TNFR1 Mediated Apoptosis Is Protective against Mycobacterium avium in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria
2.2. Mice
2.3. Animal Model of M. avium Infection
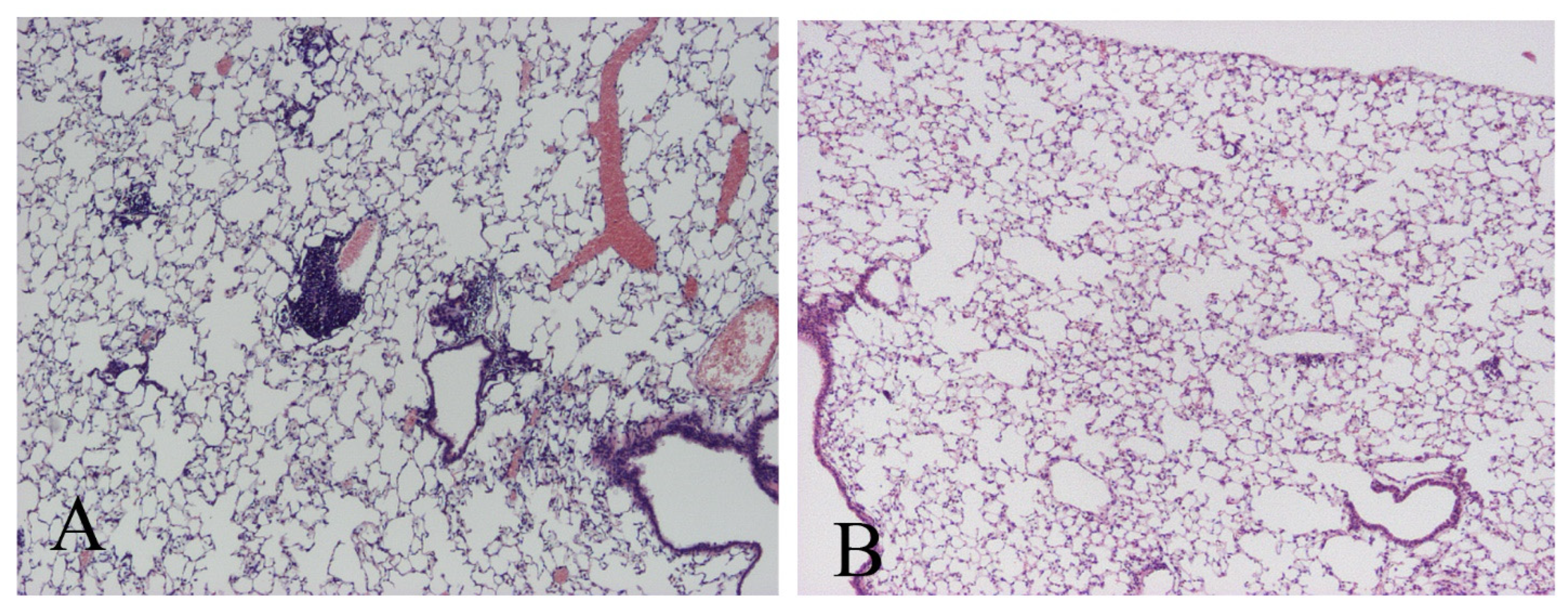
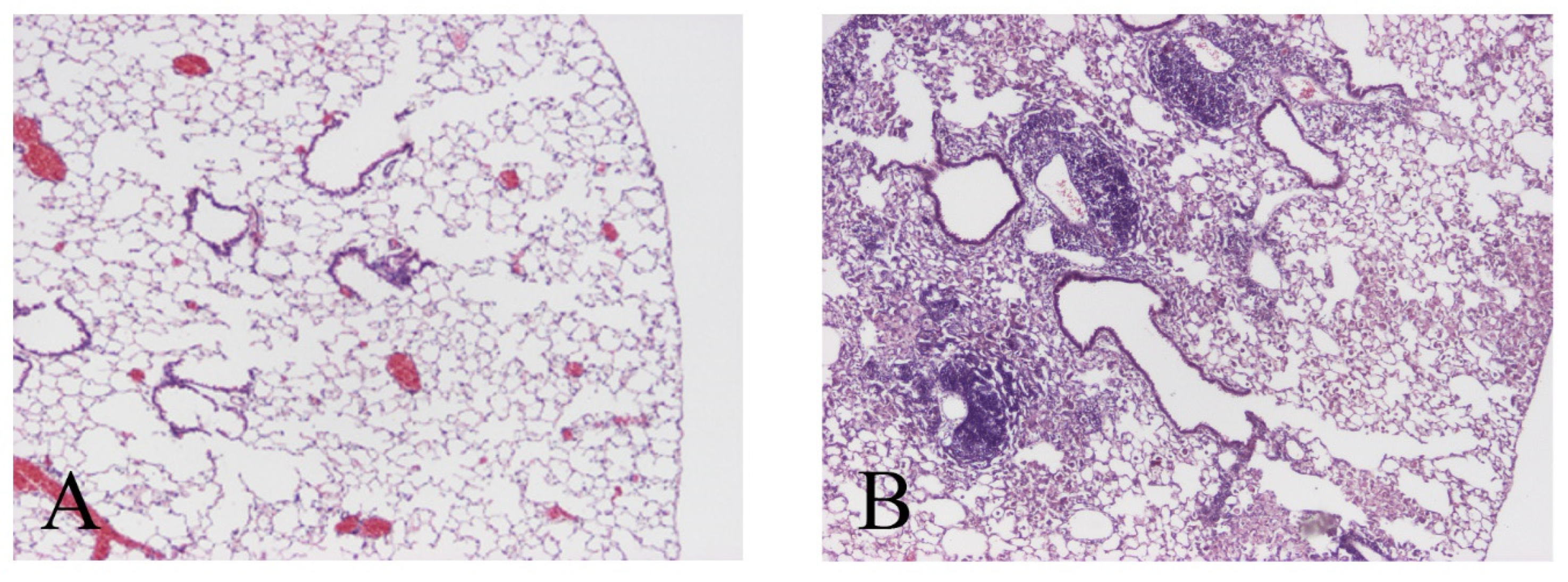
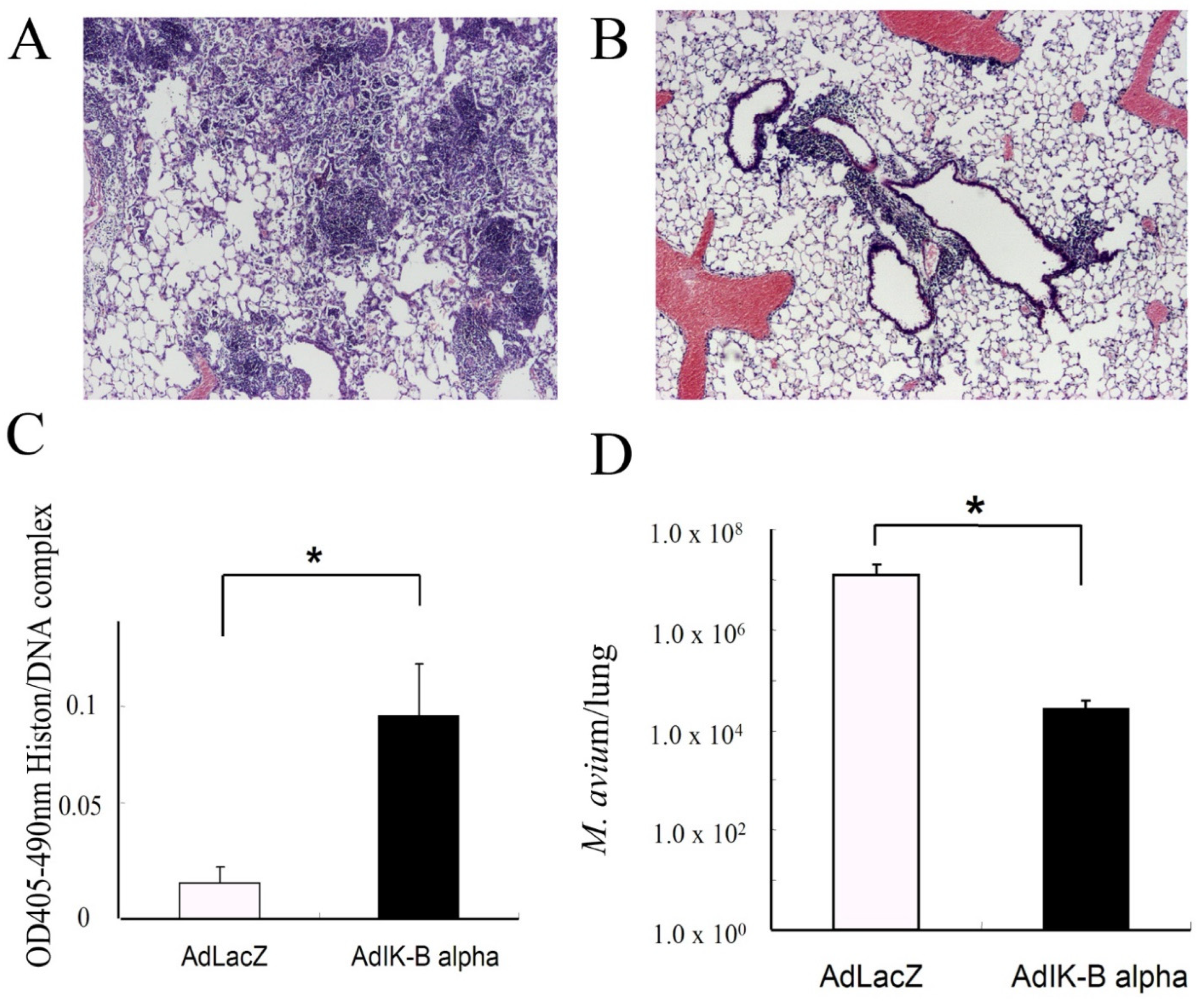
2.4. Lung Histology and Morphometry
2.5. Bronchoalveolar Lavage (BAL)
2.6. Macrophage Isolation and Infection by M. avium
2.7. Western Blotting
2.8. Apoptosis Detection
2.9. Statistical Analysis
3. Results
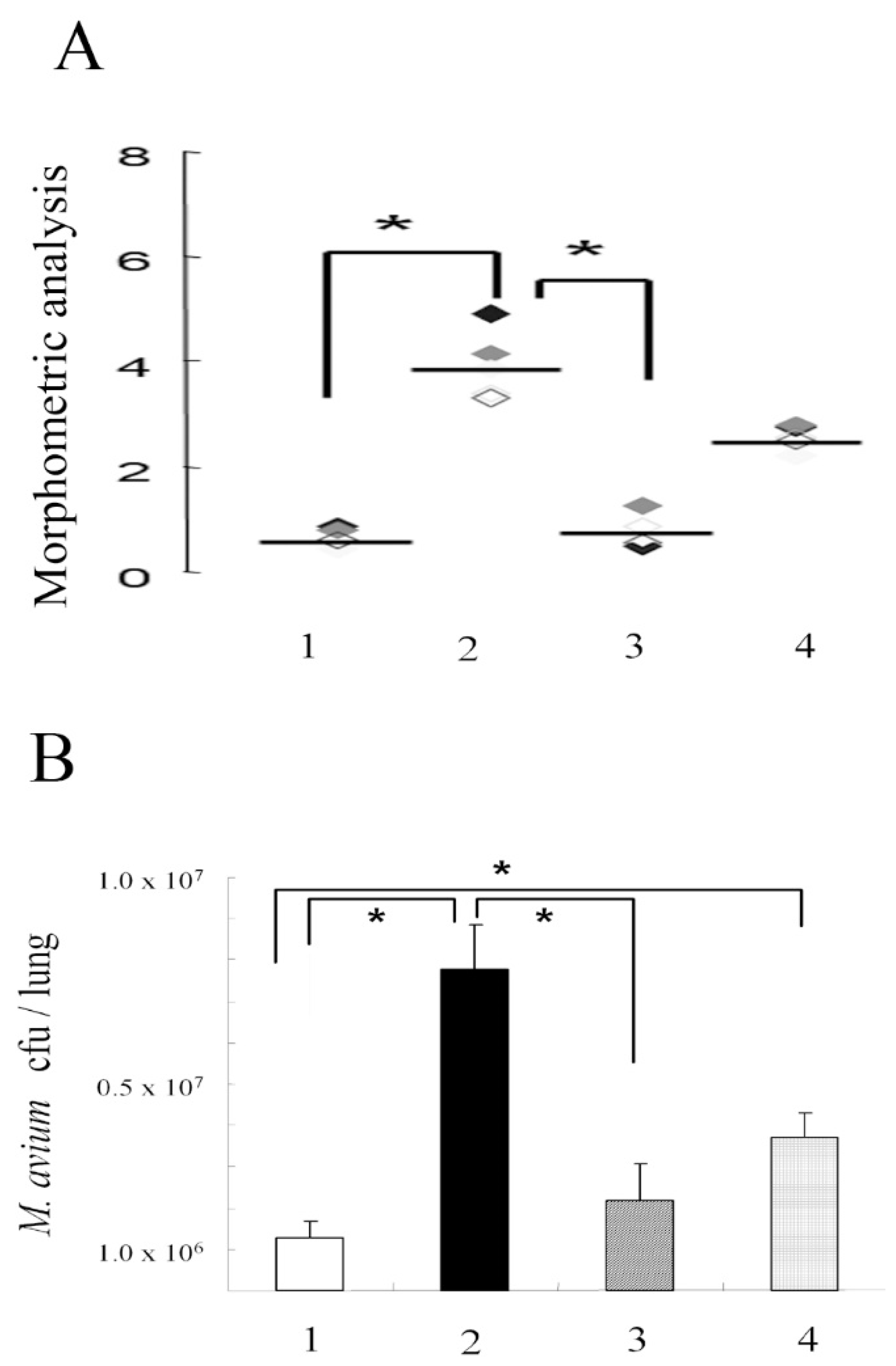
3.1. M. avium Inoculation
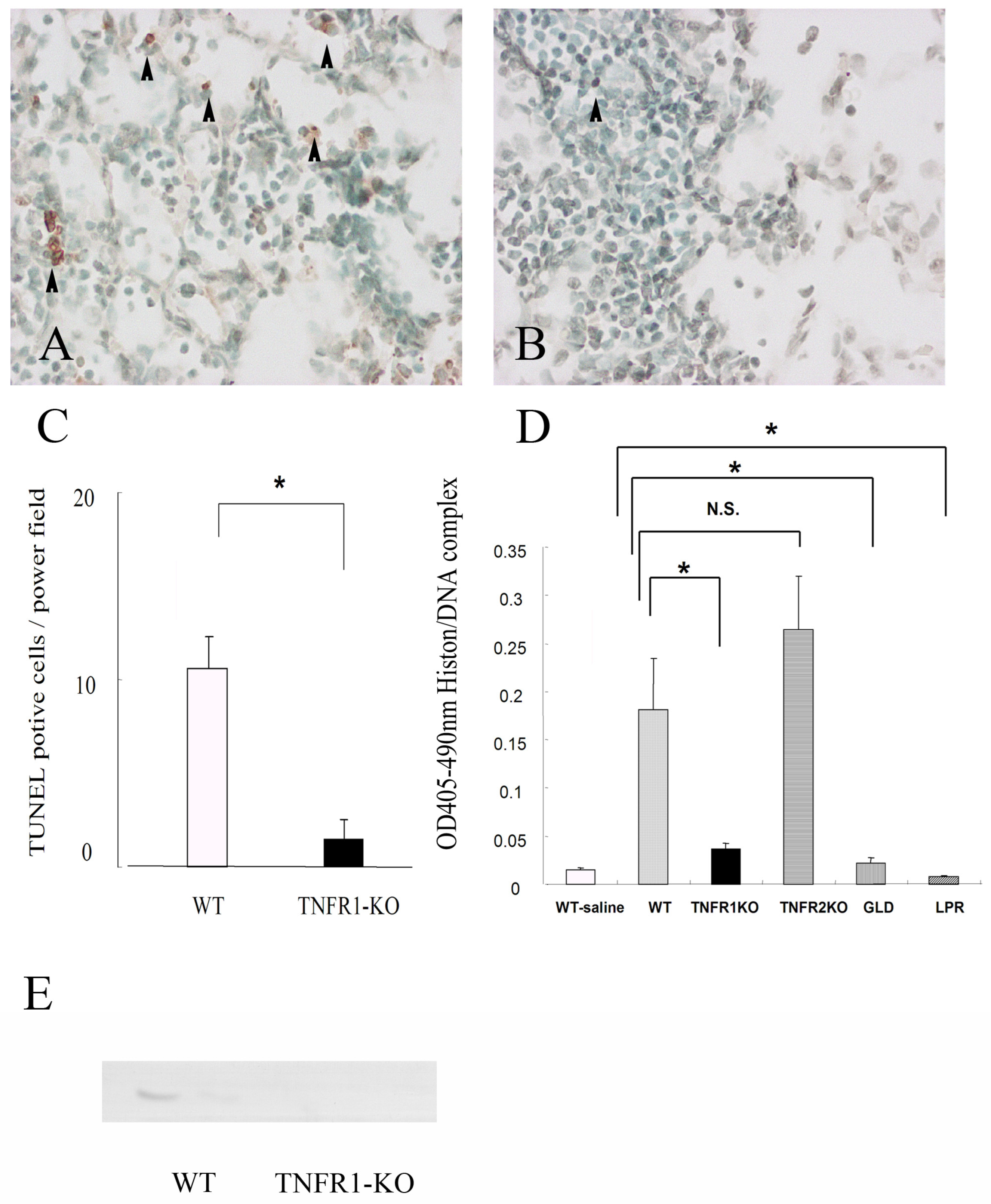
3.2. Apoptosis in the Lungs of Mice Infected with M. avium
3.3. Apoptosis Modification
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.; Andrejak, C.; Erik C Böttger, E.C.; Brozek., J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin. Infect. Dis. 2020, 71, 905–913. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [PubMed]
- Wallace, R.J., Jr.; Brown, B.A.; Griffith, D.E.; Girard, W.M.; Murphy, D.T. Clarithromycin regimens for pulmonary Mycobacterium avium complex: The first 50 patients. Am. J. Respir. Crit. Care Med. 1996, 153, 1766–1772. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown, B.A.; Girard, W.M.; Griffith, B.E.; Couch, L.A.; Wallace, R.J., Jr. Azithromycin-containing regimens for treatment of Mycobacterium avium complex lung disease. Clin. Infect. Dis. 2001, 32, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Kimoto, T.; Tsuyuguchi, K.; Watanabe, I.; Matsumoto, H.; Niimi, A.; Suzuki, K.; Murayama, T.; Amitani, R.; Kuze, F. Effect of clarithromycin regimen for Mycobacteriuim avium complex pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160, 866–872. [Google Scholar] [CrossRef]
- Corpe, R.F. Surgical management of pulmonary disease due to Mycobacterium avium-intracellulare. Rev. Infect. Dis. 1981, 3, 1064–1067. [Google Scholar] [CrossRef]
- Moran, J.F.; Alexander, L.G.; Stauh, E.W.; Young, W.G.; Sealy, W.C. Long-term results of pulmonary resection for atypical mycobacterial disease. Am. Thorac. Surg. 1983, 35, 597–604. [Google Scholar] [CrossRef]
- Hehlgans, T.; Mannel, D.N. The TNF-TNF receptor system. Biol. Chem. 2002, 383, 1581–1585. [Google Scholar] [CrossRef]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Bazzoni, F.; Beutler, B. How do tumor necrosis factor receptors work? J. Inflamm. 1995, 45, 221–238. [Google Scholar]
- Black, C.M.; Bermudez, L.E.; Young, L.S.; Remington, J.S. Co-infection of macrophages modulates interferon gamma and tumor necrosis factor-induced activation against intracellular pathogens. J. Exp. Med. 1990, 172, 977–980. [Google Scholar] [CrossRef]
- Bermudez, L.E.; Stevens, P.; Kolonoski, P.; Wu, M.; Young, L.S. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant IL-2 and tumor necrosis factor. J. Immunol. 1989, 143, 2996–3000. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, K.; Matsuyama, T.; Kundig, T.M.; Wakeham, A.; Kishihara, K.; Shahinian, A.; Wiegmann, K.; Ohashi, P.S.; Kronke, M.; Mak, T.W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock; yet succumb to L. monocytogenes infection. Cell 1993, 73, 457–467. [Google Scholar] [CrossRef]
- Rothe, J.; Lesslauer, W.; Lotscher, H.; Lang, Y.; Koebel, P.; Kontgen, F.; Althage, A.; Zinkernagel, R.; Steinmetz, M.; Bluethmann, H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 1993, 364, 798–802. [Google Scholar] [CrossRef]
- Flórido, M.; Appelberg, R. Characterization of the deregulated immune activation occurring at late stages of mycobacterial infection in TNF-deficient mice. J. Immunol. 2007, 179, 7702–7708. [Google Scholar] [CrossRef]
- Balcewicz-Sablinska, M.K.; Keane, J.; Kornfeld, H.; Remold, H.G. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 1998, 161, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.; Remold, H.G.; Kornfeld, H. Virulent Mycobacterium tuberculosis stains evade apoptosis of infected alveolar macrophages. J. Immunol. 2000, 164, 2016–2020. [Google Scholar] [CrossRef]
- Fujita, M.; Ouchi, H.; Ikegame, S.; Harada, E.; Matsumoto, T.; Uchino, J.; Nakanishi, Y.; Watanabe, K. Critical role of tumor necrosis factor receptor 1 in the pathogenesis of pulmonary emphysema in mice. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1705–1712. [Google Scholar] [CrossRef]
- Ehlers, S.; Benini, J.; Kutsch, S.; Endres, R.; Rietschel, E.T.; Pfeffer, K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect. Immun. 1999, 67, 3571–3579. [Google Scholar] [CrossRef]
- Cooper, A.; D’Souza, C.; Frank, A.; Orme, I. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin or granzyme mediated cytolytic mechanisms. Infect. Immun. 1997, 65, 1317–1320. [Google Scholar] [CrossRef] [PubMed]
- Virginia, E.; Watson; Laurie, L.; Davis, D.W.; McConkey, D.J.; Jagannath, C.; Hunter, R.L., Jr.; Actor, J.K. Apoptosis in Mycobacrerium tuberculosis infection in mice exhibiting varied immunopatthology. J. Pathol. 2000, 190, 211–220. [Google Scholar]
- Fratazzi, C.; Manjunath, N.; Arbeit, R.D.; Carini, C.; Gerken, T.A.; Ardman, B.; Remold-O’Donnell, E.; Remold, H.G. A macrophage invasion mechanism for mycobacteria implicating the extracellular domain of CD43. J. Exp. Med. 2000, 192, 183–192. [Google Scholar] [CrossRef]
- Kagi, D.; Ledermann, B.; Burki, K.; Seiler, P.; Odermatt, B.; Olsen, K.J.; Podack, E.R.; Zinkernagel, R.M.; Hengartner, H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 1994, 369, 31–37. [Google Scholar] [CrossRef]
- Kawano, S.; Kubota, T.; Monden, Y.; Kawamura, N.; Tsutsui, H.; Takeshita, A.; Sunagawa, K. Blockade of NF-kappaB ameliorates myocardial hypertrophy in response to chronic infusion of angiotensin II. Cardiovasc. Res. 2005, 67, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.C.; Liou, H.C.; Tuomanen, E.I.; Baltimore, D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 1995, 80, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Shannon, J.M.; Morikawa, O.; Gauldie, J.; Hara, N.; Mason, R.J. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am. J. Respir. Cell Mol. Biol. 2003, 29, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Harada, E.; Matsumoto, T.; Mizuta, Y.; Ikegame, S.; Ouchi, H.; Inoshima, I.; Yoshida, S.; Watanabe, K.; Nakanishi, Y. Impaired host defence against Mycobacterium avium in mice with chronic granulomatous disease. Clin. Exp. Immunol. 2010, 160, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Ye, Q.; Ouchi, H.; Harada, E.; Inoshima, I.; Kuwano, K.; Nakanishi, Y. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrob. Agents Chemother. 2006, 50, 739–743. [Google Scholar] [CrossRef]
- Fujita, M.; Ye, Q.; Ouchi, H.; Nakashima, N.; Hamada, N.; Hagimoto, N.; Kuwano, K.; Mason, R.J.; Nakanishi, N. Retinoic acid failed to reverse emphysema in adult mouse model. Thorax 2004, 59, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Shannon, J.M.; Irvin, C.G.; Fagan, K.A.; Cool, C.; Augustin, A.; Mason, R.J. Overexpression of tumor necrosis factor-a produces an increase in lung volumes and pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L39–L49. [Google Scholar] [CrossRef]
- Yoshida, S.; Goto, Y.; Mizuguchi, Y.; Nomoto, K.; Skamene, E. Genetic control of natural resistance in mouse macrophages regulating intracellular Legionella pneumophila multiplication in vitro. Infect, Immun. 1991, 59, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Kuwano, K.; Kunitake, R.; Hagimoto, N.; Miyazaki, H.; Kaneko, Y.; Kawasaki, M.; Maeyama, T.; Hara, N. Endothelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Int. Arch. Allergy Immunol. 1998, 117, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, K.; Kunitake, R.; Maeyama, T.; Hagimoto, N.; Kawasaki, M.; Matsuba, T.; Yoshimi, M.; Inoshima, I.; Yoshida, K.; Hara, N. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L316–L325. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.S.; Huang, Y. Death receptor activation complexes: It takes two to activate TNF receptor 1. Cell Cycle 2003, 2, 550–552. [Google Scholar] [CrossRef]
- Ehlers, S.; Kutsch, S.; Ehelers, E.M.; Benini, J.; Pfeffer, K. Lethal granuloma disintegration in mycobacteria-infected TNFp55-/-mice is dependent on T cells and IL-12. J. Immunol. 2000, 165, 483–492. [Google Scholar] [CrossRef]
- Spira, A.; Carroll, J.D.; Liu, G.; Aziz, Z.; Shah, V.; Kornfeld, H.; Keane, J. Apoptosis genes in human alveolar macrophages infected with virulent or attenuated Mycobacrerium tuberculosis: Apivotal role for tumor necrosis factor. Am. J. Respir. Cell Mol. Biol. 2003, 29, 545–551. [Google Scholar] [CrossRef]
- Fratazzi, C.; Arbeit, R.; Carini, C.; Remld, H.G. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added; uninfected macrophages. J. Immunol. 1997, 158, 4320–4327. [Google Scholar] [CrossRef]
- Mizgerd, J.P.; Peschon, J.J.; Doerschuk, C.M. Roles of tumor necrosis factor receptor signaling during murine Escherichia coli pneumonia. Am. J. Respir. Cell Mol. Biol. 2000, 22, 85–91. [Google Scholar] [CrossRef]
- O’Brien, D.P.; Briles, D.E.; Szalai, A.J.; Tu, A.H.; Sanz, I.; Nahm, M.H. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect. Immun. 1999, 67, 595–601. [Google Scholar] [CrossRef]
- Moore, T.A.; Perry, M.L.; Getsoian, A.G.; Monteleon, C.L.; Cogen, A.L.; Standiford, T.J. Increased mortality and dysregulated cytokine production in tumor necrosis factor receptor 1-deficient mice following systemic Klebsiella pneumoniae infection. Infect. Immun. 2003, 71, 4891–4900. [Google Scholar] [CrossRef]
- Lawrence, T.; Gilroy, D.W.; Colville-Nash, P.R.; Willoughby, D.A. Possible new role for NF-kappaB in the resolution of inflammation. Nat. Med. 2001, 7, 1291–1297. [Google Scholar]
- Bermudez, L.E.; Parker, A.; Petrofsky, M. Apoptosis of Mycobacterium avium-infected macrophages is mediated by both tumour necrosis factor (TNF) and Fas; and involves the activation of caspases. Clin. Exp. Immunol. 1999, 116, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Fratazzi, C.; Arbeit, R.D.; Carini, C.; Balcewicz-Sablinska, M.K.; Keane, J.; Kornfeld, H.; Remold, H.G. Macrophage apoptosis in mycobacterial infections. J. Leukoc. Biol. 1999, 66, 763–764. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shundo, Y.; On, R.; Matsumoto, T.; Ouchi, H.; Fujita, M. TNFR1 Mediated Apoptosis Is Protective against Mycobacterium avium in Mice. Microorganisms 2023, 11, 778. https://doi.org/10.3390/microorganisms11030778
Shundo Y, On R, Matsumoto T, Ouchi H, Fujita M. TNFR1 Mediated Apoptosis Is Protective against Mycobacterium avium in Mice. Microorganisms. 2023; 11(3):778. https://doi.org/10.3390/microorganisms11030778
Chicago/Turabian StyleShundo, Yuki, Rintaro On, Takemasa Matsumoto, Hiroshi Ouchi, and Masaki Fujita. 2023. "TNFR1 Mediated Apoptosis Is Protective against Mycobacterium avium in Mice" Microorganisms 11, no. 3: 778. https://doi.org/10.3390/microorganisms11030778
APA StyleShundo, Y., On, R., Matsumoto, T., Ouchi, H., & Fujita, M. (2023). TNFR1 Mediated Apoptosis Is Protective against Mycobacterium avium in Mice. Microorganisms, 11(3), 778. https://doi.org/10.3390/microorganisms11030778





