Chemical Constituents, Antioxidant Potential, and Antimicrobial Efficacy of Pimpinella anisum Extracts against Multidrug-Resistant Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Materials Extracts
2.2. Antibiotic Susceptibility and Bacterial Strains
2.3. Identification of Bacterial Isolates by VITEK2 System
2.4. Evaluation of Antimicrobial Susceptibility Testing, Minimum Inhibitory Concentrations (MIC), and Minimum Bactericidal Concentrations (MBC)
2.5. Time-Kill Kinetics
2.6. HPLC Analysis of Phenolic Contents
2.7. Chemical Constituents Gas Chromatography–Mass Spectroscopy (GC–MS) Analysis
2.8. Quantitative Analysis
2.8.1. Preparation of Stock Solutions, Calibration Standards, and Quality Control Samples
2.8.2. Method Validation
2.8.3. Quantitative Analysis of Estragole Using LC–MS/MS Method
2.9. Assessment of Antioxidant Activity
2.9.1. DPPH Scavenging Activity
2.9.2. ABTS Radical Cation Scavenging Activity
2.9.3. Reducing Power Scavenging Assay
2.10. Statistical Analyses
3. Results
3.1. Antibiotic Susceptibility and Bacterial Strains
3.2. Identification of Bacterial Isolates by VITEK2 System
3.3. Antimicrobial Susceptibility Testing for the Methanolic Extract of Aniseed
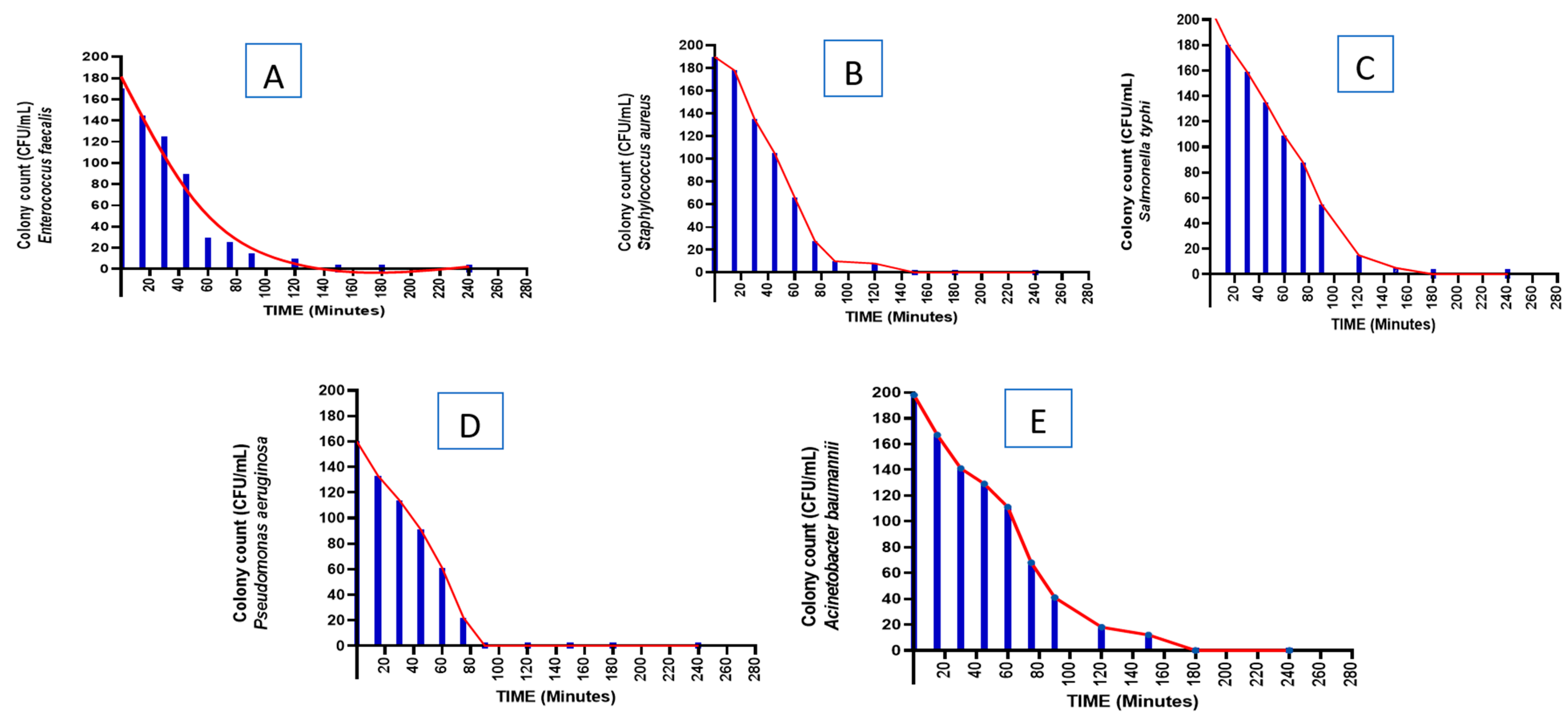
3.4. Time-Kill Kinetics
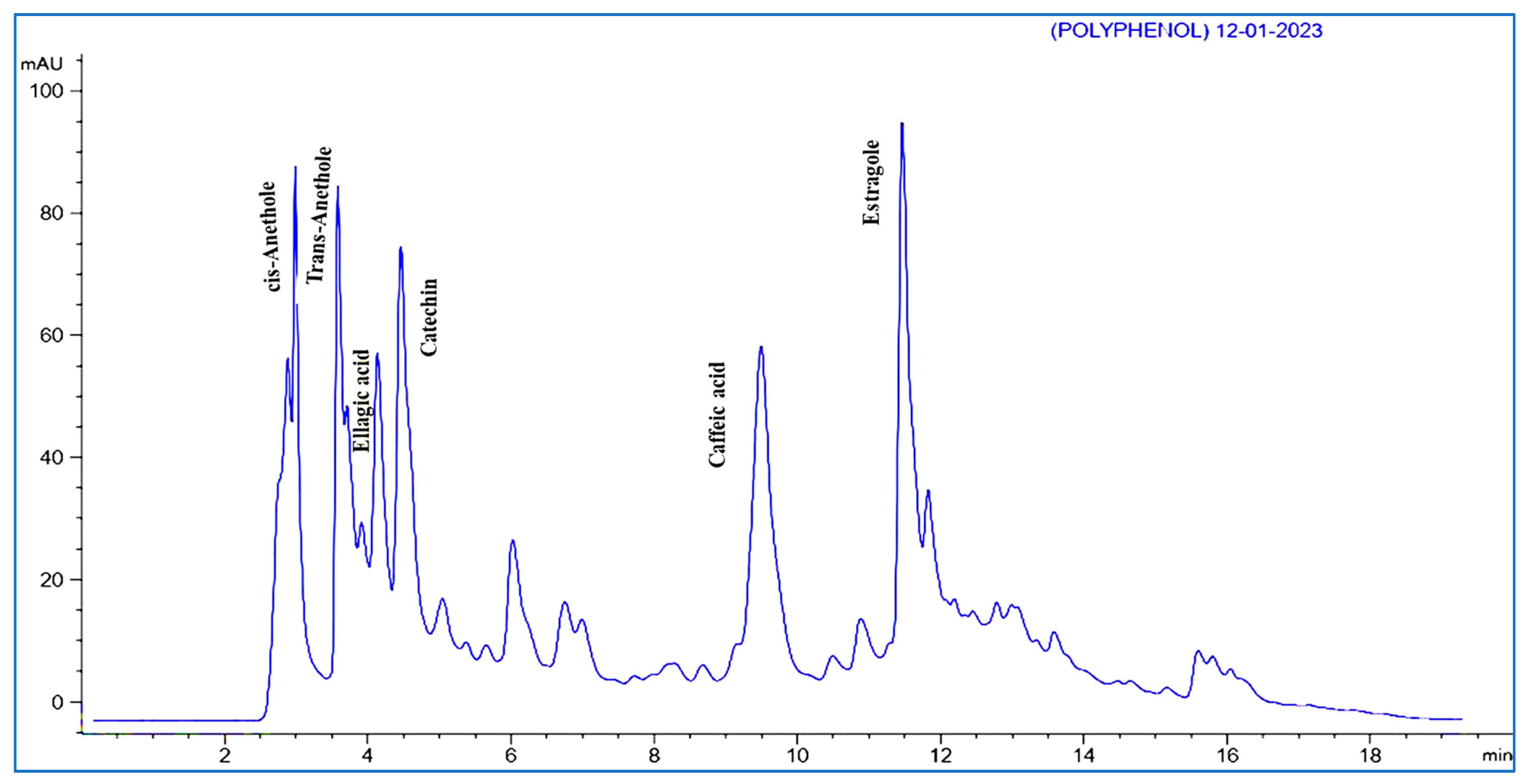
3.5. HPLC Analysis of Phenolic Contents
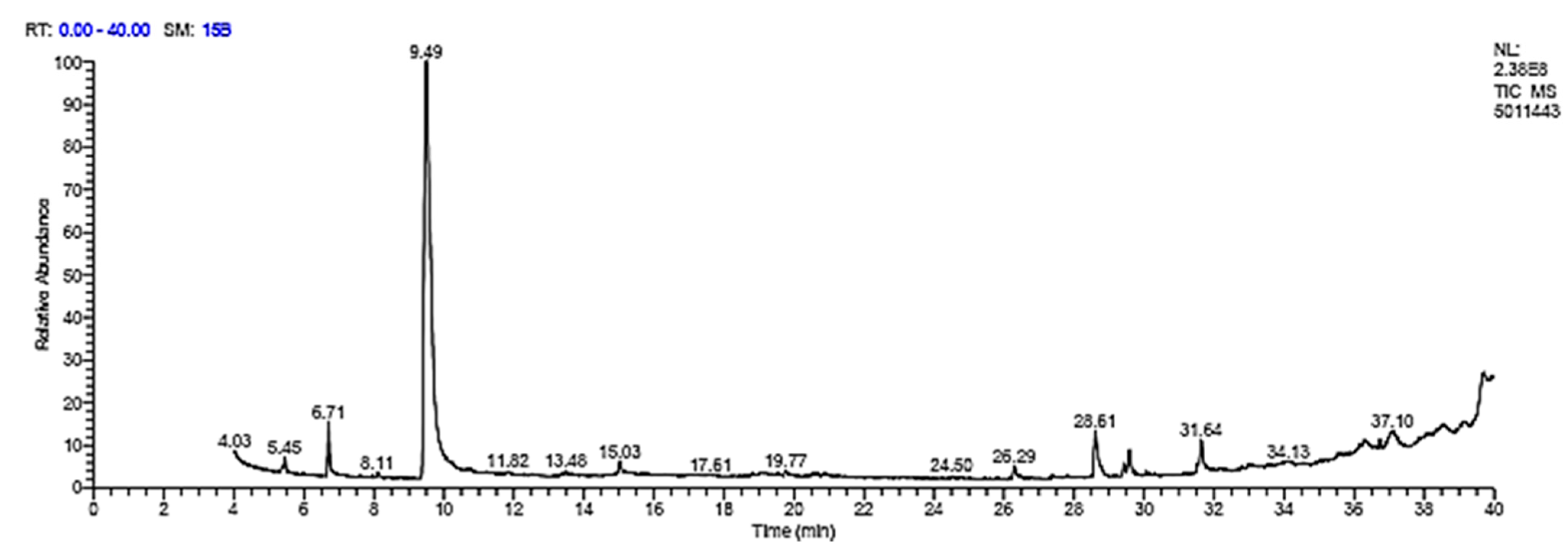
3.6. GC Analysis
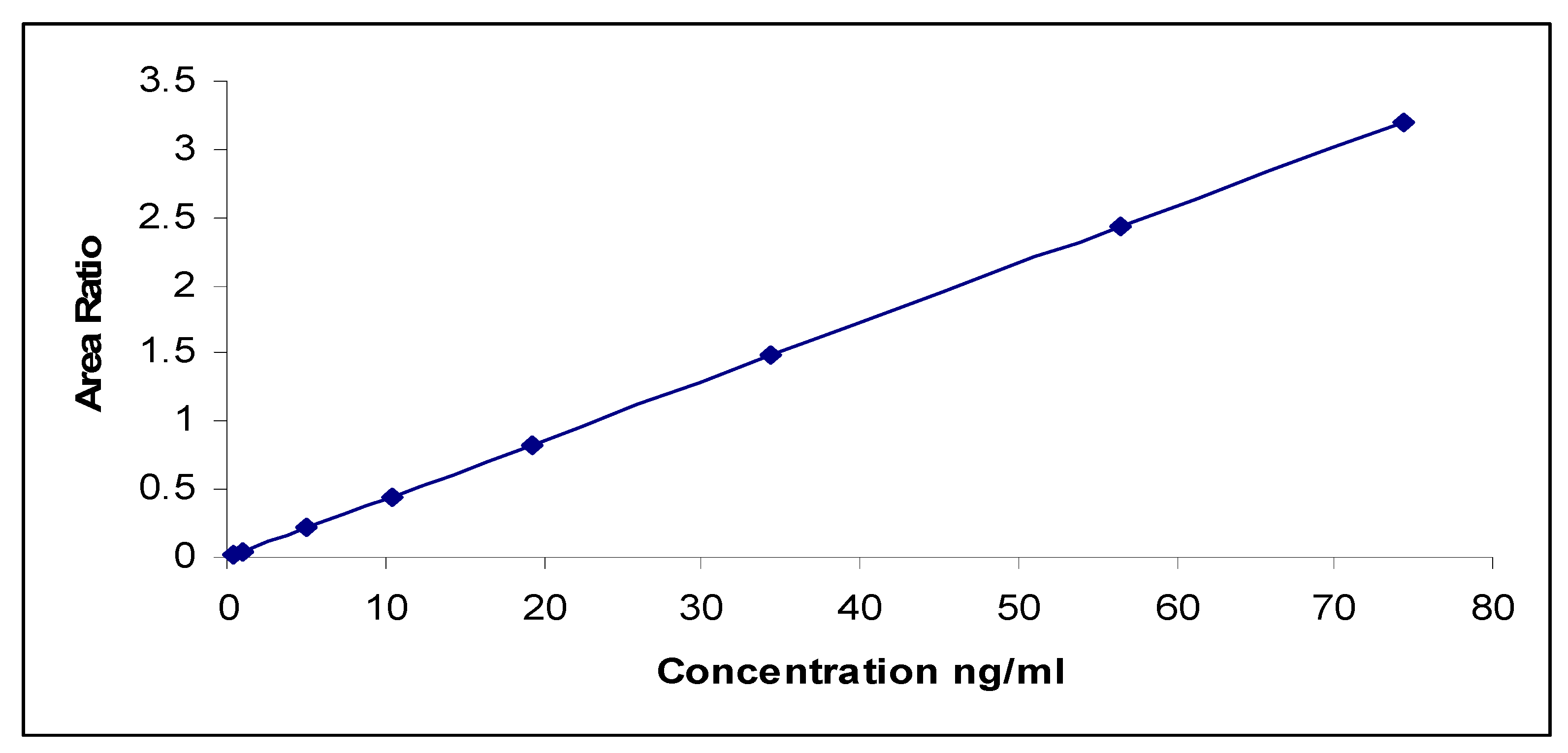
3.7. Method Validation Outcomes
| Technique | LC–MS/MS |
|---|---|
| Selectivity | No interferences from endogenous plasma samples (Figure 4). |
| Lower Quantification Limit | 0.2 ng/mL. |
| Linearity and Calibration Range | R = 0.9992 for range 0.20–20.00 ng/mL. |
| Stability%: | |
| Between 93.66% and 97.10%. |
| Mean 91.03% and 109.86% for internal standard and analyte, respectively, left for 5 h at RT. Mean 98.29% and 101.73% for internal standard and analyte, respectively, left for 27 days and in the refrigerator. |
| Between 94.87% and 98.77% for analyte and 105.38% for internal standard. |
| Between 100.53% and 101.09% for analyte and 98.96% for internal standard (IS) left dry 1.00 h at RT. Between 97.81% and 99.31% for analyte and 101.43% for IS for 50.0 h in the refrigerator. |
| Recovery | |
| Between 61.78% and 72.36% and 83.88%. |
| Between 101.78% and 104.66%. |
| Intra-Batch Precision (% CV) | Between 2.39% and 4.79% and 3.92% for LLOQ. |
| Intra-Batch Accuracy% | Between 96.22% and 103.10% and 89.33% for LLOQ. |
| Inter-Batch Precision (% CV) | Between 4.14% and 5.54% and 13.19% for LLOQ. |
| Inter-Batch Accuracy% | Between 100.00% and 104.75% and 92.33% for LLOQ. |
| Dilution Integrity | Accuracy between 92.60% and 101.17%. |
3.8. Quantitative Analysis of Estragole
3.9. Total Antioxidant Activities
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nwobodo, D.C.; Ugwu, M.C.; Anie, C.O.; Al-Ouqaili, M.T.S.; Ikem, J.C.; Chigozie, U.V.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Lamut, A.; Peterlin Mašič, L.; Kikelj, D.; Tomašič, T. Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med. Res. Rev. 2019, 39, 2460–2504. [Google Scholar] [CrossRef]
- Sameiyan, E.; Hayes, A.W.; Karimi, G. The effect of medicinal plants on multiple drug resistance through autophagy: A review of in vitro studies. Eur. J. Pharmacol. 2019, 852, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Moghtaderi, M.; Mirzaie, A.; Zabet, N.; Moammeri, A.; Mansoori-Kermani, A.; Akbarzadeh, I.; Yeganeh, F.E.; Chitgarzadeh, A.; Kashtali, A.B.; Ren, Q. Enhanced antibacterial activity of Echinacea angustifolia extract against multidrug-resistant Klebsiella pneumoniae through niosome encapsulation. Nanomaterials 2021, 11, 1573. [Google Scholar] [CrossRef] [PubMed]
- Abass, S.; Parveen, R.; Irfan, M.; Jan, B.; Husain, S.A.; Ahmad, S. Synergy Based Extracts of Medicinal Plants: Future Antimicrobials to Com-bat Multidrug Resistance. Curr. Pharm. Biotechnol. 2022, 23, 1527–1540. [Google Scholar] [PubMed]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Akhtar, N.; Mirza, B. Phytochemical analysis and comprehensive evaluation of antimicrobial and antioxidant properties of 61 medicinal plant species. Arab. J. Chem. 2018, 11, 1223–1235. [Google Scholar] [CrossRef]
- Mohr, K.I. History of antibiotics research. How Overcome Antibiot Cris. 2016, 237–272. [Google Scholar]
- Mukunda, A.; Pynadath, M.K.; Kadar, N.; Mohan, A. Cytotoxic effect of anise seed (Pimpinella anisum) extract on KB cell line-a comparative study with CISPLATIN. Oral Maxillofac. Pathol. J. 2020, 11, 6–10. [Google Scholar]
- Sarheed, N.M.; Jaffat, H.S. Protective effect of anise extract loaded by chitosan nanoparticles in mice treated with cisplatin. AIP Conf. Proc. 2022, 2450, 20026. [Google Scholar]
- Sakr, A.A.E.; Taha, K.M.; Abozid, M.M.; El-saed, H.E.Z. Comparative study between anise seeds and mint leaves (chemical composition, phenolic compounds and flavonoids). Menoufia J. Agric. Biotechnol. 2019, 4, 53–60. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Sidiropoulou, E.; Bonos, E.; Christaki, E.; Florou-Paneri, P. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In Feed Additives; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- Sengul, M.; Yildiz, H.; Gungor, N.; Cetin, B.; Eser, Z.; Ercisli, S. Total phenolic content, antioxidant and antimicrobial activities of some medicinal plants. Pak. J. Pharm. Sci. 2009, 22, 102–106. [Google Scholar]
- Bandaranayake, W.M. Quality control, screening, toxicity, and regulation of herbal drugs. Mod. Phytomedicine Turn. Med. Plants Drugs 2006, 25–57. [Google Scholar]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial activity and chemical composition of essential oil extracted from Solidago canadensis L. growing wild in Slovakia. Molecules 2019, 24, 1206. [Google Scholar] [CrossRef]
- Sen, A.; Batra, A. Evaluation of antimicrobial activity of different solvent extracts of medicinal plant: Melia azedarach L. Int. J. Curr. Pharm. Res. 2012, 4, 67–73. [Google Scholar]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The application of medicinal plants in traditional and modern medicine: A review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635. [Google Scholar] [CrossRef]
- Saad, B.; Azaizeh, H.; Said, O. Tradition and perspectives of Arab herbal medicine: A review. Evid.-Based Complement. Altern. Med. 2005, 2, 475–479. [Google Scholar] [CrossRef]
- Odey, M.O.; Iwara, I.A.; Udiba, U.U.; Johnson, J.T.; Inekwe, U.V.; Asenye, M.E.; Victor, O. Preparation of plant extracts from indigenous medicinal plants. Int. J. Sci. Technol. 2012, 1, 688–692. [Google Scholar]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- Guo, L.; Ye, L.; Zhao, Q.; Ma, Y.; Yang, J.; Luo, Y. Comparative study of MALDI-TOF MS and VITEK 2 in bacteria identification. J. Thorac. Dis. 2014, 6, 534. [Google Scholar] [PubMed]
- Zaidan, M.R.; Noor Rain, A.; Badrul, A.R.; Adlin, A.; Norazah, A.; Zakiah, I. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop. Biomed. 2005, 22, 165–170. [Google Scholar] [PubMed]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163. [Google Scholar] [CrossRef]
- Akinjogunla, O.J.; Umo, A.N.; Alozie, M.F.; Oshosanya, G.O.; Saturday, G.I. Antibacterial activity and time kill kinetics of Amlodipine, Thioridazine and Promethazine against pathogenic clinical bacterial isolates. Afr. J. Clin. Exp. Microbiol. 2021, 22, 397–406. [Google Scholar] [CrossRef]
- Michailidis, D.; Angelis, A.; Nikolaou, P.E.; Mitakou, S.; Skaltsounis, A.L. Exploitation of vitis vinifera, Foeniculum vulgare, cannabis sativa and punica granatum by-product seeds as dermo-cosmetic agents. Molecules 2021, 26, 731. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ebrahimabadi, A.H.; Djafari-Bidgoli, Z.; Batooli, H. GC/MS analysis and in vitro antioxidant activity of essential oil and methanol extracts of Thymus caramanicus Jalas and its main constituent carvacrol. Food Chem. 2009, 115, 1524–1528. [Google Scholar] [CrossRef]
- Marinova, G.; Batchvarov, V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Class, R.; Class, I.I.; BioMerieux, Inc. Jolyn Tenllado Regulatory Affairs Expert 595 Anglum Road Hazelwood, Missouri 63042. 2020. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf19/K193572.pdf (accessed on 13 March 2020).
- Said, A.; El-Gamal, M.S.; Abu-Elghait, M.; Salem, S.S. Isolation, Identification and Antibiotic Susceptibility Pattern of Urinary Tract Infection Bacterial Isolates. Lett. Appl. NanoBioSci. 2021, 10, 2820–2830. [Google Scholar]
- Davies, J. Microbes have the last word: A drastic re-evaluation of antimicrobial treatment is needed to overcome the threat of antibiotic-resistant bacteria. EMBO Rep. 2007, 8, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2011; pp. 156–167. [Google Scholar]
- Mattocks, C.J.; Morris, M.A.; Matthijs, G.; Swinnen, E.; Corveleyn, A.; Dequeker, E.; Müller, C.R.; Pratt, V.; Wallace, A. A standardized framework for the validation and verification of clinical molecular genetic tests. Eur. J. Hum. Genet. 2010, 18, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Shlaes, D.M.; Gerding, D.N.; John, J.F.; Craig, W.A.; Bornstein, D.L.; Duncan, R.A.; Eckman, M.R.; Farrer, W.E.; Greene, W.H.; Lorian, V.; et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance guidelines for the prevention of antimicrobial resistance in hospitals. Infect. Control. Hosp. Epidemiol. 1997, 18, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Machowska, A.; Stålsby Lundborg, C. Drivers of irrational use of antibiotics in Europe. Int. J. Environ. Res. Public Health 2019, 16, 27. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Kovač, J.; Gavarić, N.; Bucar, F.; Smole Možina, S. Antimicrobial and resistance modulatory activity of Alpinia katsumadai seed phenolic extract, essential oil and post-distillation extract. Food Technol. Biotechnol. 2014, 52, 248–254. [Google Scholar]
- Sharafan, M.; Jafernik, K.; Ekiert, H.; Kubica, P.; Kocjan, R.; Blicharska, E.; Szopa, A. Illicium verum (Star Anise) and Trans-Anethole as valuable raw materials for medicinal and cosmetic applications. Molecules 2022, 27, 650. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef]
- Salem, M.A.; El-Shiekh, R.A.; Hashem, R.A.; Hassan, M. In vivo antibacterial activity of star anise (Illicium verum Hook.) Extract Using Murine MRSA skin infection model in relation to its metabolite profile. Infect. Drug. Resist. 2021, 14, 33. [Google Scholar] [CrossRef]
- Bin Muhsinah, A.; Maqbul, M.S.; Mahnashi, M.H.; Jalal, M.M.; Altayar, M.A.; Saeedi, N.H.; Alshehri, O.M.; Shaikh, I.A.; Khan, A.A.L.; Iqubal, S.S.; et al. Antibacterial activity of Illicium verum essential oil against MRSA clinical isolates and determination of its phyto-chemical components. J. King Saud Univ. 2022, 34, 101800. [Google Scholar] [CrossRef]
- Yang, J.-F.; Yang, C.-H.; Chang, H.-W.; Wang, S.-M.; Hsieh, M.-C.; Chuang, L.-Y. Chemical composition and antibacterial activities of Illicium verum against antibiotic-resistant pathogens. J. Med. Food. 2010, 13, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, J.; Wang, T. Star anise essential oil: Chemical compounds, antifungal and antioxidant activities: A review. J. Essent. Oil Res. 2021, 33, 1–22. [Google Scholar] [CrossRef]
- Bohlmann, J.; Keeling, C.I. Terpenoid biomaterials. Plant. J. 2008, 54, 656–669. [Google Scholar] [CrossRef]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ornelas-Paz, J.D.J.; López-Mata, M.A.; Del-Toro-Sánchez, D.L.; Ayala-Zavala, J.F.; Márquez-Ríos, E. Total phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. Int. J. Anal. Chem. 2015, 2015, 284071. [Google Scholar] [CrossRef]
- Roy, S.; Lingampeta, P. Solid wastes of fruits peels as source of low cost broad spectrum natural antimicrobial compounds-furanone, furfural and benezenetriol. Int. J. Res. Eng. Technol. 2014, 3, 273–279. [Google Scholar]
- Welscher, Y.M.T.; van Leeuwen, M.R.; de Kruijff, B.; Dijksterhuis, J.; Breukink, E. Polyene antibiotic that inhibits membrane transport proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 11156–11159. [Google Scholar] [CrossRef]
- Grandinetti, G.; Smith, A.E.; Reineke, T.M. Membrane and nuclear permeabilization by polymeric pDNA vehicles: Efficient method for gene delivery or mechanism of cytotoxicity? Mol. Pharm. 2012, 9, 523–538. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Díaz-Maroto Hidalgo, I.J.; Sánchez-Palomo, E.; Pérez-Coello, M.S. Volatile components and key odorants of Fennel (Foeniculum vulgare Mill.) and Thyme (Thymus vulgaris L.) oil extracts obtained by simultaneous distillation− extraction and supercritical fluid extraction. J. Agric. Food Chem. 2005, 53, 5385–5389. [Google Scholar] [CrossRef]
- Anwar, F.; Ali, M.; Hussain, A.I.; Shahid, M. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. Flavour. Fragr. J. 2009, 24, 170–176. [Google Scholar] [CrossRef]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Sangeetha, K.S.S.; Umamaheswari, S.; Reddy, C.U.M.; Kalkura, S.N. Flavonoids: Therapeutic potential of natural pharmacological agents. Int. J. Pharm. Sci. Res. 2016, 7, 3924. [Google Scholar]
- Nenaah, G. Antimicrobial activity of Calotropis procera Ait. (Asclepiadaceae) and isolation of four flavonoid glycosides as the active constituents. World J. Microbiol. Biotechnol. 2013, 29, 1255–1262. [Google Scholar] [CrossRef]
- Kopel, J.; McDonald, J.; Hamood, A. An Assessment of the In Vitro Models and Clinical Trials Related to the Antimicrobial Activities of Phytochemicals. Antibiotics 2022, 11, 1838. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly) phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.M.; Izadi, M.; Abdollahi, M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Kalleli, F.; Rebey, I.B.; Wannes, W.A.; Boughalleb, F.; Hammami, M.; Tounsi, M.S.; M’Hamdi, M. Chemical composition and antioxidant potential of essential oil and methanol extract from Tunisian and French fennel (Foeniculum vulgare Mill.) seeds. J. Food Biochem. 2019, 43, e12935. [Google Scholar] [CrossRef] [PubMed]
- Rebey, I.B.; Bourgou, S.; Wannes, W.A.; Selami, I.H.; Tounsi, M.S.; Marzouk, B.; Fauconnier, M.-L.; Ksouri, R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2018, 152, 971–978. [Google Scholar]
- Nasır, A.; Yabalak, E. Investigation of antioxidant, antibacterial, antiviral, chemical composition, and traditional medicinal properties of the extracts and essential oils of the Pimpinella species from a broad perspective: A review. J. Essent. Oil Res. 2021, 33, 411–426. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- El-Kersh, D.M.; Mostafa, N.M.; Fayez, S.; Al-Warhi, T.; Abourehab, M.A.S.; Eldehna, W.M.; Salem, M.A. GC-MS metabolites profiling of anethole-rich oils by different extraction techniques: Antioxidant, cytotoxicity and in-silico enzymes inhibitory insights. J. Enzym. Inhib. Med. Chem. 2022, 37, 1974–1986. [Google Scholar] [CrossRef]
- Kimondo, J.W. An Evaluation of Antioxidant and Anti-Inflammatory Effects of Natural Foods and Medicinal Plants of the Ilkisonko Maasai Community, Kenya. 2020. Available online: http://erepository.uonbi.ac.ke/handle/11295/154650 (accessed on 30 June 2020).
- Danilenko, V.N.; Devyatkin, A.V.; Marsova, M.V.; Shibilova, M.U.; Ilyasov, R.A.; Shmyrev, V.I. Common inflammatory mechanisms in COVID-19 and Parkinson’s diseases: The role of microbiome, pharmabiotics and postbiotics in their prevention. J. Inflamm. Res. 2021, 14, 6349–6381. [Google Scholar] [CrossRef]


| Isolate Code | P | AMC | VA | AX | SAM | F | ATM | CPZ | C | DA | CN | TE | E | OFX | NOR | SXT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. faecalis n = 4 | 100 | 80 | 100 | 75 | 88 | 91 | 100 | 37 | 78 | 91 | 99 | 91 | 79 | 100 | 100 | 100 |
| S. aureus n = 11 | 100 | 95 | 92 | 94 | 79 | 49 | I00 | 74 | 71 | 87 | 97 | 79 | 84 | 99 | 100 | 100 |
| S. typhi n = 9 | 100 | 100 | 78 | 91 | 92 | 69 | 92 | 97 | 93 | 98 | 99 | 72 | 88 | 100 | 92 | 94 |
| P. aeruginosa n = 7 | 100 | 92 | 91 | 89 | 79 | 55 | 90 | 88 | 44 | 94 | 100 | 66 | 91 | 93 | 88 | 93 |
| A. baumannii n = 6 | 100 | 97 | 49 | I | 59 | 79 | 100 | 81 | 57 | 96 | 95 | 49 | 100 | 97 | 100 | 98 |
| Antimicrobial | MIC | Interpretation | Antimicrobial | MIC | Interpretation |
|---|---|---|---|---|---|
| ESBL | POS | + | Meropenem | 1 ≤ 0.25 | R |
| Ampicillin | ≥32 | R | Amikacin | ≥2 | R |
| Ampicillin/sulbactam | 4 | S | Gentamicin | ≥16 | R |
| Clindamycin | ≥64 | S | Erythromycin | 8 | R |
| Ceftriaxone | ≥64 | R | Ciprofloxacin | ≤4 | R |
| Cefepime | 2 | I | Norfloxacin | ≤64 | R |
| Aztreonam | 16 | R | Vancomycin | ≤0.5 | R |
| Ertapenem | ≤0.5 | R | Nitrofurantoin | ≤16 | R |
| Imipenem | ≤0.25 | R | Trimethoprim/sulfamethoxazole | ≤32 | R |
| Antimicrobial | MIC | Interpretation | Antimicrobial | MIC | Interpretation |
|---|---|---|---|---|---|
| ESBL | POS | + | Meropenem | 1 ≤ 0.25 | R |
| Ampicillin | ≥32 | R | Amikacin | ≥2 | R |
| Ampicillin/sulbactam | 4 | S | Gentamicin | ≥16 | R |
| Clindamycin | ≥64 | R | Erythromycin | 8 | I |
| Ceftriaxone | ≥64 | R | Ciprofloxacin | ≤4 | R |
| Cefepime | 2 | R | Norfloxacin | ≤64 | R |
| Aztreonam | 16 | R | Vancomycin | ≤0.5 | S |
| Ertapenem | ≤0.5 | R | Nitrofurantoin | ≤16 | R |
| Imipenem | ≤0.25 | R | Trimethoprim/sulfamethoxazole | ≤32 | R |
| Antimicrobial | MIC | Interpretation | Antimicrobial | MIC | Interpretation |
|---|---|---|---|---|---|
| ESBL | POS | + | Meropenem | 1 ≤ 0.25 | R |
| Ampicillin | ≥32 | R | Amikacin | ≥2 | R |
| Ampicillin/sulbactam | 4 | S | Gentamicin | ≥16 | R |
| Clindamycin | ≥64 | R | Erythromycin | 8 | R |
| Ceftriaxone | ≥64 | I | Ciprofloxacin | ≤4 | R |
| Cefepime | 2 | R | Norfloxacin | ≤64 | R |
| Aztreonam | 16 | R | Vancomycin | ≤0.5 | I |
| Ertapenem | ≤0.5 | R | Nitrofurantoin | ≤16 | R |
| Imipenem | ≤0.25 | R | Trimethoprim/sulfamethoxazole | ≤32 | R |
| Antimicrobial | MIC | Interpretation | Antimicrobial | MIC | Interpretation |
|---|---|---|---|---|---|
| ESBL | POS | + | Meropenem | 1 ≤ 0.25 | R |
| Ampicillin | ≥32 | R | Amikacin | ≥2 | R |
| Ampicillin/sulbactam | 4 | R | Gentamicin | ≥16 | R |
| Clindamycin | ≥64 | R | Erythromycin | 8 | I |
| Ceftriaxone | ≥64 | R | Ciprofloxacin | ≤4 | R |
| Cefepime | 2 | R | Norfloxacin | ≤64 | S |
| Aztreonam | 16 | R | Vancomycin | ≤0.5 | S |
| Ertapenem | ≤0.5 | R | Nitrofurantoin | ≤16 | R |
| Imipenem | ≤0.25 | R | Trimethoprim/sulfamethoxazole | ≤32 | R |
| Antimicrobial | MIC | Interpretation | Antimicrobial | MIC | Interpretation |
|---|---|---|---|---|---|
| ESBL | POS | + | Meropenem | 1 ≤ 0.25 | R |
| Ampicillin | ≥32 | R | Amikacin | ≥2 | S |
| Ampicillin/sulbactam | 4 | R | Gentamicin | ≥16 | S |
| Clindamycin | ≥64 | R | Erythromycin | 8 | R |
| Ceftriaxone | ≥64 | R | Ciprofloxacin | ≤4 | S |
| Cefepime | 2 | R | Norfloxacin | ≤64 | R |
| Aztreonam | 16 | R | Vancomycin | ≤0.5 | R |
| Ertapenem | ≤0.5 | R | Nitrofurantoin | ≤16 | R |
| Imipenem | ≤0.25 | R | Trimethoprim/sulfamethoxazole | ≤32 | R |
| Isolate | Aniseed Methanolic Extract | Amikacin (10 µg/disc) | Negative Control (DMSO, 100 µL) |
|---|---|---|---|
| Enterococcus faecalis | 18.32 ± 0.5 ab | 14.27 ± 0.2 ab | 0.0 ± 0.0 |
| Staphylococcus aureus | 19.88 ± 0.3 ab | 16.23 ± 0.1 ab | 0.0 ± 0.0 |
| Salmonella typhi | 21.33 ± 0.5 ab | 20.31 ± 0.3 ab | 0.0 ± 0.0 |
| Pseudomonas aeruginosa | 22.86 ± 0.5 ab | 18.56 ± 0.2 ab | 0.0 ± 0.0 |
| Acinetobacter baumannii | 17.56 ± 0.2 ab | 15.50 ± 0.5 ab | 0.0 ± 0.0 |
| Isolate | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|
| Enterococcus faecalis | 95 | 190 |
| Staphylococcus aureus | 125 | 250 |
| Salmonella typhi | 130 | 260 |
| Pseudomonas aeruginosa | 125 | 250 |
| Acinetobacter baumannii | 170 | 340 |
| Phenolic Compounds | Conc. (µg/g) | RT (min) |
|---|---|---|
| Cis-anethole | 3150.11 | 2.82 |
| trans-anethole | 2312.11 | 3.55 |
| Ellagic acid | 623.54 | 4.01 |
| Catechin | 357.23 | 4.52 |
| Kaempferol-O-rutinoside | 96.32 | 5.24 |
| Syringic acid | 521.49 | 5.92 |
| Pyro catechol | 578.31 | 6.04 |
| Eugenol rutinoside | 234.56 | 7.13 |
| O-coumaric acid | 578.77 | 8.11 |
| Vanillin | 52.36 | 8.31 |
| Trans-ferulic acid | 324.57 | 9.32 |
| Naringenin | 211.23 | 9.53 |
| Caffeic acid | 652.32 | 9.75 |
| Rosmarinic acid | 96.32 | 10.55 |
| Quercitin rhamnoside | 632.32 | 10.17 |
| Estragole | 4422.39 | 11.52 |
| Quercitin rhamnoside | 632.32 | 12.11 |
| Trans-cinnamic | 37.25 | 12.97 |
| Luteolin | 23.65 | 13.92 |
| Apegenin-7-O-glucoside | 55.62 | 15.72 |
| Identification | RT (min) | Area % | Peak Area |
|---|---|---|---|
| Linolenic acid | 5.38 | 0.37 | 10,147,157.35 |
| Eucalyptol | 5.45 | 1.08 | 29,892,305.40 |
| L-Fenchone | 6.70 | 3.73 | 103,381,896.04 |
| 2-9,12-Octadecadienyloxy | 8.11 | 0.42 | 11,620,299.63 |
| Estragole | 9.49 | 66.85 | 1,853,324,123.57 |
| 5,7-Dodecadiyn-1,12-diol | 13.48 | 0.36 | 9,973,126.83 |
| Caryophyllene | 15.03 | 0.95 | 26,230,030.91 |
| 1,3-Benzodioxole | 19.76 | 0.43 | 11,818,138.28 |
| Gibberellic acid | 20.63 | .46 | 12,978,627.00 |
| Pentadecanoic acid | 26.29 | 1.05 | 29,100,222.44 |
| Palmitic acid | 28.60 | 4.26 | 118,101,239.86 |
| 7,10-octadecadienoic acid | 29.42 | 0.88 | 24,453,817.51 |
| 9-octadecenoic acid | 29.58 | 2.35 | 65,019,938.64 |
| Cyclopropanebutanoic acid | 30.08 | 0.36 | 9,960,494.88 |
| 9,12-octadecadienoic acid | 31.53 | 0.57 | 15,750,740.24 |
| Petroselinic acid | 31.63 | 2.72 | 75,269,544.77 |
| Isochiapin b | 32.79 | 0.24 | 6,778,401.90 |
| 1-Heptatriacotanol | 33.00 | 0.61 | 17,018,655.01 |
| Hexadecadienoic acid | 35.52 | 0.30 | 8,369,888.17 |
| Linoleic acid ethyl ester | 35.62 | 0.33 | 9,209,906.53 |
| cis-13-Eicosenoic acid | 36.15 | 0.38 | 10,547,993.14 |
| 9-octadecenoic acid | 36.31 | 1.17 | 32,477,736.34 |
| Cyclopropane decanoic acid | 36.73 | 0.72 | 19,892,392.94 |
| 9-hexadecenoic acid | 37.11 | 3.61 | 100,140,171.41 |
| 9-Hexadecenoic acid, 9-octadecenyl ester | 38.55 | 0.96 | 26,601,291.25 |
| Oleyl oleate | 39.14 | 1.94 | 53,776,181.23 |
| Nonadecatriene-5,14-diol | 39.68 | 2.90 | 80,466,096.09 |
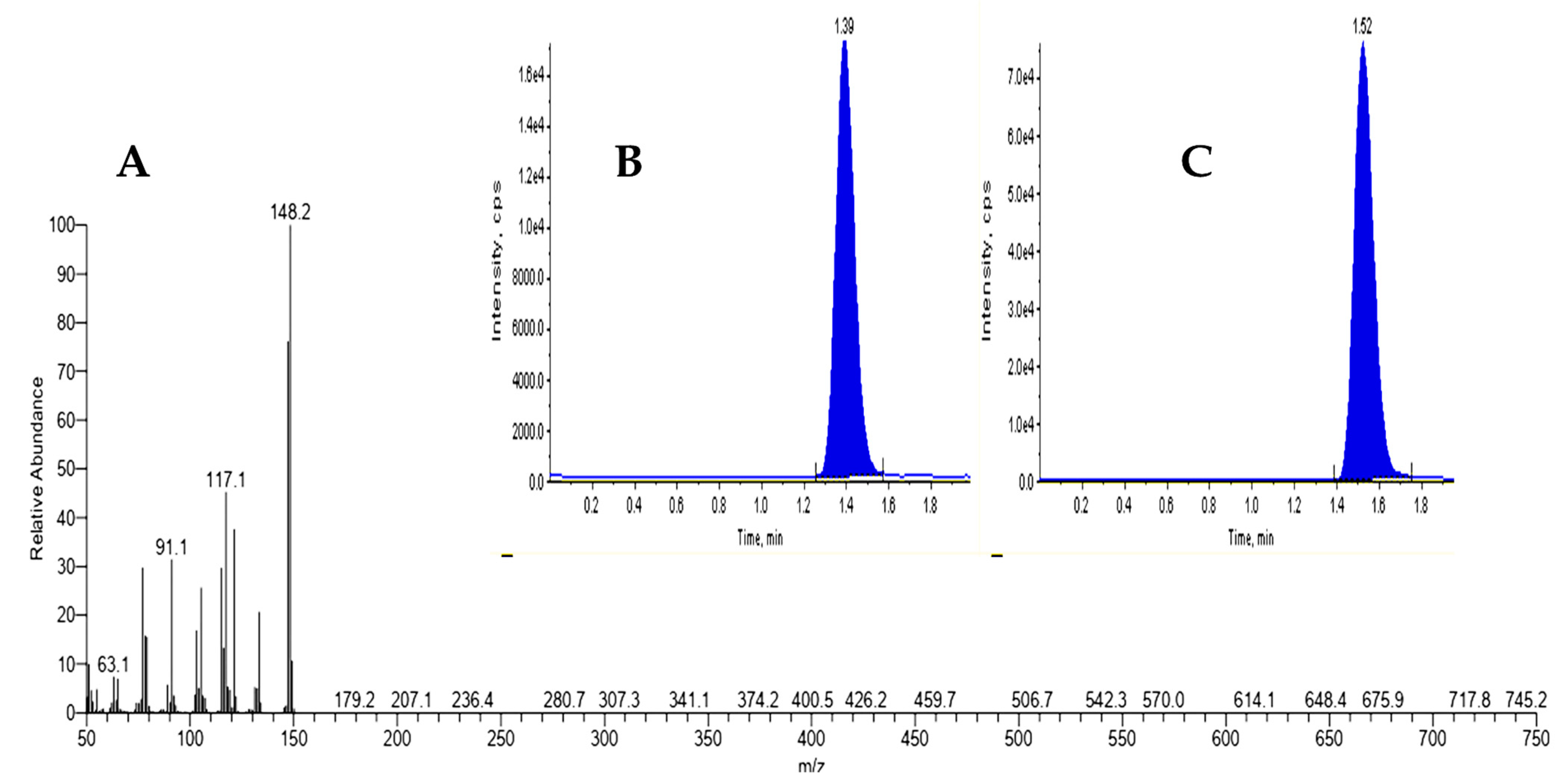
| Aniseed Retention Time and Fragment Ions for Estragole Standard | |||
|---|---|---|---|
| Compound | M.wt | (m/z) | RT (Min) |
| Estragole | 148.2 | 150 | 1.39 |
| Quantification Results of Estragole in Aniseed | |||
| Compound | Linear Range (ng/mL) | R2 | Recovery (%) |
| Estragole | 0.5–75 | 0.9992 | 101.78% to 104.66% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlBalawi, A.N.; Elmetwalli, A.; Baraka, D.M.; Alnagar, H.A.; Alamri, E.S.; Hassan, M.G. Chemical Constituents, Antioxidant Potential, and Antimicrobial Efficacy of Pimpinella anisum Extracts against Multidrug-Resistant Bacteria. Microorganisms 2023, 11, 1024. https://doi.org/10.3390/microorganisms11041024
AlBalawi AN, Elmetwalli A, Baraka DM, Alnagar HA, Alamri ES, Hassan MG. Chemical Constituents, Antioxidant Potential, and Antimicrobial Efficacy of Pimpinella anisum Extracts against Multidrug-Resistant Bacteria. Microorganisms. 2023; 11(4):1024. https://doi.org/10.3390/microorganisms11041024
Chicago/Turabian StyleAlBalawi, Aisha Nawaf, Alaa Elmetwalli, Dina M. Baraka, Hadeer A. Alnagar, Eman Saad Alamri, and Mervat G. Hassan. 2023. "Chemical Constituents, Antioxidant Potential, and Antimicrobial Efficacy of Pimpinella anisum Extracts against Multidrug-Resistant Bacteria" Microorganisms 11, no. 4: 1024. https://doi.org/10.3390/microorganisms11041024
APA StyleAlBalawi, A. N., Elmetwalli, A., Baraka, D. M., Alnagar, H. A., Alamri, E. S., & Hassan, M. G. (2023). Chemical Constituents, Antioxidant Potential, and Antimicrobial Efficacy of Pimpinella anisum Extracts against Multidrug-Resistant Bacteria. Microorganisms, 11(4), 1024. https://doi.org/10.3390/microorganisms11041024






