Exploring Bacterial and Fungal Biodiversity in Eight Mediterranean Olive Orchards (Olea europaea L.) in Tunisia
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sites, Bacterial and Fungal Isolation
2.2. Molecular Identification of Isolates
2.3. Statistical Analysis
3. Results
3.1. Microbial Community Composition
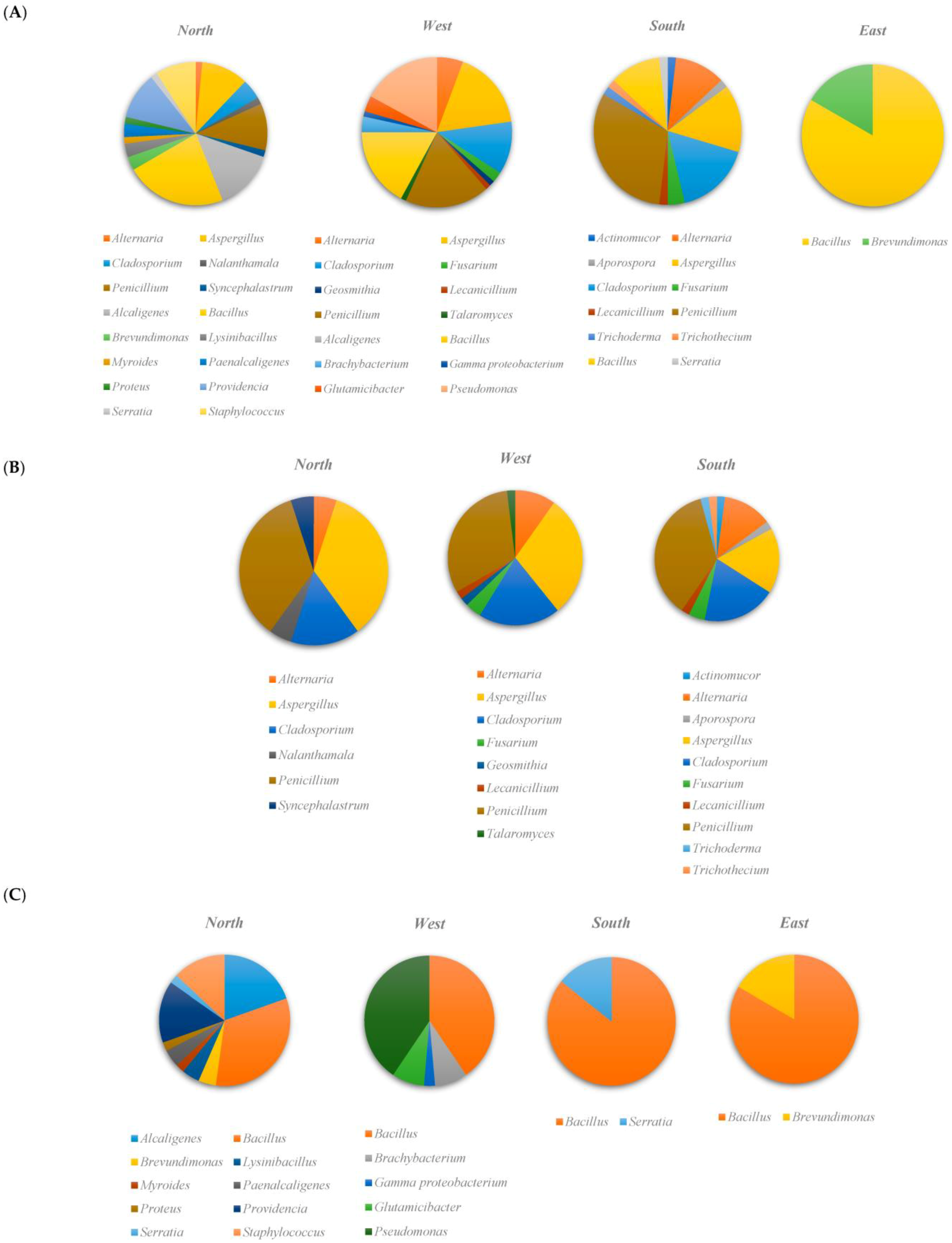
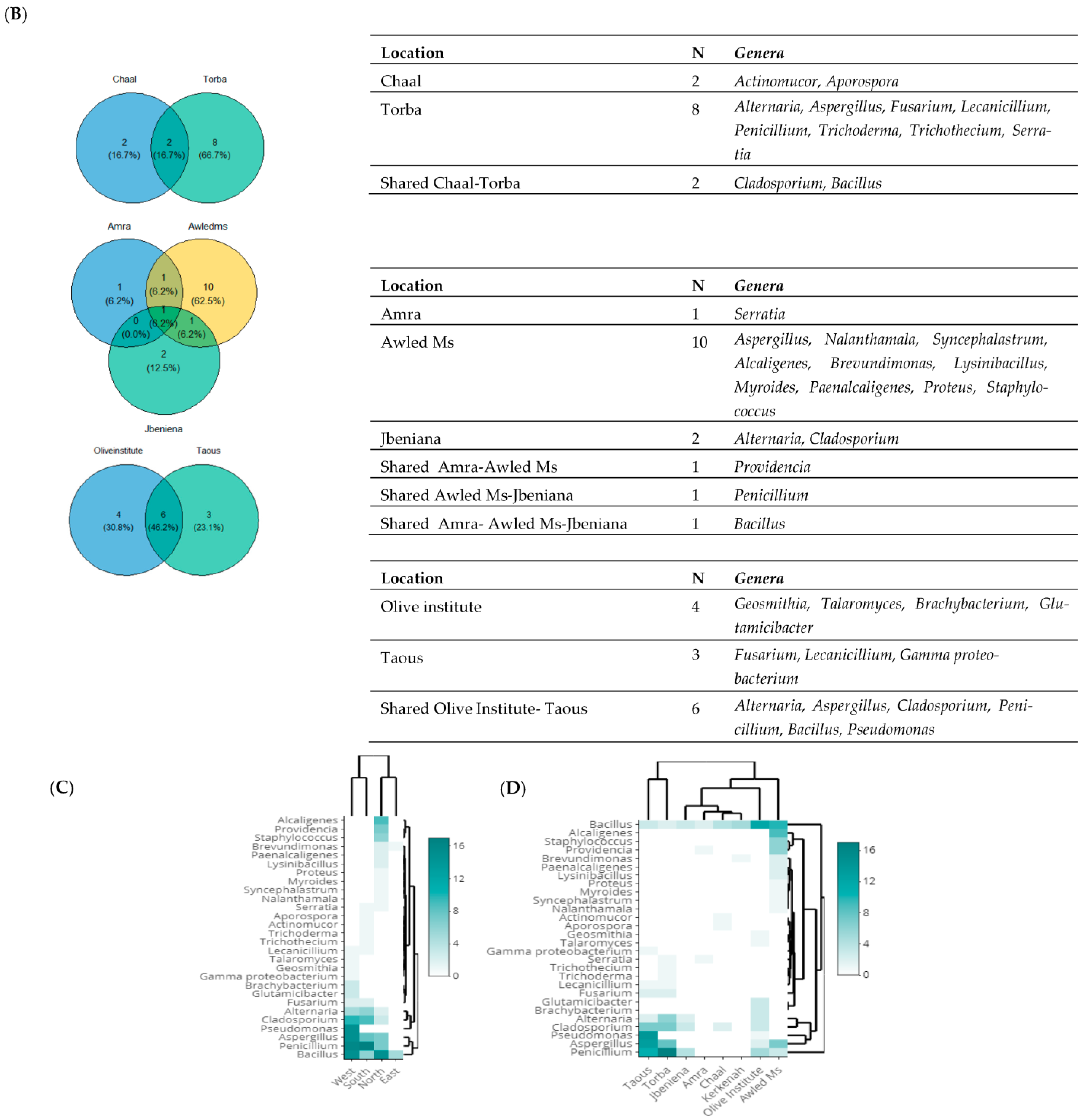
3.2. Taxonomic Diversity According to the Isolation Location
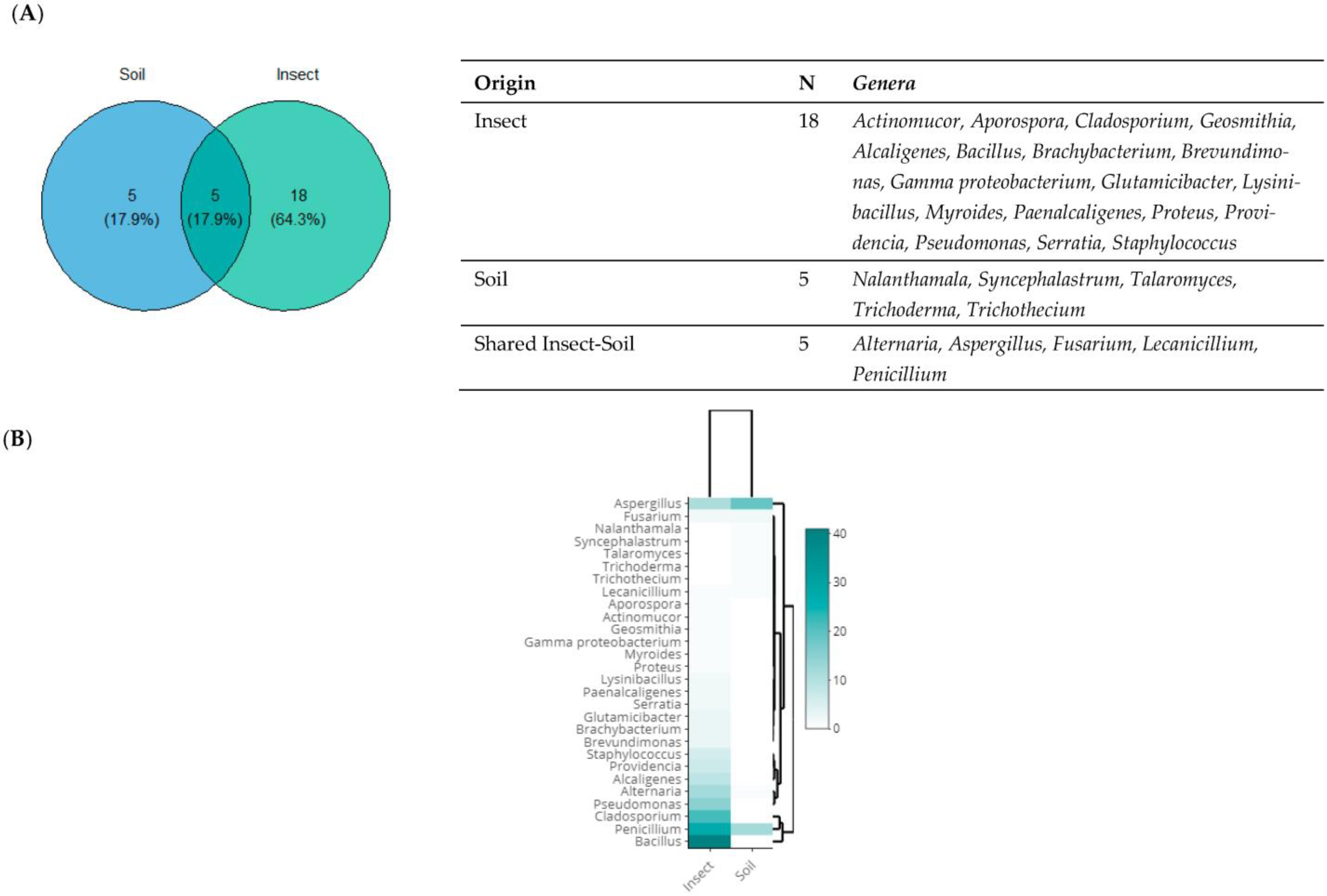
3.3. Taxonomic Diversity According to the Origin of Isolation
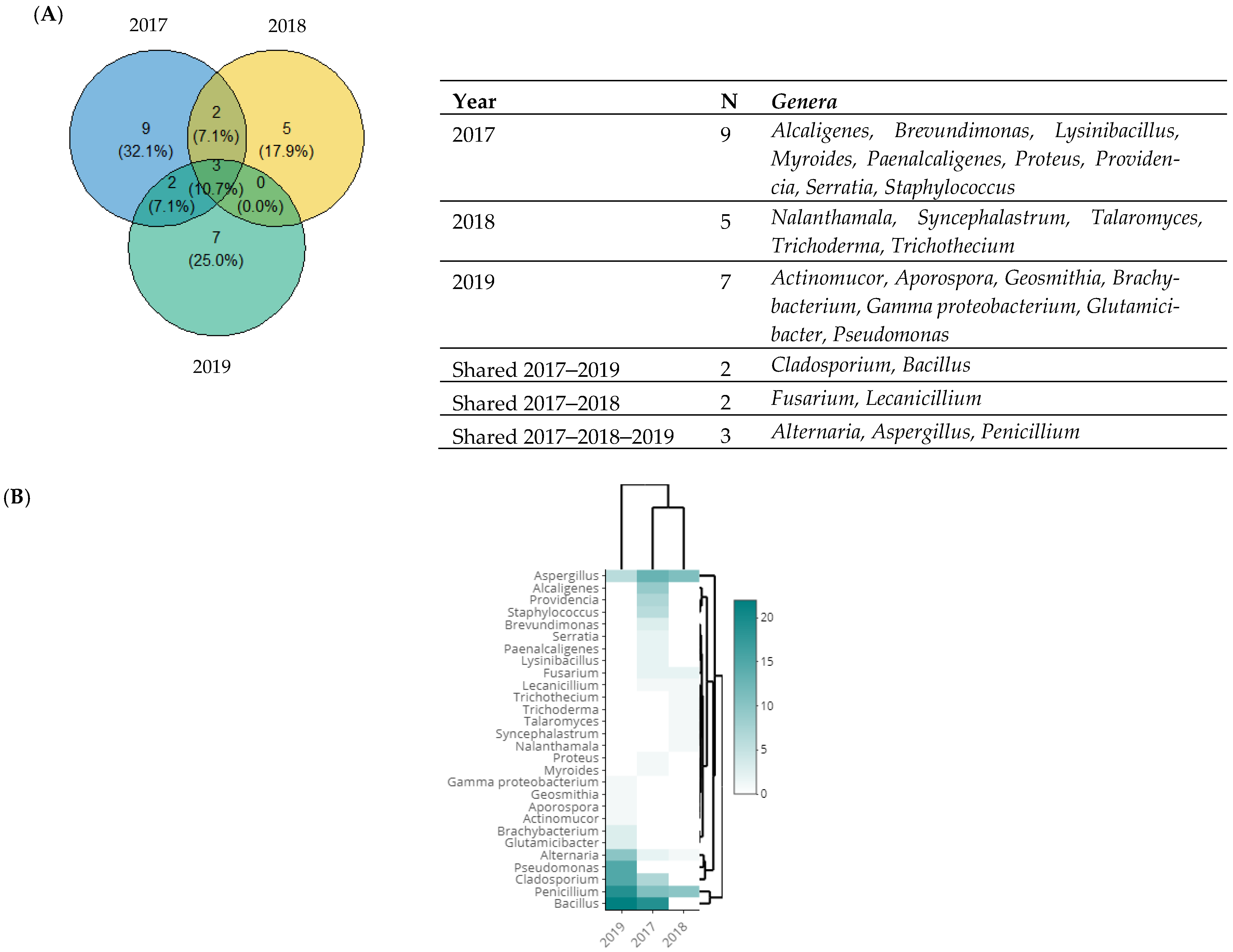
3.4. Taxonomic Diversity According to the Year of Isolation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2016 Agricultural Production Database. Available online: http://faostat.fao.org/ (accessed on 8 March 2017).
- Gucci, R.; Fereres, E. “Fruit Trees and Vines. Olive,” in Crop Yield Response to Water; 2012 FAO Irrigation and drainage paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; Volume 66, pp. 300–313. [Google Scholar]
- Tunisian Ministry of Agriculture. 2019. Available online: http://www.agridata.tn/ (accessed on 5 February 2017).
- Official Journal of the International Olive Council. 2017. Issue No 124. Available online: https://www.internationaloliveoil.org/product/olivae-124-frances/ (accessed on 5 February 2017).
- Oliveira, I.; Pereira, J.A.; Lino-Neto, T.; Bento, A.; Baptista, P. Fungal diversity associated to the olive moth, Prays oleae Bernard: A survey for potential Entomopathogenic Fungi. Microb. Ecol. 2012, 63, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.D.; Marshall, W.J. Olive Fruit Fly: Managing an Ancient Pest in Modern Times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar]
- Cardinale, M.; Luvisi, A.; Meyer, J.B.; Sabella, E.; De Bellis, L.; Cruz, A.C.; Ampatzidis, Y.; Cherubini, P. Specific fluorescence in situ hybridization (FISH) test to highlight colonization of xylem vessels by Xylella fastidiosa in naturally infected olive trees (Olea europaea L.). Front. Plan. Sci. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Giampetruzzi, A.; Baptista, P.; Morelli, M.; Cameirao, C.; Lino Neto, T.; Costa, D.; D’Attoma, G.; Abou Kubaa, R.; Altamura, G.; Saponari, M.; et al. Differences in the endophytic microbiome of olive cultivars infected by Xylella fastidiosa across seasons. Pathogens 2020, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Triki, M.A.; Rhouma, A.; Khabou, W.; Boulila, M.; Ioos, R. Recrudescence du dépérissement de l’olivier causé par les champignons telluriques en Tunisie. In Proceedings of the Olivebioteq, Sfax, Tunisia, 15–19 December 2009. [Google Scholar]
- Gharbi, Y.; Triki, M.A.; Jolodar, A.; Trabelsi, R.; Gdoura, R.; Daayf, F. Genetic diversity of Verticillium dahliae from olive trees in Tunisia based on RAMS and IGSRFLP analyses. Can. J. Plant Pathol. 2014, 36, 491–500. [Google Scholar] [CrossRef]
- Lacey, L.; Frutos, R.; Kaya, H.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; de la Rosa, L.; Leon, J.A.; Gómez, L.; Testi, F.; Orgaz, J.A.; Gil-Ribes, E.; Quesada-Moraga, A. Trapero and M. Msallem. Evolution and sustainability of the olive production systems R. Options Méditerranéennes Sé 2013, 106, 11–42. [Google Scholar]
- Schiavi, A.; Runci, A.; Maiorino, T.; Naso, F.D.; Barenys, M.; Fritsche, E.; Strappazzon, F.; Ventura, N. Cobalt chloride has beneficial effects across species through a hormetic mechanism. Front. Cell Dev. Biol. 2022, 10, 986835. [Google Scholar] [CrossRef]
- Tognaccini, L.; Ricci, M.; Gellini, C.; Feis, A.; Smulevich, G.; Becucci, M. Surface Enhanced Raman Spectroscopy for In-Field Detection of Pesticides: A Test on Dimethoate Residues in Water and on Olive Leaves. Molecules 2019, 24, 292. [Google Scholar] [CrossRef]
- Fusco, V.; Pasciuta, V.; Lumia, V.; Matere, A.; Battaglia, V.; Bertinelli, G.; Sansone, D.; Brunetti, A.; Pilotti, M. Root and stem rot, and wilting of olive tree caused by Dematophora necatrix and associated with Emmia lacerata in Central Italy European. J. Plant Pathol. 2022, 163, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Mulé, R.; Sabella, G.; Robba, L.; Manachini, B. Examen systématique des effets des insecticides chimiques sur quatre familles de papillons communs. Devant. Environ. Sci. 2017, 5, 32. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, J.A.; Benhadi, J.; Lino-Neto, T.; Baptista, P. Endophytic and epiphytic phyllosphere fungal communities are shaped by different environmental factors in a Mediterranean ecosystem. Microb. Ecol. 2018, 76, 668–679. [Google Scholar] [CrossRef]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Rei, F.; do Ros’ario F’elix, M. Spatial and temporal variation of fungal endophytic richness and diversity associated to the phyllosphere of olive cultivars. Fungal. Biol. 2019, 123, 66–76. [Google Scholar] [CrossRef]
- Mina, D.; Pereira, J.A.; Lino-Neto, T.; Baptista, P. Epiphytic and endophytic bacteria on olive tree phyllosphere: Exploring tissue and cultivar effect. Microb. Ecol. 2020, 80, 145–157. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P. Screening for novel biocontrol agents applicable in plant disease management––A review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Anguita-Maeso, M.; Olivares-García, C.; Haro, C.; Imperial, J.; Navas-Cortés, J.A.; Landa, B.B. Culture-Dependent and Culture-Independent Characterization of the Olive Xylem Microbiota: Effect of Sap Extraction Methods. Front. Plant Sci. 2020, 10, 1708. [Google Scholar] [CrossRef]
- Gharsallah, H.; Ksentini, I.; Abdelhedi, N.; Naayma, S.; Hadjtaieb, K.; Sahnoun, M.; Triki, M.A.; Ksantini, M.; Leclerque, A. Screening of bacterial isolates related to olive orchard pests in Tunisia using 16S ribosomal RNA and evaluation of their biotechnological potential. J. Appl. Microbiol. 2019, 126, 489–502. [Google Scholar] [CrossRef]
- Gharsallah, H.; Ksentini, I.; Naayma, S.; Hadjtaieb, K.; Abdelhedi, N.; Schuster, C.; Triki, M.A.; Ksantini, M.; Leclerque, A. Identification of Fungi in Tunisian Olive Orchards: Characterization and Biological Contol Potential. BMC Microbiol. 2020, 20, 307. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA, USA. 2015. Available online: http://www.rstudio.com/ (accessed on 17 December 2022).
- Müller, H.; Berg, C.; Landa, B.B.; Auerbach, A.; Moissl-Eichinger, C.; Berg, G. Plant genotype-specific archaeal and bacterial endophytes but similar Bacillus antagonists colonize Mediterranean olive trees. Front. Microbiol. 2015, 6, 138. [Google Scholar] [CrossRef]
- Fausto, C.; Mininni, A.N.; Sofo, A.; Crecchio, C.; Scagliola, M.; Dichio, B.; Xiloyannis, C. Olive orchard microbiome: Characterisation of bacterial communities in soil-plant compartments and their comparison between sustainable and conventional soil management systems. Plant Ecol. Divers. 2018, 11, 597–610. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Valverde-Corredor, A.; G_omez-Lama Caban_as, C.; Sesmero, R.; Mercado-Blanco, J. What lies beneath: Root-associated bacteria to improve the growth and health of olive trees. In Soil Biological Communities and Ecosystem Resilience, Sustainability in Plant and Crop Protection; Lukac, M., Grenni, P., Gamboni, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 189–205. [Google Scholar]
- Pascazio, S.; Crecchio, C.; Ricciuti, P.; Palese, A.M.; Xiloyannis, C.; Sofo, A. Phyllosphere and carposphere bacterial communities in olive plants subjected to different cultural practices. Int. J. Plant Biol. 2015, 6, 6011. [Google Scholar] [CrossRef]
- Vergine, M.; Meyer, J.B.; Cardinale, M.; Sabella, E.; Hartmann, M.; Cherubini, P.; de Bellis, L.; Luvisi, A. The Xylella fastidiosa-resistant olive cultivar “Leccino” has stable endophytic microbiota during the Olive Quick Decline Syndrome (OQDS). Pathogens 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Santona, M.; Sanna, M.L.; Multineddu, C.; Fancello, F.; de la Fuente, S.A.; Dettori, S.; Zara, S. Microbial biodiversity of Sardinian oleic ecosystems. Food Microbiol. 2018, 70, 65–75. [Google Scholar] [CrossRef]
- Coleman, D.; Desgarennes, D.; Fonseca-Garcia, C.; Gross, S.; Clingenpeel, S.; Woyke, T.; North, G.; Visel, A.; Partida-Martinez, L.P.; Tringe, S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytologist. 2016, 209, 798–811. [Google Scholar] [CrossRef]
- Vikram, S.; Guerrero, L.D.; Makhalanyane, T.P.; Le, P.T.; Seely, M.; Cowan, D.A. Metagenomic analysis provides insights into functional capacity in a hyperarid desert soil niche community. Environ. Microbiol. 2016, 18, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, Y.L.; Wang, H.; Zhang, T.; Yu, L.Y.; Sun, H.; Zhang, Y.Q. Diversity of bacteria and the characteristics of actinobacteria community structure in Badain Jaran desert and Tengger desert of China. Front. Microbiol. 2018, 9, 1068. [Google Scholar] [CrossRef]
- Hussain, S.S.; Mehnaz, S.; Siddique, K.H.M. Harnessing the plant microbiome for improved abiotic stress tolerance. In Plant Microbiome: Stress Response; Egamberdieva, D., Ahmad, P., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 21–43. [Google Scholar] [CrossRef]
- Rasche, F.; Trondl, R.; Naglreiter, C.; Reichenauer, T.G.; Sessitsch, A. Chilling and cultivar type affect the diversity of bacterial endophytes colonizing sweet pepper (Capsicum anuum L.). Can. J. Microbiol. 2006, 52, 1036–1045. [Google Scholar] [CrossRef]
- Rasche, F.; Velvis, H.; Zachow, C.; Berg, G.; van Elsas, J.D.; Sessitsch, A. Impact of transgenic potatoes expressing anti-bacterial agents on bacterial endophytes is comparable with the effects of plant genotype, soil type and pathogen infection. J. Appl. Ecol. 2006, 43, 555–566. [Google Scholar] [CrossRef]
- Castro, H.F.; Classen, A.T.; Austin, E.E.; Norby, R.J.; Schadt, C.W. Soil microbial community responses to multiple experimental climate change drivers. Appl. Environ. Microbiol. 2010, 76, 999–1007. [Google Scholar] [CrossRef]
- Goettel, M.S.; Johnson, D.L.; Inglis, G.D. The role of fungi in the control of grasshoppers. Can. J. Bot. 2011, 73, 71–75. [Google Scholar] [CrossRef]
- Lee, L.H.; Zainal, N.; Azman, A.S.; Eng, S.K.; Goh, B.H.; Yin, W.F. Diversity and antimicrobial activities of actinobacteria isolated from tropical mangrove sediments in Malaysia. Sci. World J. 2014, 14, 698178. [Google Scholar] [CrossRef] [PubMed]
- Olao, C.; García, I.; González, A.; Rodriguez, M.; Rozas, D.; Rubio, J.; Sánchez-Hidalgo, M.; Braña, A.F.; Méndez, C.; Salas, J.A. Activation and identification of five clusters for secondary metabolites in Streptomyces albus J1074. Microb. Biotechnol. 2014, 7, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Pereira, J.A.; Lino-Neto, T.; Bennett, A.E.; Baptista, P. Bacterial disease induced changes in fungal communities of olive tree twigs depend on host genotype. Sci. Rep. 2019, 9, 5882. [Google Scholar] [CrossRef]
- Martins, F.; Pereira, J.A.; Bota, P.; Bento, A.; Baptista, P. Fungal endophyte communities in above-and belowground olive tree organs and the effect of season and geographic location on their structures. Fungal. Ecol. 2016, 20, 193–201. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Li Destri Nicosia, M.G.; Cacciola, S.O.; Droby, S.; Schena, L. Metabarcoding Analysis of Fungal Diversity in the Phyllosphere and Carposphere of Olive (Olea europaea). PLoS ONE 2015, 10, e0131069. [Google Scholar] [CrossRef] [PubMed]
- Godinho, V.M.; Gonçalves, V.N.; Santiago, I.F.; Figueredo, H.M.; Vitoreli, G.A.; Schaefer, C.E.G.R. Diversity and bioprospection of fungal community present in oligotrophic soil of continental Antarctica. Extremophiles 2015, 19, 585–596. [Google Scholar] [CrossRef]
- Yee, T.L.; Tajuddin, R.; Mohamed Nor NM, I.; Mohd, M.H.; Zakaria, L. Filamentous ascomycete and basidiomycete fungi from beach sand. Rend. Lincei. 2016, 27, 603–607. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Sajeewa, S.N.; Maharachchikumbura, V.R.; Al-Mahrouqi, H.; Al-Sadi, A.M. Fungal Diversity in Tomato Rhizosphere Soil under Conventional and Desert Farming Systems. Front. Microbiol. 2017, 8, 1462. [Google Scholar] [CrossRef]
- Yaman, F.; Aktas, B.; Touray, M.; C_oban, P.E.; Biyik, H.H. Biodiversity of bacteria isolated from different soils. Int. J. Sec. Metabolite 2017, 4, 27–34. [Google Scholar] [CrossRef]
- Ben Abdallah, D.; Frikha-Gargouri, O.; Tounsi, S. Bacillus amyloliquefaciens strain 32a as a source of lipopeptides for biocontrol of Agrobacterium tumefaciens strains. J. Appl. Microbiol. 2015, 119, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.B.; Tounsi, S.; Gharsallah, H.; Hammami, A.; Frikha-Gargouri, O. Lipopeptides from Bacillus amyloliquefaciens strain 32a as promising biocontrol compounds against the plant pathogen Agrobacterium tumefaciens. Environ. Sci. Pollut. Res. 2018, 25, 36518–36529. [Google Scholar] [CrossRef] [PubMed]
- Pandin, C.; Védie, R.; Rousseau, T.; Le Coq, D.; Aymerich, S.; Briandet, R. Dynamics of compost microbiota during the cultivation of Agaricus bisporus in the presence of Bacillus velezensis QST713 as biocontrol agent against Trichoderma aggressivum. Biological. Control 2018, 127, 39–54. [Google Scholar] [CrossRef]
- Cheffi, M.; Bouket, A.C.; Alenezi, F.N.; Luptakova, L.; Belka, M.; Vallat, A.; Rateb, M.E.; Tounsi, S.; Triki, M.A.; Belbahri, L. Olea europaea L. Root Endophyte Bacillus velezensis OEE1 Counteracts Oomycete and Fungal Harmful Pathogens and Harbours a Large Repertoire of Secreted and Volatile Metabolites and Beneficial Functional Genes. Microorganisms 2019, 7, 314. [Google Scholar] [CrossRef]
- Zicca, S.; de Bellis, P.; Masiello, M.; Saponari, M.; Saldarelli, P.; Boscia, D.; Sisto, A. Antagonistic activity of olive endophytic bacteria and of Bacillus spp. strains against Xylella fastidiosa. Microbiol. Res. 2020, 236, 126467. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Gu, Y.Q.; Coleman-Derr, D. MetaCoMET: A web platform for discovery and visualization of the core microbiome. Bioinformatics 2016, 32, 3469–3470. [Google Scholar] [CrossRef] [PubMed]
- Syed Ab Rahmana, S.F.; Singha, E.; Pieterseb, C.M.J.; Schenka, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Rokni-Zadeh, H.; Li, W.; Yilma, E.; Sanchez-Rodriguez, A.; De Mot, R. Distinct lipopeptide production systems for WLIP (white line-inducing principle) in Pseudomonas fluorescens and Pseudomonas putida. Environ. Microbiol. Rep. 2012, 1, 12015. [Google Scholar] [CrossRef]
- Krid, S.; Rhouma, A.; Mogou, I.; Quesada, J.M.; Nesme, X.; Gargouri, A. Pseudomonas savastanoi endophytic bacteria in olive tree knots and antagonistic potential of strains of Pseudomonas fluorescens and Bacillus subtilis. J. Plant Pathol. 2010, 92, 335–341. [Google Scholar]
- Ben Amira, M.; Lopeza, D.; Triki, M.A.; Khouaja, A.; Chaar, H.; Fumanal, B.; Gousset-Dupont, A.; Bonhomme, L.; Label, P.; Goupil, P.; et al. Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biological Control. 2017, 110, 70–78. [Google Scholar] [CrossRef]
- Backman, P.A.; Sikora, R.A. Endophytes: An Emerging Tool for Biological Control. Biol. Control 2008, 46, 1–3. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, A.; Pieterse, C.M.J.; Mercado-Blanco, J. Editorial: Harnessing Useful Rhizosphere Microorganisms for Pathogen and Pest Biocontrol. Front. Microbiol. 2016, 7, 1620. [Google Scholar] [CrossRef] [PubMed]

| Sampling Sites | Coordinates | Location | |
|---|---|---|---|
| Longitude | Latitude | ||
| Amra | 10°53′09″ E | 34°59′07″ N | Sfax North |
| Ouled Msallem | 10°49′19″ E | 34°59′31″ N | Sfax North |
| Jbeniena | 10°54′16″ E | 35°03′10″ N | Sfax North |
| Torba | 10°36′47″ E | 34°55′17″ N | Sfax South |
| Olive Institute | 10°43′59″ E | 34°44′04″ N | Sfax West |
| Taous | 10°27′33.6″ E | 34°50′56.9″ N | Sfax West |
| Chaal | 10°19′19″ E | 34°27′27″ N | Sfax South |
| Kerkennah | 11°00′16″ E | 34°39′09″ N | Sfax East |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharsallah, H.; Ksentini, I.; Frikha-Gargouri, O.; Hadj Taieb, K.; Ben Gharsa, H.; Schuster, C.; Chatti-kolsi, A.; Triki, M.A.; Ksantini, M.; Leclerque, A. Exploring Bacterial and Fungal Biodiversity in Eight Mediterranean Olive Orchards (Olea europaea L.) in Tunisia. Microorganisms 2023, 11, 1086. https://doi.org/10.3390/microorganisms11041086
Gharsallah H, Ksentini I, Frikha-Gargouri O, Hadj Taieb K, Ben Gharsa H, Schuster C, Chatti-kolsi A, Triki MA, Ksantini M, Leclerque A. Exploring Bacterial and Fungal Biodiversity in Eight Mediterranean Olive Orchards (Olea europaea L.) in Tunisia. Microorganisms. 2023; 11(4):1086. https://doi.org/10.3390/microorganisms11041086
Chicago/Turabian StyleGharsallah, Houda, Ines Ksentini, Olfa Frikha-Gargouri, Karama Hadj Taieb, Haifa Ben Gharsa, Christina Schuster, Amel Chatti-kolsi, Mohamed Ali Triki, Mohieddine Ksantini, and Andreas Leclerque. 2023. "Exploring Bacterial and Fungal Biodiversity in Eight Mediterranean Olive Orchards (Olea europaea L.) in Tunisia" Microorganisms 11, no. 4: 1086. https://doi.org/10.3390/microorganisms11041086
APA StyleGharsallah, H., Ksentini, I., Frikha-Gargouri, O., Hadj Taieb, K., Ben Gharsa, H., Schuster, C., Chatti-kolsi, A., Triki, M. A., Ksantini, M., & Leclerque, A. (2023). Exploring Bacterial and Fungal Biodiversity in Eight Mediterranean Olive Orchards (Olea europaea L.) in Tunisia. Microorganisms, 11(4), 1086. https://doi.org/10.3390/microorganisms11041086








