Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks
Abstract
1. Idiopathic Pulmonary Fibrosis
1.1. Role of Immune System
1.2. Viruses
1.3. Acute Exacerbations
2. COVID-19 and Fibrotic Damage
2.1. Lung Fibrosis
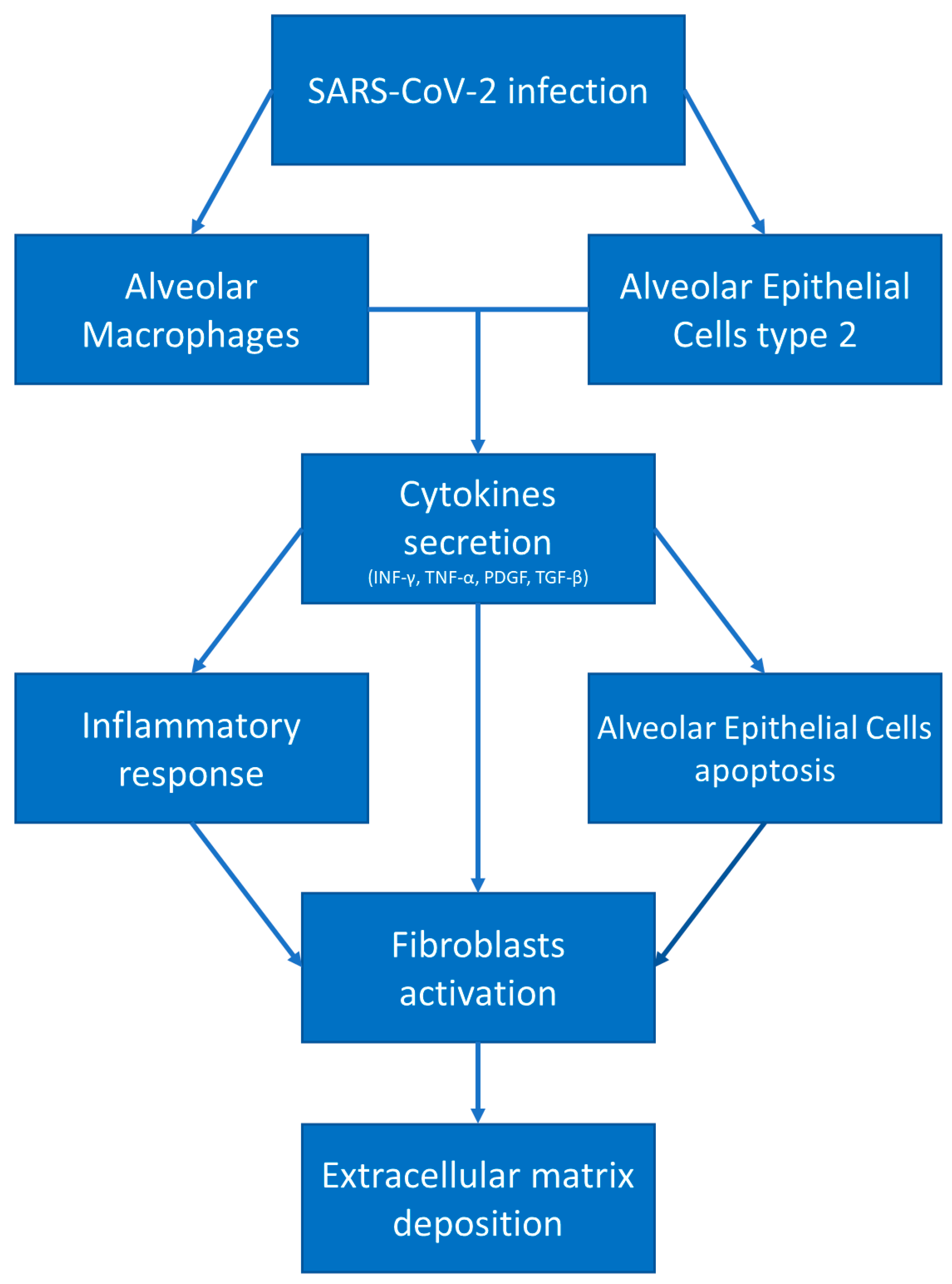
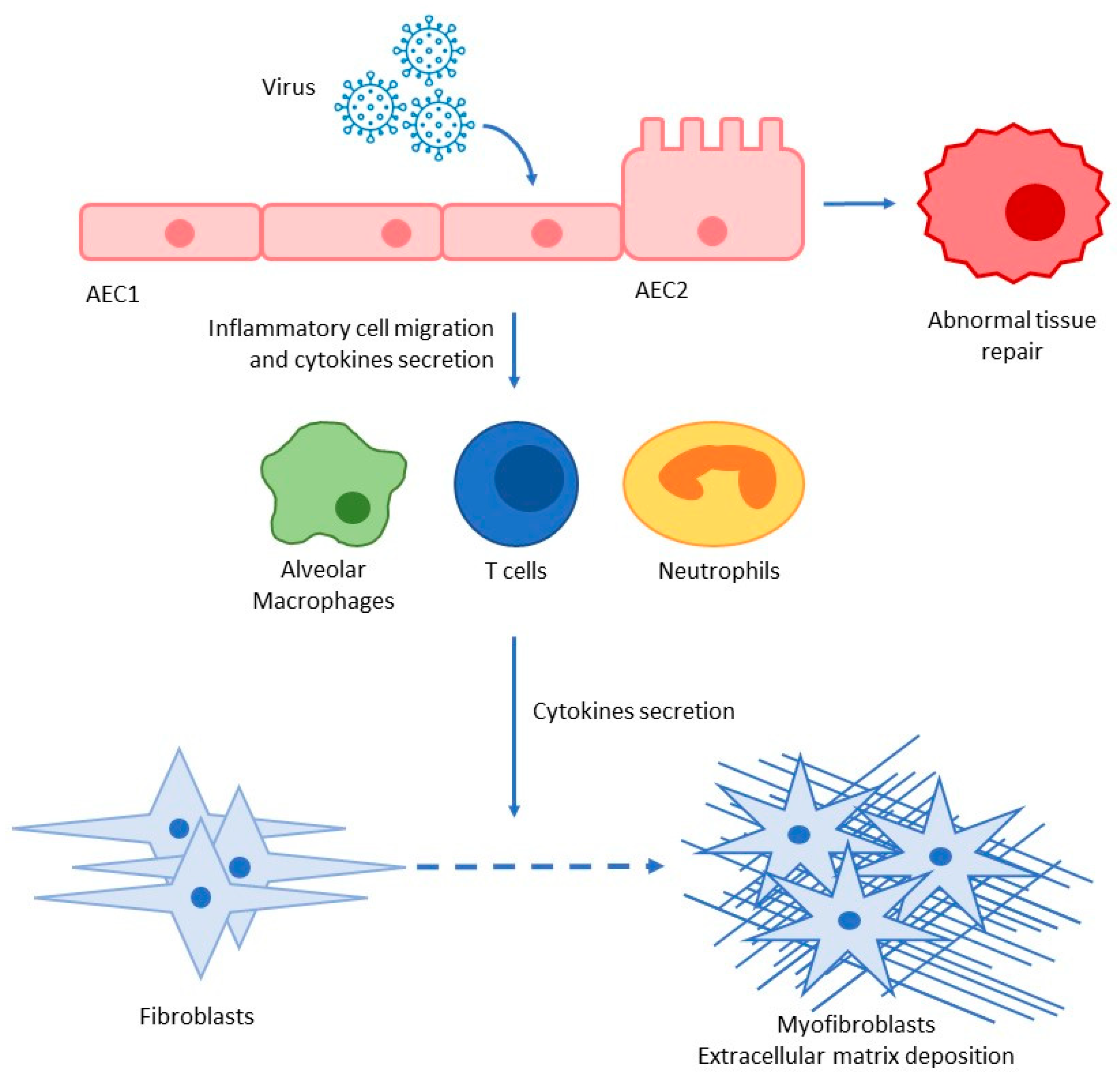
2.2. COVID-19-Mediated Lung Fibrosis
3. COVID-19 and IPF: Similarities
4. IPF and COVID-19 in Clinical Practice
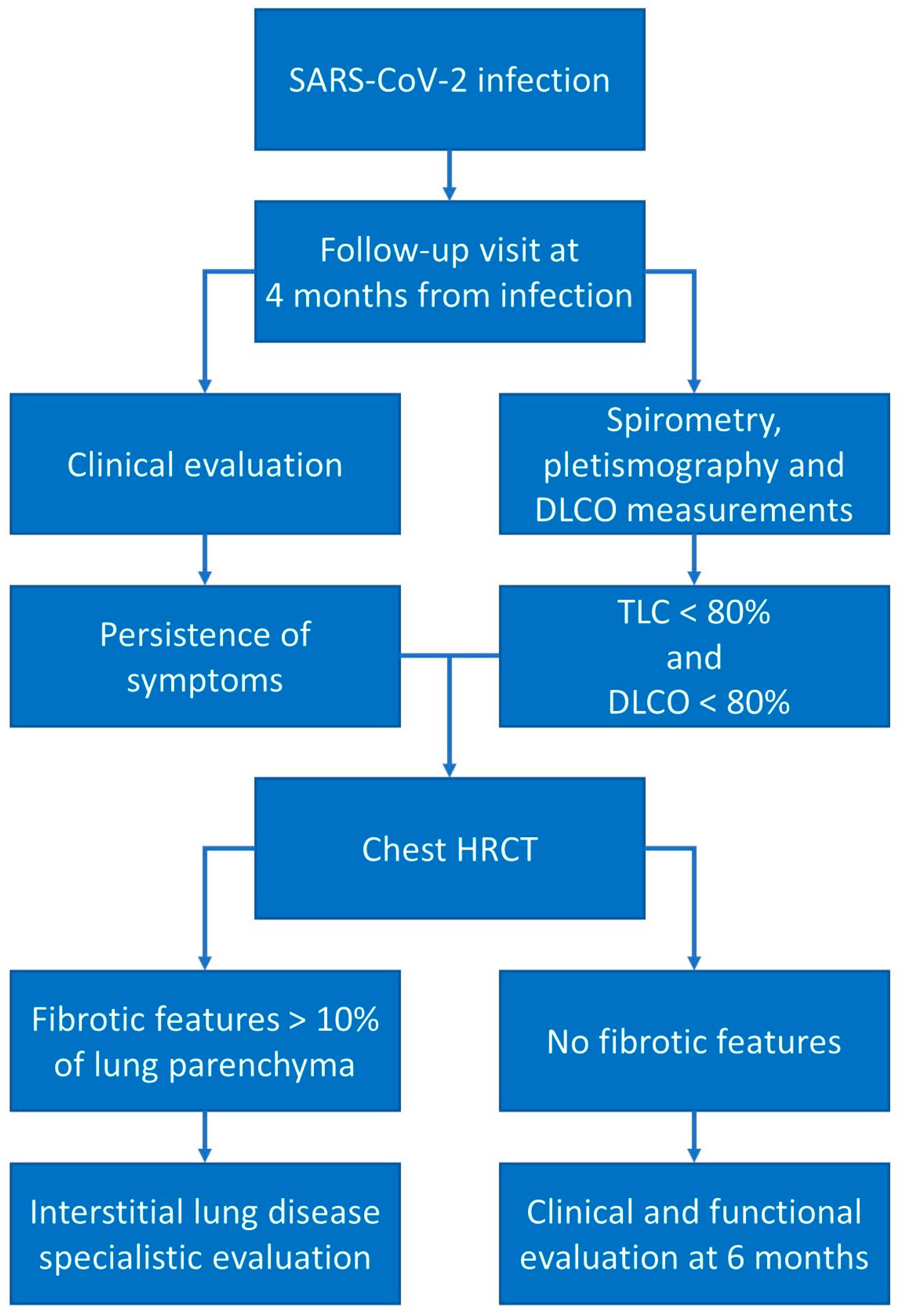
5. Post-COVID-19 Respiratory Functional Status Evaluation
6. Post-COVID-19 Follow-Up
7. Antifibrotic Agents Used in IPF for the Treatment of Post-COVID-19 Pulmonary Fibrosis: Nintedanib and Pirfenidone
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigiris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017, 3, 17074. [Google Scholar] [CrossRef]
- Schwartz, D.A. Idiopathic Pulmonary Fibrosis Is a Genetic Disease Involving Mucus and the Peripheral Airways. Ann. Am. Thorac. Soc. 2018, 15 (Suppl. S3), S192–S197. [Google Scholar] [CrossRef]
- Taskar, V.S.; Coultas, D.B. Is idiopathic pulmonary fibrosis an environmental disease? Proc. Am. Thorac. Soc. 2006, 3, 293–298. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Henke, C.A.; Horowitz, J.C.; Noble, P.W.; Roman, J.; Sime, P.J.; Zhou, Y.; Wells, R.G.; White, E.S.; Tschumperlin, D.J. Matrix biology of idiopathic pulmonary fibrosis: A workshop report of the national heart, lung, and blood institute. Am. J. Pathol. 2014, 184, 1643–1651. [Google Scholar] [CrossRef]
- Plantier, L.; Cazes, A.; Dinh-Xuan, A.-T.; Bancal, C.; Marchand-Adam, S.; Crestani, B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2018, 27, 170062. [Google Scholar] [CrossRef]
- Günther, A.; Schmidt, R.; Nix, F.; Yabut-Perez, M.; Guth, C.; Rosseau, S.; Siebert, C.; Grimminger, F.; Morr, H.; Velcovsky, H.; et al. Surfactant abnormalities in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and sarcoidosis. Eur. Respir. J. 1999, 14, 565–573. [Google Scholar] [CrossRef]
- Martinez, F.J.; Flaherty, K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc. Am. Thorac. Soc. 2006, 3, 315–321. [Google Scholar] [CrossRef]
- Cortes-Telles, A.; Forkert, L.; O’Donnell, D.E.; Morán-Mendoza, O. Idiopathic pulmonary fibrosis: New insights on functional characteristics at diagnosis. Can. Respir. J. 2014, 21, e55–e60. [Google Scholar] [CrossRef]
- Snider, G.L. Interstitial pulmonary fibrosis. Chest 1986, 89 (Suppl. 3), 115S. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.Y.; Fulmer, J.D.; Kazmierowski, J.A.; Roberts, W.C.; Frank, M.M.; Crystal, R.G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J. Clin. Investig. 1977, 59, 165–175. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Saccani, A.; Schioppa, T.; Porta, C.; Biswas, S.K.; Nebuloni, M.; Vago, L.; Bottazzi, B.; Colombo, M.P.; Mantovani, A.; Sica, A. p50 nuclear factor-kappaB overexpression in tu-mor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006, 66, 11432–11440. [Google Scholar] [CrossRef]
- Guiducci, C.; Vicari, A.P.; Sangaletti, S.; Trinchieri, G.; Colombo, M.P. Redirecting In vivo Elicited Tumor Infiltrating Macrophages and Dendritic Cells towards Tumor Rejection. Cancer Res. 2005, 65, 3437–3446. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wu, G.; Xiong, W.; Gu, W.; Wang, C.Y. Macrophages: Friend or foe in idiopathic pulmonary fibrosis? Respir. Res. 2018, 19, 170. [Google Scholar] [CrossRef]
- Zhong, B.; Yang, X.; Sun, Q.; Liu, L.; Lan, X.; Tian, J.; He, Q.; Hou, W.; Liu, H.; Jiang, C.; et al. Pdcd4 modulates markers of macrophage alternative activation and airway remodeling in antigen-induced pulmonary inflammation. J. Leukoc. Biol. 2014, 96, 1065–1075. [Google Scholar] [CrossRef]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front. Med. 2018, 5, 43. [Google Scholar] [CrossRef]
- Fireman, E.; Vardinon, N.; Burke, M.; Spizer, S.; Levin, S.; Endler, A.; Stav, D.; Topilsky, M.; Mann, A.; Schwarz, Y.; et al. Predictive value of response to treatment of T-lymphocyte subpopulations in idiopathic pulmonary fibrosis. Eur. Respir. J. 1998, 11, 706–711. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Wang, G.; Fei, G. Latent cytomegalovirus infection exacerbates experimental pulmonary fibrosis by activating TGF-β1. Mol. Med. Rep. 2016, 14, 1297–1301. [Google Scholar] [CrossRef]
- Stewart, J.P.; Egan, J.J.; Ross, A.J.; Kelly, B.G.; Lok, S.S.; Hasleton, P.S.; Woodcock, A.A. The Detection of Epstein-Barr Virus DNA in Lung Tissue from Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 1999, 159, 1336–1341. [Google Scholar] [CrossRef]
- Manika, K.; Alexiou-Daniel, S.; Papakosta, D.; Papa, A.; Kontakiotis, T.; Patakas, D.; Antoniadis, A. Epstein-Barr virus DNA in bronchoalveolar lavage fluid from patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2007, 24, 134–140. [Google Scholar]
- Sides, M.D.; Klingsberg, R.C.; Shan, B.; Gordon, K.A.; Nguyen, H.T.; Lin, Z.; Takahashi, T.; Flemington, E.K.; Lasky, J.A. The Epstein-Barr Virus Latent Membrane Protein 1 and Transforming Growth Factor–β1 Synergistically Induce Epithelial–Mesenchymal Transition in Lung Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2011, 44, 852–862. [Google Scholar] [CrossRef]
- Jafarian, A.H.; Mohamadian Roshan, N.; Ayatollahi, H.; Omidi, A.A.; Ghaznavi, M.; Gharib, M. Epstein-Barr Virus and Human Herpesvirus 8 in Idiopathic Pulmonary Fibrosis. Iran J. Pathol. 2020, 15, 30–33. [Google Scholar] [CrossRef]
- Collard, H.R.; Ryerson, C.J.; Corte, T.J.; Jenkins, G.; Kondoh, Y.; Lederer, D.J.; Lee, J.S.; Maher, T.M.; Wells, A.U.; Antoniou, K.M.; et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am. J. Respir. Crit. Care Med. 2016, 194, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Ishimoto, H.; Yamada, S.; Kushima, H.; Ishii, H.; Imanaga, T.; Harada, T.; Ishimatsu, Y.; Matsumoto, N.; Naito, K.; et al. Autopsy analyses in acute exacerbation of idiopathic pulmonary fibrosis. Respir. Res. 2014, 15, 109. [Google Scholar] [CrossRef]
- Juarez, M.M.; Chan, A.L.; Norris, A.G.; Morrissey, B.M.; Albertson, T.E. Acute exacerbation of idiopathic pulmonary fibrosis—A review of current and novel pharmacotherapies. J. Thorac. Dis. 2015, 7, 499–519. [Google Scholar] [CrossRef]
- Tomioka, H.; Sakurai, T.; Hashimoto, K.; Iwasaki, H. Acute exacerbation of idiopathic pulmonary fibrosis: Role of Chlamydophila pneumoniae infection. Respirology 2007, 12, 700–706. [Google Scholar] [CrossRef]
- Ushiki, A.; Yamazaki, Y.; Hama, M.; Yasuo, M.; Hanaoka, M.; Kubo, K. Viral infections in patients with an acute exacerbation of idiopathic interstitial pneumonia. Respir. Investig. 2014, 52, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Wootton, S.C.; Kim, D.S.; Kondoh, Y.; Chen, E.; Lee, J.S.; Song, J.W.; Huh, J.W.; Taniguchi, H.; Chiu, C.; Boushey, H.; et al. Viral Infection in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Bando, M.; Ohno, S.; Oshikawa, K.; Takahashi, M.; Okamoto, H.; Sugiyama, Y. Infection of TT virus in patients with idiopathic pulmonary fibrosis. Respir. Med. 2001, 95, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Huie, T.J.; Olson, A.L.; Cosgrove, G.P.; Janssen, W.J.; Lara, A.R.; Lynch, D.A.; Groshong, S.D.; Moss, M.; Schwarz, M.I.; Brown, K.K.; et al. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: Aetiology and outcomes. Respirology 2010, 15, 909–917. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.; Parra, E.; Stegun, F.; Cirqueira, C.; Capelozzi, V. Immunohistochemical detection of virus through its nuclear cytopathic effect in idiopathic interstitial pneumonia other than acute exacerbation. Braz. J. Med. Biol. Res. 2013, 46, 985–992. [Google Scholar] [CrossRef]
- Richeldi, L.; Du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. INPULSIS Trial Investigators. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Petnak, T.; Lertjitbanjong, P.; Thongprayoon, C.; Moua, T. Impact of Antifibrotic Therapy on Mortality and Acute Exacerbation in Idiopathic Pulmonary Fibrosis: A Systematic Review and Meta-Analysis. Chest 2021, 160, 1751–1763. [Google Scholar] [CrossRef]
- Tran, S.; Ksajikian, A.; Overbey, J.; Li, P.; Li, Y. Pathophysiology of Pulmonary Fibrosis in the Context of COVID-19 and Implications for Treatment: A Narrative Review. Cells 2022, 11, 2489. [Google Scholar] [CrossRef]
- Parimon, T.; Yao, C.; Stripp, B.R.; Noble, P.W.; Chen, P. Alveolar Epithelial Type II Cells as Drivers of Lung Fibrosis in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 2269. [Google Scholar] [CrossRef]
- Yue, X.; Shan, B.; Lasky, J.A. TGF-β: Titan of Lung Fibrogenesis. Curr. Enzym. Inhib. 2010, 6, 67–77. [Google Scholar] [CrossRef]
- Cheresh, P.; Kim, S.-J.; Tulasiram, S.; Kamp, D.W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 1028–1040. [Google Scholar] [CrossRef]
- Guarino, M.; Perna, B.; Cuoghi, F.; Spampinato, M.D.; Cesaro, A.E.; Manza, F.; Pretula, A.; Grilli, A.; Maritati, M.; Caio, G.; et al. Role of Intracellular Pulmonary Pathogens during SARS-CoV-2 Infection in the First Pandemic Wave of COVID-19: Clinical and Prognostic Significance in a Case Series of 1200 Patients. Microorganisms 2022, 10, 1636. [Google Scholar] [CrossRef]
- Cugno, M.; Meroni, P.L.; Consonni, D.; Griffini, S.; Grovetti, E.; Novembrino, C.; Torri, A.; Griffante, G.; Gariglio, M.; Varani, L.; et al. Effects of Antibody Responses to Pre-Existing Coronaviruses on Disease Severity and Complement Activation in COVID-19 Patients. Microorganisms 2022, 10, 1191. [Google Scholar] [CrossRef]
- Patrucco, F.; Carriero, A.; Falaschi, Z.; Paschè, A.; Gavelli, F.; Airoldi, C.; Bellan, M.; Sainaghi, P.P.; Solidoro, P.; Balbo, P.E. COVID-19 Diagnosis in Case of Two Negative Naso-pharyngeal Swabs: Association between Chest CT and Bronchoalveolar Lavage Results. Radiology 2021, 298, E152–E155. [Google Scholar] [CrossRef]
- Patrucco, F.; Gavelli, F.; Fagoonee, S.; Solidoro, P.; Undas, A.; Pellicano, R. Current treatment challenges in the COVID-19 pandemic. Pol. Arch. Intern. Med. 2021, 131, 854–861. [Google Scholar] [CrossRef]
- Bellan, M.; Apostolo, D.; Albè, A.; Crevola, M.; Errica, N.; Ratano, G.; Tonello, S.; Minisini, R.; D’Onghia, D.; Bricich, A.; et al. No-More COVID study group. Determinants of long COVID among adults hospitalized for SARS-CoV-2 infection: A prospective cohort study. Front. Immunol. 2022, 13, 1038227. [Google Scholar] [CrossRef]
- Camargo, L.D.N.; Righetti, R.F.; Aristóteles, L.R.D.C.R.B.; Dos Santos, T.M.; de Souza, F.C.R.; Fukuzaki, S.; Cruz, M.M.; Alonso-Vale, M.I.C.; Saraiva-Romanholo, B.M.; Prado, C.M.; et al. Effects of Anti-IL-17 on Inflammation, Remodeling, and Oxidative Stress in an Experimental Model of Asthma Exacerbated by LPS. Front. Immunol. 2018, 8, 1835. [Google Scholar] [CrossRef]
- Hama Amin, B.J.; Kakamad, F.H.; Ahmed, G.S.; Ahmed, S.F.; Abdulla, B.A.; Mohammed, S.H.; Mikael, T.M.; Salih, R.Q.; Ali, R.K.; Salh, A.M.; et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann. Med. Surg. 2022, 77, 103590. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Li, X.; Zhao, J.; An, C.; Peng, C.; Wang, L. Thin-section computed tomography findings and longitudinal variations of the residual pulmonary sequelae after discharge in patients with COVID-19: A short-term follow-up study. Eur. Radiol. 2021, 31, 7172–7183. [Google Scholar] [CrossRef]
- Barini, M.; Percivale, I.; Danna, P.S.; Longo, V.; Costantini, P.; Paladini, A.; Airoldi, C.; Bellan, M.; Saba, L.; Carriero, A. 18 Months Computed Tomography Follow-Up after Covid-19 Interstitial Pneumonia. J. Public Health Res. 2022, 11, 2782. [Google Scholar] [CrossRef]
- Allen, R.J.; Guillen-Guio, B.; Croot, E.; Kraven, L.M.; Moss, S.; Stewart, I.; Jenkins, R.G.; Wain, L.V. Genetic overlap between idiopathic pulmonary fibrosis and COVID-19. Eur. Respir. J. 2022, 60, 2103132. [Google Scholar] [CrossRef]
- Sinha, S.; Castillo, V.; Espinoza, C.R.; Tindle, C.; Fonseca, A.G.; Dan, J.M.; Katkar, G.D.; Das, S.; Sahoo, D.; Ghosh, P. COVID-19 lung disease shares driver AT2 cytopathic features with Idiopathic pulmonary fibrosis. Ebiomedicine 2022, 82, 104185. [Google Scholar] [CrossRef]
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Bendstrup, E.; Wuyts, W.; Alfaro, T.; Chaudhuri, N.; Cornelissen, R.; Kreuter, M.; Nielsen, K.M.; Münster, A.-M.B.; Myllärniemi, M.; Ravaglia, C.; et al. Nintedanib in Idiopathic Pulmonary Fibrosis: Practical Management Recommendations for Potential Adverse Events. Respiration 2019, 97, 173–184. [Google Scholar] [CrossRef]
- Richeldi, L.; Costabel, U.; Selman, M.; Kim, D.S.; Hansell, D.M.; Nicholson, A.G.; Brown, K.K.; Flaherty, K.R.; Noble, P.W.; Raghu, G.; et al. Efficacy of a Tyrosine Kinase Inhibitor in Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2011, 365, 1079–1087. [Google Scholar] [CrossRef]
- Ogata, H.; Nakagawa, T.; Sakoda, S.; Ishimatsu, A.; Taguchi, K.; Kadowaki, M.; Moriwaki, A.; Yoshida, M. Nintedanib treatment for pulmonary fibrosis after coronavirus disease 2019. Respirol. Case Rep. 2021, 9, e00744. [Google Scholar] [CrossRef]
- Umemura, Y.; Mitsuyama, Y.; Minami, K.; Nishida, T.; Watanabe, A.; Okada, N.; Yamakawa, K.; Nochioka, K.; Fujimi, S. Efficacy and safety of nintedanib for pulmonary fibrosis in severe pneumonia induced by COVID-19: An interventional study. Int. J. Infect. Dis. 2021, 108, 454–460. [Google Scholar] [CrossRef]
- Saiphoklang, N.; Patanayindee, P.; Ruchiwit, P. The Effect of Nintedanib in Post-COVID-19 Lung Fibrosis: An Observational Study. Crit. Care Res. Pract. 2022, 2022, 9972846. [Google Scholar] [CrossRef]
- Wong, A.W.; Fidler, L.; Marcoux, V.; Johannson, K.A.; Assayag, D.; Fisher, J.H.; Hambly, N.; Kolb, M.; Morisset, J.; Shapera, S.; et al. Practical Considerations for the Diagnosis and Treatment of Fibrotic Interstitial Lung Disease During the Coronavirus Disease 2019 Pandemic. Chest 2020, 158, 1069–1078. [Google Scholar] [CrossRef]
- Goto, Y.; Sakamoto, K.; Fukihara, J.; Suzuki, A.; Omote, N.; Ando, A.; Shindo, Y.; Hashimoto, N. COVID-19-Triggered Acute Exacerbation of IPF, an Un-derdiagnosed Clinical Entity With Two-Peaked Respiratory Failure: A Case Report and Literature Review. Front. Med. 2022, 9, 815924. [Google Scholar] [CrossRef]
- Lee, H.; Choi, H.; Yang, B.; Lee, S.-K.; Park, T.S.; Park, D.W.; Moon, J.-Y.; Kim, T.-H.; Sohn, J.W.; Yoon, H.J.; et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur. Respir. J. 2021, 58, 2004125. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.J.; Menon, A.A.; Ghosh, A.J.; Putman, R.K.; Fredenburgh, L.E.; El-Chemaly, S.Y.; Goldberg, H.J.; Baron, R.M.; Hunninghake, G.M.; Doyle, T.J. Increased Odds of Death for Patients with Interstitial Lung Disease and COVID-19: A Case–Control Study. Am. J. Respir. Crit. Care Med. 2020, 202, 1710–1713. [Google Scholar] [CrossRef]
- Naqvi, S.F.; Lakhani, D.A.; Sohail, A.H.; Maurer, J.; Sofka, S.; Sarwari, A.; Hadi, Y.B. Patients with idiopathic pulmonary fibrosis have poor clinical outcomes with COVID-19 disease: A propensity matched multicentre research network analysis. BMJ Open Respir. Res. 2021, 8, e000969. [Google Scholar] [CrossRef]
- Ouyang, L.; Gong, J.; Yu, M. Pre-existing interstitial lung disease in patients with coronavirus disease 2019: A meta-analysis. Int. Immunopharmacol. 2021, 100, 108145. [Google Scholar] [CrossRef]
- Faverio, P.; Conti, S.; Madotto, F.; Franco, G.; Renzoni, E.; Mantovani, L.; Luppi, F. Idiopathic pulmonary fibrosis mortality in the Italian epicenter of COVID-19 pandemic. Pulmonology 2022, 29, 85–88. [Google Scholar] [CrossRef]
- British Thoracic Society. British Thoracic Society Guidance on Respiratory Follow Up of Patients with a Clinico-Radiological Diagnosis of COVID-19 Pneumonia. 2020. Available online: https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/resp-followup-guidance-post-covid-pneumonia/ (accessed on 15 January 2023).
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solis-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 2021, 27, 328–337. [Google Scholar] [CrossRef]
- Patrucco, F.; Zeppegno, P.; Baricich, A.; Gramaglia, C.M.; Balbo, P.E.; Falaschi, Z.; Carriero, A.; Cuneo, D.; Pirisi, M.; Bellan, M. Long-lasting consequences of coronavirus disease 19 pneumonia: A systematic review. Minerva Med. 2022, 113, 158–171. [Google Scholar] [CrossRef]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and Psychophysical Sequelae Among Patients With COVID-19 Four Months After Hospital Discharge. JAMA Netw. Open 2021, 4, e2036142. [Google Scholar] [CrossRef]
- Qin, W.; Chen, S.; Zhang, Y.; Dong, F.; Zhang, Z.; Hu, B.; Zhu, Z.; Li, F.; Wang, X.; Wang, Y.; et al. Diffusion Capacity Abnormalities for Carbon Monoxide in Patients with COVID-19 At Three-Month Follow-up. Eur. Respir. J. 2021, 58, 2003677. [Google Scholar] [CrossRef]
- Bellan, M.; Baricich, A.; Patrucco, F.; Zeppegno, P.; Gramaglia, C.; Balbo, P.E.; Carriero, A.; Amico, C.S.; Avanzi, G.C.; Barini, M.; et al. Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19. Sci. Rep. 2021, 11, 22666. [Google Scholar] [CrossRef]
- Fuschillo, S.; Ambrosino, P.; Motta, A.; Maniscalco, M. COVID-19 and diffusing capacity of the lungs for carbon monoxide: A clinical biomarker in postacute care settings. Biomark. Med. 2021, 15, 537–539. [Google Scholar] [CrossRef]
- Tarraso, J.; Safont, B.; Carbonell-Asins, J.A.; Fernandez-Fabrellas, E.; Sancho-Chust, J.N.; Naval, E.; Amat, B.; Herrera, S.; Ros, J.A.; Soler-Cataluña, J.J.; et al. Lung function and radiological findings 1 year after COVID-19: A prospective follow-up. Respir. Res. 2022, 23, 242. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Vasarmidi, E.; Russell, A.-M.; Andrejak, C.; Crestani, B.; Delcroix, M.; Dinh-Xuan, A.T.; Poletti, V.; Sverzellati, N.; Vitacca, M.; et al. European Respiratory Society statement on long COVID follow-up. Eur. Respir. J. 2022, 60, 2102174. [Google Scholar] [CrossRef]
- George, P.M.; Barratt, S.L.; Condliffe, R.; Desai, S.R.; Devaraj, A.; Forrest, I.; Gibbons, M.A.; Hart, N.; Jenkins, R.G.; McAuley, D.F.; et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax 2020, 75, 1009–1016. [Google Scholar] [CrossRef]
- Han, X.; Fan, Y.; Alwalid, O.; Li, N.; Jia, X.; Yuan, M.; Li, Y.; Cao, Y.; Gu, J.; Wu, H.; et al. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 2021, 299, E177–E186. [Google Scholar] [CrossRef]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef]
- Raghu, G.; Richeldi, L. Current approaches to the management of idiopathic pulmonary fibrosis. Respir. Med. 2017, 129, 24–30. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Identifier: NCT04607928. Available online: https://clinicaltrials.gov/ct2/show/NCT04607928 (accessed on 15 January 2023).
- ClinicalTrials.gov Identifier: NCT04338802. Available online: https://clinicaltrials.gov/ct2/show/NCT04338802 (accessed on 15 January 2023).
- ClinicalTrials.gov Identifier: NCT04541680. Available online: https://clinicaltrials.gov/ct2/show/NCT04541680 (accessed on 15 January 2023).
- ClinicalTrials.gov Identifier: NCT04619680. Available online: https://clinicaltrials.gov/ct2/show/NCT04619680 (accessed on 15 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrucco, F.; Solidoro, P.; Gavelli, F.; Apostolo, D.; Bellan, M. Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks. Microorganisms 2023, 11, 895. https://doi.org/10.3390/microorganisms11040895
Patrucco F, Solidoro P, Gavelli F, Apostolo D, Bellan M. Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks. Microorganisms. 2023; 11(4):895. https://doi.org/10.3390/microorganisms11040895
Chicago/Turabian StylePatrucco, Filippo, Paolo Solidoro, Francesco Gavelli, Daria Apostolo, and Mattia Bellan. 2023. "Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks" Microorganisms 11, no. 4: 895. https://doi.org/10.3390/microorganisms11040895
APA StylePatrucco, F., Solidoro, P., Gavelli, F., Apostolo, D., & Bellan, M. (2023). Idiopathic Pulmonary Fibrosis and Post-COVID-19 Lung Fibrosis: Links and Risks. Microorganisms, 11(4), 895. https://doi.org/10.3390/microorganisms11040895






