Advances in Research on the Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases
Abstract
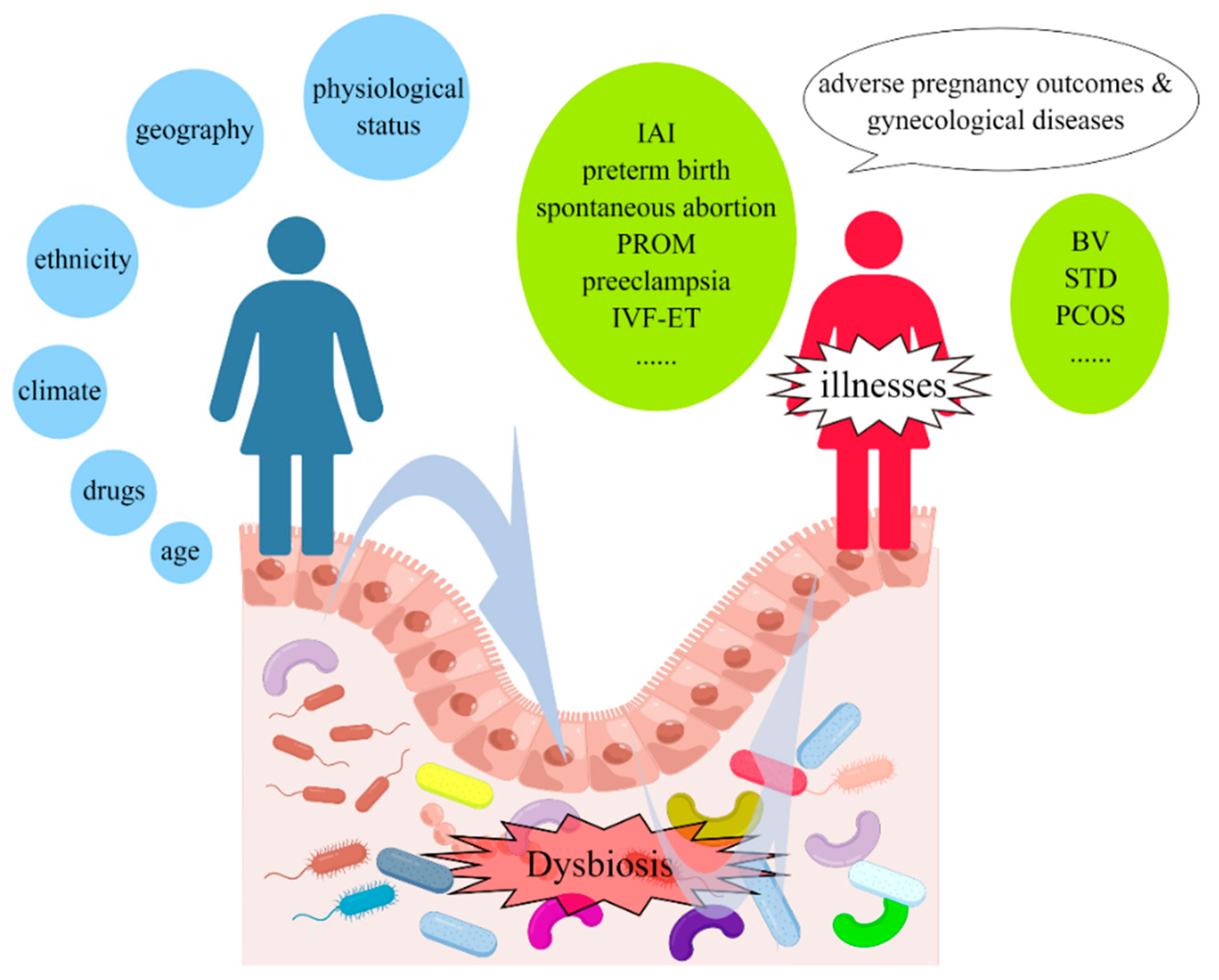
1. Introduction
2. The Normal Vaginal Microbiota
2.1. Evidence Concerning Vaginal Microbiota
2.2. Composition and Variation of Normal Vaginal Microbiota
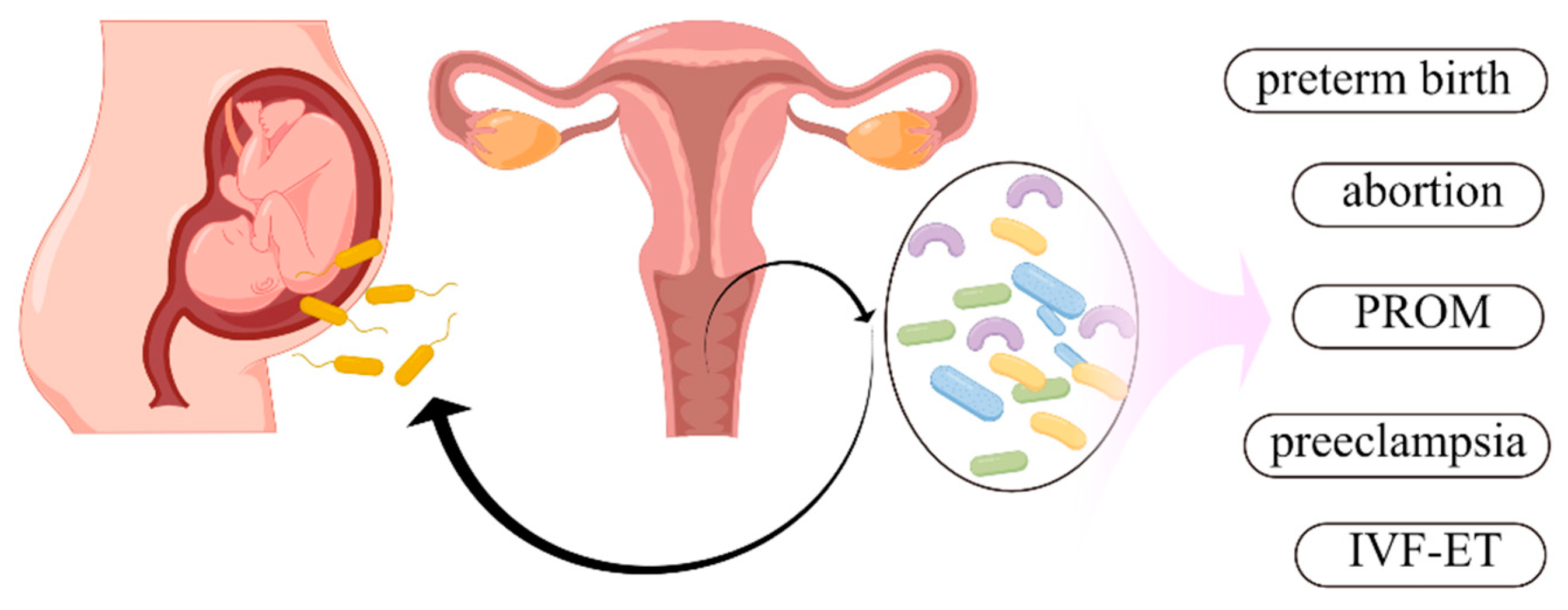
3. Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes
3.1. Vaginal Microbiota and Spontaneous Preterm Birth
3.2. Vaginal Microbiota and Premature Rupture of Membranes
3.3. Vaginal Microbiota and In Vitro Fertilization and Embryo Transfer Outcomes
3.4. Vaginal Microbiota and Spontaneous Abortion
3.5. Vaginal Microbiota and Intra-Amniotic Infection
3.6. Vaginal Microbiota and Preeclampsia
4. Relationship between Vaginal Microbiota and Gynecological Diseases
5. Deficiencies and Perspectives of Vaginal Microbiota Studies
5.1. Identify Pregnant Women at High Risk and Search for Effective Treatment
5.2. Deficiencies and Perspectives of Research Techniques
5.3. Deficiencies and Perspectives of New Research Area
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. mBio 2020, 11, e00218-20. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Nikoopour, E.; Singh, B. Reciprocity in microbiome and immune system interactions and its implications in disease and health. Inflamm. Allergy Drug Targets 2014, 13, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Mazmanian, S.K. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe 2010, 7, 265–276. [Google Scholar] [CrossRef]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef]
- Achilles, S.L.; Austin, M.N.; Meyn, L.A.; Mhlanga, F.; Chirenje, Z.M.; Hillier, S.L. Impact of contraceptive initiation on vaginal microbiota. Am. J. Obstet. Gynecol. 2018, 218, 622.e1–622.e10. [Google Scholar] [CrossRef]
- Turpin, R.; Brotman, R.M.; Miller, R.S.; Klebanoff, M.A.; He, X.; Slopen, N. Perceived stress and incident sexually transmitted infections in a prospective cohort. Ann. Epidemiol. 2019, 32, 20–27. [Google Scholar] [CrossRef]
- Abdelmaksoud, A.A.; Girerd, P.H.; Garcia, E.M.; Brooks, J.P.; Leftwich, L.M.; Sheth, N.U.; Bradley, S.P.; Serrano, M.G.; Fettweis, J.M.; Huang, B.; et al. Association between statin use, the vaginal microbiome, and Gardnerella vaginalis vaginolysin-mediated cytotoxicity. PLoS ONE 2017, 12, e0183765. [Google Scholar] [CrossRef]
- Muzny, C.A.; Schwebke, J.R. Pathogenesis of Bacterial Vaginosis: Discussion of Current Hypotheses. J. Infect. Dis. 2016, 214 (Suppl. S1), S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Gupta, A. Influence of Early Life, Diet, and the Environment on the Microbiome. Clin. Gastroenterol. Hepatol. 2019, 17, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Lehne, B.; Kindinger, L.M.; Terzidou, V.; Holmes, E.; Nicholson, J.K.; Bennett, P.R.; et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gao, J.; Wu, Y.; Li, Y.; Chen, Y.; Zhao, F.; Li, C.; Ying, C. Characterization of vaginal microbiota in Chinese women with cervical squamous intra-epithelial neoplasia. Int. J. Gynecol. Cancer 2020, 30, 1500–1504. [Google Scholar] [CrossRef]
- Champer, M.; Wong, A.M.; Champer, J.; Brito, I.L.; Messer, P.W.; Hou, J.Y.; Wright, J.D. The role of the vaginal microbiome in gynaecological cancer. BJOG 2018, 125, 309–315. [Google Scholar] [CrossRef]
- Sun, S.; Serrano, M.G.; Fettweis, J.M.; Basta, P.; Rosen, E.; Ludwig, K.; Sorgen, A.A.; Blakley, I.C.; Wu, M.C.; Dole, N.; et al. Race, the Vaginal Microbiome, and Spontaneous Preterm Birth. mSystems 2022, 7, e0001722. [Google Scholar] [CrossRef]
- Kosti, I.; Lyalina, S.; Pollard, K.S.; Butte, A.J.; Sirota, M. Meta-Analysis of Vaginal Microbiome Data Provides New Insights Into Preterm Birth. Front. Microbiol. 2020, 11, 476. [Google Scholar] [CrossRef]
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. mBio 2020, 11, e03242-19. [Google Scholar] [CrossRef]
- Hong, X.; Qin, P.; Huang, K.; Ding, X.; Ma, J.; Xuan, Y.; Zhu, X.; Peng, D.; Wang, B. Association between polycystic ovary syndrome and the vaginal microbiome: A case-control study. Clin. Endocrinol. 2020, 93, 52–60. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Zapata, H.J.; Quagliarello, V.J. The microbiota and microbiome in aging: Potential implications in health and age-related diseases. J. Am. Geriatr. Soc. 2015, 63, 776–781. [Google Scholar] [CrossRef]
- Plummer, E.L.; Vodstrcil, L.A.; Fairley, C.K.; Tabrizi, S.N.; Garland, S.M.; Law, M.G.; Hocking, J.S.; Fethers, K.A.; Bulach, D.M.; Murray, G.L.; et al. Sexual practices have a significant impact on the vaginal microbiota of women who have sex with women. Sci. Rep. 2019, 9, 19749. [Google Scholar] [CrossRef] [PubMed]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of Surface Exopolysaccharides From Bifidobacterium and Lactobacillus Within the Intestinal Environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.; Radjabzadeh, D.; Hassing, R.J.; Heeringa, J.; Uitterlinden, A.G.; Kraaij, R.; Stricker, B.H.; Verbon, A. The effect of antimicrobial drug use on the composition of the genitourinary microbiota in an elderly population. BMC Microbiol. 2019, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Drell, T.; Lillsaar, T.; Tummeleht, L.; Simm, J.; Aaspõllu, A.; Väin, E.; Saarma, I.; Salumets, A.; Donders, G.G.; Metsis, M. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PLoS ONE 2013, 8, e54379. [Google Scholar] [CrossRef]
- Guo, R.; Zheng, N.; Lu, H.; Yin, H.; Yao, J.; Chen, Y. Increased diversity of fungal flora in the vagina of patients with recurrent vaginal candidiasis and allergic rhinitis. Microb. Ecol. 2012, 64, 918–927. [Google Scholar] [CrossRef]
- Theis, K.R.; Florova, V.; Romero, R.; Borisov, A.B.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N. Sneathia: An emerging pathogen in female reproductive disease and adverse perinatal outcomes. Crit. Rev. Microbiol. 2021, 47, 517–542. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lin, C.Y.; Yeh, Y.M.; Yang, L.Y.; Lee, Y.S.; Chao, A.; Chin, C.Y.; Chao, A.S.; Yang, C.Y. Severe preeclampsia is associated with a higher relative abundance of Prevotella bivia in the vaginal microbiota. Sci. Rep. 2020, 10, 18249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Tao, Z.; Edupuganti, L.; Serrano, M.G.; Buck, G.A. Roles of the Microbiota of the Female Reproductive Tract in Gynecological and Reproductive Health. Microbiol. Mol. Biol. Rev. 2022, 86, e0018121. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e5. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef]
- Ardissone, A.N.; de la Cruz, D.M.; Davis-Richardson, A.G.; Rechcigl, K.T.; Li, N.; Drew, J.C.; Murgas-Torrazza, R.; Sharma, R.; Hudak, M.L.; Triplett, E.W.; et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS ONE 2014, 9, e90784. [Google Scholar] [CrossRef]
- Hammerschlag, M.R.; Alpert, S.; Rosner, I.; Thurston, P.; Semine, D.; McComb, D.; McCormack, W.M. Microbiology of the vagina in children: Normal and potentially pathogenic organisms. Pediatrics 1978, 62, 57–62. [Google Scholar] [CrossRef]
- Hickey, R.J.; Zhou, X.; Settles, M.L.; Erb, J.; Malone, K.; Hansmann, M.A.; Shew, M.L.; Van Der Pol, B.; Fortenberry, J.D.; Forney, L.J. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio 2015, 6, e00097-15. [Google Scholar] [CrossRef]
- Feng, T.; Liu, Y. Microorganisms in the reproductive system and probiotic’s regulatory effects on reproductive health. Comput. Struct. Biotechnol. J. 2022, 20, 1541–1553. [Google Scholar] [CrossRef]
- Neuman, H.; Koren, O. The Pregnancy Microbiome. Nestlé Nutr. Inst. Workshop Ser. 2017, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kumar, N.; Singhal, N.; Kaur, R.; Manektala, U. Vaginal microflora in postmenopausal women on hormone replacement therapy. Indian J. Pathol. Microbiol. 2006, 49, 457–461. [Google Scholar] [PubMed]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Noyes, N.; Cho, K.C.; Ravel, J.; Forney, L.J.; Abdo, Z. Associations between sexual habits, menstrual hygiene practices, demographics and the vaginal microbiome as revealed by Bayesian network analysis. PLoS ONE 2018, 13, e0191625. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, Y.; Gao, J.; Wu, M.; Li, C.; Wang, Z.; Huang, N.; Cui, L.; Du, M.; Ying, C. Characterization of Vaginal Microbiota in Women With Recurrent Spontaneous Abortion That Can Be Modified by Drug Treatment. Front. Cell Infect. Microbiol. 2021, 11, 680643. [Google Scholar] [CrossRef]
- Goodfellow, L.; Verwijs, M.C.; Care, A.; Sharp, A.; Ivandic, J.; Poljak, B.; Roberts, D.; Bronowski, C.; Gill, A.C.; Darby, A.C.; et al. Vaginal bacterial load in the second trimester is associated with early preterm birth recurrence: A nested case-control study. BJOG 2021, 128, 2061–2072. [Google Scholar] [CrossRef]
- Payne, M.S.; Newnham, J.P.; Doherty, D.A.; Furfaro, L.L.; Pendal, N.L.; Loh, D.E.; Keelan, J.A. A specific bacterial DNA signature in the vagina of Australian women in midpregnancy predicts high risk of spontaneous preterm birth (the Predict1000 study). Am. J. Obstet. Gynecol. 2021, 224, 206.e201–206.e223. [Google Scholar] [CrossRef]
- Witkin, S.S. Vaginal microbiome studies in pregnancy must also analyse host factors. BJOG 2019, 126, 359. [Google Scholar] [CrossRef]
- Hillier, S.L.; Nugent, R.P.; Eschenbach, D.A.; Krohn, M.A.; Gibbs, R.S.; Martin, D.H.; Cotch, M.F.; Edelman, R.; Pastorek, J.G., 2nd; Rao, A.V.; et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 1995, 333, 1737–1742. [Google Scholar] [CrossRef]
- Meis, P.J.; Goldenberg, R.L.; Mercer, B.; Moawad, A.; Das, A.; McNellis, D.; Johnson, F.; Iams, J.D.; Thom, E.; Andrews, W.W. The preterm prediction study: Significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obstet. Gynecol. 1995, 173, 1231–1235. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Shen, T.; Chung, P.; Buhimschi, I.A.; Buhimschi, C.S. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J. Clin. Microbiol. 2009, 47, 38–47. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef]
- Kan, H.; He, Y.; Li, Q.; Mu, Y.; Dong, Y.; Fan, W.; Zhang, M.; Wang, T.; Li, Y.; Liu, H.; et al. Differential Effect of Vaginal Microbiota on Spontaneous Preterm Birth among Chinese Pregnant Women. Biomed Res. Int. 2022, 2022, 3536108. [Google Scholar] [CrossRef] [PubMed]
- Elovitz, M.A.; Gajer, P.; Riis, V.; Brown, A.G.; Humphrys, M.S.; Holm, J.B.; Ravel, J. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat. Commun. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Bennett, P.R.; Lee, Y.S.; Kundu, S.; Teoh, T.G.; Adan, M.; Ahmed, S.; Brown, R.G.; David, A.L.; Lewis, H.V.; et al. Microbial-driven preterm labour involves crosstalk between the innate and adaptive immune response. Nat. Commun. 2022, 13, 975. [Google Scholar] [CrossRef]
- Practice Bulletin No. 172: Premature Rupture of Membranes. Obstet. Gynecol. 2016, 128, e165–e177. [CrossRef]
- Bennett, P.R.; Brown, R.G.; MacIntyre, D.A. Vaginal Microbiome in Preterm Rupture of Membranes. Obstet. Gynecol. Clin. N. Am. 2020, 47, 503–521. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Chen, Y.; Jiang, S.; Xu, H.; Zhan, H.; Ren, Y.; Xu, D.; Xu, Z.; Chen, D. Characterization of Vaginal Microbiota in Third Trimester Premature Rupture of Membranes Patients through 16S rDNA Sequencing. Pathogens 2022, 11, 847. [Google Scholar] [CrossRef]
- Brown, R.G.; Al-Memar, M.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Chan, D.; Lewis, H.; Kindinger, L.; Terzidou, V.; Bourne, T.; et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res. 2019, 207, 30–43. [Google Scholar] [CrossRef]
- Yan, C.; Hong, F.; Xin, G.; Duan, S.; Deng, X.; Xu, Y. Alterations in the vaginal microbiota of patients with preterm premature rupture of membranes. Front. Cell Infect. Microbiol. 2022, 12, 858732. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos Borges, L.G.; Pastuschek, J.; Heimann, Y.; Dawczynski, K.; Schleußner, E.; Pieper, D.H.; Zöllkau, J. Vaginal and neonatal microbiota in pregnant women with preterm premature rupture of membranes and consecutive early onset neonatal sepsis. BMC Med. 2023, 21, 92. [Google Scholar] [CrossRef]
- Paramel Jayaprakash, T.; Wagner, E.C.; van Schalkwyk, J.; Albert, A.Y.; Hill, J.E.; Money, D.M. High Diversity and Variability in the Vaginal Microbiome in Women following Preterm Premature Rupture of Membranes (PPROM): A Prospective Cohort Study. PLoS ONE 2016, 11, e0166794. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Hu, A.; Kan, H.; Li, Y.; He, Y.; Fan, W.; Liu, H.; Li, Q.; Zheng, Y. Preterm Prelabor Rupture of Membranes Linked to Vaginal Bacteriome of Pregnant Females in the Early Second Trimester: A Case-Cohort Design. Reprod. Sci. 2023, 1–12. [Google Scholar] [CrossRef]
- Hyman, R.W.; Herndon, C.N.; Jiang, H.; Palm, C.; Fukushima, M.; Bernstein, D.; Vo, K.C.; Zelenko, Z.; Davis, R.W.; Giudice, L.C. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J. Assist. Reprod. Genet. 2012, 29, 105–115. [Google Scholar] [CrossRef]
- Moore, D.E.; Soules, M.R.; Klein, N.A.; Fujimoto, V.Y.; Agnew, K.J.; Eschenbach, D.A. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil. Steril. 2000, 74, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Haahr, T.; Jensen, J.S.; Thomsen, L.; Duus, L.; Rygaard, K.; Humaidan, P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients. Hum. Reprod. 2016, 31, 795–803. [Google Scholar] [CrossRef]
- Ji, L.; Peng, C.; Bao, X. Effect of vaginal flora on clinical outcome of frozen embryo transfer. Front. Cell Infect. Microbiol. 2022, 12, 987292. [Google Scholar] [CrossRef]
- Toth, B.; Würfel, W.; Bohlmann, M.K.; Gillessen-Kaesbach, G.; Nawroth, F.; Rogenhofer, N.; Tempfer, C.; Wischmann, T.; von Wolff, M. Recurrent Miscarriage: Diagnostic and Therapeutic Procedures. Guideline of the DGGG (S1-Level, AWMF Registry No. 015/050, December 2013). Geburtshilfe Frauenheilkd. 2015, 75, 1117–1129. [Google Scholar] [CrossRef]
- Penta, M.; Lukic, A.; Conte, M.P.; Chiarini, F.; Fioriti, D.; Longhi, C.; Pietropaolo, V.; Vetrano, G.; Villaccio, B.; Degener, A.M.; et al. Infectious agents in tissues from spontaneous abortions in the first trimester of pregnancy. New Microbiol. 2003, 26, 329–337. [Google Scholar] [PubMed]
- Kuon, R.J.; Togawa, R.; Vomstein, K.; Weber, M.; Goeggl, T.; Strowitzki, T.; Markert, U.R.; Zimmermann, S.; Daniel, V.; Dalpke, A.H.; et al. Higher prevalence of colonization with Gardnerella vaginalis and gram-negative anaerobes in patients with recurrent miscarriage and elevated peripheral natural killer cells. J. Reprod. Immunol. 2017, 120, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Al-Memar, M.; Bobdiwala, S.; Fourie, H.; Mannino, R.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Timmerman, D.; Bourne, T.; Bennett, P.R.; et al. The association between vaginal bacterial composition and miscarriage: A nested case-control study. BJOG 2020, 127, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Grewal, K.; Lee, Y.S.; Smith, A.; Brosens, J.J.; Bourne, T.; Al-Memar, M.; Kundu, S.; MacIntyre, D.A.; Bennett, P.R. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhao, X.; Pan, Q.; Li, F.; Gao, B.; Zhang, A.; Huang, H.; Xu, D.; Cheng, C. The association between vaginal microbiota disorders and early missed abortion: A prospective study. Acta Obstet. Gynecol. Scand. 2022, 101, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Yang, S.; Yang, Z.; Zhou, P.; Peng, T.; Yin, J.; Ye, Z.; Shan, H.; Yu, Y.; Li, R. An Altered Microbiota in the Lower and Upper Female Reproductive Tract of Women with Recurrent Spontaneous Abortion. Microbiol. Spectr. 2022, 10, e0046222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, T.; Ma, Y.; Huang, Z.; He, Y.; Pan, H.; Fang, M.; Ding, H. Alteration of vaginal microbiota in patients with unexplained recurrent miscarriage. Exp. Ther. Med. 2019, 17, 3307–3316. [Google Scholar] [CrossRef]
- Best, K.A.; McKinney, J.; Alkhasawneh, A.; McCullough, D.C.; Sanchez-Ramos, L. Amniotic fluid “sludge” due to intra-amniotic infection/inflammation with Candidaalbicans. Am. J. Obstet. Gynecol. 2022, 227, 329–330. [Google Scholar] [CrossRef]
- Vani, K.T.; Wolfe, D.S. Intra-amniotic Infection. Neoreviews 2020, 21, e772–e777. [Google Scholar] [CrossRef]
- Romero, R.; Gomez-Lopez, N.; Winters, A.D.; Jung, E.; Shaman, M.; Bieda, J.; Panaitescu, B.; Pacora, P.; Erez, O.; Greenberg, J.M.; et al. Evidence that intra-amniotic infections are often the result of an ascending invasion—A molecular microbiological study. J. Perinat. Med. 2019, 47, 915–931. [Google Scholar] [CrossRef]
- Kindinger, L.M.; Bennett, P.R.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.G.; MacIntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Doyle, R.M.; Harris, K.; Kamiza, S.; Harjunmaa, U.; Ashorn, U.; Nkhoma, M.; Dewey, K.G.; Maleta, K.; Ashorn, P.; Klein, N. Bacterial communities found in placental tissues are associated with severe chorioamnionitis and adverse birth outcomes. PLoS ONE 2017, 12, e0180167. [Google Scholar] [CrossRef] [PubMed]
- Chaemsaithong, P.; Lertrut, W.; Kamlungkuea, T.; Santanirand, P.; Singsaneh, A.; Jaovisidha, A.; Pakdeeto, S.; Mongkolsuk, P.; Pongchaikul, P. Maternal septicemia caused by Streptococcus mitis: A possible link between intra-amniotic infection and periodontitis. Case report and literature review. BMC Infect. Dis. 2022, 22, 562. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B.; Romero, R.; Amogan, H.P.; Kusanovic, J.P.; Bik, E.M.; Gotsch, F.; Kim, C.J.; Erez, O.; Edwin, S.; Relman, D.A. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: A molecular and culture-based investigation. PLoS ONE 2008, 3, e3056. [Google Scholar] [CrossRef] [PubMed]
- Mendz, G.L.; Kaakoush, N.O.; Quinlivan, J.A. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front. Cell Infect. Microbiol. 2013, 3, 58. [Google Scholar] [CrossRef]
- Severgnini, M.; Morselli, S.; Camboni, T.; Ceccarani, C.; Salvo, M.; Zagonari, S.; Patuelli, G.; Pedna, M.F.; Sambri, V.; Foschi, C.; et al. Gardnerella vaginalis clades in pregnancy: New insights into the interactions with the vaginal microbiome. PLoS ONE 2022, 17, e0269590. [Google Scholar] [CrossRef]
- Amarasekara, R.; Jayasekara, R.W.; Senanayake, H.; Dissanayake, V.H. Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of pre-eclampsia. J. Obstet. Gynaecol. Res. 2015, 41, 662–669. [Google Scholar] [CrossRef]
- Huang, L.; Cai, M.; Li, L.; Zhang, X.; Xu, Y.; Xiao, J.; Huang, Q.; Luo, G.; Zeng, Z.; Jin, C.; et al. Gut microbiota changes in preeclampsia, abnormal placental growth and healthy pregnant women. BMC Microbiol. 2021, 21, 265. [Google Scholar] [CrossRef]
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef]
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 2005, 353, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Borgdorff, H.; Tsivtsivadze, E.; Verhelst, R.; Marzorati, M.; Jurriaans, S.; Ndayisaba, G.F.; Schuren, F.H.; van de Wijgert, J.H. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014, 8, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Ziklo, N.; Vidgen, M.E.; Taing, K.; Huston, W.M.; Timms, P. Dysbiosis of the Vaginal Microbiota and Higher Vaginal Kynurenine/Tryptophan Ratio Reveals an Association with Chlamydia trachomatis Genital Infections. Front. Cell Infect. Microbiol. 2018, 8, 1. [Google Scholar] [CrossRef]
- Wee, B.A.; Thomas, M.; Sweeney, E.L.; Frentiu, F.D.; Samios, M.; Ravel, J.; Gajer, P.; Myers, G.; Timms, P.; Allan, J.A.; et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust. N. Zealand J. Obstet. Gynaecol. 2018, 58, 341–348. [Google Scholar] [CrossRef]
- Ahmadi, M.H.; Mirsalehian, A.; Bahador, A. Association of Chlamydia trachomatis with infertility and clinical manifestations: A systematic review and meta-analysis of case-control studies. Infect. Dis. 2016, 48, 517–523. [Google Scholar] [CrossRef]
- Lozano, F.M.; Bernabeu, A.; Lledo, B.; Morales, R.; Diaz, M.; Aranda, F.I.; Llacer, J.; Bernabeu, R. Characterization of the vaginal and endometrial microbiome in patients with chronic endometritis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, C.P.; Sallas, M.L.; Nunes, R.A.L.; Lorenzi, N.P.C.; Termini, L. Microbiome and Cervical Cancer. Pathobiology 2021, 88, 187–197. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial communities in women with bacterial vaginosis: High resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef]
- Chen, C.H.; Lin, Y.L.; Chen, K.H.; Chen, W.P.; Chen, Z.F.; Kuo, H.Y.; Hung, H.F.; Tang, C.Y.; Liou, M.L. Bacterial diversity among four healthcare-associated institutes in Taiwan. Sci. Rep. 2017, 7, 8230. [Google Scholar] [CrossRef]
- Dotters-Katz, S. Antibiotics for Prophylaxis in the Setting of Preterm Prelabor Rupture of Membranes. Obstet. Gynecol. Clin. N. Am. 2020, 47, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Ronzoni, S.; Cobo, T.; D’Souza, R.; Asztalos, E.; O’Rinn, S.E.; Cao, X.; Herranz, A.; Melamed, N.; Ferrero, S.; Barrett, J.; et al. Individualized treatment of preterm premature rupture of membranes to prolong the latency period, reduce the rate of preterm birth, and improve neonatal outcomes. Am. J. Obstet. Gynecol. 2022, 227, 296.e1–296.e18. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef]
- Reichman, O.; Akins, R.; Sobel, J.D. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex. Transm. Dis. 2009, 36, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Salmonella infection—Prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 2018, 164, 1327–1344. [Google Scholar] [CrossRef] [PubMed]
- Tita, A.T.; Andrews, W.W. Diagnosis and management of clinical chorioamnionitis. Clin. Perinatol. 2010, 37, 339–354. [Google Scholar] [CrossRef]
- Zöllkau, J.; Pieper, D.H.; Pastuschek, J.; Makarewicz, O.; Mentzel, H.J.; Dawczynski, K.; Schleußner, E. Lethal Neonatal Respiratory Failure by Perinatal Transmission of Ureaplasma Parvum after Maternal PPROM. Z. Geburtshilfe Neonatol. 2021, 225, 361–365. [Google Scholar] [CrossRef]
- Li, W.; Shrivastava, M.; Lu, H.; Jiang, Y. Calcium-calcineurin signaling pathway in Candida albicans: A potential drug target. Microbiol. Res. 2021, 249, 126786. [Google Scholar] [CrossRef]
- Bradford, L.L.; Ravel, J. The vaginal mycobiome: A contemporary perspective on fungi in women’s health and diseases. Virulence 2017, 8, 342–351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Hu, X.; Ying, C. Advances in Research on the Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases. Microorganisms 2023, 11, 991. https://doi.org/10.3390/microorganisms11040991
Zhao F, Hu X, Ying C. Advances in Research on the Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases. Microorganisms. 2023; 11(4):991. https://doi.org/10.3390/microorganisms11040991
Chicago/Turabian StyleZhao, Fuju, Xianyang Hu, and Chunmei Ying. 2023. "Advances in Research on the Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases" Microorganisms 11, no. 4: 991. https://doi.org/10.3390/microorganisms11040991
APA StyleZhao, F., Hu, X., & Ying, C. (2023). Advances in Research on the Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases. Microorganisms, 11(4), 991. https://doi.org/10.3390/microorganisms11040991




