Expression and Purification of Glycosyltransferase DnmS from Streptomyces peucetius ATCC 27952 and Study on Catalytic Characterization of Its Reverse Glycosyltransferase Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Materials
2.2. Molecular Cloning and Construction of Recombinant Plasmids
2.3. Overexpression of dnmS and dnmQ in E. coli Strains
2.4. Purification and Isolation of DnmS and DnmQ
2.5. Enzyme Assay of DnmS and DnmQ
3. Results
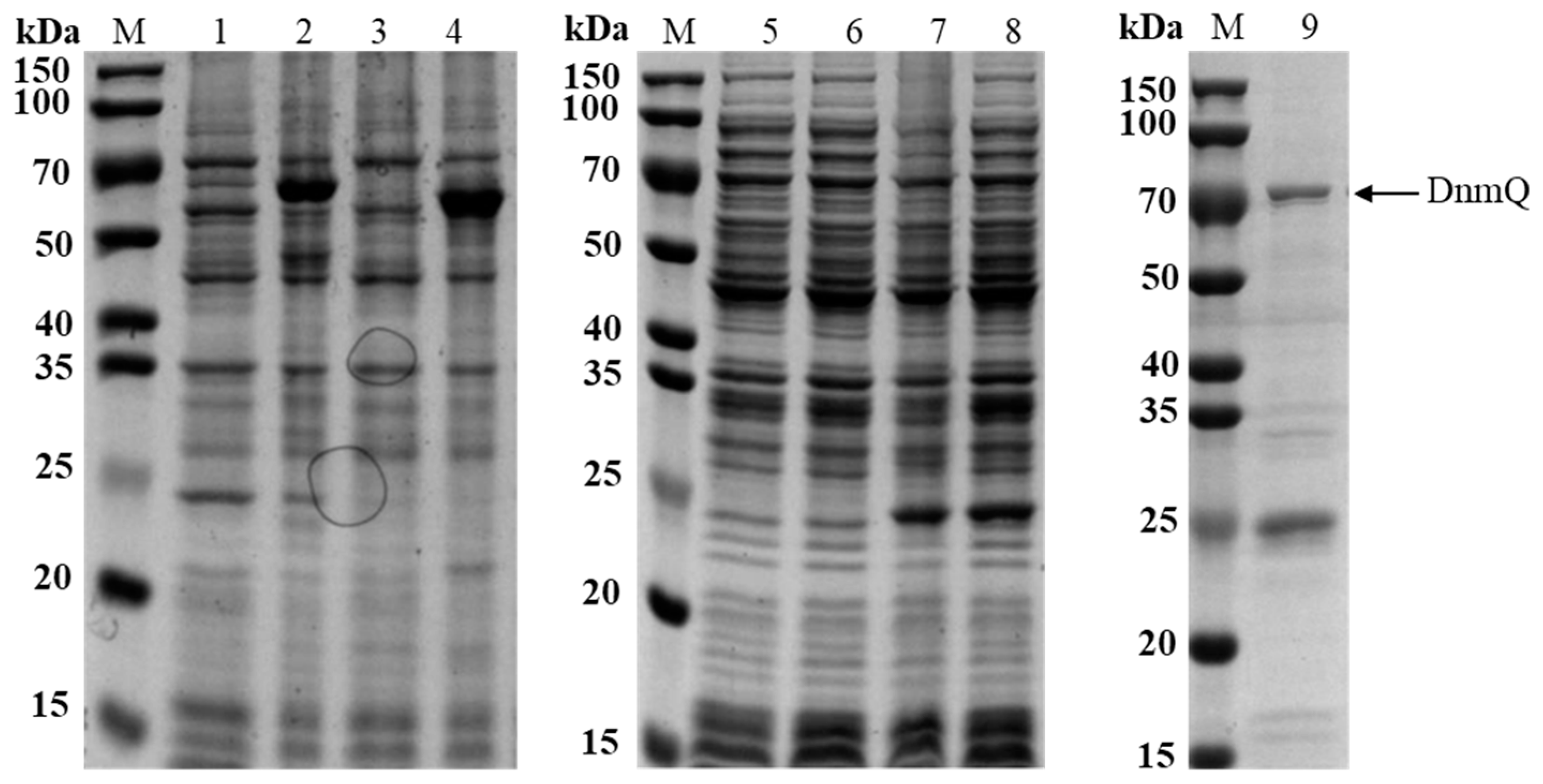
3.1. The Expression of DnmQ in pET22b and pET32a
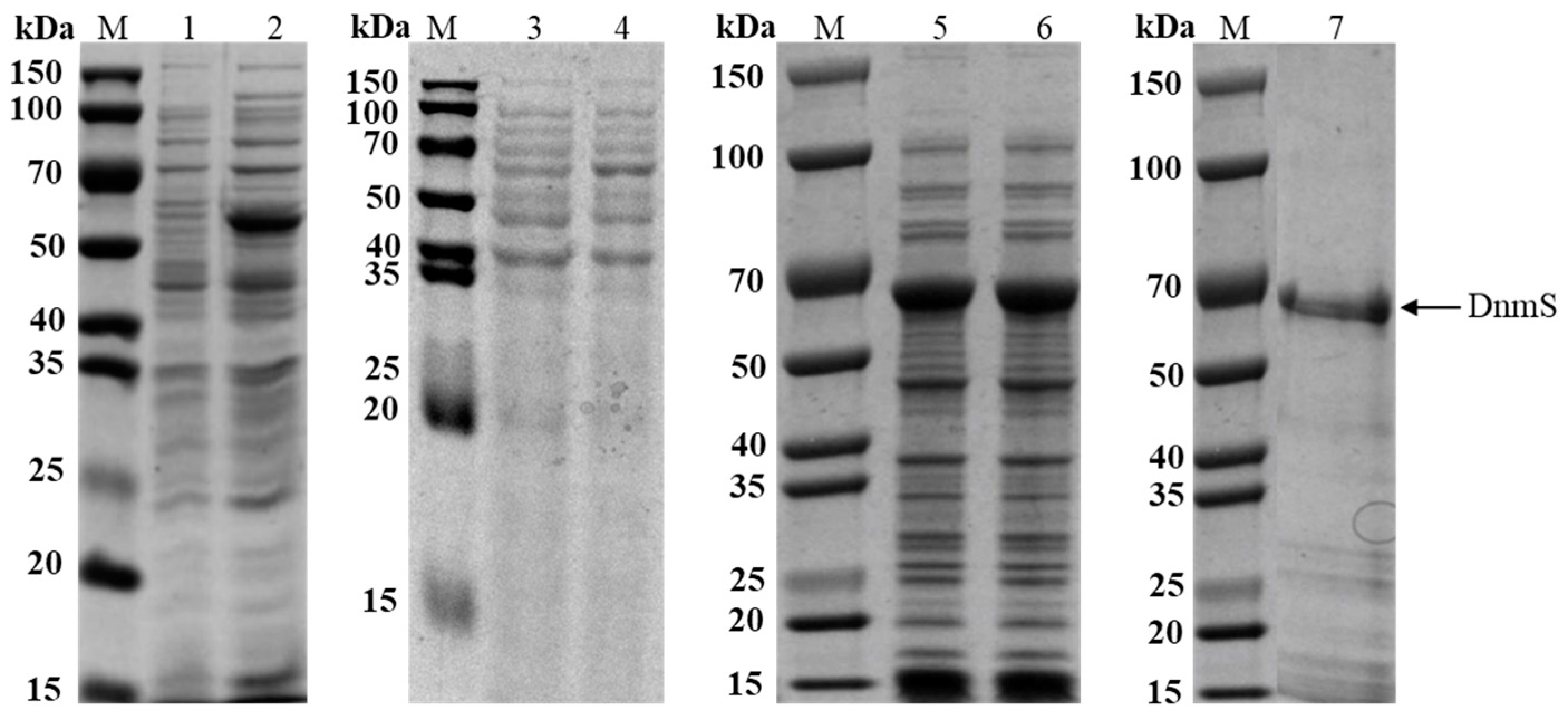
3.2. The Expression of DnmS in Different Expression Vectors and Hosts
3.3. Enhanced Soluble Expression of DnmS in E. coli BL21 (DE3) by Chaperone Plasmid
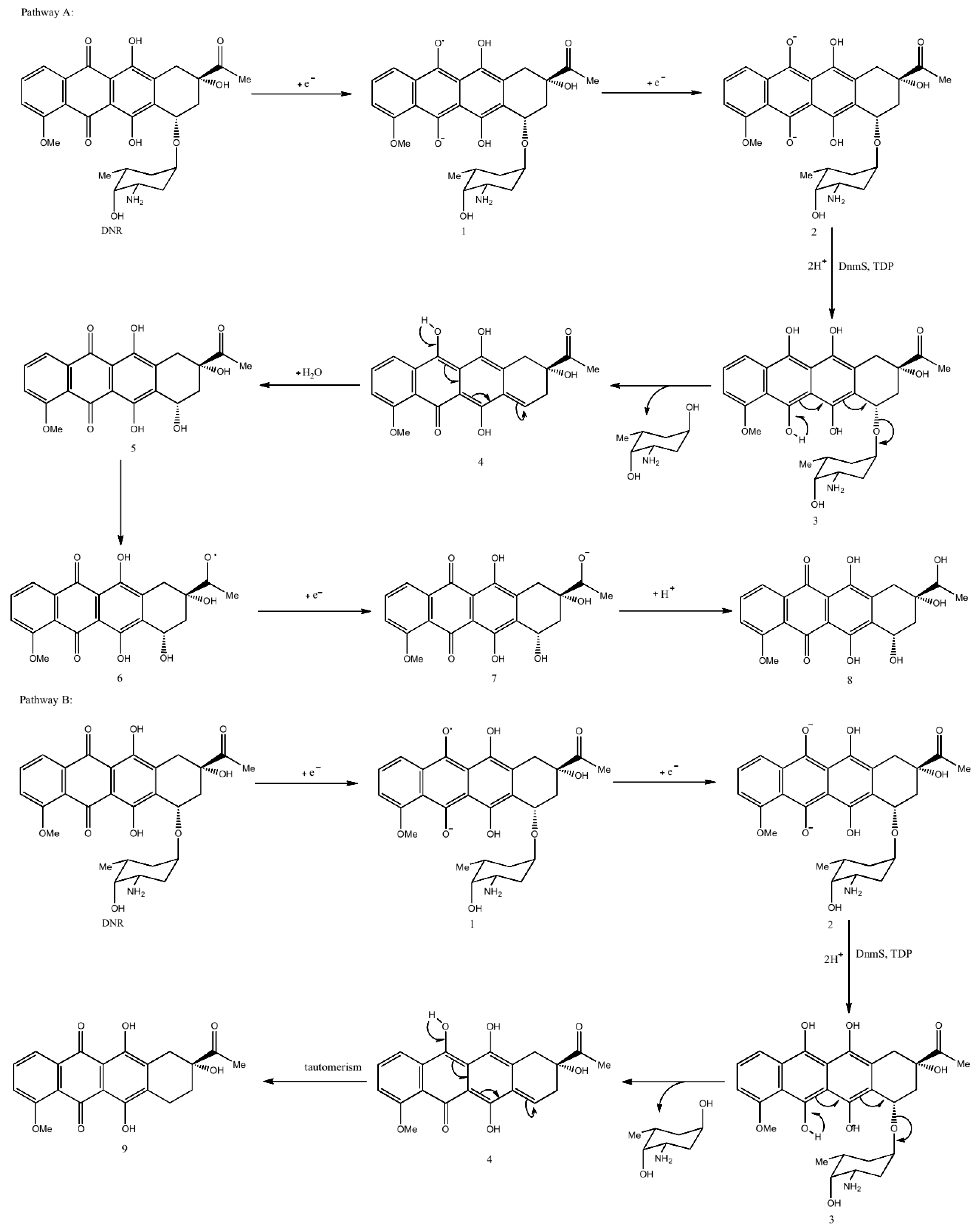
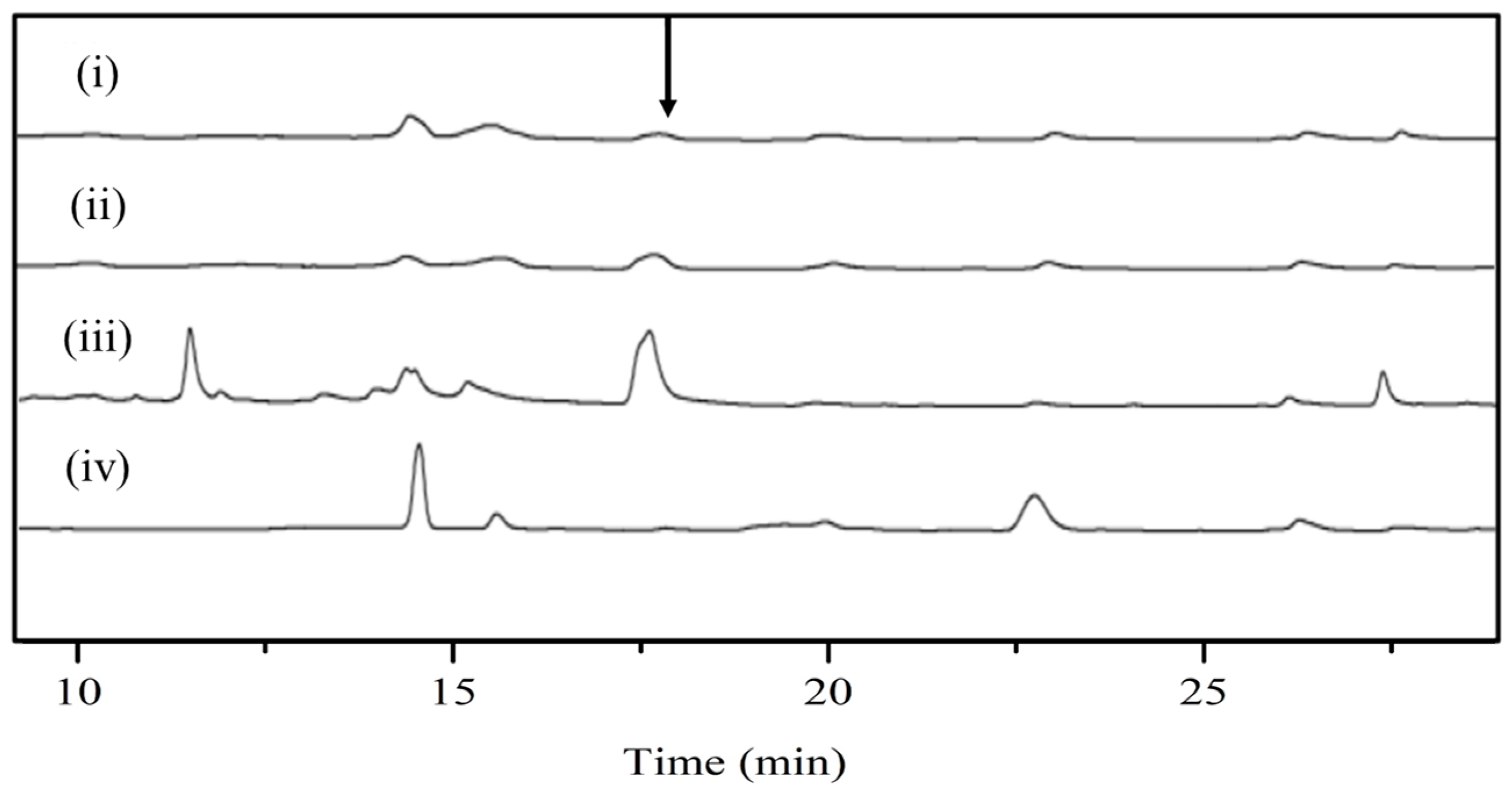
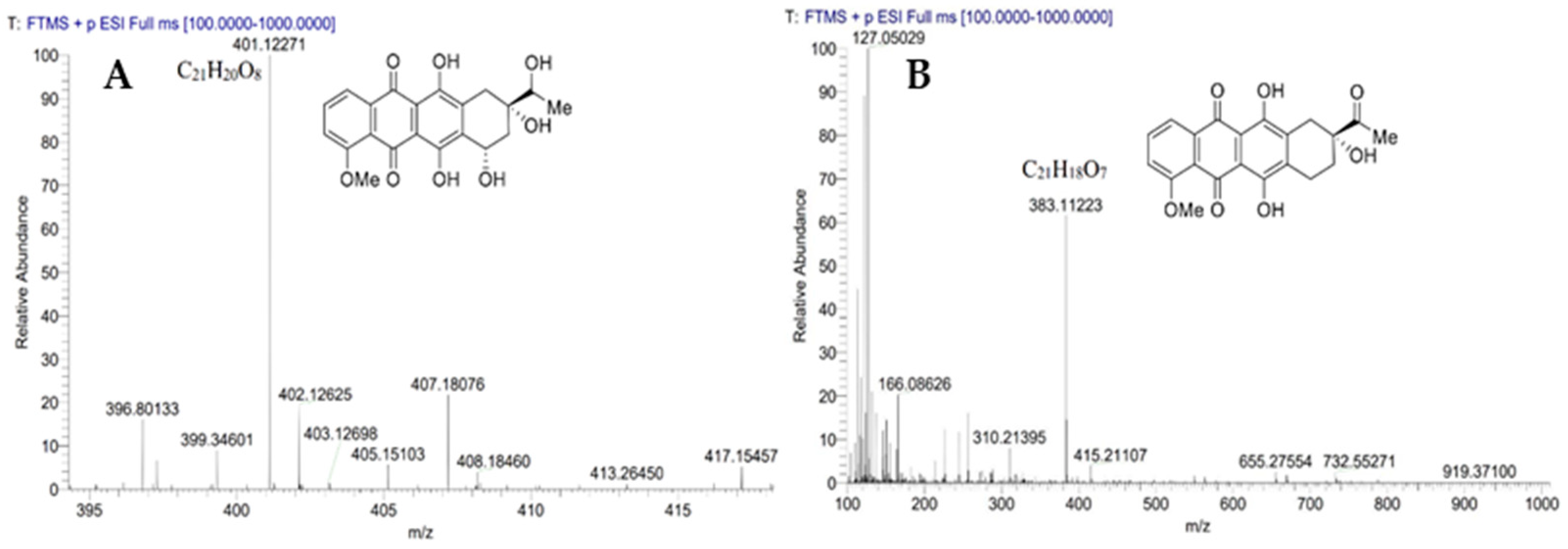
3.4. Reverse Catalytic Reaction of DnmS and DnmQ In Vitro
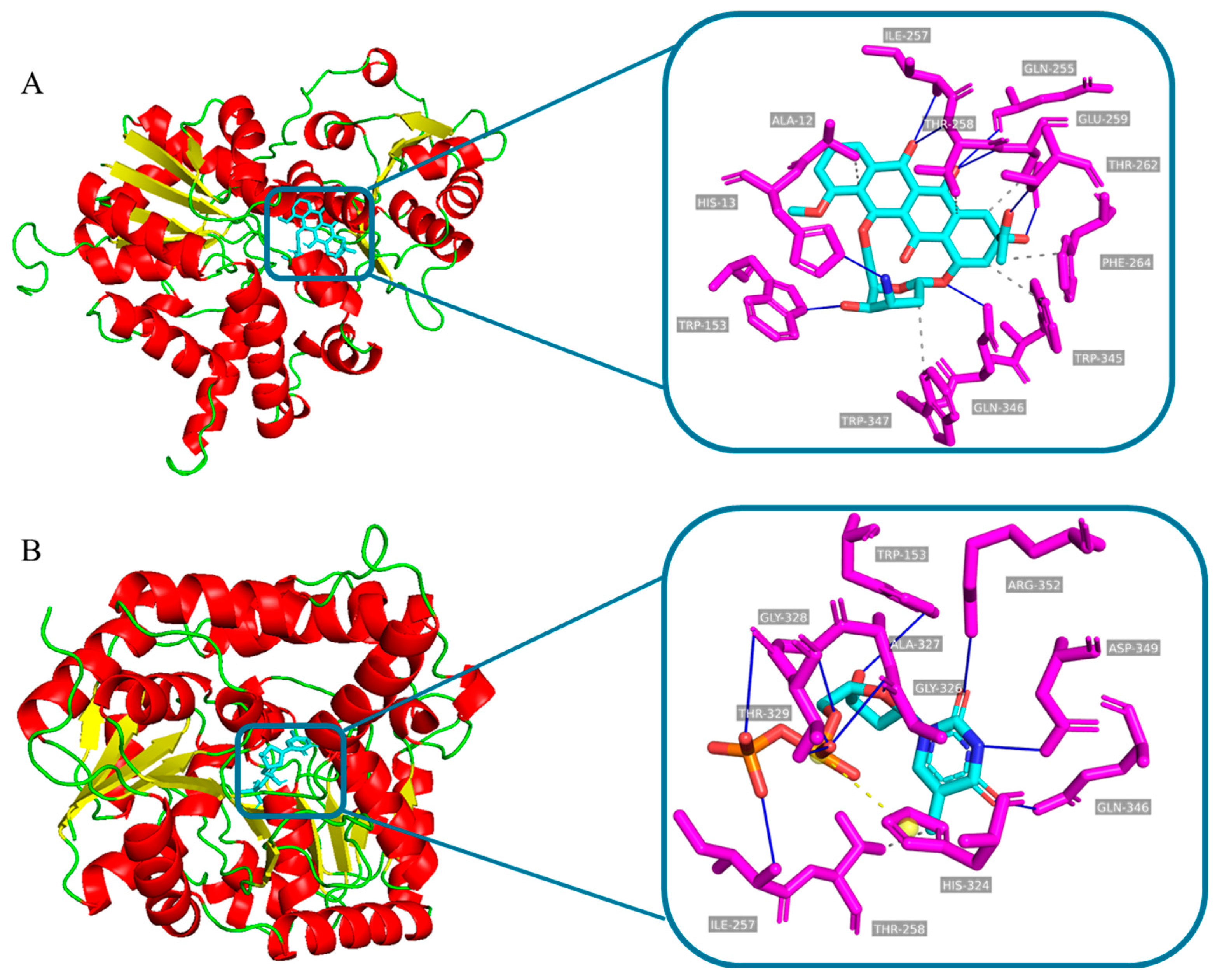
3.5. Sequence Analysis, 3D-Structure Prediction and Molecular-Docking Studies of DnmS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lown, J.W. Discovery and development of anthracycline antitumour antibiotics. Chem. Soc. Rev. 1993, 22, 165–176. [Google Scholar] [CrossRef]
- Di Marco, A.; Gaetani, M.; Orezzi, P.; Scarpinato, B.M.; Silvestrini, R.; Soldati, M.; Dasdia, T.; Valentini, L. Daunomycin, a New Antibiotic of the Rhodomycin Group. Nature 1964, 201, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, F.; Franceschi, G.; Orezzi, P.; Penco, S.; Mondelli, R. The structure of daunomycin. Tetrahedron Lett. 1968, 9, 3349–3352. [Google Scholar] [CrossRef]
- Ascensao, A.; Oliveira, P.J.; Magalhaes, J. Exercise as a beneficial adjunct therapy during Doxorubicin treatment—Role of mitochondria in cardioprotection. Int. J. Cardiol. 2012, 156, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaimomycin, a new antitumor antibiotic from S. peucetius var. Caesius. Biotechnol. Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Fujii, I.; Ebizuka, Y. Anthracycline Biosynthesis in Streptomyces galilaeus. Chem. Rev. 1997, 97, 2511–2524. [Google Scholar] [CrossRef]
- Dickens, M.L.; Strohl, W.R. Isolation and characterization of a gene from Streptomyces sp. strain C5 that confers the ability to convert daunomycin to doxorubicin on Streptomyces lividans TK24. J. Bacteriol. 1996, 178, 3389–3395. [Google Scholar] [CrossRef]
- Dickens, M.L.; Priestley, N.D.; Strohl, W.R. In Vivo and in vitro bioconversion of epsilon-rhodomycinone glycoside to doxorubicin: Functions of DauP, DauK, and DoxA. J. Bacteriol. 1997, 179, 2641–2650. [Google Scholar] [CrossRef]
- Stutzman-Engwall, K.J.; Hutchinson, C.R. Multigene families for anthracycline antibiotic production in Streptomyces peucetius. Proc. Natl. Acad. Sci. USA 1989, 86, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Otten, S.L.; Stutzman-Engwall, K.J.; Hutchinson, C.R. Cloning and expression of daunorubicin biosynthesis genes from Streptomyces peucetius and S. peucetius subsp. caesius. J. Bacteriol. 1990, 172, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the Molecular Basis of Doxorubicin Induced Cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; van der Zanden, S.Y.; Wander, D.P.A.; Borràs, D.M.; Song, J.Y.; Li, X.; van Duikeren, S.; van Gils, N.; Rutten, A.; van Herwaarden, T.; et al. Uncoupling DNA Damage from Chromatin Damage to Detoxify Doxorubicin. Proc. Natl. Acad. Sci. USA 2020, 117, 15182–15192. [Google Scholar] [CrossRef]
- Guidi, C.; Biarnés, X.; Planas, A.; De Mey, M. Controlled processivity in glycosyltransferases: A way to expand the enzymatic toolbox. Biotechnol. Adv. 2022, 63, 108081. [Google Scholar] [CrossRef]
- Sahdev, S.; Khattar, S.K.; Saini, K.S. Production of active eukaryotic proteins through bacterial expression systems: A review of the existing biotechnology strategies. Mol. Cell. Biochem. 2008, 307, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Hutchinson, C.R. Enhanced antibiotic production by manipulation of the Streptomyces peucetius dnrH and dnmT genes involved in doxorubicin (Adriamycin) biosynthesis. J. Bacteriol. 1996, 178, 7316–7321. [Google Scholar] [CrossRef]
- Olano, C.; Lomovskaya, N.; Fonstein, L.; Roll, J.T.; Hutchinson, C.R. A two-plasmid system for the glycosylation of polyketide antibiotics: Bioconversion of ε-rhodomycinone to rhodomycin D. Chem. Biol. 1999, 6, 845–855. [Google Scholar] [CrossRef]
- Tong, Y.; Feng, S.; Xin, Y.; Yang, H.; Zhang, L.; Wang, W.; Chen, W. Enhancement offusion expression of codon-optimized Thermomicrobium roseum sarcosine oxidase in Escherichia coli via chaperone co-expression. J. Biotechnol. 2016, 218, 75–84. [Google Scholar] [CrossRef]
- Betancor, L.; FernáNdez, M.J.; Weissman, K.J.; Leadlay, P.F. Improved catalytic activity of a purified multienzyme from a modular polyketide synthase after coexpression with Streptomyces chaperonins in Escherichia coli. ChemBioChem 2008, 9, 2962–2966. [Google Scholar] [CrossRef]
- Moncrieffe, M.C.; Fernandez, M.J.; Spiteller, D.; Matsumura, H.; Gay, N.J.; Luisi, B.F.; Leadlay, P.F. Structure of the Glycosyltransferase EryCIII in Complex with its Activating P450 Homologue EryCII. J. Mol. Biol. 2012, 415, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Chung, H.S.; Hang, C.; Khosla, C.; Walsh, C.T.; Kahne, D.; Walker, S. Reconstitution and characterization of a new desosaminyl transferase, EryCIII, from the erythromycin biosynthetic pathway. J. Am. Chem. Soc. 2004, 126, 9924–9925. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chung, H.S.; Leimkuhler, C.; Walsh, C.T.; Kahne, D.; Walker, S. In Vitro reconstitution of EryCIII activity for the preparation of unnatural macrolides. J. Am. Chem. Soc. 2005, 127, 14128–14129. [Google Scholar] [CrossRef]
- Zhang, C.; Griffith, B.R.; Fu, Q.; Albermann, C.; Fu, X.; Lee, I.-K.; Li, L.; Thorson, J.S. Exploiting the reversibility of natural product glycosyltransferase-catalyzed reactions. Science 2006, 313, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Singh, S.; Helmich, K.E.; Goff, R.D.; Bingman, C.A.; Thorson, J.S.; Phillips, G.N., Jr. Complete set of glycosyltransferase structures in the calicheamicin biosynthetic pathway reveals the origin of regiospecificity. Proc. Natl. Acad. Sci. USA 2011, 108, 17649–17654. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, C.J.; Melançon, C.E.; Liu, H.W. Natural-Product Sugar Biosynthesis and Enzymatic Glycodiversification. Angew Chem. Int. Ed. Engl. 2008, 47, 9814–9859. [Google Scholar] [CrossRef]
- Haider, S.; Tarasov, A.I.; Craig, T.J.; Sansom, M.S.P.; Ashcroft, F.M. Identification of the PIP2-binding site on Kir6.2 by molecular modelling and functional analysis. EMBO J. 2007, 26, 3749–3759. [Google Scholar] [CrossRef]
- Imberty, A.; Wimmerová, M.; Koca, J.; Breton, C. Molecular modeling of glycosyltransferases. Methods Mol. Biol. 2006, 347, 145–156. [Google Scholar]
- Saibil, H. Chaperone machines for protein folding, unfolding and disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef]
- Niwa, T.; Ying, B.W.; Saito, K.; Jin, W.; Takada, S.; Ueda, T.; Taguchi, H. Bimodal protein solubility distribution revealed by an aggregation analysis of the entire ensemble of Escherichia coli proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 4201–4206. [Google Scholar] [CrossRef]
- Gottesman, S.; Wickner, S.; Maurizi, M.R. Protein quality control: Triage by chaperones and proteases. Genes Dev. 1997, 11, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Bukau, B.; Deuerling, E.; Pfund, C.; Craig, E.A. Getting newly synthesized proteins into shape. Cell 2000, 101, 119–122. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Deuerling, E.; Mogk, A.; Tomoyasu, T.; Bukau, B. Chaperone-based procedure to increase yields of soluble recombinant proteins produced in E. coli. BMC Biotechnol. 2007, 7, 32. [Google Scholar] [CrossRef]
- Mahamad, P.; Boonchird, C.; Panbangred, W. High level accumulation of soluble diphtheria toxin mutant (CRM197) with co-expression of chaperones in recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 6319–6330. [Google Scholar] [CrossRef]
- Borisova, S.A.; Liu, H.W. Characterization of glycosyltransferase DesVII and its auxiliary partner protein DesVIII in the methymycin/pikromycin biosynthetic pathway. Biochemistry 2010, 49, 8071–8084. [Google Scholar] [CrossRef]
- Lu, W.; Leimkuhler, C.; Gatto, G.J.; Kruger, R.G.; Oberthür, M.; Kahne, D.; Walsh, C.T. AknT is an activating protein for the glycosyltransferase AknS in L-aminodeoxysugar transfer to the aglycone of aclacinomycin A. Chem. Biol. 2005, 12, 527–534. [Google Scholar] [CrossRef]
- Thibodeaux, C.J.; Melançon, C.E.; Liu, H.-W. Unusual sugar biosynthesis and natural product glycodiversification. Nature 2007, 446, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Erb, A.; Weiss, H.; Härle, J.; Bechthold, A. A bacterial glycosyltransferase gene toolbox: Generation and applications. Phytochemistry 2009, 70, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
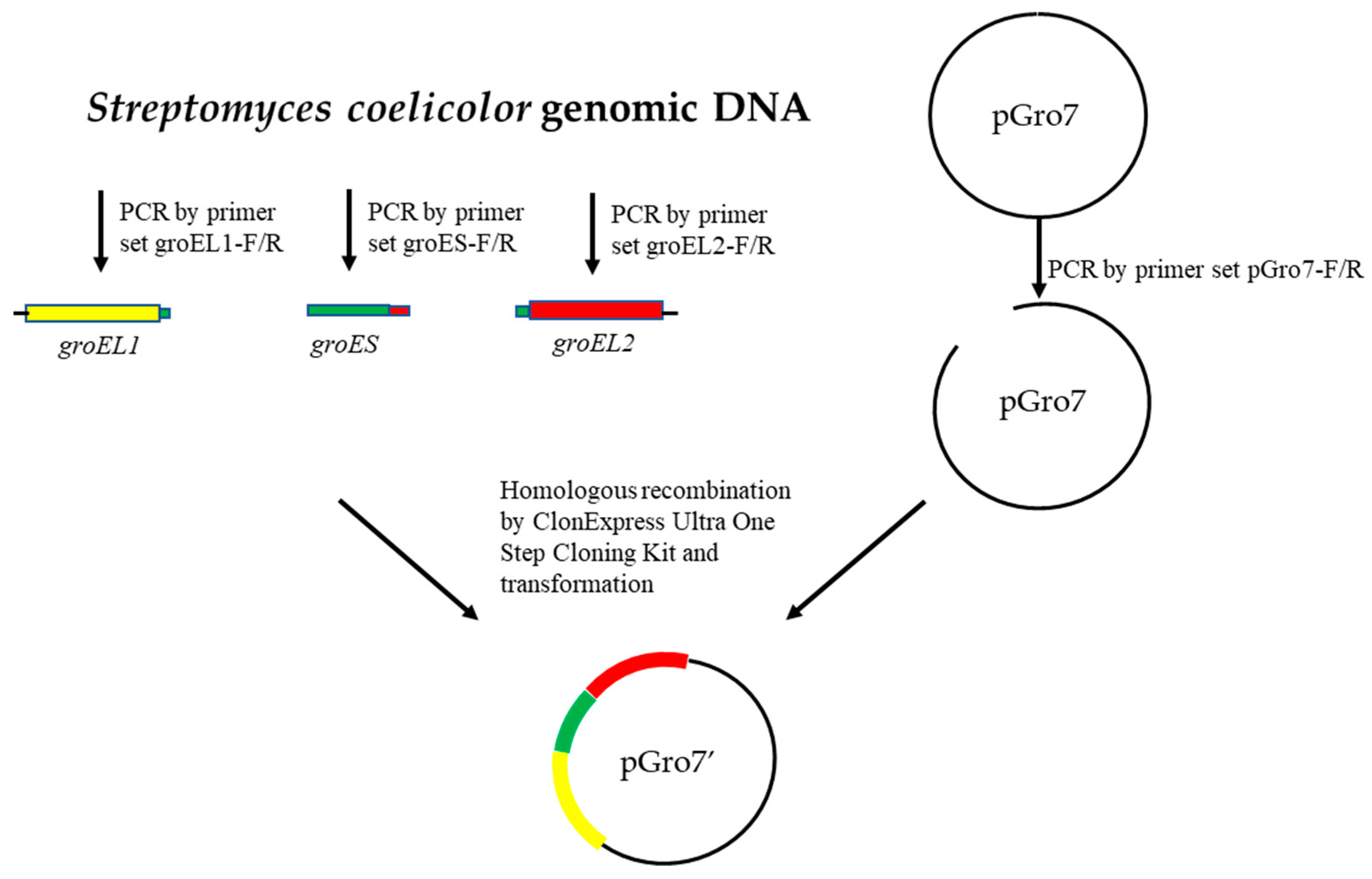

| Strains or Plasmids | Relevant Characteristics | Reference or Source |
|---|---|---|
| Escherichia coli | ||
| DH5α | F− Φ80lacZΔM15Δ(lacZYA-argF) U169 recA1 endA1 hsdR17(rk−,mk+) phoA supE44 thi-1 gyrA96 relA1 λ− | Gibco BRL, Life Technologies |
| BL21 (DE3) | F− ompT hsdS(rB− mB−) dcm+ galλ(DE3) | Novagen |
| BL21 Codon plus (DE3) RIL | F− ompT hsdS(rB− mB−) dcm+ galλ(DE3) endA Hte [argU ileY leuW Camr] | Novagen |
| 22bS/DE3 | BL21 (DE3) harboring 22bS, Ampr | This work |
| 22bS/RIL | BL21 Codon plus (DE3) RIL harboring 22bS, Ampr, Camr | This work |
| 32aS/DE3 | BL21 (DE3) harboring 32aS, Ampr | This work |
| 32aS/RIL | BL21 Codon plus (DE3) RIL harboring 32aS, Ampr, Camr | This work |
| 32aS/pGro7 | BL21 (DE3) harboring 32aS and pGro7, Ampr, Camr | This work |
| 32aS/pGro7′ | BL21 (DE3) harboring 32aS and pGro7′, Ampr, Camr | This work |
| 22bQ/DE3 | BL21 (DE3) harboring 22bQ, Ampr | This work |
| 22bQ/RIL | BL21 Codon plus (DE3) RIL harboring 22bQ, Ampr, Camr | This work |
| 32aQ/DE3 | BL21 (DE3) harboring 32aQ, Ampr | This work |
| 32aQ/RIL | BL21 Codon plus (DE3) RIL harboring 32aQ, Ampr, Camr | This work |
| Plasmids | ||
| pET22b | Expression vector, C-terminal 6×His-tagged sequences, Ampr | Novagen |
| pET32a | Expression vector, N-terminal 6×His-tagged sequences, Ampr | Novagen |
| pGro7 | Chaperone plasmid, Camr | Takara |
| pGro7′ | Chaperone plasmid with molecular chaperone gene of Streptomyces, Camr | This work |
| 22bS | pET22b containing dnmS coding region, Ampr | This work |
| 32aS | pET32a containing dnmS coding region, Ampr | This work |
| 22bQ | pET22b containing dnmQ coding region, Ampr | This work |
| 32aQ | pET32a containing dnmQ coding region, Ampr | This work |
| Primer Name | Sequence |
|---|---|
| 32a-dnmS-F | gccatggctgatatcggatccatgaaggtgctcgtgacggc |
| 32a-dnmS-R | ctcgagtgcggccgcaagcttctagtgccggacgccctg |
| 22b-dnmS-F | taagaaggagatatacatatgatgaaggtgctcgtgacggc |
| 22b-dnmS-R | gtggtggtggtggtgctcgaggtgccggacgccctgccc |
| 32a-dnmQ-F | Gccatggctgatatcggatccatgcccacacccacgtcc |
| 32a-dnmQ-R | ctcgagtgcggccgcaagctttcacttctgggccagccg |
| 22b-dnmQ-F | taagaaggagatatacatatgatgcccacacccacgtcc |
| 22b-dnmQ-R | gtggtggtggtggtgctcgagcttctgggccagccgcag |
| groEL1-F | ttctcaaaggagagttatcaatggcgaagatcctgaagttcg |
| groEL1-R | ttggagctggtggtcgtcacgtgggagtggccgtggct |
| groES-F | gtgacgaccaccagctccaa |
| groES-R | atcttggccatcttctcgacgatcgcgagc |
| groEL2-F | gtcgagaagatggccaagatcatcgcgt |
| groEL2-R | ttctgcgaggtgcagggcaatcagaagtccatgtcaccaccc |
| pGro7-F | ttgccctgcacctcgcag |
| pGro7-R | tgataactctcctttgagaaagtccg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhou, H.; Chen, G.; Li, H.; Yang, D.; Pan, L. Expression and Purification of Glycosyltransferase DnmS from Streptomyces peucetius ATCC 27952 and Study on Catalytic Characterization of Its Reverse Glycosyltransferase Reaction. Microorganisms 2023, 11, 762. https://doi.org/10.3390/microorganisms11030762
Yang L, Zhou H, Chen G, Li H, Yang D, Pan L. Expression and Purification of Glycosyltransferase DnmS from Streptomyces peucetius ATCC 27952 and Study on Catalytic Characterization of Its Reverse Glycosyltransferase Reaction. Microorganisms. 2023; 11(3):762. https://doi.org/10.3390/microorganisms11030762
Chicago/Turabian StyleYang, Liyan, Huimin Zhou, Guiguang Chen, Hongliang Li, Dengfeng Yang, and Lixia Pan. 2023. "Expression and Purification of Glycosyltransferase DnmS from Streptomyces peucetius ATCC 27952 and Study on Catalytic Characterization of Its Reverse Glycosyltransferase Reaction" Microorganisms 11, no. 3: 762. https://doi.org/10.3390/microorganisms11030762
APA StyleYang, L., Zhou, H., Chen, G., Li, H., Yang, D., & Pan, L. (2023). Expression and Purification of Glycosyltransferase DnmS from Streptomyces peucetius ATCC 27952 and Study on Catalytic Characterization of Its Reverse Glycosyltransferase Reaction. Microorganisms, 11(3), 762. https://doi.org/10.3390/microorganisms11030762






