Lyme Neuroborreliosis—Significant Local Variations in Incidence within a Highly Endemic Region in Sweden
Abstract
1. Introduction
2. Materials and Methods
2.1. Exclusions
2.2. Ethics
2.3. Statistics
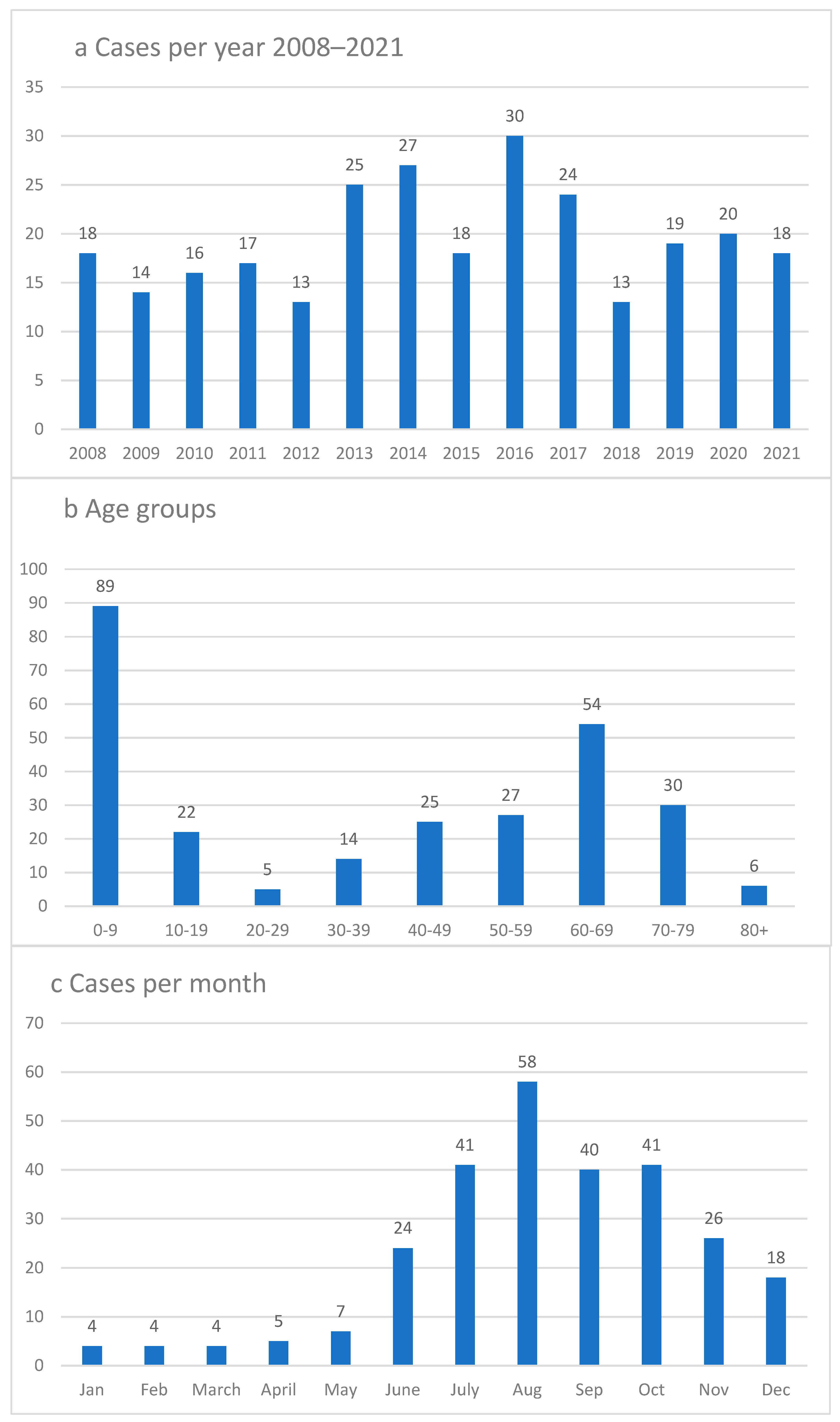
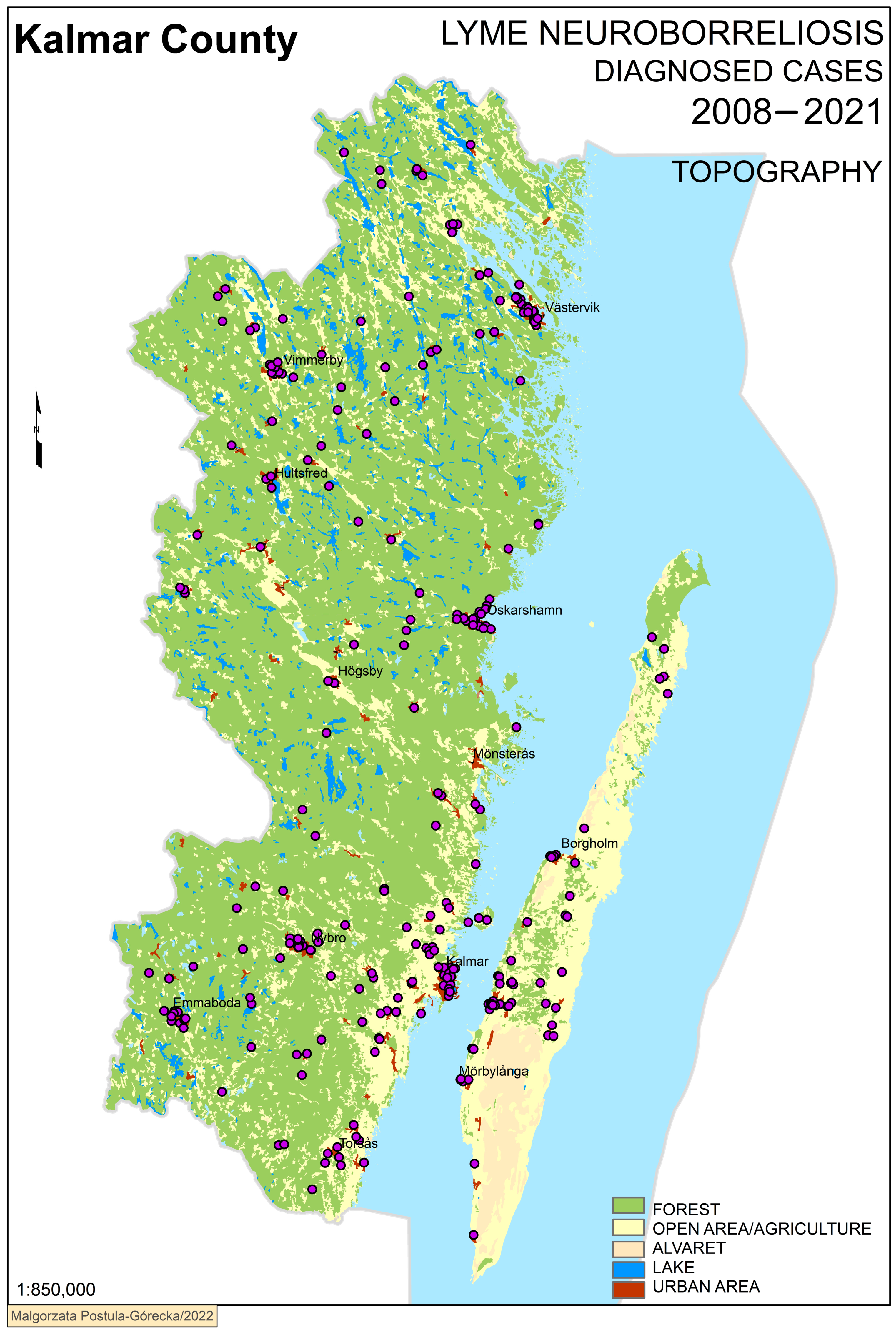
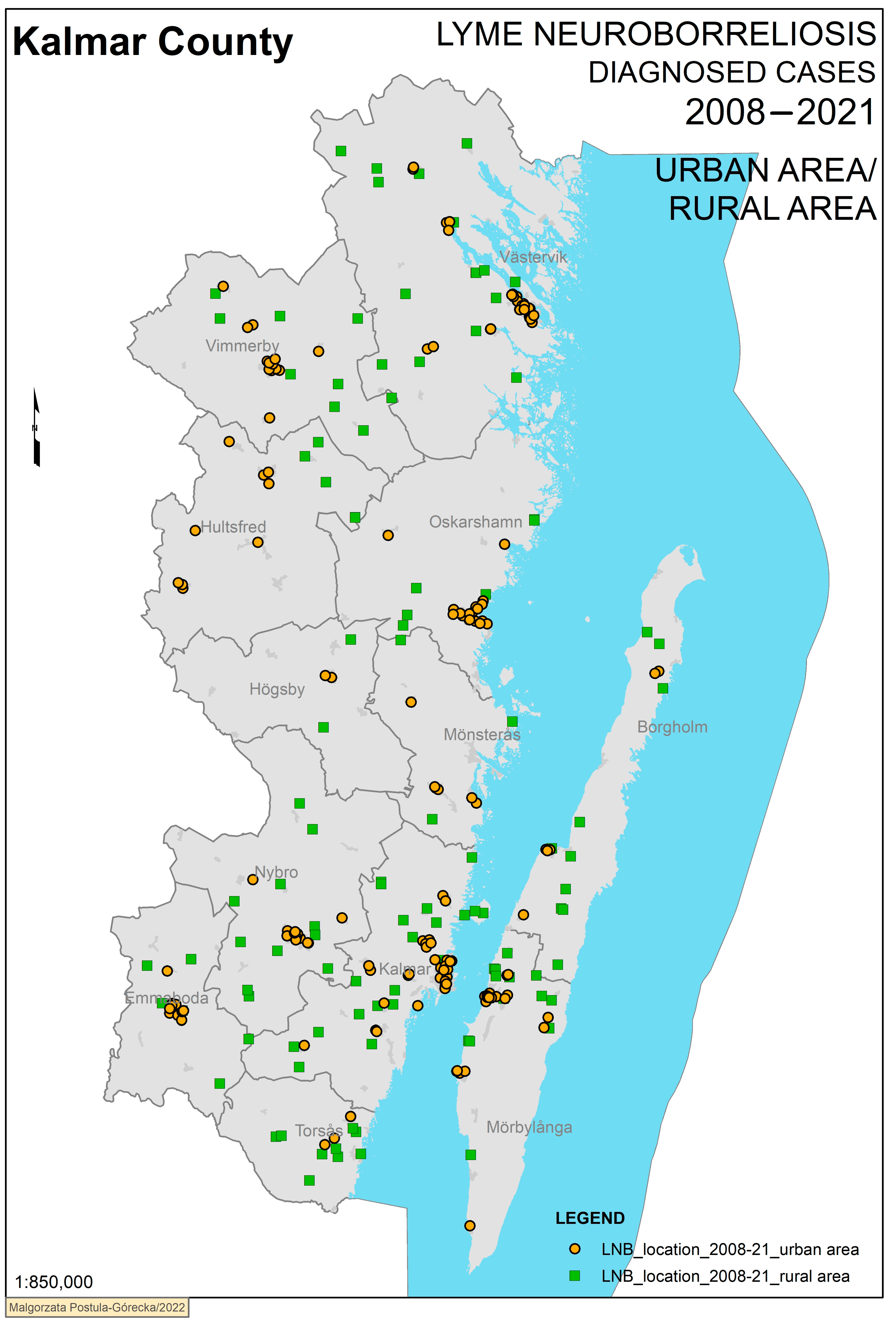
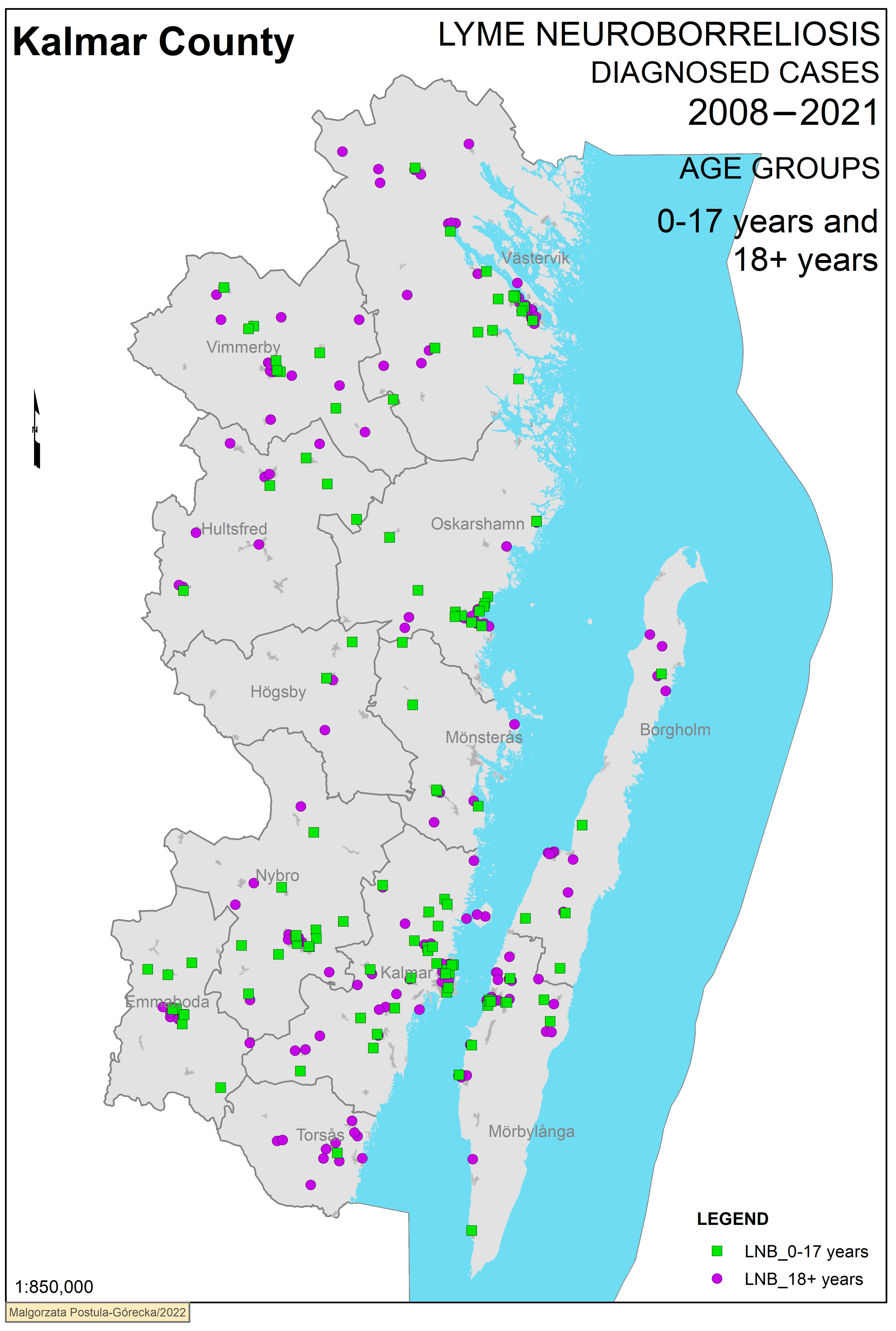
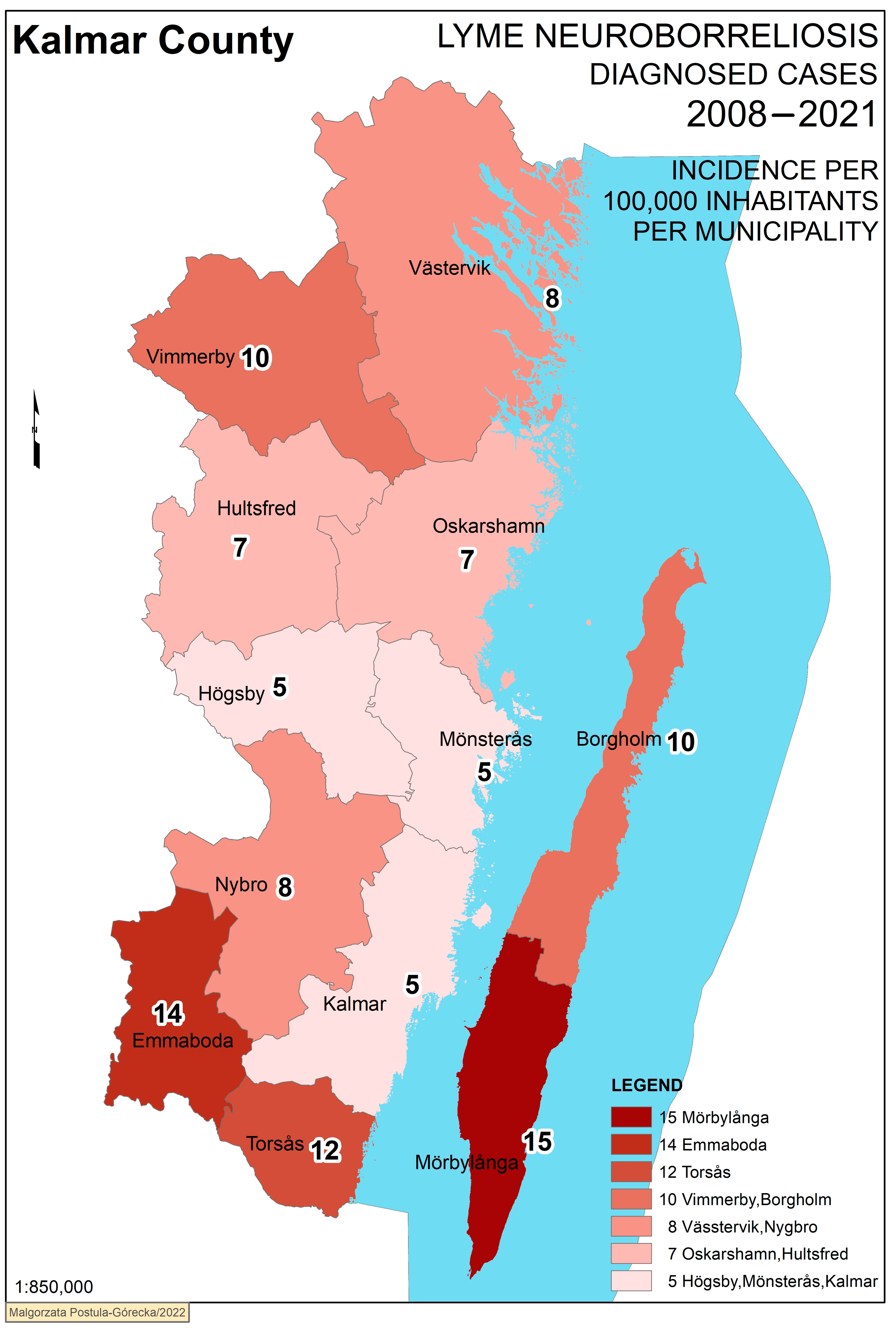
3. Results
Geographical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumic, I.; Severnini, E. “Ticking Bomb”: The Impact of Climate Change on the Incidence of Lyme Disease. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 5719081. [Google Scholar] [CrossRef]
- Stanek, G.; Strle, F. Lyme borreliosis-from tick bite to diagnosis and treatment. FEMS Microbiol. Rev. 2018, 42, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Cinco, M.; Trevisini, S.; di Meo, N.; Chersi, K.; Ruscio, M.; Forgione, P.; Bonin, S. Borreliae Part 1: Borrelia Lyme Group and Echidna-Reptile Group. Biology 2021, 10, 1036. [Google Scholar] [CrossRef]
- Carlsson, H.; Ekerfelt, C.; Henningsson, A.J.; Brudin, L.; Tjernberg, I. Subclinical Lyme borreliosis is common in south-eastern Sweden and may be distinguished from Lyme neuroborreliosis by sex, age and specific immune marker patterns. Ticks Tick Borne Dis. 2018, 9, 742–748. [Google Scholar] [CrossRef]
- Ogrinc, K.; Maraspin, V. Nervous system involvement in Lyme borreliosis. Open Dermatol. J. 2016, 10, 44–54. [Google Scholar] [CrossRef]
- Trevisan, G.; Bonin, S.; Ruscio, M. A Practical Approach to the Diagnosis of Lyme Borreliosis: From Clinical Heterogeneity to Laboratory Methods. Front. Med. 2020, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Kindstrand, E.; Nilsson, B.Y.; Hovmark, A.; Pirskanen, R.; Asbrink, E. Peripheral neuropathy in acrodermatitis chronica atrophicans—Effect of treatment. Acta Neurol. Scand. 2002, 106, 253–257. [Google Scholar] [CrossRef]
- Berglund, J.; Eitrem, R.; Ornstein, K.; Lindberg, A.; Ringer, A.; Elmrud, H.; Carlsson, M.; Runehagen, A.; Svanborg, C.; Norrby, R. An epidemiologic study of Lyme disease in southern Sweden. N. Engl. J. Med. 1995, 333, 1319–1327. [Google Scholar] [CrossRef]
- Södermark, L.; Sigurdsson, V.; Näs, W.; Wall, P.; Trollfors, B. Neuroborreliosis in Swedish Children: A Population-based Study on Incidence and Clinical Characteristics. Pediatr. Infect. Dis. J. 2017, 36, 1052–1056. [Google Scholar] [CrossRef]
- Bennet, L.; Halling, A.; Berglund, J. Increased incidence of Lyme borreliosis in southern Sweden following mild winters and during warm, humid summers. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 426–432. [Google Scholar] [CrossRef]
- Mygland, A.; Ljostad, U.; Fingerle, V.; Rupprecht, T.; Schmutzhard, E.; Steiner, I. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur. J. Neurol. 2010, 17, 8-e4. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Nikoskelainen, J.; Hiekkanen, H.; Lauhio, A.; Peltomaa, M.; Pitkäranta, A.; Nyman, D.; Granlund, H.; Carlsson, S.-A.; Seppälä, I.; et al. Duration of antibiotic treatment in disseminated Lyme borreliosis: A double-blind, randomized, placebo-controlled, multicenter clinical study. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Ljøstad, U.; Skogvoll, E.; Eikeland, R.; Midgard, R.; Skarpaas, T.; Berg, A.; Mygland, A. Oral doxycycline versus intravenous ceftriaxone for European Lyme neuroborreliosis: A multicentre, non-inferiority, double-blind, randomised trial. Lancet Neurol. 2008, 7, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Bremell, D.; Dotevall, L. Oral doxycycline for Lyme neuroborreliosis with symptoms of encephalitis, myelitis, vasculitis or intracranial hypertension. Eur. J. Neurol. 2014, 21, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Berglund, J.; Stjernberg, L.; Ornstein, K.; Tykesson-Joelsson, K.; Walter, H. 5-y Follow-up study of patients with neuroborreliosis. Scand. J. Infect. Dis. 2002, 34, 421–425. [Google Scholar] [CrossRef]
- Keith, K.; Årestedt, K.; Tjernberg, I. The relationship between the laboratory diagnosis of Lyme neuroborreliosis and climate factors in Kalmar County Sweden—An overview between 2008 and 2019. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 253–261. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Lindgren, E. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis. 2011, 2, 44–49. [Google Scholar] [CrossRef]
- Stjernberg, L.; Berglund, J. Risk of acquiring tick bites in south-eastern Sweden. Scand. J. Infect. Dis. 2002, 34, 840–844. [Google Scholar] [CrossRef]
- Strle, F.; Maraspin, V.; Furlan-Lotric, S.; Cimperman, J. Epidemiological study of a cohort of adult patients with Erythema migrans registered in Slovenia in 1993. Eur. J. Epidemiol. 1996, 12, 503–507. [Google Scholar] [CrossRef]
- Mannelli, A.; Bertolotti, L.; Gern, L.; Gray, J. Ecology of Borrelia burgdorferi sensu lato in Europe: Transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol. Rev. 2012, 36, 837–861. [Google Scholar] [CrossRef]
- Comstedt, P.; Bergström, S.; Olsen, B.; Garpmo, U.; Marjavaara, L.; Mejlon, H.; Barbour, A.G.; Bunikis, J. Migratory passerine birds as reservoirs of Lyme borreliosis in Europe. Emerg. Infect. Dis. 2006, 12, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Kurtenbach, K.; De Michelis, S.; Etti, S.; Schäfer, S.M.; Sewell, H.S.; Brade, V.; Kraiczy, P. Host association of Borrelia burgdorferi sensu lato--the key role of host complement. Trends Microbiol. 2002, 10, 74–79. [Google Scholar] [CrossRef]
- Johansson, M.; Manfredsson, L.; Wistedt, A.; Serrander, L.; Tjernberg, I. Significant variations in the seroprevalence of C6 ELISA antibodies in a highly endemic area for Lyme borreliosis: Evaluation of age, sex and seasonal differences. APMIS 2017, 125, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Lebech, A.M. Lyme neuroborreliosis: A new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi--specific immunoglobulin G, A, and M. Ann. Neurol. 1991, 30, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Statistics Sweden. Folkmängd i riket, län och Kommuner 31 December 2021 och Befolkningsförändringar 2021; Statistics Sweden: Örebro, Sweden, 2022. [Google Scholar]
- Dahl, V.; Wisell, K.T.; Giske, C.G.; Tegnell, A.; Wallensten, A. Lyme neuroborreliosis epidemiology in Sweden 2010 to 2014: Clinical microbiology laboratories are a better data source than the hospital discharge diagnosis register. Eurosurveillance 2019, 24, 1800453. [Google Scholar] [CrossRef]
- Dessau, R.B.; Espenhain, L.; Mølbak, K.; Krause, T.G.; Voldstedlund, M. Improving national surveillance of Lyme neuroborreliosis in Denmark through electronic reporting of specific antibody index testing from 2010 to 2012. Eurosurveillance 2015, 20, 21184. [Google Scholar] [CrossRef]
- Henningsson, A.J.; Malmvall, B.E.; Ernerudh, J.; Matussek, A.; Forsberg, P. Neuroborreliosis--an epidemiological, clinical and healthcare cost study from an endemic area in the south-east of Sweden. Clin. Microbiol. Infect. 2010, 16, 1245–1251. [Google Scholar] [CrossRef]
- Andreasen, A.M.; Dehlendorff, P.B.; Knudtzen, F.C.; Bødker, R.; Kjær, L.J.; Skarphedinsson, S. Spatial and temporal patterns of Lyme Neuroborreliosis on Funen, Denmark from 1995–2014. Sci. Rep. 2020, 10, 7796. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.E. Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: A seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasites Vectors 2020, 13, 607. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Fryland, L.; Borjesson, S.; Nordgren, J.; Bergstrom, S.; Ernerudh, J.; Forsberg, P.; Lindgren, P.E. Prevalence and Diversity of Borrelia Species in Ticks that have Bitten Humans in Sweden. J. Clin. Microbiol. 2010, 48, 4169–4176. [Google Scholar] [CrossRef]
- Hofmeester, T.R.; Sprong, H.; Jansen, P.A.; Prins, H.H.T.; van Wieren, S.E. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasites Vectors 2017, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the Communicable Diseases and Related Special Health Issues to be Covered by Epidemiological Surveillance as well as Relevant Case Definitions; European Commission: Brussels, Belgium, 2018. [Google Scholar]
| Patient Group and Symptoms | |||
|---|---|---|---|
| Age Group, Years | Children, 0–17 (n = 111) | Adults, 18+ (n = 161) | p-Value |
| Median age (range), years | 6 (0–17) | 61 (20–91) | |
| Female/male, n (% female) | 59/52 (53%) | 73/88 (46%) | 0.205 |
| Duration of symptoms before | 7 (1–90) | 16 (1–150) | <0.001 |
| treatment days, median (range) | |||
| Facial palsy, n (%) | 63 (57%) | 78 (48%) | 0.178 |
| Other cranial nerve palsy, n (%) | 3 (3%) | 10 (6%) | 0.183 |
| Radiculitis, n (%) | 5 (5%) | 77 (48%) | <0.001 |
| Meningitis, n (%) | 25 (23%) | 25 (16%) | 0.143 |
| Fever, n (%) | 44 (40%) | 9 (6%) | <0.001 |
| Fatigue, n (%) | 61 (55%) | 40 (25%) | <0.001 |
| Headache, n (%) | 58 (52%) | 42 (26%) | <0.001 |
| Muscle/joint pain, n (%) | 36 (32%) | 79 (49%) | 0.006 |
| Nausea, n (%) | 18 (16%) | 14 (9%) | 0.059 |
| Erythema migrans, n (%) | 19 (17%) | 22 (14%) | 0.434 |
| Follow-up of treatment, n (%) | 97 (87%) | 113 (70%) | <0.001 |
| Symptoms at follow-up, n (%) | 9 (8%) | 44 (27%) | <0.001 |
| Incidence Annually per | Population | Mean | ||||
|---|---|---|---|---|---|---|
| Cases | Population | 100,000 Inhabitants | p-Value | Urban/Rural (%) | Age (Years) | |
| Kalmar County | 272 | 247,175 | 7.8 | 80.1/19.9 | 44.4 | |
| Sex | 0.741 | |||||
| Male | 140 | 124,754 | 8.0 | |||
| Female | 132 | 122,421 | 7.7 | |||
| Age group | <0.001 | |||||
| Children (0–17) | 111 | 48,879 | 16 | |||
| Adults (18+) | 161 | 198,296 | 5.8 | |||
| Urban/Rural | <0.001 | |||||
| Urban | 162 | 197,906 | 5.8 | |||
| Rural | 110 | 49,263 | 16 | |||
| Municipality | <0.001 | |||||
| Borgholm | 16 | 10,895 | 10 | 51.7/48.3 | 51.4 | |
| Emmaboda | 18 | 9329 | 14 | 69/31 | 46.1 | |
| Hultsfred | 13 | 14,056 | 6.6 | 81.8/18.2 | 45.6 | |
| Högsby | 4 | 5645 | 5.1 | 64.6/35.4 | 45.6 | |
| Kalmar | 51 | 71,328 | 5.1 | 89.3/10.7 | 41.1 | |
| Mönsterås | 9 | 13,258 | 4.8 | 75.8/24.2 | 45.1 | |
| Mörbylånga | 34 | 15,722 | 15 | 78.4/21.6 | 45.2 | |
| Nybro | 24 | 20,284 | 8.4 | 79/21 | 44.3 | |
| Oskarshamn | 26 | 27,220 | 6.8 | 85.3/14.7 | 43.8 | |
| Torsås | 12 | 7113 | 12 | 58.7/41.3 | 46.5 | |
| Vimmerby | 22 | 15,578 | 10 | 73.2/26.8 | 44.6 | |
| Västervik | 43 | 36,747 | 8.4 | 81.1/18.9 | 46.7 | |
| Total | 0–17 | 18+ | Urban | Rural | |
|---|---|---|---|---|---|
| Kalmar County, overall | 7.8 | 16 | 5.8 | 5.8 | 16 |
| Municipality | |||||
| Borgholm | 10 | 22 | 8.4 | 7.6 | 14 |
| Emmaboda | 14 | 36 | 8.5 | 13 | 16 |
| Hultsfred | 6.6 | 13 | 5.1 | 5.6 | 11 |
| Högsby | 5.0 | 13 | 3.2 | 3.9 | 7.2 |
| Kalmar | 5.1 | 12 | 3.4 | 3.4 | 20 |
| Mönsterås | 4.8 | 14 | 2.7 | 4.3 | 6.7 |
| Mörbylånga | 15 | 25 | 13 | 12 | 27 |
| Nybro | 8.4 | 22 | 4.9 | 5.8 | 18 |
| Oskarshamn | 6.8 | 16 | 4.6 | 6.2 | 11 |
| Torsås | 12 | 5.3 | 17 | 5.1 | 22 |
| Vimmerby | 10 | 21 | 7.4 | 8.1 | 15 |
| Västervik | 8.4 | 15 | 6.9 | 6.5 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilsson, P.-O.; Tjernberg, I. Lyme Neuroborreliosis—Significant Local Variations in Incidence within a Highly Endemic Region in Sweden. Microorganisms 2023, 11, 917. https://doi.org/10.3390/microorganisms11040917
Nilsson P-O, Tjernberg I. Lyme Neuroborreliosis—Significant Local Variations in Incidence within a Highly Endemic Region in Sweden. Microorganisms. 2023; 11(4):917. https://doi.org/10.3390/microorganisms11040917
Chicago/Turabian StyleNilsson, Per-Olof, and Ivar Tjernberg. 2023. "Lyme Neuroborreliosis—Significant Local Variations in Incidence within a Highly Endemic Region in Sweden" Microorganisms 11, no. 4: 917. https://doi.org/10.3390/microorganisms11040917
APA StyleNilsson, P.-O., & Tjernberg, I. (2023). Lyme Neuroborreliosis—Significant Local Variations in Incidence within a Highly Endemic Region in Sweden. Microorganisms, 11(4), 917. https://doi.org/10.3390/microorganisms11040917





