Metagenomic Analysis of Bacterial, Archaeal and Fungal Diversity in Two-Stage Anaerobic Biodegradation for Production of Hydrogen and Methane from Corn Steep Liquor
Abstract
1. Introduction
2. Materials and Methods
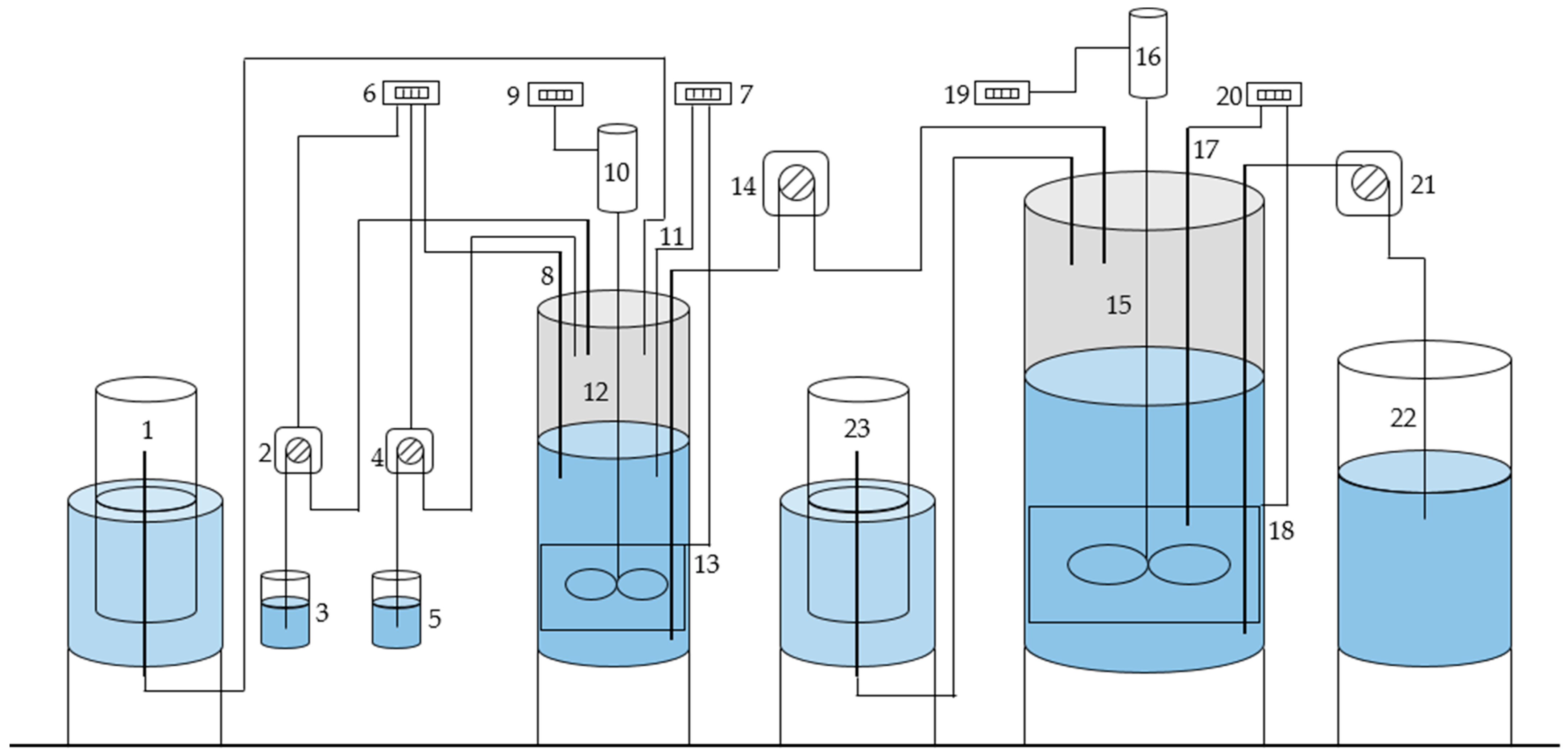
2.1. Two Stage Bioreactor System
2.2. Substrate
2.3. Inoculum
2.4. Analytical Methods
2.5. Sample Collection and DNA Extraction
2.6. Metagenome Sequencing and Bioinformatics Analysis
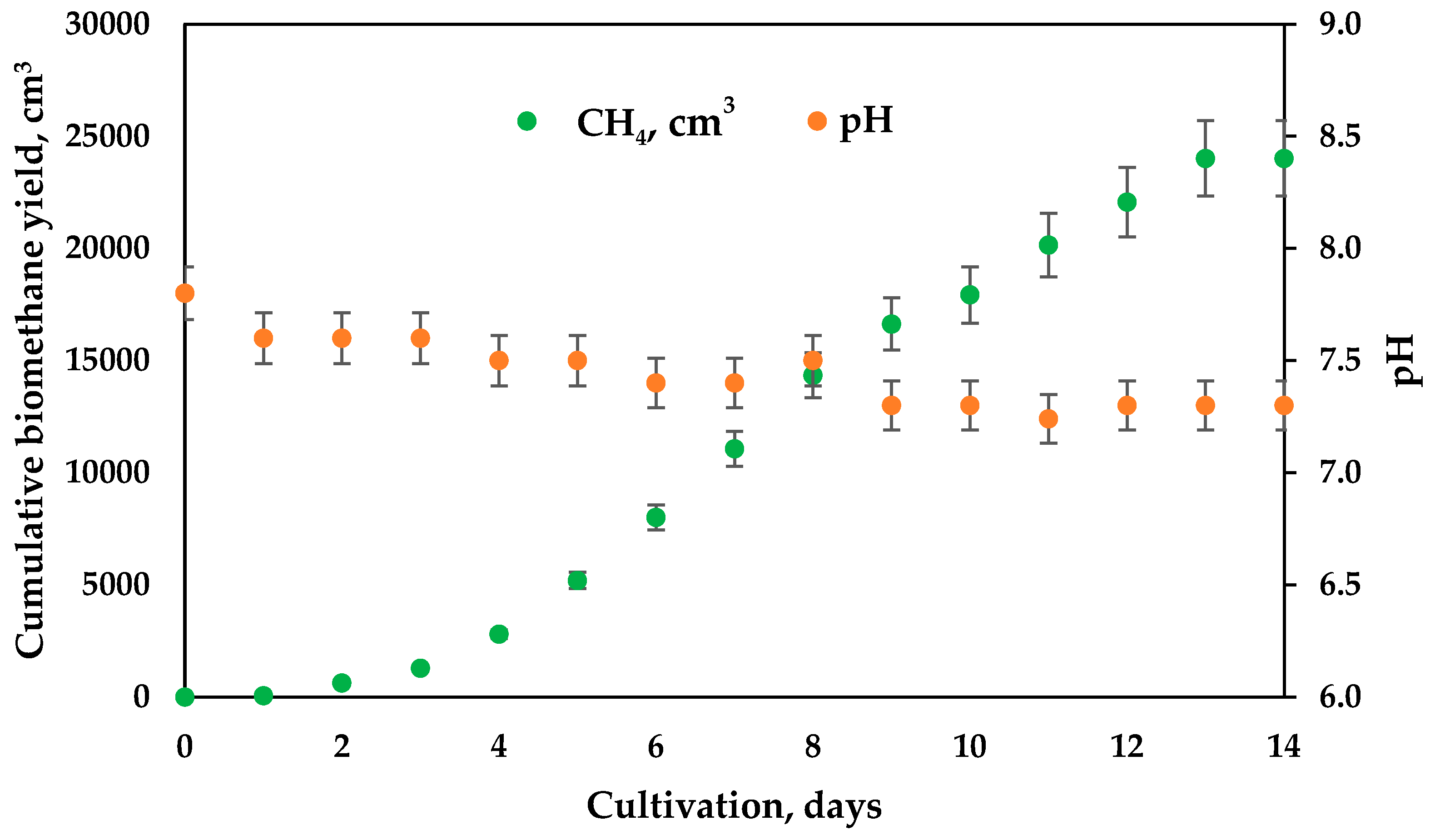
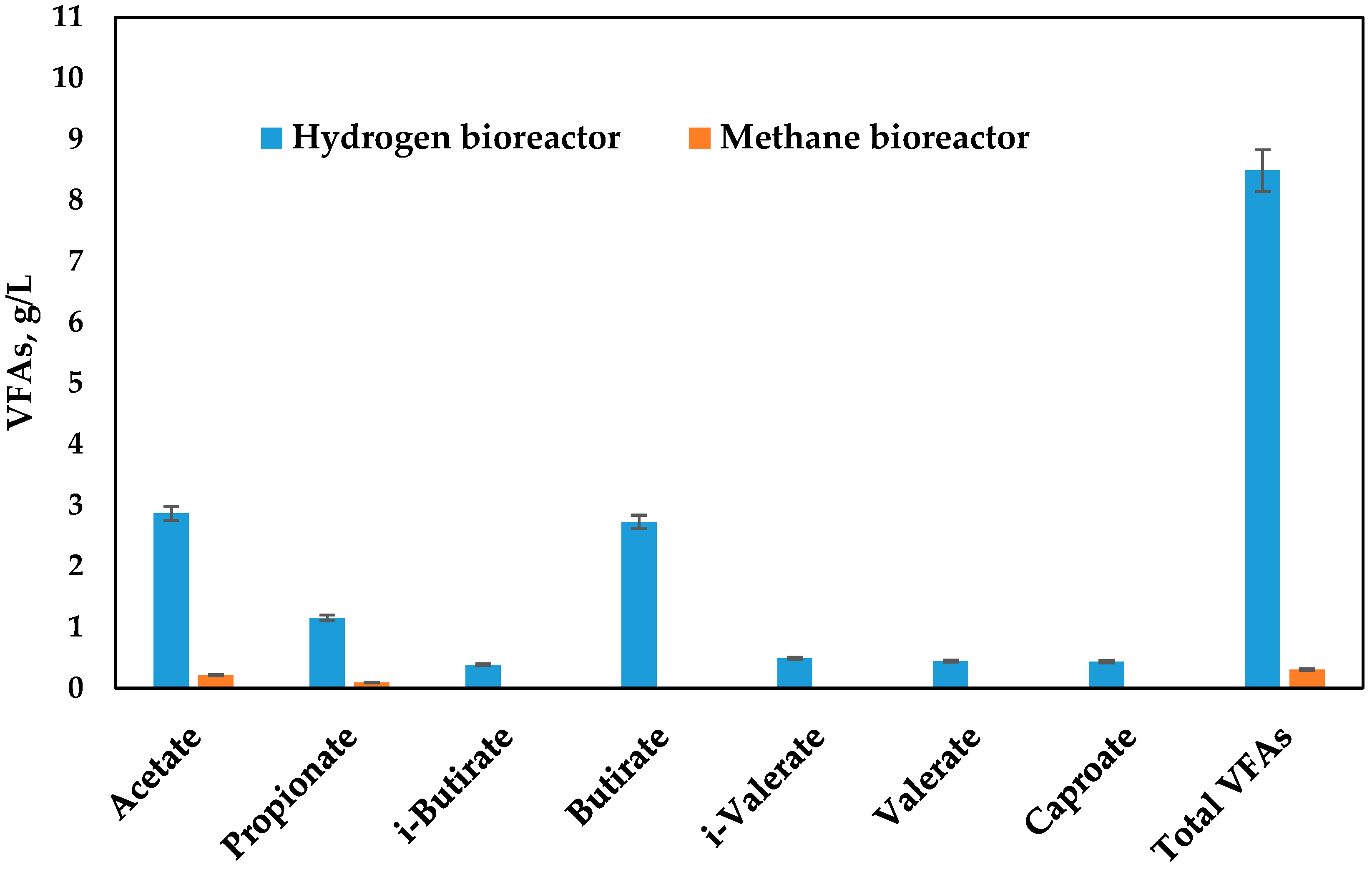
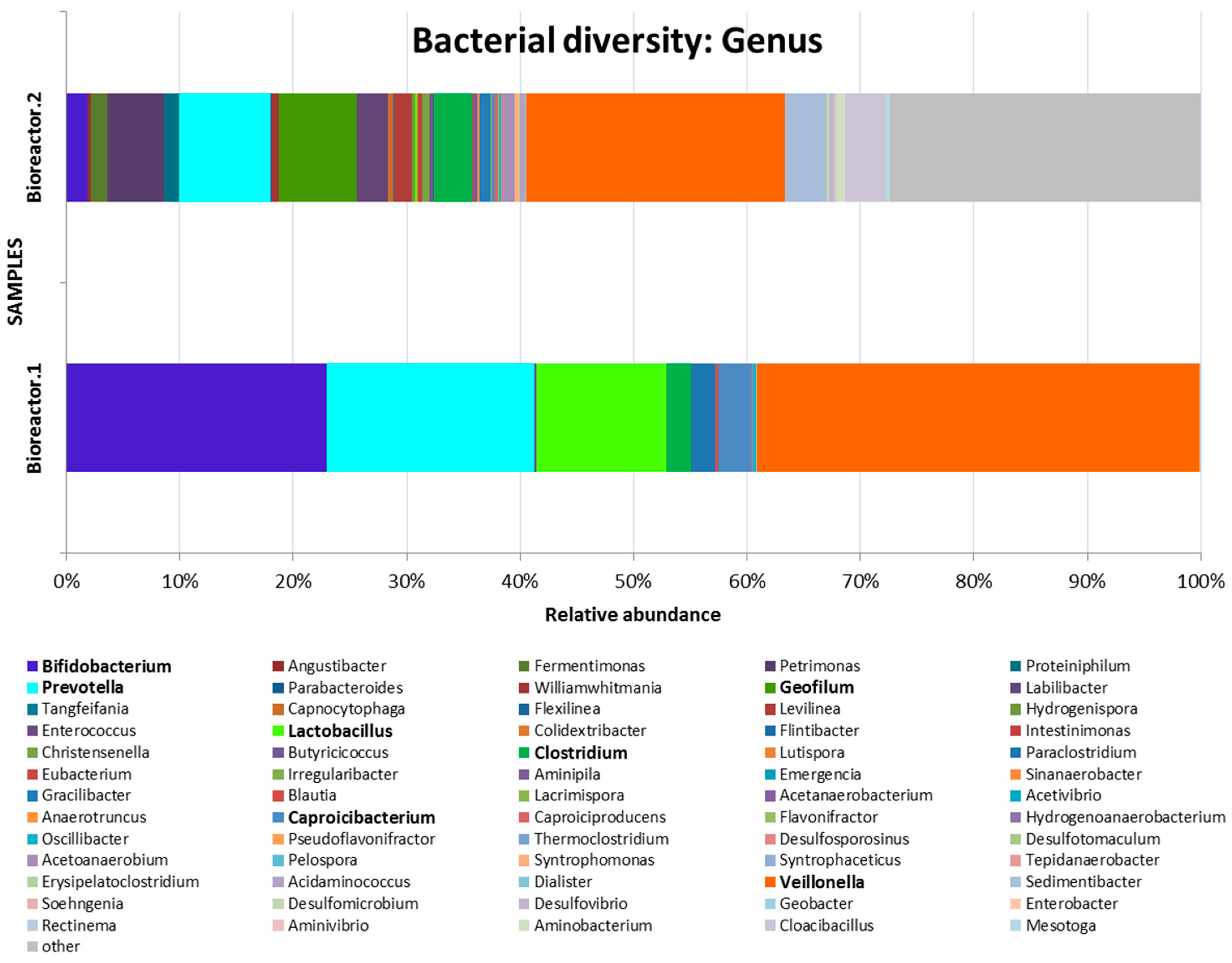
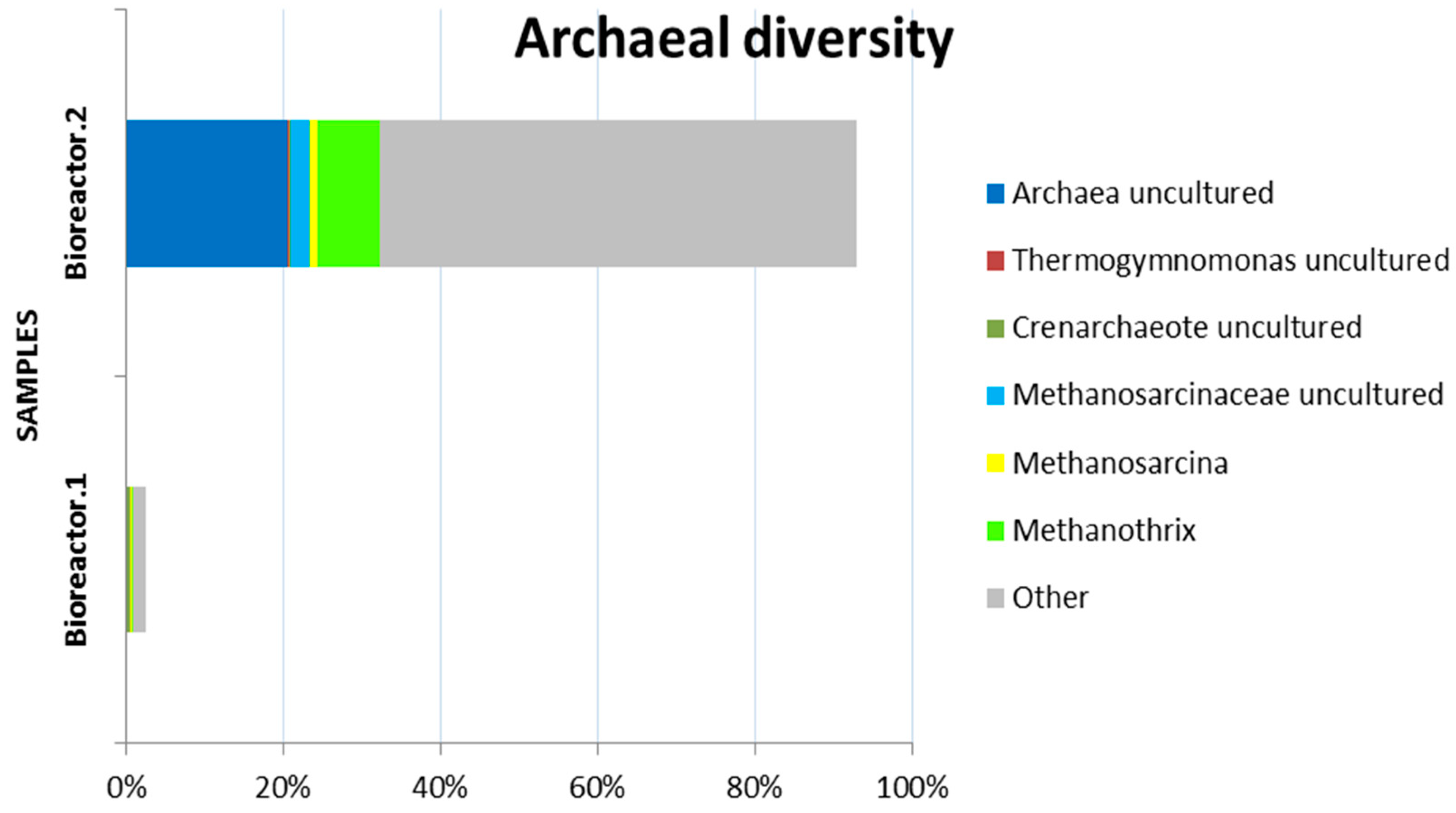
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spišáková, M.; Mandičák, T.; Mésároš, P.; Špak, M. Waste Management in a Sustainable Circular Economy as a Part of Design of Construction. Appl. Sci. 2022, 12, 4553. [Google Scholar] [CrossRef]
- Lins, M.; Puppin Zandonadi, R.; Raposo, A.; Ginani, V.C. Food Waste on Foodservice: An Overview through the Perspective of Sustainable Dimensions. Foods 2021, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Holmes, D.E.; Smith, J.A. Biologically Produced Methane as a Renewable Energy Source. Adv. Appl. Microbiol. 2016, 97, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Yang, J.; Zhao, H.; Xin, H.; Zhang, Y. Effect of different levels of corn steep liquor addition on fermentation characteristics and aerobic stability of fresh rice straw silage. Anim. Nutr. 2016, 2, 345–350. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Sydney, E.B.; Rodrigues de Paula, D.; Medeirosa, A.B.P.; Cesar de Carvalho, J.; Molina, D.; Soccol, C.R. Hydrogen production by dark fermentation using a new low-cost culture medium composed of corn steep liquor and cassava processing water: Process optimization and scale-up. Bioresour. Technol. 2021, 320, 124370. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Khan, M.Z.; Xiao, J.; Alugongo, G.M.; Liu, S.; Wang, J.; Cao, Z. Effect of the Combining Corn Steep Liquor and Urea Pre-treatment on Biodegradation and Hydrolysis of Rice Straw. Front. Microbiol. 2022, 13, 916195. [Google Scholar] [CrossRef] [PubMed]
- Mitra, J.; Pramodbabu, R.; Patil, P.; Khanna, N.; Fosso-Kankeu, E.; Pandit, S. Role of the microbial community in the anaerobic digester for biomethane production. In Wastewater Treatment Reactors; Shah, M.P., Rodriguez-Couto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 65–89. ISBN 9780128239919. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Wang, D.S.; Yu, X.J.; Zhu, X.Y.; Wang, Z.; Li, H.J.; Wang, Z.P. Transcriptome Mechanism of Utilizing Corn Steep Liquor as the Sole Nitrogen Resource for Lipid and DHA Biosynthesis in Marine Oleaginous Protist Aurantiochytrium sp. Biomolecules 2019, 9, 695. [Google Scholar] [CrossRef]
- Uddin, M.N.; Siddiki, S.Y.A.; Mofijur, M.; Djavanroodi, F.; Hazrat, M.A.; Show, P.L.; Ahmed, S.F.; Chu, Y.-M. Prospects of Bioenergy Production from Organic Waste Using Anaerobic Digestion Technology: A Mini Review. Front. Energy Res. 2021, 9, 627093. [Google Scholar] [CrossRef]
- Murphy, R.; Woods, J.; Black, M.; McManus, M. Global developments in the competition for land from biofuels. Food Policy 2011, 36, S52–S61. [Google Scholar] [CrossRef]
- Pagliaccia, P.; Gallipoli, A.; Gianico, A.; Montecchio, D.; Braguglia, C.M. Single stage anaerobic bioconversion of food waste in mono and co-digestion with olive husks: Impact of thermal pretreatment on hydrogen and methane production. Int. J. Hydrogen Energy 2016, 41, 905–915. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Nguyen, A.Q.; Nghiem, L.D. Microbial Community in Anaerobic Digestion System: Progression in Microbial Ecology. In Water and Wastewater Treatment Technologies. Energy, Environment, and Sustainability; Bui, X.T., Chiemchaisri, C., Fujioka, T., Varjani, S., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Szulc, J.; Okrasa, M.; Majchrzycka, K.; Sulyok, M.; Nowak, A.; Szponar, B.; Górczyńska, A.; Ryngajłło, M.; Gutarowska, B. Microbiological and toxicological hazard assessment in a waste sorting plant and proper respiratory protection. J. Environ. Manag. 2022, 303, 114257. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Sakami, T.; Watanabe, T.; Taniuchi, Y.; Kuwata, A.; Kakehi, S.; Engkong, T.; Igarashi, Y.; Kinoshita, S.; Asakawa, S.; et al. Metagenomic analysis provides functional insights into seasonal change of a non-cyanobacterial prokaryotic community in temperate coastal waters. PLoS ONE 2021, 16, e0257862. [Google Scholar] [CrossRef]
- Chorukova, E.; Hubenov, V.; Gocheva, Y.; Simeonov, I. Two-Phase Anaerobic Digestion of Corn Steep Liquor in Pilot Scale Biogas Plant with Automatic Control System with Simultaneous Hydrogen and Methane Production. Appl. Sci. 2022, 12, 6274. [Google Scholar] [CrossRef]
- Hubenov, V.; Miteva-Staleva, J.; Eneva, R.; Boteva, N.; Kabaivanova, L. Two-stage anaerobic digestion of wheat straw using immobilized microbial consortia. Ecol. Eng. Environ. Prot. 2021, 3, 35–44. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for Examination of Water and Waste Water, 23rd ed.; American Public Health Association (APHA): Washington, DC, USA, 2003. [Google Scholar]
- Chatterjee, B.; Mazumder, D. Performance evaluation of three-stage AD for stabilization of fruit and vegetable waste. J. Indian Chem. Soc. 2018, 95, 65–80. [Google Scholar]
- Schievano, A.; Tenca, A.; Lonati, S.; Manzini, E.; Adani, F. Can two-stage instead one-stage anaerobic digestion really increase energy recovery from biomass? Appl. Energy 2014, 124, 335–342. [Google Scholar] [CrossRef]
- Regueiro, L.; Veiga, P.; Figueroa, M.; Alonso-Gutierrez, J.; Stams, A.J.M.; Lema, J.M.; Carballa, M. Relationship between microbial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol. Res. 2012, 167, 581–589. [Google Scholar] [CrossRef]
- Xiao, K.; Maspolim, Y.; Zhou, Y.; Guo, C.; Ng, W.J. Effect of Sodium on Methanogens in a Two-Stage Anaerobic System. Appl. Sci. 2022, 12, 956. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas Upgrading via Hydrogenotrophic Methanogenesis in Two-Stage Continuous Stirred Tank Reactors at Mesophilic and Thermophilic Conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Aguilar, F.A.; Longoria, A.; Sebastian, J.; Santos, A.S.D.; Pantoja, L.; Sebastian, P.J. Optimization of Hydrogen Yield from the Anaerobic Digestion of Crude Glycerol and Swine Manure. Catalysts 2019, 9, 316. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Flores-Larios, A.; González-Álvarez, V.; Isela Corona-González, R.; Oscar Méndez-Acosta, H. Single and two-stage anaerobic digestion for hydrogen and methane production from acid and enzymatic hydrolysates of Agave tequilana bagasse. Int. J. Hydrogen Energy 2016, 41, 897–904. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Li, Q.; Liu, C.Z. Coproduction of hydrogen and methane via anaerobic fermentation of cornstalk waste in continuous stirred tank reactor integrated with up-flow anaerobic sludge bed. Bioresour. Technol. 2012, 114, 327–333. [Google Scholar] [CrossRef]
- Park, M.J.; Jo, J.H.; Park, D.; Lee, D.S.; Park, J.M. Comprehensive study on a two-stage anaerobic digestion process for the sequential production of hydrogen and methane from cost-effective molasses. Int. J. Hydrogen Energy 2010, 35, 6194–6202. [Google Scholar] [CrossRef]
- Choudhury, A.; Lepine, C.; Good, C. Methane and Hydrogen Sulfide Production from the Anaerobic Digestion of Fish Sludge from Recirculating Aquaculture Systems: Effect of Varying Initial Solid Concentrations. Fermentation 2023, 9, 94. [Google Scholar] [CrossRef]
- Sulbarán-Rangel, B.; Alarcón Aguirre, J.S.; Breton-Deval, L.; del Real-Olvera, J.; Gurubel Tun, K.J. Improvement of Anaerobic Digestion of Hydrolysed Corncob Waste by Organosolv Pretreatment for Biogas Production. Appl. Sci. 2020, 10, 2785. [Google Scholar] [CrossRef]
- Klocke, M.; Mahnert, P.; Mundt, K.; Souidi, K.; Linke, B. Microbial community analysis of a biogas-producing completely stirred tank reactor fed continuously with fodder beet silage as mono-substrate. Syst. Appl. Microbiol. 2007, 30, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Tukanghan, W.; Hupfauf, S.; Gómez-Brandón, M.; Insam, H.; Salvenmoser, W.; Prasertsan, P.; Cheirsilp, B.; O-Thong, S. Symbiotic Bacteroides and Clostridium-rich methanogenic consortium enhanced biogas production of high-solid anaerobic digestion systems. Bioresour. Technol. Rep. 2021, 14, 100685. [Google Scholar] [CrossRef]
- Kampmann, K.; Ratering, S.; Kramer, I.; Schmidt, M.; Zerr, W.; Schnell, S. Unexpected stability of Bacteroidetes and Firmicutes communities in laboratory biogas reactors fed with different defined substrates. Appl. Environ. Microbiol. 2012, 78, 2106–2119. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Li, Y.; Zhu, T.; Yu, Q.; Wang, T.; Liang, S.; Zhang, Y. Why do DIETers like drinking: Metagenomic analysis for methane and energy metabolism during anaerobic digestion with ethanol. Water Res. 2020, 171, 115425. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.R.; Yang, B.Y.; Venzke, D.; Kulhavy, K.; Montgomery, R. Composition of corn steep water during steeping. J. Agric. Food Chem. 1996, 44, 1857–1863. [Google Scholar] [CrossRef]
- Loy, D.D.; Lundy, E.L. Nutritional properties and feeding value of corn and its coproducts. In Corn; AACC International Press: St. Paul, MN, USA, 2019; pp. 633–659. [Google Scholar]
- Kabaivanova, L.; Hubenov, V.; Dimitrova, L.; Simeonov, I.; Wang, H.; Petrova, P. Archaeal and Bacterial Content in a Two-Stage Anaerobic System for Efficient Energy Production from Agricultural Wastes. Molecules 2022, 27, 1512. [Google Scholar] [CrossRef]
- Seon, J.; Lee, T.; Lee, S.C.; Pham, H.D.; Woo, H.C.; Song, M. Bacterial community structure in maximum volatile fatty acids production from alginate in acidogenesis. Bioresour. Technol. 2014, 157, 22–27. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Khan, A.; Xiong, J.; Tian, F.; Li, X. A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion. Bioresour. Technol. 2018, 247, 1119–1127. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, H.; Yu, H.; Chen, D.; Li, X.; Wang, W.; Piao, R.; Cui, Z. Evaluation of biogas production performance and archaeal microbial dynamics of corn straw during anaerobic co-digestion with cattle manure liquid. J. Microbiol. Biotechnol. 2016, 26, 739–747. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, D.; Li, X.; Lu, W.; Li, J. Impact of long-term industrial contamination on the bacterial communities in urban river sediments. BMC Microbiol. 2020, 20, 254. [Google Scholar] [CrossRef]
- Shin, S.G.; Lee, S.; Lee, C.; Hwang, K.; Hwang, S. Qualitative and quantitative assessment of microbial community in batch anaerobic digestion of secondary sludge. Bioresour. Technol. 2010, 101, 9461–9470. [Google Scholar] [CrossRef] [PubMed]
- Prasse, D.; Thomsen, J.; De Santis, R.; Muntel, J.; Becher, D.; Schmitz, R.A. First description of small proteins encoded by spRNAs in Methanosarcina mazei strain Gö1. Biochimie 2015, 117, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Krzycki, J.A. Function of genetically encoded pyrrolysine in corrinoid-dependent methylamine methyltransferases. Curr. Opin. Chem. Biol. 2004, 8, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.; Deere, T.M.; Chanderban, M.; McIntosh, G.J.; Lessner, D.J. Methanosarcina acetivorans . Trends Microbiol. 2023, 31, 320–321. [Google Scholar] [CrossRef]
- Ming, G.; Shuang, Z.; Xinxin, M.; Weijie, G.; Na, S.; Qunhui, W.; Chuanfu, W. Effect of yeast addition on the biogas production performance of a food waste anaerobic digestion system. R. Soc. Open Sci. 2020, 7, 200443200443. [Google Scholar] [CrossRef]



| Parameter | Value |
|---|---|
| pH | 4.2 ± 0.1 |
| TS, % | 50.5 ± 0.2 |
| VS, % | 91 ± 0.3 |
| Reducing sugars, g/L | 70.6 ± 2.4 |
| Cellulose, g/L | 2.46 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyancheva, G.; Kabaivanova, L.; Hubenov, V.; Chorukova, E. Metagenomic Analysis of Bacterial, Archaeal and Fungal Diversity in Two-Stage Anaerobic Biodegradation for Production of Hydrogen and Methane from Corn Steep Liquor. Microorganisms 2023, 11, 1263. https://doi.org/10.3390/microorganisms11051263
Stoyancheva G, Kabaivanova L, Hubenov V, Chorukova E. Metagenomic Analysis of Bacterial, Archaeal and Fungal Diversity in Two-Stage Anaerobic Biodegradation for Production of Hydrogen and Methane from Corn Steep Liquor. Microorganisms. 2023; 11(5):1263. https://doi.org/10.3390/microorganisms11051263
Chicago/Turabian StyleStoyancheva, Galina, Lyudmila Kabaivanova, Venelin Hubenov, and Elena Chorukova. 2023. "Metagenomic Analysis of Bacterial, Archaeal and Fungal Diversity in Two-Stage Anaerobic Biodegradation for Production of Hydrogen and Methane from Corn Steep Liquor" Microorganisms 11, no. 5: 1263. https://doi.org/10.3390/microorganisms11051263
APA StyleStoyancheva, G., Kabaivanova, L., Hubenov, V., & Chorukova, E. (2023). Metagenomic Analysis of Bacterial, Archaeal and Fungal Diversity in Two-Stage Anaerobic Biodegradation for Production of Hydrogen and Methane from Corn Steep Liquor. Microorganisms, 11(5), 1263. https://doi.org/10.3390/microorganisms11051263







