Insights into Antimicrobial and Anti-Inflammatory Applications of Plant Bioactive Compounds
Abstract
:1. Introduction
2. Gut Microbiome as a Target of Aromatic Plant Bioactive Derivatives
2.1. Gut Microbiome in Brief
2.2. The Most Known Actions of Medicinal Plants on Intestinal Microbiota
2.3. The Influence of the Drastic Substances on Immune Response
3. Herbal Plants and Antimicrobial–Antiviral Activity
3.1. Mode of Action
3.2. Antimicrobial Resistance (AMR)—The “Superbugs”
3.3. Examples of Plant Derivatives and Their Targets
4. Anti-Inflammatory Applications of Herbal Compounds
4.1. Inflammation as the Hidden Flame in a Variety of Diseases
4.2. EOs as an Alternative Weapon against Inflammatory Pathologies (IBD, TDM2, Obesity)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- An, X.; Bao, Q.; Di, S.; Zhao, Y.; Zhao, S.; Zhang, H.; Lian, F.; Tong, X. The interaction between the gut Microbiota and herbal medicines. Biomed. Pharmacother. 2019, 118, 109252. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, P.; Sharma, R.; Gupta, G.; Chaudhary, A. Immunomodulators: Role of medicinal plants in immune system. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 1. [Google Scholar] [CrossRef]
- Wadood, A.; Ghufran, M.; Jamal, S.B.; Naeem, M.; Khan, A.; Ghaffar, R.; Asnad. Phytochemical Analysis of Medicinal Plants Occurring in Local Area of Mardan. Biochem. Anal. Biochem. 2013, 2, 1–4. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.; Stavropoulou, E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe 2011, 17, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Voidarou, C.; Konstantinidis, T.; Bezirtzoglou, E. Unraveling the Interconnection Patterns across Lung Microbiome, Respiratory Diseases, and COVID-19. Front. Cell. Infect. Microbiol. 2021, 10, 619075. [Google Scholar] [CrossRef]
- Chao, Y.-X.; Gulam, M.Y.; Chia, N.S.J.; Feng, L.; Rotzschke, O.; Tan, E.-K. Gut–Brain Axis: Potential Factors Involved in the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2020, 11, 849. [Google Scholar] [CrossRef]
- Wilasrusmee, C.; Siddiqui, J.; Bruch, D.; Wilasrusmee, S.; Kittur, S.; Kittur, D.S. In Vitro Immunomodulatory Effects of Herbal Products. Am. Surg. 2002, 68, 860–864. [Google Scholar] [CrossRef]
- Sultan, M.T.; Buttxs, M.S.; Qayyum, M.M.N.; Suleria, H.A.R. Immunity: Plants as Effective Mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H. The gut microbiota and host innate immunity: Regulators of host metabolism and metabolic diseases in poultry? J. Appl. Poult. Res. 2013, 22, 637–646. [Google Scholar] [CrossRef]
- Baccala, R.; Gonzalez-Quintial, R.; Lawson, B.R.; Stern, M.E.; Kono, D.H.; Beutler, B.; Theofilopoulos, A.N. Sensors of the innate immune system: Their mode of action. Nat. Rev. Rheumatol. 2009, 5, 448–456. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, S.T.; Betancur, J.G. Viral Recognition by the Innate Immune System: The Role of Pattern Recognition Receptors. Colomb. Med. 2010, 41, 377–387. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Pircalabioru, G.G.; Bezirtzoglou, E. The Role of Cytochromes P450 in Infection. Front. Immunol. 2018, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Enwa, F.O. Mechanisms of Antimicrobial Actions of Phytochemicals against Enteric Pathogens Review. Res. J. Pharm. Biol. Chem. Sci. 2014, 2, 77–85. [Google Scholar]
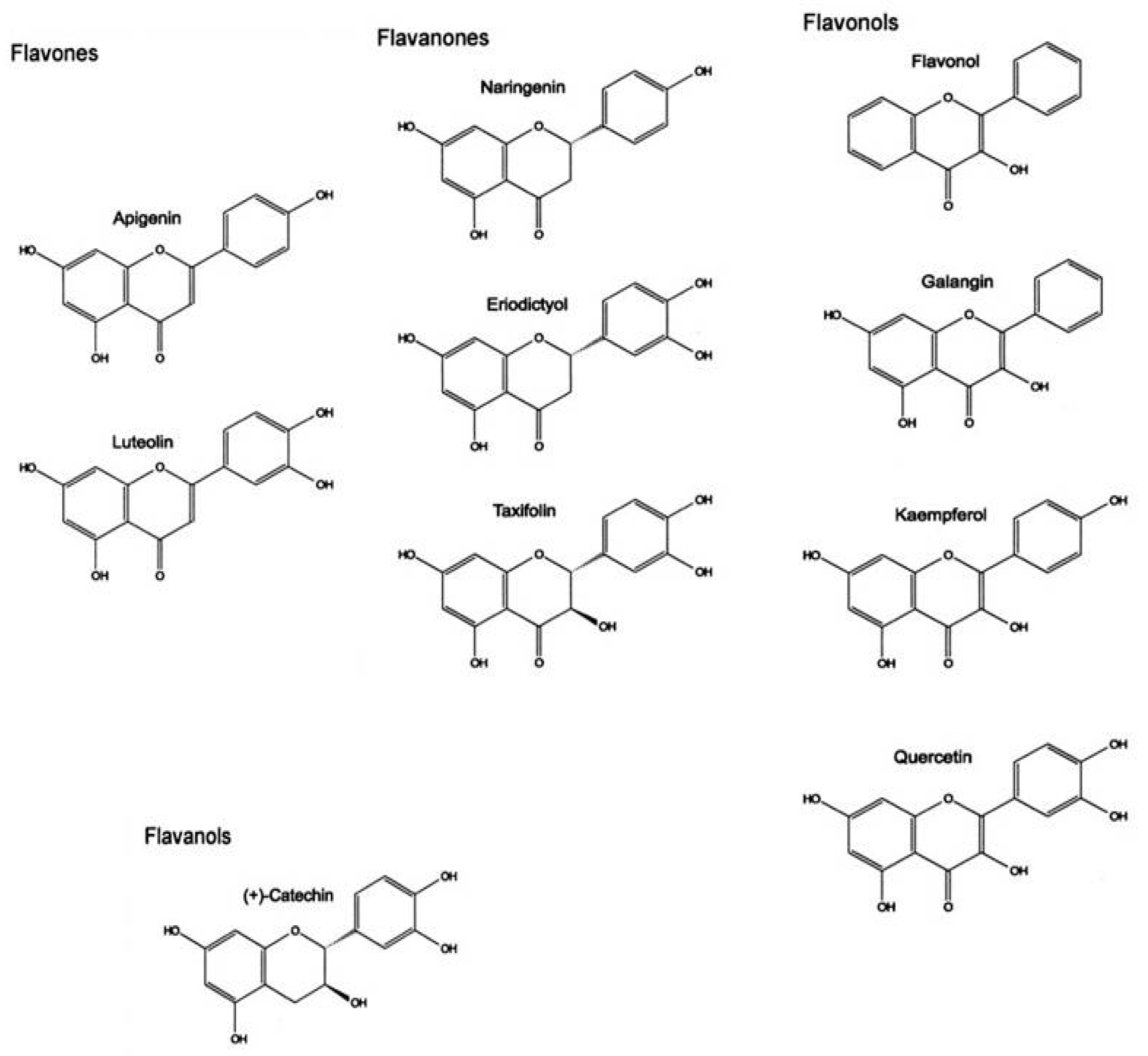
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Kar, A. Pharmacognosy and Pharmacobiotechnology; New Age International Publishers: New Delhi, India, 2007. [Google Scholar]
- Enwa, F. A Review on the A Review on the Phytochemical Profile and the Antibacterial Susceptibility Pattern of Some Clinical Isolates to the Ethanolic Leaves Extract of Moringa oleifera LAM (Moringaceae). Int. J. Adv. Res. 2013, 1, 226–238. [Google Scholar]
- Pérez-Rodríguez, F.; Taban, B.M. A State-of-Art Review on Multi-Drug Resistant Pathogens in Foods of Animal Origin: Risk Factors and Mitigation Strategies. Front. Microbiol. 2019, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Thanner, S.; Drissner, D.; Walsh, F. Antimicrobial Resistance in Agriculture. Mbio 2016, 7, e02227-15. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
- Nüesch-Inderbinen, M.; Zurfluh, K.; Peterhans, S.; Hächler, H.; Stephan, R. Assessment of the Prevalence of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Ready-to-Eat Salads, Fresh-Cut Fruit, and Sprouts from the Swiss Market. J. Food Prot. 2015, 78, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, K.; Helmke, K.; Hölzel, C.S.; Bauer, J. Antibiotic resistance in bacteria isolated from vegetables with regards to the marketing stage (farm vs. supermarket). Int. J. Food Microbiol. 2011, 148, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ruimy, R.; Brisabois, A.; Bernede, C.; Skurnik, D.; Barnat, S.; Arlet, G.; Momcilovic, S.; Elbaz, S.; Moury, F.; Vibet, M.-A.; et al. Organic and conventional fruits and vegetables contain equivalent counts of Gram-negative bacteria expressing resistance to antibacterial agents. Environ. Microbiol. 2010, 12, 608–615. [Google Scholar] [CrossRef]
- Bezanson, G.S.; MacInnis, R.; Potter, G.; Hughes, T. Presence and potential for horizontal transfer of antibiotic resistance in oxidase-positive bacteria populating raw salad vegetables. Int. J. Food Microbiol. 2008, 127, 37–42. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.; Alexopoulos, A.; Voidarou, C. Apparent antibiotic misuse in environmental ecosystems and food. Microb. Ecol. Health Dis. 2008, 20, 197–198. [Google Scholar] [CrossRef]
- Mora-Gamboa, M.P.C.; Rincón-Gamboa, S.M.; Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. Impact of Antibiotics as Waste, Physical, Chemical, and Enzymatical Degradation: Use of Laccases. Molecules 2022, 27, 4436. [Google Scholar] [CrossRef]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015, 26, 23289. [Google Scholar] [CrossRef] [PubMed]
- Koutroumanidou, E.; Kimbaris, A.; Kortsaris, A.; Bezirtzoglou, E.; Polissiou, M.; Charalabopoulos, K.; Pagonopoulou, O. Increased Seizure Latency and Decreased Severity of Pentylenetetrazol-Induced Seizures in Mice after Essential Oil Administration. Epilepsy Res. Treat. 2013, 2013, 532657. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Setzer, W.N. Aromatic Herbs, Medicinal Plant-Derived Essential Oils, and Phytochemical Extracts as Potential Therapies for Coronaviruses: Future Perspectives. Plants 2020, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Renard-Nozaki, J.; Kim, T.; Imakura, Y.; Kihara, M.; Kobayashi, S. Effect of alkaloids isolated from Amaryllidaceae on herpes simplex virus. Res. Virol. 1989, 140, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Notka, F.; Meier, G.; Wagner, R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antivir. Res. 2004, 64, 93–102. [Google Scholar] [CrossRef]
- Clark, K.; Grant, P.; Sarr, A.; Belakere, J.; Swaggerty, C.; Phillips, T.; Woode, G. An in vitro study of theaflavins extracted from black tea to neutralize bovine rotavirus and bovine coronavirus infections. Veter. Microbiol. 1998, 63, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef]
- Li, Z.-H.; Cai, M.; Liu, Y.-S.; Sun, P.L.; Luo, S.-L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Plessas, S.; Kimbaris, A.; Varvatou, M.; Mantzourani, I.; Fournomiti, M.; Tzouti, V.; Nerantzaki, A.; Bezirtzoglou, E. Mode of Antimicrobial Action of Origanum vulgare Essential Oil against Clinical Pathogens. Curr. Res. Nutr. Food Sci. J. 2017, 5, 109–115. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.; Čmiková, N.; Kačániová, M. Thymus vulgaris Essential Oil and Its Biological Activity. Plants 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, B.A.; Noshad, M.; Falah, F. Cumin essential oil: Phytochemical analysis, antimicrobial activity and investigation of its mechanism of action through scanning electron microscopy. Microb. Pathog. 2019, 136, 103716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Z.; Li, G.; Fu, F.; Liang, Z.; Zhu, H.; Shan, Y. Antimicrobial and antibiofilm efficacy and mechanism of essential oil from Citrus Changshan-huyou Y. B. chang against Listeria monocytogenes. Food Control. 2019, 105, 256–264. [Google Scholar] [CrossRef]
- Xiang, F.; Bai, J.; Tan, X.; Chen, T.; Yang, W.; He, F. Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Ind. Crop. Prod. 2018, 125, 582–587. [Google Scholar] [CrossRef]
- Oliveira-Tintino, C.D.D.M.; Tintino, S.R.; Limaverde, P.W.; Figueredo, F.G.; Campina, F.F.; da Cunha, F.A.; da Costa, R.H.; Pereira, P.S.; Lima, L.F.; de Matos, Y.M.; et al. Inhibition of the essential oil from Chenopodium ambrosioides L. and α-terpinene on the NorA efflux-pump of Staphylococcus aureus. Food Chem. 2018, 262, 72–77. [Google Scholar] [CrossRef]
- Kovács, J.K.; Felső, P.; Horváth, G.; Schmidt, J.; Dorn, Á.; Ábrahám, H.; Cox, A.; Márk, L.; Emődy, L.; Kovács, T.; et al. Stress Response and Virulence Potential Modulating Effect of Peppermint Essential Oil in Campylobacter jejuni. BioMed Res. Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control. 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Chen, X.; Cui, H.; Lin, L. The Interference Mechanism of Basil Essential Oil on the Cell Membrane Barrier and Respiratory Metabolism of Listeria monocytogenes. Front. Microbiol. 2022, 13, 855905. [Google Scholar] [CrossRef]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A novel interpretation of the Fractional Inhibitory Concentration Index: The case Origanum vulgare L. and Leptospermum scoparium J. R. et G. Forst essential oils against Staphylococcus aureus strains. Microbiol Res. 2017, 195, 11–17. [Google Scholar] [CrossRef]
- György, É.; Laslo, É.; Hajnalka Kuzman, I.; Dezső András, C. The effect of essential oils and their combinations on bacteria from the surface of fresh vegetables. Food Sci. Nutr. 2020, 8, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The Biosynthesis of C5–C25 Terpenoid Compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. The 1-Deoxy-d-Xylulose-5-Phosphate Pathway of Isoprenoid Biosynthesis in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef] [PubMed]
- de Lavor, É.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.E.B.P.; de Oliveira Júnior, R.G.; Gama e Silva, M.; de Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araújo, M.T.; Coutinho, H.D.M.; et al. Essential Oils and Their Major Compounds in the Treatment of Chronic Inflammation: A Review of Antioxidant Potential in Preclinical Studies and Molecular Mechanisms. Oxid. Med. Cell. Longev. 2018, 2018, 6468593. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free Radicals, Oxidative Stress, and Antioxidants in Human Health and Disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Halliwell, B. What Nitrates Tyrosine? Is Nitrotyrosine Specific as a Biomarker of Peroxynitrite Formation In Vivo? FEBS Lett. 1997, 411, 157–160. [Google Scholar] [CrossRef]
- Chen, P.C.; Wheeler, D.S.; Malhotra, V.; Odoms, K.; Denenberg, A.G.; Wong, H.R. A Green Tea-Derived Polyphenol, Epigallocatechin-3-Gallate, Inhibits IκB Kinase Activation and IL-8 Gene Expression in Respiratory Epithelium. Inflammation 2002, 26, 233–241. [Google Scholar] [CrossRef]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular Pharmacology of Inflammation: Medicinal Plants as Anti-Inflammatory Agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef]
- Ejaz Ahmed, M.; Khan, M.M.; Javed, H.; Vaibhav, K.; Khan, A.; Tabassum, R.; Ashafaq, M.; Islam, F.; Safhi, M.M.; Islam, F. Amelioration of Cognitive Impairment and Neurodegeneration by Catechin Hydrate in Rat Model of Streptozotocin-Induced Experimental Dementia of Alzheimer’s Type. Neurochem. Int. 2013, 62, 492–501. [Google Scholar] [CrossRef]
- Kodydkova, J.; Vavrova, L.; Stankova, B.; Macasek, J.; Krechler, T.; Zak, A. Antioxidant Status and Oxidative Stress Markers in Pancreatic Cancer and Chronic Pancreatitis. Pancreas 2013, 42, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, J.J.; Xu, J.; Feng, F.; Qu, W. Medicinal Uses, Phytochemistry and Pharmacology of the Genus Uncaria. J. Ethnopharmacol. 2015, 173, 48–80. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Kelber, O.; Bauer, R.; Kubelka, W. Phytotherapy in Functional Gastrointestinal Disorders. Dig. Dis. 2017, 35 (Suppl. S1), 36–42. [Google Scholar] [CrossRef]
- Schilcher, H.; Kammerer, S.; Wegener, T. Leitfaden Phytotherapie, 4th ed.; Urban & Fischer in Elsevier: Munich, Germany, 2010. [Google Scholar]
- Bürger, M.; Lange, K.; Stallmach, A. Intestinales Mikrobiom und chronisch-entzündliche Darmerkrankungen: Feindschaft oder Freundschaft? Gastroenterologe 2015, 10, 87–101. [Google Scholar] [CrossRef]
- Thumann, T.A.; Pferschy-Wenzig, E.-M.; Moissl-Eichinger, C.; Bauer, R. The Role of Gut Microbiota for the Activity of Medicinal Plants Traditionally Used in the European Union for Gastrointestinal Disorders. J. Ethnopharmacol. 2019, 245, 112153. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, E.; Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Voidarou, C.; Mitropoulou, G.; Prapa, I.; Santarmaki, V.; Kompoura, V.; Yanni, A.E.; et al. Maintaining Digestive Health in Diabetes: The Role of the Gut Microbiome and the Challenge of Functional Foods. Microorganisms 2021, 9, 516. [Google Scholar] [CrossRef]
- Sell, H.; Habich, C.; Eckel, J. Adaptive Immunity in Obesity and Insulin Resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Reports on Diabetes. Available online: https://www.who.int/publications-detail-redirect/97 (accessed on 29 December 2021).
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the Gut Microbiota on Inflammation, Obesity, and Metabolic Disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Acharya, A.; Thakur, S.; Muddapur, M. Evaluation of Serum Interleukin-10 Levels as a Predictor of Glycemic Alteration in Chronic Periodontitis and Type 2 Diabetes Mellitus. J. Indian Soc. Periodontol. 2015, 19, 388. [Google Scholar] [CrossRef] [PubMed]
- Gérard, P. Gut Microbiota and Obesity. Cell. Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Kalekar, S.; Munshi, R.; Bhalerao, S.; Thatte, U. Insulin Sensitizing Effect of 3 Indian Medicinal Plants: An In Vitro Study. Indian J. Pharmacol. 2013, 45, 30. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. Medicinal Plants with Antidiabetic Effects—An Overview (Part 1). IOSRPHR 2019, 9, 9–46. [Google Scholar]
- Dong, H.; Wang, N.; Zhao, L.; Lu, F. Berberine in the Treatment of Type 2 Diabetes Mellitus: A Systemic Review and Meta-Analysis. Evid. Based. Complement. Alternat. Med. 2012, 2012, 591654. [Google Scholar] [CrossRef]
- Hajimonfarednejad, M.; Nimrouzi, M.; Heydari, M.; Zarshenas, M.M.; Raee, M.J.; Jahromi, B.N. Insulin Resistance Improvement by Cinnamon Powder in Polycystic Ovary Syndrome: A Randomized Double-Blind Placebo Controlled Clinical Trial. Phytother. Res. 2018, 32, 276–283. [Google Scholar] [CrossRef]
- Hamza, N.; Berke, B.; Cheze, C.; Agli, A.-N.; Robinson, P.; Gin, H.; Moore, N. Prevention of Type 2 Diabetes Induced by High Fat Diet in the C57BL/6J Mouse by Two Medicinal Plants Used in Traditional Treatment of Diabetes in the East of Algeria. J. Ethnopharmacol. 2010, 128, 513–518. [Google Scholar] [CrossRef]
- Jiang, B.; Le, L.; Zhai, W.; Wan, W.; Hu, K.; Yong, P.; He, C.; Xu, L.; Xiao, P. Protective Effects of Marein on High Glucose-Induced Glucose Metabolic Disorder in HepG2 Cells. Phytomedicine 2016, 23, 891–900. [Google Scholar] [CrossRef]
- Mata-Torres, G.; Andrade-Cetto, A.; Espinoza-Hernández, F.A.; Cárdenas-Vázquez, R. Hepatic Glucose Output Inhibition by Mexican Plants Used in the Treatment of Type 2 Diabetes. Front. Pharmacol. 2020, 11, 215. [Google Scholar] [CrossRef]
- Mao, Z.-J.; Lin, M.; Zhang, X.; Qin, L.-P. Combined Use of Astragalus Polysaccharide and Berberine Attenuates Insulin Resistance in IR-HepG2 Cells via Regulation of the Gluconeogenesis Signaling Pathway. Front. Pharmacol. 2019, 10, 1508. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, Z.; Huang, Y.-Y.; Zhuang, Z.-Z.; Jin, Y.; Ye, H.-Y.; Lin, X.-J.; Zheng, Q.; Wang, Y.-L. Protective Effect and Possible Mechanisms of Astragaloside IV in Animal Models of Diabetic Nephropathy: A Preclinical Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 988. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Tzeng, T.-F.; Chang, Y.-S.; Liu, I.-M. Beneficial Impact of Zingiber Zerumbeton Insulin Sensitivity in Fructose-Fed Rats. Planta Med. 2012, 78, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Eddouks, M.; Andrade-Cetto, A.; Heinrich, M.; De Feo, V.; Cho, W.C. Editorial: Mechanisms of Traditional Medicinal Plants Used to Control Type 2 Diabetes or Metabolic Syndrome. Front. Pharmacol. 2021, 11, 617018. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 29 December 2021).
- Haaz, S.; Fontaine, K.R.; Cutter, G.; Limdi, N.; Perumean-Chaney, S.; Allison, D.B. Citrus Aurantium and Synephrine Alkaloids in the Treatment of Overweight and Obesity: An Update. Obes. Rev. 2006, 7, 79–88. [Google Scholar] [CrossRef]
- Moro, C.O.; Basile, G. Obesity and Medicinal Plants. Fitoterapia 2000, 71, S73–S82. [Google Scholar] [CrossRef]
- Han, L.-K.; Takaku, T.; Li, J.; Kimura, Y.; Okuda, H. Anti-Obesity Action of Oolong Tea. Int. J. Obes. 1999, 23, 98–105. [Google Scholar] [CrossRef]
- de Freitas Junior, L.M.; de Almeida, E.B., Jr. Medicinal Plants for the Treatment of Obesity: Ethnopharmacological Approach and Chemical and Biological Studies. Am. J. Transl. Res. 2017, 9, 2050–2064. [Google Scholar]
- Shang, A.; Gan, R.-Y.; Xu, X.-Y.; Mao, Q.-Q.; Zhang, P.-Z.; Li, H.-B. Effects and Mechanisms of Edible and Medicinal Plants on Obesity: An Updated Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2061–2077. [Google Scholar] [CrossRef]
- Mhatre, S.; Srivastava, T.; Naik, S.; Patravale, V. Antiviral Activity of Green Tea and Black Tea Polyphenols in Prophylaxis and Treatment of COVID-19: A Review. Phytomedicine 2021, 85, 153286. [Google Scholar] [CrossRef]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef]
- Becker, K.; Schwaiger, S.; Waltenberger, B.; Fuchs, D.; Pezzei, C.K.; Schennach, H.; Stuppner, H.; Gostner, J.M. Immunomodulatory Effects of Diterpene Quinone Derivatives from the Roots of Horminum pyrenaicum in Human PBMC. Oxid. Med. Cell. Longev. 2018, 2018, 2980295. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Owais, F.; Anwar, S.; Saeed, F.; Muhammad, S.; Ishtiaque, S.; Mohiuddin, O. Analgesic, Anti-Inflammatory and Neuropharmacological Effects of Atropa belladonna. Pak. J. Pharm. Sci. 2014, 27, 2183–2187. [Google Scholar] [PubMed]
- Wickramasinghe, A.S.D.; Kalansuriya, P.; Attanayake, A.P. Herbal Medicines Targeting the Improved β-Cell Functions and β-Cell Regeneration for the Management of Diabetes Mellitus. Evid. Based. Complement. Alternat. Med. 2021, 2021, 2920530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV Derived from Astragalus Membranaceus: A Research Review on the Pharmacological Effects. In Pharmacological Advances in Natural Product Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–112. [Google Scholar]
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K. A Pilot, Randomized, Double-blind, Placebo-controlled Trial to Assess the Safety and Efficacy of a Novel Boswellia serrata Extract in the Management of Osteoarthritis of the Knee. Phytother. Res. 2019, 33, 1457–1468. [Google Scholar] [CrossRef]
- Srinivasan, K. Cumin (Cuminum cyminum) and Black Cumin (Nigella sativa) Seeds: Traditional Uses, Chemical Constituents, and Nutraceutical Effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Liu, L.L.; Liu, Q.; Li, P.; Liu, E.H. Discovery of synergistic anti-inflammatory compound combination from herbal formula GuGe FengTong Tablet. Chin. J. Nat. Med. 2018, 16, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Theanphong, O.; Somwong, P. Combination of selected Thai traditional pain relief medicinal plants with anti-inflammatory abilities in a protein denaturation assay. Pharmacia 2022, 69, 745–753. [Google Scholar] [CrossRef]

| Plant Species/Derivative | Microbial Species | Mode of Action | Reference |
|---|---|---|---|
| Citrus medica L. var. sarcodactylis EO | Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Micrococcus luteus | bacteria morphology, permeability of cell, and membrane integrity | [40] |
| Oreganum vulgare EO | 26 E.coli strains and 24 S. aureus strains | cell wall and cytoplamic membrane disruption | [41] |
| Thymus vulgaris EO | B. subtilis, E. faecalis, and S. aureus. Pseudomonas aeruginosa, Yersinia enterocolitica, Salmonella enterica subsp. enterica ser. Enteritidis, Serratia marcescens | changes in protein profile | [42] |
| Cumin EO | Escherichia coli, Listeria innocua | increase in the permeabilization of the cells and disruption of the membrane integrity | [43] |
| Cinnamomum zeylanycum EO | Escherichia coli, Staphylococcus aureus | release of intracellular contents with increased levels of nucleic acids and extracellular proteins | [44] |
| Citrus chagshan—huyou EO | Listeria monocytogenes | morphological changes, such as wrinkled and collapsing surfaces, cavities, and fragmented cells | [45] |
| Artemisia argyi EO | Staphylococcus aureus | increased permeability of the cytoplasmic membrane and the extravasation of soluble proteins and intracellular potassium ions | [46] |
| Chenopodium ambrosioides L.EO and a-terpinene | Staphylococcus aureus | efflux pump inhibition | [47] |
| Mentha piperita EO | Campylobacter jejuni | elevation in the expression of general stress genes such as dnaK, groEL, and groES | [48] |
| Clove oil | Listeria monocytogenes | leakage of three biological macromolecules (protein, ATP, and DNA) and the reduction in two intracellular enzymes (β-galactosidase and AKP) activities | [49] |
| Ocimum basilicum L. EO | Listeria monocytogenes | increased cell membrane permeability, thereby causing the leakage of intracellular proteins and DNA | [50] |
| Origanum vulgare L. EO and Leptospermum scoparium J. R. et G. Forst EO | 14 Staphylococcus aureus strains | collapse of the protonmotive force and depletion of the ATP pool | [51] |
| Thymus spp. EO and Juniperus spp. EO | Pseudomonas hibiscicola, Brevibacillus agri, Acinetobacter calcoaceticus | damage to the outer membrane or metabolic activities | [52] |
| Plant Species | Active Compound | Disease | Reference |
|---|---|---|---|
| Glycyrrhiza glabra Scuttelaria baicalensis | glycyrrhizin baicalin | coronaviruses | [35] |
| Camellia sinensis (L.) Kuntze | epigallocatechin-3-gallate (EGCG) theaflavins | SARS-CoV-2 | [93] |
| Fabaceae species | catechins | chronic diseases, inflammatory bowel disease (IBD), | [94] |
| Horminum pyrenaicum | diterpene quinones | suppress central Th1-type immunometabolic pathways | [95] |
| Curcuma longa | curcumin | oxidative stress | [96] |
| Atropa belladona | alcaloid atropine | anti-inflammatory, analgesic and neuro-pharmacological activities | [97] |
| Centaurium erythraea Hibiscus rosa sinensis Panax ginseng | secoiridoids, polyphenols myricetin, syringic acid ginsenosides | regulation of diabetes mellitus | [98] |
| Coreopsis tinctoria | marein | improvement of insulin resistance | [81] |
| Astragalus membranaceus | Astragaloside IV (AS-IV) | improvement of chemosensitivity of chemotherapy drugs | [99] |
| Boswellia serrata | 3-acetyl-11-keto-β-boswellic acid (AKBBA), β-boswellic acid (BBA). | osteoarthritis | [100] |
| Nigella sativa | thymoquinone | allergic rhinitis, metabolic disorders, diabetes mellitus | [101] |
| Spatholobus suberectus, Dioscorea nipponica, and Zingiber officinale | biochanin A and 6-gingerol | pro-inflammatory cytokines and MAPKs signaling pathway | [102] |
| Crateva adansonii, Maerua siamensis, and Mallotus repandus | lupeol | analgesic | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitropoulou, G.; Stavropoulou, E.; Vaou, N.; Tsakris, Z.; Voidarou, C.; Tsiotsias, A.; Tsigalou, C.; Taban, B.M.; Kourkoutas, Y.; Bezirtzoglou, E. Insights into Antimicrobial and Anti-Inflammatory Applications of Plant Bioactive Compounds. Microorganisms 2023, 11, 1156. https://doi.org/10.3390/microorganisms11051156
Mitropoulou G, Stavropoulou E, Vaou N, Tsakris Z, Voidarou C, Tsiotsias A, Tsigalou C, Taban BM, Kourkoutas Y, Bezirtzoglou E. Insights into Antimicrobial and Anti-Inflammatory Applications of Plant Bioactive Compounds. Microorganisms. 2023; 11(5):1156. https://doi.org/10.3390/microorganisms11051156
Chicago/Turabian StyleMitropoulou, Gregoria, Elisavet Stavropoulou, Natalia Vaou, Zacharias Tsakris, Chrysa Voidarou, Arsenis Tsiotsias, Christina Tsigalou, Birce Mercanoglou Taban, Yiannis Kourkoutas, and Eugenia Bezirtzoglou. 2023. "Insights into Antimicrobial and Anti-Inflammatory Applications of Plant Bioactive Compounds" Microorganisms 11, no. 5: 1156. https://doi.org/10.3390/microorganisms11051156
APA StyleMitropoulou, G., Stavropoulou, E., Vaou, N., Tsakris, Z., Voidarou, C., Tsiotsias, A., Tsigalou, C., Taban, B. M., Kourkoutas, Y., & Bezirtzoglou, E. (2023). Insights into Antimicrobial and Anti-Inflammatory Applications of Plant Bioactive Compounds. Microorganisms, 11(5), 1156. https://doi.org/10.3390/microorganisms11051156











