J.M.; Sutter, R.W.; Bahl, S.; Verma, H.; Ahmad, M.; Kunwar, A.; Vishwakarma, R.; Agarwal, A.; Jain, S.; et al. Polio eradication. Efficacy of inactivated poliovirus vaccine in India. Science 2014, 345, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Ciapponi, A.; Bardach, A.; Rey Ares, L.; Glujovsky, D.; Cafferata, M.L.; Cesaroni, S.; Bhatti, A. Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis. Cochrane Database Syst. Rev. 2019, 12, CD011260. [Google Scholar] [CrossRef]
- Sutter, R.W.; Prevots, D.R.; Cochi, S.L. Poliovirus vaccines. Progress toward global poliomyelitis eradication and changing routine immunization recommendations in the United States. Pediatr. Clin. N. Am. 2000, 47, 287–308. [Google Scholar] [CrossRef]
- Korotkova, E.A.; Gmyl, A.P.; Yakovenko, M.L.; Ivanova, O.E.; Eremeeva, T.P.; Kozlovskaya, L.I.; Shakaryan, A.K.; Lipskaya, G.Y.; Parshina, I.L.; Loginovskikh, N.V.; et al. A Cluster of Paralytic Poliomyelitis Cases Due to Transmission of Slightly Diverged Sabin 2 Vaccine Poliovirus. J. Virol. 2016, 90, 5978–5988. [Google Scholar] [CrossRef]
- Tuma, J.N.; Wilkinson, A.L.; Diop, O.M.; Jorba, J.; Gardner, T.; Snider, C.J.; Anand, A.; Ahmed, J. Surveillance to Track Progress Toward Polio Eradication-Worldwide, 2019–2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Cochi, S.L.; Pallansch, M.A. The Long and Winding Road to Eradicate Vaccine-Related Polioviruses. J. Infect. Dis. 2021, 223, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.; Yeh, M.T.; Zinger, T.; Smith, M.; Wright, C.; Ling, G.; Nielsen, R.; Macadam, A.; Andino, R. The Evolutionary Pathway to Virulence of an RNA. Virus. Cell 2017, 169, 35–46.e19. [Google Scholar] [PubMed]
- Jorgensen, D.; Pons-Salort, M.; Shaw, A.G.; Grassly, N.C. The role of genetic sequencing and analysis in the polio eradication programme. Virus Evol. 2020, 6, veaa040. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Zheng, D.P.; Zhang, L.B.; Oberste, M.S.; Kew, O.M.; Pallansch, M.A. Serial recombination during circulation of type 1 wild-vaccine recombinant polioviruses in China. J. Virol. 2003, 77, 10994–11005. [Google Scholar] [CrossRef]
- Burns, C.C.; Diop, O.M.; Sutter, R.W.; Kew, O.M. Vaccine-derived polioviruses. J. Infect. Dis. 2014, 210, S283–S293. [Google Scholar] [CrossRef]
- Macklin, G.; Diop, O.M.; Humayun, A.; Shahmahmoodi, S.; El-Sayed, Z.A.; Triki, H.; Rey, G.; Avagyan, T.; Grabovac, V.; Jorba, J.; et al. Update on Immunodeficiency-Associated Vaccine-Derived Polioviruses-Worldwide, July 2018–December 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 913–917. [Google Scholar] [CrossRef]
- Burns, C.C.; Shaw, J.; Jorba, J.; Bukbuk, D.; Adu, F.; Gumede, N.; Pate, M.A.; Abanida, E.A.; Gasasira, A.; Iber, J.; et al. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J. Virol. 2013, 87, 4907–4922. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Naguib, T.; Yang, S.J.; Nasr, E.; Jorba, J.; Ahmed, N.; Campagnoli, R.; van der Avoort, H.; Shimizu, H.; Yoneyama, T.; et al. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt from 1983 to 1993. J. Virol. 2003, 77, 8366–8377. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Shimizu, H. The Molecular Evolution of Type 2 Vaccine-Derived Polioviruses in Individuals with Primary Immunodeficiency Diseases. Viruses 2021, 13, 1407. [Google Scholar] [CrossRef]
- Delpeyroux, F.; Colbère-Garapin, F.; Razafindratsimandresy, R.; Sadeuh-Mba, S.; Joffret, M.L.; Rousset, D.; Blondel, B. Éradication de la poliomyélite et émergence de poliovirus pathogènes dérivés du vaccin-De Madagascar au Cameroun [Eradication of poliomyelitis and emergence of pathogenic vaccine-derived polioviruses: From Madagascar to Cameroon]. Med. Sci. 2013, 29, 1034–1041. [Google Scholar]
- Kew, O.; Morris-Glasgow, V.; Landaverde, M.; Burns, C.; Shaw, J.; Garib, Z.; André, J.; Blackman, E.; Freeman, C.J.; Jorba, J.; et al. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 2002, 296, 356–359. [Google Scholar] [CrossRef]
- Rousset, D.; Rakoto-Andrianarivelo, M.; Razafindratsimandresy, R.; Randriamanalina, B.; Guillot, S.; Balanant, J.; Mauclère, P.; Delpeyroux, F. Recombinant vaccine-derived poliovirus in Madagascar. Emerg. Infect. Dis. 2003, 9, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Thorley, B.; Paladin, F.J.; Brussen, K.A.; Stambos, V.; Yuen, L.; Utama, A.; Tano, Y.; Arita, M.; Yoshida, H.; et al. Circulation of type 1 vaccine-derived poliovirus in the Philippines in 2001. J. Virol. 2004, 78, 13512–13521. [Google Scholar] [CrossRef]
- World Health Organization. Vaccine-derived polioviruses—Update. Wkly. Epidemiol. Rec. 2006, 81, 398–404, (In English, French). [Google Scholar]
- World Health Organization. Global update on vaccine-derived polioviruses, January 2006–August 2007. Wkly. Epidemiol. Rec. 2007, 82, 337–343. [Google Scholar]
- Adu, F.; Iber, J.; Bukbuk, D.; Gumede, N.; Yang, S.J.; Jorba, J.; Campagnoli, R.; Sule, W.F.; Yang, C.F.; Burns, C.; et al. Isolation of recombinant type 2 vaccine-derived poliovirus (VDPV) from a Nigerian child. Virus. Res. 2007, 127, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wassilak, S.; Pate, M.A.; Wannemuehler, K.; Jenks, J.; Burns, C.; Chenoweth, P.; Abanida, E.A.; Adu, F.; Baba, M.; Gasasira, A.; et al. Outbreak of type 2 vaccine-derived poliovirus in Nigeria: Emergence and widespread circulation in an underimmunized population. J. Infect. Dis. 2011, 203, 898–909. [Google Scholar] [CrossRef]
- Jenkins, H.E.; Aylward, R.B.; Gasasira, A.; Donnelly, C.A.; Mwanza, M.; Corander, J.; Garnier, S.; Chauvin, C.; Abanida, E.; Pate, M.A.; et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N. Engl. J. Med. 2010, 362, 2360–2369. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Progress toward poliomyelitis eradication-Nigeria, January 2009–June 2010. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 802–807. [Google Scholar]
- Arita, M.; Zhu, S.L.; Yoshida, H.; Yoneyama, T.; Miyamura, T.; Shimizu, H. A Sabin 3-derived poliovirus recombinant contained a sequence homologous with indigenous human enterovirus species C in the viral polymerase coding region. J. Virol. 2005, 79, 12650–12657. [Google Scholar] [CrossRef] [PubMed]
- Rakoto-Andrianarivelo, M.; Gumede, N.; Jegouic, S.; Balanant, J.; Andriamamonjy, S.N.; Rabemanantsoa, S.; Birmingham, M.; Randriamanalina, B.; Nkolomoni, L.; Venter, M.; et al. Reemergence of recombinant vaccine-derived poliovirus outbreak in Madagascar. J. Infect. Dis. 2008, 197, 1427–1435. [Google Scholar] [CrossRef]
- Estívariz, C.F.; Watkins, M.A.; Handoko, D.; Rusipah, R.; Deshpande, J.; Rana, B.J.; Irawan, E.; Widhiastuti, D.; Pallansch, M.A.; Thapa, A.; et al. A large vaccine-derived poliovirus outbreak on Madura Island--Indonesia, 2005. J. Infect. Dis. 2008, 197, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Combelas, N.; Holmblat, B.; Joffret, M.L.; Colbère-Garapin, F.; Delpeyroux, F. Recombination between poliovirus and coxsackie A viruses of species C: A model of viral genetic plasticity and emergence. Viruses 2011, 3, 1460–1484. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly-Polio-Analyses-cVDPV-20230425. 2023. Available online: https://polioeradication.org/wp-con (accessed on 9 May 2023).
- Li, L.; Ivanova, O.; Driss, N.; Tiongco-Recto, M.; da Silva, R.; Shahmahmoodi, S.; Sazzad, H.M.; Mach, O.; Kahn, A.L.; Sutter, R.W. Poliovirus excretion among persons with primary immune deficiency disorders: Summary of a seven-country study series. J. Infect. Dis. 2014, 210, S368–S372. [Google Scholar] [CrossRef]
- Macklin, G.; Liao, Y.; Takane, M.; Dooling, K.; Gilmour, S.; Mach, O.; Kew, O.M.; Sutter, R.W.; iVDPV Working Group. Prolonged Excretion of Poliovirus among Individuals with Primary Immunodeficiency Disorder: An Analysis of the World Health Organization Registry. Front. Immunol. 2017, 25, 1103. [Google Scholar] [CrossRef]
- Kew, O.M.; Sutter, R.W.; Nottay, B.K.; McDonough, M.J.; Prevots, D.R.; Quick, L.; Pallansch, M.A. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 1998, 36, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Buttinelli, G.; Donati, V.; Fiore, S.; Marturano, J.; Plebani, A.; Balestri, P.; Soresina, A.R.; Vivarelli, R.; Delpeyroux, F.; Martin, J.; et al. Nucleotide variation in Sabin type 2 poliovirus from an immunodeficient patient with poliomyelitis. J. Gen. Virol. 2003, 84, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Liu, Y.; Xu, J.W.; Wang, Q.; Yin, Z.D.; Wen, N.; Yang, H.; Rodewald, L.E.; Zhang, Z.Y. Detection of a Highly Divergent Type 3 Vaccine-Derived Poliovirus in a Child with a Severe Primary Immunodeficiency Disorder-Chongqing, China, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Jorba, J.; Diop, O.M.; Iber, J.; Henderson, E.; Zhao, K.; Sutter, R.W.; Wassilak, S.G.F.; Burns, C.C. Update on Vaccine-Derived Polioviruses-Worldwide, January 2017–June 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Polio Eradication Strategy 2022–2026: Delivering on a Promise; World Health Organization: Geneva, Switzerland, 2021.
- Modlin, J.F.; Bandyopadhyay, A.S.; Sutter, R. Immunization Against Poliomyelitis and the Challenges to Worldwide Poliomyelitis Eradication. J. Infect. Dis. 2021, 224, S398–S404. [Google Scholar] [CrossRef]
- Norton, E.B.; Lawson, L.B.; Freytag, L.C.; Clements, J.D. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 2011, 18, 546–551. [Google Scholar] [CrossRef]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, e00215–e00218. [Google Scholar] [CrossRef]
- Norton, E.B.; Bauer, D.L.; Weldon, W.C.; Oberste, M.S.; Lawson, L.B.; Clements, J.D. The novel adjuvant dmLT promotes dose sparing, mucosal immunity and longevity of antibody responses to the inactivated polio vaccine in a murine model. Vaccine 2015, 33, 1909–1915. [Google Scholar] [CrossRef]
- White, J.A.; Blum, J.S.; Hosken, N.A.; Marshak, J.O.; Duncan, L.; Zhu, C.; Norton, E.B.; Clements, J.D.; Koelle, D.M.; Chen, D.; et al. Serum and mucosal antibody responses to inactivated polio vaccine after sublingual immunization using a thermoresponsive gel delivery system. Hum. Vaccin Immunother. 2014, 10, 3611–3621. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Gutiérrez, R.L.; Maciel, M.; Poole, S.; Testa, K.J.; Trop, S.; Duplessis, C.; Lane, A.; Riddle, M.S.; Hamer, M.; et al. Safety and immunogenicity of intramuscularly administered CS6 subunit vaccine with a modified heat-labile enterotoxin from enterotoxigenic Escherichia coli. Vaccine 2021, 39, 5548–5556. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Pasetti, M.F.; Brady, R.; Buskirk, A.D.; Wahid, R.; Dickey, M.; Cohen, M.; Baughman, H.; El-Khorazaty, J.; Maier, N.; et al. A Phase 1 dose escalating study of double mutant heat-labile toxin LTR192G/L211A (dmLT) from Enterotoxigenic Escherichia coli (ETEC) by sublingual or oral immunization. Vaccine 2019, 37, 602–611. [Google Scholar] [CrossRef]
- Svennerholm, A.M.; Qadri, F.; Lundgren, A.; Kaim, J.; Rahman Bhuiyan, T.; Akhtar, M.; Maier, N.; Louis Bourgeois, A.; Walker, R.I. Induction of mucosal and systemic immune responses against the common O78 antigen of an oral inactivated ETEC vaccine in Bangladeshi children and infants. Vaccine 2022, 40, 380–389. [Google Scholar] [CrossRef]
- Okayasu, H.; Sein, C.; Chang Blanc, D.; Gonzalez, A.R.; Zehrung, D.; Jarrahian, C.; Macklin, G.; Sutter, R.W. Intradermal Administration of Fractional Doses of Inactivated Poliovirus Vaccine: A Dose-Sparing Option for Polio Immunization. J. Infect. Dis. 2017, 216, S161–S167. [Google Scholar] [CrossRef]
- Crothers, J.W.; Ross Colgate, E.; Cowan, K.J.; Dickson, D.M.; Walsh, M.; Carmolli, M.; Wright, P.F.; Norton, E.B.; Kirkpatrick, B.D. Intradermal fractional-dose inactivated polio vaccine (fIPV) adjuvanted with double mutant Enterotoxigenic Escherichia coli heat labile toxin (dmLT) is well-tolerated and augments a systemic immune response to all three poliovirus serotypes in a randomized placebo-controlled trial. Vaccine 2022, 40, 2705–2713. [Google Scholar] [PubMed]
- Bessaud, M. Le nouveau vaccin antipoliomyélitique oral: Un tournant décisif pour le programme d’éradication? [New oral polio vaccine: A turning point for the global polio eradication initiative?]. Med. Trop. Sante Int. 2021, 1, 191. [Google Scholar]
- De Coster, I.; Leroux-Roels, I.; Bandyopadhyay, A.S.; Gast, C.; Withanage, K.; Steenackers, K.; De Smedt, P.; Aerssens, A.; Leroux-Roels, G.; Oberste, M.S.; et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in healthy adults: Two clinical trials. Lancet 2021, 397, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Llorens, X.; Bandyopadhyay, A.S.; Gast, C.; Leon, T.; DeAntonio, R.; Jimeno, J.; Caballero, M.I.; Aguirre, G.; Oberste, M.S.; Weldon, W.C.; et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: Two clinical trials. Lancet 2021, 397, 27–38. [Google Scholar] [CrossRef]
- Mirzoev, A.; Macklin, G.R.; Zhang, Y.; Mainou, B.A.; Sadykova, U.; Olsavszky, V.S.; Huseynov, S.; Ruziev, M.; Saidzoda, F.; Bobokhonova, M.; et al. Assessment of serological responses following vaccination campaigns with type 2 novel oral polio vaccine: A population-based study in Tajikistan in 2021. Lancet Glob. Health. 2022, 10, e1807–e1814. [Google Scholar] [CrossRef]
- Zaman, K.; Bandyopadhyay, A.S.; Hoque, M.; Gast, C.; Yunus, M.; Jamil, K.M.; Mainou, B.A.; Konopka-Anstadt, J.L.; Hendley, W.S.; Vincent, A.; et al. Evaluation of the safety, immunogenicity, and faecal shedding of novel oral polio vaccine type 2 in healthy newborn infants in Bangladesh: A randomised, controlled, phase 2 clinical trial. Lancet 2023, 401, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Polioeradication. Available online: https://polioeradication.org/news-post/gpei-statement-on-cvdpv2-detections-in-burundi-and-democratic-republic-of-the-congo/ (accessed on 10 May 2023).
- Yang, H.; Qi, Q.; Zhang, Y.; Wen, N.; Cao, L.; Liu, Y.; Fan, C.; Yan, D.; Zhu, X.; Hao, L.; et al. Analysis of a Sabin-Strain Inactivated Poliovirus Vaccine Response to a Circulating Type 2 Vaccine-Derived Poliovirus Event in Sichuan Province, China 2019–2021. JAMA Netw. Open. 2023, 6, e2249710. [Google Scholar] [CrossRef]
- Viktorova, E.G.; Khattar, S.K.; Kouiavskaia, D.; Laassri, M.; Zagorodnyaya, T.; Dragunsky, E.; Samal, S.; Chumakov, K.; Belov, G.A. Newcastle Disease Virus-Based Vectored Vaccine against Poliomyelitis. J. Virol. 2018, 92, e00976-18. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.O.; Nicol, C.; Stonehouse, N.J.; Rowlands, D.J. Increasing Type 1 Poliovirus Capsid Stability by Thermal Selection. J. Virol. 2017, 91, e01586-16. [Google Scholar] [CrossRef] [PubMed]
- Ansardi, D.C.; Porter, D.C.; Morrow, C.D. Coinfection with recombinant vaccinia viruses expressing poliovirus P1 and P3 proteins results in polyprotein processing and formation of empty capsid structures. J. Virol. 1991, 65, 2088–2092. [Google Scholar] [CrossRef]
- Bahar, M.W.; Porta, C.; Fox, H.; Macadam, A.J.; Fry, E.E.; Stuart, D.I. Mammalian expression of virus-like particles as a proof of principle for next generation polio vaccines. NPJ Vaccines 2021, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, S.; Snezhkov, E.; Bishop, D.H. Formation of poliovirus-like particles by recombinant baculoviruses expressing the individual VP0, VP3, and VP1 proteins by comparison to particles derived from the expressed poliovirus polyprotein. Virology 1993, 192, 512–524. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Huang, Y.; Chen, F.; Chen, L.; Ding, D.; Zheng, Y.; Li, H.; Xiao, J.; Feng, J.; et al. Virus-like particle vaccines for poliovirus types 1, 2, and 3 with enhanced thermostability expressed in insect cells. Vaccine 2019, 37, 2340–2347. [Google Scholar] [CrossRef]
- Marsian, J.; Fox, H.; Bahar, M.W.; Kotecha, A.; Fry, E.E.; Stuart, D.I.; Macadam, A.J.; Rowlands, D.J.; Lomonossoff, G.P. Plant-made polio type 3 stabilized VLPs-a candidate synthetic polio vaccine. Nat. Commun. 2017, 8, 245. [Google Scholar] [CrossRef]
- Sherry, L.; Grehan, K.; Swanson, J.J.; Bahar, M.W.; Porta, C.; Fry, E.E.; Stuart, D.I.; Rowlands, D.J.; Stonehouse, N.J. Production and Characterisation of Stabilised PV-3 Virus-like Particles Using Pichia pastoris. Viruses 2022, 14, 2159. [Google Scholar] [CrossRef]
- Hummeler, K.; Anderson, T.F.; Brown, R.A. Identification of poliovirus particles of different antigenicity by specific agglutination as seen in the electron microscope. Virology 1962, 16, 84–90. [Google Scholar] [CrossRef]
- Urakawa, T.; Ferguson, M.; Minor, P.D.; Cooper, J.; Sullivan, M.; Almond, J.W.; Bishop, D.H. Synthesis of immunogenic, but non-infectious, poliovirus particles in insect cells by a baculovirus expression vector. J. Gen. Virol. 1989, 70, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, W.H.; Canning, B.; Wilton, T.; Kidd, M.; Klapsa, D.; Majumdar, M.; Sooriyakumar, K.; Martin, J.; Huissoon, A.P. Case report: Clearance of longstanding, immune-deficiency-associated, vaccine-derived polio virus infection following remdesivir therapy for chronic SARS-CoV-2 infection. Front Immunol. 2023, 14, 1135834. [Google Scholar] [CrossRef] [PubMed]
| VDPV | Countries | Number of Cases | Environmental Samples | |
|---|---|---|---|---|
| AFP Cases | Non-AFP Cases | |||
| cVDPV1 | ||||
| Madagascar | 36 | 37 | 168 | |
| Malawi | 4 | 1 | 0 | |
| DRC | 156 | 5 | 0 | |
| Mozambique | 25 | 1 | 0 | |
| Congo | 1 | 0 | ||
| Yemen | 3 | 0 | ||
| cVDPV2 | ||||
| Benin | 16 | 3 | 12 | |
| Burkina Faso | 2 | 0 | 1 | |
| Guinea | 6 | 0 | 2 | |
| Guinea-Bissau | 3 | 1 | 0 | |
| Burundi | 1 | 2 | 13 | |
| Botswana | 0 | 0 | 5 | |
| Algeria | 3 | 5 | 26 | |
| Central African Republic | 10 | 1 | 9 | |
| Cameroon | 6 | 3 | 1 | |
| Chad | 50 | 7 | 6 | |
| Côte d’Ivoire | 1 | 0 | 3 | |
| Democratic Republic of the Congo | 412 | 37 | 13 | |
| Congo | 2 | 0 | 3 | |
| Eritrea | 2 | 0 | 0 | |
| Ethiopia | 11 | 0 | 0 | |
| Ghana | 3 | 4 | 19 | |
| Mauritania | 0 | 4 | 7 | |
| Mozambique | 6 | 0 | 0 | |
| Malawi | 0 | 0 | 1 | |
| Niger | 33 | 4 | 15 | |
| Nigeria | 467 | 234 | 397 | |
| Senegal | 17 | 34 | 15 | |
| Zambia | 0 | 0 | 3 | |
| United States of America | 1 | 0 | 14 | |
| Canada | 0 | 0 | 2 | |
| Djibouti | 0 | 0 | 19 | |
| Egypt | 0 | 0 | 18 | |
| Mali | 2 | 0 | 0 | |
| Somalia | 8 | 4 | 7 | |
| Soudan | 1 | 0 | 1 | |
| Yemen | 228 | 50 | 38 | |
| Israel | 1 | 0 | 55 | |
| United kingdom | 0 | 0 | 6 | |
| Ukraine | 2 | 18 | 0 | |
| Indonesia | 4 | 10 | 0 | |
| Uganda | 0 | 0 | 2 | |
| Gambia | 0 | 0 | 9 | |
| Pakistan | 8 | 0 | 35 | |
| Tajikistan | 35 | 22 | 17 | |
| Afghanistan | 43 | 2 | 40 | |
| Sierra Leone | 5 | 8 | 9 | |
| Liberia | 3 | 5 | 14 | |
| South Sudan | 9 | 5 | 0 | |
| Iran | 0 | 0 | 0 | |
| Kenya | 0 | 2 | 1 | |
| Togo | 2 | 0 | 2 | |
| cVDPV3 | ||||
| Israel | 1 | 3 | 30 | |
| Occupied Palestinian territory | 0 | 0 | 16 | |
| China | 0 | 0 | 1 | |
| TOTAL | 1629 | 512 | 1055 | |
| VDPV | Countries | Years | Number of Cases | Environmental Samples | |
|---|---|---|---|---|---|
| iVDPV | AFP | Non-AFP | |||
| iVDPV1 | Egypt | 2018–2019 | 1 | 4 | 0 |
| Iran | 2018–2019 | 2 | 1 | 0 | |
| iVDPV2 | Philippines | 2018–2019 | 1 | 0 | 0 |
| iVDPV3 | Egypt | 2018–2019 | 1 | 4 | 0 |
| Tunisia | 2018–2019 | 1 | 0 | 0 | |
| Argentina | 2018 | 1 | 0 | 0 | |
| TOTAL | 7 | 9 | 0 | ||
| aVDPV | |||||
| aVDPV1 | Democratic Republic of the Congo | 2017–2018 | 1 | 0 | 0 |
| aVDPV2 | Nigeria | 2017–2018 | 0 | 1 | 12 |
| Pakistan | 2017–2018 | 0 | 0 | 5 | |
| Somalia | 2017–2018 | 0 | 0 | 1 | |
| India | 2017–2018 | 0 | 0 | 1 | |
| Democratic Republic of the Congo | 2017–2018 | 2 | 0 | 0 | |
| Australia | 2017–2018 | 0 | 0 | 1 | |
| aVDPV3 | China | 2017–2018 | 1 | 0 | 2 |
| India | 2017–2018 | 0 | 0 | 1 | |
| TOTAL | 4 | 1 | 23 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbani, C.J.; Nekoua, M.P.; Moukassa, D.; Hober, D. The Fight against Poliovirus Is Not Over. Microorganisms 2023, 11, 1323. https://doi.org/10.3390/microorganisms11051323
Mbani CJ, Nekoua MP, Moukassa D, Hober D. The Fight against Poliovirus Is Not Over. Microorganisms. 2023; 11(5):1323. https://doi.org/10.3390/microorganisms11051323
Chicago/Turabian StyleMbani, Chaldam Jespère, Magloire Pandoua Nekoua, Donatien Moukassa, and Didier Hober. 2023. "The Fight against Poliovirus Is Not Over" Microorganisms 11, no. 5: 1323. https://doi.org/10.3390/microorganisms11051323
APA StyleMbani, C. J., Nekoua, M. P., Moukassa, D., & Hober, D. (2023). The Fight against Poliovirus Is Not Over. Microorganisms, 11(5), 1323. https://doi.org/10.3390/microorganisms11051323







