Comprehensive Bioinformatics Analysis of the Biodiversity of Lsm Proteins in the Archaea Domain
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria for Lsm Proteins from the Archaea Domain
2.2. Prediction of Physicochemical Properties
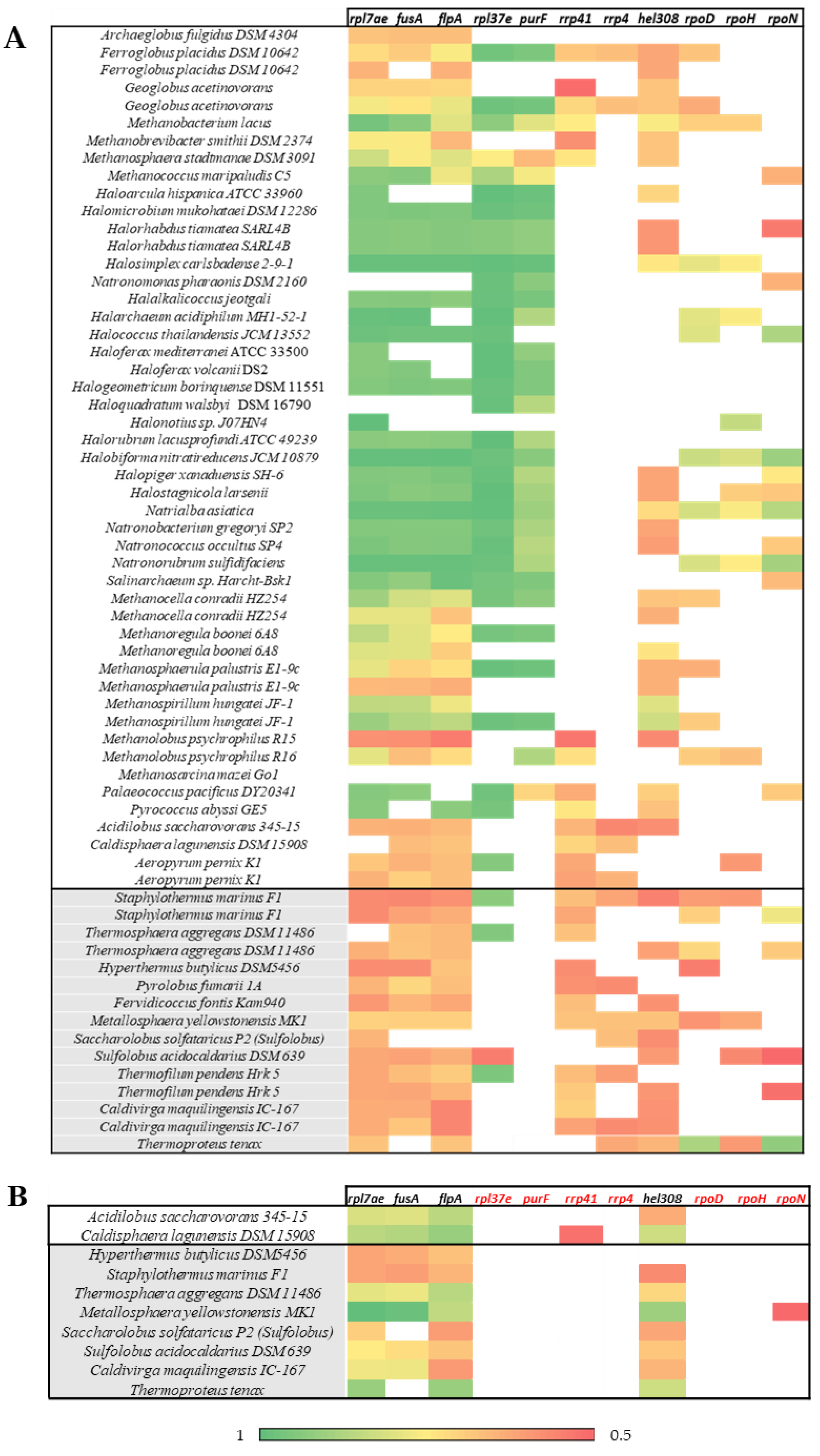
2.3. Gene-Environment Analysis
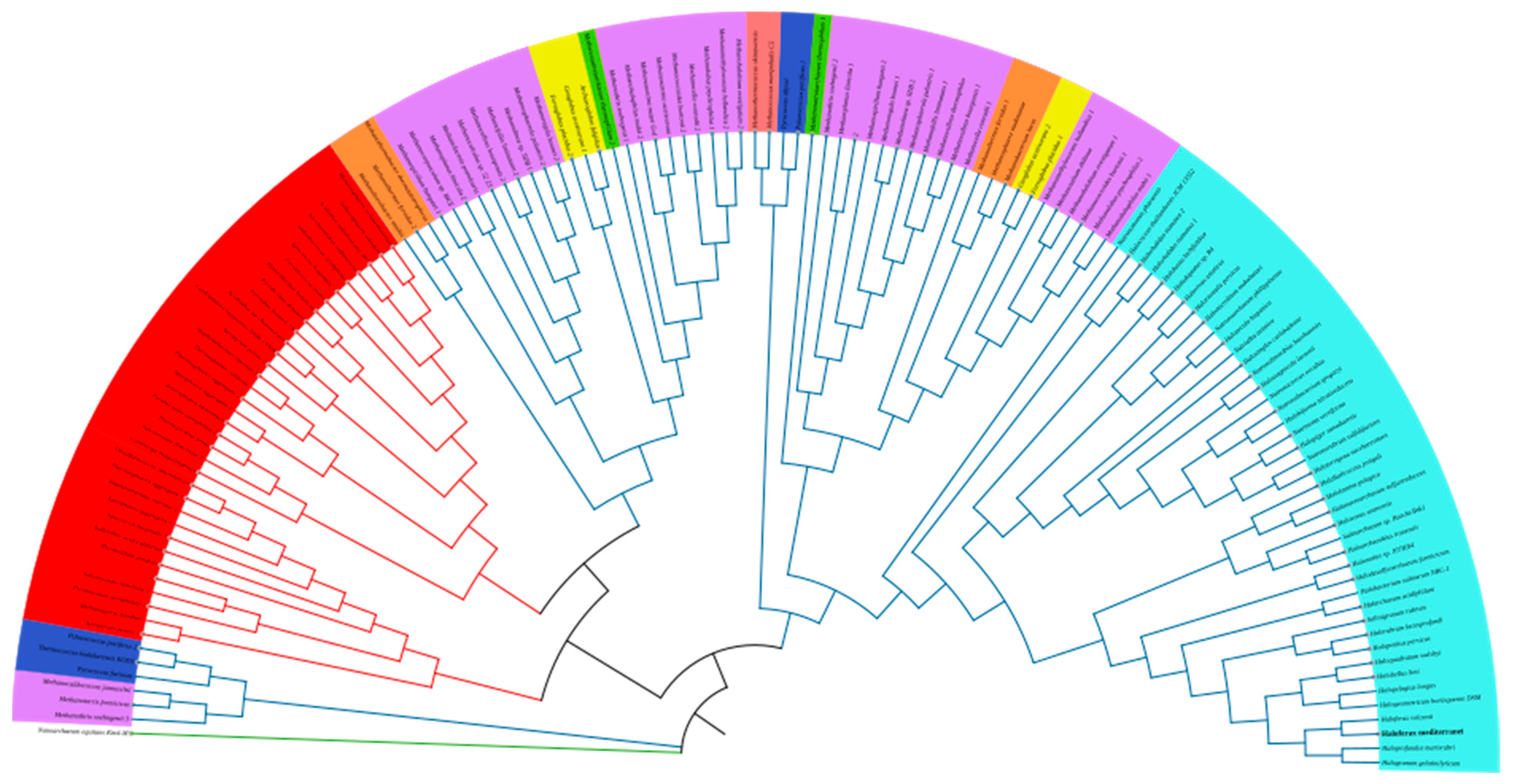
2.4. Phylogenetic Analysis of the Lsm Proteins of the Archaea Domain
2.5. Multiple Alignments of Lsm Protein Sequences
2.6. Analysis of Protein–Protein Interaction Networks of Lsm Proteins
3. Results and Discussion
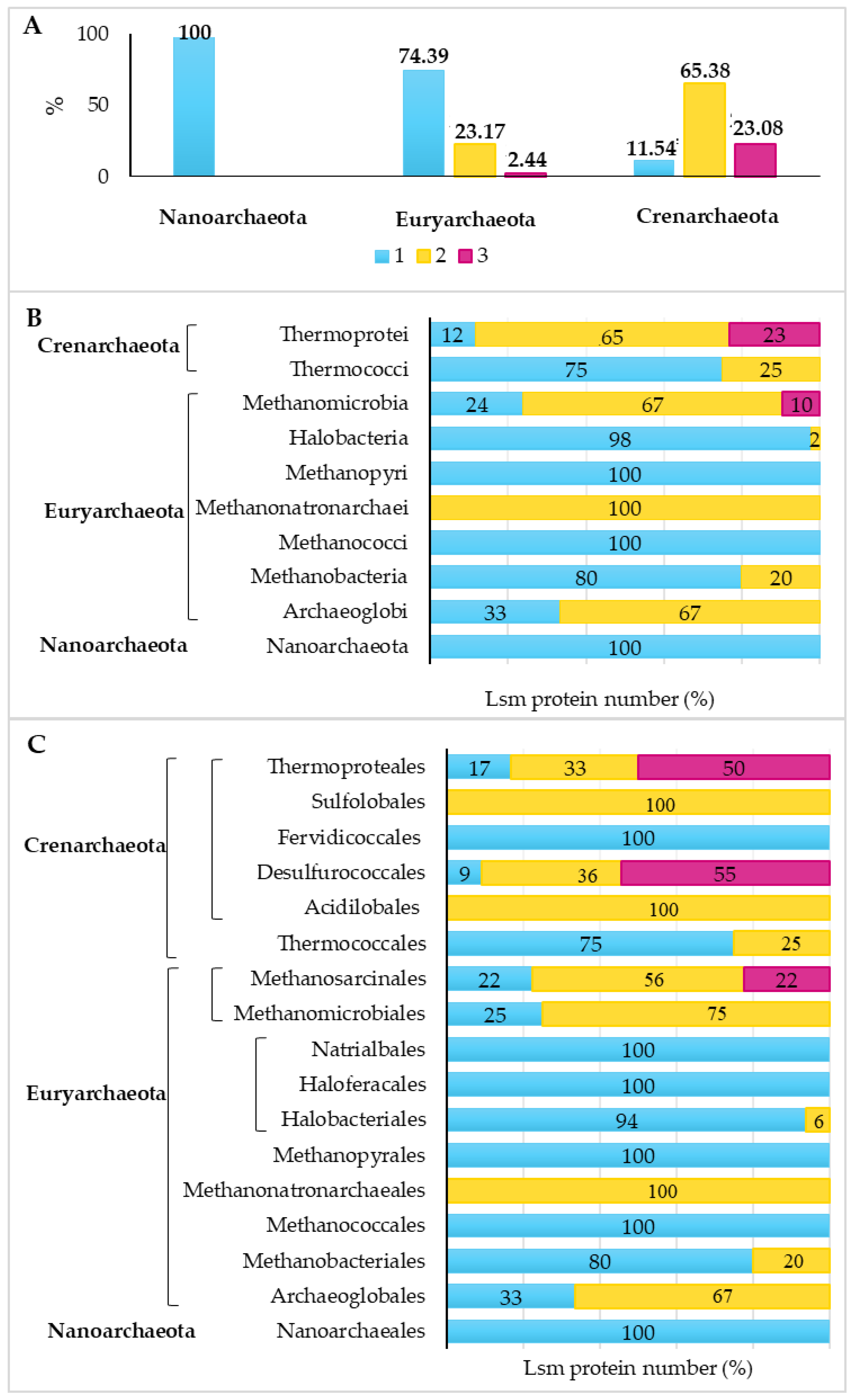
3.1. Selected Lsm Proteins and Their Distribution in the Archaea Domain
3.2. Prediction of Physicochemical Properties
3.3. Gene-Environment Analysis
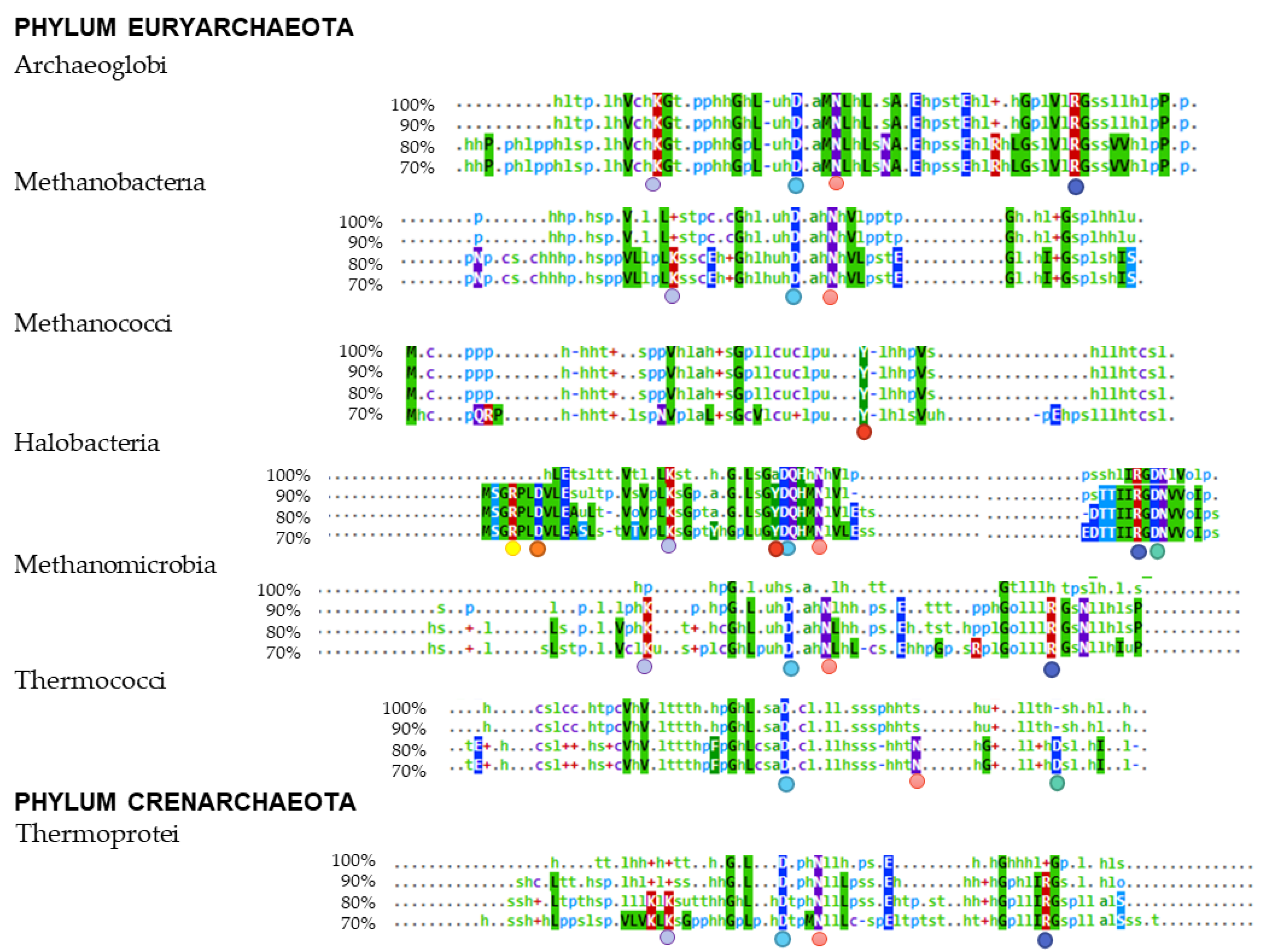
3.4. Multiple Alignments of Lsm Protein Sequences
3.5. Phylogenetic Analysis of the Lsm Proteins of the Archaea Domain
3.6. Analysis of Protein–Protein Interaction Networks of Lsm Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Séraphin, B. Sm and Sm-like proteins belong to a large family: Identification of proteins of the U6 as well as the U1, U2, U4 and U5 snRNPs. EMBO J. 1995, 14, 2089–2098. [Google Scholar] [CrossRef]
- Scofield, D.G.; Lynch, M. Evolutionary diversification of the Sm family of RNA-associated proteins. Mol. Biol. Evol. 2008, 25, 2255–2267. [Google Scholar] [CrossRef]
- Vogel, J.; Luisi, B.F. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 2011, 9, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Sauter, C.; Basquin, J.; Suck, D. Sm-like proteins in Eubacteria: The crystal structure of the Hfq protein from Escherichia coli. Nucleic Acids Res. 2003, 31, 4091–4098. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; Bøggild, A.; Andersen, C.B.; Nielsen, G.; Boysen, A.; Brodersen, D.E.; Valentin-Hansen, P. An Hfq-like protein in archaea: Crystal structure and functional characterization of the Sm protein from Methanococcus jannaschii. RNA 2007, 13, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Valentin-Hansen, P.; Eriksen, M.; Udesen, C. The bacterial Sm-like protein Hfq: A key player in RNA transactions. Mol. Microbiol. 2004, 51, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, C.; Wilusz, J. Eukaryotic Lsm proteins: Lessons from bacteria. Nat. Struct. Mol. Biol. 2005, 12, 1031–1036. [Google Scholar] [CrossRef]
- Törö, I.; Thore, S.; Mayer, C.; Basquin, J.; Séraphin, B.; Suck, D. RNA binding in an Sm core domain: X-ray structure and functional analysis of an archaeal Sm protein complex. EMBO J. 2001, 20, 2293–2303. [Google Scholar] [CrossRef]
- Kazimierz, T.T.; Nikolay, G.K.; Nicholas, K.C.; Victor, F.; Steitz, J.A. The evergrowing world of small nuclear ribonucleoproteins. In The RNA World, 3rd ed.; Cold Spring Harbor Monographs; Cold Spring Harbor Laboratory Press: Plainview, NY, USA, 2006; Volume 43, pp. 327–368. [Google Scholar]
- Salgado-Garrido, J.; Bragado-Nilsson, E.; Kandels-Lewis, S.; Séraphin, B. Sm and Sm-like proteins assemble in two related complexes of deep evolutionary origin. EMBO J. 1999, 18, 3451–3462. [Google Scholar] [CrossRef]
- Mura, C.; Randolph, P.S.; Patterson, J.; Cozen, A.E. Archaeal and eukaryotic homologs of Hfq: A structural and evolutionary perspective on Sm function. RNA Biol. 2013, 10, 636–651. [Google Scholar] [CrossRef]
- Törö, I.; Basquin, J.; Teo-Dreher, H.; Suck, D. Archaeal Sm proteins form heptameric and hexameric complexes: Crystal structures of the Sm1 and Sm2 proteins from the hyperthermophile Archaeoglobus fulgidus. J. Mol. Biol. 2002, 320, 129–142. [Google Scholar] [CrossRef]
- Collins, B.M.; Harrop, S.J.; Kornfeld, G.D.; Dawes, I.W.; Curmi, P.M.; Mabbutt, B.C. Crystal structure of a heptameric Sm-like protein complex from archaea: Implications for the structure and evolution of snRNPs. J. Mol. Biol. 2001, 309, 915–923. [Google Scholar] [CrossRef]
- Mura, C.; Cascio, D.; Sawaya, M.R.; Eisenberg, D.S. The crystal structure of a heptameric archaeal Sm protein: Implications for the eukaryotic snRNP core. Proc. Natl. Acad. Sci. USA 2001, 98, 5532–5537. [Google Scholar] [CrossRef] [PubMed]
- Franze de Fernandez, M.T.; Eoyang, L.; August, J.T. Factor fraction required for the synthesis of bacteriophage Qbeta-RNA. Nature 1968, 219, 588–590. [Google Scholar] [CrossRef]
- Carmichael, G.G.; Weber, K.; Niveleau, A.; Wahba, A.J. The host factor required for RNA phage Qbeta RNA replication in vitro. Intracellular location, quantitation, and purification by polyadenylate-cellulose chromatography. J. Biol. Chem. 1975, 250, 3607–3612. [Google Scholar] [CrossRef] [PubMed]
- Schuppli, D.; Miranda, G.; Tsui, H.C.; Winkler, M.E.; Sogo, J.M.; Weber, H. Altered 3’-terminal RNA structure in phage Qbeta adapted to host factor-less Escherichia coli. Proc. Natl. Acad. Sci. USA 1997, 94, 10239–10242. [Google Scholar] [CrossRef]
- Wassarman, K.M.; Repoila, F.; Rosenow, C.; Storz, G.; Gottesman, S. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 2001, 15, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Fando, M.S.; Mikhaylina, A.O.; Lekontseva, N.V.; Tishchenko, S.V.; Nikulin, A.D. Structure and RNA-Binding Properties of Lsm Protein from Halobacterium salinarum. Biochemistry 2021, 86, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Benz, J.; Späth, B.; Maier, L.K.; Straub, J.; Granzow, M.; Raabe, M.; Urlaub, H.; Hoffmann, J.; Brutschy, B.; et al. The archaeal Lsm protein binds to small RNAs. J. Biol. Chem. 2010, 285, 34429–34438. [Google Scholar] [CrossRef]
- Märtens, B.; Bezerra, G.A.; Kreuter, M.J.; Grishkovskaya, I.; Manica, A.; Arkhipova, V.; Djinovic-Carugo, K.; Bläsi, U. The Heptameric SmAP1 and SmAP2 Proteins of the Crenarchaeon Sulfolobus solfataricus Bind to Common and Distinct RNA Targets. Life 2015, 5, 1264–1281. [Google Scholar] [CrossRef]
- Märtens, B.; Hou, L.; Amman, F.; Wolfinger, M.T.; Evguenieva-Hackenberg, E.; Bläsi, U. The SmAP1/2 proteins of the crenarchaeon Sulfolobus solfataricus interact with the exosome and stimulate A-rich tailing of transcripts. Nucleic Acids Res. 2017, 45, 7938–7949. [Google Scholar] [CrossRef] [PubMed]
- Payá, G.; Bautista, V.; Camacho, M.; Bonete, M.J.; Esclapez, J. Functional analysis of Lsm protein under multiple stress conditions in the extreme haloarchaeon Haloferax mediterranei. Biochimie 2021, 187, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Brazma, A.; Jonassen, I.; Vilo, J.; Ukkonen, E. Predicting gene regulatory elements in silico on a genomic scale. Genome Res. 1998, 8, 1202–1215. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, 480–489. [Google Scholar] [CrossRef]
- Gill, S.C.; Von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Guruprasad, K.; Reddy, B.B.; Pandit, M.W. Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. Des. Sel. 1990, 4, 155–161. [Google Scholar] [CrossRef]
- Ikai, A. Thermostability and aliphatic index of globular proteins. J. Biochem. 1980, 88, 1895–1898. [Google Scholar]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Drozdetskiy, A.; Cole, C.; Procter, J.; Barton, G.J. JPred4: A protein secondary structure prediction server. Nucleic Acids Res. 2015, 43, 389–394. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539–544. [Google Scholar] [CrossRef]
- Soppa, J.; Baumann, A.; Brenneis, M.; Dambeck, M.; Hering, O.; Lange, C. Genomics and functional genomics with haloarchaea. Arch. Microbiol. 2008, 190, 197–215. [Google Scholar] [CrossRef]
- Oren, A. Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Syst. 2008, 4, 2. [Google Scholar] [CrossRef]
- Palanca, C.; Pedro-Roig, L.; Llácer, J.L.; Camacho, M.; Bonete, M.-J.; Rubio, V. The structure of a PII signaling protein from a halophilic archaeon reveals novel traits and high-salt adaptations. FEBS J. 2014, 281, 3299–3314. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.L.; Baker, P.J.; Fisher, M.; Ruzheinikov, S.; Gilmour, D.J.; Bonete, M.-J.; Ferrer, J.; Pire, C.; Esclapez, J.; Rice, D.W. Analysis of protein solvent interactions in glucose dehydrogenase from the extreme halophile Haloferax mediterranei. Proc. Natl. Acad. Sci. USA 2006, 103, 4846–4851. [Google Scholar] [CrossRef] [PubMed]
- Amblee, V.; Jeffery, C.J. Physical Features of Intracellular Proteins that Moonlight on the Cell Surface. PLoS ONE 2015, 10, e0130575. [Google Scholar] [CrossRef]
- Calvo, J.M.; Matthews, R.G. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol. Rev. 1994, 58, 466–490. [Google Scholar] [CrossRef]
- Deng, W.; Wang, H.; Xie, J. Regulatory and Pathogenesis Roles of Mycobacterium Lrp/AsnC Family Transcriptional Factors. J. Cell. Biochem. 2011, 112, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Kyrpides, N.C.; Ouzounis, C.A. The eubacterial transcriptional activator Lrp is present in the Archaeon Pyrococcus furiosus. Trends Biochem. Sci. 1995, 20, 140–141. [Google Scholar] [CrossRef]
- Kyrpides, N.C.; Ouzounis, C.A. Transcription in archaea. Proc. Natl. Acad. Sci. USA 1999, 96, 8545–8550. [Google Scholar] [CrossRef] [PubMed]
- Napoli, A.; Van der Oost, J.; Sensen, C.W.; Charlebois, R.L.; Rossi, M.; Ciaramella, M. An Lrp-like protein of the hyperthermophilic archaeon Sulfolobus solfataricus which binds to its own promoter. J. Bacteriol. 1999, 181, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Leonard, P.M.; Smits, S.H.J.; Sedelnikova, S.E.; Brinkman, A.B.; de Vos, W.M.; Van der Oost, J.; Rice, D.W.; Rafferty, J.B. Crystal structure of the Lrp-like transcriptional regulator from the archaeon Pyrococcus furiosus. EMBO J. 2001, 20, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Albers, S.V.; Vassart, A.; Driessen, A.J.M.; Charlier, D. Ss-LrpB, a transcriptional regulator from Sulfolobus solfataricus, regulates a gene cluster with a pyruvate ferredoxin oxidoreductaseencoding operon and permease genes. Mol. Microbiol. 2009, 71, 972–988. [Google Scholar] [CrossRef]
- Peeters, E.; Charlier, D. The Lrp family of transcription regulators in Archaea. Archaea 2010, 2010, 750457. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, R.; Schwarz, C.; Furtwängler, K.; Tarasov, V.; Wende, A.; Oesterhelt, D. Transcriptional control by two leucine-responsive regulatory proteins in Halobacterium salinarum R1. BMC Mol. Biol. 2010, 11, 40. [Google Scholar] [CrossRef]
- Matarredona, L.; Camacho, M.; García-Bonete, M.J.; Esquerra, B.; Zafrilla, B.; Esclapez, J.; Bonete, M.J. Analysis of Haloferax mediterranei Lrp Transcriptional Regulator. Genes 2021, 12, 802. [Google Scholar] [CrossRef]
- Matarredona, L.; Camacho, M.; Bautista, V.; Bonete, M.J.; Esclapez, J. Lrp as a potential transcriptional regulator involved in stress response in Haloferax mediterranei. Biochimie 2023, 209, 61–72. [Google Scholar] [CrossRef]
- Sulavik, M.C.; Gambino, L.F.; Miller, P.F. The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: Prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol. Med. 1995, 1, 436–446. [Google Scholar] [CrossRef]
- Deochand, D.K.; Grove, A. MarR family transcription factors: Dynamic variations on a common scaffold. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.J.; Moore, P.B.; Steitz, T.A. The roles of ribosomal proteins in the structure assembly, and evolution of the large ribosomal subunit. J. Mol. Biol. 2004, 340, 141–177. [Google Scholar] [CrossRef] [PubMed]
- Even, S.; Pellegrini, O.; Zig, L.; Labas, V.; Vinh, J.; Bréchemmier-Baey, D.; Putzer, H. Ribonucleases J1 and J2: Two novel endoribonucleases in B. subtilis with functional homology to E. coli RNase E. Nucleic Acids Res. 2005, 33, 2141–2152. [Google Scholar] [CrossRef]
- Dominski, Z.; Carpousis, A.J.; Clouet-d’Orval, B. Emergence of the β-CASP ribonucleases: Highly conserved and ubiquitous metallo-enzymes involved in messenger RNA maturation and degradation. Biochim. Biophys. Acta 2013, 1829, 532–551. [Google Scholar] [CrossRef]
- Clouet-d’Orval, B.; Rinaldi, D.; Quentin, Y.; Carpousis, A.J. Euryarchaeal beta-CASP proteins with homology to bacterial RNase J Have 5’- to 3’-exoribonuclease activity. J. Biol. Chem. 2010, 285, 17574–17583. [Google Scholar] [CrossRef]
- Lu, Z.J.; Markham, G.D. Enzymatic properties of S-adenosylmethionine synthetase from the archaeon Methanococcus jannaschii. J. Biol. Chem. 2002, 277, 16624–16631. [Google Scholar] [CrossRef] [PubMed]
- Thore, S.; Mayer, C.; Sauter, C.; Weeks, S.; Suck, D. Crystal structures of the Pyrococcus abyssi Sm core and its complex with RNA. Common features of RNA binding in archaea and eukarya. J. Biol. Chem. 2003, 278, 1239–1247. [Google Scholar] [CrossRef]
- Suryadi, J.; Tran, E.J.; Maxwell, E.S.; Brown, B.A. The crystal structure of the Methanocaldococcus jannaschii multifunctional L7Ae RNA-binding protein reveals an induced-fit interaction with the box C/D RNAs. Biochemistry 2005, 44, 9657–9672. [Google Scholar] [CrossRef]
- Atkinson, G.C.; Baldauf, S.L. Evolution of elongation factor G and the origins of mitochondrial and chloroplast forms. Mol. Biol. Evol. 2011, 28, 1281–1292. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef]
- Amiri, K.A. Fibrillarin-like proteins occur in the domain Archaea. J. Bacteriol. 1994, 176, 2124–2127. [Google Scholar] [CrossRef] [PubMed]
- Evguenieva-Hackenberg, E.; Hou, L.; Glaeser, S.; Klug, G. Structure and function of the archaeal exosome. Wiley Interdiscip. Rev. RNA 2014, 5, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovic, M.A.; Wurm, J.P.; Audin, M.J.; Schütz, S.; Sprangers, R. The Rrp4-exosome complex recruits and channels substrate RNA by a unique mechanism. Nat. Chem. Biol. 2017, 13, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Woodman, I.L.; Bolt, E.L. Molecular biology of Hel308 helicase in archaea. Biochem. Soc. Trans. 2009, 37, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Murakami, K.S. Archaeal RNA polymerase. Curr. Opin. Struct. Biol. 2009, 19, 724–731. [Google Scholar] [CrossRef] [PubMed]
 ) and percentage of positively charged residues (Arg + Lys) (
) and percentage of positively charged residues (Arg + Lys) ( ) (C); aliphatic index (D); and GRAVY (E), for each species (represented on the X-axis and listed in Table S1).
) (C); aliphatic index (D); and GRAVY (E), for each species (represented on the X-axis and listed in Table S1).
 ) and percentage of positively charged residues (Arg + Lys) (
) and percentage of positively charged residues (Arg + Lys) ( ) (C); aliphatic index (D); and GRAVY (E), for each species (represented on the X-axis and listed in Table S1).
) (C); aliphatic index (D); and GRAVY (E), for each species (represented on the X-axis and listed in Table S1).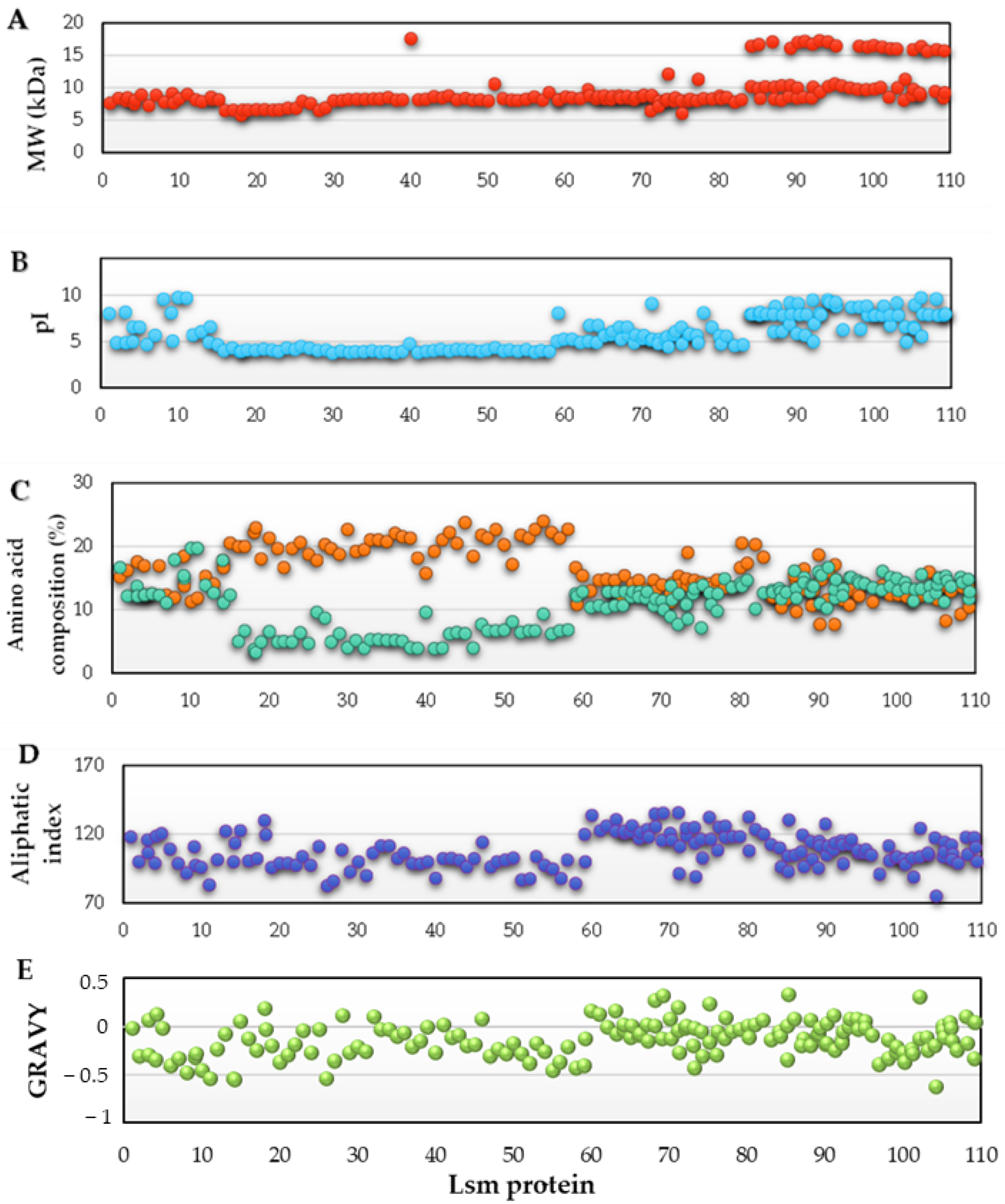
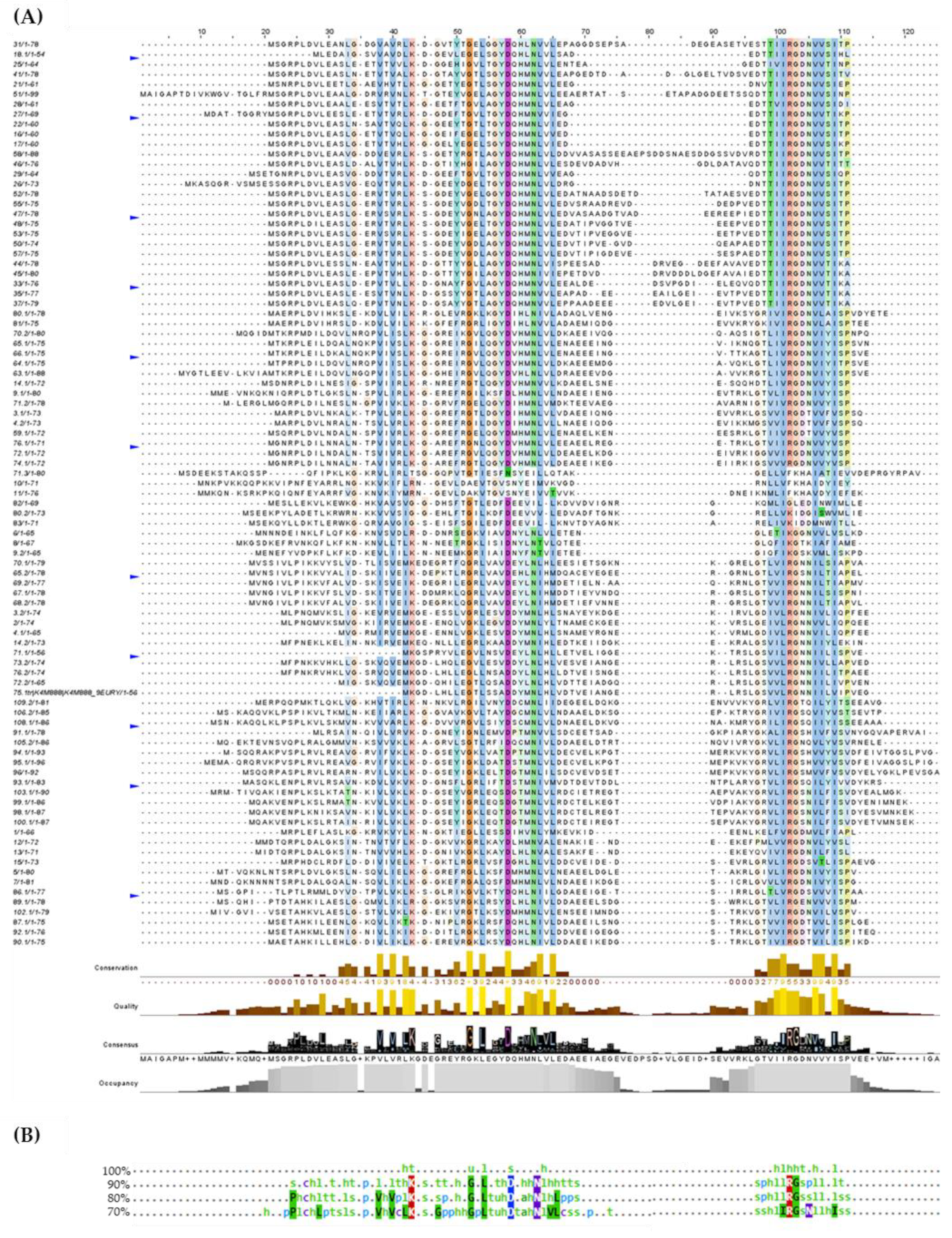
 ), Asp-7 (
), Asp-7 ( ), Tyr-34 (
), Tyr-34 ( ). The internal RNA binding site residues: Lys-22 (
). The internal RNA binding site residues: Lys-22 ( ), Asp-35 (
), Asp-35 ( ), Asn-39 (
), Asn-39 ( ), Arg-63 (
), Arg-63 ( ), and Asp-65 (
), and Asp-65 ( ).
).
 ), Asp-7 (
), Asp-7 ( ), Tyr-34 (
), Tyr-34 ( ). The internal RNA binding site residues: Lys-22 (
). The internal RNA binding site residues: Lys-22 ( ), Asp-35 (
), Asp-35 ( ), Asn-39 (
), Asn-39 ( ), Arg-63 (
), Arg-63 ( ), and Asp-65 (
), and Asp-65 ( ).
).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payá, G.; Bautista, V.; Camacho, M.; Esclapez, J.; Bonete, M.-J. Comprehensive Bioinformatics Analysis of the Biodiversity of Lsm Proteins in the Archaea Domain. Microorganisms 2023, 11, 1196. https://doi.org/10.3390/microorganisms11051196
Payá G, Bautista V, Camacho M, Esclapez J, Bonete M-J. Comprehensive Bioinformatics Analysis of the Biodiversity of Lsm Proteins in the Archaea Domain. Microorganisms. 2023; 11(5):1196. https://doi.org/10.3390/microorganisms11051196
Chicago/Turabian StylePayá, Gloria, Vanesa Bautista, Mónica Camacho, Julia Esclapez, and María-José Bonete. 2023. "Comprehensive Bioinformatics Analysis of the Biodiversity of Lsm Proteins in the Archaea Domain" Microorganisms 11, no. 5: 1196. https://doi.org/10.3390/microorganisms11051196
APA StylePayá, G., Bautista, V., Camacho, M., Esclapez, J., & Bonete, M.-J. (2023). Comprehensive Bioinformatics Analysis of the Biodiversity of Lsm Proteins in the Archaea Domain. Microorganisms, 11(5), 1196. https://doi.org/10.3390/microorganisms11051196








