The Preventive Effects of Probiotic Prevotella histicola on the Bone Loss of Mice with Ovariectomy-Mediated Osteoporosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics, Preparation and Disposal Related to Animal Experiments
2.2. Construction of Mice Models with OVX-Mediated OP
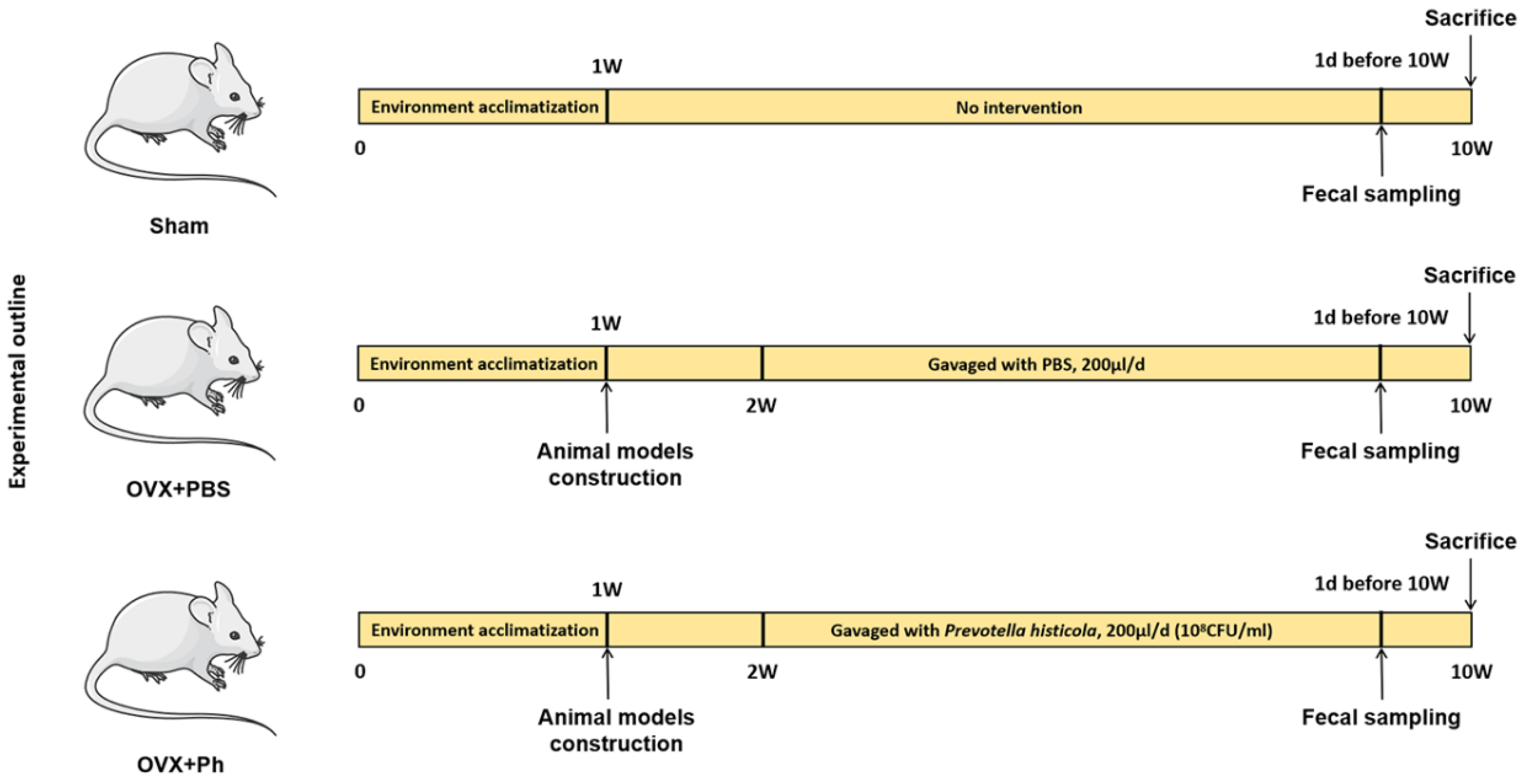
2.3. Animals Grouping and Sample Extraction
2.4. Culture and Preparation of Ph
2.5. The Detection of Micro-Computed Tomography (Micro-CT)
2.6. Histological Staining and Assessment
2.7. Immunohistochemistry (IHC) and Assessment
2.8. 16S rRNA High Throughput Sequencing and Bioinformatics Assessment
2.9. Statistical Evaluation
3. Results
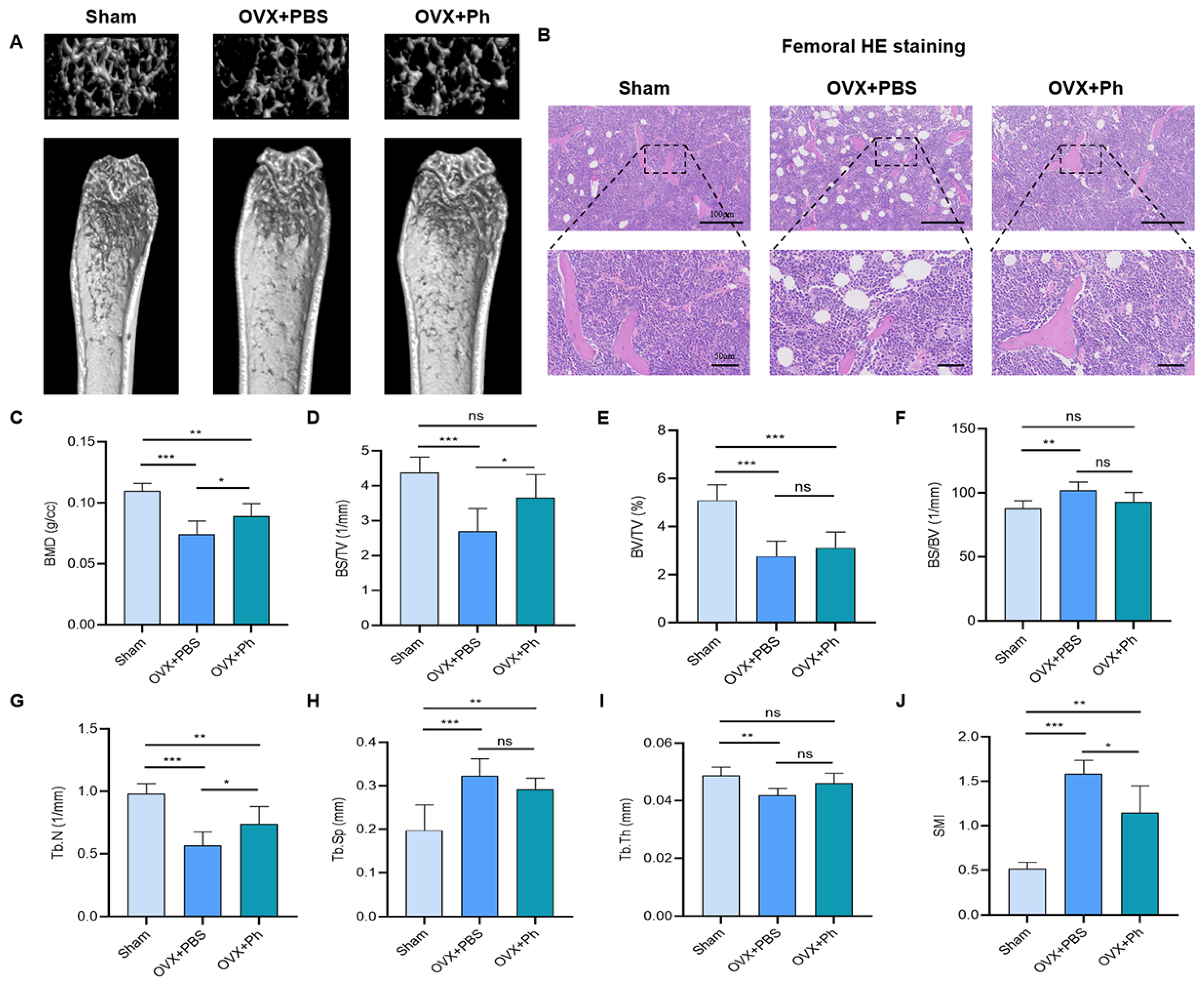
3.1. The Perfusion of Ph Mitigated the Bone Loss in Mice with OVX-Mediated OP
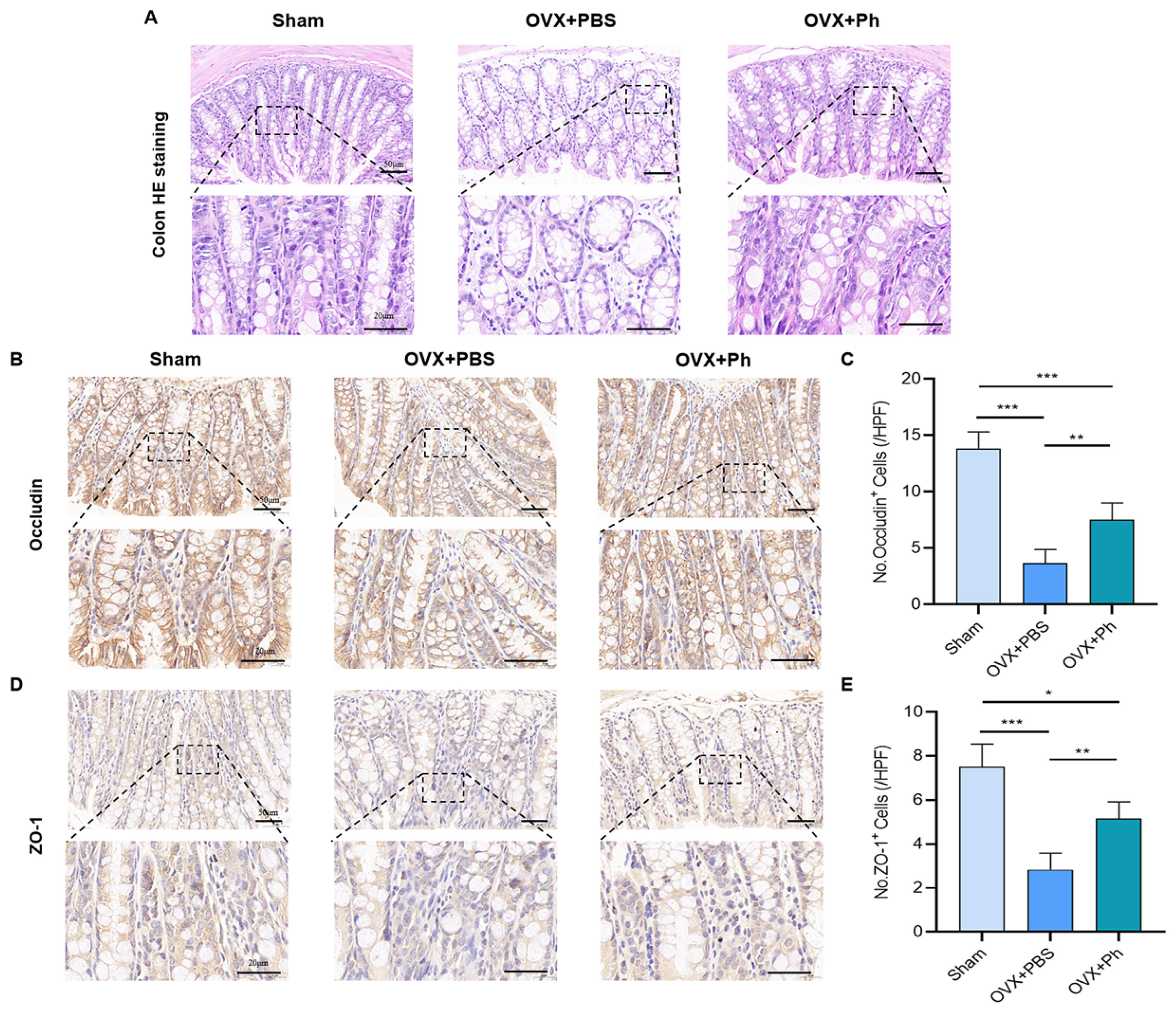
3.2. The Perfusion of Ph Reversed OVX-Mediated Intestinal Mucosal Barrier Damage
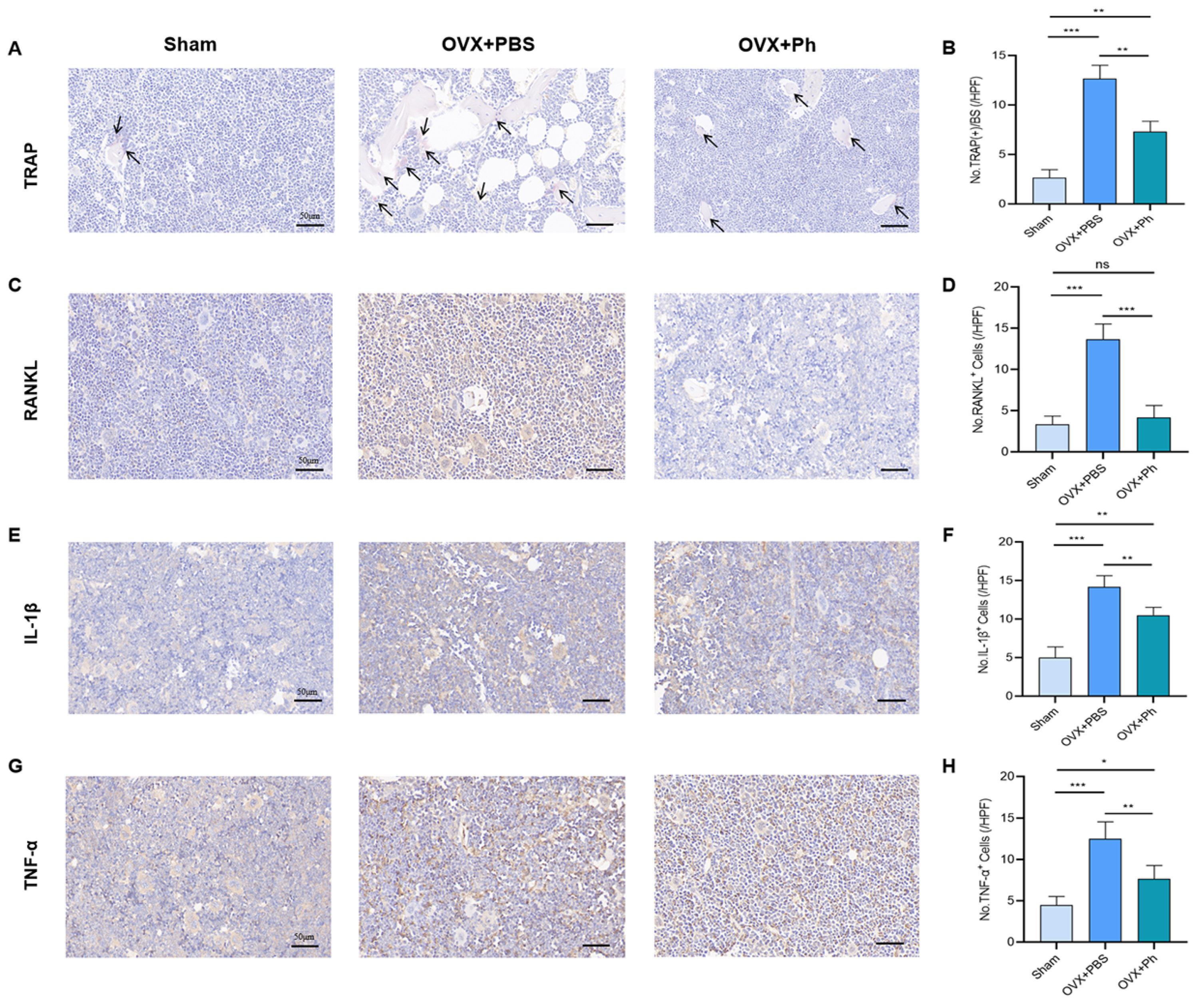
3.3. The Perfusion of Ph Decreased the OVX-Mediated Intestinal Inflammation
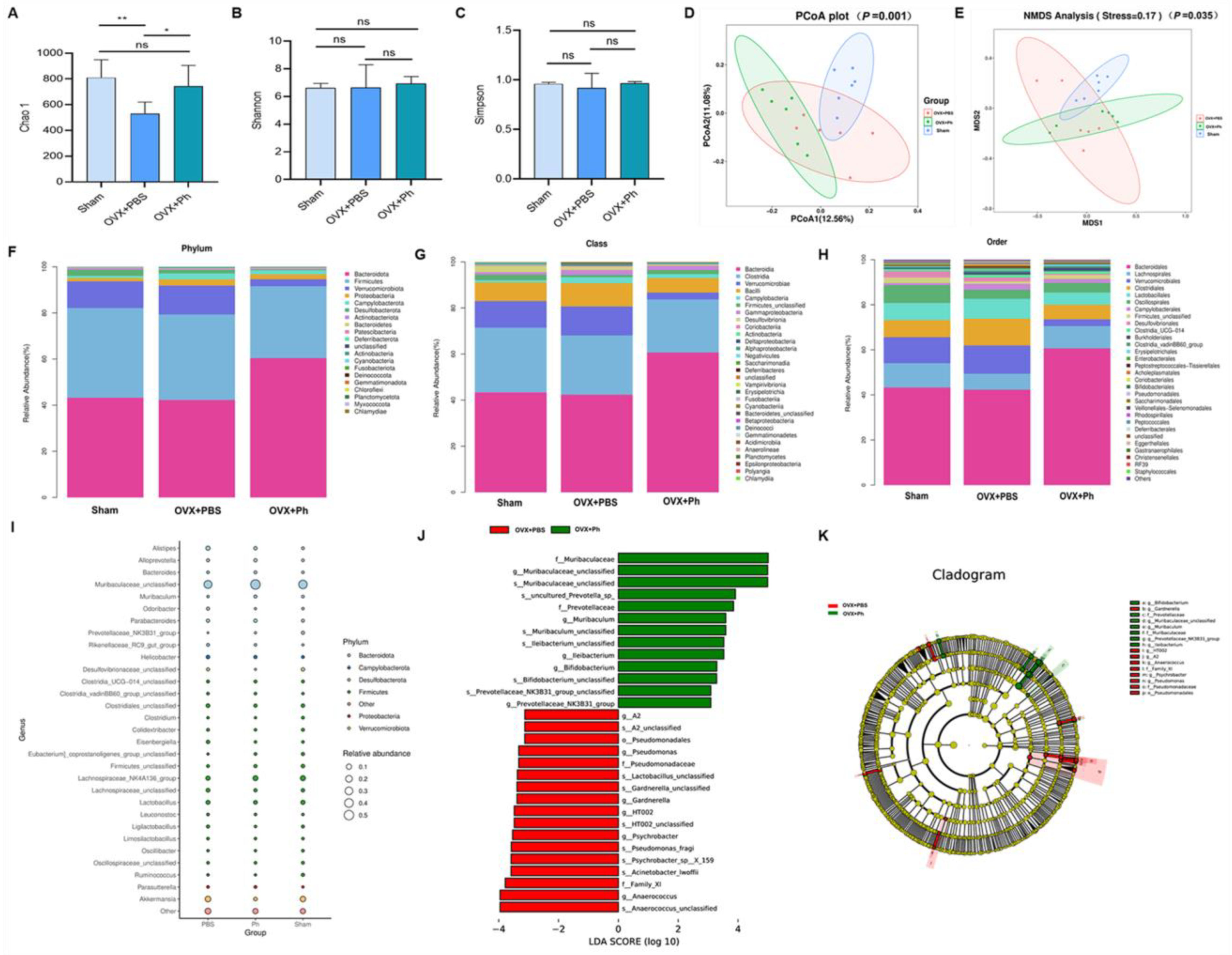
3.4. The Perfusion of Ph Ameliorated the Disturbance of GM in Mice with OVX-Mediated OP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallander, M.; Axelsson, K.F.; Lundh, D.; Lorentzon, M. Patients with prostate cancer and androgen deprivation therapy have increased risk of fractures-a study from the fractures and fall injuries in the elderly cohort (FRAILCO). Osteoporos. Int. 2019, 30, 115–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.B.; Chen, H.; Xu, H.W.; Yi, Y.Y.; Fang, X.Y.; Wang, S.J. Computed tomography-based paravertebral muscle density predicts subsequent vertebral fracture risks independently of bone mineral density in postmenopausal women following percutaneous vertebral augmentation. Aging Clin. Exp. Res. 2022, 34, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Li, W.; Zhang, W.; Yang, C.; Zhang, C.; Liang, X.; Yin, J.; Bai, J.; Ge, G.; Zhang, H.; et al. Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-κB activated pyroptosis pathways. Pharmacol. Res. 2021, 174, 105967. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Lu, P.P.; Li, Y.J.; Dai, G.C.; Cao, M.M.; Xie, T.; Zhang, C.; Shi, L.; Rui, Y.F. Low dietary choline intake is associated with the risk of osteoporosis in elderly individuals: A population-based study. Food Funct. 2021, 12, 6442–6451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Lu, P.P.; Li, Y.J.; Dai, G.C.; Chen, M.H.; Zhao, Y.K.; Cao, M.M.; Rui, Y.F. Prevalence, Characteristics, and Associated Risk Factors of the Elderly with Hip Fractures: A Cross-Sectional Analysis of NHANES 2005–2010. Clin. Interv. Aging 2021, 16, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, O.; Cheng, Z.; Xia, P.; Wang, L.; Shen, J.; Kong, X.; Zeng, Y.; Chao, A.; Yan, L.; et al. Jintiange combined with alfacalcidol improves muscle strength and balance in primary osteoporosis: A randomized, double-blind, double-dummy, positive-controlled, multicenter clinical trial. J. Orthop. Transl. 2022, 35, 53–61. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, C.; Ma, C.; Qi, H.; Yang, Z.H.; Wu, H.Y.; Yang, K.D.; Lin, J.Y.; Wong, T.M.; Li, Z.Y.; et al. Automatic phantom-less QCT system with high precision of BMD measurement for osteoporosis screening: Technique optimisation and clinical validation. J. Orthop. Transl. 2022, 33, 24–30. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Dai, G.C.; Lu, P.P.; Zhang, M.; Bai, L.Y.; Chen, X.X.; Shi, L.; Zhang, C.; et al. Dietary Protein Intake in Relation to the Risk of Osteoporosis in Middle-Aged and Older Individuals: A Cross-Sectional Study. J. Nutr. Health Aging 2022, 26, 252–258. [Google Scholar] [CrossRef]
- Merlotti, D.; Falchetti, A.; Chiodini, I.; Gennari, L. Efficacy and safety of abaloparatide for the treatment of post-menopausal osteoporosis. Expert Opin. Pharmacother. 2019, 20, 805–811. [Google Scholar] [CrossRef]
- Tuck, S.; Little, E.A.; Aspray, T.J. Implications of guidelines for osteoporosis and its treatment. Age Ageing 2018, 47, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Wang, F.; Hu, Y.; Luo, Y.; Wei, Y.; Xu, K.; Zhang, H.; Liu, H.; Bo, L.; Lv, S.; et al. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Rep. Med. 2023, 4, 100881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Li, Y.J.; Lu, P.P.; Dai, G.C.; Chen, X.X.; Rui, Y.F. The modulatory effect and implication of gut microbiota on osteoporosis: From the perspective of “brain-gut-bone” axis. Food Funct. 2021, 12, 5703–5718. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Tan, Q.; Wei, Z.; Chen, Q.; Wang, C.; Qi, L.; Wen, L.; Zhang, C.; Yao, C. Toll-like receptor 9 deficiency induces osteoclastic bone loss via gut microbiota-associated systemic chronic inflammation. Bone Res. 2022, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, A.; Zong, W.; Dai, L.; Liu, Y.; Luo, R.; Ge, S.; Dong, G. Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J. Biol. Sci. 2021, 28, 40–49. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Chen, X.X.; Yu, Q.; Rui, Y.F. A narrative review of the moderating effects and repercussion of exercise intervention on osteoporosis: Ingenious involvement of gut microbiota and its metabolites. J. Transl. Med. 2022, 20, 490. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, T.; Meng, Y.; Hu, M.; Shu, L.; Jiang, H.; Gao, R.; Ma, J.; Wang, C.; Zhou, X. FOS/GOS attenuates high-fat diet induced bone loss via reversing microbiota dysbiosis, high intestinal permeability and systemic inflammation in mice. Metabolism 2021, 119, 154767. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Dai, G.C.; Lu, P.P.; Zhang, M.; Bai, L.Y.; Chen, X.X.; Zhang, C.; Shi, L.; et al. The regulative effect and repercussion of probiotics and prebiotics on osteoporosis: Involvement of brain-gut-bone axis. Crit. Rev. Food Sci. Nutr. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Liao, Y.C.; Lee, M.C.; Lin, K.J.; Hsu, H.Y.; Chiou, S.Y.; Young, S.L.; Lin, J.S.; Huang, C.C.; Watanabe, K. Lactobacillus plantarum TWK10 Attenuates Aging-Associated Muscle Weakness, Bone Loss, and Cognitive Impairment by Modulating the Gut Microbiome in Mice. Front. Nutr. 2021, 8, 708096. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, J.Y.; Kim, B.K.; Lee, I.O.; Park, N.H.; Kim, S.H. Lactobacillus-fermented milk products attenuate bone loss in an experimental rat model of ovariectomy-induced post-menopausal primary osteoporosis. J. Appl. Microbiol. 2021, 130, 2041–2062. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, J. Bacteroides vulgatus diminishes colonic microbiota dysbiosis ameliorating lumbar bone loss in ovariectomized mice. Bone 2021, 142, 115710. [Google Scholar] [CrossRef]
- Shahi, S.K.; Freedman, S.N.; Murra, A.C.; Zarei, K.; Sompallae, R.; Gibson-Corley, K.N.; Karandikar, N.J.; Murray, J.A.; Mangalam, A.K. Prevotella histicola, A Human Gut Commensal, Is as Potent as COPAXONE® in an Animal Model of Multiple Sclerosis. Front. Immunol. 2019, 10, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Liu, X.; Xu, S.; Hu, S.; Wang, S.; Shi, D.; Wang, K.; Wang, Z.; Lin, Q.; Li, S.; et al. Prevotella histicola Mitigated Estrogen Deficiency-Induced Depression via Gut Microbiota-Dependent Modulation of Inflammation in Ovariectomized Mice. Front. Nutr. 2021, 8, 805465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, K.; Wu, C.; Chen, J.; Pan, H.; Liu, Y.; Wu, P.; Yuan, J.; Huang, F.; Lang, J.; et al. An emerging role of Prevotella histicola on estrogen deficiency-induced bone loss through the gut microbiota-bone axis in postmenopausal women and in ovariectomized mice. Am. J. Clin. Nutr. 2021, 114, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Lu, P.P.; Dai, G.C.; Zhang, M.; Wang, H.; Rui, Y.F. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J. Orthop. Transl. 2022, 37, 46–60. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Hu, Y.; Cui, J.; Zhi, X.; Li, X.; Jiang, H.; Wang, Y.; Gu, Z.; Qiu, Z.; et al. Lactulose Suppresses Osteoclastogenesis and Ameliorates Estrogen Deficiency-Induced Bone Loss in Mice. Aging Dis. 2020, 11, 629–641. [Google Scholar] [CrossRef]
- Sun, K.; Zhu, J.; Deng, Y.; Xu, X.; Kong, F.; Sun, X.; Huan, L.; Ren, C.; Sun, J.; Shi, J. Gamabufotalin Inhibits Osteoclastgenesis and Counteracts Estrogen-Deficient Bone Loss in Mice by Suppressing RANKL-Induced NF-κB and ERK/MAPK Pathways. Front. Pharmacol. 2021, 12, 629968. [Google Scholar] [CrossRef]
- Tirelle, P.; Breton, J.; Riou, G.; Déchelotte, P.; Coëffier, M.; Ribet, D. Comparison of different modes of antibiotic delivery on gut microbiota depletion efficiency and body composition in mouse. BMC Microbiol. 2020, 20, 340. [Google Scholar] [CrossRef]
- Bogatyrev, S.R.; Rolando, J.C.; Ismagilov, R.F. Self-reinoculation with fecal flora changes microbiota density and composition leading to an altered bile-acid profile in the mouse small intestine. Microbiome 2020, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Luckey, D.; Bodhke, R.; Chen, J.; Marietta, E.; Jeraldo, P.; Murray, J.; Taneja, V. Prevotella histicola Protects From Arthritis by Expansion of Allobaculum and Augmenting Butyrate Production in Humanized Mice. Front. Immunol. 2021, 12, 609644. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Lu, P.P.; Li, Y.J.; Wang, H.; Zhao, Y.K.; Chen, H.; Rui, Y.F. Short report: Relationship between self-reported sleep characteristics and falls-associated fractures in elderly individuals: A population-based study. Psychol. Health Med. 2023, 28, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Zhang, R.L.; Wu, M.T.; Yu, Q.; Rui, Y.F. Fecal microbiota transplantation as a promising treatment option for osteoporosis. J. Bone Miner. Metab. 2022, 40, 874–889. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ho, W.T.P.; Liu, C.; Chow, S.K.; Ip, M.; Yu, J.; Wong, H.S.; Cheung, W.H.; Sung, J.J.Y.; Wong, R.M.Y. The role of gut microbiota in bone homeostasis. Bone Joint Res. 2021, 10, 51–59. [Google Scholar] [CrossRef] [PubMed]
- McCabe, L.; Britton, R.A.; Parameswaran, N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr. Osteoporos. Rep. 2015, 13, 363–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCabe, L.R.; Parameswaran, N. Advances in Probiotic Regulation of Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef]
- Lopes, M.P.; Cruz, Á.A.; Xavier, M.T.; Stöcker, A.; Carvalho-Filho, P.; Miranda, P.M.; Meyer, R.J.; Soledade, K.R.; Gomes-Filho, I.S.; Trindade, S.C. Prevotella intermedia and periodontitis are associated with severe asthma. J. Periodontol. 2020, 91, 46–54. [Google Scholar] [CrossRef]
- Mangalam, A.K.; Murray, J. Microbial monotherapy with Prevotella histicola for patients with multiple sclerosis. Expert. Rev. Neurother. 2019, 19, 45–53. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Fehlner-Peach, H.; Magnabosco, C.; Raghavan, V.; Scher, J.U.; Tett, A.; Cox, L.M.; Gottsegen, C.; Watters, A.; Wiltshire-Gordon, J.D.; Segata, N.; et al. Distinct Polysaccharide Utilization Profiles of Human Intestinal Prevotella copri Isolates. Cell Host Microbe 2019, 26, 680–690.e5. [Google Scholar] [CrossRef]
- Shi, T.; Shi, Y.; Gao, H.; Ma, Y.; Wang, Q.; Shen, S.; Shao, X.; Gong, W.; Chen, X.; Qin, J.; et al. Exercised accelerated the production of muscle-derived kynurenic acid in skeletal muscle and alleviated the postmenopausal osteoporosis through the Gpr35/NFκB p65 pathway. J. Orthop. Translat 2022, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Batoon, L.; Millard, S.M.; Raggatt, L.J.; Wu, A.C.; Kaur, S.; Sun, L.W.H.; Williams, K.; Sandrock, C.; Ng, P.Y.; Irvine, K.M.; et al. Osteal macrophages support osteoclast-mediated resorption and contribute to bone pathology in a postmenopausal osteoporosis mouse model. J. Bone Miner. Res. 2021, 36, 2214–2228. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Zhao, Z.; Gu, Y.; Han, J.; Ye, K.; Zhang, Y. 7,8-Dihydroxyflavone modulates bone formation and resorption and ameliorates ovariectomy-induced osteoporosis. Elife 2021, 10, e64872. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, R.; Liu, J.; Fang, J.; Wang, X.; Cui, Y.; Zhang, P.; Du, B. Linarin Protects against Cadmium-Induced Osteoporosis Via Reducing Oxidative Stress and Inflammation and Altering RANK/RANKL/OPG Pathway. Biol. Trace Elem. Res. 2022, 200, 3688–3700. [Google Scholar] [CrossRef]
- Macari, S.; Sharma, L.A.; Wyatt, A.; da Silva, J.M.; Dias, G.J.; Silva, T.A.; Szawka, R.E.; Grattan, D.R. Lactation induces increases in the RANK/RANKL/OPG system in maxillary bone. Bone 2018, 110, 160–169. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Z.; Kwok, L.Y.; Zhao, Z.; Wang, K.; Li, Y.; Sun, Z.; Zhao, J.; Zhang, H. Bifidobacterium lactis Probio-M8 improves bone metabolism in patients with postmenopausal osteoporosis, possibly by modulating the gut microbiota. Eur. J. Nutr. 2023, 62, 965–976. [Google Scholar] [CrossRef]
- Morato-Martínez, M.; López-Plaza, B.; Santurino, C.; Palma-Milla, S.; Gómez-Candela, C. A Dairy Product to Reconstitute Enriched with Bioactive Nutrients Stops Bone Loss in High-Risk Menopausal Women without Pharmacological Treatment. Nutrients 2020, 12, 2203. [Google Scholar] [CrossRef]
- Parvaneh, K.; Ebrahimi, M.; Sabran, M.R.; Karimi, G.; Hwei, A.N.; Abdul-Majeed, S.; Ahmad, Z.; Ibrahim, Z.; Jamaluddin, R. Probiotics (Bifidobacterium longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. Biomed. Res. Int. 2015, 2015, 897639. [Google Scholar] [CrossRef] [Green Version]
- Lan, H.; Liu, W.H.; Zheng, H.; Feng, H.; Zhao, W.; Hung, W.L.; Li, H. Bifidobacterium lactis BL-99 protects mice with osteoporosis caused by colitis via gut inflammation and gut microbiota regulation. Food Funct. 2022, 13, 1482–1494. [Google Scholar] [CrossRef]
- Yeom, J.; Ma, S.; Lim, Y.H. Probiotic Propionibacterium freudenreichii MJ2 Enhances Osteoblast Differentiation and Mineralization by Increasing the OPG/RANKL Ratio. Microorganisms 2021, 9, 673. [Google Scholar] [CrossRef]
- Yang, L.C.; Lin, S.W.; Li, I.C.; Chen, Y.P.; Tzu, S.Y.; Chou, W.; Chen, C.C.; Lin, W.C.; Chen, Y.L.; Lin, W.H. Lactobacillus plantarum GKM3 and Lactobacillus paracasei GKS6 Supplementation Ameliorates Bone Loss in Ovariectomized Mice by Promoting Osteoblast Differentiation and Inhibiting Osteoclast Formation. Nutrients 2020, 12, 1914. [Google Scholar] [CrossRef] [PubMed]
- Wear, E.K.; Wilbanks, E.G.; Nelson, C.E.; Carlson, C.A. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environ. Microbiol. 2018, 20, 2709–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-W.; Cao, M.-M.; Li, Y.-J.; Sheng, R.-W.; Zhang, R.-L.; Wu, M.-T.; Chi, J.-Y.; Zhou, R.-X.; Rui, Y.-F. The Preventive Effects of Probiotic Prevotella histicola on the Bone Loss of Mice with Ovariectomy-Mediated Osteoporosis. Microorganisms 2023, 11, 950. https://doi.org/10.3390/microorganisms11040950
Zhang Y-W, Cao M-M, Li Y-J, Sheng R-W, Zhang R-L, Wu M-T, Chi J-Y, Zhou R-X, Rui Y-F. The Preventive Effects of Probiotic Prevotella histicola on the Bone Loss of Mice with Ovariectomy-Mediated Osteoporosis. Microorganisms. 2023; 11(4):950. https://doi.org/10.3390/microorganisms11040950
Chicago/Turabian StyleZhang, Yuan-Wei, Mu-Min Cao, Ying-Juan Li, Ren-Wang Sheng, Ruo-Lan Zhang, Meng-Ting Wu, Jia-Yu Chi, Rui-Xin Zhou, and Yun-Feng Rui. 2023. "The Preventive Effects of Probiotic Prevotella histicola on the Bone Loss of Mice with Ovariectomy-Mediated Osteoporosis" Microorganisms 11, no. 4: 950. https://doi.org/10.3390/microorganisms11040950
APA StyleZhang, Y.-W., Cao, M.-M., Li, Y.-J., Sheng, R.-W., Zhang, R.-L., Wu, M.-T., Chi, J.-Y., Zhou, R.-X., & Rui, Y.-F. (2023). The Preventive Effects of Probiotic Prevotella histicola on the Bone Loss of Mice with Ovariectomy-Mediated Osteoporosis. Microorganisms, 11(4), 950. https://doi.org/10.3390/microorganisms11040950



