Links between Soil Bacteriobiomes and Fungistasis toward Fungi Infecting the Colorado Potato Beetle
Abstract
1. Introduction
2. Methods
2.1. Study Locations and Sampling
2.2. Fungi and Insects
2.3. Metarhizium and Beauveria CFU Counts in Soils
2.4. Fungistatic Activity of Soils toward M. robertsii and B. bassiana
2.5. 16S rDNA Metabarcoding
2.6. Bacterial DNA Quantification
2.7. Isolation and Identification of Soil Bacteria
2.8. Antagonism of Soil Bacteria against Entomopathogenic Fungi
2.9. Fungal Infection of the CPB in Soils with Different Fungistasis Levels
2.10. The Influence of Soil Bacteria on Fungal Infection in the CPB
2.11. Statistics
3. Results
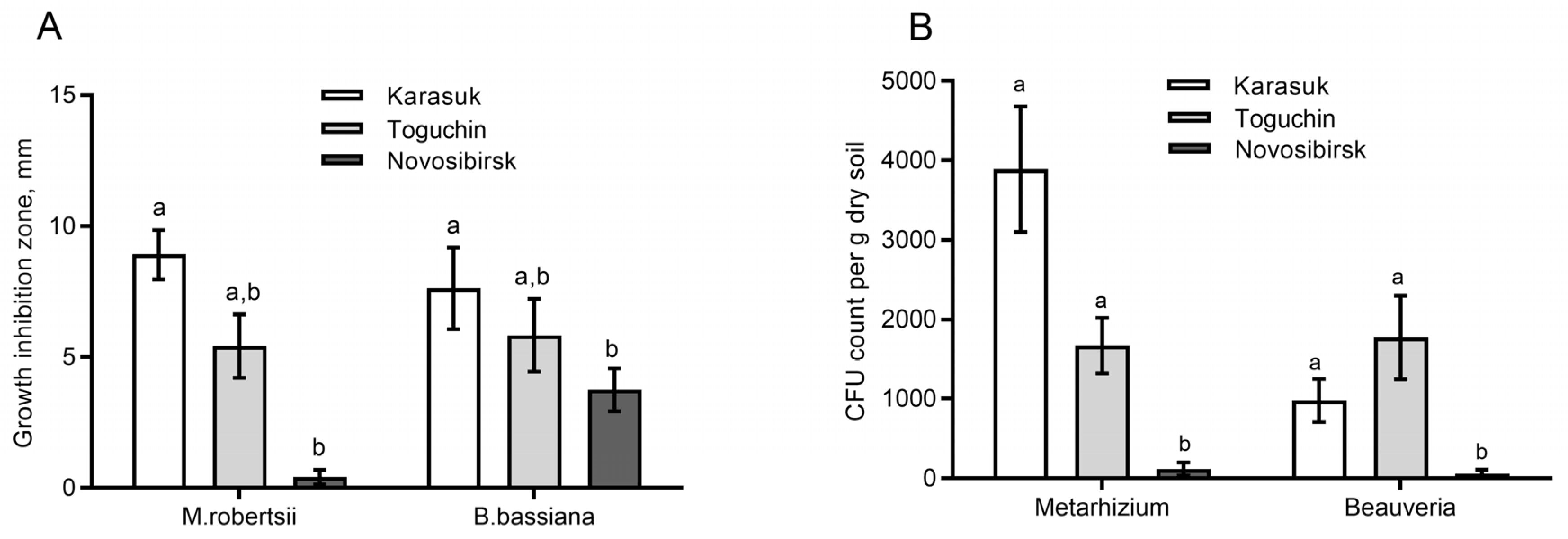
3.1. Fungistatic Activity of Soil Extracts and Fungal CFU Counts
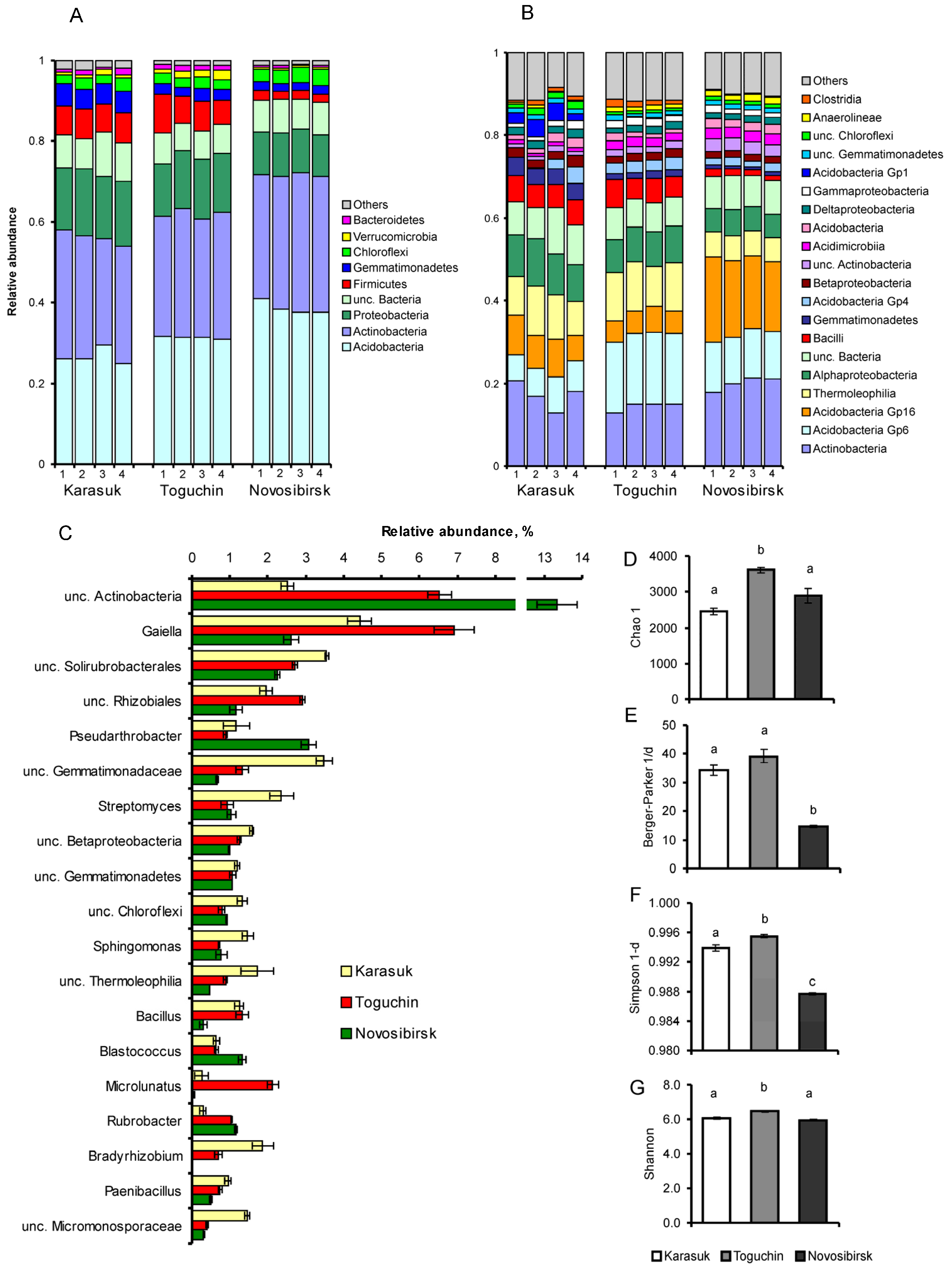
3.2. 16S rDNA Metabarcoding Analysis
3.3. Total Bacterial Load
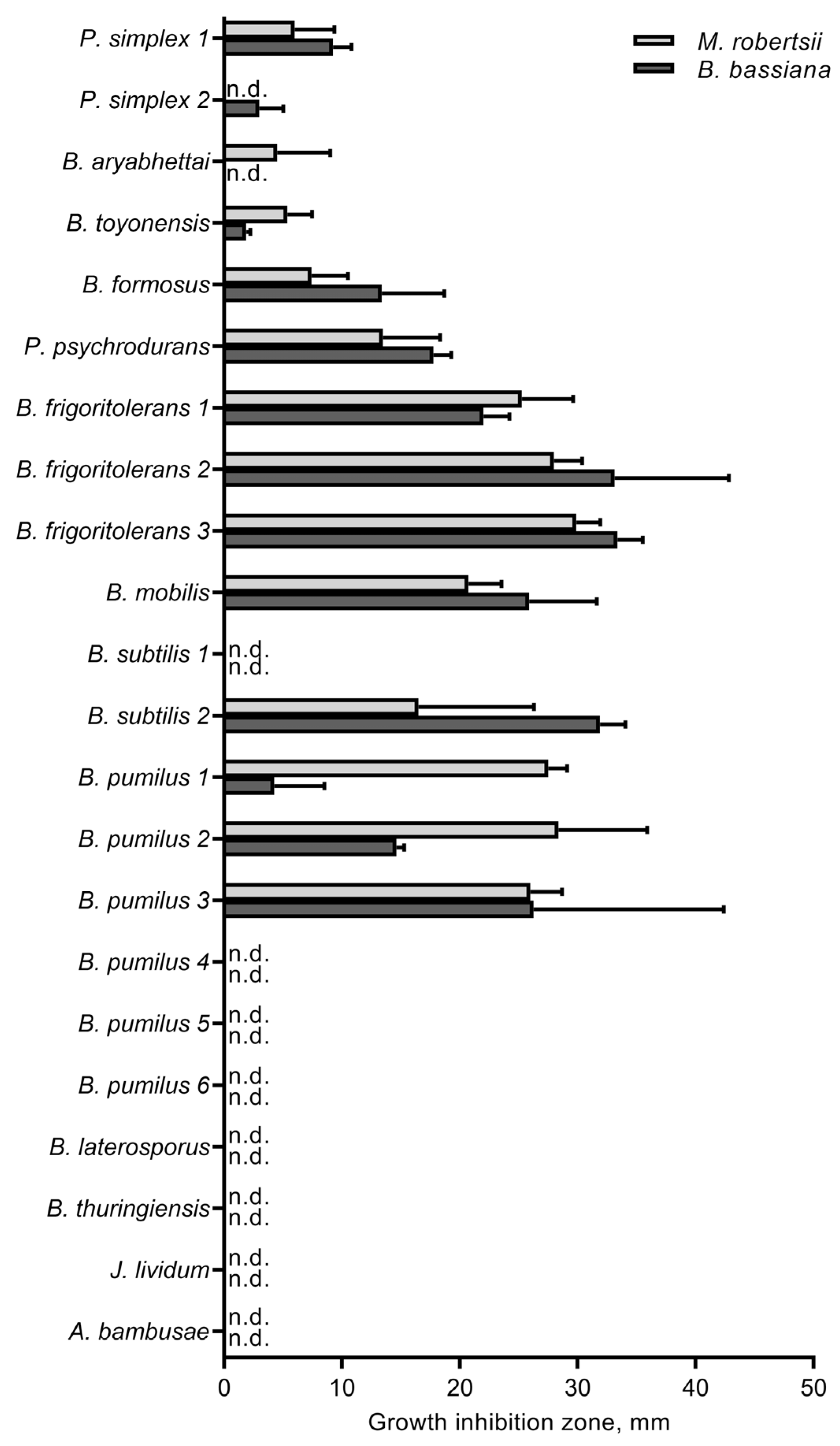
3.4. The Influence of Cultivable Bacteria on Fungi
3.5. Effects of Different Soils on Fungal Infection in the CPB
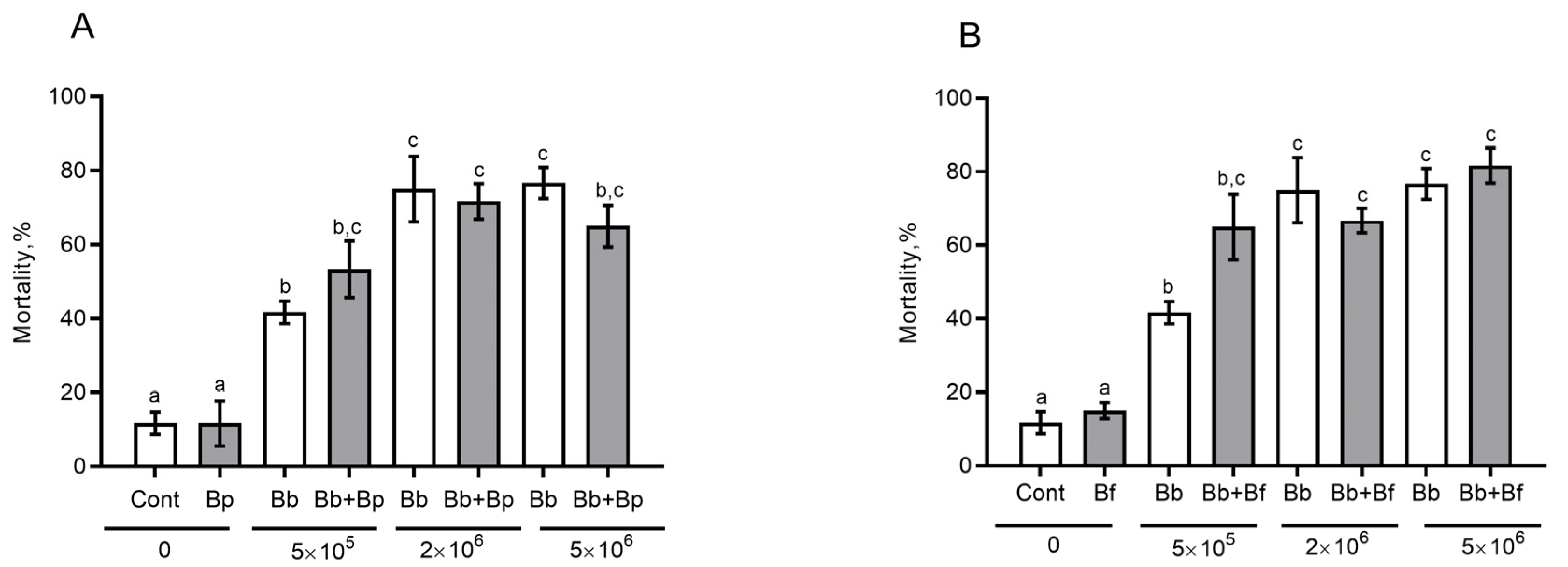
3.6. The Effect of Antagonistic Bacteria on Fungal Infection in the CPB in Sterile Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Dobbs, C.G.; Hinson, W.H.; Anderson, T.H.A. Widespread fungistasis in soils. Nature 1953, 172, 197–199. [Google Scholar] [CrossRef]
- Chuankun, X.; Minghe, M.; Leming, Z.; Keqin, Z. Soil volatile fungistasis and volatile fungistatic compounds. Soil Biol. Biochem. 2004, 36, 1997–2004. [Google Scholar] [CrossRef]
- De Boer, W.; Verheggen, P.; Klein Gunnewiek, P.J.A.; Kowalchuk, G.A.; van Veen, J.A. Microbial community composition affects soil fungistasis. Appl. Environ. Microbiol. 2003, 69, 835–844. [Google Scholar] [CrossRef]
- Zou, C.S.; Mo, M.H.; Gu, Y.Q.; Zhou, J.P.; Zhang, K.Q. Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol. Biochem. 2007, 39, 2371–2379. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef] [PubMed]
- Bamisile, B.S.; Siddiqui, J.A.; Akutse, K.S.; Ramos Aguila, L.C.; Xu, Y. General limitations to endophytic entomopathogenic fungi use as plant growth promoters, pests and pathogens biocontrol agents. Plants 2021, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Litwin, A.; Nowak, M.; Rozalska, S. Entomopathogenic fungi: Unconventional applications. Rev. Environ. Sci. Bio/Technol. 2020, 19, 23–42. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Butt, T.M. Natural and released inoculum levels of entomopathogenic fungal biocontrol agents in soil in relation to risk assessment and in accordance with EU regulations. Biocontrol Sci. Technol. 2010, 20, 503–552. [Google Scholar] [CrossRef]
- Hu, S.; Bidochka, M.J. Root colonization by endophytic insect-pathogenic fungi. J. Appl. Microbiol. 2019, 130, 570–581. [Google Scholar] [CrossRef]
- St. Leger, R.J.; Wang, J.B. Metarhizium: Jack of all trades, master of many. Open Biol. 2020, 10, 200307. [Google Scholar] [CrossRef] [PubMed]
- Jaronski, S.T. Soil ecology of the entomopathogenic ascomycetes: A critical examination of what we (think) we know. In Use of Entomopathogenic Fungi in Biological Pest Management; Ekesi, S., Maniania, N.K., Eds.; Research Signpost: Thiruvananthapuram, India, 2007; pp. 91–144. [Google Scholar]
- Popowska-Nowak, E. Interactions between soil microorganisms: Bacteria, actinomycetes and entomopathogenic fungi of the genera Beauveria and Paecilomyces. Pol. J. Ecol. 2003, 51, 85–90. [Google Scholar]
- Sharapov, V.M.; Kalvish, T.K. Effect of soil fungistasis on zoopathogenic fungi. Mycopathologia 1984, 85, 121–128. [Google Scholar] [CrossRef]
- Kraut-Cohen, J.; Zolti, A.; Shaltiel-Harpaz, L.; Argaman, E.; Rabinovich, R.; Green, S.J.; Minz, D. Effects of tillage practices on soil microbiome and agricultural parameters. Sci. Total Environ. 2020, 705, 135791. [Google Scholar] [CrossRef]
- Bziuk, N.; Maccario, L.; Douchkov, D.; Lueck, S.; Babin, D.; Sørensen, S.J.; Schikora, A.; Smalla, K. Tillage shapes the soil and rhizosphere microbiome of barley—But not its susceptibility towards Blumeria graminis f. sp. hordei. FEMS Microbiol. Ecol. 2021, 97, fiab018. [Google Scholar] [CrossRef] [PubMed]
- Srour, A.Y.; Ammar, H.A.; Subedi, A.; Pimentel, M.; Cook, R.L.; Bond, J.; Fakhoury, A.M. Microbial communities associated with long-term tillage and fertility treatments in a corn-soybean cropping system. Front. Microbiol. 2020, 11, 1363. [Google Scholar] [CrossRef]
- Naumova, N.; Baturina, O.; Nechaeva, T.; Kabilov, M. Root and rhizosphere microbiome of tomato plants grown in the open field in the south of West Siberia under mineral fertilization. Horticulturae 2022, 8, 1051. [Google Scholar] [CrossRef]
- Frøslev, T.G.; Nielsen, I.B.; Santos, S.S.; Barnes, C.J.; Bruun, H.H.; Ejrnæs, R. The biodiversity effect of reduced tillage on soil microbiota. Ambio 2022, 51, 1022–1033. [Google Scholar] [CrossRef]
- Alyokhin, A.; Kryukov, V. Ecology of a potato field. In Insect Pests of Potato, 2nd ed.; Alyokhin, A., Rondon, S.I., Gao, Y., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 451–462. ISBN 9780128212370. [Google Scholar] [CrossRef]
- Tyurin, M.; Kabilov, M.R.; Smirnova, N.; Tomilova, O.G.; Yaroslavtseva, O.; Alikina, T.; Glupov, V.V.; Kryukov, V.Y. Can Potato Plants Be Colonized with the Fungi Metarhizium and Beauveria under Their Natural Load in Agrosystems? Microorganisms 2021, 9, 1373. [Google Scholar] [CrossRef]
- Wraight, S.P.; Lacey, L.A.; Kabaluk, J.T.; Goettel, M.S. Potential for microbial biological control of coleopteran and hemipteranpests of potato. In Fruit, Vegetable and Cereal Science and Biotechnology 3 (Special Issue 1); Tennant, P., Benkeblia, N., Eds.; GlobalScience Book: Ikenobe, Japan, 2009; pp. 25–38. [Google Scholar]
- Sikura, A.I.; Sikura, L.V. Entomopathogenous fungi, bacteria, protozoa, viroses. In Colorado Potato Beetle, Leptinotarsa decemlineata Say Phylogeny, Morphology, Physiology, Ecology, Adaptation, Natural Enemies; Nauka Publishers: Moscow, Russia, 1981; pp. 299–313. (In Russian) [Google Scholar]
- Groden, E.; Lockwood, J.L. Effects of soil fungistasis on Beauveria bassiana and its relationship to disease incidence in the Colorado potato beetle, Leptinotarsa decemlineata, in Michigan and Rhode Island soils. J. Invertebr. Pathol. 1991, 57, 7–16. [Google Scholar] [CrossRef]
- Dutch Potato Growing Technology. Available online: https://en.blabto.com/1565-dutch-potato-growing-technology.html (accessed on 15 February 2023).
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UNOISE2: Improved error-correction for Illumina 16S and ITS amplicon reads. bioRxiv 2016. [Google Scholar] [CrossRef]
- Edgar, R.C. SINTAX, a Simple Non-Bayesian Taxonomy Classifier for 16S and ITS Sequences. bioRxiv 2016. [Google Scholar] [CrossRef]
- Edgar, R.C. Accuracy of taxonomy prediction for 16S rRNA and fungal ITS sequences. PeerJ 2018, 6, e4652. [Google Scholar] [CrossRef]
- Wang, Q.G.; Garrity, M.J.; Tiedje, M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Suzuki, M.T.; Taylor, L.T.; DeLong, E.F. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl. Environ. Microbiol. 2000, 66, 4605–4614. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16s ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- National Library of Medicine. BLAST: Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 12 March 2023).
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Scheirer, C.J.; Ray, W.S.; Hare, N. The analysis of ranked data derived from completely randomized factorial designs. Biometrics 1976, 32, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Lupatini, M.; Korthals, G.W.; de Hollander, M.; Janssens, T.K.S.; Kuramae, E.E. Soil microbiome is more heterogeneous in organic than in conventional farming system. Front. Microbiol. 2017, 7, 2064. [Google Scholar] [CrossRef] [PubMed]
- Jamalizadeh, M.; Etebarian, H.R.; Aminian, H.; Alizadeh, A. Biological control of Botrytis mali on apple fruit by use of Bacillus bacteria, isolated from the rhizosphere of wheat. Arch. Phytopathol. Plant Prot. 2010, 43, 1836–1845. [Google Scholar] [CrossRef]
- Mardanova, A.M.; Hadieva, G.F.; Lutfullin, M.T.; Khilyas, I.V.E.; Minnullina, L.F.; Gilyazeva, A.G.; Bogomolnaya, L.M.; Sharipova, M.R. Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agric. Sci. 2016, 8, 1–20. [Google Scholar] [CrossRef]
- Ji, S.H.; Paul, N.C.; Deng, J.X.; Kim, Y.S.; Yun, B.; Yu, S.H. Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant disease. Mycobiology 2013, 41, 234–242. [Google Scholar] [CrossRef]
- Karim, H.; Azis, A.A.; Jumadi, O. Antagonistic activity and characterization of indigenous soil isolates of bacteria and fungi against onion wilt incited by Fusarium sp. Arch. Microbiol. 2022, 204, 68. [Google Scholar] [CrossRef]
- Toledo, A.V.; Alippi, A.M.; de Remes Lenicov, A.M.M. Growth inhibition of Beauveria bassiana by bacteria isolated from the cuticular surface of the corn leafhopper, Dalbulus maidis and the planthopper, Delphacodes kuscheli, two important vectors of maize pathogens. J. Insect Sci. 2011, 11, 29. [Google Scholar] [CrossRef]
- Sivakumar, G.; Rangeshwaran, R.; Yandigeri, M.S.; Mohan, M.; Venkatesan, T.; Ballal, C.R.; Ramanujam, B.; Yalashetti, S.; Kumari, S.; Verghese, A. Characterization and role of gut bacterium Bacillus pumilus on nutrition and defense of leafhopper (Amrasca biguttula biguttula) of cotton. Indian J. Agric. Sci. 2017, 87, 534–539. [Google Scholar] [CrossRef]
- Altahtawy, M.M. Effect of Bacillus subtilis (Cohn.) on Bacillus thuringiensis Berliner and Beauveria bassiana (Balsamo) Vuillemin. Bull. Soc. Entomol. D’egypte 1970, 54, 249–257. [Google Scholar]
- Ordonez-Beltran, V.; Orduno-Cruz, N.; Rios-Velasco, C.; Jacobo-Cuellar, J.L.; Hernandez-Dominguez, C.; Acosta-Muniz, C.H. Characterization of rhizobacteria associated with Vitis vinifera and its interaction in vitro with entomopathogenic fungi. Eurasian Soil Sci. 2020, 53, 1469–1479. [Google Scholar] [CrossRef]
- Effmert, U.; Kalderas, J.; Warnke, R.; Piechulla, B. Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R. Bacterial inhibition of fungal growth and pathogenicity. Microb. Ecol. Health Dis. 1999, 11, 129–142. [Google Scholar] [CrossRef]
- Augustyniuk-Kram, A.; Mandrik, M.N.; Romanovskaya, T.V.; Kolomiets, E.I.; Kuptsov, V.N. Survival rate, insecticidal and fungistatic activity of antagonistic actinomycete Streptomyces griseoviridis and entomopathogenic fungus Beauveria bassiana in separate and combined introductions to the soil. J. Plant Prot. Res. 2007, 47, 179–186. [Google Scholar]
- Mattoso, T.C.; Moreira, D.D.O.; Samuels, R.I. Symbiotic bacteria on the cuticle of the leaf-cutting ant Acromyrmex subterraneus subterraneus protect workers from attack by entomopathogenic fungi. Biol. Lett. 2012, 8, 461–464. [Google Scholar] [CrossRef]
- Boya, P.C.A.; Fernández-Marín, H.; Mejía, L.C.; Spadafora, C.; Dorrestein, P.C.; Gutiérrez, M. Imaging mass spectrometry and MS/MS molecular networking reveals chemical interactions among cuticular bacteria and pathogenic fungi associated with fungus-growing ants. Sci. Rep. 2017, 7, 5604. [Google Scholar] [CrossRef]
- Chouvenc, T.; Efstathion, C.A.; Elliott, M.L.; Su, N.Y. Extended disease resistance emerging from the faecal nest of a subterranean termite. Proc. R. Soc. Lond. Ser. B 2013, 280, 20131885. [Google Scholar] [CrossRef]
- Li, J.; Sang, M.; Jiang, Y.; Wei, J.; Shen, Y.; Huang, Q.; Li, Y.; Ni, J. Polyene-producing Streptomyces spp. from the fungus-growing termite Macrotermes barneyi exhibit high inhibitory activity against the antagonistic fungus Xylaria. Front. Microbiol. 2021, 12, 649962. [Google Scholar] [CrossRef]
- Das, K.; Prasanna, R.; Saxena, A.K. Rhizobia: A potential biocontrol agent for soilborne fungal pathogens. Folia Microbiol. 2017, 62, 425–435. [Google Scholar] [CrossRef]
- Berg, G.; Ballin, G. Bacterial Antagonists to Verticillium dahliae Kleb. J. Phytopathol. 1994, 141, 99–110. [Google Scholar] [CrossRef]
- Borisov, B.A. Zooparasitic cordycipitoid fungi (Ascomycota, Hypocreales): ROLE of edaphic factor in the emergence and extinction of epizootics in populations of arthropods and nematodes. In Parasitological Research in Siberia and the Far East. Materials of the Interregional Scientific Conference of Parasitologists of Siberia and the Far East, Novosibirsk-Artybash, Russia, 15–20 September 2009; Taler-Press: Novosibirsk, Russia, 2009; pp. 36–39. (In Russian) [Google Scholar]
- Quintela, E.D.; Lord, J.C.; Alves, S.B.; Roberts, D.W. Persistencia de Beauveria bassian em solo de cerrado e sua interaçao com microorganismos do solo. An. Soc. Entomol. Bras. 1992, 21, 69–82. [Google Scholar] [CrossRef]
- Ho, W.C.; Ko, W.H. Microbiostasis by nutrient deficiency shown in natural and synthetic soils. J. Gen. Microbiol. 1986, 132, 2807–2815. [Google Scholar] [CrossRef]
- Selvakumar, G.; Sushil, S.N.; Stanley, J.; Mohan, M.; Deol, A.; Rai, D.; Ramkewal; Bhatt, J.C.; Gupta, H.S. Brevibacterium frigoritolerans a novel entomopathogen of Anomala dimidiata and Holotrichia longipennis (Scarabaeidae: Coleoptera). Biocontrol Sci. Technol. 2011, 21, 821–827. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Penetration of the cuticular layers of elaterid larvae (Coleoptera) by the fungus Metarhizium anisopliae, and notes on a bacterial invasion. J. Invertebr. Pathol. 1973, 21, 101–106. [Google Scholar] [CrossRef]
- Lednev, G.R.; Kryukov, V.Y.; Khodyrev, V.P.; Levchenko, M.A.; Duisembekov, B.A.; Sagitov, A.O.; Glupov, V.V. Dynamics of mortality of the migratory locust under synchronous infection with entomopathogenic fungi (Beauveria bassiana, Metarhizium anisopliae) and bacteria Pseudomonas sp. Contemp. Probl. Ecol. 2008, 1, 210–213. [Google Scholar] [CrossRef]
- Noskov, Y.A.; Kabilov, M.R.; Polenogova, O.V.; Yurchenko, Y.A.; Belevich, O.E.; Yaroslavtseva, O.N.; Alikina, T.Y.; Byvaltsev, A.M.; Rotskaya, U.N.; Morozova, V.V.; et al. A neurotoxic insecticide promotes fungal infection in Aedes aegypti larvae by altering the bacterial community. Microb. Ecol. 2021, 81, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Boucias, D.G.; Zhou, Y.; Huang, S.; Keyhani, N.O. Microbiota in insect fungal pathology. Appl. Microbiol. Biotechnol. 2018, 102, 5873–5888. [Google Scholar] [CrossRef]

| Location | Total Amount of Bacterial DNA, ng/mg | Tukey’s Test | ||
|---|---|---|---|---|
| Karasuk | Toguchin | Novosibirsk | ||
| Karasuk | 5.7 ± 0.48 | - | 0.0001 | 0.003 |
| Toguchin | 19.22 ± 1.3 | 0.0001 | - | 0.0001 |
| Novosibirsk | 0.23 ± 0.09 | 0.003 | 0.0001 | - |
| Isolate and Location of Soil | Nearest Isolate from GenBank | Accession Number in GenBank | Identity, (%) |
|---|---|---|---|
| 30720(T) | Peribacillus simplex NBRC 15720 = DSM 1321 | NR_042136 | 99.77 |
| 30920(N) | Peribacillus simplex NBRC 15720 = DSM 1321 | NR_042136 | 99.65 |
| 30220(T) | Bacillus aryabhattai strain B8W22 | NR_115953 | 100 |
| 11022(K) | Bacillus toyonensis strain BCT-7112/B. thuringiensis strain IAM 12077 | NR_121761.1 NR_043403.1 | 99.93 |
| 10222(K) | Brevibacillus formosus strain DSM 9885 | NR_040979.1 | 99.57 |
| 29720(N) | Psychrobacillus psychrodurans strain 68E3 | NR_025409 | 99.93 |
| 31320(K) | Bacillus frigoritolerans comb. nov. strain DSM 8801 | NR_115064 | 100 |
| 31120(K) | Bacillus frigoritolerans comb. nov. strain DSM 8801 | NR_117474.1 | 99.93 |
| 30020(N) | Bacillus frigoritolerans comb. nov. strain DSM 8801 | NR_115064 | 100 |
| 30420(T) | Bacillus mobilis strain MCCC 1A05942 | NR_157731 | 100 |
| 9922(K) | Bacillus subtilis strain DSM 10 | NR_027552.1 | 100 |
| 31520(K) | Bacillus subtilis strain DSM 10 | NR_027552 | 100 |
| 31420(K) | Bacillus pumilus strain ATCC 7061 | NR_043242.1 | 100 |
| 30620(T) | Bacillus pumilus strain ATCC 7061 | NR_043242 | 99.86 |
| 29820(N) | Bacillus pumilus strain NBRC 12092 | NR_112637 | 100 |
| 30820(T) | Bacillus pumilus strain NBRC 15720 = DSM 1321 | NR_112726 | 99.79 |
| 30320(T) | Bacillus pumilus strain NBRC 12092 | NR_112637 | 100 |
| 30520(T) | Bacillus pumilus strain NBRC 12092 | NR_112637 | 100 |
| 10322(K) | Brevibacillus laterosporus strain DSM 25 | NR_112212.1 | 99.78 |
| 31220(K) | Bacillus thuringiensis strain IAM 12077 | NR_043403 | 100 |
| 8722(K) | Janthinobacterium lividum strain DSM 1522 | NR_026365.1 | 99.93 |
| 30120(N) | Arthrobacter bambusae strain THG-GM18 | NR_133968 | 99.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chertkova, E.; Kabilov, M.R.; Yaroslavtseva, O.; Polenogova, O.; Kosman, E.; Sidorenko, D.; Alikina, T.; Noskov, Y.; Krivopalov, A.; Glupov, V.V.; et al. Links between Soil Bacteriobiomes and Fungistasis toward Fungi Infecting the Colorado Potato Beetle. Microorganisms 2023, 11, 943. https://doi.org/10.3390/microorganisms11040943
Chertkova E, Kabilov MR, Yaroslavtseva O, Polenogova O, Kosman E, Sidorenko D, Alikina T, Noskov Y, Krivopalov A, Glupov VV, et al. Links between Soil Bacteriobiomes and Fungistasis toward Fungi Infecting the Colorado Potato Beetle. Microorganisms. 2023; 11(4):943. https://doi.org/10.3390/microorganisms11040943
Chicago/Turabian StyleChertkova, Ekaterina, Marsel R. Kabilov, Olga Yaroslavtseva, Olga Polenogova, Elena Kosman, Darya Sidorenko, Tatyana Alikina, Yury Noskov, Anton Krivopalov, Viktor V. Glupov, and et al. 2023. "Links between Soil Bacteriobiomes and Fungistasis toward Fungi Infecting the Colorado Potato Beetle" Microorganisms 11, no. 4: 943. https://doi.org/10.3390/microorganisms11040943
APA StyleChertkova, E., Kabilov, M. R., Yaroslavtseva, O., Polenogova, O., Kosman, E., Sidorenko, D., Alikina, T., Noskov, Y., Krivopalov, A., Glupov, V. V., & Kryukov, V. Y. (2023). Links between Soil Bacteriobiomes and Fungistasis toward Fungi Infecting the Colorado Potato Beetle. Microorganisms, 11(4), 943. https://doi.org/10.3390/microorganisms11040943









