A Bibliometric Analysis on the Research Trend of Exercise and the Gut Microbiome
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Description of Study Selection and Characteristics
3.2. Analysis of Active Countries/Regions, Institutions and Authors
3.3. Analysis of Relevant Journals and Highly Influential Publications
3.4. Analysis of Keywords
4. Discussion
4.1. General Information
4.2. Hotspots and Frontiers
4.2.1. Effect of Exercise on the Gut Microbiome
Cross-Sectional Studies
Longitudinal Studies
| Author | Publication Year | Subjects | Exercise | Effects on Composition and/or Metabolite of Gut Microbiota | Physiological Metabolite/ Functional Change |
|---|---|---|---|---|---|
| Wang, R.H. et al. [112] | 2023 | 30 young adolescents aged 12–14 years | Moderate-intensity exercise, comprised of 30 min of running per day, 4 days a week for 3 months | After training: Genus: Coprococcus ↑, Blautia ↑, Dorea ↑, Tyzzerella ↑ Species: Tyzzerella nexilis ↑, Ruminococcus obeum ↑ | The improvement of depressive symptoms ↑, KEGG pathways belonging to the neurodegenerative diseases ↓ |
| Wang, Y.J. et al. [113] | 2023 | Chronic mouse model of Parkinson’s disease, induced by MPTP | Rotarod walking training (5 times a week at 25 rpm for 20 min for 8 weeks) | After training: Family: Oscillospiraceae ↑, Ruminococcaceae↑ Genus: Lachnospiraceae_NK4A136_group ↑, unclassfied_f__Oscillospiraceae ↑, Alloprevotella ↓ | DA and TH in the striatum ↑, BDNF gene expression ↑, IL-1β gene expression in the striatum ↓ |
| Craven, J. et al. [114] | 2021 | Middle-distance runners | Three weeks of NormTr + three weeks of HVolTr + a one-week TaperTr | Following HVolTr: Pasterellaceae ↓, Lachnoclostridium ↓, Haemophilus ↓, S. parasagunis ↓, and H. parainfluenzae ↓, R. callidus ↑ | - |
| Bielik, V. et al. [115] | 2022 | Young competitive male and female swimmers | 7-week high-intensity swimming training program | HIT group: Firmicutes ↓, Bacteroidota ↑, Actinobacteriota ↑ HITB group: Firmicutes ↓, Bacteroidota ↑, Actinobacteriota ↓ | Uric acid ↓, alanine transaminase ↓, vitamin D ↑, lactate ↑, pyruvate ↑, acetate ↓, butyrate ↓ |
| Zhong, F. et al. [85] | 2022 | 14 elderly women | 60 min exercise program: 10 min warm-up + 20 min aerobic exercise (20 min) + 25 min resistance exercise + 5 min cool down | After training: Order: Coriobacteriales ↑, Family: Coriobacteriaceae ↑, Asaccharobacter ↑, Collinsella ↑, Fusicatenibacter ↑ | The skeletal muscle mass ↑, fasting blood glucose ↓, CVD risk ↓ |
| Qiu, L.W. et al. [116] | 2022 | 22 university students with/without sleep disorders | Jog three times per week, at a speed of 8–9 km/h, for a distance of 4–7 km each time | After training: Blautia ↑, Eubacterium hallii↑, Agathobacter ↓ | Sleep quality ↑ |
| Dupuit, M. et al. [117] | 2022 | Postmenopausal women who were overweight or obese | HIIT + RT (3 times per week for 12 weeks) | FM ↑: Bifidobacteriaceae ↑, Paraprevotellaceae ↓, Prevotellaceae ↓ Muscle mass ↑: Bifidobacteriaceae ↓, Paraprevotellaceae ↑, Prevotellaceae ↑ HDL-C ↑: Bifidobacteriaceae ↓, Paraprevotellaceae ↓, Prevotellaceae ↓, Streptococcaceae ↓, Desulfovibrionaceae ↓ | Physical fitness (maximal oxygen consumption, peak power output) ↑, segmental muscle mass ↑, total abdominal and visceral fat mass ↓ |
| Sato, M. et al. [118] | 2022 | Ultramarathon runners | Run 96.102 km or 99.12 km within 38–44 h | After training: Butyrate-producing bacteria, such as F. prausnitzii↓, C. aerofaciens ↑ | Butyrate levels in the intestine ↓ |
| Fernández, J. et al. [86] | 2021 | 8-week-old C57BL6N male mice | Four-week endurance training/resistance training | After training: Ruminococcus gnavus ↓, Parabacteroides ↑ Endurance performance ↑: Lachnospiraceae family ↓, Lactobacillaceae family ↓, Prevotellaceae family ↑, Prevotella genus ↑, Akkermansia muciniphila ↑ Resistance performance ↑: Desulfovibrio sp. ↓, Proteobacteria taxon ↓, Alistipes ↑ | H2S production by Desulfovibrio ↑, anti-inflammatory Parabacteroides ↑, pro-inflammatory bacterium ↓ |
| Chen, H. et al. [119] | 2021 | 3–4-week-old C57BL/6J WT female mice | Four-week SE training + 7 days post-immunization SE training | After training: Anaerotruncus ↓, Jeotgalicoccus ↓, Anaerotruncus ↓, Alistipes ↓, Ruminococcus ↓, Desulfovibrio ↓, Clostridium ↑, Parabacteroides ↑, Christensenella ↑, Dorea ↑, Roseburia ↑, Paraprevotella ↑ | Th17 responses ↓, Treg responses ↑, intestinal mucosal permeability ↓, MS disease severity ↓, neuropathology scores ↓ |
| Moitinho-Silva, L. et al. [120] | 2021 | 42 healthy physically inactive German male and female volunteers aged 20 to 45 years | Endurance training, strength training | After training: Coprococcus genera ↑, Parasutterella genera ↑, Ruminococcaceae family ↑, Dialister genera ↓, Odoribacter ↓, Phascolarctobacterium ↓ | Strength intervention group: Lymphocytes ↑, MCHC ↓ Endurance group: Hip circumference ↓, physical working capacity ↑ |
| Resende, A.S. et al. [121] | 2021 | 24 previously sedentary men | 10-week moderate aerobic exercise, 150 min per week of supervised moderate (60–65% of VO2 peak) aerobic exercise | After training: Streptococcus genus ↑, Clostridiales-order genus ↓ VO2 peak ↑: Odoribacter ↑, Roseburia ↑, Sutterella ↑ BMI ↑: Desulfovibrio ↓ Body fat ↑: Faecalibacterium ↓ | VO2 peak ↑, Wpeak ↑, AT1 ↑, cardiorespiratory fitness ↑ |
| Li, K.F. et al. [122] | 2021 | 54 male C57BL/6J mice at 12 weeks of age | 4-week wheel-running exercise | After training: Gut microbial diversity ↑ Phyla: Firmicutes ↓, Proteobacteria ↓, Bacteriodetes ↑, Firmicutes/ Bacteriodetes ratio ↓ Family: Bacteroidales_S24-7 ↑, Prevotellaceae ↑, Desulfovibrionaceae ↓, Peptostreptococcaceae ↓ | LPS levels in the blood ↓, synovial fluid ↓, TLR4 and MMP-13 expression levels ↓, cartilage degeneration ↓ |
| Verheggen, R.J.H.M. et al. [123] | 2021 | 20 inactive participants with obesity (BMI > 30 kg/m2) | 8-week exercise intervention (2 to 4 times per week, on 65–85% of heart rate reserve). | After training: Genus: Ruminococcus gauvreauii ↑, Lachnospiraceae FCS020 ↑, Anaerostipes ↑ M-value ↑: R. gauvreauii ↑ VO2 max ↑: R. gauvreauii ↑, Anaerostipes ↑ | Insulin sensitivity ↑, visceral adiposity ↓, cardiorespiratory fitness levels ↑ |
| Ahrens, A.P. et al. [124] | 2021 | 73 adults (37 male, 36 female) | One-week, in-house, lifestyle-based “immersion program”, including dedicated fitness | After training: Lachnospiraceae ↑, Oscillospirales ↑, Ruminococcaceae ↑, Faecalibacterium ↑, Roseburia ↑, Blautia ↑, Anaerostipes ↑, Subdoligranulum ↑, Bacteroidaceae ↓, Phascolarctobacterium ↓ | Blood pressure ↓, total cholesterol ↓, triglycerides ↓, cardiovascular risk ↓ |
| Related Diseases | Author | Publication Year | Objects (Materials) | Results | |
|---|---|---|---|---|---|
| Cancer | Breast cancer | Sampsell, K. et al. [110] | 2022 | Breast cancer patient survivors, C57BL/6 mice | Exercise and prebiotic fiber demonstrated adjuvant action, potentially via an enhanced anti-tumor immune response modulated by advantageous gut microbial shifts. |
| Colitis-associated cancer (CAC) | Wang, W.Y. et al. [111] | 2022 | C57BL/6 mice | Swimming pretreatment can protect mice from CAC, possibly through regulating gut microbiota and intestinal SCFAs, and affecting the function of colonic lipid metabolites and choline metabolism in cancer. | |
| Lung cancer | Marfil-Sánchez, A. et al. [125] | 2021 | Early-stage lung cancer patients | There are some beneficial bacterial species for improving recovery of overall physical condition and lung capacity in patients one year after lung resection surgery. Some bacterial metabolic pathways might be associated with increased oxygen uptake and exercise tolerance. | |
| Prostate cancer | Frugé, A.D. et al. [126] | 2020 | Prostate cancer patients | Relative abundance of Bifidobacterium was associated with Cathepsin L (CTSL) and free fatty acids (FFAs); Firmicutes was positively related with change in physical activity. Although glucose metabolism improved, it was inversely related with Ki67 and CTSL. | |
| Metabolic endocrine diseases | Obesity | Verheggen, R. et al. [123] | 2021 | Obese patients | Eight-week exercise training in obese humans leads to marked improvements in insulin sensitivity and body composition, accompanied by modest changes in Ruminococcus gauvreauii, Lachnospiraceae FCS020 group, and Anaerostipes, which all belong to the Firmicutes phylum. |
| Childhood obesity | Quiroga, R. et al. [102] | 2020 | Obese pediatric patients | Exercise training could be considered an efficient nonpharmacological therapy, reducing inflammatory signaling pathways induced by obesity in children via microbiota modulation. The existence of obesity-related deleterious microbiota profiles can be positively modified by exercise. | |
| Nonalcoholic fatty liver disease (NAFLD) | Cheng, R.T. et al. [99] | 2022 | NAFLD and prediabetes patients | Combined aerobic exercise and diet intervention are associated with diversified and stabilized keystone taxa. A personalized gut microbial network at baseline could predict the individual responses in liver fat, to exercise intervention. | |
| Obesity, insulin resistance, obstructive sleep apnea | Khalyfa, A. et al. [127] | 2021 | C57BL/6 mice | Intermittent hypoxia (IH) exposures induce changes in gut microbiota (GM), increase gut permeability, and alter plasma exosome cargo, the latter inducing adipocyte dysfunction (increased insulin resistance). GM alterations can be improved with physical activity. | |
| Prediabetes | Liu, Y. et al. [104] | 2020 | Medication-naive men with prediabetes, C57BL/6J mice | Exercise-induced alterations in the gut microbiota correlated closely with improvements in glucose homeostasis and insulin sensitivity. The microbiome of responders exhibited an enhanced capacity for biosynthesis of short-chain fatty acids and catabolism of branched-chain amino acids. | |
| Immune diseases | Multiple sclerosis | Mokhtarzade, M. et al. [128] | 2021 | Multiple sclerosis (MS) patients | Home-based exercise significantly increased prevotella counts, and decreased akkermansia muciniphila counts, which can probably have a beneficial effect on MS disease pathology and course. These changes were associated with changes in IL-10. |
| Celiac disease | Warbeck, C. et al. [129] | 2021 | Adults with celiac disease | Following 12-week high-intensity interval training (HIIT) plus lifestyle education intervention, the group showed beneficial changes in the gut microbiota of adults with celiac disease. | |
| Neurological and psychotic disorders | Insomnia symptoms | Magzal, F. et al. [130] | 2022 | Older adults with insomnia | Different microbiota taxa in each physical activity group, increased SCFAs in feces of less active individuals, and significant associations among physical activity, gut microbiota, SCFAs, and sleep parameters were observed. |
| Alzheimer’s disease | Abraham, D. et al. [131] | 2019 | APP/PS1 transgenic (APP/PS1TG) mice | Exercise and probiotic treatment can decrease the progress of Alzheimer’s disease and the beneficial effects could be partly mediated by alteration of the microbiome. | |
4.2.2. Relationship between Exercise and Gut-Brain Axis (GBA)
4.2.3. Impact of Gut Microbiome on Exercise
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cataldi, S.; Poli, L.; Sahin, F.N.; Patti, A.; Santacroce, L.; Bianco, A.; Greco, G.; Ghinassi, B.; Di Baldassarre, A.; Fischetti, F. The Effects of Physical Activity on the Gut Microbiota and the Gut-Brain Axis in Preclinical and Human Models: A Narrative Review. Nutrients 2022, 14, 3293. [Google Scholar] [CrossRef]
- Belizario, J.E.; Napolitano, M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015, 6, 1050. [Google Scholar] [CrossRef]
- Jones, R.B.; Zhu, X.; Moan, E.; Murff, H.J.; Ness, R.M.; Seidner, D.L.; Sun, S.; Yu, C.; Dai, Q.; Fodor, A.A.; et al. Inter-niche and inter-individual variation in gut microbial community assessment using stool, rectal swab, and mucosal samples. Sci. Rep. 2018, 8, 4139. [Google Scholar] [CrossRef]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Clauss, M.; Gerard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Aziz, Q.; Dore, J.; Emmanuel, A.; Guarner, F.; Quigley, E.M. Gut microbiota and gastrointestinal health: Current concepts and future directions. Neurogastroenterol. Motil. 2013, 25, 4–15. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Turroni, S.; Brigidi, P.; Cavalli, A.; Candela, M. Microbiota-Host Transgenomic Metabolism, Bioactive Molecules from the Inside. J. Med. Chem. 2018, 61, 47–61. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Pedersini, P.; Turroni, S.; Villafane, J.H. Gut microbiota and physical activity: Is there an evidence-based link? Sci. Total Environ. 2020, 727, 138648. [Google Scholar] [CrossRef]
- Bibbo, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Juarez-Fernandez, M.; Porras, D.; Garcia-Mediavilla, M.V.; Roman-Saguillo, S.; Gonzalez-Gallego, J.; Nistal, E.; Sanchez-Campos, S. Aging, Gut Microbiota and Metabolic Diseases: Management through Physical Exercise and Nutritional Interventions. Nutrients 2020, 13, 16. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Keohane, D.M.; Woods, T.; O’Connor, P.; Underwood, S.; Cronin, O.; Whiston, R.; O’Sullivan, O.; Cotter, P.; Shanahan, F.; Molloy, M.G.M. Four men in a boat: Ultra-endurance exercise alters the gut microbiome. J. Sci. Med. Sport 2019, 22, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; Rochester, L. Mobilizing Parkinson’s Disease: The Future of Exercise. J. Park. Dis. 2018, 8, S95–S100. [Google Scholar] [CrossRef]
- Kim, Y.; Lai, B.; Mehta, T.; Thirumalai, M.; Padalabalanarayanan, S.; Rimmer, J.H.; Motl, R.W. Exercise Training Guidelines for Multiple Sclerosis, Stroke, and Parkinson Disease: Rapid Review and Synthesis. Am. J. Phys. Med. Rehabil. 2019, 98, 613–621. [Google Scholar] [CrossRef]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef]
- Klement, R.J.; Pazienza, V. Impact of Different Types of Diet on Gut Microbiota Profiles and Cancer Prevention and Treatment. Medicina 2019, 55, 84. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Therap. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Durk, R.P.; Castillo, E.; Marquez-Magana, L.; Grosicki, G.J.; Bolter, N.D.; Lee, C.M.; Bagley, J.R. Gut Microbiota Composition Is Related to Cardiorespiratory Fitness in Healthy Young Adults. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.; Wiklund, P.; Tan, X.; Wu, N.; Zhang, X.; Tikkanen, O.; Zhang, C.; Munukka, E.; Cheng, S. The Association between Cardiorespiratory Fitness and Gut Microbiota Composition in Premenopausal Women. Nutrients 2017, 9, 792. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Al-Jabi, S.W.; Amer, R.; Shakhshir, M.; Shahwan, M.; Jairoun, A.A.; Akkawi, M.; Abu Taha, A. Global research trends on the links between the gut microbiome and cancer: A visualization analysis. J. Transl. Med. 2022, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zheng, H.; Guo, Z.; Deng, R.; Yu, W.; Song, Y.; Ding, S. Effect of Helicobacter pylori on immunotherapy is gaining more attention. Helicobacter 2022, 27, e12925. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.K.; Hwang, J.W. Global Trends in Immunotherapy Research on Breast Cancer over the Past 10 Years. J. Oncol. 2020, 2020, 4708394. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhu, Z.; Huang, L.; Zeng, C.; Zhang, L.; Wu, W.K.; Lu, N.; Xie, C. Research Trends on Clinical Helicobacter pylori Eradication: A Bibliometric Analysis from 1983 to 2020. Helicobacter 2021, 26, e12835. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, S.; Ye, Y.; Jia, L.; Lou, Y. Global research trends on the links between gut microbiota and cancer immunotherapy: A bibliometric analysis (2012–2021). Front. Immunol. 2022, 13, 952546. [Google Scholar] [CrossRef] [PubMed]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Hirsch, J.E. An index to quantify an individual’s scientific research output. Proc. Natl. Acad. Sci. USA 2005, 102, 16569–16572. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, Q.; Zhu, Z. Evolutionary Overview of Land Consolidation Based on Bibliometric Analysis in Web of Science from 2000 to 2020. Int. J. Environ. Res. Public Health 2022, 19, 3218. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.E. Does the H index have predictive power? Proc. Natl. Acad. Sci. USA 2007, 104, 19193–19198. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Choi, J.J.; Eum, S.Y.; Rampersaud, E.; Daunert, S.; Abreu, M.T.; Toborek, M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ. Health Perspect. 2013, 121, 725–730. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Marcos, A.; Warnberg, J.; Marti, A.; Martin-Matillas, M.; Campoy, C.; Moreno, L.A.; Veiga, O.; Redondo-Figuero, C.; Garagorri, J.M.; et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity 2009, 17, 1906–1915. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Cobo, M.J.; Lopez-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. An approach for detecting, quantifying, and visualizing the evolution of a research field: A practical application to the Fuzzy Sets Theory field. J. Informetr. 2011, 5, 146–166. [Google Scholar] [CrossRef]
- Ogunsakin, R.E.; Ebenezer, O.; Ginindza, T.G. A Bibliometric Analysis of the Literature on Norovirus Disease from 1991–2021. Int. J. Environ. Res. Public Health 2022, 19, 2508. [Google Scholar] [CrossRef]
- Sun, H.L.; Bai, W.; Li, X.H.; Huang, H.; Cui, X.L.; Cheung, T.; Su, Z.H.; Yuan, Z.; Ng, C.H.; Xiang, Y.T. Schizophrenia and Inflammation Research: A Bibliometric Analysis. Front. Immunol. 2022, 13, 907851. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, K.; Guo, Q.; Yang, W.; Tong, L.; Wang, Y.; Sun, Z. Mapping Knowledge Structure and Themes Trends of Osteoporosis in Rheumatoid Arthritis: A Bibliometric Analysis. Front. Med. 2021, 8, 787228. [Google Scholar] [CrossRef] [PubMed]
- Wosinska, L.; Walsh, C.J.; O’Connor, P.M.; Lawton, E.M.; Cotter, P.D.; Guinane, C.M.; O’Sullivan, O. In Vitro and In Silico Based Approaches to Identify Potential Novel Bacteriocins from the Athlete Gut Microbiome of an Elite Athlete Cohort. Microorganisms 2022, 10, 701. [Google Scholar] [CrossRef]
- O’Donovan, C.M.; Madigan, S.M.; Garcia-Perez, I.; Rankin, A.; O’Sullivan, O.; Cotter, P.D. Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. J. Sci. Med. Sport 2020, 23, 63–68. [Google Scholar] [CrossRef]
- CM, O.D.; Connor, B.; Madigan, S.M.; Cotter, P.D.; O’Sullivan, O. Instances of altered gut microbiomes among Irish cricketers over periods of travel in the lead up to the 2016 World Cup: A sequencing analysis. Travel. Med. Infect. Dis. 2020, 35, 101553. [Google Scholar] [CrossRef]
- Cronin, O.; Barton, W.; Skuse, P.; Penney, N.C.; Garcia-Perez, I.; Murphy, E.F.; Woods, T.; Nugent, H.; Fanning, A.; Melgar, S.; et al. A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems 2018, 3, e00044-18. [Google Scholar] [CrossRef]
- Barton, W.; Cronin, O.; Garcia-Perez, I.; Whiston, R.; Holmes, E.; Woods, T.; Molloy, C.B.; Molloy, M.G.; Shanahan, F.; Cotter, P.D.; et al. The effects of sustained fitness improvement on the gut microbiome: A longitudinal, repeated measures case-study approach. Transl. Sports Med. 2021, 4, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Z.; Hu, B.; Huang, W.; Yuan, C.; Zou, L. Response of Gut Microbiota to Metabolite Changes Induced by Endurance Exercise. Front. Microbiol. 2018, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Hsu, Y.J.; Liao, C.C.; Ho, S.T.; Huang, C.C.; Huang, W.C. Physiological and Biochemical Effects of Intrinsically High and Low Exercise Capacities Through Multiomics Approaches. Front. Physiol. 2019, 10, 1201. [Google Scholar] [CrossRef]
- Huang, W.C.; Tung, C.L.; Yang, Y.S.H.; Lin, I.H.; Ng, X.E.; Tung, Y.T. Endurance exercise ameliorates Western diet-induced atherosclerosis through modulation of microbiota and its metabolites. Sci. Rep. 2022, 12, 3612. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Huang, Y.T.; Huang, C.C.; Chuang, H.L. Effect of intestinal microbiota on exercise performance in mice. J. Strength Cond. Res. 2015, 29, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, Y.H.; Chuang, H.L.; Chiu, C.C.; Huang, C.C. Investigation of the Effects of Microbiota on Exercise Physiological Adaption, Performance, and Energy Utilization Using a Gnotobiotic Animal Model. Front. Microbiol. 2019, 10, 1906. [Google Scholar] [CrossRef]
- Huang, W.C.; Hsu, Y.J.; Huang, C.C.; Liu, H.C.; Lee, M.C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients 2020, 12, 1145. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, H.; Chen, Z.; Wang, T. Bibliometric Analysis of Dendritic Epidermal T Cell (DETC) Research From 1983 to 2019. Front. Immunol. 2020, 11, 259. [Google Scholar] [CrossRef]
- Steves, C.J.; Bird, S.; Williams, F.M.; Spector, T.D. The Microbiome and Musculoskeletal Conditions of Aging: A Review of Evidence for Impact and Potential Therapeutics. J. Bone Miner. Res. 2016, 31, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.S.B.; Raes, J.; Bork, P. The Human Gut Microbiome: From Association to Modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Jang, L.G.; Choi, G.; Kim, S.W.; Kim, B.Y.; Lee, S.; Park, H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: An observational study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Aya, V.; Florez, A.; Perez, L.; Ramirez, J.D. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef] [PubMed]
- Tarracchini, C.; Fontana, F.; Lugli, G.A.; Mancabelli, L.; Alessandri, G.; Turroni, F.; Ventura, M.; Milani, C. Investigation of the Ecological Link between Recurrent Microbial Human Gut Communities and Physical Activity. Microbiol. Spectr. 2022, 10, e0042022. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yang, K.; Yang, P.; Zhong, C.; Chen, C.; Wang, S.; Lu, Q.; Ning, K. Stratification of athletes’ gut microbiota: The multifaceted hubs associated with dietary factors, physical characteristics and performance. Gut Microbes 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Fontana, F.; Longhi, G.; Tarracchini, C.; Mancabelli, L.; Lugli, G.A.; Alessandri, G.; Turroni, F.; Milani, C.; Ventura, M. The human gut microbiome of athletes: Metagenomic and metabolic insights. Microbiome 2023, 11, 27. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, F.; Zheng, X.; Lai, H.Y.; Wu, C.; Huang, C. Disparity of Gut Microbiota Composition Among Elite Athletes and Young Adults With Different Physical Activity Independent of Dietary Status: A Matching Study. Front. Nutr. 2022, 9, 843076. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Hughes, R.L. A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Front. Nutr. 2019, 6, 191. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Tana, C.; Nouvenne, A.; Ridolo, E.; Meschi, T. Exercise and immune system as modulators of intestinal microbiome: Implications for the gut-muscle axis hypothesis. Exerc. Immunol. Rev. 2019, 25, 84–95. [Google Scholar]
- Fehrenbach, E.; Niess, A.M.; Schlotz, E.; Passek, F.; Dickhuth, H.H.; Northoff, H. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J. Appl. Physiol. 2000, 89, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Rosa, J.C.; Pimentel, G.D.; Souza, H.A.; Caperuto, E.C.; Carnevali, L.C., Jr.; Seelaender, M.; Damaso, A.R.; Oyama, L.M.; de Mello, M.T.; et al. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 2010, 9, 82. [Google Scholar] [CrossRef]
- Dokladny, K.; Moseley, P.L.; Ma, T.Y. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G204–G212. [Google Scholar] [CrossRef]
- Kulecka, M.; Fraczek, B.; Balabas, A.; Czarnowski, P.; Zeber-Lubecka, N.; Zapala, B.; Baginska, K.; Glowienka, M.; Szot, M.; Skorko, M.; et al. Characteristics of the gut microbiome in esports players compared with those in physical education students and professional athletes. Front. Nutr. 2022, 9, 1092846. [Google Scholar] [CrossRef] [PubMed]
- Hintikka, J.E.; Munukka, E.; Valtonen, M.; Luoto, R.; Ihalainen, J.K.; Kallonen, T.; Waris, M.; Heinonen, O.J.; Ruuskanen, O.; Pekkala, S. Gut Microbiota and Serum Metabolome in Elite Cross-Country Skiers: A Controlled Study. Metabolites 2022, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Soltys, K.; Lendvorsky, L.; Hric, I.; Baranovicova, E.; Penesova, A.; Mikula, I.; Bohmer, M.; Budis, J.; Vavrova, S.; Grones, J.; et al. Strenuous Physical Training, Physical Fitness, Body Composition and Bacteroides to Prevotella Ratio in the Gut of Elderly Athletes. Front. Physiol. 2021, 12, 670989. [Google Scholar] [CrossRef]
- Babszky, G.; Torma, F.; Aczel, D.; Bakonyi, P.; Gombos, Z.; Feher, J.; Szabo, D.; Ligeti, B.; Pongor, S.; Balogh, L.; et al. COVID-19 Infection Alters the Microbiome: Elite Athletes and Sedentary Patients Have Similar Bacterial Flora. Genes 2021, 12, 1577. [Google Scholar] [CrossRef] [PubMed]
- Kulecka, M.; Fraczek, B.; Mikula, M.; Zeber-Lubecka, N.; Karczmarski, J.; Paziewska, A.; Ambrozkiewicz, F.; Jagusztyn-Krynicka, K.; Cieszczyk, P.; Ostrowski, J. The composition and richness of the gut microbiota differentiate the top Polish endurance athletes from sedentary controls. Gut Microbes 2020, 11, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Fart, F.; Rajan, S.K.; Wall, R.; Rangel, I.; Ganda-Mall, J.P.; Tingo, L.; Brummer, R.J.; Repsilber, D.; Schoultz, I.; Lindqvist, C.M. Differences in Gut Microbiome Composition between Senior Orienteering Athletes and Community-Dwelling Older Adults. Nutrients 2020, 12, 2610. [Google Scholar] [CrossRef]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Loyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Silva, M.A.; Lyssa, V.; Marques, G.; Lima, H.K.; Franco, O.L.; Petriz, B. The molecular signaling of exercise and obesity in the microbiota-gut-brain axis. Front. Endocrinol. 2022, 13, 927170. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Alvarez, L.; Xu, H.; Martinez-Tellez, B. Influence of Exercise on the Human Gut Microbiota of Healthy Adults: A Systematic Review. Clin. Transl. Gastroenterol. 2020, 11, e00126. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Xu, Y.; Lai, H.Y.; Yang, M.; Cheng, L.; Liu, X.; Sun, X.; Yang, Y.; Wang, J.; Lv, W.; et al. Effects of combined aerobic and resistance training on gut microbiota and cardiovascular risk factors in physically active elderly women: A randomized controlled trial. Front. Physiol. 2022, 13, 1004863. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Fernandez-Sanjurjo, M.; Iglesias-Gutierrez, E.; Martinez-Camblor, P.; Villar, C.J.; Tomas-Zapico, C.; Fernandez-Garcia, B.; Lombo, F. Resistance and Endurance Exercise Training Induce Differential Changes in Gut Microbiota Composition in Murine Models. Front. Physiol. 2021, 12, 748854. [Google Scholar] [CrossRef]
- de Sire, A.; de Sire, R.; Petito, V.; Masi, L.; Cisari, C.; Gasbarrini, A.; Scaldaferri, F.; Invernizzi, M. Gut-Joint Axis: The Role of Physical Exercise on Gut Microbiota Modulation in Older People with Osteoarthritis. Nutrients 2020, 12, 574. [Google Scholar] [CrossRef]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701. [Google Scholar] [CrossRef]
- Valdes-Ramos, R.; Martinez-Carrillo, B.E.; Aranda, G., II; Guadarrama, A.L.; Pardo-Morales, R.V.; Tlatempa, P.; Jarillo-Luna, R.A. Diet, exercise and gut mucosal immunity. Proc. Nutr. Soc. 2010, 69, 644–650. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Crowley, E.K.; Brown, J.R.; O’Sullivan, O.; O’Leary, O.F.; Timmons, S.; Nolan, Y.M.; Clarke, D.J.; Hyland, N.P.; Joyce, S.A.; et al. Nigral overexpression of alpha-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol. Motil. 2020, 32, e13726. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Sohail, M.U.; Yassine, H.M.; Sohail, A.; Thani, A.A.A. Impact of Physical Exercise on Gut Microbiome, Inflammation, and the Pathobiology of Metabolic Disorders. Rev. Diabet. Stud. 2019, 15, 35–48. [Google Scholar] [CrossRef]
- Rowell, L.B.; Brengelmann, G.L.; Blackmon, J.R.; Twiss, R.D.; Kusumi, F. Splanchnic blood flow and metabolism in heat-stressed man. J. Appl. Physiol. 1968, 24, 475–484. [Google Scholar] [CrossRef]
- van Wijck, K.; Lenaerts, K.; van Loon, L.J.; Peters, W.H.; Buurman, W.A.; Dejong, C.H. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef]
- Otte, J.A.; Oostveen, E.; Geelkerken, R.H.; Groeneveld, A.B.; Kolkman, J.J. Exercise induces gastric ischemia in healthy volunteers: A tonometry study. J. Appl. Physiol. 2001, 91, 866–871. [Google Scholar] [CrossRef]
- Munukka, E.; Ahtiainen, J.P.; Puigbo, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietila, S.; Hollmen, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, L.; Le, S.; Yang, Y.; Zhao, C.; Zhang, X.; Yang, X.; Xu, T.; Xu, L.; Wiklund, P.; et al. A randomized controlled trial for response of microbiome network to exercise and diet intervention in patients with nonalcoholic fatty liver disease. Nat. Commun. 2022, 13, 2555. [Google Scholar] [CrossRef]
- Lee, C.J.; Sears, C.L.; Maruthur, N. Gut microbiome and its role in obesity and insulin resistance. Ann. N. Y. Acad. Sci. 2020, 1461, 37–52. [Google Scholar] [CrossRef]
- John, G.K.; Mullin, G.E. The Gut Microbiome and Obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, R.; Nistal, E.; Estebanez, B.; Porras, D.; Juarez-Fernandez, M.; Martinez-Florez, S.; Garcia-Mediavilla, M.V.; de Paz, J.A.; Gonzalez-Gallego, J.; Sanchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Fang, Y.; Deng, H.; Yin, H.; Shen, B.; Hu, M. Six-Week Exercise Training With Dietary Restriction Improves Central Hemodynamics Associated With Altered Gut Microbiota in Adolescents With Obesity. Front. Endocrinol. 2020, 11, 569085. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91.e5. [Google Scholar] [CrossRef] [PubMed]
- Toohey, K. Exercise in Cancer Care. Semin. Oncol. Nurs. 2020, 36, 151066. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine-evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- Odynets, T.; Briskin, Y.; Todorova, V. Effects of Different Exercise Interventions on Quality of Life in Breast Cancer Patients: A Randomized Controlled Trial. Integr. Cancer Ther. 2019, 18, 1534735419880598. [Google Scholar] [CrossRef]
- Cavalheri, V.; Granger, C.L. Exercise training as part of lung cancer therapy. Respirology 2020, 25 (Suppl. S2), 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker, R.; Meichtry, A.; Eicher, M.; Nilsson Balfe, L.; Knols, R.H.; Verra, M.L.; Taeymans, J. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect-comparisons meta-analysis. Br. J. Sports Med. 2018, 52, 651–658. [Google Scholar] [CrossRef]
- Sampsell, K.; Wang, W.; Ohland, C.; Mager, L.F.; Pett, N.; Lowry, D.E.; Sales, K.M.; McNeely, M.L.; McCoy, K.D.; Culos-Reed, S.N.; et al. Exercise and Prebiotic Fiber Provide Gut Microbiota-Driven Benefit in a Survivor to Germ-Free Mouse Translational Model of Breast Cancer. Cancers 2022, 14, 2722. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Wang, X.; Chu, Y.; Zhang, H.; Zhou, L.; Zhu, H.; Li, J.; Kuai, R.; Zhou, F.; et al. Swimming Impedes Intestinal Microbiota and Lipid Metabolites of Tumorigenesis in Colitis-Associated Cancer. Front. Oncol. 2022, 12, 929092. [Google Scholar] [CrossRef]
- Wang, R.; Cai, Y.; Lu, W.; Zhang, R.; Shao, R.; Yau, S.Y.; Stubbs, B.; McIntyre, R.S.; Su, K.P.; Xu, G.; et al. Exercise effect on the gut microbiota in young adolescents with subthreshold depression: A randomized psychoeducation-controlled Trial. Psychiatry Res. 2023, 319, 115005. [Google Scholar] [CrossRef]
- Wang, Y.; Pu, Z.; Zhang, Y.; Du, Z.; Guo, Z.; Bai, Q. Exercise training has a protective effect in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice model with improved neural and intestinal pathology and modified intestinal flora. Behav. Brain Res. 2023, 439, 114240. [Google Scholar] [CrossRef]
- Craven, J.; Cox, A.J.; Bellinger, P.; Desbrow, B.; Irwin, C.; Buchan, J.; McCartney, D.; Sabapathy, S. The influence of exercise training volume alterations on the gut microbiome in highly-trained middle-distance runners. Eur. J. Sport Sci. 2022, 22, 1222–1230. [Google Scholar] [CrossRef]
- Bielik, V.; Hric, I.; Ugrayova, S.; Kubanova, L.; Putala, M.; Grznar, L.; Penesova, A.; Havranova, A.; Sardzikova, S.; Grendar, M.; et al. Effect of High-intensity Training and Probiotics on Gut Microbiota Diversity in Competitive Swimmers: Randomized Controlled Trial. Sports Med. Open. 2022, 8, 64. [Google Scholar] [CrossRef]
- Qiu, L.; Gong, F.; Wu, J.; You, D.; Zhao, Y.; Xu, L.; Cao, X.; Bao, F. Exercise Interventions Improved Sleep Quality through Regulating Intestinal Microbiota Composition. Int. J. Environ. Res. Public Health 2022, 19, 12385. [Google Scholar] [CrossRef]
- Dupuit, M.; Rance, M.; Morel, C.; Bouillon, P.; Boscaro, A.; Martin, V.; Vazeille, E.; Barnich, N.; Chassaing, B.; Boisseau, N. Effect of Concurrent Training on Body Composition and Gut Microbiota in Postmenopausal Women with Overweight or Obesity. Med. Sci. Sports Exerc. 2022, 54, 517–529. [Google Scholar] [CrossRef]
- Sato, M.; Suzuki, Y. Alterations in intestinal microbiota in ultramarathon runners. Sci. Rep. 2022, 12, 6984. [Google Scholar] [CrossRef]
- Chen, H.; Shen, L.; Liu, Y.; Ma, X.; Long, L.; Ma, X.; Ma, L.; Chen, Z.; Lin, X.; Si, L.; et al. Strength Exercise Confers Protection in Central Nervous System Autoimmunity by Altering the Gut Microbiota. Front. Immunol. 2021, 12, 628629. [Google Scholar] [CrossRef]
- Moitinho-Silva, L.; Wegener, M.; May, S.; Schrinner, F.; Akhtar, A.; Boysen, T.J.; Schaeffer, E.; Hansen, C.; Schmidt, T.; Ruhlemann, M.C.; et al. Short-term physical exercise impacts on the human holobiont obtained by a randomised intervention study. BMC Microbiol. 2021, 21, 162. [Google Scholar] [CrossRef]
- Resende, A.S.; Leite, G.S.F.; Lancha Junior, A.H. Changes in the Gut Bacteria Composition of Healthy Men with the Same Nutritional Profile Undergoing 10-Week Aerobic Exercise Training: A Randomized Controlled Trial. Nutrients 2021, 13, 2839. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, A.; Zong, W.; Dai, L.; Liu, Y.; Luo, R.; Ge, S.; Dong, G. Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi. J. Biol. Sci. 2021, 28, 40–49. [Google Scholar] [CrossRef]
- Verheggen, R.; Konstanti, P.; Smidt, H.; Hermus, A.; Thijssen, D.H.J.; Hopman, M.T.E. Eight-week exercise training in humans with obesity: Marked improvements in insulin sensitivity and modest changes in gut microbiome. Obesity 2021, 29, 1615–1624. [Google Scholar] [CrossRef]
- Ahrens, A.P.; Culpepper, T.; Saldivar, B.; Anton, S.; Stoll, S.; Handberg, E.M.; Xu, K.; Pepine, C.; Triplett, E.W.; Aggarwal, M. A Six-Day, Lifestyle-Based Immersion Program Mitigates Cardiovascular Risk Factors and Induces Shifts in Gut Microbiota, Specifically Lachnospiraceae, Ruminococcaceae, Faecalibacterium prausnitzii: A Pilot Study. Nutrients 2021, 13, 3459. [Google Scholar] [CrossRef] [PubMed]
- Marfil-Sanchez, A.; Seelbinder, B.; Ni, Y.; Varga, J.; Berta, J.; Hollosi, V.; Dome, B.; Megyesfalvi, Z.; Dulka, E.; Galffy, G.; et al. Gut microbiome functionality might be associated with exercise tolerance and recurrence of resected early-stage lung cancer patients. PLoS ONE 2021, 16, e0259898. [Google Scholar] [CrossRef]
- Fruge, A.D.; Smith, K.S.; Bail, J.R.; Rais-Bahrami, S.; Demark-Wahnefried, W. Biomarkers Associated With Tumor Ki67 and Cathepsin L Gene Expression in Prostate Cancer Patients Participating in a Presurgical Weight Loss Trial. Front. Oncol. 2020, 10, 544201. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Ericsson, A.; Qiao, Z.; Almendros, I.; Farre, R.; Gozal, D. Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: Effects of physical activity. EBioMedicine 2021, 64, 103208. [Google Scholar] [CrossRef]
- Mokhtarzade, M.; Molanouri Shamsi, M.; Abolhasani, M.; Bakhshi, B.; Sahraian, M.A.; Quinn, L.S.; Negaresh, R. Home-based exercise training influences gut bacterial levels in multiple sclerosis. Complement. Ther. Clin. Pract. 2021, 45, 101463. [Google Scholar] [CrossRef]
- Warbeck, C.; Dowd, A.J.; Kronlund, L.; Parmar, C.; Daun, J.T.; Wytsma-Fisher, K.; Millet, G.Y.; Schick, A.; Reimer, R.A.; Fung, T.; et al. Feasibility and effects on the gut microbiota of a 12-week high-intensity interval training plus lifestyle education intervention on inactive adults with celiac disease. Appl. Physiol. Nutr. Metab. 2021, 46, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Magzal, F.; Shochat, T.; Haimov, I.; Tamir, S.; Asraf, K.; Tuchner-Arieli, M.; Even, C.; Agmon, M. Increased physical activity improves gut microbiota composition and reduces short-chain fatty acid concentrations in older adults with insomnia. Sci. Rep. 2022, 12, 2265. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef]
- Lensu, S.; Pekkala, S. Gut Microbiota, Microbial Metabolites and Human Physical Performance. Metabolites 2021, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Junges, V.M.; Closs, V.E.; Nogueira, G.M.; Gottlieb, M.G.V. Crosstalk between Gut Microbiota and Central Nervous System: A Focus on Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Royes, L.F.F. Cross-talk between gut and brain elicited by physical exercise. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165877. [Google Scholar] [CrossRef] [PubMed]
- Agirman, G.; Hsiao, E.Y. Gut microbes shape athletic motivation. Nature 2022, 612, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef]
- de Sire, R.; Rizzatti, G.; Ingravalle, F.; Pizzoferrato, M.; Petito, V.; Lopetuso, L.; Graziani, C.; de Sire, A.; Mentella, M.C.; Mele, M.C.; et al. Skeletal muscle-gut axis: Emerging mechanisms of sarcopenia for intestinal and extra intestinal diseases. Minerva. Gastroenterol. Dietol. 2018, 64, 351–362. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Moller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Dohnalova, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.P.; Litichevskiy, L.; Descamps, H.C.; et al. A microbiome-dependent gut-brain pathway regulates motivation for exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef]
- Cerovic, M.; Forloni, G.; Balducci, C. Neuroinflammation and the Gut Microbiota: Possible Alternative Therapeutic Targets to Counteract Alzheimer’s Disease? Front. Aging Neurosci. 2019, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Barres, B.A. The mystery and magic of glia: A perspective on their roles in health and disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jager, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Marlicz, W.; Loniewski, I. The effect of exercise and diet on gut microbial diversity. Gut 2015, 64, 519–520. [Google Scholar] [CrossRef]
- Peters, H.P.; De Vries, W.R.; Vanberge-Henegouwen, G.P.; Akkermans, L.M. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 2001, 48, 435–439. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sports Med. 2014, 44 (Suppl. S1), S79–S85. [Google Scholar] [CrossRef]
- Kim, K.H.; Chung, Y.; Huh, J.W.; Park, D.J.; Cho, Y.; Oh, Y.; Jeong, H.; Yoon, J.; Kang, J.H.; Shin, H.S.; et al. Gut microbiota of the young ameliorates physical fitness of the aged in mice. Microbiome 2022, 10, 238. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Peake, J.M.; Cripps, A.W. Probiotics, immunity and exercise: A review. Exerc. Immunol. Rev. 2009, 15, 107–126. [Google Scholar] [PubMed]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Wosinska, L.; Cotter, P.D.; O’Sullivan, O.; Guinane, C. The Potential Impact of Probiotics on the Gut Microbiome of Athletes. Nutrients 2019, 11, 2270. [Google Scholar] [CrossRef]
- Imaoka, A.; Shima, T.; Kato, K.; Mizuno, S.; Uehara, T.; Matsumoto, S.; Setoyama, H.; Hara, T.; Umesaki, Y. Anti-inflammatory activity of probiotic Bifidobacterium: Enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J. Gastroenterol. 2008, 14, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef]
- West, N.P.; Horn, P.L.; Pyne, D.B.; Gebski, V.J.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin. Nutr. 2014, 33, 581–587. [Google Scholar] [CrossRef]
- Santibanez-Gutierrez, A.; Fernandez-Landa, J.; Calleja-Gonzalez, J.; Delextrat, A.; Mielgo-Ayuso, J. Effects of Probiotic Supplementation on Exercise with Predominance of Aerobic Metabolism in Trained Population: A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 622. [Google Scholar] [CrossRef]
- Salleh, R.M.; Kuan, G.; Aziz, M.N.A.; Rahim, M.R.A.; Rahayu, T.; Sulaiman, S.; Kusuma, D.W.Y.; Adikari, A.; Razam, M.S.M.; Radhakrishnan, A.K.; et al. Effects of Probiotics on Anxiety, Stress, Mood and Fitness of Badminton Players. Nutrients 2021, 13, 1783. [Google Scholar] [CrossRef]
- Furber, M.J.W.; Young, G.R.; Holt, G.S.; Pyle, S.; Davison, G.; Roberts, M.G.; Roberts, J.D.; Howatson, G.; Smith, D.L. Gut Microbial Stability is Associated with Greater Endurance Performance in Athletes Undertaking Dietary Periodization. mSystems 2022, 7, e0012922. [Google Scholar] [CrossRef]
- Liu, G.; Zhuang, H.B. Probiotic Supplements for Endurance Exercise Performance and Immune Function. Rev. Bras. Med. Esporte 2022, 28, 99–102. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Wu, M.F.; Lee, M.C.; Huang, C.C. Exercise training combined with Bifidobacterium longum OLP-01 treatment regulates insulin resistance and physical performance in db/db mice. Food. Funct. 2021, 12, 7728–7740. [Google Scholar] [CrossRef]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Kuo, Y.W.; Lin, W.Y.; Tsai, S.Y.; Chen, W.L.; Lin, C.L.; Huang, C.C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci. Rep. 2021, 11, 19469. [Google Scholar] [CrossRef]
- Fu, S.K.; Tseng, W.C.; Tseng, K.W.; Lai, C.C.; Tsai, Y.C.; Tai, H.L.; Hsu, C.C. Effect of Daily Oral Lactobacillus plantarum PS128 on Exercise Capacity Recovery after a Half-Marathon. Nutrients 2021, 13, 4023. [Google Scholar] [CrossRef]
- Valentino, T.R.; Vechetti, I.J., Jr.; Mobley, C.B.; Dungan, C.M.; Golden, L.; Goh, J.; McCarthy, J.J. Dysbiosis of the gut microbiome impairs mouse skeletal muscle adaptation to exercise. J. Physiol. 2021, 599, 4845–4863. [Google Scholar] [CrossRef]
- Quero, C.D.; Manonelles, P.; Fernandez, M.; Abellan-Aynes, O.; Lopez-Plaza, D.; Andreu-Caravaca, L.; Hinchado, M.D.; Galvez, I.; Ortega, E. Differential Health Effects on Inflammatory, Immunological and Stress Parameters in Professional Soccer Players and Sedentary Individuals after Consuming a Synbiotic. A Triple-Blinded, Randomized, Placebo-Controlled Pilot Study. Nutrients 2021, 13, 1321. [Google Scholar] [CrossRef]

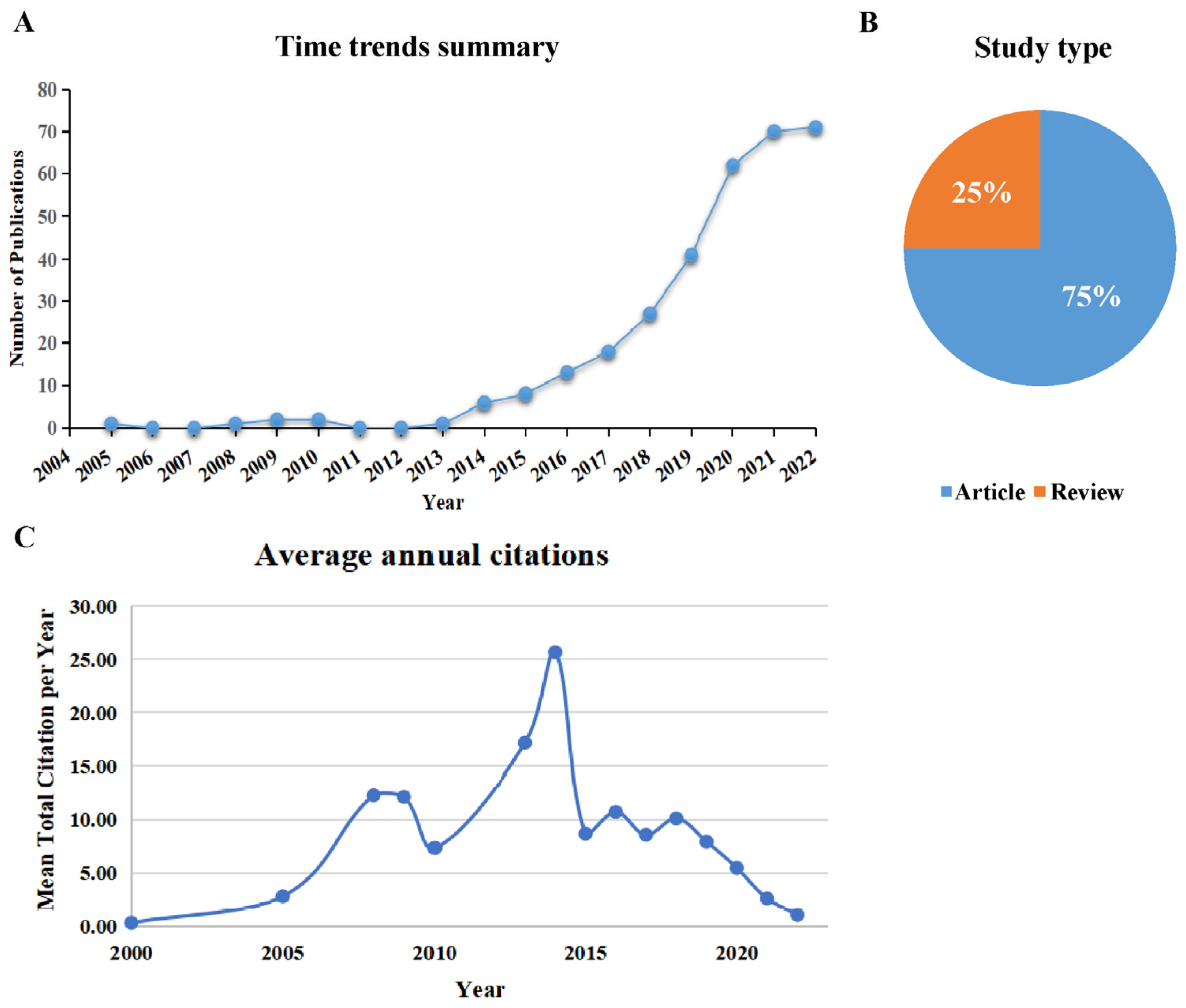

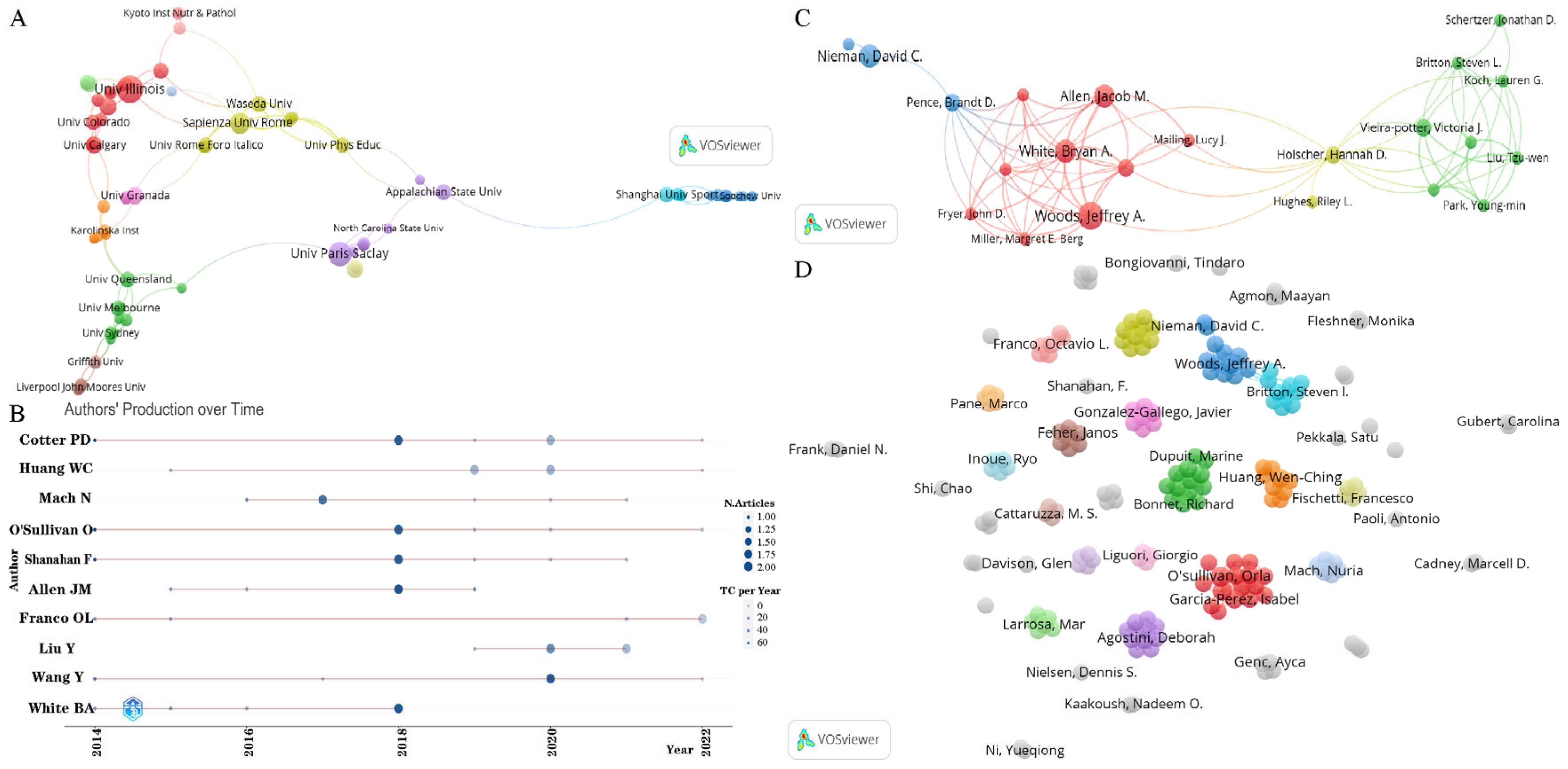

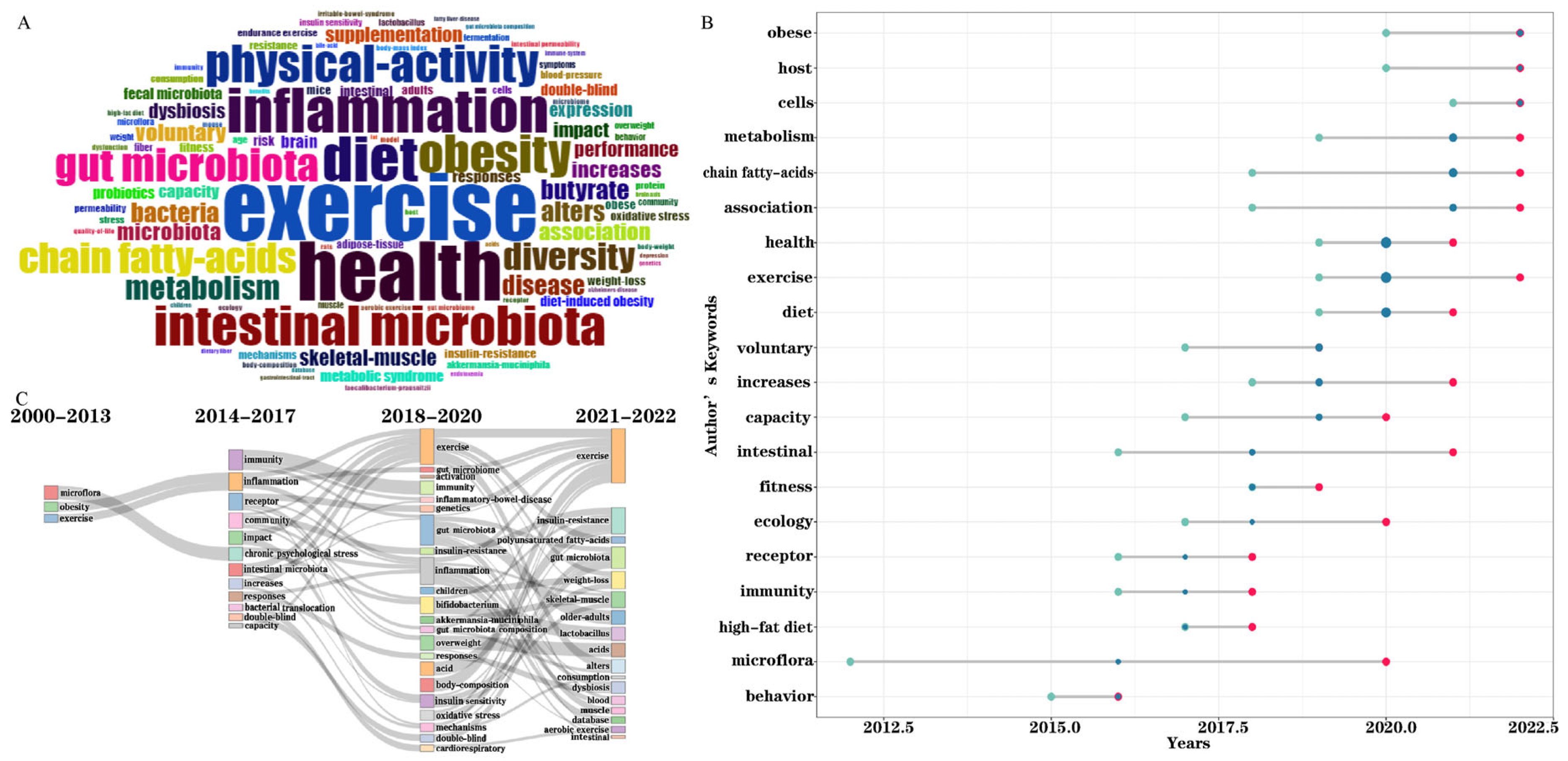
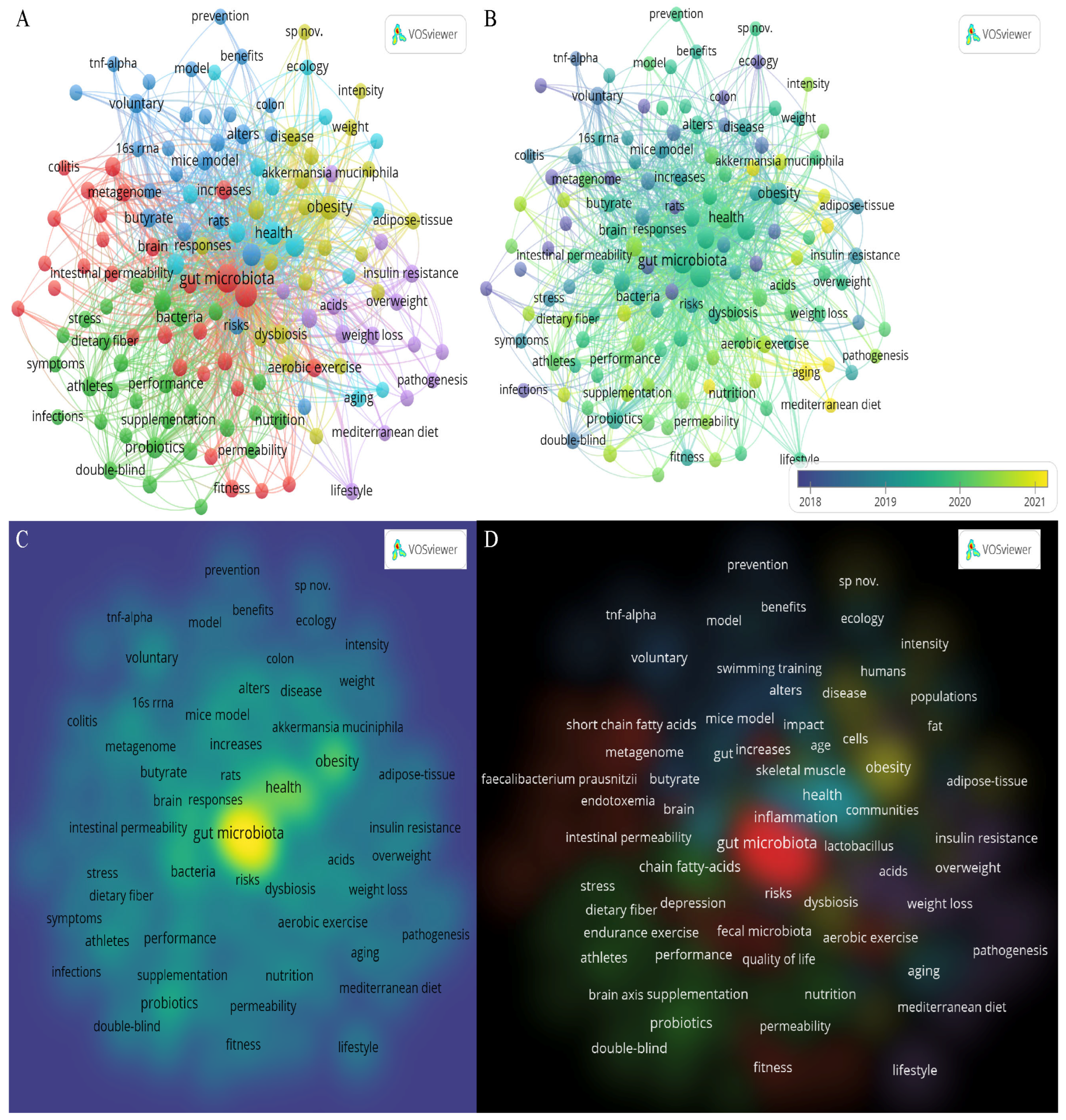
| No. | Countries/Regions | Publications | Citations | Average Article Citations | h-Index | Total Link Strength |
|---|---|---|---|---|---|---|
| 1 | USA | 81 | 3218 | 53.63 | 30 | 50 |
| 2 | China | 64 | 1254 | 18.72 | 18 | 20 |
| 3 | Italy | 38 | 664 | 21.42 | 14 | 34 |
| 4 | Spain | 26 | 1166 | 77.73 | 14 | 30 |
| 5 | England | 24 | 159 | 19.88 | 11 | 32 |
| 6 | Canada | 21 | 419 | 41.90 | 9 | 30 |
| 7 | Japan | 19 | 481 | 34.36 | 11 | 13 |
| 8 | Australia | 18 | 482 | 34.43 | 10 | 15 |
| 9 | Brazil | 17 | 332 | 23.71 | 8 | 8 |
| 10 | France | 16 | 130 | 13.00 | 8 | 16 |
| No. | Institutions | Publications | Citations | Time Cited (per Article) | Total Link Strength | Countries/Regions |
|---|---|---|---|---|---|---|
| 1 | Univ. Illinois | 11 | 1043 | 95 | 11 | USA |
| 2 | Univ. Paris Saclay | 9 | 556 | 62 | 10 | France |
| 3 | Sapienza Univ. Rome | 7 | 138 | 20 | 17 | Italy |
| 4 | Univ. Coll. Cork | 7 | 127 | 18 | 9 | Canada |
| 5 | Teagasc Food Res. Ctr. | 6 | 926 | 154 | 11 | Ireland |
| 6 | Imperial Coll. London | 5 | 362 | 72 | 13 | England |
| 7 | Natl. Taipei Univ. Nursing and Hlth. Sci. | 5 | 83 | 17 | 11 | Taiwan |
| 8 | Natl. Taiwan Sport Univ. | 5 | 173 | 35 | 11 | Taiwan |
| 9 | Univ. Calgary | 5 | 17 | 3 | 7 | Canada |
| 10 | Univ. Catolica Brasilia | 5 | 323 | 65 | 12 | Brazil |
| No. | Authors | Publications | Total Citations | h-Index | g-Index | m-Index | Total Link Strength |
|---|---|---|---|---|---|---|---|
| 1 | Cotter, P.D. | 7 | 1101 | 5 | 7 | 0.500 | 41 |
| 2 | Huang, W.C. | 6 | 189 | 5 | 6 | 0.556 | 11 |
| 3 | Mach, N. | 6 | 566 | 6 | 6 | 0.750 | 12 |
| 4 | O’Sullivan, O. | 6 | 1065 | 5 | 6 | 0.500 | 43 |
| 5 | Shanahan, F. | 6 | 1073 | 5 | 6 | 0.500 | 35 |
| 6 | Allen, J.M. | 5 | 780 | 5 | 5 | 0.556 | 19 |
| 7 | Franco, O.L. | 5 | 339 | 3 | 5 | 0.300 | 9 |
| 8 | Liu, Y. | 5 | 191 | 5 | 5 | 1.000 | 0 |
| 9 | Wang, Y. | 5 | 654 | 4 | 5 | 0.400 | 0 |
| 10 | White, B.A. | 5 | 790 | 5 | 5 | 0.500 | 22 |
| Journal | Publications | Impact Factor | Quartile in Category | h-Index | g-Index | m-Index | Total Citations |
|---|---|---|---|---|---|---|---|
| Nutrients | 29 | 6.706 | Q1 | 13 | 21 | 2.167 | 467 |
| PLoS ONE | 12 | 3.752 | Q2 | 10 | 12 | 1 | 829 |
| Frontiers in Microbiology | 9 | 6.064 | Q1 | 6 | 9 | 0.857 | 421 |
| Frontiers in Nutrition | 7 | 6.590 | Q1 | 3 | 7 | 0.750 | 99 |
| Scientific Report | 7 | 4.997 | Q2 | 4 | 7 | 0.667 | 125 |
| Frontiers in Physiology | 6 | 4.755 | Q1 | 5 | 6 | 0.625 | 356 |
| Gut Microbes | 6 | 9.434 | Q1 | 6 | 6 | 1 | 207 |
| Physiological Reports | 6 | 0.720 | Q3 | 4 | 6 | 0.667 | 108 |
| Microorganisms | 5 | 4.926 | Q2 | 3 | 5 | 0.750 | 45 |
| Msystems | 5 | 7.328 | Q1 | 4 | 5 | 0.571 | 155 |
| No. | Author | Publication Year | Document Type | Journal | Title | Times Cited in WOSCC Database |
|---|---|---|---|---|---|---|
| 1 | Siobhan, C.F. et al. [33] | 2014 | Article | Gut | Exercise and associated dietary extremes impact on gut microbial diversity | 644 |
| 2 | Christian, E.C. et al. [44] | 2014 | Article | PLoS ONE | Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity | 347 |
| 3 | Santacruz, A. et al. [43] | 2009 | Article | Obesity | Interplay Between Weight Loss and Gut Microbiota Composition in Overweight Adolescents | 317 |
| 4 | Jacob, A.M. et al. [17] | 2018 | Article | Medicine and Science in Sports and Exercise | Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans | 279 |
| 5 | Scheiman, J. et al. [42] | 2019 | Article | Nature Medicine | Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism | 251 |
| 6 | Barton, W. et al. [41] | 2018 | Article | Gut | The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level | 236 |
| 7 | Monda, V. et al. [15] | 2017 | Review | Oxidative Medicine and Cellular Longevity | Exercise Modifies the Gut Microbiota with Positive Health Effects | 213 |
| 8 | Estaki, M. et al. [40] | 2016 | Article | Microbiome | Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions | 199 |
| 9 | Clark, A. et al. [39] | 2016 | Review | Journal of the International Society of Sports Nutrition | Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes | 187 |
| 10 | Jeong, C.J. et al. [38] | 2013 | Article | Environmental Health Perspectives | Exercise Attenuates PCB-Induced Changes in the Mouse Gut Microbiome | 187 |
| Author | Publication Year | Subjects | Results |
|---|---|---|---|
| Fontana, F. et al. [67] | 2023 | 254 healthy athletes vs. 164 healthy sedentary adults | SCFAs microbial producers including Faecalibacterium, Eubacterium, Blautia, and Ruminococcus species, are statistically associated with athletes’ samples. The EFC related to athletes was positively linked to 752 enzymes (EC numbers) and 73 HIBS. The EFC related to sedentary adults was positively linked only to 105 EC numbers and 14 HIBS. |
| Kulecka, M. et al. [75] | 2023 | 109 well-characterized Polish male e-sports players, 25 endurance athletes, 36 healthy students of physical education | The differences of lifestyle and dietary habits appear to have little effect on most gut microbiota parameters. Several metabolic pathways including fermentation, amino acid biosynthesis and degradation, carbohydrate biosynthesis and degradation, fatty acid biosynthesis and degradation, and the TCA cycle pathways, were over-represented in professional athletes compared with e-sports players and students. |
| Hintikka, J.E. et al. [76] | 2022 | 27 national team cross-country skiers vs. 27 normally physically active people | Phylogenetic diversity and the abundance of mucin-degrading gut microbial taxa were lower among athletes. Butyricicoccus is associated positively with HDL cholesterol, HDL2 cholesterol, and HDL particle size. The R. torques group was less abundant in the athlete group and positively associated with total cholesterol and VLDL and LDL particles. |
| Xu, Y.J. et al. [68] | 2022 | 22 elite athletes vs. 44 general young adults | No significant difference was observed in both alpha and beta diversity. Compared to general young adults, elite athletes had a significantly higher abundance of Clostridiaceae and Megamonas_rupellensis. Inflammation-related bacteria, such as Bilophila and Faecalicoccus, were enriched in physically inactive young adults compared to two other groups. |
| Šoltys, K. et al. [77] | 2021 | 13 elderly endurance athletes vs. 9 healthy controls | Lifelong endurance training does not bring significant benefit regarding overall gut microbiota. Continual exercise by elderly endurance athletes is associated with favorable gut microbiota composition on the lower taxonomy level. The Bacteroides to Prevotella ratio seems to distinguish the endurance trained elderly from healthy controls. |
| Babszky, G. et al. [78] | 2021 | 20 actively competing athletes vs. 20 sedentary subjects | No significant differences in the fecal microbiome flora of trained and sedentary subjects who were diagnosed as positive for COVID-19 was detected. The levels of Bacteroidetes were enhanced during mild COVID-19 infection, supporting the immune system by suppressing the activation of TLR4 and ACE2 receptors. |
| Kulecka, M. et al. [79] | 2020 | 25 endurance athletes (14 marathon runners and 11 cross-country skiers) vs. 46 sedentary healthy controls | Excessive training is associated with both large alterations to microbial community composition and promotion of higher bacterial diversity. Endurance athletes presented a lowered abundance of major gut microbiota genus, Bacteroidetes and higher abundance of complex carbohydrates fermenters (from Prevotella genus and Firmicutes phylum). |
| Han, M.Z. et al. [66] | 2020 | 19 professional female rowing athletes (7 AE + 6 YE) vs. 6 YN | The microbial diversities, taxonomical, functional, and phenotypic compositions, of AE, YE, and YN were significantly different. SCFA-producing bacteria, such as Clostridiales, Ruminococcaceae and Faecalibacterium, are dominant in the microbial community of elite athletes. ATP metabolism, multiple sugar transport systems, and carbohydrate metabolism are enriched in the microbial community of elite athletes. |
| O’Donovan, C.M. et al. [50] | 2020 | 37 elite Irish athletes across 16 different sports | Streptococcus suis, Clostridium bolteae, Lactobacillus phage LfeInf, and Anaerostipes hadrus were found to be associated with a moderate dynamic component, including sports such as fencing. Bifidobacterium animalis, Lactobacillus acidophilus, Prevotella intermedia and F. prausnitzii were found to be associated with the high dynamic and low static components, including sports such as field hockey, while Bacteroides caccae was found to be associated with the high dynamic and static components, including sports such as rowing. |
| Fart, F. et al. [80] | 2020 | 28 physically active senior orienteering athletes vs. 70 community-dwelling older adults and | Compared to the community-dwelling older adults, the physically active senior orienteers had a more homogeneous microbiota within the group, a higher abundance of Faecalibacterium prausnitzii, and a lower abundance of Parasutterella excrementihominis and Bilophila unclassified. |
| Author | Publication Year | Subjects | Intervention | Results |
|---|---|---|---|---|
| Kim, K.H. et al. [150] | 2022 | 5-week-old young mice, 12-month-old mice | Transfer the gut microbiota from the young mice to the old mice | The young-mice-derived gut microbiota rejuvenated the physical fitness of the old mice, by altering the microbial composition of the gut and gene expression in muscle and skin. After the transfer, the old mice’s muscle fiber thickness, grip strength increased, and the water retention ability of the skin was enhanced, with a thickened stratum corneum. The host fitness of the old mice significantly improved. |
| Furber, M.J.W. et al. [159] | 2022 | 16 highly trained endurance runners | Isocaloric HPD or HCD | HCD improved time-trial performance by 16.5%, and was associated with expansion of Ruminococcus and Collinsella bacterial spp. HPD led to a reduction in performance by 223.3%, accompanied by significantly reduced diversity and altered composition of the gut phageome, as well as enrichment of both free and inducible Sk1virus and Leuconostoc bacterial populations. Gut microbial stability during acute dietary periodization was associated with greater athletic performance. |
| Liu, G. et al. [160] | 2022 | 7 health professional athletes (2 males and 5 females) | Probiotic supplements after exercise | The increase in the number of E. coli in the athlete’s gastrointestinal tract, is likely to destroy the quantitative structure of the gastrointestinal flora, potentially ruining the stability and physiological function of the athlete’s gastrointestinal tract. Long-term oral probiotics for athletes can reduce inflammation in the body, reduce damage to the body during exercise, and effectively improve the gastrointestinal tract’s immune function. |
| Salleh, R.M. et al. [158] | 2021 | 30 university badminton players aged from 19 to 22 years old | Probiotic supplements containing Lactobacillus casei Shirota | Supplementation of probiotics improved aerobic capacity in PG players by 5.9%, relieved anxiety and stress, but did not influence the speed, strength, leg power, and agility. |
| Hsu, Y.J. et al. [161] | 2021 | 24 C57BL/6 J db/db male mice (20 weeks old) | Bifidobacterium longum OLP-01 (OLP-01) | Grip strength and exhaustive swimming time were significantly higher in mice that received exercise training combined with Bifidobacterium longum OLP-01 supplementation (EX OLP-01), comapred to mice in the other groups. EX OLP-01 showed effects on improved physical activity, lowered blood glucose, increased insulin sensitivity, decreased total body fat, and protection against liver injury, without any adverse effects. |
| Lee, M.C. et al. [162] | 2021 | 40 six-week-old male ICR mice | Human-origin strain L. plantarum PL-02 supplementation | Four weeks of PL-02 supplementation could significantly improve grip and endurance exercise performance, and increase muscle mass, and hepatic and muscular glycogen storage. PL-02 could significantly decrease the level of fatigue indicators after exercise, such as lactate, BUN, ammonia, and CK. |
| Fu, S.K. et al. [163] | 2021 | 8 recreational HM runners | 4-week PS128 supplement administration | PS128 supplementation was related to an improvement in muscle damage, renal damage, and oxidative stress caused by HM, through microbiota modulation and related metabolites, but not in exercise capacity. PS128 may neutralize the ROS caused by exhaustive and prolonged exercise. |
| Valentino, T.R. et al. [164] | 2021 | 42 four-month-old female C57BL/6J mice | Antibiotic cocktail treatment, consisting of 100 μg/mL each of metronidazole, neomycin, and ampicillin, and 50 μg/mL each of vancomycin and streptomycin | Gut microbiome dysbiosis caused by antibiotics treatment impaired the ability of skeletal muscle to adapt to exercise training. Dysbiosis caused blunted hypertrophy in both the soleus and plantaris muscles following PoWeR training, and was associated with a loss of PoWeR-induced myonuclei accretion in the plantaris muscle. |
| Quero, C.D. et al. [165] | 2021 | 13 professional soccer players, 14 sedentary students | Synbiotic Gasteel Plus® supplementation | Synbiotic nutritional supplements can improve anxiety, stress, and sleep quality, particularly in sportspeople, which appears to be associated with an improved immuno-neuroendocrine response involving IL-1β, CRH, and dopamine. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, R.; Wang, M.; Song, Y.; Shi, Y. A Bibliometric Analysis on the Research Trend of Exercise and the Gut Microbiome. Microorganisms 2023, 11, 903. https://doi.org/10.3390/microorganisms11040903
Deng R, Wang M, Song Y, Shi Y. A Bibliometric Analysis on the Research Trend of Exercise and the Gut Microbiome. Microorganisms. 2023; 11(4):903. https://doi.org/10.3390/microorganisms11040903
Chicago/Turabian StyleDeng, Ruiyi, Mopei Wang, Yahan Song, and Yanyan Shi. 2023. "A Bibliometric Analysis on the Research Trend of Exercise and the Gut Microbiome" Microorganisms 11, no. 4: 903. https://doi.org/10.3390/microorganisms11040903
APA StyleDeng, R., Wang, M., Song, Y., & Shi, Y. (2023). A Bibliometric Analysis on the Research Trend of Exercise and the Gut Microbiome. Microorganisms, 11(4), 903. https://doi.org/10.3390/microorganisms11040903






