Phytochemicals of Withania somnifera as a Future Promising Drug against SARS-CoV-2: Pharmacological Role, Molecular Mechanism, Molecular Docking Evaluation, and Efficient Delivery
Abstract
1. Introduction
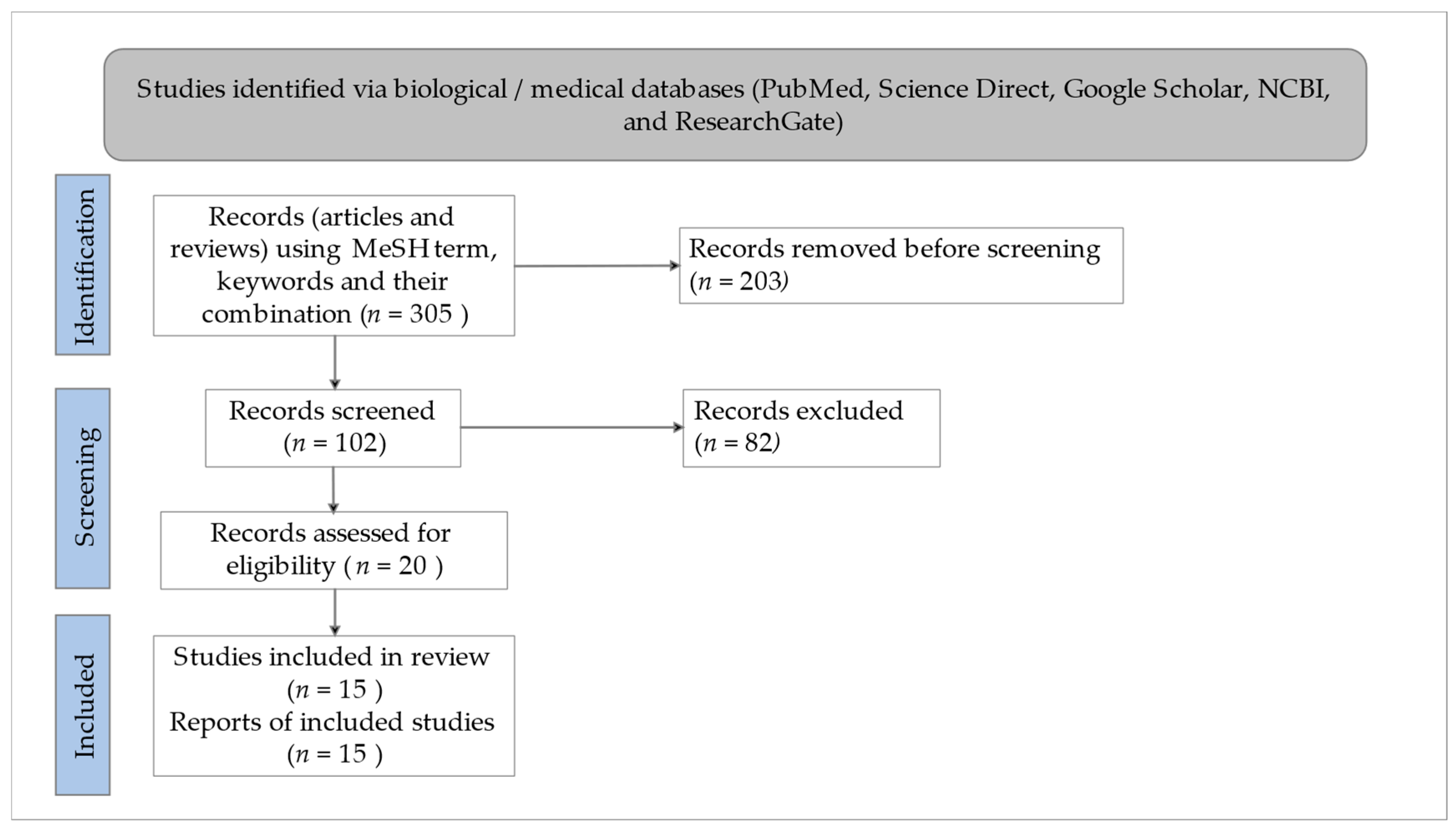
2. Methodology for Literature Search/Selection
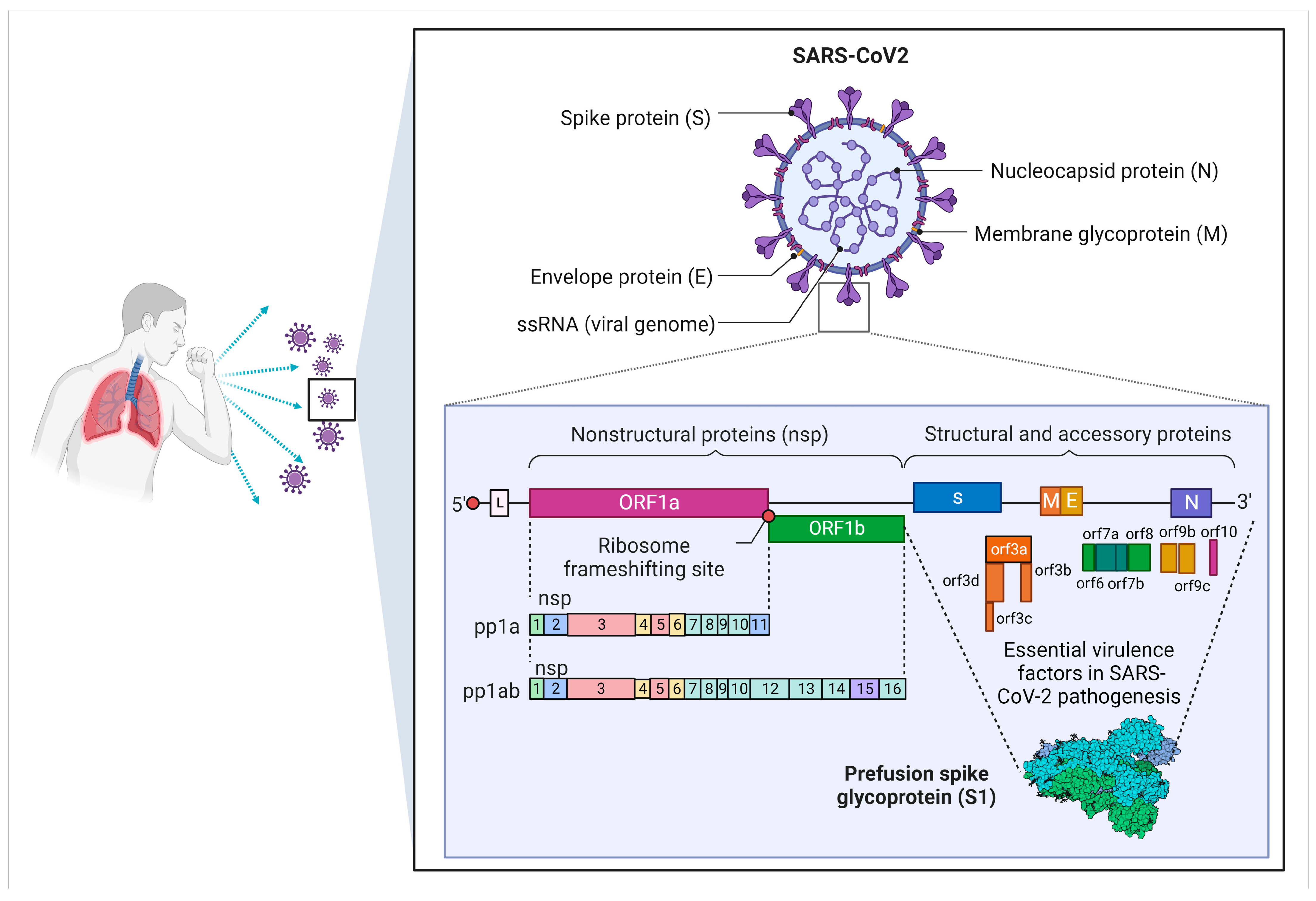
3. Overview of SARS-CoV-2
4. Pathogenesis and Clinical Manifestations of SARS-CoV-2
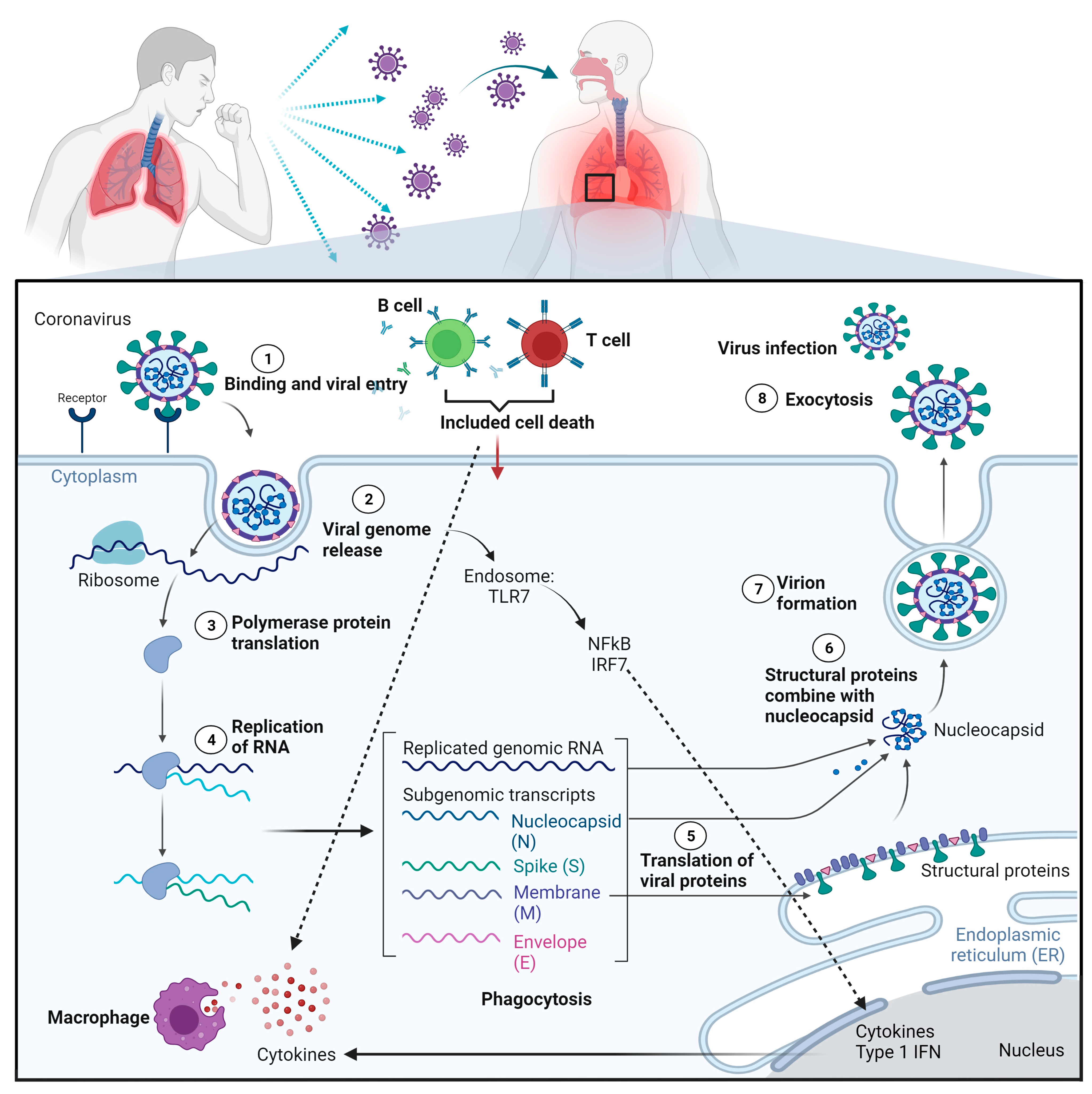
4.1. SARS-CoV-2 Pathogenesis
4.2. Clinical Manifestations of SARS-CoV-2
5. COVID-19 Vaccines against SARS-CoV-2
6. Efficiency of Antiviral Treatment in COVID-19
7. Withania somnifera and Its Traditional and Medicinal Uses
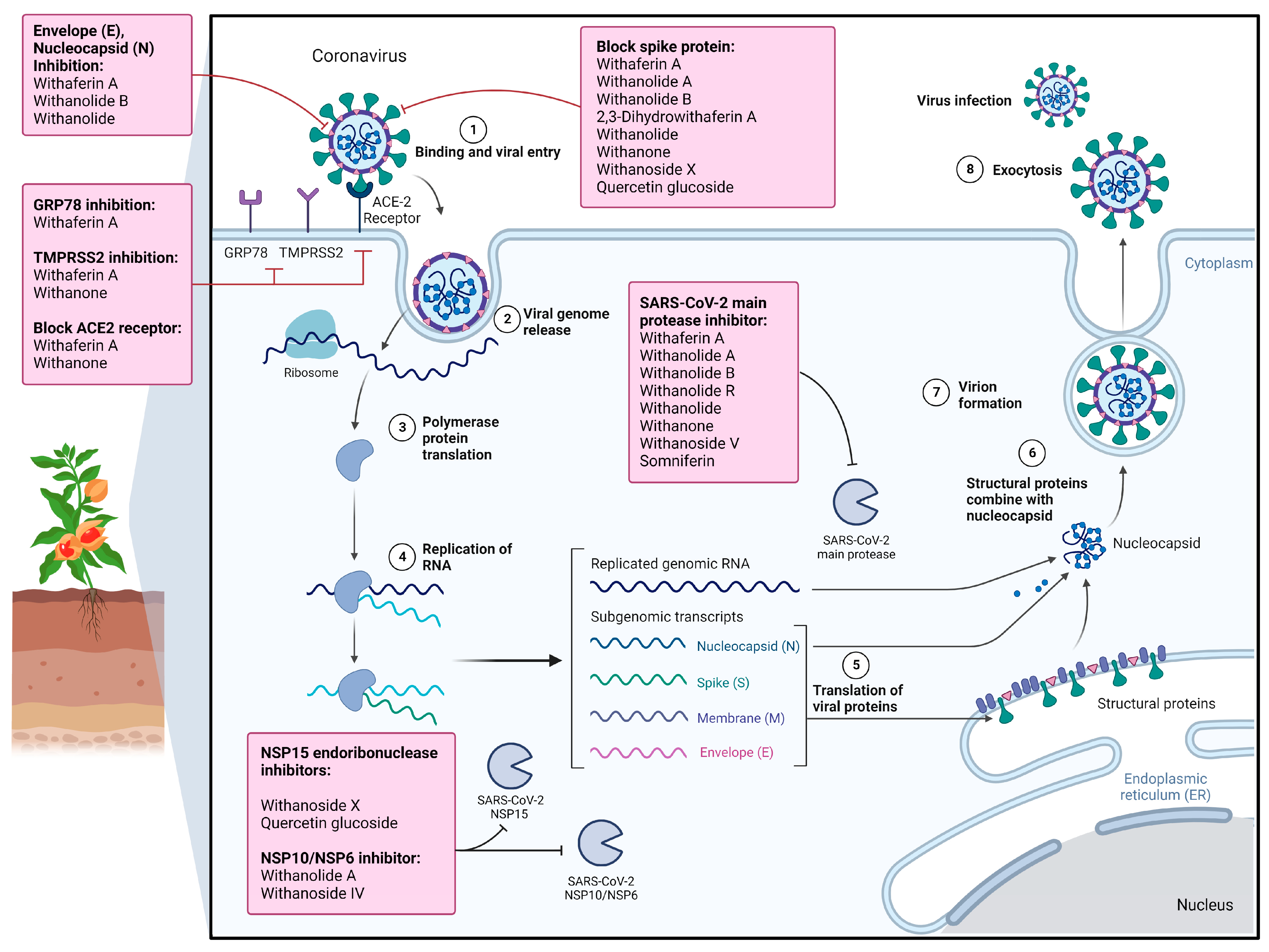
8. Therapeutic Effects and Associated Molecular Mechanisms of Withania somnifera and Its Phytochemicals against COVID-19
8.1. Antiviral Potentials of WS Crude Extracts against SARS-CoV-2
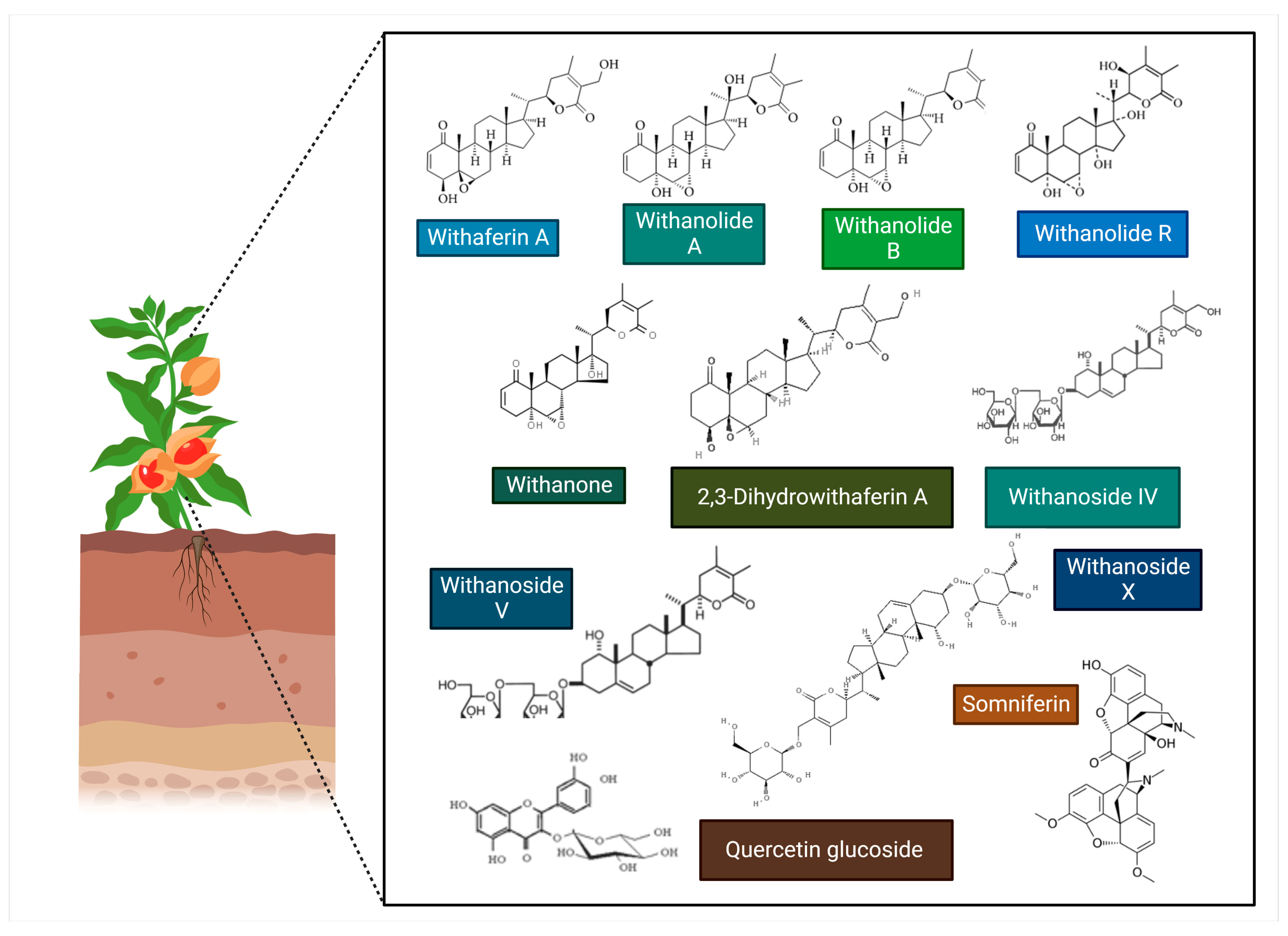
8.2. Antiviral Potentials of Phytochemicals of Withania somnifera against SARS-CoV-2
8.2.1. Withaferin A
8.2.2. Withanolide A
8.2.3. Withanolide R
8.2.4. 2,3-Dihydrowithaferin A
8.2.5. Withanone
8.2.6. Withanoside IV
8.2.7. Withanoside V
8.2.8. Withanoside X
9. Identification of Anti-SARS-CoV-2 Inhibitors from Withania somnifera Using an In Silico Molecular Docking Approach
10. Strategies for Efficient Delivery of Withania somnifera and Its Phytochemicals
11. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19) Pandemic. 2022. Available online: https://www.who.int/covid-19 (accessed on 15 May 2022).
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Rome, B.N.; Avorn, J. Drug Evaluation during the COVID-19 Pandemic. N. Engl. J. Med. 2020, 382, 2282–2284. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhães, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front. Pharm. 2020, 11, 1479. [Google Scholar] [CrossRef]
- Nugraha, R.V.; Ridwansyah, H.; Ghozali, M.; Khairani, A.F.; Atik, N. Traditional herbal medicine candidates as complementary treatments for COVID-19: A review of their mechanisms, pros and cons. Evid. Based Complement. Altern. Med. 2020, 2020, 2560645. [Google Scholar] [CrossRef]
- Srivastava, M.; Gupta, S.; Dixit, S.; Yadav, N.; Yadav, V.; Singh, H.; Kanaujia, P.; Sharma, Y. Withania somnifera (Ashwagandha): A wonder herb with multiple medicinal properties. Asian J. Pharm. Pharmacol. 2018, 4, 123–130. [Google Scholar] [CrossRef]
- Bungau, S.; Popa, V.-C. Between Religion and Science: Some Aspects: Concerning Illness and Healing in Antiquity. Transylv. Rev. 2015, XXIV, 3–19. [Google Scholar]
- Bungau, S.; Vesa, C.M.; Abid, A.; Behl, T.; Tit, D.M.; Purza, A.L.; Pasca, B.; Todan, L.M.; Endres, L. Withaferin A—A Promising Phytochemical Compound with Multiple Results in Dermatological Diseases. Molecules 2021, 26, 2407. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives. Biomedicines 2020, 8, 571. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Madhu Kumar Nair, R.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of withaferin A, an active constituent of the Indian ginseng Withania somnifera. Environ. Sci. Pollut. Res. 2020, 27, 26025–26035. [Google Scholar] [CrossRef] [PubMed]
- Negru, P.A.; Miculas, D.C.; Behl, T.; Bungau, A.F.; Marin, R.-C.; Bungau, S.G. Virtual screening of substances used in the treatment of SARS-CoV-2 infection and analysis of compounds with known action on structurally similar proteins from other viruses. Biomed. Pharmacother. 2022, 153, 113432. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e819. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.E.; Paul, P.; MacCannell, D.R.; Johansson, M.A.; Brooks, J.T.; MacNeil, A.; Slayton, R.B.; Tong, S.; Silk, B.J.; Armstrong, G.L.; et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage—United States, December 29, 2020-January 12, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á.; et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef]
- Davies, N.G.; Jarvis, C.I.; Edmunds, W.J.; Jewell, N.P.; Diaz-Ordaz, K.; Keogh, R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 2021, 593, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Werner, A.P.; Moliva, J.I.; Koch, M.; Choi, A.; Stewart-Jones, G.B.E.; Bennett, H.; Boyoglu-Barnum, S.; Shi, W.; Graham, B.S.; et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv 2021. [Google Scholar] [CrossRef]
- Walensky, R.P.; Walke, H.T.; Fauci, A.S. SARS-CoV-2 Variants of Concern in the United States-Challenges and Opportunities. J. Am. Med. Assoc. 2021, 325, 1037–1038. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef]
- Wibmer, C.K.; Ayres, F.; Hermanus, T.; Madzivhandila, M.; Kgagudi, P.; Oosthuysen, B.; Lambson, B.E.; de Oliveira, T.; Vermeulen, M.; van der Berg, K.; et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021, 27, 622–625. [Google Scholar] [CrossRef]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.D.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and epidemiology of a novel SARS-CoV-2 lineage in Manaus, Brazil. Medrxiv Prepr. Serv. Health Sci. 2021. [Google Scholar] [CrossRef]
- Mwenda, M.; Saasa, N.; Sinyange, N.; Busby, G.; Chipimo, P.J.; Hendry, J.; Kapona, O.; Yingst, S.; Hines, J.Z.; Minchella, P.; et al. Detection of B.1.351 SARS-CoV-2 Variant Strain—Zambia, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 280–282. [Google Scholar] [CrossRef]
- Wang, P.; Casner, R.G.; Nair, M.S.; Wang, M.; Yu, J.; Cerutti, G.; Liu, L.; Kwong, P.D.; Huang, Y.; Shapiro, L.; et al. Increased Resistance of SARS-CoV-2 Variant P.1 to Antibody Neutralization. Cell Host Microbe 2021, 29, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.; Cheung, M.-C.; Perera, R.A.P.M.; Ng, K.-C.; Bui, C.; Ho, J.; Ng, M.; Kuok, I.T.; Shih, K.C.; Tsao, S.-W.; et al. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: An analysis in ex-vivo and in-vitro cultures. Lancet Respir. Med. 2020, 8, 687–695. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011, 85, 873–882. [Google Scholar] [CrossRef]
- Upton, J.W.; Chan, F.K. Staying alive: Cell death in antiviral immunity. Mol. Cell 2014, 54, 273–280. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019, 73, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Hur, S. Double-Stranded RNA Sensors and Modulators in Innate Immunity. Annu. Rev. Immunol. 2019, 37, 349–375. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Chen, J.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020, 53, 38–42. [Google Scholar] [CrossRef]
- McBride, R.; van Zyl, M.; Fielding, B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro. Surveill. Bull. Eur. Sur. Les Mal. Transm.=Eur. Commun. Dis. Bull. 2020, 25, 2000180. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H.; Kobayashi, T.; Miyama, T.; Suzuki, A.; Jung, S.M.; Hayashi, K.; Kinoshita, R.; Yang, Y.; Yuan, B.; Akhmetzhanov, A.R.; et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 94, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.; Mantovani, A.J.C.D. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.J.N. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.; Cyranoski, D.; Mallapaty, S.J.N. A leading coronavirus vaccine trial is on hold: Scientists react. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Negru, P.A.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Abdel-Daim, M.M.; Nechifor, A.C.; Endres, L.; Stoicescu, M.; Pasca, B.; Tit, D.M.; et al. Therapeutic dilemmas in addressing SARS-CoV-2 infection: Favipiravir versus Remdesivir. Biomed. Pharmacother. 2022, 147, 112700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S. Remdesivir for the treatment of COVID-19. N. Engl. J. Med. 2020, 383, 1813–1836. [Google Scholar] [CrossRef] [PubMed]
- Olender, S.A.; Perez, K.K.; Go, A.S.; Balani, B.; Price-Haywood, E.G.; Shah, N.S.; Wang, S.; Walunas, T.L.; Swaminathan, S.; Slim, J.J.C.I.D. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin. Infect. Dis. 2021, 73, e4166–e4174. [Google Scholar] [CrossRef] [PubMed]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate use of remdesivir for patients with severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Negrut, N.; Codrean, A.; Hodisan, I.; Bungau, S.; Tit, D.M.; Marin, R.; Behl, T.; Banica, F.; Diaconu, C.C.; Nistor-Cseppento, D.C. Efficiency of antiviral treatment in COVID-19. Exp. Ther. Med. 2021, 21, 648. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Joseph, B.A.; Dibas, M.; Evanson, K.W.; Paranjape, G.; Vegivinti, C.T.R.; Selvan, P.T.; Saravu, K.; Gupta, N.; Pulakurthi, Y.S.; Keesari, P.R. Efficacy and safety of lopinavir/ritonavir in the treatment of COVID-19: A systematic review. Expert Rev. Anti-Infect. Ther. 2021, 19, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Depcinski, S.; Sharma, M. Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs. Clin. Infect. Dis. 2023, 76, 165–171. [Google Scholar] [CrossRef]
- Saggam, A.; Limgaokar, K.; Borse, S.; Chavan-Gautam, P.; Dixit, S.; Tillu, G.; Patwardhan, B. Withania somnifera (L.) Dunal: Opportunity for Clinical Repurposing in COVID-19 Management. Front. Pharm. 2021, 12, 835. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, S.; Tagde, P.; Bhattacharya, T.; Monzur, S.M.; Rahman, M.H.; Otrisal, P.; Behl, T.; ul Hassan, S.S.; Abdel-Daim, M.M.; et al. Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2. Biomedicines 2021, 9, 1266. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef]
- Mukherjee, T.; Behl, T.; Sharma, S.; Sehgal, A.; Singh, S.; Sharma, N.; Mathew, B.; Kaur, J.; Kaur, R.; Das, M.; et al. Anticipated pharmacological role of Aviptadil on COVID-19. Environ. Sci. Pollut. Res. 2022, 29, 8109–8125. [Google Scholar] [CrossRef]
- Pastick, K.A.; Okafor, E.C.; Wang, F.; Lofgren, S.M.; Skipper, C.P.; Nicol, M.R.; Pullen, M.F.; Rajasingham, R.; McDonald, E.G.; Lee, T.C. Hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19). In Proceedings of the Open Forum Infectious Diseases; Oxford University Press: Oxford, MS, USA, 2020; p. ofaa130. [Google Scholar]
- Rosenberg, E.S.; Dufort, E.M.; Udo, T.; Wilberschied, L.A.; Kumar, J.; Tesoriero, J.; Weinberg, P.; Kirkwood, J.; Muse, A.; DeHovitz, J.J.J. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. Jama 2020, 323, 2493–2502. [Google Scholar] [CrossRef]
- Magagnoli, J.; Narendran, S.; Pereira, F.; Cummings, T.H.; Hardin, J.W.; Sutton, S.S.; Ambati, J.J.M. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med 2020, 1, 114–127.e113. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T. Hydroxychloroquine with or without azithromycin in mild-to-moderate COVID-19. N. Engl. J. Med. 2020, 383, 2041–2052. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Rane, M.; Solano, J.; Alter, S.; Johnson, H.; Krishnaswamy, S.; Shih, R.; Maki, D.; DeMets, D.L. Updates on Hydroxychloroquine in Prevention and Treatment of COVID-19. Am. J. Med. 2022, 135, 7–9. [Google Scholar] [CrossRef]
- Mirjalili, M.H.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazón, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef]
- Mishra, L.-C.; Singh, B.B.; Dagenais, S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): A review. Altern. Med. Rev. 2000, 5, 334–346. [Google Scholar] [PubMed]
- Singh, N. Stress disease and herbal medicine. J. Biotech. Med. Plant Res. 1993, 3, 2–4. [Google Scholar]
- Nasimi Doost Azgomi, R.; Zomorrodi, A.; Nazemyieh, H.; Fazljou, S.M.B.; Sadeghi Bazargani, H.; Nejatbakhsh, F.; Moini Jazani, A.; Ahmadi AsrBadr, Y. Effects of Withania somnifera on Reproductive System: A Systematic Review of the Available Evidence. BioMed Res. Int. 2018, 2018, 4076430. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.J.; MuzamilAhmad. Neurodegenerative diseases and Withania somnifera (L.): An update. J. Ethnopharmacol. 2020, 256, 112769. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.; Arya, D.S.; Dinda, A.; Talwar, K.K.; Joshi, S.; Gupta, S.K. Mechanisms of Cardioprotective Effect of Withania somnifera in Experimentally Induced Myocardial Infarction. Basic Clin. Pharm. Toxicol. 2004, 94, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Afewerky, H.K.; Ayodeji, A.E.; Tiamiyu, B.B.; Orege, J.I.; Okeke, E.S.; Oyejobi, A.O.; Bate, P.N.N.; Adeyemi, S.B. Critical review of the Withania somnifera (L.) Dunal: Ethnobotany, pharmacological efficacy, and commercialization significance in Africa. Bull. Natl. Res. Cent. 2021, 45, 176. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, R.; Garg, N. Withania somnifera—A magic plant targeting multiple pathways in cancer related inflammation. Phytomedicine 2022, 101, 154137. [Google Scholar] [CrossRef]
- Khan, A.M.; Ahmed, S.R.; Chandra, N.; Arora, K.V.; Ali, A. In vivo, Extract from Withania somnifera Root Ameliorates Arthritis via Regulation of Key Immune Mediators of Inflammation in Experimental Model of Arthritis. Antiinflamm. Antiallergy Agents Med. Chem. 2019, 18, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.K.; Dhasmana, A.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. Withania somnifera as a potential future drug molecule for COVID-19. Future Drug Discov. 2020, 2, FDD50. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, K. An insight to curative effects of Ashwagandha (Withania somnifera), an Ayurveda herb. J. Med. Plants 2020, 8, 227–235. [Google Scholar]
- Singh, P.A.; Bajwa, N.; Baldi, A. Possible role of traditional systems of medicine to manage COVID-19: A review. Isr J. Plant Sci. 2021, 68, 3–28. [Google Scholar] [CrossRef]
- Chopra, A.; Srikanth, N.; Patwardhan, B. Withania somnifera as a safer option to hydroxychloroquine in the chemoprophylaxis of COVID-19: Results of interim analysis. Complement. Ther. Med. 2021, 62, 102768. [Google Scholar] [CrossRef]
- Tharakan, A.; Shukla, H.; Benny, I.R.; Tharakan, M.; George, L.; Koshy, S. Immunomodulatory Effect of Withania somnifera (Ashwagandha) Extract-A Randomized, Double-Blind, Placebo Controlled Trial with an Open Label Extension on Healthy Participants. J. Clin. Med. 2021, 10, 3644. [Google Scholar] [CrossRef]
- Parwe, S.D.; Nisargandha, M.A.; Morey, D.T. Role of Ashwagandha (Withania somnifera) as Immunomodulator in Coronavirus in a pandemic—A systemic review. Int. J. Res. Pharm. Sci. 2020, 11, 1649–1654. [Google Scholar] [CrossRef]
- Kanyaiya, M.; Digambar, S.P.; Arora, S.; Kapila, S.; Singh, R.R.B. In vivo, effect of herb (Withania somnifera) on immunomodulatory and antioxidative potential of milk in mice. Food Agric. Immunol. 2014, 25, 443–452. [Google Scholar] [CrossRef]
- Mikolai, J.; Erlandsen, A.; Murison, A.; Brown, K.A.; Gregory, W.L.; Raman-Caplan, P.; Zwickey, H.L. In vivo effects of Ashwagandha (Withania somnifera) extract on the activation of lymphocytes. J. Altern. Complement. Med. 2009, 15, 423–430. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Malik, F.; Singh, J.; Khajuria, A.; Suri, K.A.; Satti, N.K.; Singh, S.; Kaul, M.K.; Kumar, A.; Bhatia, A.; Qazi, G.N. A standardized root extract of Withania somnifera and its major constituent withanolide-A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci. 2007, 80, 1525–1538. [Google Scholar] [CrossRef]
- Malik, F.; Kumar, A.; Bhushan, S.; Mondhe, D.M.; Pal, H.C.; Sharma, R.; Khajuria, A.; Singh, S.; Singh, G.; Saxena, A.K.; et al. Immune modulation and apoptosis induction: Two sides of antitumoural activity of a standardised herbal formulation of Withania somnifera. Eur. J. Cancer 2009, 45, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Alharbi, R. Structure insights of SARS-CoV-2 open state envelope protein and inhibiting through active phytochemical of ayurvedic medicinal plants from Withania somnifera. Saudi J. Biol. Sci. 2021, 28, 3594–3601. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Kim, S.J. Production of reactive oxygen species by withaferin A causes loss of type collagen expression and COX-2 expression through the PI3K/Akt, p38, and JNK pathways in rabbit articular chondrocytes. Exp. Cell Res. 2013, 319, 2822–2834. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chakraborty, M.; Chandra, A.; Alam, M.P. Structure-activity relationship (SAR) and molecular dynamics study of withaferin-A fragment derivatives as potential therapeutic lead against main protease (Mpro) of SARS-CoV-2. J. Mol. Model. 2021, 27, 97. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Parmar, M.; Sharun, K.; Tiwari, R.; Bilal, M.; Dhama, K. Medicinal and therapeutic potential of withanolides from Withania somnifera against COVID-19. J. Appl. Pharm. Sci. 2021, 11, 6–13. [Google Scholar] [CrossRef]
- Matsuda, H.; Murakami, T.; Kishi, A.; Yoshikawa, M. Structures of withanosides I, II, III, IV, V, VI, and VII, new withanolide glycosides, from the roots of Indian Withania somnifera DUNAL. and inhibitory activity for tachyphylaxis to clonidine in isolated guinea-pig ileum. Bioorg. Med. Chem. 2001, 9, 1499–1507. [Google Scholar] [CrossRef]
- Maurya, D.K.; Sharma, D. Evaluation of Traditional Ayurvedic Preparation for Prevention and Management of the Novel Coronavirus (SARS-CoV-2) Using Molecular Docking Approach. Available online: http://doi.org/10.26434/chemrxiv.12110214.v1 (accessed on 15 May 2022).
- Chakraborty, S.; Mallick, D.; Goswami, M.; Guengerich, F.P.; Chakrabarty, A.; Chowdhury, G. The Natural Products Withaferin A and Withanone from the Medicinal Herb Withania somnifera Are Covalent Inhibitors of the SARS-CoV-2 Main Protease. J. Nat. Prod. 2022, 85, 2340–2350. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanjal, J.K.; Bhargava, P.; Kaul, A.; Wang, J.; Zhang, H.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J. Biomol. Struct. Dyn. 2020, 40, 1–13. [Google Scholar] [CrossRef]
- Sudeep, H.; Gouthamchandra, K.; Shyamprasad, K. Molecular docking analysis of Withaferin A from Withania somnifera with the Glucose regulated protein 78 (GRP78) receptor and the SARS-CoV-2 main protease. Bioinformation 2020, 16, 411. [Google Scholar] [CrossRef]
- Srivastava, A.; Siddiqui, S.; Ahmad, R.; Mehrotra, S.; Ahmad, B.; Srivastava, A.N. Exploring nature’s bounty: Identification of Withania somnifera as a promising source of therapeutic agents against COVID-19 by virtual screening and in silico evaluation. J. Biomol. Struct. Dyn. 2020, 40, 1858–1908. [Google Scholar] [CrossRef] [PubMed]
- Parida, P.K.; Paul, D.; Chakravorty, D. The natural way forward: Molecular dynamics simulation analysis of phytochemicals from Indian medicinal plants as potential inhibitors of SARS-CoV-2 targets. Phytother. Res. 2020, 34, 3420–3433. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Hupparage, V.B.; Malgi, A.P.; Deshpande, S.H.; Patil, S.A.; Mallapur, S.P. Dual inhibition of COVID-19 spike glycoprotein and main protease 3CLpro by Withanone from Withania somnifera. Chin. Herb. Med. 2021, 13, 359–369. [Google Scholar] [CrossRef]
- Dhanjal, J.K.; Kumar, V.; Garg, S.; Subramani, C.; Agarwal, S.; Wang, J.; Zhang, H.; Kaul, A.; Kalra, R.S.; Kaul, S.C. Molecular mechanism of anti-SARS-CoV2 activity of Ashwagandha-derived withanolides. Int. J. Biol. Macromol. 2021, 184, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dhanjal, J.K.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (M(pro)) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2021, 39, 3842–3854. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Pokhrel, S.; Singh, H.; Joshi, M.; Mulay, V.P.; Haldar, S.; Varshney, A. Withanone from Withania somnifera Attenuates SARS-CoV-2 RBD and Host ACE2 Interactions to Rescue Spike Protein Induced Pathologies in Humanized Zebrafish Model. Drug Des. Dev. 2021, 15, 1111–1133. [Google Scholar] [CrossRef]
- Vuai, S.A.; Onoka, I.; Sahini, M.G.; Swai, H.S.; Shadrack, D.M. Abrogating the nsp10–nsp16 switching mechanisms in SARS-CoV-2 by phytochemicals from Withania somnifera: A molecular dynamics study. Mol. Simul. 2021, 47, 1372–1380. [Google Scholar] [CrossRef]
- Shree, P.; Mishra, P.; Selvaraj, C.; Singh, S.K.; Chaube, R.; Garg, N.; Tripathi, Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants—Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)—A molecular docking study. J. Biomol. Struct. Dyn. 2022, 40, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Singh, P.; Sharma, S.; Singh, T.P.; Ethayathulla, A.S.; Kaur, P. Identification of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor. J. Biomol. Struct. Dyn. 2021, 39, 5668–5681. [Google Scholar] [CrossRef] [PubMed]
- Chikhale, R.V.; Gurav, S.S.; Patil, R.B.; Sinha, S.K.; Prasad, S.K.; Shakya, A.; Shrivastava, S.K.; Gurav, N.S.; Prasad, R.S. SARS-CoV-2 host entry and replication inhibitors from Indian ginseng: An in-silico approach. J. Biomol. Struct. Dyn. 2021, 39, 4510–4521. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Kakar, S.S.; Parte, S.; Carter, K.; Joshua, I.G.; Worth, C.; Rameshwar, P.; Ratajczak, M.Z. Withaferin A (WFA) inhibits tumor growth and metastasis by targeting ovarian cancer stem cells. Oncotarget 2017, 8, 74494–74505. [Google Scholar] [CrossRef]
- Straughn, A.R.; Kakar, S.S. Withaferin A: A potential therapeutic agent against COVID-19 infection. J. Ovarian Res. 2020, 13, 79. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elshahat, M.E.; Elfiky, A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J. Infect. 2020, 80, 554–562. [Google Scholar] [CrossRef]
- Pillon, M.C.; Frazier, M.N.; Dillard, L.B.; Williams, J.G.; Kocaman, S.; Krahn, J.M.; Perera, L.; Hayne, C.K.; Gordon, J.; Stewart, Z.D.; et al. Cryo-EM structures of the SARS-CoV-2 endoribonuclease Nsp15 reveal insight into nuclease specificity and dynamics. Nat. Commun. 2021, 12, 636. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Prabakaran, P.; Gan, J.; Feng, Y.; Zhu, Z.; Choudhry, V.; Xiao, X.; Ji, X.; Dimitrov, D.S. Structure of severe acute respiratory syndrome coronavirus receptor-binding domain complexed with neutralizing antibody. J. Biol. Chem. 2006, 281, 15829–15836. [Google Scholar] [CrossRef]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, A.S. Stress induction of GRP78/BiP and its role in cancer. Curr. Mol. Med. 2006, 6, 45–54. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.; Tan, J.; Chen, J.; Du, L.; Sun, T.; Shen, J.; Chen, K.; Jiang, H.; Shen, X. Severe acute respiratory syndrome coronavirus 3C-like proteinase N terminus is indispensable for proteolytic activity but not for enzyme dimerization: Biochemical and thermodynamic investigation in conjunction with molecular dynamics simulations. J. Biol. Chem. 2005, 280, 164–173. [Google Scholar] [CrossRef]
- Yang, H.; Yang, M.; Ding, Y.; Liu, Y.; Lou, Z.; Zhou, Z.; Sun, L.; Mo, L.; Ye, S.; Pang, H. The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc. Natl. Acad. Sci. USA 2003, 100, 13190–13195. [Google Scholar] [CrossRef]
- Xue, X.; Yang, H.; Shen, W.; Zhao, Q.; Li, J.; Yang, K.; Chen, C.; Jin, Y.; Bartlam, M.; Rao, Z. Production of authentic SARS-CoV Mpro with enhanced activity: Application as a novel tag-cleavage endopeptidase for protein overproduction. J. Mol. Biol. 2007, 366, 965–975. [Google Scholar] [CrossRef]
- Khanal, P.; Chikhale, R.; Dey, Y.N.; Pasha, I.; Chand, S.; Gurav, N.; Ayyanar, M.; Patil, B.; Gurav, S. Withanolides from Withania somnifera as an immunity booster and their therapeutic options against COVID-19. J. Biomol. Struct. Dyn. 2022, 40, 5295–5308. [Google Scholar] [CrossRef]
- Verma, S.; Patel, C.N.; Chandra, M. Identification of novel inhibitors of SARS-CoV-2 main protease (Mpro) from Withania sp. by molecular docking and molecular dynamics simulation. J. Comput. Chem. 2021, 42, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Shweta, P. Anti-viral activity of Withania somnifera phytoconstituents against corona virus (SARS-CoV-2). J. Pharm. Drug Res. 2022, 3, 22–26. [Google Scholar] [CrossRef]
- Kalra, R.S.; Kumar, V.; Dhanjal, J.K.; Garg, S.; Li, X.; Kaul, S.C.; Sundar, D.; Wadhwa, R. COVID19-inhibitory activity of withanolides involves targeting of the host cell surface receptor ACE2: Insights from computational and biochemical assays. J. Biomol. Struct. Dyn. 2022, 40, 7885–7898. [Google Scholar] [CrossRef]
- Velmurugan, R.; Kumar, P.M.; Keerthi, G.S.N. Nebulizer, Inhaled Remdesivir Nanoparticle Co-administered with Withania somnifera may Minimize the Hepatotoxicity in COVID-19. Mater. Highlights 2021, 2, 68–70. [Google Scholar] [CrossRef]
- Chinembiri, T.N.; Gerber, M.; Du Plessis, L.H.; Du Preez, J.L.; Hamman, J.H.; Du Plessis, J. Topical delivery of Withania somnifera crude extracts in niosomes and solid lipid nanoparticles. Pharm. Mag. 2017, 13, S663. [Google Scholar]
- Tiwari, D.K.; Mishra, K.; Mishra, N.; Upmanyu, N. Formulation and optimization of water soluble granules of Withania somnifera. J. Drug Deliv. Ther. 2021, 11, 26–30. [Google Scholar] [CrossRef]
- Joon, M.; Garg, M. Formulation and evaluation of standardised Withania somnifera leaf extract loaded transdermal gel. J. Med. Sci. 2013, 13, 814. [Google Scholar] [CrossRef]
- Marslin, G.; Franklin, G.; Sarmento, B.; Dias, A. Withania somnifera leaf extract delivery as a nanoparticle protect the glioma cells from oxidative damage. Planta Med. 2015, 81, PW_131. [Google Scholar] [CrossRef]
- Ramli, S.; Sim, M.S.; Guad, R.M.; Gopinath, S.C.B.; Subramaniyan, V.; Fuloria, S.; Fuloria, N.K.; Choy, K.W.; Rana, S.; Wu, Y.S. Long Noncoding RNA UCA1 in Gastrointestinal Cancers: Molecular Regulatory Roles and Patterns, Mechanisms, and Interactions. J. Oncol. 2021, 2021, 5519720. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Z.U.; Sim, S.M.; Guad, M.R.; Subramaniyan, V.; Sekar, M.; Fuloria, K.N.; Fuloria, S.; Choy, W.K.; Fareez, M.I.; Bonam, R.S.; et al. Molecular Regulatory Roles of Long Non-coding RNA HOTTIP: An Overview in Gastrointestinal Cancers. Curr. Mol. Med. 2022, 22, 478–490. [Google Scholar] [CrossRef]

| Antiviral | Mechanism of Action | Adverse Effects/Issue | Reference |
|---|---|---|---|
| Remdesivir |
|
| [51,52,53,54] |
| Remdesivir |
|
| [50,51,52,53,54] |
| Lopinavir/Ritonavir (LPV/r) |
| Does not improve clinical outcome, mortality, time to RT-PCR negativity, or chest CT clearance | [56,57] |
| Molnupiravir |
|
| [58] |
| Nirmatrelvir/Ritonavir |
|
| [58] |
| Hydroxychloroquine |
|
| [63,64,65,66] |
| Phytochemical | Source | Molecular Mechanism | Reference |
|---|---|---|---|
| Withaferin A | Not Available | Inhibited SARS-CoV-2 Mpro, thus preventing SARS-CoV-2 replication | [90] |
| Not Available | Inhibited SARS-CoV-2 Mpro and structural proteins, including S, E, and N, thus impeding viral replication and entry into host cells | [93] | |
| Purchased from Sigma-Aldrich (now Merck, St. Louis, MO, USA) | Inhibited SARS-CoV-2 Mpro, thus disrupting SARS-CoV-2 replication | [94] | |
| Not Available | Inhibited TMPRSS2 receptor, thereby preventing viral entry into host cells | [95] | |
| Not Available | Inhibited SARS-CoV-2 Mpro and GRP78, thus impeding viral replication and SARS-CoV-2 recognition by host cells | [96] | |
| Withanolide A | Leaves, stems, roots, and flowers extract | Inhibited SARS-CoV and SARS-CoV-2 S protein, SARS-CoV 3CL-pro main protease, and SARS-CoV-2 NSP10/NSP-16 complex, thus decreasing viral recognition and replication in host cells | [97] |
| Withanolide B | Not Available | Inhibited SARS-CoV-2 Mpro and structural proteins, including S, E, and N. | [93] |
| Withanolide R | Not Available | Inhibited main protease (NSP5) of SARS-CoV-2, consequently reducing the survival of SARS-CoV-2 | [98] |
| 2,3-Dihydrowithaferin A | Not Available | Inhibited SARS-CoV-2 S protein and acted as the most potent S protein inhibitor as compared with the other WS phytochemicals, preventing viral entry into host cells | [98] |
| Withanone | Not Available | Inhibited TMPRSS2 receptor and downregulated TMPRSS2 mRNA, thereby impeding SARS-CoV-2 entry into host cells | [95] |
| Not Available | Inhibited SARS-CoV-2 main protease 3CL-pro and S protein, thus decreasing viral entry and replication in host cells | [99] | |
| Leaves and stem extract | Inhibited SARS-CoV-2 TMPRSS2 and Mpro, thus prohibiting SARS-CoV-2 entry and replication in host cells | [100] | |
| Not Available | Inhibited SARS-CoV-2 Mpro, thus halting SARS-CoV-2 replication in host cells | [101] | |
| Root extract | Inhibited viral entry by attenuating the interaction between the host ACE2 receptor and RBD located in the SARS-CoV-2 S-protein | [102] | |
| Purchased from Sigma-Aldrich (now Merck, St. Louis, MO, USA) (>98% purity) | Inhibited SARS-CoV-2 Mpro, thus disrupting SARS-CoV-2 replication | [94] | |
| Withanoside IV | Root extract | Abrogated NSP10-NSP16 switching mechanisms in SARS-CoV-2, thus promoting fatality of SARS-CoV-2 | [103] |
| Withanoside V | Not Available | Inhibited SARS-CoV-2 Mpro, thus attenuating SARS-CoV-2 replication in host cell | [104] |
| Not Available | Inhibited SARS-CoV-2 Mpro, thus attenuating SARS-CoV-2 replication in host cells | [105] | |
| Withanoside X | Not Available | Inhibited SARS-CoV-2 NSP15 endoribonuclease and SARS-CoV-2 S protein, thus preventing SARS-CoV-2 entry and replications in host cells | [106] |
| Quercetin glucoside | Not Available | Inhibited SARS-CoV-2 NSP15 endoribonuclease and SARS-CoV-2 S proteins, thus reducing SARS-CoV-2 entry and replication in host cells | [106] |
| Somniferin | Not Available | Inhibited SARS-CoV-2 Mpro, thus attenuating SARS-CoV-2 replication in host cells | [104] |
| Phytochemical | Target Protein | PDB ID | Binding Energy (Kcal/mol) |
|---|---|---|---|
| Sitoindoside IX | Mpro | 6LU7 | −8.37 |
| Withaferin A | ACE2-RBD | 6M17 | −9.1 |
| Withaferin A | GRP78 | 5E84 | −8.7 |
| Withaferin A | Mpro | 6LU7 | −9.83 |
| Withanolide A | ACE2-RBD | 6M17 | −9.6 |
| Withanolide B | ACE2-RBD | 6M17 | −9.4 |
| Withanone | ACE2-RBD | 6M17 | −9.4 |
| Withanoside II | Mpro | 6LU7 | −11.3 |
| Withanoside IV | Mpro | 6LU7 | −11.02 |
| Withanoside V | Mpro | 6LU7 | −8.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramli, S.; Wu, Y.S.; Batumalaie, K.; Guad, R.M.; Choy, K.W.; Kumar, A.; Gopinath, S.C.B.; Rahman Sarker, M.M.; Subramaniyan, V.; Sekar, M.; et al. Phytochemicals of Withania somnifera as a Future Promising Drug against SARS-CoV-2: Pharmacological Role, Molecular Mechanism, Molecular Docking Evaluation, and Efficient Delivery. Microorganisms 2023, 11, 1000. https://doi.org/10.3390/microorganisms11041000
Ramli S, Wu YS, Batumalaie K, Guad RM, Choy KW, Kumar A, Gopinath SCB, Rahman Sarker MM, Subramaniyan V, Sekar M, et al. Phytochemicals of Withania somnifera as a Future Promising Drug against SARS-CoV-2: Pharmacological Role, Molecular Mechanism, Molecular Docking Evaluation, and Efficient Delivery. Microorganisms. 2023; 11(4):1000. https://doi.org/10.3390/microorganisms11041000
Chicago/Turabian StyleRamli, Suaidah, Yuan Seng Wu, Kalaivani Batumalaie, Rhanye Mac Guad, Ker Woon Choy, Ashok Kumar, Subash C. B. Gopinath, Md. Moklesur Rahman Sarker, Vetriselvan Subramaniyan, Mahendran Sekar, and et al. 2023. "Phytochemicals of Withania somnifera as a Future Promising Drug against SARS-CoV-2: Pharmacological Role, Molecular Mechanism, Molecular Docking Evaluation, and Efficient Delivery" Microorganisms 11, no. 4: 1000. https://doi.org/10.3390/microorganisms11041000
APA StyleRamli, S., Wu, Y. S., Batumalaie, K., Guad, R. M., Choy, K. W., Kumar, A., Gopinath, S. C. B., Rahman Sarker, M. M., Subramaniyan, V., Sekar, M., Fuloria, N. K., Fuloria, S., Chinni, S. V., & Ramachawolran, G. (2023). Phytochemicals of Withania somnifera as a Future Promising Drug against SARS-CoV-2: Pharmacological Role, Molecular Mechanism, Molecular Docking Evaluation, and Efficient Delivery. Microorganisms, 11(4), 1000. https://doi.org/10.3390/microorganisms11041000











