Abstract
This review provides background and perspective for the articles contributing to the Special Issue of MDPI Micro-organisms on Paramecium as a Modern Model Organism. The six articles cover a variety of topics, each taking advantage of an important aspect of Paramecium biology: peripheral surface proteins that are developmentally regulated, endosymbiont algae and bacteria, ion channel regulation by calmodulin, regulation of cell mating reactivity and senescence, and the introns that dwell in the large genome. Each article highlights a significant aspect of Paramecium and its versatility.
Keywords:
Paramecium; ciliate; calcium; calmodulin; symbiont; intron; peripheral protein; mating reactivity 1. Introduction
Reviews about ciliates often cast back to the first documented studies of these cells by the earliest microscopists, who were fascinated with their swimming behavior [1,2]. However, ciliates can be large—Paramecium species, for example, ranging from 50–300 μm long—making them easily accessible to a greater number of observers. They thus became endearing to many of us as we observed their acrobatic swimming with low-powered microscopes. This endearment inspired the effective use of ciliates in research, which has grown in popularity and recently been the subject of reviews and two special issues of this journal [3,4,5].
We focus here on the ciliate Paramecium, which Denis Lyn called the “white rat” of the Ciliophora due to their usefulness in research. Almost concurrent with a description of a “renaissance” in P. tetrauralia research [6] came an exciting discovery of a link between cilia and some human diseases, now known as ciliopathies [7]. As this study will show, ciliates, including Paramecium, gained even more momentum as useful model organisms for the study of cilia and provided insights into ciliopathies. Paramecium is now the subject of this special journal issue [8], which compiles resources from recent reviews to provide guidance into the use of Paramecium in modern research [6,9,10,11,12,13,14,15,16].
Not only are cells of Paramecium species large (50–300 μm long), but also, as holotrichous ciliates, they are covered in thousands of cilia. While not multicellular, they are bona fide organisms carrying out functions assigned to multiple different cell types in metazoans (see Preer’s Forward [17] for more details). Within their pond or stream environment, ciliates find food, mate, divide to multiply in numbers, swim by beating their cilia, respond to many kinds of stimuli, control their water balance, release waste and secrete trichocysts [18]. The cells that we feature in this review are in the Phylum Ciliophora, Class Ciliatea, Subclass Euciliata and Order Holotrichida, meaning that we can expect them to be single cells covered all over in cilia. They are of the genus Paramecium, which comprises many subspecies [19]. Nyberg and Vivier [20] explain that, over time, the subspecies were divided into two complexes (aurelia and caudatum), each with their sets of sibling species. P. tetraurelia, for example, is in the aurelia complex’s subspecies and formerly was P. aurelia syngen 4, hence the name “tetraurelia”. In addition to sibling species within the two complexes, there are other types, such as multi-micronucleatum, bursaria, trichium, calkinsi, polycaryum, woodruffi, utrinium [20]. All are aquatic free swimming and found in fresh water ponds and streams. Several past studies [21] and an article discussed in this special issue [22] contain a select phylogenetic tree. Species diversity of Paramecium may be higher than we portray here due to the existence of cryptic species [23]. For example, P. fokini n.sp. may be a cryptic species to P. multimicronucleatum, which can be distinguished only by molecular methods. However, cryptic species are not among the species described in this special issue.
Species discussed in this special issue are P. tetraurelia [24], P. bursaria [25], P. caudatum [26,27], 6 Paramecium species referenced by [28] and even more species in discussed by [22].
Hausmann and Allen [29] have documented Paramecium with beautiful microscopic images (Figure 1A,B,D). Some of their images and diagrams in Figure 1A–C show Paramecium as an asymmetrical cell with two star-shaped contractile vacuoles (cv) for osmotic control. The cilia lining the gullet sweep microscopic cells (usually bacteria, yeast or algae) into the vacuole that forms at the base of the gullet. Structures called trichocysts (tr) that are docked at the cell surface can be forcefully secreted, triggered by the touch of a predator. Figure 1A–C also show two kinds of nuclei: the large polyploid macronucleus (Mac), which is the site of transcription, and the smaller diploid nucleus (Mic), which serves germline transmission. P. tetraurelia has two Mics and its large Mac is 800 ploid. As the cells grow and divide, Mics divide by mitosis and move to the poles of the cell. The Mac divides roughly in half, but not by mitosis. Berger, in his review of Görtz’s book titled Cell Cycle, Regulation of Cell Mass and Macronuclear DNA Content [30], refers to the division of the Mac as elongation followed by “amitotic” division and resulting in 5–10% difference in DNA from parental content between the sister Macs. Beisson et al. refer to the Mac division as non-mitotic [31]. Therefore, the new cells receive identical Mics but not necessarily identical Macs.
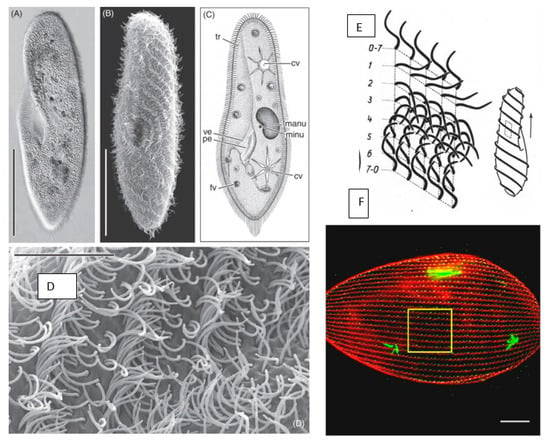
Figure 1.
Panel 1 (A,B): light microscopic appearance of Paramecium caudatum. Panel 1 (C): drawing of Paramecium illustrating light microscopic features: cv contractile vacuoles, fv food vacuoles, manu macronucleus, mino micronucleus, pe peristome, tr trichocysts and ve vestibulum. Panel 1 (D): higher magnification of the metachronal waves of the cilia. Scale bar in (A) and (B) 100 μm; (D) 10 μm. from Figure 1 in [29] and used with permission. Panel (E): form of ciliary stroke and metachrony. Diagrams of instantaneously fixed surface area with five ciliary rows with 0–2 effective, 2–7 recovery stroke. Cell diagram at left shows source of metachronal waves in forward movement. From Grell [32] and used with permission. Panel (F): immunofluorescence image of P. tetraurelia. Basal bodies green (1D5 antibody) and striated rootlets of the basal bodies red (anti-–SR). Straight rows extend between the anterior and posterior poles. [33]; used with permission.
In the laboratory, paramecia feed on bacteria and grow into large, dense cultures. To avoid contamination in experiments for protein identification and mass spectrometry, for example, the cells must be washed and treated with antibiotics [9,34]. Alternatively, when just a few cells are needed for crosses or RNA Interference (RNAi), cells can be washed by allowing them to swim upward (negative gravitaxis) several times in tubes filled with sterile buffers, or by purging the bacteria from their food vacuole system by letting them rest in buffer.
As beautifully described by Jennings more than a century ago, Paramecium lives in a rich sensory environment [35]. Dryl described in great detail the swimming behavior and motor responses of Paramecium [36]. Paramecia find food, primarily bacteria, by detecting the metabolites and by-products produced by bacterial decomposition in the pond [35,37,38]. In addition, cells move toward the water surface by negative gravitaxis. Upon mechanical stimulation of the anterior caused by bumping into an object or the touch of a predator, the cells back up. Upon stimulation of the posterior, they zoom away from predators [39,40]. Paramecium swims against water currents (rheotaxis) and avoids bright light [41] and extremes of temperature. These cells typically swim in helical paths interrupted by abrupt spontaneous changes in direction that Jennings named avoiding reactions. As we will later discuss, the modulation of the frequency of these turns as well as speed modulation form the bases of their biased random walk behavior [42,43,44,45,46]. Paramecium is prey for other micro-organisms, such as Didinium [47] and Dileptus, but Paramecium trichocysts appear to provide some protection mechanism against being swallowed by these predators [18,48].
2. Results
2.1. Peripheral Surface Proteins
The most abundant membrane proteins of Paramecium, known as surface antigens (SAgs), are peripheral proteins tethered to the cell surface by a glycosylphosphatidyl inositol (GPI) anchor [49]. Ciliary and cell body membranes display these very large proteins that coat the cell and make the cell look fuzzy in transmission electron microscopy [50]. Their functions could include buffering or protection of the cell from environmental insults. Similar proteins in parasitic protozoa are part of strategies to elude host defenses by trypanosomes or Plasmodium by expressing a large number of antigens or switching antigens to avoid the host’s antibodies [51]. In Paramecium, one strategy of interest, similar to those of antigen switching in parasitic protozoans, is programmed antigenic variation as you will see below.
Paramecium surface GPI-anchored proteins are primarily large SAgs, but also include smaller proteins. Among this latter group is a chemoreceptor [52], the evidence for which is both indirect and direct. Indirect evidence has come from antisera: antibodies against Paramecium surface antigens specifically block chemoresponse to folate. Presumably, the antisera raised against the GPI-anchored proteins included antibodies against this smaller protein as well. Secondly, antibodies against the mammalian GPI-anchored folate receptor blocks chemoresponse to folate [53]. More recently, RNAi reduction in the first enzyme in the GPI anchor synthesis, PIG-A, has selectively reduced chemoresponse to folate and glutamate [54]. A physical protein that binds folate and acts as a chemoreceptor for the attractant folate has been identified among the GPI-anchored proteins of the cilia and cell surface [52,53,55]. While the folate receptor clearly is part of a signal transduction pathway governing a chemoresponse, the mechanistic details for signaling by these peripheral proteins still need to be determined. The study by [28] discusses further receptor possibilities.
These GPI-anchored proteins, especially surface antigen (SAg), have come to be known as immobilization antigens because antibodies against them cross-link and prevent the cell from moving [56]. These antigens also relatively quickly change across the entire cell surface and in a particular order after environmental perturbations of temperature or pH; this process is known as programmed antigenic variation [57]. Since there is mutually exclusive expression of these SAg genes in the multigene family, these programmed changes must involve controls at the genome level. Only a few of the genes for surface antigens in P. tetraurelia and P. primaurelia had until now been studied, making it difficult to understand how SAg genes are transcribed and how the feat of programmed changes in the antigens is accomplished.
Additionally, the release of the SAgs from the surface for replacement by a new group of proteins seems to be accomplished by Phospholipase C isoforms that are selective for GPI protein cleavage and do not cleave all GPI-anchored proteins at once, which would lead to non-specific shedding of all the SAgs [58]. The SAgs complicate the membrane biochemistry protocols of ciliary and cell body membranes because SAGs are so numerous and the target proteins of interest are much less abundant. Therefore, it is imperative to separate the SAgs from channel proteins or transmembrane receptor proteins of cilia, as shown in [15]. A fortuitous aspect of SAgs comes to the rescue: they are easily cleaved from their anchors. During the membrane isolation process, treatment of the preparation with detergents or exogenous Phospholipase C releases the GPI-anchored proteins into the aqueous phase; they can then be separated from membranes by differential centrifugation [15,34].
Pirritano et al. in this special issue [28] present a deep dive into the SAg genes in the genomes of six Paramecium species. Their data provide insights into subfamilies, the evolution of these genes and the consensus motifs across species. The motifs that they identified hint at potential functions for these interesting proteins.
2.2. Endosymbionts
Some Paramecium species harbor algae or bacteria as endosymbionts. Two of our special issue articles address endosymbiosis.
P. bursaria cells become a beautiful green when they are inhabited by the algae Chlorella. These P. bursaria can be studied with or without the Chlorella, setting up an interesting comparison and control. Iwatsuki and Naitoh [41] showed that P. bursaria are intrinsically photosensitive, even without symbionts like Chorella that are photosynthetic. They point out that Chlorella symbionts alter the intrinsic responses of P. bursaria to changes in light; Without Chlorella, P. bursaria react to the step up in light with an avoiding reaction (AR) and transient depolarization, which help to keep the cells in the shade and out of harmful radiation. P. bursaria with Chorella show an AR upon step down to lower light, often sending them back to the lighted area where photosynthesis is supported. This behavior is a form of klinokinesis and results in accumulation of cells in the shade, as explained in more detail below.
Likewise, Matsuoka and Nakaoka [59] observed that P. bursaria accumulated in lighted regions; however, in their studies both Chorella-containing and Chorella-free ciliates responded to a step up in light with a steady depolarization. This steady depolarization translated into a slowing of cell swimming speed that would keep ciliates in the area of light. This slowing resulting in accumulation of a population is called orthokinesis and is discussed below. The cells also show an AR upon entering shade that tends to send them back into the lighted areas, thus trapping them in areas of light.
As discussed by Sommaruga and Sonntag [60], these previous studies are difficult to compare because of different cell preparations and protocols for adaptation. What is clear is that P. bursaria with Chlorella execute behaviors that are familiar to those who analyze Paramecium swimming behavior and the physiological correlates associated with electrophysiology. We will further discuss this aspect below under the section on swimming behavior.
In this special edition, Takahashi [61] takes advantage of what is known about the algae of P. bursaria to analyze the effects of environmental stress upon the host and its endosymbionts. One goal was to provide a monitoring method for endosymbiotic systems beyond P. bursaria. Temperature rise and toxins, such as paraquat cause corals, lose their algal symbionts and bleach. The system being developed by Takahashi could help to monitor and anticipate the collapse of endosymbiotic systems under stress.
The topic of endosymbiosis in Paramecium also has a long history in the study of bacteria such as the ones responsible for the Killer phenotype, in which infected cells kill non-infected ones and become immune to the effects of the Killer bacteria [62,63]. The source of the factor has been identified as the obligate symbiont bacterium Caedibacter taeniospiralis [64] and identification of the toxin(s) among secreted candidates is underway.
Fujishima et al. describe in this special journal issue [27] a different symbiotic system in which a bacterium specifically infects the macronucleus of P. caudatum. Holospora obtusa is a gram-negative bacterium that endows its host with heat-shock and high salt tolerance by modifying host cell transcription [65] (read references 1–6 in Fujishima et al. in this special issue for information on Holospora species [27]). To investigate how Mac transcription is hijacked by this bacterial symbiont, Fujishima et al. focused on the macronucleus and a 63 kDa protein, showing that it binds to P. caudatum DNA.
2.3. Mating
A characteristic of ciliates is the presence of two kinds of nuclei: one is diploid and has the expected 2n number of chromosomes for germline transmission of the genome, while the other is highly polyploid (800 ploid in Paramecium tetraurelia) for gene expression. In P. tetraurelia, there are two diploid micronuclei (Mic) and one polyploid macronucleus (Mac); in P. caudatum, there is one Mic tucked next to the large polypoid Mac (reviewed in [6]).
During the life cycle of P. tetraurelia, during the vegetative cell division cycle the cell duplicates its micronuclei by mitosis and elongates its macronucleus. The duplicated micronuclei move to the poles of the cell—two to each pole—to prepare for two new cells that will form from cytokinesis. The macronucleus also divides, though not by mitosis; the products are, therefore, not identical. DNA synthesis in Paramecium’s nuclei does not neatly follow rules of other eukaryotes. For example, Mac and Mic DNA replication follow different time regimens in the cell cycle for vegetative cell division and reproduction [30]. Nanney refers to the timing of Mac and Mic DNA synthesis as uncoupled from each other [66]. After cytokinesis there are two cells each, with two micronuclei and one macronucleus (read [31,66] for a review of this subject).
Other important features of the P. tetraurelia are the two sexual processes of mating and autogamy. Starvation of the P. tetraurelia cells (and others only of the P. aurelia complex) results in a nuclear reorganization; this can progress to mating of complementary cells or, if no complementary cells are available, to autogamy. During mating, starved complementary mating type cells will fuse over part of their surface and exchange haploid nuclei; these nuclei are the products of the multistep process by which the diploid Mic divides by meiosis twice, forming four products of which three disintegrate. The last haploid nucleus divides again, providing one nucleus to donate to the conjugant mate and one to retain. After the cells have exchanged the haploid nuclei, within each cell, the nuclei fuse to form a diploid nucleus. These further divide by mitoses, ultimately providing the two that become micronuclei for the cell and two that will contribute to the process of forming a new Mac. The old Mac disintegrates and a new Mac is formed with directions from the new Mic. In cells entering into conjugation and sexual reproduction, the Mic initiates DNA replication and meiosis late in the cell cycle while the cells are in G2 [30]. Fujishima describes the preparation of Mics for entry into meiosis [67]. At 2–2.5 h after mixing, during the Mic pre-meiotic S phase, the mating reactive cells commit to the degradation of the Mac. The DNA synthesis for the new Mac development (or duplication for adjusting Mac DNA content after vegetative or sexual cycle) is not coupled in time to the Mic DNA S phases (read Section 2.4 for a discussion of Mac development).
In the case of autogamy, the species of Paramecium that go through this process become homozygous at all loci in one manipulation in the laboratory (read the excellent diagrams in [6] and [66] that visualize both the nuclear steps in crosses and autogamy and how autogamy makes a second set of crosses to produce an F2 unnecessary). A downside of autogamy is that it fixes in place any mutations that creep into stocks and are selected for if they provide an advantage to the cells in culture [68]. Autogamy starts in the starving vegetative cell with the macronucleus breaking down and fragmenting, while the micronuclei divide by meiosis to produce haploid nuclei followed by one more mitotic division. All but one of these haploid micronuclei disintegrate; the remaining one divides and fuses to form a diploid micronucleus and, after another division, divides again to produce four diploid nuclei. The two at the posterior of the cell differentiate into macronuclei; the ones at the anterior pole remain micronuclei. During the following cytokinesis, these nuclei distribute to the two daughter cells, with each receiving two identical micronuclei and one macronucleus. Fragments of the old macronucleus continue to disintegrate. The important take away is that these new cells are now homozygous at all loci [11].
Following mating, the cells are “immature” and unable to mate again for a species-specific time period [69]. In P. caudatum, this immature period lasts for about 50 fissions. Using the important technique of microinjection, Haga and Hiwatashi [70] identified a cytoplasmic factor that inhibits mating by mature cells. From the cytoplasm used to inhibit mating, Haga and Hiwatashi isolated a small protein they called immaturin. P. caudatum cells age and, when they reach senescence, are unable to mate [70]. Immaturin also has the property of reversing senility in aging cells. This protein is the topic of the article in this special issue written by Haga et al. [26] Their research takes the study of immaturity to the genomic level (for more information about P. tetraurelia that has the capacity to undergo autogamy as well as mating, see the recent article by Haga [71]). This article also describes in detail the method and discoveries in Paramecium biology using microinjection, including identification of calmodulin as a critical component of ion channels (see below). See [12] for more protocols for microinjection.
2.4. Intronization
The Paramecium genome is physically different in each of its two forms of nuclei (read [6,72] for excellent overviews, as well as Berger’s review for the timing of DNA replication during the cell cycle [30]). The germline micronucleus of P. tetraurelia is diploid with about 50–60 small chromosomes. After each sexual cycle (mating or autogamy), a new macronucleus develops from the micronucleus by amplification of the micronucleus to about 800 ploid. However, not all the sequences of the micronucleus are found in the macronucleus. Instead, many are internally eliminated sequences (IESs) that have been precisely excised from the micronuclear DNA or imprecisely excised to create the Mac. The processes involved in these conversions of micronuclear into macronuclear genomes have been extensively documented. The precise IES removal involves transposases and comparisons of sequences by non-coding RNAs [72]. PiggyMac is a transposase that was acquired, domesticated and now utilized in the precise excision of IES in Mac development [73]. Five additional PiggyMac like proteins interact and participate in the IES excision genome-wide [74]. Imprecise elimination of the germline sequences from the new macronucleus seems to involve the fragmentation of the micronuclear genome [75] to create 200 compact chromosomes of about 50 kb to 1 Mb and characterized by little spacing between genes and coding regions with short introns. The estimated 30,000 genes of Paramecium [72,76] are closely packed into the macronuclear genome. This rather high number of genes is due to three genome-wide duplications [77].
The introns within the gene exons are very small [78]; however, they should be precisely spliced from mRNAs. Ryll et al. [24] in their contributed article ask us to consider introns, which in some systems are retained in mature mRNA 50% of the time. They use Paramecium tetraurelia as a model, which they examine on a genome-wide scale as a snapshot of ongoing evolutionary processes, such as exon-to-intron conversion (and vice versa). The authors remind us that there can be variations in splicing that are important in processes such as the development of immune cells; some intron sequences are retained in exons and serve as opportunities for evolutionary change. They also suggest how introns are gained and eventually expressed, beginning with a splicing error. Other authors would point out that an intron located between the two exons can be either excised or retained; if retained, this intron could be a by-product of alternative splicing. Clearly Ryll et al. challenge our ideas of RNA splicing, introns and exons.
2.5. Calcium, Calmodulin and Cilia in Swimming Behavior
2.5.1. We Should Not Leave Paramecium without Discussing Their Contribution to Our Knowledge of Cilia and the Spectacular Genetic Dissection of the Roles of Calmodulin
Because cilia structure and function are highly conserved, it is possible to use many different organisms to study the fundamentals of cilia and even the diseases (ciliopathies) arising from their malfunction [79]. While it is expected that many studies of cilia employ mammalian tissues (e.g., kidney cells or lung epithelia), many informative studies also come from a variety of other organisms, such as zebra fish, the algae Chlamydomonas reinhardtii, the nematode worm Caenorhabditis elegans, trypanosomes and ciliates, such as Tetrahymena and Paramecium, among many others. Paramecium studies benefit from the large number of cilia that can be harvested for biochemical studies and proteomics, compared to the single cilium per cell of the tissues with primary cilia. There are other important research approaches, such as amenability to electrophysiology and generation of mutants, for which Paramecium is advantageous, as we explain below.
Paramecia are propelled by thousands of motile cilia that are long (10 µm), thin, membrane-covered organelles protruding from the cell surface. The many cilia beat in metachronal waves (Figure 1D), as well as being physically entrained and not coordinated by any physical or electrical connections [29,80]. The basal bodies from which cilia arise are spaced in a regimented pattern of longitudinal rows from pole to pole across the cell surface (Figure 1F), which is critical to maintain the spacing needed for cilia to beat in metachronal waves. We also noted that the scanning electron microscope images of the waves (Figure 1B,D) capture the cilia in stages of the ciliary beat over time. During forward swimming, the cilia beat with their power stroke toward the posterior of the cell, driving the cell ahead. The return stroke is slower and repositions the cilium for the next power stroke. In Figure 1B,E, the curled cilia are most abundant since the cilia spend the most time in this slow recovery stage of the beat. We can compare the cilia in Figure 1D with the diagram rendered by Parducz in Figure 1E [32,81] to see the stages of the ciliary beat within the metachronal waves. They are also illustrated by Satir [82].
The cells also are able to turn and swim backward. In general, the turn consists of a short backward movement, twirling in place and renewed forward swimming in a new direction. This is the basis of the avoiding reaction described by Jennings [35] and mentioned above in the behavior of P. bursaria with Chlorella as they leave a lighted area. In the change between backward and forward movement, the sweep of the cilium toward the anterior becomes the strong power stroke, and the stroke toward the posterior becomes the lazier return stroke. The cells pivot in place with the cilia straight out as they transition between power stroke patterns. Machemer thoroughly reviews cilia movements during beating [83]. The molecular and physiological bases of these ciliary motions are discussed below.
The cilia that cover Paramecium are remarkably similar to cilia across many phyla [10,11,84]. As with other cilia, they grow from base to tip by transport of proteins from a basal body (BB), which docks at the cell membrane to the tip. The BB is characterized by triplets of microtubules and a transition zone between the axoneme and the BB for sorting, through which proteins enter and leave the cilium [85,86] (read the review by Tassin and workers [87] and more recently [10,88] for more details). The cytoskeleton of the cilium is the axoneme, with nine doublet microtubules and two singlet microtubules in the center. The microtubules slide relative to one another, allowing the cilium to bend and beat [89]. Special motor dynein Mg-ATPases interact with the microtubule doublets to move the cilia in their graceful arcs [89]. Paramecium cilia beat at about 10–20 Hz.
2.5.2. Neuronal Properties of Cilia
Physiologists from Japan, Europe and the United States developed the insight that Paramecium swimming behavior and the ciliary beat underlying it are under bioelectric control [83,90,91,92,93]. They went on to characterize channels of the cilia that are the fundamental bases for this bioelectric control (reviewed in [94,95,96]). Inevitably, the cells were given the nickname of “little swimming neuron” [97].
Figure 2 summarizes the understanding of the physiology underlying Paramecium forward and backward ciliary beating and the AR. Forward swimming, associated with a power stroke toward the posterior, is associated with the resting membrane potential (Figure 2a). A turn in the swimming path requires a short change in the power stroke causing an AR (Figure 2b). This change is initiated by a depolarization large enough to open the Ca2+ channels of the cilia, transiently allowing the Ca2+ to reach the axoneme. The power stroke changes transiently toward the anterior and the cell swims backward, usually for a short time. Since the cell is asymmetrical, the new direction of swimming after an AR is usually in a new random direction.
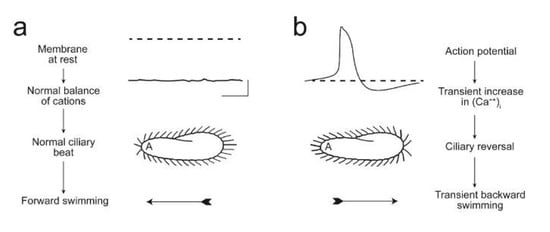
Figure 2.
(a) These images illustrate that the resting membrane potential of Paramecium is negative (about −25 to −40 mV); the corresponding ciliary beat is toward the posterior of the cell and the cell swims forward. (b) In depolarizing solutions, such as high K+ or Ba2+, the cell’s plasma membrane depolarizes and reaches threshold for the action potential. During the action potential, Ca2+ enters the cilia through voltage-gated channels; the high levels of Ca2+ change the power stroke of the cilia, which now beat most strongly toward the anterior and move the cell backward. The action potential is quickly terminated and the Ca2+ removed from or sequestered in the cilia, allowing ciliary beat and swimming to return to normal. From [94] Figure 1; used with permission.
During the AR, the return of the membrane potential to rest is accomplished by two K+ conductances: a rapid voltage activated K+ conductance (I(KV)) and a slower Ca2+ activated K+ conductance (I(KCa)). The depolarization phase of the action potential activates the fast voltage dependent ciliary K channel (KV) and the Ca2+ that enters the cilium through the CaV channels during the action potential activates the slower responding calcium-dependent ciliary K channel (KCa) [98,99]. These two types of K channels, similar to the special ciliary Ca2+ channels [100], appear to be concentrated in the cilia and reduced or absent from the soma [91,101]. The Ca2+ feed-back to inactivate the CaV channel also contributes to the end of the action potential [101].
Even though the membrane potential returns to rest, cells will continue to swim backward as long as Ca2+ remains high in the cilium. The removal or sequestration of ciliary Ca2+ is a matter of discussion, further discussion on the role of calmodulin-regulated Ca2+ pumps in this process [10,95,102].
The speed of swimming is also under bioelectric control. It depends upon the resting membrane potential, which, in turn, controls ciliary beat frequency [91,103,104]. Small hyperpolarizing stimuli increase beat frequency and swimming speed; small depolarizing stimuli do the opposite.
These basic swimming behaviors (speed and turning) underly more complex swimming feats of populations of cells. For example, paramecia swim to accumulate in or disperse from chemicals in their environment. They do not orient and swim directly toward or away from the source of chemicals by a chemotaxis. Instead, they employ more indirect methods of chemokinesis. Paramecia move in their environment in a random walk, a combination of forward swimming runs and turns due to ARs. If they enter an area with attractant molecules, they move up gradients of attractants by biasing this random walk. They accumulate in attractants by suppressing turns from ARs and increasing speed as they make their way up gradients [45]. In this complex behavior, they employ klinokinesis (turning) and orthokinesis (speed) modulation. Their behavior as they encounter repellents is the converse. If they start to go up a gradient of repellent (or begin to go down a gradient of attractant), they immediately start to turn and swim more slowly and, eventually, move out of the repellent or back into the attractant.
The fast smooth swimming with few turns in attractants results from hyperpolarization, which increases ciliary beating frequency and suppresses action potentials and turns. In repellents, cells depolarize, which increases the frequency of action potentials and turns and decreases ciliary beating frequency, slowing the swimming speed [105] (read the [38,45] for more details).
(Read Fraenkel and Gunn for an extensive description of behavior of micro-organisms including orthokinesis and klinokinesis [106]; read Manson for a description of biased random walks in bacterial chemoaccumulation [107]; read [38,42,44,108] for more information on Paramecium chemoresponse).
Returning briefly to the behavior of the P. bursaria with and without Chlorella endosymbionts, we see that the cells’ reaction of an AR and an action potential, which is to a step up in light and transient depolarization, keeps these cells in the shade by a klino-kinesis mechanism [41]. Likewise, when cells with Chlorella experience a step down to lower light, they have an AR that can send them back into the lighted area.
Matsuoka and Nakaoka [59] observed accumulation of P. bursaria (with and without Chlorella) in lighted regions by associated with a steady depolarization. Such a depolarization would slow down the cells and keep them, perhaps trapped, in the areas of light by orthokinesis. Upon entering shade, the subsequent AR would reinforce their accumulation in light by a klinokinesis. These behaviors and underlying membrane potential changes are very much like those of P. tetraurelia in attractants and repellents.
2.5.3. Calmodulin, Channels and Swimming Behavior
In this special issue, Villalobo et al. [22] provide an extensive review of Paramecium calmodulin, including the Paramecium behavior described below.
Another advantage of Paramecium that was very successfully exploited for the analysis of swimming behavior is the generation of mutants. Kung named one group of mutants Pawn because, like the chess piece, they could not move backward (reviewed in [109]). These mutants were found to lack the CaV conductance and, more recently, to lack the protein that is the cilia-specific voltage gated Ca channel in their cilia [110].
Other behavioral mutants showed prolonged backward swimming upon depolarization because they lacked the conductance from the KCa channel to quickly repolarize the cell. Others showed hypoactive response to stimuli that should depolarize, such as Na solutions. Their failure to swim backward stemmed from the failure of Ca to activate NaCa channels. Genetic analysis showed that these two apparently opposite phenotypes were due to different mutations in the same gene, while microinjection studies showed that the gene product was calmodulin [35,71]. Analysis of a large number of alleles showed that mutations with changes in the C terminal lobe were associated with the hyperactive phenotype and the changes in the N terminal lobe resulted in the hypoactive phenotype. The Kung lab had, remarkably, accomplished a genetic dissection of a molecule that is crucial in the control of ion channels.
We advise readers to refer to the reviews by [12,22,92,104] for discussions of the conductances, channels and receptors for chemoreception in P. tetraurelia.
3. Conclusions
This collection of articles gives some important insights into the use of Paramecium species in research:
- Regulation of gene exclusion and developmentally regulated sets of genes, such as for the surface antigens;
- Harboring eukaryotic and prokaryotic symbionts, which can be evaluated for their impact on their host’s physiology, gene expression and behavior;
- The most conspicuous feature of Paramecium is its lively swimming powered by cilia, which, in turn, are controlled by ion channels. Calmodulin is an important player in the Ca2+ dependence of many of these channels and other Ca2+-dependent cellular processes;
- Paramecium cells mate and exchange nuclei, and they also age and senesce. The timing and control of these processes are becoming clear;
- Lastly, the very large genomes and high ploidy provide a great source of material for examination of genome structures and development of introns.
The above studies depend upon some aspects of Paramecium that make it a handy modern model organism: large cultures for biochemistry and proteomics; genetic and molecular manipulation (e.g., RNAi); availability of large high ploidy genomes for analysis of genome rearrangements; access to ion channels in cilia and cell body membranes using electrophysiology; visualization of intracellular and surface structures by scanning and transmission of electron microscopy and cryo-tomography; expression of fluorescent-tagged proteins using microinjection and immunofluorescence microscopy techniques to follow location and trafficking; and forward mutant generation followed by whole genome sequencing to identify genes.
There is a rich assortment of resources available for research on Paramecium, such as ParameciumDB, which is a community resource that integrates the Paramecium tetraurelia genome sequence with genetic data [2,3,5,6,7].
Funding
This paper received no external funding.
Data Availability Statement
This is a review. Data are available from the primary sources.
Conflicts of Interest
The author declares no conflict of interest.
References
- Allen, S.E.; Nowacki, M. Necessity Is the Mother of Invention: Ciliates, Transposons, and Transgenerational Inheritance. Trends Genet. 2017, 33, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz, O.; Cain, S.; Cohen, J.; Sperling, L. ParameciumDB: A community resource that integrates the Paramecium tetraurelia genome sequence with genetic data. Nucleic Acids Res. 2007, 35, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz, O.; Malinowska, A.; Klotz, C.; Sperling, L.; Dadlez, M.; Koll, F.; Cohen, J. Cildb: A knowledgebase for centrosomes and cilia. Database 2009, 2009, bap022. [Google Scholar] [CrossRef]
- Arnaiz, O.; Mathy, N.; Baudry, D.; Malinsky, S.; Aury, J.-M.D.W.; Garnier, O.; Labadie, K.; Lauderdale, B.; LeMouel, A.; Marmington, A.; et al. The Paramecium germline genome provides a niche for intragenic parasitic DNA: Evolutionary dynamics of internal eliminated sequences. PLoS Genet 2012, 8, e1002984. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz, O.; Meyer, E.; Sperling, L. ParameciumDB 2019: Integrating genomic data across the genus for functional and evolutionary biology. Nucleic Acids Res. 2020, 48, D599–D605. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz, O.; Sperling, L. Paramecium DB in 2011: New tools and new data for functional and comparative genomics of the model ciliate Paramecium tetraurelia. Nucleic Acids Res. 2011, 39, D632–D636. [Google Scholar] [CrossRef]
- Arnaiz, O.; Van Dijk, V.E.; Bétermier, M.; Lhuillier-Akakpo, M.; de Vanssay, A.; Duharcourt, S.; Sallet, E.; Gouzy, J.; Sperling, L. Improved methods and resources for paramecium genomics: Transcription units, gene annotation and gene expression. BMC Genom. 2017, 18, 483. [Google Scholar] [CrossRef] [PubMed]
- Aubusson-Fleury, A.; Lemullois, M.; Bengueddach, H.; Abdallah, S.; Shi, L.; Cohen, J. Transition zone: The sequential assembly of its components parallels its dual role in basal body anchoring and ciliary function. Cilia 2015, 4, P26. [Google Scholar] [CrossRef]
- Aury, J.-M.; Jaillon, O.; Duret, L.; Noel, B.; Jubin, C.; Porcel, B.; Segurens, B.; Daubin, V.; Anthouard, V.; Aiach, N.; et al. Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 2006, 444, 171–178. [Google Scholar] [CrossRef]
- Baudry, C.; Malinsky, S.; Restituito, M.; Kapusta, A.; Rosa, S.; Meyer, E.; Betermier, M. PiggyMac a domesticated piggyBac transposase involved in programmed genome rearrangements in the ciliate Paramecium tetraurelia. Gene Dev. 2009, 23, 2478–2483. [Google Scholar] [CrossRef]
- Beisson, J.; Bétermier, M.; Bré, M.-H.; Cohen, J.; Duharcourt, S.; Duret, L.; Kung, C.; Malinsky, S.; Meyer, E.; Preer, J.R.; et al. Paramecium tetraurelia: The Renaissance of an Early Unicellular Model. Cold Spring Harb. Protoc 2010, 2010, pdb.emo140. [Google Scholar] [CrossRef] [PubMed]
- Beisson, J.; Bétermier, M.; Bré, M.-H.; Cohen, J.; Duharcourt, S.; Duret, L.; Kung, C.; Malinsky, S.; Meyer, E.; Preer, J.R., Jr.; et al. DNA microinjection into the macronucleus of Paramecium. Cold Spring Harb. Protoc. 2010, 2, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.D. The Cell cycle and Regulation of Cell Mass and Macronuclear DNA Content. In Paramecium; Gortz, H.-D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 97–119. [Google Scholar]
- Bouhouche, K.; Valentine, M.S.; Le Borgne, P.; Lemullois, M.; Yano, J.; Lodh, S.; Nabi, A.; Tassin, A.M.; Van Houten, J.L. Paramecium a Model to Study Ciliary Beating and Ciliogenesis: Insights From Cutting-Edge Approaches. Front. Cell Dev. Biol. 2022, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Hildebrandt, F. Ciliopathies. Cold Spring Harb. Perspect. Biol. 2017, 9, a028191. [Google Scholar] [CrossRef]
- Brehm, P.; Eckert, R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science 1978, 202, 1203–1206. [Google Scholar] [CrossRef]
- Brehm, P.; Eckert, R. An electrophysiological study of the regulation of ciliary beating frequency in Paramecium. J. Physiol 1978, 283, 557–568. [Google Scholar] [CrossRef]
- Brette, R. Integrative Neuroscience of Paramecium, a Swimming Neuron. eNeuro 2021, 8, 1–21. [Google Scholar] [CrossRef]
- Butzel, H. Mating Type Determination and Development in Paramecium aurelia. In Paramecium A Current Survey; Van Wagentendonk, W., Ed.; Elsevier: Amsterdam, The Netherlands, 1974; pp. 118–122. [Google Scholar]
- Capdeville, Y.; Benwakrim, A. The major ciliary membrane proteins in Paramecium primaurelia are all glycosylphosphatidylinositol-anchored proteins. Eur. J. Cell Biol. 1996, 70, 339–346. [Google Scholar]
- Catania, F.; Wurmser, F.; Potekhin, A.A.; Przybos, E.; Lynch, M. Genetic Diversity in the Paramecium aurelia Species Complex. Mol. Biol. Evol. 2009, 26, 421–431. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Van Houten, J.; Robles, L.J.; Lui, S.; Kung, C. An extensive behavioural and genetic analysis of Parn mutants in Paramecium aurelia. Gene. Res. 1976, 23, 165–173. [Google Scholar] [CrossRef]
- Deitsch, K.; Lukehart, S.; Stringer, J. Common strategies for antigenic variation by bacterial, fungal and protozoan pathogens. Nat. Rev. Microbiol. 2009, 7, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Dryl, S. Behavior and Motor Response of Paramecium. In Paramecium a Current Survey; Van Wagtendonk, W., Ed.; Elsevier: Amsterdam, The Netherlands, 1974; pp. 165–218. [Google Scholar]
- Dunlap, K. Localization of calcium channels in Paramecium caudatum. J. Physiol. 1977, 271, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R. Bioelectric control of ciliary activity. Science 1972, 176, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.; Naitoh, Y. Passive electrical properties of paramecium and problems of ciliary coordination. J. Gen. Physiol. 1970, 55, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.; Naitoh, Y. Bioelectric control of locomotion in the ciliates. J. Protozool. 1972, 19, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Fraenkel, G.S.; Gunn, D.L. The Orientation of Animals; Dover Publications: New York, NY, USA, 1961. [Google Scholar]
- Fujishima, M. Conjugation. In Paramecium; Görtz, H.-D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 70–84. [Google Scholar]
- Fujishima, M.; Kawai, M.; Yamamoto, R. Paramecium caudatum acquires heat-shock resistance in ciliary movement by with the endonuclear symbiotic bacterium Holospora obtusa. FEMS Microbiol. Lett. 2005, 243, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.; Kawano, H.; Miyakawa, I. A 63-kDa Periplasmic Protein of the Endonuclear Symbiotic Bacterium Holospora obtusa Secreted to the Outside of the Bacterium during the Early Infection Process Binds Weakly to the Macronuclear DNA of the Host Paramecium caudatum. Microorganisms 2023, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Gortz, H.-D. Paramecium; Springer-Verlag: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Grell, K. Protozoology. Springer-Verlag: Berlin/Heidelberg, Germany, 1973; ISBN 978-3-642-61958-8. [Google Scholar]
- Haga, N. Micromanipulation in Paramecium: From non-mendelian inheritance to the outlook for versatile micromachines. J. Eukaryot. Microbiol. 2022, 69, e12909. [Google Scholar] [CrossRef]
- Haga, N.; Hiwatashi, K. A protein called immaturin controlling sexual maturity in Paramecium. Nature 1981, 289, 177–179. [Google Scholar] [CrossRef]
- Haga, N.; Saimi, Y.; Takahashi, M.; Kung, C. Intra- and interspecific complementation of membrane-inexcitable mutants of Paramecium. J. Cell Biol. 1983, 97, 378–382. [Google Scholar] [CrossRef]
- Haga, N.; Usui, T.; Takenaka, Y.; Chiba, Y.; Abe, T. Immaturin-Nuclease as a Model System for a Gene-Programmed Sexual Development and Rejuvenescence in Paramecium Life History. Microorganisms 2023, 11, 82. [Google Scholar] [CrossRef]
- Hausmann, k.; Allen, R.D. Electron Microscopy of Paramecium (Ciliata). Methods Cell Biol. 2010. [Google Scholar]
- Hill, J. An History of Animals; John Osborne Grays-Inn.: London, UK, 1727. [Google Scholar]
- Howard, A.E.; Kar, P.; Fabritius, A.; Winey, M. Recent advances in ciliate biology. Annu. Rev. Cell Dev.Biol. 2022, 38, 75–102. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Naitoh, Y. The Role of Symbiotic Chlorella in Photoresponses of Paramecium bursaria. Proc. Jpn. Acad. 1981, 57, 318–323. [Google Scholar] [CrossRef]
- Jennings, H. Behavior of the Lower Organisms; Indiana University Press: Bloomington, IN, USA, 1906. [Google Scholar]
- Kleene, S.J.; Van Houten, J.L. Electrical Signaling in Motile and Primary Cilia. BioScience 2014, 64, 1092–1102. [Google Scholar] [CrossRef]
- Knoll, G.; Haacke-Bell, B.; Plattner, H. Local trichocyst exocytosis provides an efficient escape mechanism for Paramecium cells. Eur. J. Protistol. 1991, 27, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.; Chang, S.; Satow, Y.; Van Houten, J.; Hansma, H. Genetic dissection of behavior in Paramecium. Science 1975, 188, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.; Preston, R.R.; Maley, M.E.; Ling, K.Y.; Kanabrocki, J.A.; Seavey, B.R.; Saimi, Y. In vivo Paramecium mutants show that calmodulin orchestrates membrane responses to stimuli. Cell Calcium 1992, 13, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.; Saimi, Y. The physiological basis of taxes in Paramecium. Annu. Rev. Physiol. 1982, 44, 519–534. [Google Scholar] [CrossRef]
- Kutomi, O.; Takemura, M.; Kamachi, H.; Noguchi, M. Estimation of Effective Concentrations of ATP-Regenerating Enzymes in Cilia of Paramecium caudatum. J. Eukaryot. Microbiol. 2012, 59, 49–53. [Google Scholar] [CrossRef]
- Le Mouel, A.; Butler, A.; Caron, F.; Meyer, E. Developmentally regulated chromosome fragmentation linked to imprecise elimination of repeated sequences in paramecia. Eukaryot. Cell 2003, 2, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenhoek, A. Observation, communicated to the publisher by Mr. Antony van Leewenhoek, in a Dutch letter of the 9 Octob. 1676. Philos. Trans. R. Soc. Lond. 1677, 12, 821–831. [Google Scholar]
- LeGuennec, M.; Klena, N.; Aeschlimann, G.; Hamel, V.; Guichard, P. Overview of the centriole architecture. Curr. Opin. Struct. Biol. 2021, 66, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Lodh, S.; Yano, J.; Valentine, M.S.; Van Houten, J.L. Voltage-gated calcium channels of Paramecium cilia. J. Exp. Biol. 2016, 219, 3028–3038. [Google Scholar] [CrossRef]
- Machemer, H. Electrophysiology. In Paramecium; Gortz, H.-D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 186–215. [Google Scholar]
- Machemer, H. Motor Control of cilia. In Paramecium; Gortz, H.-D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 216–235. [Google Scholar]
- Machemer, H. Cellular behavior modulated by ions: Electrophysiological implications. J. Protozool. 1989, 36, 463–487. [Google Scholar] [CrossRef]
- Machemer, H.; Machemer-Rohnisch, S.; Braucker, R.; Takahashi, K. Gravikinesis in Paramecium: Theory and isolation of the physiological response to the natural gravity vector. J. Comp. Physiol. A 1991, 168, 1–12. [Google Scholar] [CrossRef]
- Manson, M.D. Howard Berg’s Random Walk through Biology. J. Bacteriol. 2020, 202, e00494-20. [Google Scholar] [CrossRef]
- Matsuoka, K.; Nakaoka, Y. Photoreceptor Potential Causing Phototaxis of Paramecium Bursaria. J. Exp. Biol. 1988, 137, 477–485. [Google Scholar] [CrossRef]
- Melekhin, M.; Yakovleva, Y.; Lebedeva, N.; Nekrasova, I.; Nikitashina, L.; Castelli, M.; Mayén-Estrada, R.; Romanovich, A.E.; Petroni, G.; Potekhin, A. Potekhin Cryptic Diversity in Paramecium multimicronucleatum Revealed with a Polyphasic Approach. Microorganisms 2022, 10, 974. [Google Scholar] [CrossRef]
- Miceli, C.; Valessi, A.; Pearlman, R.E. Ciliates as Model Organisms: From ‘omics’ to Genetics, Ecology and Signaling. Microorganisms 2023. [Google Scholar]
- Muller, A.; Kloppel, C.; Smith-Valentine, M.; Van Houten, J.; Simon, M. Selective and programmed cleavage of GPI-anchored proteins from the surface membrane by phospholipase C. Biochim. Et Biophys. Acta (BBA) Biomembr. 2012, 1818, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Nabi, A.; Yano, J.; Valentine, M.S.; Picariello, T.; Van Houten, J.L. SF-Assemblin genes in Paramecium: Phylogeny and phenotypes of RNAi silencing on the ciliary-striated rootlets and surface organization. Cilia 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Nanney, D. Experimental Ciliatology; Wiley-Interscience, John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Nyberg, D. The species comcept and breeding systems. In Paramecium; Gortz, H.-D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 41–58. [Google Scholar]
- Ogura, A.; Machemer, H. Distribution of mechanoreceptor channels in the Paramecium surface membrane. J. Comp. Physiol. 1980, 13, 233–242. [Google Scholar] [CrossRef]
- Paquette, C.; Rakochy, V.; Bush, A.; Van Houten, J.L. Glycophosphatidylinositol-anchored proteins in Paramecium tetraurelia: Possible role in chemoresponse. J. Exp. Biol. 2001, 204, 2899–2910. [Google Scholar] [CrossRef]
- Parducz, B. Ciliary Movement and Coordination in Ciliates. Int. Rev. Cytol. 1967, 21, 91–128. [Google Scholar]
- Pazour, G.J.; Rosenbaum, J.L. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002, 12, 551–555. [Google Scholar] [CrossRef]
- Pirritano, M.; Yakovleva, A.; Potekhin, A.; Simon, M. Species-Specific Duplication of Surface Antigen Genes in Paramecium. Microorganisms 2022, 10, 2378. [Google Scholar] [CrossRef]
- Pirritano, M.; Zaburannyi, N.; Grosser, K.; Gasparoni, G.; Müller, R.; Simon, M.; Schrallhammer, M. Dual-Seq reveals genome and transcriptome of Caedibacter taeniospiralis, obligate encosymbiont of Paramecium. Nat. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Plattner, H. Trichocysts—Paramecium’s Projectile-like Secretory Organelles. J. Eukaryot. Microbiol. 2017, 64, 106–133. [Google Scholar] [CrossRef]
- Plattner, H. Ciliate research: From myth to trendsetting science. J. Eukaryot. Microbiol. 2022, 69, e12926. [Google Scholar] [CrossRef]
- Plattner, H. Membrane traffic and Ca2+ signals in ciliates. J. Eukaryot. Microbiol. 2022, 69, e12895. [Google Scholar] [CrossRef] [PubMed]
- Pond, F.R.; Gibson, I.; Lalucat, J.; Quackenbush, R.L. R-body-producing bacteria. Microbiol. Rev. 1989, 53, 25–67. [Google Scholar] [CrossRef]
- Quackenbush, R.L. Endosymbionts of killer paramecia. In Paramecium; Gortz, H.-D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 406–418. [Google Scholar]
- Ramanathan, R.; Adoutte, A.; Dute, R. Biochemical studies of the excitable membrane of Paramecium tetrarurelia. V. Effects of proteases on the ciliary membrane. Biochim. Biophys. Acta 1981, 6441, 349–365. [Google Scholar] [CrossRef]
- Ramanathan, R.; Saimi, Y.; Peterson, J.B.; Nelson, D.L.; Kung, C. Antibodies to the ciliary membrane of Paramecium tetraurelia alter membrane excitability. J. Cell Biol. 1983, 97, 1421–1428. [Google Scholar] [CrossRef]
- Russell, C.B.; Fraga, D.; Hinrichsen, R.D. Extremely short 20-33 nucleotide introns are the standard length in Paramecium tetraurelia. Nucleic Acids Res 1994, 22, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Ryll, J.; Rothering, R.; Catania, F. Intronization Signatures in Coding Exons Reveal the Evolutionary Fluidity of Eukaryotic Gene Architecture. Microorganisms 2022, 10, 1901. [Google Scholar] [CrossRef] [PubMed]
- Saimi, Y.; Hinrichesen, M.F.; Kung, C. Mutant analysis shows that the Ca2+-induced K+ current shuts off one type of excitation in Paramecium. Proc. Natl. Acad. Sci. USA 1983, 80, 5112–5116. [Google Scholar] [CrossRef]
- Saimi, Y.; Kung, C. Behavioral genetics of paramecium. Annu. Rev. Genet. 1987, 21, 47–65. [Google Scholar] [CrossRef]
- Satir, P.; Heuser, T.; Sale, W. A structural basis for how motile cilia beat. BioScience 2014, 64, 1073–1083. [Google Scholar] [CrossRef]
- Satow, Y.; Kung, C. Ca-induced K+ outward current in Paramecium tetraurelia. J. Exp. Biol. 1980, 88, 293–303. [Google Scholar] [CrossRef]
- Simon, M.C.; Schmidt, H.J. Antigenic variation in ciliates: Antigen structure, function, expression. J. Eukaryot. Microbiol. 2007, 54, 1–7. [Google Scholar] [CrossRef]
- Sommaruga, R.; Sonntag, B. Photobiological Aspects of the Mutualistic Association between Paramecium bursaria and Chlorella. Endosymbionts Paramecium 2009, 12, 111–130. [Google Scholar]
- Takahashi, M. BEhavioral mutants in Paramecium caudatum. GEnetics 1979, 91, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Method for stress assessment of endocymbiotic algae in Paramecium bursaria as a model system for endosymbiosis. Microorganisms 2022, 10, 1248. [Google Scholar] [CrossRef]
- Tassin, A.-M.; Lemullois, M.; Aubusson-Fleury, A. Paramecium tetraurelia basal body structure. Cilia 2015, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.; Rajendran, A.; Yano, J.; Weeraratne, S.; Beisson, J.; Cohen, J.; Koll, F.; Van Houten, J. Paramecium BBS genes are key to presence of channels in Cilia. Cilia 2012, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.; Van Houten, J. Using paramecium as a model for ciliopathies. Genes 2021, 12, 1493. [Google Scholar] [CrossRef]
- Valentine, M.; Van Houten, J. Ion Channels in Cilia: Paramecium as a Model. J. Eukaryot. Microbiol. 2022, 69, e12884. [Google Scholar] [CrossRef]
- Valentine, M.; Van Houten, J. Methods for Paramecium tetraurelia ciliary membrane protein identification and function: Using Paramecium as a model for ciliopathies. In Methods in Cell Biology: Cilia from Pathogenesis to Disease.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Valentine, M.; Yano, J.; Van Houten, J. Chemosensory Transduction in paramecium. Jpn. J. Protozool. 2008, 41, 1–7. [Google Scholar]
- Van Houten, J.L. Chemosensory transduction in Paramecium. Eur. J. Protistlogy 1998, 34, 301–307. [Google Scholar] [CrossRef]
- Van Houten, J.L. Paramecium Biology. In Evo-Devo: Non-Model Species in Cell and Developmental Biology, Results and Problems in Differentiarion 68; Tworzydlo, W., Bilinski, S.M., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 68, pp. 291–318. [Google Scholar]
- Van Houten, J.L. Paramecium as a modern model system. Microorganisms 2023. Available online: https://www.mdpi.com/journal/microorganisms/special_issues/Paramecium (accessed on 28 March 2023).
- Houten, V.J.; Martel, E.; Kasch, T. Kinetic analysis of chemokinesis of Paramecium. J. Protozool. 1982, 29, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Van Houten, V.J.; Van Houten, J. Computer analysis of Paramecium chemokinesis behavior. J. Theor. Biol. 1982, 98, 4453–4468. [Google Scholar]
- Van Houten, J.L. Two mechanisms of chemotaxis in Paramecium. J. Comp. Physiol. A 1978, 127, 167–174. [Google Scholar] [CrossRef]
- Van Houten, J.L. Membrane potential changes during chemokinesis in Paramecium. Science 1979, 204, 1100–1103. [Google Scholar] [CrossRef]
- Van Houten, J.L. Chemoresponse in Microorganisms. Ann. Rev. Physiol. 1992, 54, 639–663. [Google Scholar] [CrossRef]
- Van Houten, J.L.; Preston, R. Chemokinesis. In Paramecium; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Villalobo, E.; Gutiérrez, G.; Villalobo, A. Calmodulin in Paramecium: Focus on Genomic Data. Microorganisms 2022, 10, 1915. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E. Morphology, Taxonomy and General Biology of the Genus Paramecium. In Paramecium, a Current Survey; Wagtendonk, W.J.V., Ed.; Elsevier: Amsterdam, The Netherlands, 1974; pp. 1–90. [Google Scholar]
- Weeraratne, S. GPI-anchored chemoreceptors in folate chemosensory transduction in Paramecium tetraurelia. Ph.D. Thesis, University of Vermont, Burlington, VT, USA, 2007. [Google Scholar]
- Wessenberg, H.; Antipa, G. Capture and Ingestion of Paramecium by Didinium nasutum. J. Protozool. 1970, 17, 250–270. [Google Scholar] [CrossRef]
- Yano, J.; Rajendran, A.; Valentine, M.S.; Saha, M.; Ballif, B.A.; Van Houten, J.L. Proteomic analysis of the cilia membrane of Paramecium tetraurelia. J. Proteomics 2013, 78, 113–122. [Google Scholar] [CrossRef]
- Yano, J.; Rakochy, V.; Van Houten, J.L. Glycosyl phosphatidylinositol-anchored proteins in chemosensory signaling: Antisense manipulation of Paramecium tetraurelia PIG-A gene expression. Eukaryot. Cell 2003, 2, 1211–1219. [Google Scholar] [CrossRef]
- Yano, J.; Wells, R.; Lam, Y.-W.; Van Houten, J.L. Ciliary Ca2+ pumps regulate intraciliary Ca2+ from the action potential and may co-localize with ciliary voltage-gated Ca2+ channels. J. Exp. Biol. 2021, 224, jeb232074. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).