In Vitro and In Vivo Study of Combined Effect of Some Algerian Medicinal Plants and Probiotics against Helicobacter pylori
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.1.1. Plant Material
Determination of Total Phenolic Content
Determination of Total Flavonoids Content
Determination of Condensed Tannins Content
Determination of Hydrolysable Tannins Content
2.1.2. Probiotic Strains
2.1.3. H. pylori Strains
2.1.4. Animals
2.2. Methods
2.2.1. Polymerase Chain Reaction Identification of H. pylori
2.2.2. Evaluation of Anti-H. pylori Effect of Plant Extracts
Evaluation of the Diameter of Inhibition Zones (DIZ) of Plant Extracts Using Disc Diffusion Method
Evaluation of the Minimum Inhibitory Concentration (MIC) of Plant Extracts with the Agar Dilution Method
Evaluation of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Plant Extracts with the Broth Dilution Method
Evaluation of Growth Kinetics of H. pylori in the Presence of Plant Extracts
2.3. Evaluation of Anti-H. pylori Effect of Probiotics (Well Diffusion Assay)
2.3.1. Preparation of Cell-Free Supernatant (CFS) of Probiotics
2.3.2. Evaluation of Growth Kinetics of H. pylori in the Presence of Probiotics
Influence of Organic Acids (Lactic and Acetic Acids)
Influence of Bacteriocins
Influence of Hydrogen Peroxide
2.4. Combined Effect of Medicinal Plants with Probiotics on H. pylori
2.4.1. Determination of DIZ Using Disc Diffusion Method
2.4.2. Evaluation of Growth Kinetics of H. pylori in the Presence of Combined Solutions (Plant Extracts with Probiotics)
2.5. In Vivo Study Protocol
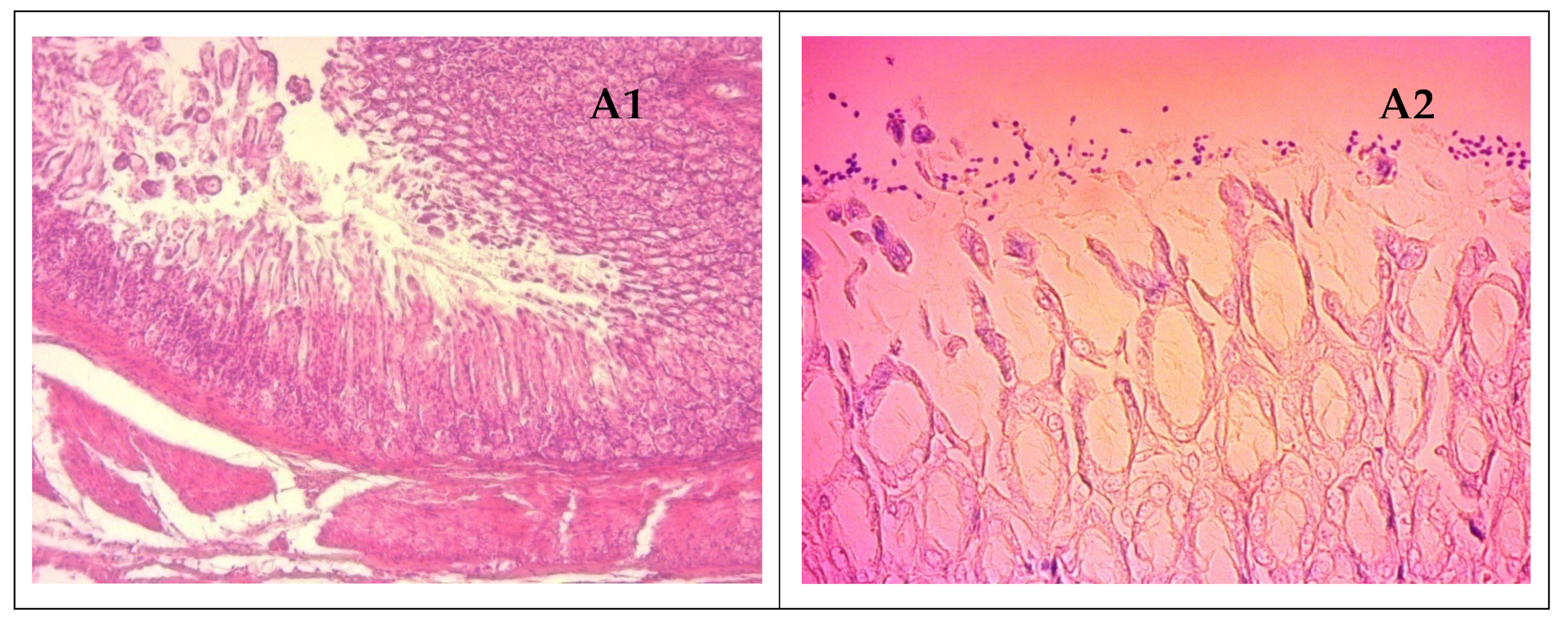
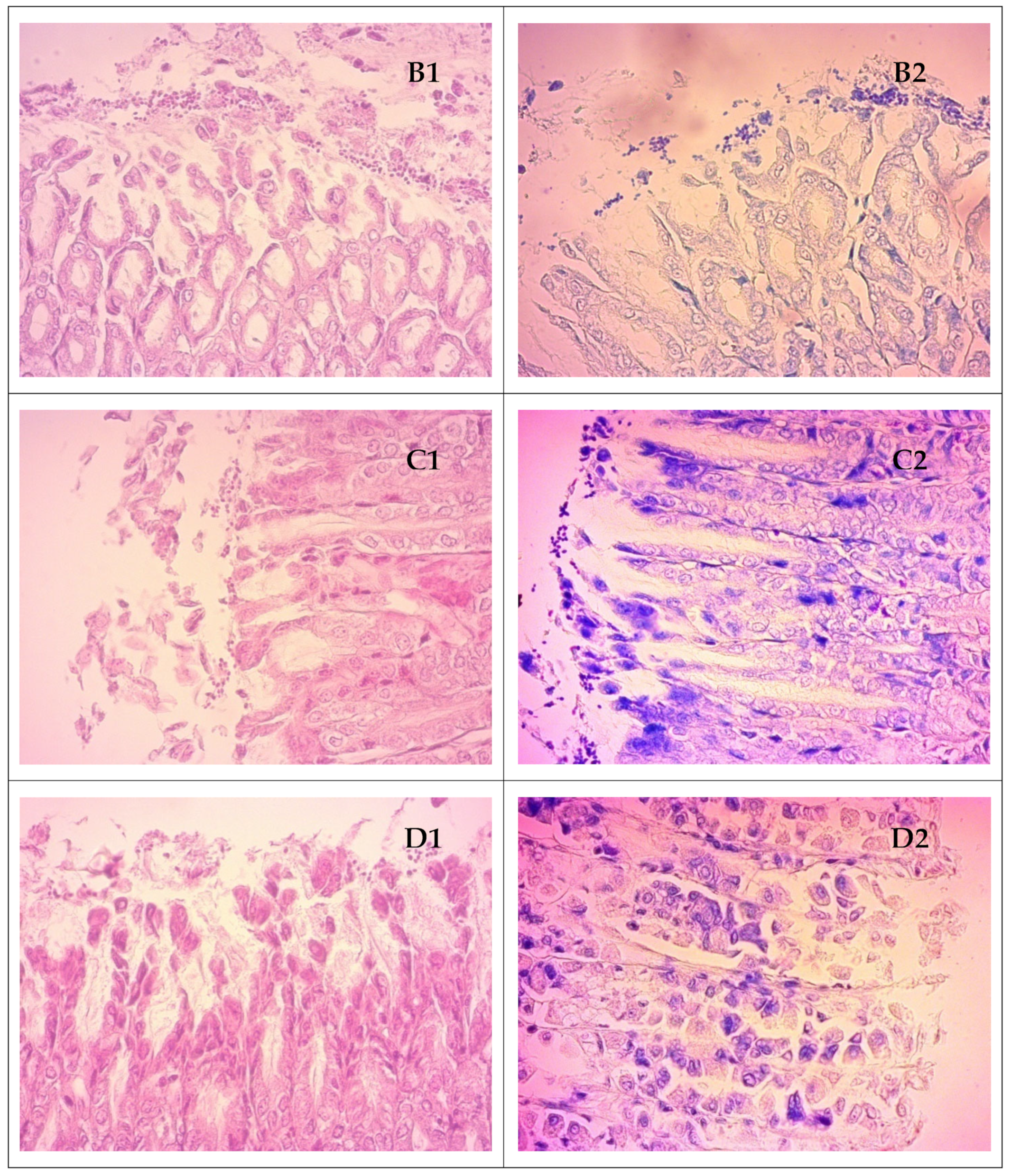
2.6. Histopathologic Analysis of Gastric Tissue Samples
2.7. Statistical Analysis
3. Results and Discussion
3.1. Results of Phytochemical Analysis and Screening
3.2. Results of Isolation and Identification of H. pylori
3.2.1. Results of Biochemical Identification
3.2.2. Results of H. pylori Identification by PCR
3.3. Results of Evaluation of Anti-H. pylori Effect of the Plant Extracts
3.3.1. Results of Evaluation of DIZ of Plant Extracts Using the Disc Diffusion Method
3.3.2. Evaluation of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Plant Extracts
3.3.3. Results of Evaluation of Growth Kinetics of H. pylori in the Presence of Plant Extracts
3.4. Results of Evaluation of Probiotics Effect on H. pylori
3.4.1. Evaluation of DIZ of Probiotics against H. pylori Using Well Diffusion Assay
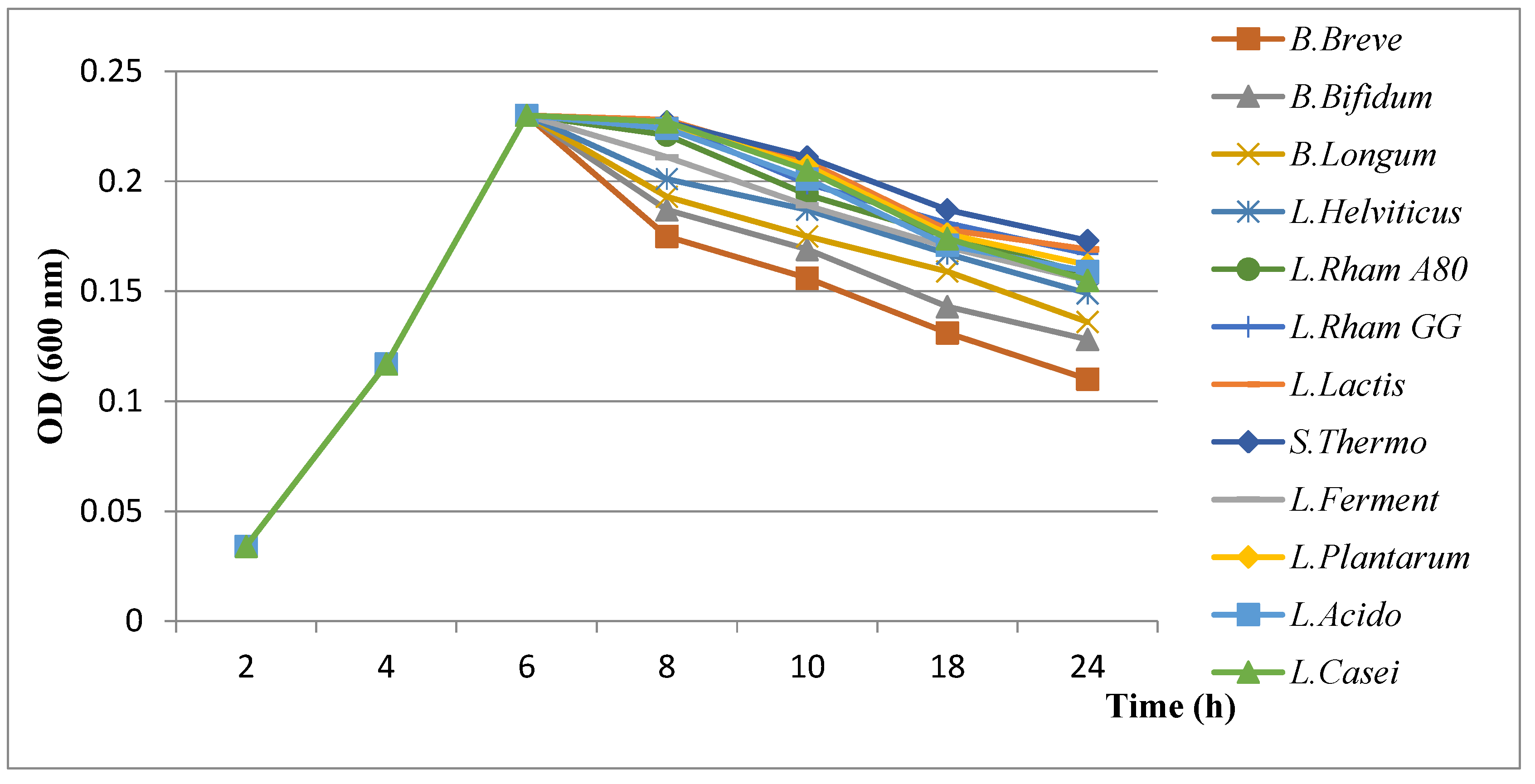
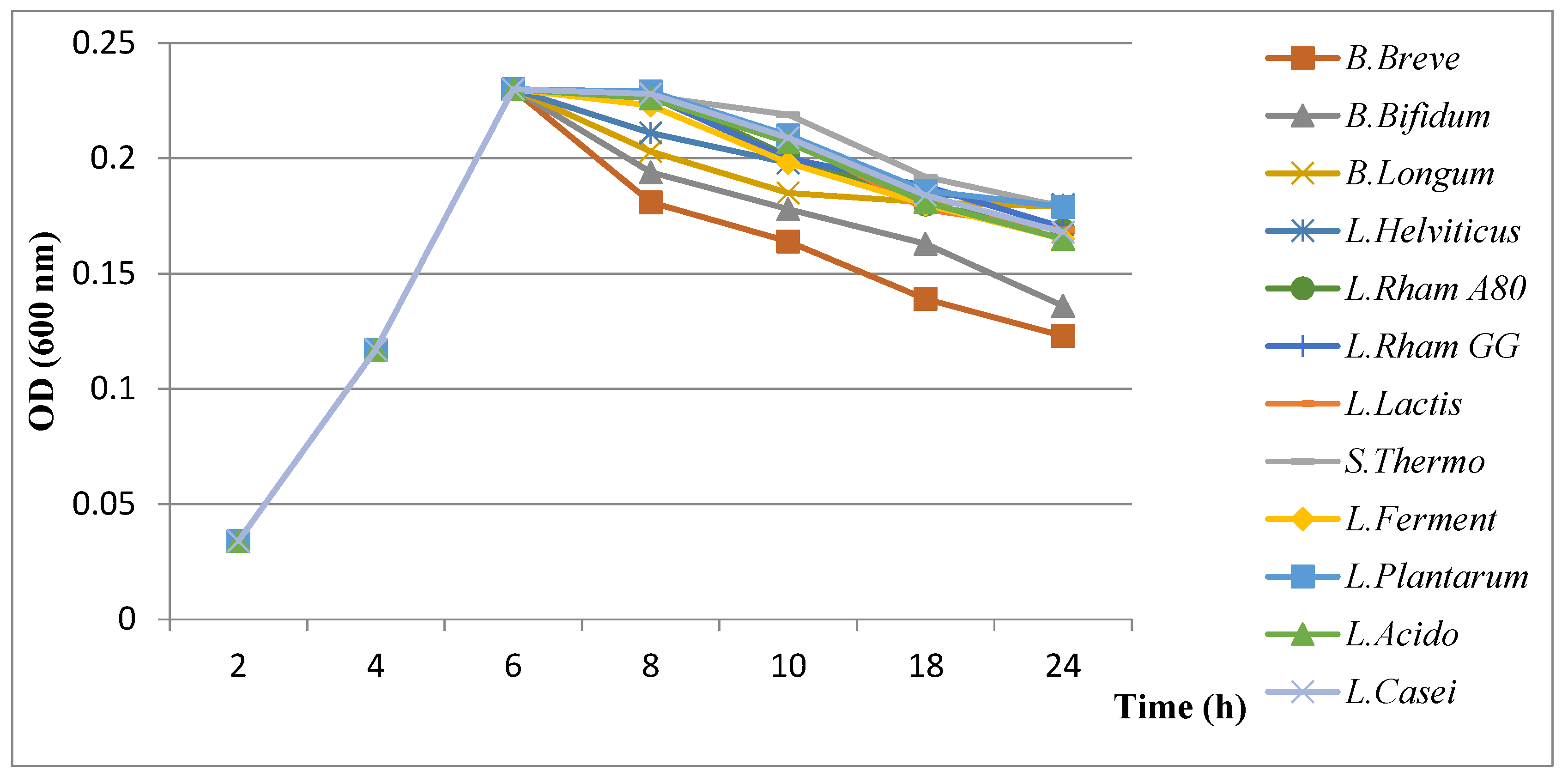
3.4.2. Results of Evaluation of Growth Kinetics of H. pylori in the Presence of Probiotics
- ✓
- Influence of organic acids (lactic and acetic acids)
- ✓
- Influence of bacteriocins
- ✓
- Influence of hydrogen peroxide (H2O2)
3.5. Results of Combined Effects of Medicinal Plants with Probiotics on H. pylori
3.5.1. Quantifying the DIZ Using the Disc Diffusion Method
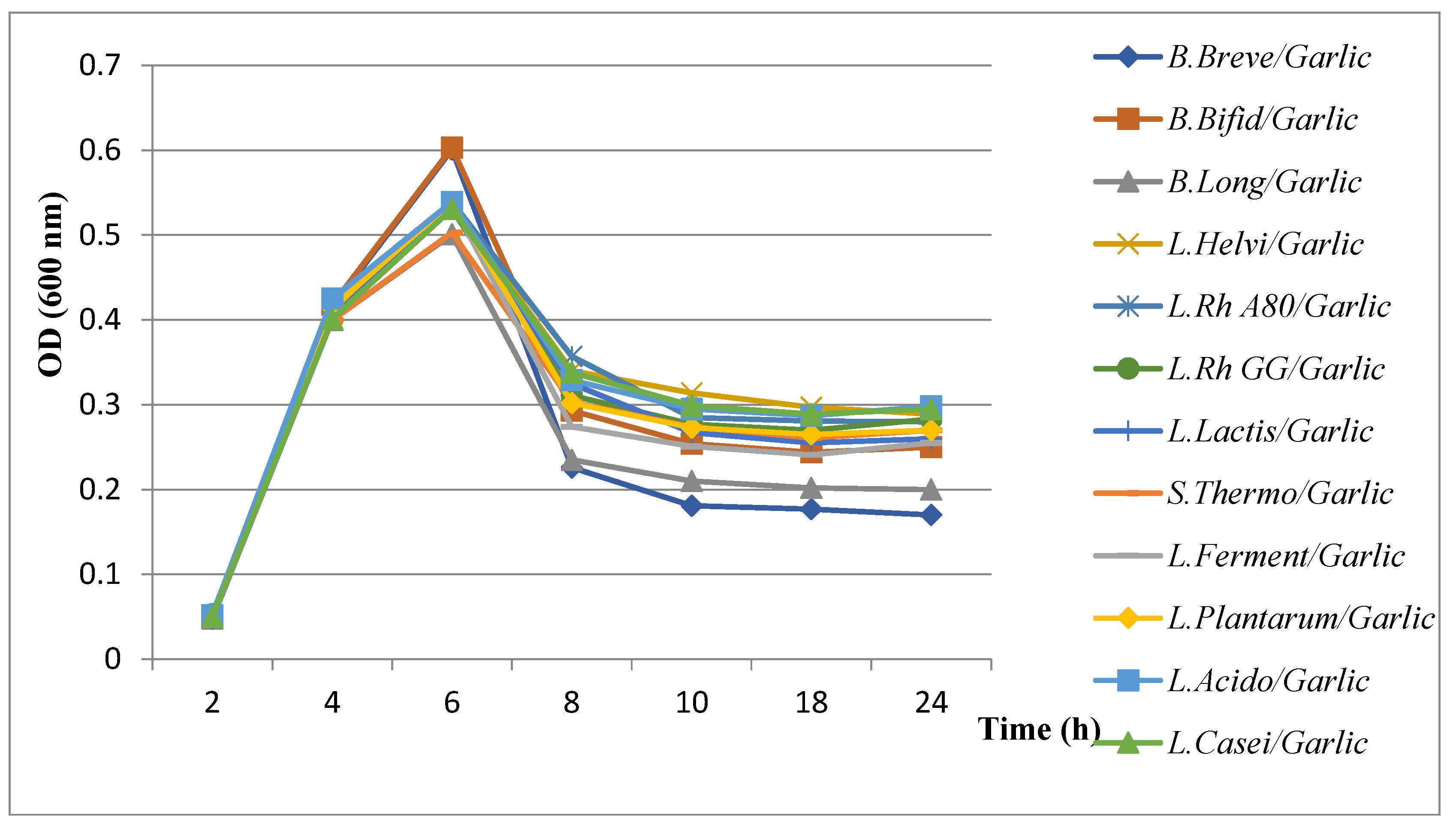
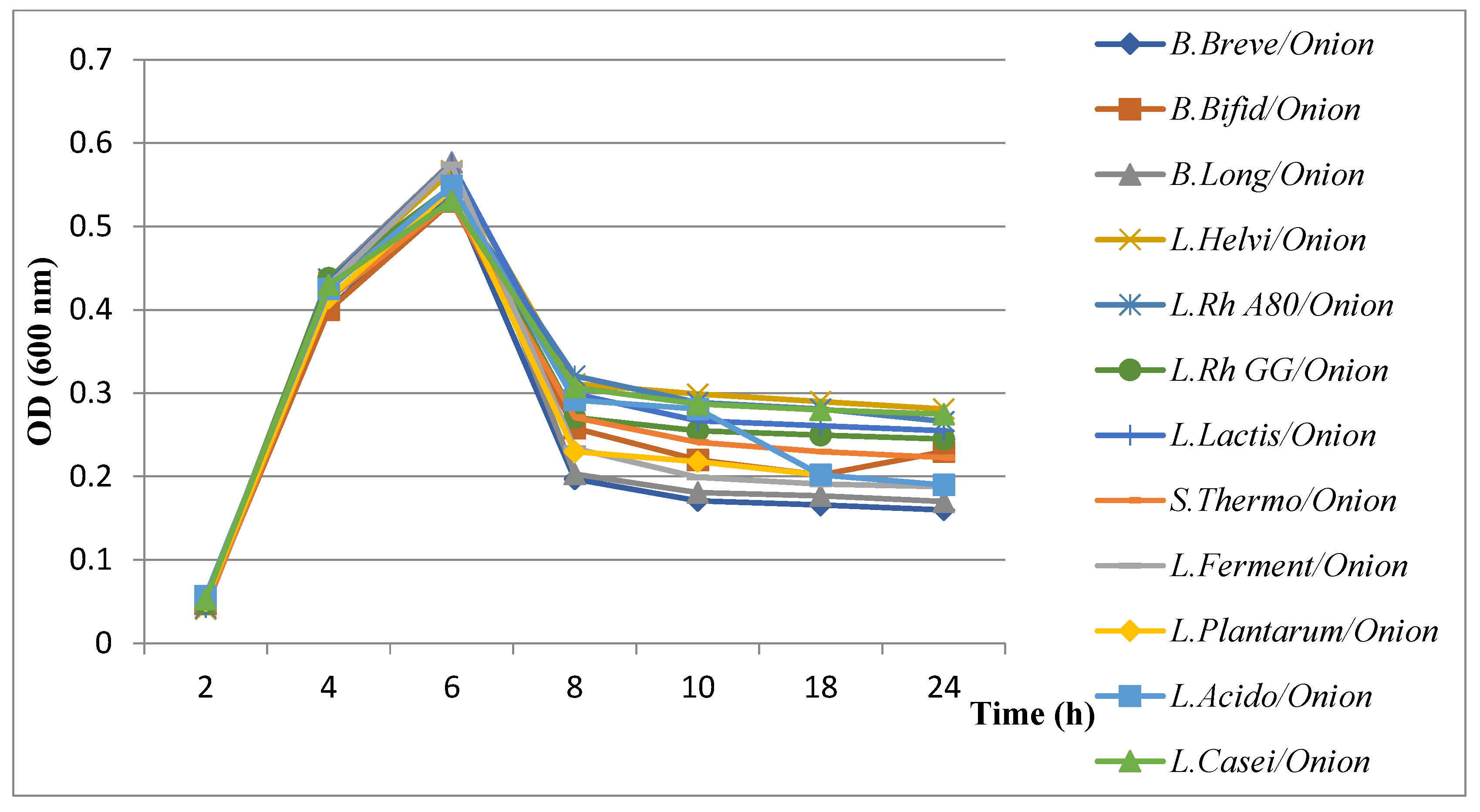
3.5.2. Evaluation of Growth Kinetics of H. pylori in the Presence of Combined Extract Plants with Probiotics
3.6. Results of the In Vivo Study
3.7. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crowe, S.E. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr. Opin. Gastroenterol. 2005, 21, 32–38. [Google Scholar] [PubMed]
- Haley, K.P.; Gaddy, J.A. Helicobacter pylori: Genomic insight into the host-pathogen interaction. Int. J. Genom. 2015, 2015, 386905. [Google Scholar] [CrossRef] [PubMed]
- Gotteland, M.; Brunser, O.; Cruchet, S. Systematic review: Are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment. Pharmacol. Ther. 2006, 23, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- The IMW Group. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: International Agency for Research on Cancer. In Part B: Biological Agents; 2011; Volume 100. [Google Scholar]
- Kasmi, H.; Doukani, K.; Ali, A.; Tabak, S.; Bouhenni, H. Epidemiological profile of Helicobacter pylori infection in patients with digestive symptoms in Algeria. J. Epidemiol. Glob. Health 2020, 10, 293. [Google Scholar] [CrossRef]
- Raaf, N.; Amhis, W.; Saoula, H.; Abid, A.; Nakmouche, M.; Balamane, A.; Ali Arous, N.; Ouar-Korichi, M.; Vale, F.F.; Bénéjat, L. Prevalence, antibiotic resistance, and MLST typing of Helicobacter pylori in Algiers, Algeria. Helicobacter 2017, 22, e12446. [Google Scholar] [CrossRef]
- Goderska, K.; Agudo Pena, S.; Alarcon, T. Helicobacter pylori treatment: Antibiotics or probiotics. Appl. Microbiol. Biotechnol. 2018, 102, 1–7. [Google Scholar] [CrossRef]
- Garrido-Treviño, L.; López-Martínez, M.; Flores-Hinojosa, J.; Tijerina-Rodríguez, L.; Bosques-Padilla, F. Empiric treatment vs susceptibility-guided treatment for eradicating H. pylori: Is it possible to change that paradigm using modern molecular methods? Rev. Gastroenterol. 2022, 87, 330–341. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382.e1317. [Google Scholar] [CrossRef]
- Abadi, A.T.B. Helicobacter pylori treatment: New perspectives using current experience. J. Glob. Antimicrob. Resist. 2017, 8, 123–130. [Google Scholar] [CrossRef]
- Elrais, A.M.; Arab, W.S.; Sallam, K.I.; Elmegid, W.A.; Elgendy, F.; Elmonir, W.; Imre, K.; Morar, A.; Herman, V.; Elaadli, H. Prevalence, Virulence Genes, Phylogenetic Analysis, and Antimicrobial Resistance Profile of Helicobacter Species in Chicken Meat and Their Associated Environment at Retail Shops in Egypt. Foods 2022, 11, 1890. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Dung, P.P.; Nhi, N.V.T.Y.; Hoang, N.v.M.; Hieu, T.T. Antibacterial activity of ethanolic extracts of some Vietnamese medicinal plants against Helicobacter pylori. In Proceedings of the AIP Conference Proceedings, Phuket, Thailand, 6–8 June 2017; AIP Publishing LLC: College Park, MD, USA, 2017; p. 020030. [Google Scholar]
- Bi, W.-P.; Man, H.-B.; Man, M.-Q. Efficacy and safety of herbal medicines in treating gastric ulcer: A review. World J. Gastroenterol. WJG 2014, 20, 17020. [Google Scholar] [CrossRef]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Matkowski, A.; Chodaczek, G.; Płusa, T.; Gościniak, G. Antibiofilm and antimicrobial-enhancing activity of Chelidonium majus and Corydalis cheilanthifolia extracts against multidrug-resistant Helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef]
- Mintah, S.O.; Asafo-Agyei, T.; Archer, M.-A.; Junior, P.A.-A.; Boamah, D.; Kumadoh, D.; Appiah, A.; Ocloo, A.; Boakye, Y.D.; Agyare, C. Medicinal plants for treatment of prevalent diseases. Pharmacogn. Med. Plants 2019, 1–19. [Google Scholar]
- Spînu, M.; Niculae, M.; Paştiu, A.I.; Şandru, C.D.; Pall, E.; Vasiu, A. Vegetal extracts influence in vitro on the cell-mediated immunity in carnivores depending on health status, target species and plant taxonomy. Ind. Crops Prod. 2016, 88, 44–47. [Google Scholar] [CrossRef]
- O’Gara, E.A.; Hill, D.J.; Maslin, D.J. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000, 66, 2269–2273. [Google Scholar] [CrossRef]
- Yordanov, D.; Boyanova, L.; Markovska, R.; Ilieva, J.; Andreev, N.; Gergova, G.; Mitov, I. Influence of dietary factors on Helicobacter pylori and CagA seroprevalence in Bulgaria. Gastroenterol. Res. Pract. 2017, 2017, 9212143. [Google Scholar] [CrossRef]
- Nakhaei, M.; Khajeh, K.A.M.; Ramezani, M. Inhibition of Helicobacter pylori growth in vitro by saffron (Crocus sativus L.). Iran. J. Basic Med. Sci. 2008, 11, 91–96. [Google Scholar]
- Belagihalli, S.M.; Dharmesh, S.M. Anti-Helicobacter pylori, proton pump inhibitory and antioxidant properties of selected dietary/medicinal plants. Int. J. Phytomed. 2012, 4, 573. [Google Scholar]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef]
- Vítor, J.M.; Vale, F.F. Alternative therapies for Helicobacter pylori: Probiotics and phytomedicine. FEMS Immunol. Med. Microbiol. 2011, 63, 153–164. [Google Scholar] [CrossRef]
- Peres, C.M.; Alves, M.; Hernandez-Mendoza, A.; Moreira, L.; Silva, S.; Bronze, M.R.; Vilas-Boas, L.; Peres, C.; Malcata, F.X. Novel isolates of lactobacilli from fermented Portuguese olive as potential probiotics. LWT-Food Sci. Technol. 2014, 59, 234–246. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef] [PubMed]
- Sgouras, D.; Maragkoudakis, P.; Petraki, K.; Martinez-Gonzalez, B.; Eriotou, E.; Michopoulos, S.; Kalantzopoulos, G.; Tsakalidou, E.; Mentis, A. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl. Environ. Microbiol. 2004, 70, 518–526. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Mitchell, D.J.; Avitzur, Y.; Galindo-Mata, E.; Jones, N.L.; Sherman, P.M. Probiotics reduce bacterial colonization and gastric inflammation in H. pylori-infected mice. Dig. Dis. Sci. 2004, 49, 1095–1102. [Google Scholar] [CrossRef]
- Zou, J.; Dong, J.; Yu, X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter 2009, 14, 449–459. [Google Scholar] [CrossRef]
- Zheng, X.; Lyu, L.; Mei, Z. Lactobacillus-containing probiotic supplementation increases Helicobacter pylori eradication rate: Evidence from a meta-analysis. Rev. Esp. Enferm. Dig. 2013, 105, 445–453. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Zelaya, H.; Ilabaca, A.; Espinoza-Monje, M.; Komatsu, R.; Albarracín, L.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. Lactobacillus fermentum UCO-979C beneficially modulates the innate immune response triggered by Helicobacter pylori infection in vitro. Benef. Microbes 2018, 9, 829–841. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Apostolidis, E.; Shetty, K. Antimicrobial activity of an Amazon medicinal plant (Chancapiedra) (Phyllanthus niruri L.) against Helicobacter pylori and lactic acid bacteria. Phytother. Res. 2012, 26, 791–799. [Google Scholar] [CrossRef]
- Bouhenni, H.; Doukani, K.; Şekeroğlu, N.; Gezici, S.; Tabak, S. Comparative study on chemical composition and antibacterial activity of fenugreek (Trigonella foenum graecum L.) and cumin (Cuminum cyminum L.) seeds. Ukr. Food J. 2019, 8, 755–767. [Google Scholar] [CrossRef]
- Bouhenni, H.; Doukani, K.; Sekeroglu, N.; Tabak, S. Proximate composition and antibacterial potentials of cultivated garlic (Allium sativum L.) and onion (Allium cepa L.) in Algeria. Int. J. Ecosyst. Ecol. Sci. 2019, 9, 383–394. [Google Scholar] [CrossRef]
- Oberg, T.S.; McMahon, D.J.; Culumber, M.D.; McAuliffe, O.; Oberg, C.J. Invited review: Review of taxonomic changes in dairy-related lactobacilli. J. Dairy Sci. 2022, 105, 2750–2770. [Google Scholar] [CrossRef]
- Lage, A.P.; Godfroid, E.; Fauconnier, A.; Burette, A.; Butzler, J.-P.; Bollen, A.; Glupczynski, Y. Diagnosis of Helicobacter pylori infection by PCR: Comparison with other invasive techniques and detection of cagA gene in gastric biopsy specimens. J. Clin. Microbiol. 1995, 33, 2752–2756. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.; Crisafi, G.; Musolino, A.; Procopio, F.; Alonzo, V. Modifications of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett. Appl. Microbiol. 2004, 38, 423–427. [Google Scholar] [CrossRef]
- Arbyn, M.; Anttila, A.; Jordan, J.; Ronco, G.; Schenck, U.; Segnan, N.; Wiener, H.; Herbert, A.; Von Karsa, L. European guidelines for quality assurance in cervical cancer screening—Summary document. Ann. Oncol. 2010, 21, 448–458. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Huang, T.-L. Anti-Helicobacter pylori activity of Plumbago zeylanica L. FEMS Immunol. Med. Microbiol. 2005, 43, 407–412. [Google Scholar] [CrossRef]
- Barefoot, S.F.; Klaenhammer, T.R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl. Environ. Microbiol. 1983, 45, 1808–1815. [Google Scholar] [CrossRef]
- Kim, M.-R.; Lee, S.-J.; Seul, K.-J.; Park, Y.-M.; Ghim, S.-Y. Characterization of antimicrobial substance produced by Lactobacillus paraplantarum KNUC25 isolated from kimchi. Microbiol. Biotechnol. Lett. 2009, 37, 24–32. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lee, A.; O’Rourke, J.; De Ungria, M.C.; Robertson, B.; Daskalopoulos, G.; Dixon, M.F. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. Gastroenterology 1997, 112, 1386–1397. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis: The updated Sydney system. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Cellini, L.; Di Campli, E.; Masulli, M.; Di Bartolomeo, S.; Allocati, N. Inhibition of Helicobacter pylori by garlic extract (Allium sativum). FEMS Immunol. Med. Microbiol. 1996, 13, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Matsuura, H.; Pendland, S.L.; Allixin, A. Phytoalexin From Garlic, Inhibits The Growth of Helicobacter Pylori in Vitro. Off. J. Am. Coll. Gastroenterol. ACG 2001, 96, 3454–3455. [Google Scholar] [CrossRef] [PubMed]
- Sivam, G.P.; Lampe, J.W.; Ulness, B.; Swanzy, S.R.; Potter, J.D. Helicobacter pylori—In vitro susceptibility to garlic (Allium sativum) extract. Nutr. Cancer 1997, 27, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Ngan, L.; Tan, M.; Hoang, N.; Thanh, D.; Linh, N.; Hoa, T.; Nuong, N.; Hieu, T. Antibacterial activity of Hibiscus rosa-sinensis L. red flower against antibiotic-resistant strains of Helicobacter pylori and identification of the flower constituents. Braz. J. Med. Biol. Res. 2021, 54. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.; Escobedo-Hinojosa, W.I.; de la Cruz-Herrera, C.F.; Romero, I. Exploring alternative treatments for Helicobacter pylori infection. World J. Gastroenterol. WJG 2014, 20, 1450. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Alibi, S.; Crespo, D.; Navas, J. Plant-derivatives small molecules with antibacterial activity. Antibiotics 2021, 10, 231. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sundaramurthi, J.C.; Sarada, D.V. Antibacterial activity of flavonoid isolated from Trianthema decandra against Pseudomonas aeruginosa and molecular docking study of FabZ. Microb. Pathog. 2018, 121, 87–92. [Google Scholar] [CrossRef]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 39. [Google Scholar] [CrossRef]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. BioMed Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef]
- Dey, D.; Ghosh, S.; Ray, R.; Hazra, B. Polyphenolic secondary metabolites synergize the activity of commercial antibiotics against clinical isolates of β-Lactamase-producing Klebsiella pneumoniae. Phytother. Res. 2016, 30, 272–282. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef]
- Wang, S.-S.; Wang, D.-M.; Pu, W.-J.; Li, D.-W. Phytochemical profiles, antioxidant and antimicrobial activities of three Potentilla species. BMC Complement. Altern. Med. 2013, 13, 1–11. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sakharkar, M.K.; Lim, C.S.; Tang, T.H.; Sakharkar, K.R. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int. J. Biol. Sci. 2010, 6, 556. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Pearson, R.; Steigbigel, R.; Davis, H.; Chapman, S. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 1980, 18, 699–708. [Google Scholar] [CrossRef]
- Bae, E.-A.; Kim, D.-H.; Han, M.-J. Anti-Helicobacter pylori activity of Bifidobacterium spp. J. Microbiol. Biotechnol. 2000, 10, 532–534. [Google Scholar]
- Boyanova, L.; Stephanova-Kondratenko, M.; Mitov, I. Anti-Helicobacter pylori activity of Lactobacillus delbrueckii subsp. bulgaricus strains: Preliminary report. Lett. Appl. Microbiol. 2009, 48, 579–584. [Google Scholar] [CrossRef]
- Boyanova, L.; Gergova, G.; Markovska, R.; Yordanov, D.; Mitov, I. Bacteriocin-like inhibitory activities of seven Lactobacillus delbrueckii subsp. bulgaricus strains against antibiotic susceptible and resistant Helicobacter pylori strains. Lett. Appl. Microbiol. 2017, 65, 469–474. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Tsai, W.-H.; Wu, H.-Y.; Chen, C.-Y.; Yeh, W.-L.; Chen, Y.-H.; Hsu, H.-Y.; Chen, W.-W.; Chen, Y.-W.; Chang, W.-W. Probiotic Lactobacillus spp. act against Helicobacter pylori-induced inflammation. J. Clin. Med. 2019, 8, 90. [Google Scholar] [CrossRef]
- Paucar-Carrión, C.; Espinoza-Monje, M.; Gutiérrez-Zamorano, C.; Sánchez-Alonzo, K.; Carvajal, R.I.; Rogel-Castillo, C.; Sáez-Carrillo, K.; García-Cancino, A. Incorporation of Limosilactobacillus fermentum UCO-979C with Anti-Helicobacter pylori and Immunomodulatory Activities in Various Ice Cream Bases. Foods 2022, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Chenoll, E.; Casinos, B.; Bataller, E.; Astals, P.; Echevarría, J.; Iglesias, J.R.; Balbarie, P.; Ramon, D.; Genovés, S. Novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacterium Helicobacter pylori. Appl. Environ. Microbiol. 2011, 77, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Lorca, G.L.; Wadström, T.; Font de Valdez, G.; Ljungh, Å. Lactobacillus acidophilus autolysins inhibit Helicobacter pylori in vitro. Curr. Microbiol. 2001, 42, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.-P.; Lovitt, R. Bacteriocins produced by lactic acid bacteria a review article. Apcbee Procedia 2012, 2, 50–56. [Google Scholar] [CrossRef]
- Drider, D.; Bendali, F.; Naghmouchi, K.; Chikindas, M.L. Bacteriocins: Not only antibacterial agents. Probiotics Antimicrob. Proteins 2016, 8, 177–182. [Google Scholar] [CrossRef]
- Lee, H.-A.; Kim, J.-Y.; Kim, J.; Nam, B.; Kim, O. Anti-Helicobacter pylori activity of acomplex mixture of Lactobacillus paracasei HP7 including the extract of Perilla frutescens var. acuta and Glycyrrhiza glabra. Lab. Anim. Res. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Behrad, S.; Yusof, M.; Goh, K.; Baba, A. Manipulation of probiotics fermentation of yogurt by cinnamon and licorice: Effects on yogurt formation and inhibition of Helicobacter pylori growth in vitro. World Acad. Sci. Eng. Technol. 2009, 60, 590–594. [Google Scholar]
- Kang, S.; Guo, Y.; Rao, J.; Jin, H.; You, H.J.; Ji, G.E. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus plantarum pH3A, monolaurin, and grapefruit seed extract. Food Funct. 2021, 12, 11024–11032. [Google Scholar] [CrossRef]
- Sadeghi, A.R.; Pourahmad, R.; Mokhtare, M. Enrichment of probiotic yogurt with broccoli sprout extract and its effect on Helicobacter pylori. Appl. Food Biotechnol. 2017, 4, 55–59. [Google Scholar]
- Figer, B.; Pissurlenkar, R.; Ambre, P.; Kalekar, S.; Munshi, R.; Gatne, M.; Shirsat, V. Treatment of gastric ulcers with fenugreek seed extract; in vitro, in vivo and in silico approaches. Indian J. Pharm. Sci. 2017, 79, 724–730. [Google Scholar] [CrossRef]
- Pandian, R.S.; Anuradha, C.; Viswanathan, P. Gastroprotective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J. Ethnopharmacol. 2002, 81, 393–397. [Google Scholar] [CrossRef]
- Tan, P.V.; Boda, M.; Etoa, F.-X. In vitro and in vivo anti-Helicobacter/Campylobacter activity of the aqueous extract of Enantia chlorantha. Pharm. Biol. 2010, 48, 349–356. [Google Scholar] [CrossRef]
- Lesbros-Pantoflickova, D.; Corthesy-Theulaz, I.; Blum, A.L. Helicobacter pylori and probiotics. J. Nutr. 2007, 137, 812S–818S. [Google Scholar] [CrossRef]
- Michetti, P.; Dorta, G.; Wiesel, P.; Brassart, D.; Verdu, E.; Herranz, M.; Felley, C.; Porta, N.; Rouvet, M.; Blum, A. Effect of whey-based culture supernatant of Lactobacillus acidophilus (johnsonii) La1 on Helicobacter pylori infection in humans. Digestion 1999, 60, 203–209. [Google Scholar] [CrossRef]
- Coconnier, M.-H.; Lievin, V.; Hemery, E.; Servin, A.L. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl. Environ. Microbiol. 1998, 64, 4573–4580. [Google Scholar] [CrossRef]
- Bernet, M.-F.; Brassart, D.; Neeser, J.-R.; Servin, A. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 1994, 35, 483–489. [Google Scholar] [CrossRef]
- Nasr, N.M.; Khider, M.; Metry, W.; Atallah, K. Antibacterial Activity of Lactic Acid Bacteria against Helicobacter pylori Evidence by in vivo and in vitro Studies. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 4235–4247. [Google Scholar] [CrossRef]





| Probiotic Strains | Source | Origin |
|---|---|---|
| Lactobacillus acidophilus | Local dairy product | Laboratory of Sciences and Technics of Animal Production, Mostaganem, Algeria |
| Limosilactobacillusfermentum | Local dairy product | |
| Lactiplantibacillusplantarum | Local dairy product | |
| Lacticaseibacilluscasei | Local dairy product | |
| Lactococcus lactis | Local dairy product | |
| Streptococcus thermophilus | Local dairy product | |
| Lacticaseibacillusrhamnosus LA180 | LACTIBIANE | Pharmacy, Lille, France |
| Lacticaseibacillusrhamnosus GG | PROBIOLOG | |
| Lactobacillus helviticus | LAXATRANSIT | |
| Bifidobacterium longum | BENEFLORA | |
| Bifidobacterium bifidum | BENEFLORA | |
| Bifidobacterium breve | Local dairy product | Laboratory of Natural and Local Bioresources, University of Hassiba Benbouali, Chlef-Algeria |
| DIZ (mm) | Garlic Extract | Onion Extract | Cumin Extract | Fenugreek Extract | |
|---|---|---|---|---|---|
| Concentrations (µg) | |||||
| 10 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | |
| 20 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | |
| 30 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | |
| 40 | 6.67 ± 0.58 | 6.67 ± 0.58 | 6.67 ± 0.58 | 6.67 ± 0.58 | |
| 50 | 7.00 ± 0.00 | 7.00 ± 0.00 | 7.00 ± 0.00 | 7.00 ± 0.00 | |
| 60 | 7.33 ± 0.58 | 7.33 ± 0.58 | 7.33 ± 0.58 | 7.33 ± 0.58 | |
| 70 | 7.67 ± 0.58 | 8.00 ± 0.00 | 8.00 ± 0.00 | 8.33 ± 0.58 | |
| 80 | 8.00 ± 0.00 | 8.33 ± 0.58 | 8.67 ± 0.58 | 9.00 ± 0.00 | |
| 90 | 8.33 ± 0.58 | 8.67 ± 0.58 | 9.33 ± 0.58 | 9.67 ± 0.58 | |
| 100 | 9.00 ± 0.00 | 7.79 ± 0.58 | 10.33 ± 0.58 | 10.67 ± 0.58 | |
| 150 | 9.67 ± 0.58 | 10.67 ± 0.58 | 11.00 ± 1.00 | 11.33 ± 1.15 | |
| 250 | 10.33 ± 0.58 | 11.33 ± 0.58 | 12.33 ± 0.58 | 12.67 ± 0.58 | |
| 500 | 11.67 ± 0.58 | 12.67 ± 0.58 | 14.33 ± 0.58 | 14.67 ± 0.58 | |
| 1000 | 13.33 ± 0.58 | 14.67 ± 0.58 | 15.67 ± 0.58 | 16.00 ± 0.00 | |
| Plant Extract | Garlic | Onion | Cumin | Fenugreek |
|---|---|---|---|---|
| MIC (µg/mL) | 500 | 250 | 150 | 100 |
| MBC (µg/mL) | 1000 | 500 | 250 | 150 |
| Probiotic Strains | DIZ (mm) |
|---|---|
| B. breve | 20.33 ± 0.58 |
| B. bifidum | 12.67 ± 0.58 |
| B. longum | 11.33 ± 0.58 |
| L. rhamnosus LA80 | 10.67 ± 0.58 |
| L. rhamnosus GG | 10.67 ± 0.58 |
| L. helviticus | 10.00 ± 0.00 |
| L. lactis | 10.67 ± 0.58 |
| S. thermophilus | 10.33 ± 0.58 |
| L. plantarum | 10.67 ± 0.58 |
| L. acidophilus | 10.67 ± 0.58 |
| L. fermentum | 10.33 ± 0.58 |
| L. casei | 10.00 ± 0.00 |
| DIZ (mm) | Garlic | Onion | Cumin | Fenugreek | |
|---|---|---|---|---|---|
| Probiotic Strains | |||||
| B. breve | 22.67 ± 0.58 | 24.67 ± 0.58 | 26.67 ± 0.58 | 28.67 ± 0.58 | |
| B. bifidum | 14.33 ± 0.58 | 15.67 ± 0.58 | 16.33 ± 0.58 | 17.67 ± 0.58 | |
| B. longum | 13.67 ± 0.58 | 14.33 ± 0.58 | 15.33 ± 0.58 | 16.33 ± 0.58 | |
| L. rhamnosusLA80 | 11.67 ± 0.58 | 12.67 ± 0.58 | 13.67 ± 0.58 | 14.33 ± 0.58 | |
| L. rhamnosusLA80GG | 11.67 ± 0.58 | 12.00 ± 0.00 | 12.33 ± 0.58 | 13.00 ± 0.00 | |
| L. helveticus | 11.00 ± 0.00 | 11.67 ± 0.58 | 12.00 ± 0.00 | 12.33 ± 0.58 | |
| L. lactis | 12.00 ± 0.00 | 12.33 ± 0.58 | 12.67 ± 0.58 | 13.67 ± 0.58 | |
| S. thermophilus | 11.67 ± 0.58 | 12.33 ± 0.58 | 13.33 ± 0.58 | 13.67 ± 0.58 | |
| L. plantarum | 11.33 ± 0.58 | 12.33 ± 0.58 | 13.00 ± 0.00 | 13.67 ± 0.58 | |
| L. acidophilus | 11.00 ± 0.00 | 11.67 ± 0.58 | 12.00 ± 0.00 | 12.67 ± 0.58 | |
| L. fermentum | 11.33 ± 0.58 | 12.33 ± 0.58 | 13.00 ± 0.00 | 13.33 ± 0.58 | |
| L. casei | 11.33 ± 0.58 | 12.33 ± 0.58 | 13.33 ± 0.58 | 13.67 ± 0.58 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasna, B.; Houari, H.; Koula, D.; Marina, S.; Emilia, U.; Assia, B. In Vitro and In Vivo Study of Combined Effect of Some Algerian Medicinal Plants and Probiotics against Helicobacter pylori. Microorganisms 2023, 11, 1242. https://doi.org/10.3390/microorganisms11051242
Hasna B, Houari H, Koula D, Marina S, Emilia U, Assia B. In Vitro and In Vivo Study of Combined Effect of Some Algerian Medicinal Plants and Probiotics against Helicobacter pylori. Microorganisms. 2023; 11(5):1242. https://doi.org/10.3390/microorganisms11051242
Chicago/Turabian StyleHasna, Bouhenni, Hemida Houari, Doukani Koula, Spinu Marina, Ungureanu Emilia, and Boumezrag Assia. 2023. "In Vitro and In Vivo Study of Combined Effect of Some Algerian Medicinal Plants and Probiotics against Helicobacter pylori" Microorganisms 11, no. 5: 1242. https://doi.org/10.3390/microorganisms11051242
APA StyleHasna, B., Houari, H., Koula, D., Marina, S., Emilia, U., & Assia, B. (2023). In Vitro and In Vivo Study of Combined Effect of Some Algerian Medicinal Plants and Probiotics against Helicobacter pylori. Microorganisms, 11(5), 1242. https://doi.org/10.3390/microorganisms11051242








