SYNBIO® Probiotic and Antioxidant Dietary Supplementation: Clinical Trial Evaluation of Potential Effects on Airline Flight Crew Members’ Well-Being
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Production, and Stability of the Dietary Supplement
2.2. Microbiological and Physico-Chemical Analysis
2.2.1. Viability of Probiotic Strains in Dietary Supplements
2.2.2. Water Activity and Moisture Content
2.2.3. Antioxidant Activity
2.3. In Vivo Study
2.3.1. Subjects
2.3.2. Dietary Supplement
2.3.3. Study Design
2.3.4. Sample Collection
2.3.5. Outcome Measures
Bowel Well-Being
Health-Related Quality of Life
Gastrointestinal Tolerance
Modulation of Gut Microbiota Composition
DNA Extraction from Fecal Samples
Quantitative Real-Time PCR
Recovery of Probiotic Strains from Fecal Samples
Determination of sIgA in Saliva
2.3.6. Statistical Analysis
3. Results
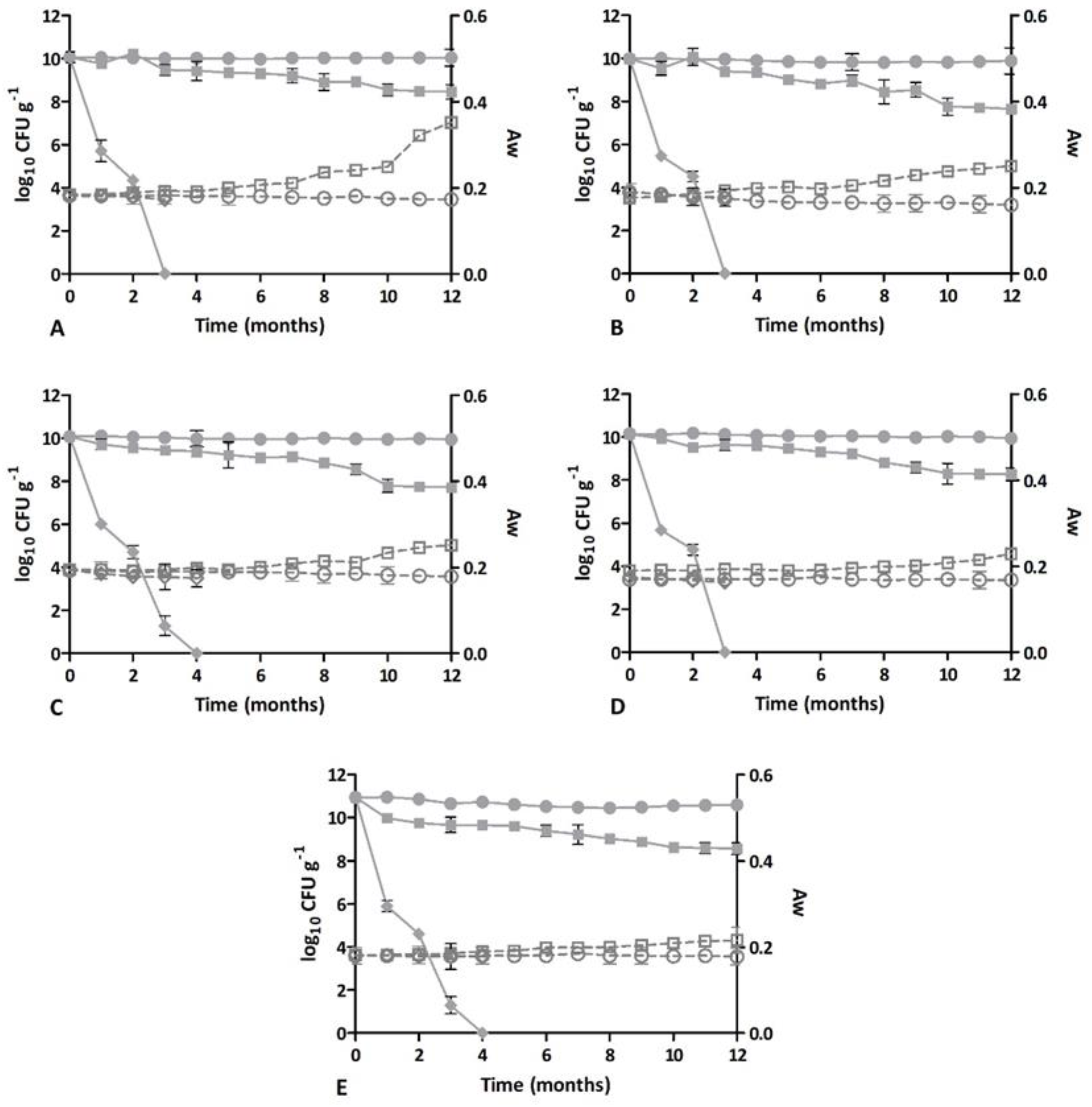
3.1. Dietary Supplement Stability: Microbiological and Physico-Chemical Analysis
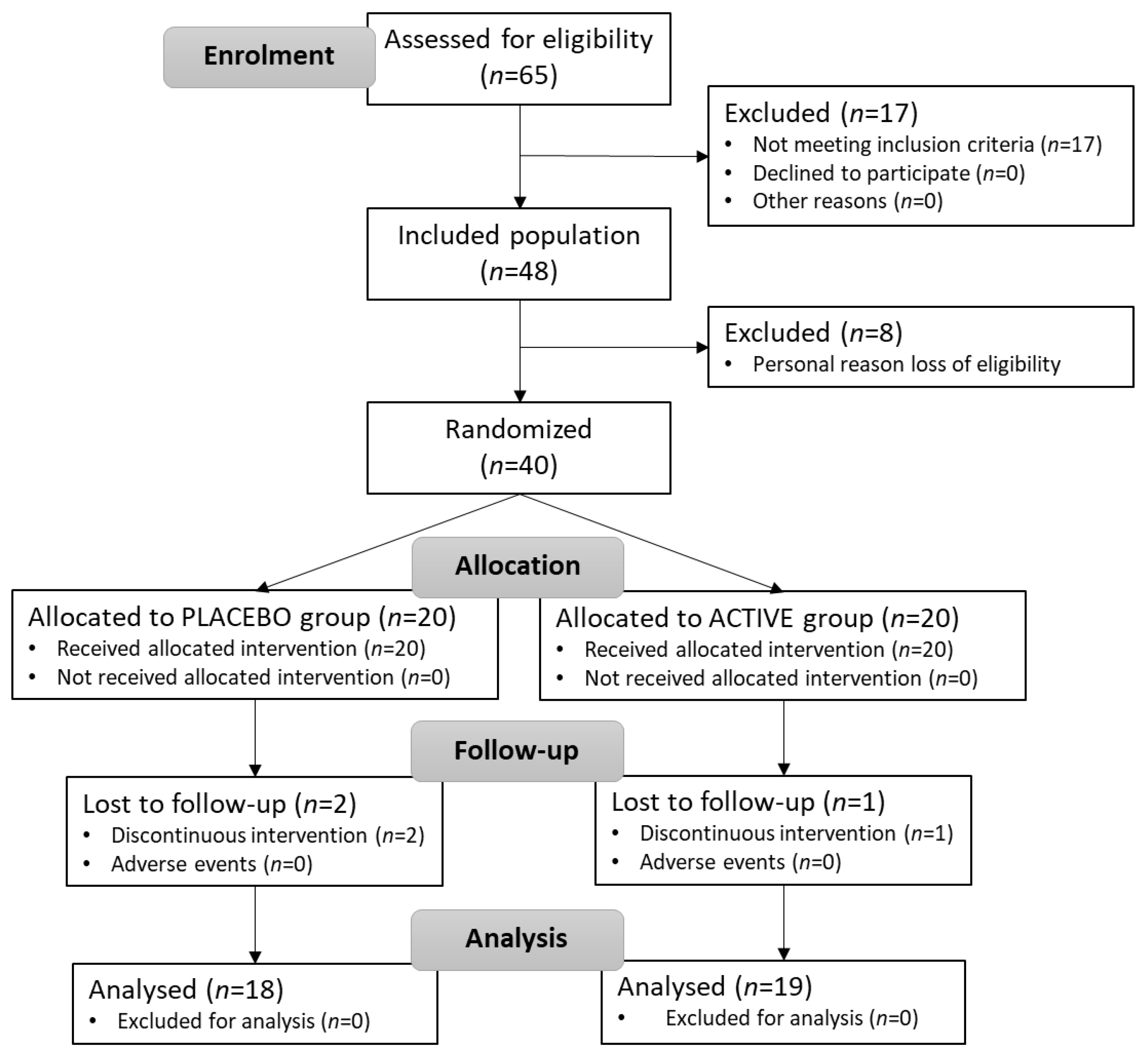
3.2. Participant Flow
3.3. Outcome Measures
3.3.1. Bowel Well-Being
3.3.2. Health-Related Quality of Life
3.3.3. Gastrointestinal Tolerance
3.4. Gut Microbiota Modulation
3.4.1. Bacterial Quantification by RT-PCR from Fecal Samples
3.4.2. Recovery of Probiotic Strains from Fecal Samples
3.5. Concentration of sIgA in Saliva
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinkerton, L.E.; Hein, M.J.; Anderson, J.L.; Christianson, A.; Little, M.P.; Sigurdson, A.J.; Schubauer-Berigan, M.K. Melanoma, thyroid cancer, and gynecologic cancers in a cohort of female flight attendants. Am. J. Ind. Med. 2018, 61, 572–581. [Google Scholar] [CrossRef]
- Scheibler, C.; Toprani, S.M.; Mordukhovich, I.; Schaefer, M.; Staffa, S.; Nagel, Z.D.; McNeely, E. Cancer risks from cosmic radiation exposure in flight: A review. Front. Public. Health. 2022, 10, 947068. [Google Scholar] [CrossRef] [PubMed]
- Grajewski, B.; Yong, L.C.; Bertke, S.J.; Bhatti, P.; Little, M.P.; Ramsey, M.J.; Tucker, J.D.; Ward, E.M.; Whelan, E.A.; Sigurdson, A.J.; et al. Chromosome Translocations and Cosmic Radiation Dose in Male, U.S. Commercial Airline Pilots. Aerosp. Med. Hum. Perform. 2018, 89, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Espey, M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013, 55, 130–140. [Google Scholar] [CrossRef]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H.; et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014, 147, 1055–1063.e8. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, V.A.; Brudastov Iu, A.; Zhurlov, O.S.; Chertkobv, K.L. The properties of Escherichia isolated from the bodies of mice in bacterial translocation after immobilization stress. Zh. Mikrobiol. Epidemiol. Immunobiol. 2000, 1, 37–41. [Google Scholar]
- Patricia, M.; Heavey, I.R.R. The Gut Microflora of the Developing Infant: Microbiology and Metabolism. Microb. Ecol. Health Dis. 1999, 11, 75–83. [Google Scholar] [CrossRef]
- Holy, O.; Chmelar, D. Oxygen tolerance in anaerobic pathogenic bacteria. Folia. Microbiol. 2012, 57, 443–446. [Google Scholar] [CrossRef]
- Mohler, S.R. Air crews face stomach and intestinal illness risks at many layover sites around the world. Flight Saf. Foundation Hum. Factor Aviaation. Med. 1994, 41, 5. [Google Scholar]
- Ito, H. Are intestinal microbes our friend or foe? J. Gastroenterol. 2005, 40, 111–112. [Google Scholar] [CrossRef]
- Kaye, D. Effect of hyperbaric oxygen on Clostridia in vitro and in vivo. Proc. Soc. Exp. Biol. Med. 1967, 124, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Bezirtzoglou, K.E.; Mättö, J.B. Culture-Based Knowledge on Biodiversity, Development and Stability of Human Gastrointestinal Microflora. Microb. Ecol. Health Dis. 2000, 12, 53–63. [Google Scholar] [CrossRef]
- Loesche, W.J. Oxygen sensitivity of various anaerobic bacteria. Appl. Microbiol. 1969, 18, 723–727. [Google Scholar] [CrossRef]
- Carco, C.; Young, W.; Gearry, R.B.; Talley, N.J.; McNabb, W.C.; Roy, N.C. Increasing Evidence That Irritable Bowel Syndrome and Functional Gastrointestinal Disorders Have a Microbial Pathogenesis. Front. Cell Infect. Microbiol. 2020, 10, 468. [Google Scholar] [CrossRef]
- Vaughan, T.L.; Daling, J.R.; Starzyk, P.M. Fetal death and maternal occupation. An analysis of birth records in the State of Washington. J. Occup. Med. 1984, 26, 676–678. [Google Scholar]
- Radon, K.; Aberl, S.; Nowak, D.; Volkenandt, M.; Przybilla, B.J.; Siebeck, M.; Mutschler, W. Incidence of cancer among commercial airline pilots. Occup. Env. Med. 2000, 57, 843. [Google Scholar] [CrossRef]
- Ali, Y.F.; Cucinotta, F.A.; Ning-Ang, L.; Zhou, G. Cancer Risk of Low Dose Ionizing Radiation. Front. Phys. 2020, 8, 234. [Google Scholar] [CrossRef]
- Strigari, L.; Strolin, S.; Morganti, A.G.; Bartoloni, A. Dose-Effects Models for Space Radiobiology: An Overview on Dose-Effect Relationships. Front. Public Health 2021, 9, 733337. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Roy, P.; Tomassoni, D.; Traini, E.; Martinelli, I.; Micioni Di Bonaventura, M.V.; Cifani, C.; Amenta, F.; Tayebati, S.K. Natural Antioxidant Application on Fat Accumulation: Preclinical Evidence. Antioxidants 2021, 10, 858. [Google Scholar] [CrossRef]
- Liu, D.; He, X.Q.; Wu, D.T.; Li, H.B.; Feng, Y.B.; Zou, L.; Gan, R.Y. Elderberry (Sambucus nigra L.): Bioactive Compounds, Health Functions, and Applications. J. Agric. Food. Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef]
- Rakel, D. Integrative Medicine, 4th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Dominguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.I.; Van-Buren, L.; Kroner, C.I.; Koning, M.M. Herbal medicines as diuretics: A review of the scientific evidence. J. Ethnopharmacol. 2007, 114, 1–31. [Google Scholar] [CrossRef]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. A systematic review on the sambuci fructus effect and efficacy profiles. Phytother. Res. 2010, 24, 1–8. [Google Scholar] [CrossRef]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food. Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Silvi, S.; Verdenelli, M.C.; Cecchini, C.; Coman, M.M.; Bernabei, M.S.; Rosati, J.; De Leone, R.; Orpianesi, C.; Cresci, A. Probiotic-enriched foods and dietary supplement containing SYNBIO positively affects bowel habits in healthy adults: An assessment using standard statistical analysis and Support Vector Machines. Int. J. Food. Sci. Nutr. 2014, 65, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Verdenelli, M.C.; Ghelfi, F.; Silvi, S.; Orpianesi, C.; Cecchini, C.; Cresci, A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur. J. Nutr. 2009, 48, 355–363. [Google Scholar] [CrossRef]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Dupuy, H.J. The General Well-being Schedule. In Measuring Health: A Guide to Rating Scales and Questionnaire, 2nd ed.; McDowell, I., Newell, C., Eds.; Oxford University Press: New York, NY, USA, 1977; pp. 206–213. [Google Scholar]
- Revicki, D.A.; Wood, M.; Wiklund, I.; Crawley, J. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual. Life Res. 1998, 7, 75–83. [Google Scholar] [CrossRef]
- Nasuti, C.; Coman, M.M.; Olek, R.A.; Fiorini, D.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Fedeli, D.; Gabbianelli, R. Changes on fecal microbiota in rats exposed to permethrin during postnatal development. Environ. Sci. Pollut. Res. Int. 2016, 23, 10930–10937. [Google Scholar] [CrossRef]
- Hutt, P.; Shchepetova, J.; Loivukene, K.; Kullisaar, T.; Mikelsaar, M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J. Appl. Microbiol. 2006, 100, 1324–1332. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Antonopoulos, D.A.; Kalra, A.; Tonelli, A.; Khalife, W.T.; Schmidt, T.M.; Young, V.B. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J. Infect. Dis. 2008, 197, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Coman, M.M.; Miorelli, L.; Micioni Di Bonaventura, M.V.; Cifani, C.; Salvesi, C.; Amedei, A.; Silvi, S.; Verdenelli, M.C. Effects of probiotic Lactiplantibacillus plantarum IMC 510 supplementation on metabolic factors in otherwise healthy overweight and obese individuals. J. Appl. Microbiol. 2022, 133, 1956–1968. [Google Scholar] [CrossRef]
- Micioni Di Bonaventura, M.V.; Coman, M.M.; Tomassoni, D.; Micioni Di Bonaventura, E.; Botticelli, L.; Gabrielli, M.G.; Rossolini, G.M.; Di Pilato, V.; Cecchini, C.; Amedei, A.; et al. Supplementation with Lactiplantibacillus plantarum IMC 510 Modifies Microbiota Composition and Prevents Body Weight Gain Induced by Cafeteria Diet in Rats. Int. J. Mol. Sci. 2021, 22, 11171. [Google Scholar] [CrossRef]
- Verdenelli, M.C.; Silvi, S.; Cecchini, C.; Orpianesi, C.; Cresci, A. Influence of a combination of two potential probiotic strains, Lactobacillus rhamnosus IMC 501(R) and Lactobacillus paracasei IMC 502(R) on bowel habits of healthy adults. Lett. Appl. Microbiol. 2011, 52, 596–602. [Google Scholar] [CrossRef]
- Coman, M.M.; Verdenelli, M.C.; Silvi, S.; Cecchini, C.; Gabbianelli, R.; Amadio, E.; Orpianesi, C.; Cresci, A. Knowledge and acceptance of functional foods: A preliminary study on influence of a synbiotic fermented milk on athlete health. Int. J. Probiotics Prebiotics 2017, 12, 33. [Google Scholar]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Boyko, N.; Cresci, A. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501((R)), Lactobacillus paracasei IMC 502((R)) and SYNBIO((R)) against pathogens. J. Appl. Microbiol. 2014, 117, 518–527. [Google Scholar] [CrossRef]
- Ishikawa, T.; Nanjo, F. Dietary cycloinulooligosaccharides enhance intestinal immunoglobulin A production in mice. Biosci. Biotechnol. Biochem. 2009, 73, 677–682. [Google Scholar] [CrossRef]
- Tomasi, T.B., Jr. Structure and function of mucosal antibodies. Annu. Rev. Med. 1970, 21, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Corthesy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef]
- Newburg, D.S.; Walker, W.A. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr. Res. 2007, 61, 2–8. [Google Scholar] [CrossRef]
- Tsujita, S.; Morimoto, K. Secretory IgA in saliva can be a useful stress marker. Environ. Health Prev. Med. 1999, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jemmott, J.B., 3rd; McClelland, D.C. Secretory IgA as a measure of resistance to infectious disease: Comments on Stone, Cox, Valdimarsdottir, and Neale. Behav. Med. 1989, 15, 63–71. [Google Scholar] [CrossRef]
- Bonneau, R.H.; Kiecolt-Glaser, J.K.; Glaser, R. Stress-induced modulation of the immune response. Ann. N. Y. Acad. Sci. 1990, 594, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; McGuire, L.; Robles, T.F.; Glaser, R. Psychoneuroimmunology: Psychological influences on immune function and health. J. Consult. Clin. Psychol. 2002, 70, 537–547. [Google Scholar] [CrossRef] [PubMed]
 T0 and
T0 and  T30 days) relative to the two groups of volunteers (PLACEBO and ACTIVE). * Significantly different (p < 0.05) from T0 and # from the placebo group at the same time point according to Tukey’s test following one-way ANOVA.
T30 days) relative to the two groups of volunteers (PLACEBO and ACTIVE). * Significantly different (p < 0.05) from T0 and # from the placebo group at the same time point according to Tukey’s test following one-way ANOVA.
 T0 and
T0 and  T30 days) relative to the two groups of volunteers (PLACEBO and ACTIVE). * Significantly different (p < 0.05) from T0 and # from the placebo group at the same time point according to Tukey’s test following one-way ANOVA.
T30 days) relative to the two groups of volunteers (PLACEBO and ACTIVE). * Significantly different (p < 0.05) from T0 and # from the placebo group at the same time point according to Tukey’s test following one-way ANOVA.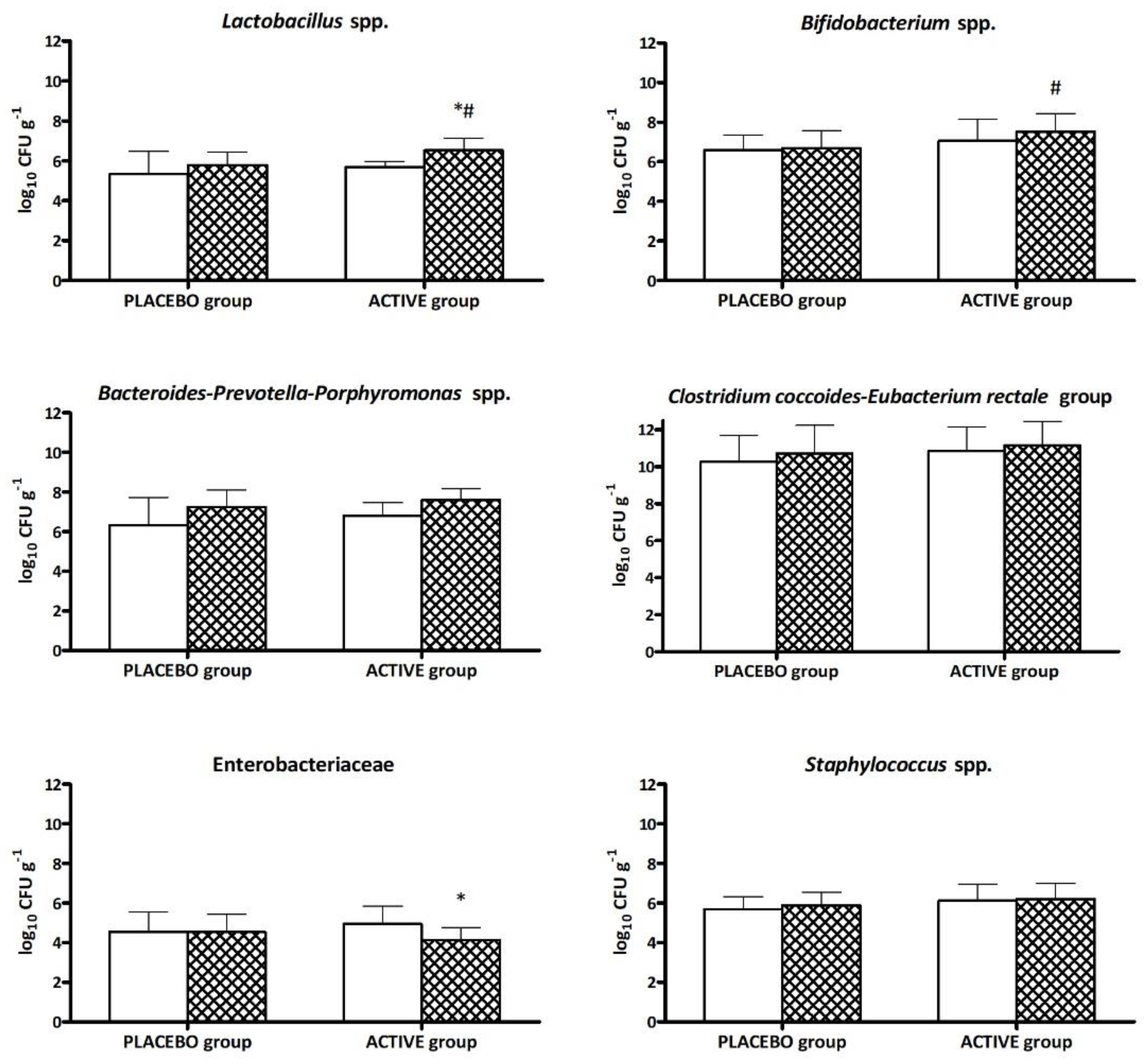
| Characteristics | PLACEBO (n = 18) | ACTIVE (n = 19) | p * |
|---|---|---|---|
| Age, years (mean ± confidence limits) | 44.3 ± 1.8 | 46.6 ± 3.0 | >0.05 |
| Male | 45.6 ± 1.6 | 47.9 ± 4.1 | >0.05 |
| Female | 42.4 ± 3.5 | 43.7 ± 3.1 | >0.05 |
| Gender | |||
| Male (n [%]) | 11 (61) | 13 (68) | >0.05 |
| Female (n [%]) | 7 (39) | 6 (32) | >0.05 |
| Current smokers | |||
| No (n [%]) | 15 (83) | 12 (63) | >0.05 |
| Yes (n [%]) | 3 (17) | 7 (37) | >0.05 |
| Overweight/obese | |||
| No (n [%]) | 17 (94) | 16 (84) | >0.05 |
| Yes (n [%]) | 1 (6) | 3 (16) | >0.05 |
| Bowel Habits and Other Health Parameters * | PLACEBO (n = 18) (Mean ± Confidence Limits) | ACTIVE (n = 19) (Mean ± Confidence Limits) |
|---|---|---|
| Intestinal regularity | 0.4 ± 0.3 | 1.2 ± 0.2 † |
| Stool volume | 0.1 ± 0.2 | 1.2 ± 0.3 † |
| Stool consistency | 0.4 ± 0.3 | 0.9 ± 0.2 † |
| Ease of defecation | 0.6 ± 0.3 | 1.3 ± 0.2 † |
| Rumbling of the stomach | 0.1 ± 0.2 | 0.7 ± 0.2 † |
| Swelling | 0.3 ± 0.2 | 0.7 ± 0.1 † |
| Flatulence | 0.5 ± 0.3 | 0.8 ± 0.2 † |
| Constipation | −0.1 ± 0.3 | 0.8 ± 0.2 † |
| Diarrhea | 0.1 ± 0.1 | 0.5 ± 0.1 † |
| Abdominal pains | 0.2 ± 0.1 | 0.3 ± 0.1 |
| Intestinal cramps | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Headaches | −0.1 ± 0.0 | 0.1 ± 0.1 |
| Toothache | 0.1 ± 0.3 | 0.3 ± 0.0 |
| Dermatitis | 0.0 ± 0.0 | 0.3 ± 0.0 |
| Skin rash | 0.1 ± 0.0 | 0.2 ± 0.0 |
| Itch | −0.1 ± 0.1 | 0.4 ± 0.1 |
| Cold | 0.1 ± 0.2 | 0.6 ± 0.1 † |
| Fever | 0.1 ± 0.0 | 0.5 ± 0.1 |
| Fatigue | 0.2 ± 0.2 | 0.7 ± 0.1 † |
| Weight variations | 0.1 ± 0.0 | 0.5 ± 0.1 † |
| Vomit | 0.0 ± 0.0 | 0.2 ± 0.0 |
| Nausea | 0.0 ± 0.0 | 0.1 ± 0.0 |
| Incontinence | 0.0 ± 0.0 | 0.2 ± 0.0 |
| Halitosis | 0.1 ± 0.0 | 0.3 ± 0.0 † |
| Food intolerance | 0.2 ± 0.0 | 0.3 ± 0.0 |
| Stool consistency # | ||
| Type | 4 ± 0.5 | 4 ± 0.4 |
| Global Score * (Mean Values ± Confidence Limits) | ||||||
|---|---|---|---|---|---|---|
| PLACEBO | ACTIVE | |||||
| Baseline | Week 4 | Change Δ | Baseline | Week 4 | Change Δ | |
| Psychological–emotional health (negative aspects) | ||||||
| Management of anxiety | 73.8 ± 3.4 | 73.3 ± 3.3 | −0.4 ± 0.3 | 75.8 ± 2.2 | 81.0 ± 2.4 | 5.2 ± 1.5 † |
| Management of depression | 85.3 ± 2.5 | 82.5 ± 2.7 | −2.8 ± 0.8 † | 90.8 ± 1.6 | 94.9 ± 1.2 | 4.1 ± 1.2 †‡ |
| General health (intermediate aspects) | ||||||
| General health | 87.1 ± 1.0 | 85.9 ± 1.1 | −1.2 ± 0.4 | 82.1 ± 1.4 | 88.1 ± 1.9 | 6.0 ± 1.8 † |
| Vitality | 66.7 ± 2.1 | 66.9 ± 2.0 | 0.3 ± 0.5 | 71.8 ± 2.1 | 77.6 ± 2.1 | 5.8 ± 1.4 †‡ |
| Psychological–emotional health (positive aspects) | ||||||
| Positivity | 66.4 ± 2.5 | 65.8 ± 2.5 | −0.6 ± 0.2 | 69.3 ± 2.4 | 72.4 ± 2.1 | 3.1 ± 1.5 |
| Self-control | 83.3 ± 3.0 | 82.2 ± 3.0 | −1.2 ± 0.4 | 82.5 ± 2.9 | 90.7 ± 1.4 | 8.2 ± 2.3 † |
| General state of health (total score) | 77.1 ± 2.0 | 76.1 ± 2.0 | −1.0 ± 0.2 † | 78.7 ± 1.7 | 84.1 ± 1.6 | 5.4 ± 0.5 †‡ |
| Symptom * | PLACEBO (n = 18) | ACTIVE (n = 19) | ||||
|---|---|---|---|---|---|---|
| Median | Range | Mean ± SD | Median | Range | Mean ± SD | |
| Stomach ache or pain | 1 | 1–3 | 1.17 ± 0.51 | 1 | 1–2 | 1.05 ± 0.23 |
| Heartburn | 1 | 1–2 | 1.11 ± 0.32 | 1 | 1 | 1.00 ± 0.00 |
| Acid reflux | 1 | 1–2 | 1.11 ± 0.32 | 1 | 1-2 | 1.11 ± 0.32 |
| Hunger pains in the stomach/belly | 1 | 1–4 | 1.17 ± 0.71 | 1 | 1 | 1.00 ± 0.00 |
| Nausea | 1 | 1–2 | 1.06 ± 0.24 | 1 | 1 | 1.00 ± 0.00 |
| Rumbling stomach/belly | 1 | 1–4 | 1.39 ± 0.85 | 1 | 1 | 1.00 ± 0.00 |
| Bloated stomach/belly | 1 | 1–5 | 1.61 ± 1.33 | 1 | 1–3 | 1.16 ± 0.50 |
| Burping | 1 | 1–3 | 1.22 ± 0.55 | 1 | 1–2 | 1.11 ± 0.32 |
| Passing gas or flatus | 1 | 1–7 | 2.11 ± 1.71 | 1 | 1–3 | 1.42 ± 0.69 |
| Constipation | 1 | 1–2 | 1.11 ± 0.32 | 1 | 1–2 | 1.11 ± 0.32 |
| Diarrhea | 1 | 1–3 | 1.39 ± 1.04 | 1 | 1–2 | 1.05 ± 0.23 |
| Loose stools | 1 | 1–6 | 1.44 ± 1.25 | 1 | 1–2 | 1.05 ± 0.23 |
| Hard stools | 1 | 1–3 | 1.11 ± 0.47 | 1 | 1–2 | 1.05 ± 0.23 |
| Urgent need (bowel movement) | 1 | 1–6 | 1.50 ± 1.20 | 1 | 1–2 | 1.11 ± 0.32 |
| Feeling of not completely emptying | 1 | 1–7 | 1.83 ± 1.86 | 1 | 1–3 | 1.21 ± 0.54 |
| PLACEBO | ACTIVE | |||
|---|---|---|---|---|
| T0 | T30 | T0 | T30 | |
| sIgA (ng mL−1) * | 11.30 ± 6.39 | 11.18 ± 6.77 | 10.95 ± 7.19 | 15.25 ± 6.73 †‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coman, M.M.; Micioni Di Bonaventura, M.V.; Cifani, C.; Silvi, S.; Verdenelli, M.C. SYNBIO® Probiotic and Antioxidant Dietary Supplementation: Clinical Trial Evaluation of Potential Effects on Airline Flight Crew Members’ Well-Being. Microorganisms 2023, 11, 924. https://doi.org/10.3390/microorganisms11040924
Coman MM, Micioni Di Bonaventura MV, Cifani C, Silvi S, Verdenelli MC. SYNBIO® Probiotic and Antioxidant Dietary Supplementation: Clinical Trial Evaluation of Potential Effects on Airline Flight Crew Members’ Well-Being. Microorganisms. 2023; 11(4):924. https://doi.org/10.3390/microorganisms11040924
Chicago/Turabian StyleComan, Maria Magdalena, Maria Vittoria Micioni Di Bonaventura, Carlo Cifani, Stefania Silvi, and Maria Cristina Verdenelli. 2023. "SYNBIO® Probiotic and Antioxidant Dietary Supplementation: Clinical Trial Evaluation of Potential Effects on Airline Flight Crew Members’ Well-Being" Microorganisms 11, no. 4: 924. https://doi.org/10.3390/microorganisms11040924
APA StyleComan, M. M., Micioni Di Bonaventura, M. V., Cifani, C., Silvi, S., & Verdenelli, M. C. (2023). SYNBIO® Probiotic and Antioxidant Dietary Supplementation: Clinical Trial Evaluation of Potential Effects on Airline Flight Crew Members’ Well-Being. Microorganisms, 11(4), 924. https://doi.org/10.3390/microorganisms11040924







