Synthesis of Novel Benzenesulfonamide-Bearing Functionalized Imidazole Derivatives as Novel Candidates Targeting Multidrug-Resistant Mycobacterium abscessus Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment Used for Synthesis and Characterization of Compounds
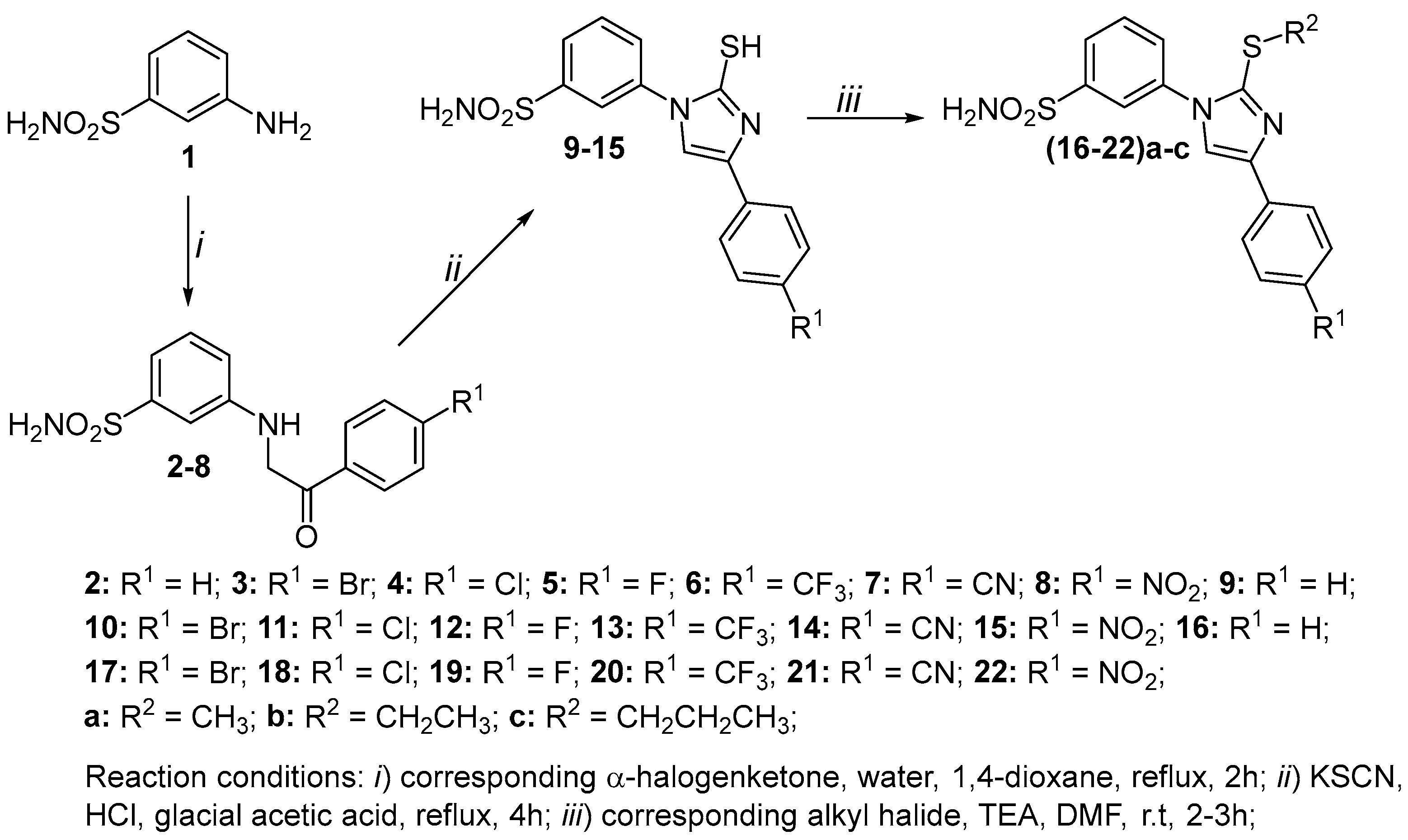
2.2. Synthesis
2.3. Minimal Inhibitory Concentration Determination
2.3.1. Preparation of Assay Microplates
2.3.2. Antibacterial Activity Characterization Using Gram-Positive and Gram-Negative Pathogens
2.3.3. Antifungal Activity Characterization
2.3.4. Antimycobacterial Activity Determination
3. Results and Discussion
3.1. Chemistry
3.2. Benzenesulfonamide Derivatives 2-22a-c Demonstrated Structure-Depended Antimicrobial Activity against Multidrug-Resistant Non-Tuberculous Mycobacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelaal, H.F.M.; Chan, E.D.; Young, L.; Baldwin, S.L.; Coler, R.N. Mycobacterium Abscessus: It’s Complex. Microorganisms 2022, 10, 1454. [Google Scholar] [CrossRef]
- Shiraishi, K.; Kasai, H.; Saito, M.; Kawaguchi, H.; Kinoshita, T.; Suzuki, T.; Shikano, K.; Takagi, K.; Sakao, S.; Hanazawa, T.; et al. Case of a Deep Neck Abscess During Treatment for COVID-19. Am. J. Case Rep. 2022, 23, e936034-1. [Google Scholar] [CrossRef] [PubMed]
- Furuuchi, K.; Morimoto, K.; Yoshiyama, T.; Tanaka, Y.; Fujiwara, K.; Okumura, M.; Izumi, K.; Shiraishi, Y.; Mitarai, S.; Ogata, H.; et al. Interrelational Changes in the Epidemiology and Clinical Features of Nontuberculous Mycobacterial Pulmonary Disease and Tuberculosis in a Referral Hospital in Japan. Respir. Med. 2019, 152, 74–80. [Google Scholar] [CrossRef]
- Shin, S.H.; Jhun, B.W.; Kim, S.-Y.; Choe, J.; Jeon, K.; Huh, H.J.; Ki, C.-S.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Nontuberculous Mycobacterial Lung Diseases Caused by Mixed Infection with Mycobacterium Avium Complex and Mycobacterium Abscessus Complex. Antimicrob. Agents Chemother. 2018, 62, e01105-18. [Google Scholar] [CrossRef] [PubMed]
- Philley, J.V.; Griffith, D.E. Medical Management of Pulmonary Nontuberculous Mycobacterial Disease. Thorac. Surg. Clin. 2019, 29, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Wang, W.; Zhao, W.; Zuo, L.; Wang, D.; Chai, X.; Cui, J. Differentiating Nontuberculous Mycobacterium Pulmonary Disease from Pulmonary Tuberculosis through the Analysis of the Cavity Features in CT Images Using Radiomics. BMC Pulm. Med. 2022, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Daley, C.L.; Griffith, D.E.; Loebinger, M.R. Management of Mycobacterium Avium Complex and Mycobacterium Abscessus Pulmonary Disease: Therapeutic Advances and Emerging Treatments. Eur. Respir. Rev. 2022, 31, 210212. [Google Scholar] [CrossRef]
- Togo, T.; Atsumi, J.; Hiramatsu, M.; Shimoda, K.; Morimoto, K.; Shiraishi, Y. Outcomes of Surgical Treatment for Mycobacterium Abscessus Complex Pulmonary Disease. Ann. Thorac. Surg. 2022, 113, 949–956. [Google Scholar] [CrossRef]
- Qin, R.; Wang, P.; Wang, B.; Fu, L.; Batt, S.M.; Besra, G.S.; Wu, C.; Wang, Y.; Huang, H.; Lu, Y.; et al. Identification of Thiophene-Benzenesulfonamide Derivatives for the Treatment of Multidrug-Resistant Tuberculosis. Eur. J. Med. Chem. 2022, 231, 114145. [Google Scholar] [CrossRef]
- Du, J.; Liu, P.; Zhu, Y.; Wang, G.; Xing, S.; Liu, T.; Xia, J.; Dong, S.; Lv, N.; Li, Z. Novel Tryptanthrin Derivatives with Benzenesulfonamide Substituents: Design, Synthesis, and Anti-Inflammatory Evaluation. Eur. J. Med. Chem. 2023, 246, 114956. [Google Scholar] [CrossRef]
- Audat, S.A.; Al-Shar’i, N.A.; Al-Oudat, B.A.; Alnabulsi, S. Design, Synthesis, and Biological Evaluation of SMYD3 Inhibitors Possessing N-Thiazole Benzenesulfonamide Moiety as Potential Anti-Cancer Agents. J. Saudi Chem. Soc. 2022, 26, 101482. [Google Scholar] [CrossRef]
- Muthukumar, R.; Karnan, M.; Elangovan, N.; Karunanidhi, M.; Thomas, R. Synthesis, Spectral Analysis, Antibacterial Activity, Quantum Chemical Studies and Supporting Molecular Docking of Schiff Base (E)-4-((4-Bromobenzylidene) Amino)Benzenesulfonamide. J. Indian Chem. Soc. 2022, 99, 100405. [Google Scholar] [CrossRef]
- Dhumad, A.M.; Jassem, A.M.; Alharis, R.A.; Almashal, F.A. Design, Cytotoxic Effects on Breast Cancer Cell Line (MDA-MB 231), and Molecular Docking of Some Maleimide-Benzenesulfonamide Derivatives. J. Indian Chem. Soc. 2021, 98, 100055. [Google Scholar] [CrossRef]
- Kanagavalli, A.; Jayachitra, R.; Thilagavathi, G.; Padmavathy, M.; Elangovan, N.; Sowrirajan, S.; Thomas, R. Synthesis, Structural, Spectral, Computational, Docking and Biological Activities of Schiff Base (E)-4-Bromo-2-Hydroxybenzylidene) Amino)-N-(Pyrimidin-2-Yl) Benzenesulfonamide from 5-Bromosalicylaldehyde and Sulfadiazine. J. Indian Chem. Soc. 2023, 100, 100823. [Google Scholar] [CrossRef]
- Carrión, M.D.; Rubio-Ruiz, B.; Franco-Montalban, F.; Amoia, P.; Zuccarini, M.C.; De Simone, C.; Camacho, M.E.; Amoroso, R.; Maccallini, C. New Amidine-Benzenesulfonamides as INOS Inhibitors for the Therapy of the Triple Negative Breast Cancer. Eur. J. Med. Chem. 2023, 248, 115112. [Google Scholar] [CrossRef] [PubMed]
- El-Azab, A.S.; Alkahtani, H.M.; AlSaif, N.A.; Al-Suwaidan, I.A.; Obaidullah, A.J.; Alanazi, M.M.; Al-Obaid, A.M.; Al-Agamy, M.H.M.; Abdel-Aziz, A.A.-M. Synthesis, Antiproliferative and Enzymatic Inhibition Activities of Quinazolines Incorporating Benzenesulfonamide: Cell Cycle Analysis and Molecular Modeling Study. J. Mol. Struct. 2023, 1278, 134928. [Google Scholar] [CrossRef]
- Balandis, B.; Šimkūnas, T.; Paketurytė-Latvė, V.; Michailovienė, V.; Mickevičiūtė, A.; Manakova, E.; Gražulis, S.; Belyakov, S.; Kairys, V.; Mickevičius, V.; et al. Beta and Gamma Amino Acid-Substituted Benzenesulfonamides as Inhibitors of Human Carbonic Anhydrases. Pharmaceuticals 2022, 15, 477. [Google Scholar] [CrossRef]
- Kakakhan, C.; Türkeş, C.; Güleç, Ö.; Demir, Y.; Arslan, M.; Özkemahlı, G.; Beydemir, Ş. Exploration of 1,2,3-Triazole Linked Benzenesulfonamide Derivatives as Isoform Selective Inhibitors of Human Carbonic Anhydrase. Bioorganic Med. Chem. 2023, 77, 117111. [Google Scholar] [CrossRef]
- Vaškevičienė, I.; Paketurytė, V.; Zubrienė, A.; Kantminienė, K.; Mickevičius, V.; Matulis, D. N-Sulfamoylphenyl- and N-Sulfamoylphenyl-N-Thiazolyl-β-Alanines and Their Derivatives as Inhibitors of Human Carbonic Anhydrases. Bioorganic Chem. 2017, 75, 16–29. [Google Scholar] [CrossRef]
- Aspatwar, A.; Winum, J.-Y.; Carta, F.; Supuran, C.T.; Hammaren, M.; Parikka, M.; Parkkila, S. Carbonic Anhydrase Inhibitors as Novel Drugs against Mycobacterial β-Carbonic Anhydrases: An Update on In Vitro and In Vivo Studies. Molecules 2018, 23, 2911. [Google Scholar] [CrossRef]
- Stahl, D.A.; Urbance, J.W. The Division between Fast- and Slow-Growing Species Corresponds to Natural Relationships among the Mycobacteria. J. Bacteriol. 1990, 172, 116–124. [Google Scholar] [CrossRef]
- Aspatwar, A.; Hammarén, M.; Koskinen, S.; Luukinen, B.; Barker, H.; Carta, F.; Supuran, C.T.; Parikka, M.; Parkkila, S. β-CA-Specific Inhibitor Dithiocarbamate Fc14–584B: A Novel Antimycobacterial Agent with Potential to Treat Drug-Resistant Tuberculosis. J. Enzym. Inhib. Med. Chem. 2017, 32, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, I.; Minakuchi, T.; Vullo, D.; Scozzafava, A.; Innocenti, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors. Cloning, Characterization, and Inhibition Studies of a New β-Carbonic Anhydrase from Mycobacterium Tuberculosis. J. Med. Chem. 2009, 52, 3116–3120. [Google Scholar] [CrossRef]
- Muhammed, M.T.; Er, M.; Akkoc, S. Molecular Modeling and in Vitro Antiproliferative Activity Studies of Some Imidazole and Isoxazole Derivatives. J. Mol. Struct. 2023, 1282, 135066. [Google Scholar] [CrossRef]
- Çetiner, G.; Acar Çevik, U.; Celik, I.; Bostancı, H.E.; Özkay, Y.; Kaplancıklı, Z.A. New Imidazole Derivatives as Aromatase Inhibitor: Design, Synthesis, Biological Activity, Molecular Docking, and Computational ADME-Tox Studies. J. Mol. Struct. 2023, 1278, 134920. [Google Scholar] [CrossRef]
- Roy, D.; Anas, M.; Manhas, A.; Saha, S.; Kumar, N.; Panda, G. Synthesis, Biological Evaluation, Structure—Activity Relationship Studies of Quinoline-Imidazole Derivatives as Potent Antimalarial Agents. Bioorganic Chem. 2022, 121, 105671. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Yang, Y.-D.; Liu, W.-Q.; Du, T.-T.; Wang, R.; Ji, M.; Yang, B.-B.; Li, L.; Chen, X.-G. Benzobis(Imidazole) Derivatives as STAT3 Signal Inhibitors with Antitumor Activity. Bioorganic Med. Chem. 2022, 65, 116757. [Google Scholar] [CrossRef] [PubMed]
- Badura, A.; Krysiński, J.; Nowaczyk, A.; Buciński, A. Application of Artificial Neural Networks to the Prediction of Antifungal Activity of Imidazole Derivatives against Candida Albicans. Chemom. Intell. Lab. Syst. 2022, 222, 104501. [Google Scholar] [CrossRef]
- Mickevičienė, K.; Voskienė, A.; Mickevičius, V. Synthesis of Some 1- and 2-Carboxyalkyl Substituted Benzimidazoles and Their Derivatives. Res. Chem. Intermed. 2014, 40, 1619–1631. [Google Scholar] [CrossRef]
- Tumosienė, I.; Peleckis, A.; Jonuškienė, I.; Vaickelionienė, R.; Kantminienė, K.; Šiugždaitė, J.; Beresnevičius, Z.J.; Mickevičius, V. Synthesis of Novel 1,2- and 2-Substituted Benzimidazoles with High Antibacterial and Antioxidant Activity. Monatsh. Chem. 2018, 149, 577–594. [Google Scholar] [CrossRef]
- Strelciunaite, V.; Jonuskiene, I.; Anusevicius, K.; Tumosiene, I.; Siugzdaite, J.; Ramanauskaite, I.; Mickevicius, V. Synthesis of Novel Benzimidazoles 2-Functionalized with Pyrrolidinone and γ-Amino Acid with a High Antibacterial Activity. Heterocycles 2016, 92, 235. [Google Scholar] [CrossRef]
- Kuzu, B.; Tan, M.; Taslimi, P.; Gülçin, İ.; Taşpınar, M.; Menges, N. Mono- or Di-Substituted Imidazole Derivatives for Inhibition of Acetylcholine and Butyrylcholine Esterases. Bioorganic Chem. 2019, 86, 187–196. [Google Scholar] [CrossRef]
- Perevalov, V.P.; Mityanov, V.S.; Lichitsky, B.V.; Komogortsev, A.N.; Kuz’mina, L.G.; Koldaeva, T.Y.; Miroshnikov, V.S.; Kutasevich, A.V. Synthesis of Highly Functional Imidazole Derivatives via Assembly of 2-Unsubstituted Imidazole N-Oxides with CH-Acids and Arylglyoxals. Tetrahedron 2020, 76, 130947. [Google Scholar] [CrossRef]
- Cirillo, D.; Angelucci, F.; Bjørsvik, H.-R. Functionalization of the Imidazole Backbone by Means of a Tailored and Optimized Oxidative Heck Cross-Coupling. Adv. Synth. Catal. 2020, 362, 5079–5092. [Google Scholar] [CrossRef]
- Balandis, B.; Mickevičius, V.; Petrikaitė, V. Exploration of Benzenesulfonamide-Bearing Imidazole Derivatives Activity in Triple-Negative Breast Cancer and Melanoma 2D and 3D Cell Cultures. Pharmaceuticals 2021, 14, 1158. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and Outcomes of Invasive Candidiasis Due to Non-Albicans Species of Candida in 2,496 Patients: Data from the Prospective Antifungal Therapy (PATH) Registry 2004–2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef]
- Peano, A.; Beccati, M.; Chiavassa, E.; Pasquetti, M. Evaluation of the Antifungal Susceptibility of Malassezia Pachydermatis to Clotrimazole, Miconazole and Thiabendazole Using a Modified CLSI M27-A3 Microdilution Method. Vet. Dermatol. 2012, 23, 131-e29. [Google Scholar] [CrossRef]
- Novak, I.; Klasinc, L.; McGlynn, S.P. Electronic Structure and Tautomerism of Thioamides. J. Electron Spectrosc. Relat. Phenom. 2016, 209, 62–65. [Google Scholar] [CrossRef]
- Oh, S.; Park, Y.; Engelhart, C.A.; Wallach, J.B.; Schnappinger, D.; Arora, K.; Manikkam, M.; Gac, B.; Wang, H.; Murgolo, N.; et al. Discovery and Structure–Activity-Relationship Study of N-Alkyl-5-Hydroxypyrimidinone Carboxamides as Novel Antitubercular Agents Targeting Decaprenylphosphoryl-β-d-Ribose 2′-Oxidase. J. Med. Chem. 2018, 61, 9952–9965. [Google Scholar] [CrossRef]
- de Faria, C.F.; Moreira, T.; Lopes, P.; Costa, H.; Krewall, J.R.; Barton, C.M.; Santos, S.; Goodwin, D.; Machado, D.; Viveiros, M.; et al. Designing New Antitubercular Isoniazid Derivatives with Improved Reactivity and Membrane Trafficking Abilities. Biomed. Pharmacother. 2021, 144, 112362. [Google Scholar] [CrossRef]
- Yong, Y.; Xiao-li, Y.; Xue-gang, L.; Jing, Z.; Baoshun, Z.; Lujiang, Y. Synthesis and Antimicrobial Activity of 8-Alkylberberine Derivatives with a Long Aliphatic Chain. Planta Med. 2007, 73, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Ashry, E.S.H. Synthesis, Molecular Structure and Spectroscopic Studies of Some New Quinazolin-4(3H)-One Derivatives; an Account on the N- versus S-Alkylation. J. Mol. Struct. 2016, 1108, 667–679. [Google Scholar] [CrossRef]
- Spears, R.J.; McMahon, C.; Chudasama, V. Cysteine Protecting Groups: Applications in Peptide and Protein Science. Chem. Soc. Rev. 2021, 50, 11098–11155. [Google Scholar] [CrossRef] [PubMed]
| Compound | Minimal Inhibitory Concentration (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| M. abscessus MA1884 | M. abscessus MA1753 | M. abscessus MA1836 | M. abscessus MA1704 | M. abscessus MA0040 | M. bovis BCG | M. tuberculosis H37Ra | |
| 2 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 3 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 4 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 5 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 6 | 64 | 64 | >4 | 64 | 64 | >64 | >64 |
| 7 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 8 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 9 | 64 | 64 | 64 | >64 | >64 | 32 | 32 |
| 10 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 11 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 12 | 64 | 64 | 32 | 64 | >64 | >64 | >64 |
| 13 | 1 | 4 | 4 | 4 | 4 | 1 | 0.5 |
| 14 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 15 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 16a | 64 | 64 | >64 | >64 | >64 | >64 | >64 |
| 16b | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 16c | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 17a | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 17b | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 17c | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 18a | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 18b | 64 | 64 | >64 | >64 | 32 | >64 | >64 |
| 18c | 64 | 64 | 32 | 16 | 32 | 32 | 16 |
| 19a | 8 | 8 | 4 | 4 | 8 | 4 | 4 |
| 19b | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 19c | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 20a | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 20b | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 20c | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 21a | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 21b | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 21c | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 22a | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 22b | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 22c | 64 | 64 | >64 | >64 | 64 | >64 | >64 |
| Rifampin | 64 | >64 | 32 | 16 | 32 | ≤0.5 | 0.5 |
| Isoniazid | 8 | 4 | 16 | 32 | 32 | 0.5 | ≤0.5 |
| Amikacin | 32 | 32 | 16 | 16 | 32 | ≤0.5 | ≤0.5 |
| Levofloxacin | 16 | 8 | 16 | 32 | 32 | 1 | 1 |
| Meropenem | 8 | 8 | 32 | 32 | 64 | 8 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balandis, B.; Kavaliauskas, P.; Grybaitė, B.; Petraitis, V.; Petraitienė, R.; Naing, E.; Garcia, A.; Grigalevičiūtė, R.; Mickevičius, V. Synthesis of Novel Benzenesulfonamide-Bearing Functionalized Imidazole Derivatives as Novel Candidates Targeting Multidrug-Resistant Mycobacterium abscessus Complex. Microorganisms 2023, 11, 935. https://doi.org/10.3390/microorganisms11040935
Balandis B, Kavaliauskas P, Grybaitė B, Petraitis V, Petraitienė R, Naing E, Garcia A, Grigalevičiūtė R, Mickevičius V. Synthesis of Novel Benzenesulfonamide-Bearing Functionalized Imidazole Derivatives as Novel Candidates Targeting Multidrug-Resistant Mycobacterium abscessus Complex. Microorganisms. 2023; 11(4):935. https://doi.org/10.3390/microorganisms11040935
Chicago/Turabian StyleBalandis, Benas, Povilas Kavaliauskas, Birutė Grybaitė, Vidmantas Petraitis, Rūta Petraitienė, Ethan Naing, Andrew Garcia, Ramunė Grigalevičiūtė, and Vytautas Mickevičius. 2023. "Synthesis of Novel Benzenesulfonamide-Bearing Functionalized Imidazole Derivatives as Novel Candidates Targeting Multidrug-Resistant Mycobacterium abscessus Complex" Microorganisms 11, no. 4: 935. https://doi.org/10.3390/microorganisms11040935
APA StyleBalandis, B., Kavaliauskas, P., Grybaitė, B., Petraitis, V., Petraitienė, R., Naing, E., Garcia, A., Grigalevičiūtė, R., & Mickevičius, V. (2023). Synthesis of Novel Benzenesulfonamide-Bearing Functionalized Imidazole Derivatives as Novel Candidates Targeting Multidrug-Resistant Mycobacterium abscessus Complex. Microorganisms, 11(4), 935. https://doi.org/10.3390/microorganisms11040935







