Characterization of Two Novel Rumen-Derived Exo-Polygalacturonases: Catalysis and Molecular Simulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
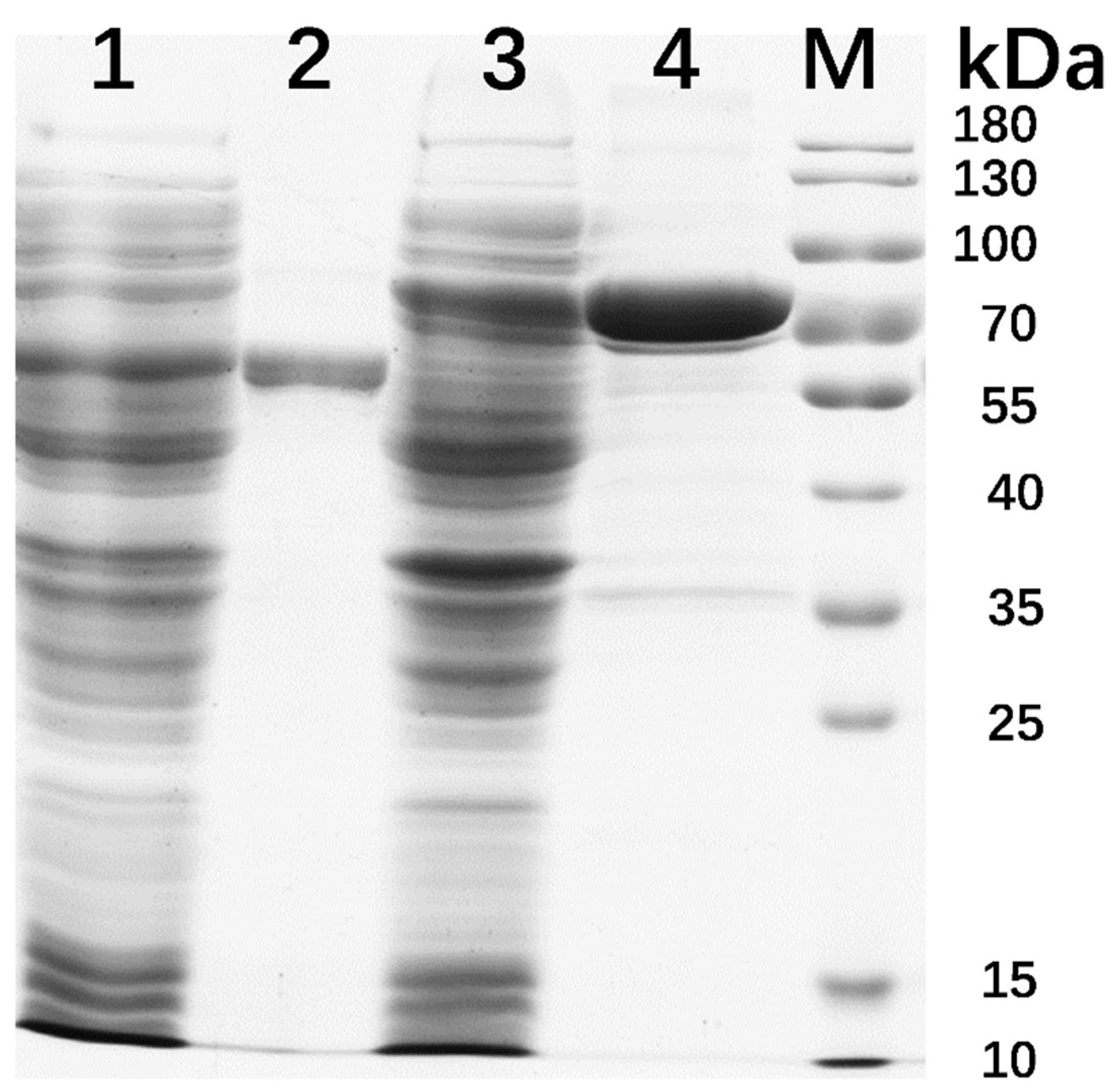
2.2. Gene Cloning, Expression, and Protein Purification
2.3. Sequence Analysis
2.4. Enzyme Activity Assay
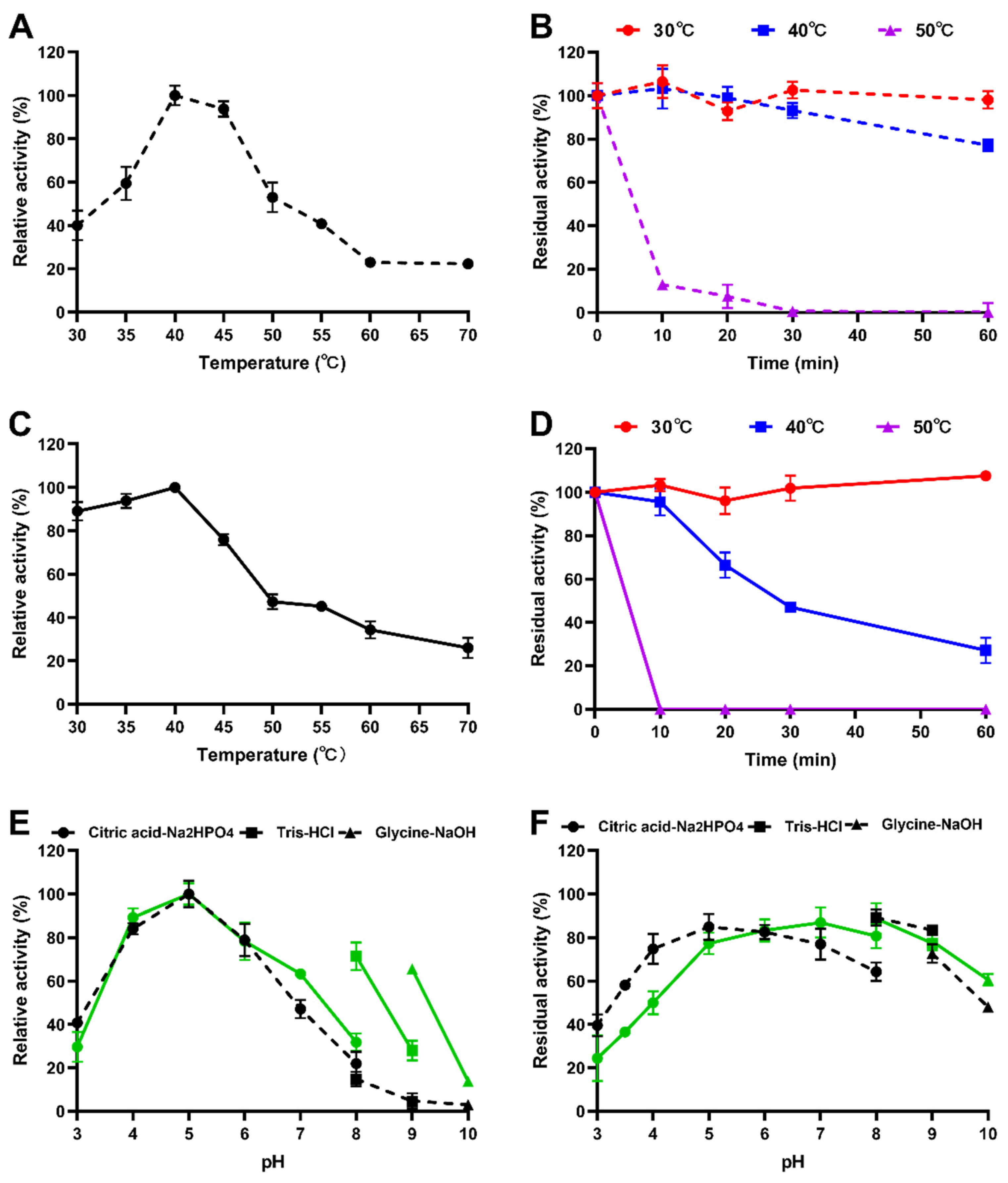
2.5. Characterization of Purified Recombinant Enzymes
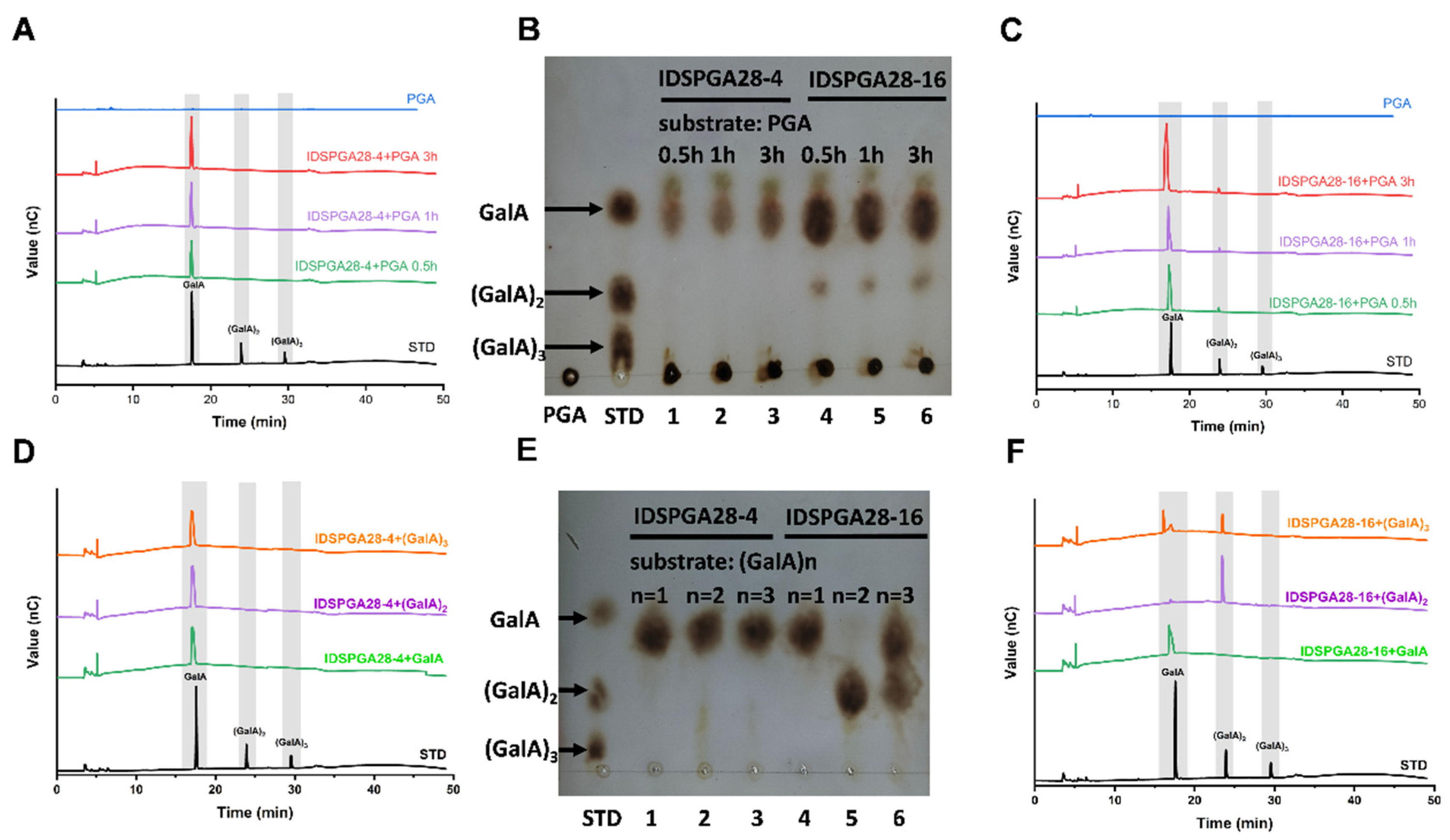
2.6. Hydrolysis Product Analysis
2.7. Molecular Docking and Molecular Dynamics (MD) Simulation
2.8. Fruit Juice Clarification with Exo-Polygalacturonase
2.9. Statistical Analysis
3. Results and Discussion
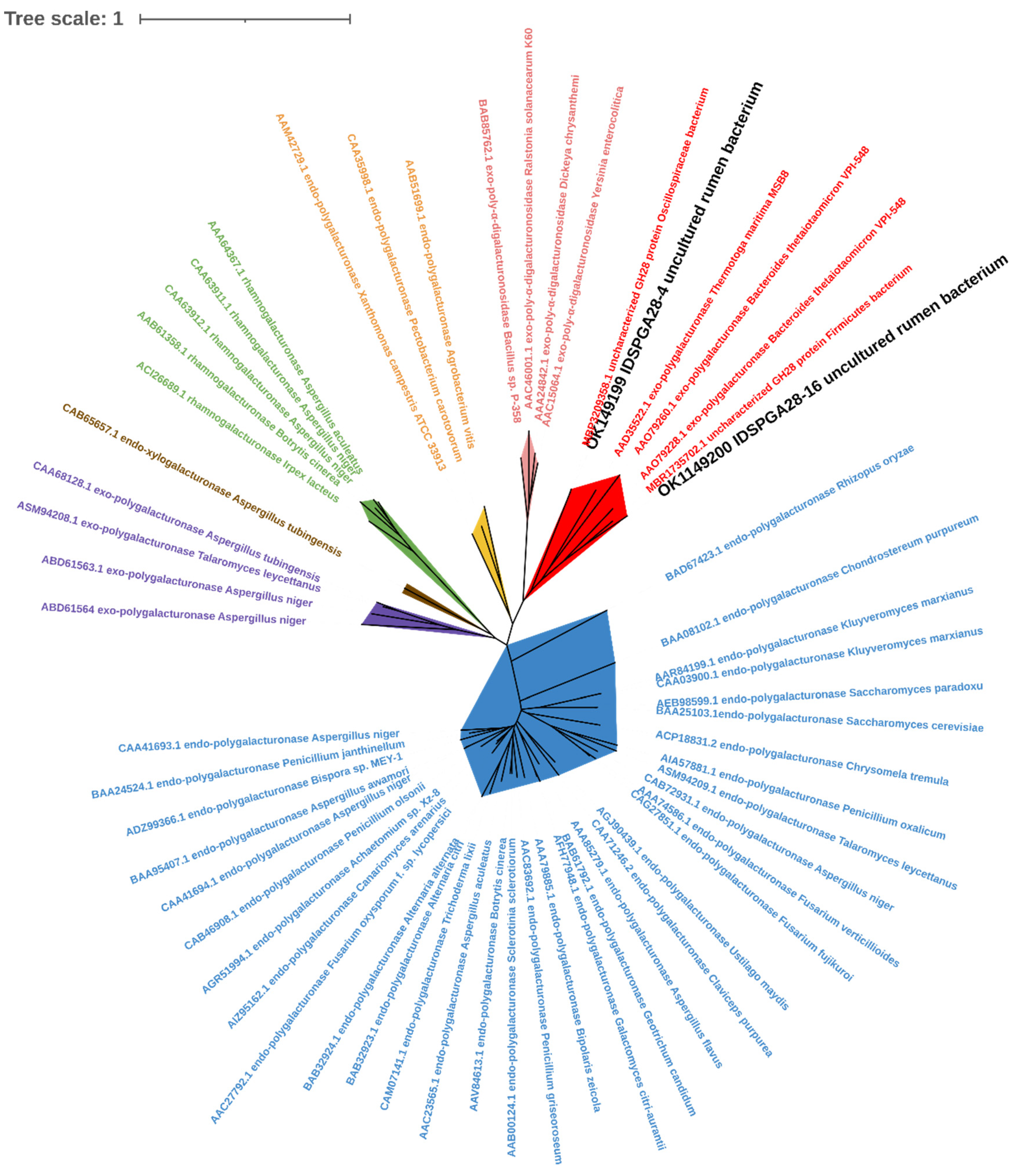
3.1. Gene Cloning and Sequence Analysis
3.2. Protein Expression and Enzymatic Properties
3.3. Hydrolytic Products and Hydrolysis Pattern Analyses
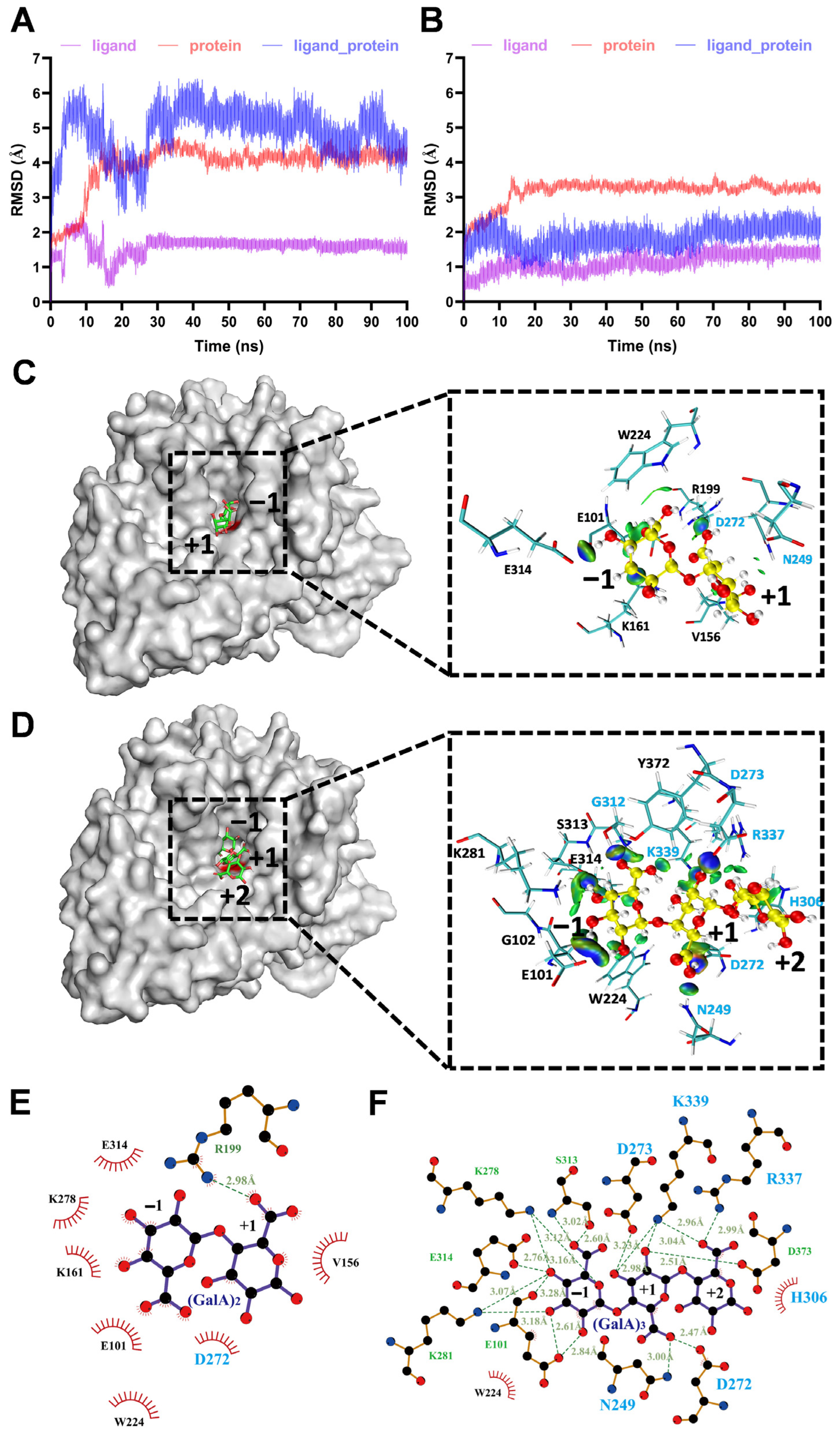
3.4. Molecular Dynamics (MD) Simulation
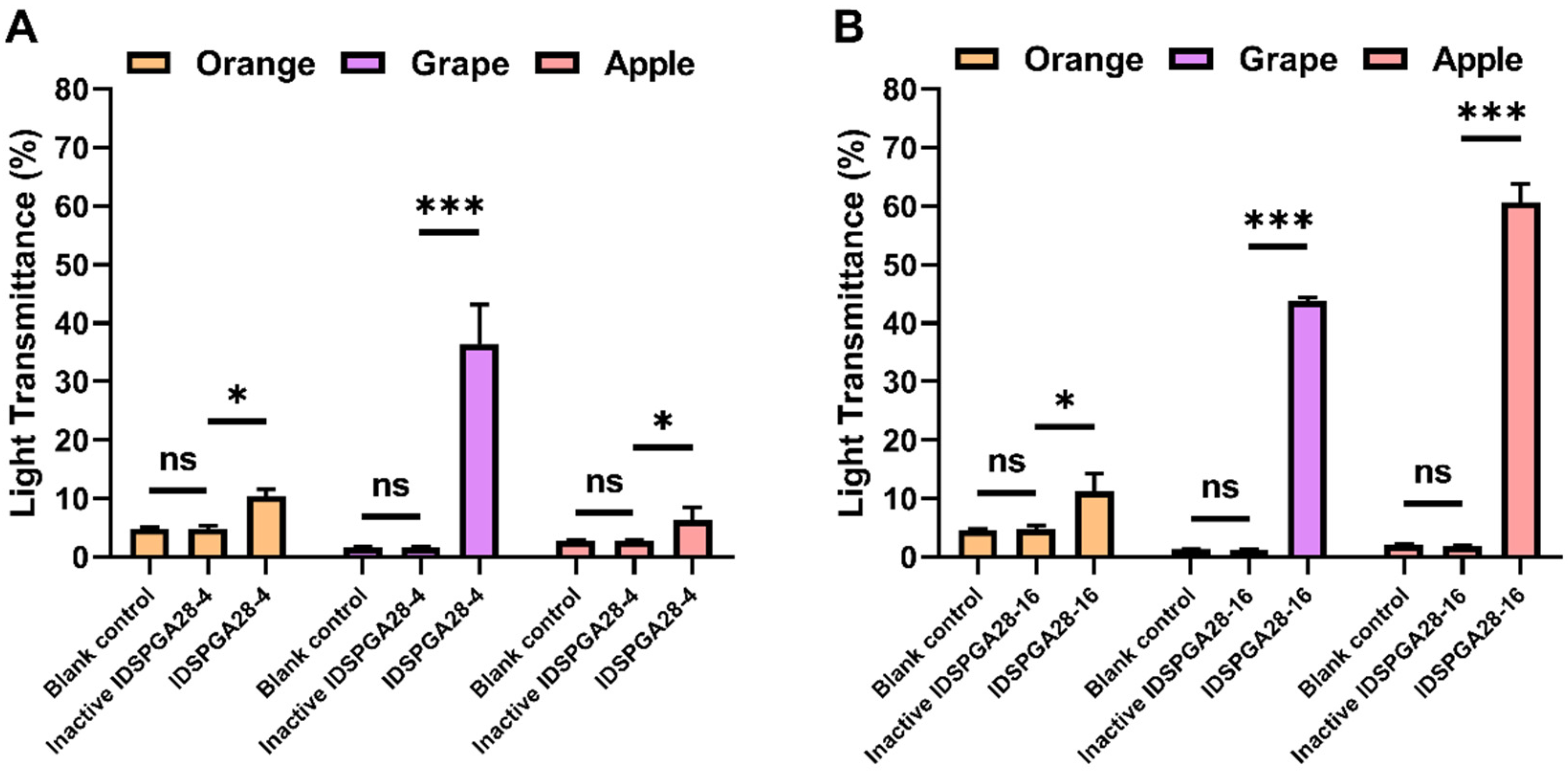
3.5. Effect of Recombinant Enzymes on Fruit Juice Clarification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef]
- Hamouda, H.I.; Ali, N.; Su, H.; Feng, J.; Lu, M.; Li, F.L. Exploration of two pectate lyases from Caldicellulosiruptor bescii reveals that the CBM66 module has a crucial role in pectic biomass degradation. Appl. Environ. Microbiol. 2020, 86, e00787-20. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Tu, T.; Zhang, D.; Ma, R.; You, S.; Wang, X.; Yao, B.; Luo, H.; Xu, B. Two acidic, thermophilic GH28 polygalacturonases from Talaromyces leycettanus JCM 12802 with application potentials for grape juice clarification. Food Chem. 2017, 237, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.W.; Boraston, A.B. The structural basis for exopolygalacturonase activity in a family 28 glycoside hydrolase. J. Mol. Biol. 2007, 368, 1215–1222. [Google Scholar] [CrossRef]
- Tu, T.; Meng, K.; Luo, H.; Turunen, O.; Zhang, L.; Cheng, Y.; Su, X.; Ma, R.; Shi, P.; Wang, Y.; et al. New insights into the role of T3 Loop in determining catalytic efficiency of GH28 endo-polygalacturonases. PLoS ONE 2015, 10, e0135413. [Google Scholar] [CrossRef] [PubMed]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Pan, X.; Li, K.; Ma, R.; Shi, P.; Huang, H.; Yang, P.; Meng, K.; Yao, B. Biochemical characterization of three distinct polygalacturonases from Neosartorya fischeri p1. Food Chem. 2015, 188, 569–575. [Google Scholar] [CrossRef]
- Kars, I.; Krooshof, G.H.; Wagemakers, L.; Joosten, R.; Benen, J.A.E.; Van Kan, J.A.L. Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J. 2005, 43, 213–225. [Google Scholar] [CrossRef]
- Shimizu, T.; Nakatsu, T.; Miyairi, K.; Okuno, T.; Kato, H. Active-site architecture of endopolygalacturonase I from Stereum purpureum revealed by crystal structures in native and ligand-bound forms at atomic resolution. Biochemistry 2002, 41, 6651–6659. [Google Scholar] [CrossRef]
- Van Santen, Y.; Benen, J.A.E.; Schröter, K.-H.; Kalk, K.H.; Armand, S.; Visser, J.; Dijkstra, B.W. 1.68-Å crystal structure of endopolygalacturonase II from Aspergillus niger and identification of active site residues by site-directed mutagenesis. J. Biol. Chem. 1999, 274, 30474–30480. [Google Scholar] [CrossRef]
- Kluskens, L.D.; van Alebeek, G.-J.W.M.; Walther, J.; Voragen, A.G.J.; de Vos, W.M.; van der Oost, J. Characterization and mode of action of an exopolygalacturonase from the hyperthermophilic bacterium Thermotoga maritima. FEBS J. 2005, 272, 5464–5473. [Google Scholar] [CrossRef]
- Martens-Uzunova, E.S.; Zandleven, J.S.; Benen, J.A.E.; Awad, H.; Kools, H.J.; Beldman, G.; Voragen, A.G.J.; Van Den Berg, J.A.; Schaap, P.J. A new group of exo-acting family 28 glycoside hydrolases of Aspergillus niger that are involved in pectin degradation. Biochem. J. 2006, 400, 43–52. [Google Scholar] [CrossRef]
- Sawada, K.; Suzumatsu, A.; Kobayashi, T.; Ito, S. Molecular cloning and sequencing of the gene encoding an exopolygalacturonase of a Bacillus isolate and properties of its recombinant enzyme. Biochim. Biophys. Acta 2001, 1568, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ariaeenejad, S.; Sheykh Abdollahzadeh Mamaghani, A.; Maleki, M.; Kavousi, K.; Foroozandeh Shahraki, M.; Hosseini Salekdeh, G. A novel high performance in-silico screened metagenome-derived alkali-thermostable endo-β-1,4-glucanase for lignocellulosic biomass hydrolysis in the harsh conditions. BMC Biotechnol. 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.W.; Deng, Q.; Gao, D.Y.; He, B.; Yin, S.J.; Qian, L.C.; Wang, J.K.; Wang, Q. A novel bifunctional glucanase exhibiting high production of glucose and cellobiose from rumen bacterium. Int. J. Biol. Macromol. 2021, 173, 136–145. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Jin, S.W.; Cao, J.W.; Mi, L.; Wang, J.K. Metatranscriptomics of the Hu sheep rumen microbiome reveals novel cellulases. Biotechnol. Biofuels 2019, 12, 153. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; He, B.; Jiang, L.S.; Liu, J.X.; Wang, J.K. Characterization of a novel xylanase gene from rumen content of Hu sheep. Appl. Biochem. Biotechnol. 2015, 177, 1424–1436. [Google Scholar] [CrossRef]
- Luis, A.S.; Briggs, J.; Zhang, X.; Farnell, B.; Ndeh, D.; Labourel, A.; Baslé, A.; Cartmell, A.; Terrapon, N.; Stott, K.; et al. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat. Microbiol. 2018, 3, 210–219. [Google Scholar] [CrossRef]
- Ndeh, D.; Rogowski, A.; Cartmell, A.; Luis, A.S.; Baslé, A.; Gray, J.; Venditto, I.; Briggs, J.; Zhang, X.; Labourel, A.; et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 2017, 544, 65–70. [Google Scholar] [CrossRef]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: AutoDock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14sb: Improving the accuracy of protein side chain and backbone parameters from ff99sb. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef]
- Hess, M.; Sczyrba, A.; Egan, R.; Kim, T.-W.; Chokhawala, H.; Schroth, G.; Luo, S.; Clark, D.S.; Chen, F.; Zhang, T.; et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 2011, 331, 463–467. [Google Scholar] [CrossRef]
- Pijning, T.; van Pouderoyen, G.; Kluskens, L.; van der Oost, J.; Dijkstra, B.W. The crystal structure of a hyperthermoactive exopolygalacturonase from Thermotoga maritima reveals a unique tetramer. FEBS Lett. 2009, 583, 3665–3670. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.C.; Lee, J.H.; Han, Y.; Choi, J.H.; Song, J.J. A novel multifunctional cellulolytic enzyme screened from metagenomic resources representing ruminal bacteria. Biochem. Biophys. Res. Commun. 2013, 441, 567–572. [Google Scholar] [CrossRef]
- Dadheech, T.; Shah, R.; Pandit, R.; Hinsu, A.; Chauhan, P.S.; Jakhesara, S.; Kunjadiya, A.; Rank, D.; Joshi, C. Cloning, molecular modeling and characterization of acidic cellulase from buffalo rumen and its applicability in saccharification of lignocellulosic biomass. Int. J. Biol. Macromol. 2018, 113, 73–81. [Google Scholar] [CrossRef]
- Song, Y.H.; Lee, K.T.; Baek, J.Y.; Kim, M.J.; Kwon, M.R.; Kim, Y.J.; Park, M.R.; Ko, H.; Lee, J.S.; Kim, K.S. Isolation and characterization of a novel endo-β-1,4-glucanase from a metagenomic library of the black-goat rumen. Braz. J. Microbiol. 2017, 48, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, D.A.; Pepe, O.; Pennacchio, A.; Fagnano, M.; Faraco, V. Directed evolution of the bacterial endo-β-1,4-glucanase from Streptomyces sp. G12 towards improved catalysts for lignocellulose conversion. AMB Express 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.Y.; Sun, X.B.; Liu, M.Q.; Liu, Y.N.; Zhang, H.E.; Shi, X.L.; Li, Y.N.; Wang, J.K.; Yin, S.J.; Wang, Q. Characterization of thermostable and chimeric enzymes via isopeptide bond-mediated molecular cyclization. J. Agric. Food Chem. 2019, 67, 6837–6846. [Google Scholar] [CrossRef] [PubMed]
- Kester, H.C.M.; Someren, M.A.K.-V.; Müller, Y.; Visser, J. Primary structure and characterization of an exopolygalacturonase from Aspergillus tubingensis. Eur. J. Biochem. 1996, 240, 738–746. [Google Scholar] [CrossRef]
- Pařenicová, L.; Kester, H.C.M.; Benen, J.A.E.; Visser, J. Characterization of a novel endopolygalacturonase from Aspergillus niger with unique kinetic properties. FEBS Lett. 2000, 467, 333–336. [Google Scholar] [CrossRef]
- Maiorov, V.N.; Crippen, G.M. Significance of root-mean-square deviation in comparing three-dimensional structures of globular proteins. J. Mol. Biol. 1994, 235, 625–634. [Google Scholar] [CrossRef]
- Tu, T.; Meng, K.; Huang, H.; Luo, H.; Bai, Y.; Ma, R.; Su, X.; Shi, P.; Yang, P.; Wang, Y.; et al. Molecular Characterization of a thermophilic endo-polygalacturonase from Thielavia arenaria XZ7 with high catalytic efficiency and application potential in the food and feed industries. J. Agric. Food Chem. 2014, 62, 12686–12694. [Google Scholar] [CrossRef]
- Tu, T.; Meng, K.; Bai, Y.; Shi, P.; Luo, H.; Wang, Y.; Yang, P.; Zhang, Y.; Zhang, W.; Yao, B. High-yield production of a low-temperature-active polygalacturonase for papaya juice clarification. Food Chem. 2013, 141, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, S.; Tan, H.; Li, K.; Chen, W.; Yin, H. A highly efficient biocatalytic conversion of renewable pectic polysaccharide biomass into bioactive oligogalacturonides. ACS Food Sci. Technol. 2021, 1, 338–346. [Google Scholar] [CrossRef]
- Sieiro, C.; García-Fraga, B.; López-Seijas, J.; da Silva, A.F.; Villa, T.G. Microbial Pectic Enzymes in the Food and Wine Industry; IntechOpen: London, UK, 2012; ISBN 978-953-307-905-9. [Google Scholar]
- Wang, J.; Zhang, Y.; Qin, X.; Gao, L.; Han, B.; Zhang, D.; Li, J.; Huang, H.; Zhang, W. Efficient expression of an acidic endo-polygalacturonase from Aspergillus niger and its application in juice production. J. Agric. Food Chem. 2017, 65, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huo, W.; Dai, X.; Dang, Y. Preparation of low-molecular-weight citrus pectin by recombinant Bacillus subtilis pectate lyase and promotion of growth of Bifidobacterium longum. Catal. Commun. 2018, 107, 39–42. [Google Scholar] [CrossRef]
- Sassi, A.H.; Tounsi, H.; Trigui-Lahiani, H.; Bouzouita, R.; Romdhane, Z.B.; Gargouri, A. A low-temperature polygalacturonase from P. occitanis: Characterization and application in juice clarification. Int. J. Biol. Macromol. 2016, 91, 158–164. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
| Name | Origin | EC Number | Substrate (w/v) | Hydrolytic Products | Specific Activity (U/mg) 1 | Reference |
|---|---|---|---|---|---|---|
| PGXA | Aspergillus niger | EC 3.2.1.67 | 0.25% PGA | GalA | 7 | [13] |
| PGXB | Aspergillus niger | EC 3.2.1.67 | 0.25% PGA | GalA | 242 | [13] |
| PGXC | Aspergillus niger | EC 3.2.1.67 | 0.25% PGA | GalA | 223 | [13] |
| Exo-PG | Aspergillus tubingensis | EC 3.2.1.67 | 0.25% PGA | GalA | 230 | [13] |
| PGAX | Aspergillus tubingensis | EC 3.2.1.67 | 0.25% PGA | GalA | 255 | [37] |
| NfPGIII | Neosartorya fischeri | EC 3.2.1.67 | 0.33% PGA | GalA | 19.97 | [8] |
| TePG28a | Talaromyces leycettanus | EC 3.2.1.67 | 0.33% PGA | GalA | 280 ± 9 | [4] |
| IDSPGA28-4 | rumen bacterium | EC 3.2.1.67 | 0.25% PGA | GalA | 31.2 ± 1.5 | this study |
| IDSPGA28-16 | rumen bacterium | EC 3.2.1.67 | 0.25% PGA | GalA, (GalA)2 | 330.4 ± 12.4 | this study |
| YeGH28 | Yersinia enterocolitica | EC 3.2.1.82 | 1% PGA | (GalA)2 | 80 | [5] |
| PehK | Bacillus subtilis | EC 3.2.1.82 | 0.75% PGA | (GalA)2 | 33.1 | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Sun, X.; Gao, D.; Wang, Y.; Liu, Y.; Li, N.; Wang, Z.; Liu, M.; Wang, J.; Wang, Q. Characterization of Two Novel Rumen-Derived Exo-Polygalacturonases: Catalysis and Molecular Simulations. Microorganisms 2023, 11, 760. https://doi.org/10.3390/microorganisms11030760
Deng Q, Sun X, Gao D, Wang Y, Liu Y, Li N, Wang Z, Liu M, Wang J, Wang Q. Characterization of Two Novel Rumen-Derived Exo-Polygalacturonases: Catalysis and Molecular Simulations. Microorganisms. 2023; 11(3):760. https://doi.org/10.3390/microorganisms11030760
Chicago/Turabian StyleDeng, Qian, Xiaobao Sun, Deying Gao, Yuting Wang, Yu Liu, Nuo Li, Zhengguang Wang, Mingqi Liu, Jiakun Wang, and Qian Wang. 2023. "Characterization of Two Novel Rumen-Derived Exo-Polygalacturonases: Catalysis and Molecular Simulations" Microorganisms 11, no. 3: 760. https://doi.org/10.3390/microorganisms11030760
APA StyleDeng, Q., Sun, X., Gao, D., Wang, Y., Liu, Y., Li, N., Wang, Z., Liu, M., Wang, J., & Wang, Q. (2023). Characterization of Two Novel Rumen-Derived Exo-Polygalacturonases: Catalysis and Molecular Simulations. Microorganisms, 11(3), 760. https://doi.org/10.3390/microorganisms11030760






