Legionella pneumophila and Free-Living Nematodes: Environmental Co-Occurrence and Trophic Link
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling Campaign
2.2. Determination of the Nematode Fauna
2.3. Cultivation of Bacteria, Amoebae and Nematodes
2.3.1. Bacteria
2.3.2. Amoebae
2.3.3. Nematodes
2.4. Confocal Laser-Scanner Microscopy
2.5. Infection of A. castellanii
2.6. Pharyngeal Pumping Assay
2.6.1. Experimental Set-Up
2.6.2. Food Resources
2.6.3. Effector Proteins
2.7. Nematode Fitness
2.8. Statistics
3. Results
3.1. Co-Occurrence of Free-Living Nematodes and Legionella pneumophila in Natural and Technical Water Habitats
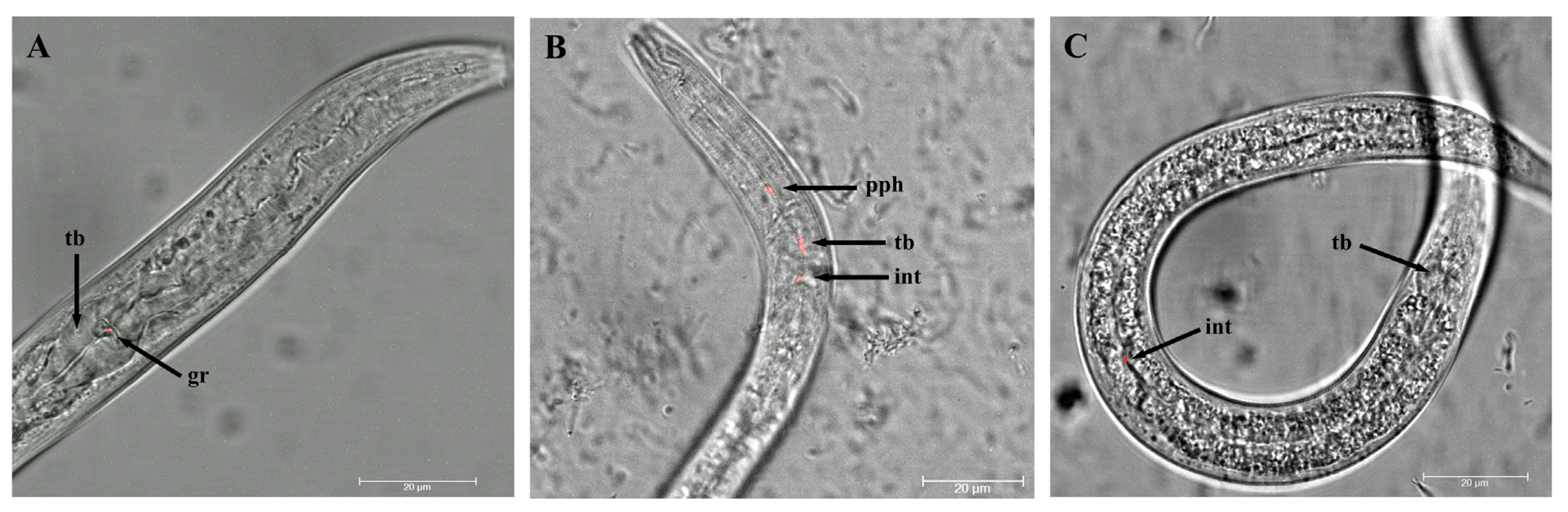
3.2. Ingestion of Legionella pneumophila by Plectus similis and Plectus sp.
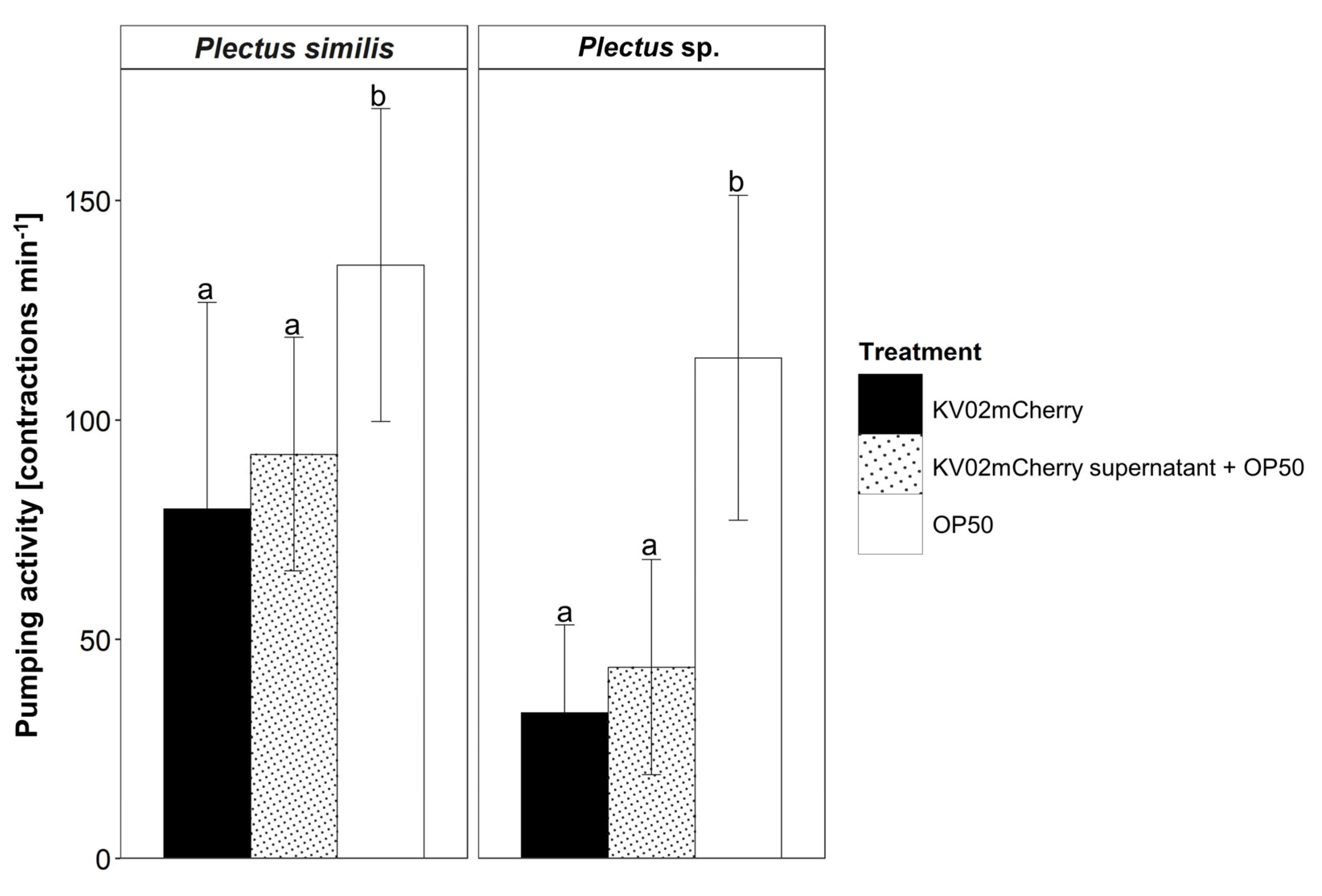
3.3. Legionella pneumophila Affects Pharyngeal Pumping Activity in Plectus similis and Plectus sp.
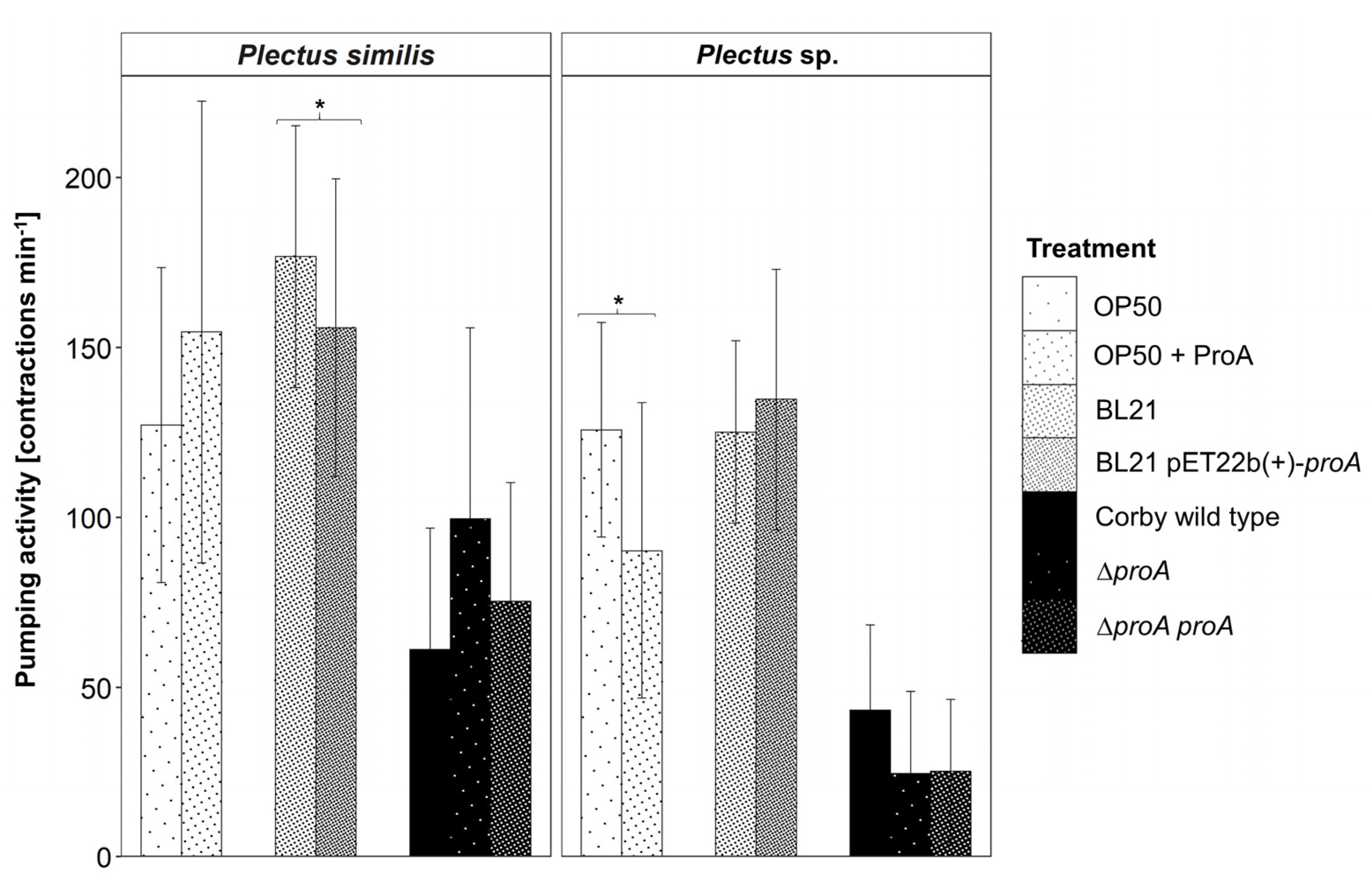
3.4. Effector Protein ProA as a Prospect Candidate for the Regulation of Pumping Activity
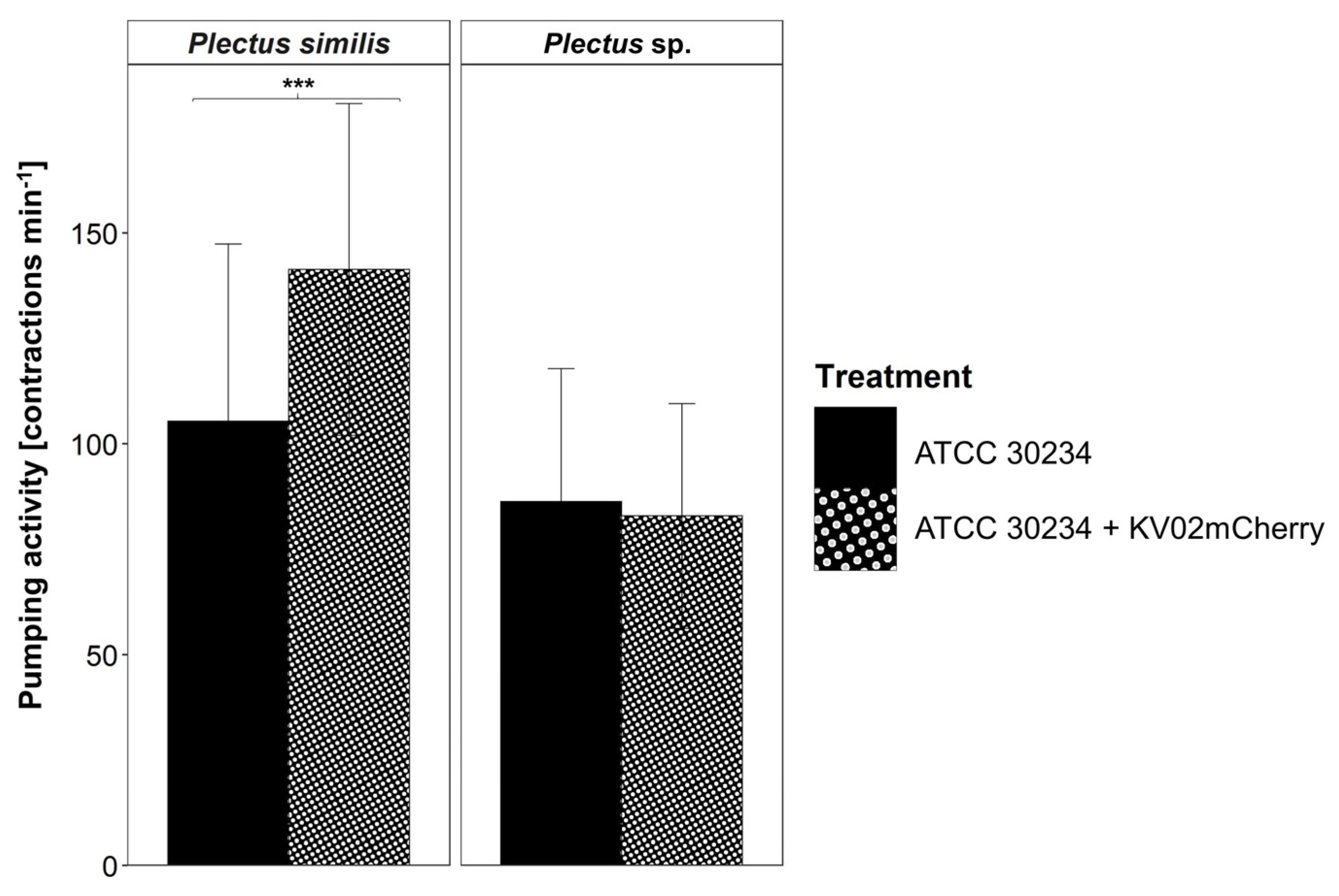
3.5. Acanthamoeba castellanii as Potential Promotor for Legionella pneumophila Ingestion
3.6. Influence of Diet on Overall Nematode Feeding Behavior
3.7. Effect of Legionella pneumophila Supernatant on Nematode Fitness
4. Discussion
4.1. Free-Living Nematodes and L. pneumophila Co-Occur in Cooling Towers
4.2. L. pneumophila Diet Reduces Feeding Activity in Free-Living Nematodes
4.3. L. pneumophila Reduces Fitness of Free-Living Nematodes
4.4. A. castellanii as Trojan Horse for L. pneumophila Transmission
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mondino, S.; Schmidt, S.; Rolando, M.; Escoll, P.; Gomez-Valero, L.; Buchrieser, C. Legionnaires’ disease: State of the art knowledge of pathogenesis mechanisms of Legionella. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 439–466. [Google Scholar]
- Horwitz, M.A.; Silverstein, S.C. Legionnaires’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Investig. 1980, 66, 441–450. [Google Scholar]
- Finsel, I.; Hilbi, H. Formation of a pathogen vacuole according to Legionella pneumophila: How to kill one bird with many stones. Cell. Microbiol. 2015, 17, 935–950. [Google Scholar]
- Atlas, R.M. Legionella: From environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1999, 1, 283–293. [Google Scholar]
- Borella, P.; Guerrieri, E.; Marchesi, I.; Bondi, M.; Messi, P. Water ecology of Legionella and protozoan: Environmental and public health perspectives. Biotechnol. Annu. Rev. 2005, 11, 355–380. [Google Scholar] [PubMed]
- Newton, H.J.; Ang, D.K.; Van Driel, I.R.; Hartland, E.L. Molecular pathogenesis of infections caused by Legionella pneumophila. Clin. Microbiol. Rev. 2010, 23, 274–298. [Google Scholar] [PubMed]
- Rogers, J.; Dowsett, A.B.; Dennis, P.J.; Lee, J.V.; Keevil, C. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl. Environ. Microbiol. 1994, 60, 1585–1592. [Google Scholar] [PubMed]
- Phin, N.; Parry-Ford, F.; Harrison, T.; Stagg, H.R.; Zhang, N.; Kumar, K.; Lortholary, O.; Zumla, A.; Abubakar, I. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect. Dis. 2014, 14, 1011–1021. [Google Scholar] [PubMed]
- Lueck, C.; Brzuszkiewicz, E.; Rydzewski, K.; Koshkolda, T.; Sarnow, K.; Essig, A.; Heuner, K. Subtyping of the Legionella pneumophila “Ulm” outbreak strain using the CRISPR–Cas system. Int. J. Med. Microbiol. 2015, 305, 828–837. [Google Scholar]
- Maisa, A.; Brockmann, A.; Renken, F.; Lück, C.; Pleischl, S.; Exner, M.; Jurke, A. Epidemiological investigation and case–control study: A Legionnaires’ disease outbreak associated with cooling towers in Warstein, Germany, August–September 2013. Eurosurveillance 2015, 20, 30064. [Google Scholar]
- Molofsky, A.B.; Swanson, M.S. Differentiate to thrive: Lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 2004, 53, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Ross, K.; Bentham, R. Legionella, protozoa, and biofilms: Interactions within complex microbial systems. Microb. Ecol. 2009, 58, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, T.J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 1980, 33, 1179–1183. [Google Scholar] [CrossRef]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef]
- Swart, A.L.; Harrison, C.F.; Eichinger, L.; Steinert, M.; Hilbi, H. Acanthamoeba and Dictyostelium as cellular models for Legionella infection. Front. Cell. Infect. Microbiol. 2018, 8, 61. [Google Scholar] [CrossRef]
- Schroeder, F.; Traunspurger, W.; Pettersson, K.; Peters, L. Temporal changes in periphytic meiofauna in lakes of different trophic states. J. Limnol. 2012, 71, e23. [Google Scholar] [CrossRef]
- Traunspurger, W.; Wilden, B.; Majdi, N. An overview of meiofaunal and nematode distribution patterns in lake ecosystems differing in their trophic state. Hydrobiologia 2020, 847, 2665–2679. [Google Scholar] [CrossRef]
- Paranjape, K.; Bédard, É.; Shetty, D.; Hu, M.; Choon, F.C.P.; Prévost, M.; Faucher, S.P. Unravelling the importance of the eukaryotic and bacterial communities and their relationship with Legionella spp. ecology in cooling towers: A complex network. Microbiome 2020, 8, 157. [Google Scholar] [CrossRef]
- Sabater, S.; Vilalta, E.; Gaudes, A.; Guasch, H.; Munoz, I.; Romani, A. Ecological implications of mass growth of benthic cyanobacteria in rivers. Aquat. Microb. Ecol. 2003, 32, 175–184. [Google Scholar] [CrossRef]
- Mathieu, M.; Leflaive, J.; Ten-Hage, L.; De Wit, R.; Buffan-Dubau, E. Free-living nematodes affect oxygen turnover of artificial diatom biofilms. Aquat. Microb. Ecol. 2007, 49, 281–291. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- McCoy-Simandle, K.; Stewart, C.R.; Dao, J.; DebRoy, S.; Rossier, O.; Bryce, P.J.; Cianciotto, N.P. Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia. Infect. Immun. 2011, 79, 1984–1997. [Google Scholar] [CrossRef]
- White, R.C.; Cianciotto, N.P. Assessing the impact, genomics and evolution of type II secretion across a large, medically important genus: The Legionella type II secretion paradigm. Microb. Genom. 2019, 5, e000273. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.Y.; Vargas, P.; Cianciotto, N.P. The novel Legionella pneumophila type II secretion substrate NttC contributes to infection of amoebae Hartmannella vermiformis and Willaertia magna. Microbiology 2014, 160, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- White, R.C.; Truchan, H.K.; Zheng, H.; Tyson, J.Y.; Cianciotto, N.P. Type II secretion promotes bacterial growth within the Legionella-containing vacuole in infected amoebae. Infect. Immun. 2019, 87, e00374-19. [Google Scholar] [CrossRef] [PubMed]
- Hell, W.; Essig, A.; Bohnet, S.; Gatermann, S.; Marre, R. Cleavage of tumor necrosis factor-α by Legionella exoprotease. Apmis 1993, 101, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.Y.; Pearce, M.M.; Vargas, P.; Bagchi, S.; Mulhern, B.J.; Cianciotto, N.P. Multiple Legionella pneumophila Type II secretion substrates, including a novel protein, contribute to differential infection of the amoebae Acanthamoeba castellanii, Hartmannella vermiformis, and Naegleria lovaniensis. Infect. Immun. 2013, 81, 1399–1410. [Google Scholar] [CrossRef]
- Conlan, J.W.; Baskerville, A.; Ashworth, L.A.E. Separation of Legionella pneumophila Proteases and Purification of a Protease Which Produces Lesions Like Those of Legionnaires Disease in Guinea Pig Lung. Microbiol. 1986, 132, 1565–1574. [Google Scholar] [CrossRef]
- Scheithauer, L.; Thiem, S.; Schmelz, S.; Dellmann, A.; Büssow, K.; Brouwer, R.M.; Ünal, C.M.; Blankenfeldt, W.; Steinert, M. Zinc metalloprotease ProA of Legionella pneumophila increases alveolar septal thickness in human lung tissue explants by collagen IV degradation. Cell. Microbiol. 2021, 23, e13313. [Google Scholar] [CrossRef]
- Scheithauer, L.; Thiem, S.; Ünal, C.M.; Dellmann, A.; Steinert, M. Zinc metalloprotease ProA from Legionella pneumophila inhibits the pro-inflammatory host response by degradation of bacterial flagellin. Biomolecules 2022, 12, 624. [Google Scholar] [CrossRef]
- Rossier, O.; Dao, J.; Cianciotto, N.P. The type II secretion system of Legionella pneumophila elaborates two aminopeptidases, as well as a metalloprotease that contributes to differential infection among protozoan hosts. Appl. Environ. Microbiol. 2008, 74, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Hellinga, J.R.; Garduño, R.A.; Kormish, J.D.; Tanner, J.R.; Khan, D.; Buchko, K.; Jimenez, C.; Pinette, M.M.; Brassinga, A.K.C. Identification of vacuoles containing extraintestinal differentiated forms of Legionella pneumophila in colonized Caenorhabditis elegans soil nematodes. Microbiol. Open 2015, 4, 660–681. [Google Scholar] [CrossRef] [PubMed]
- Rasch, J.; Krüger, S.; Fontvieille, D.; Ünal, C.M.; Michel, R.; Labrosse, A.; Steinert, M. Legionella-protozoa-nematode interactions in aquatic biofilms and influence of Mip on Caenorhabditis elegans colonization. Int. J. Med. Microbiol. 2016, 306, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Brassinga, A.K.C.; Kinchen, J.M.; Cupp, M.E.; Day, S.R.; Hoffman, P.S.; Sifri, C.D. Caenorhabditis is a metazoan host for Legionella. Cell. Microbiol. 2010, 12, 343–361. [Google Scholar] [CrossRef]
- Kurz, C.L.; Ewbank, J.J. Caenorhabditis elegans for the study of host–pathogen interactions. Trends Microbiol. 2000, 8, 142–144. [Google Scholar] [CrossRef]
- Anderson, G.L.; Caldwell, K.N.; Beuchat, L.R.; Williams, P.L. Interaction of a free-living soil nematode, Caenorhabditis elegans, with surrogates of foodborne pathogenic bacteria. J. Food Prot. 2003, 66, 1543–1549. [Google Scholar] [CrossRef]
- Kroupitski, Y.; Pinto, R.; Bucki, P.; Belausov, E.; Ruess, L.; Spiegel, Y.; Sela, S. Acrobeloides buetschlii as a potential vector for enteric pathogens. Nematology 2015, 17, 447–457. [Google Scholar] [CrossRef]
- Walters, J.V.; Holcomb, R.R. Isolation of enteric pathogen from sewage-borne nematode. Nematologica 1967, 13, 155. [Google Scholar]
- Wasilewska, L.; Webster, J.M. Free-living nematodes as disease factors of man and his crops. Int. J. Environ. Stud. 1975, 7, 201–204. [Google Scholar] [CrossRef]
- Moens, T.; Van Gansbeke, D.; Vincx, M. Linking estuarine nematodes to their suspected food. A case study from the Westerschelde Estuary (south-west Netherlands). J. Mar. Biol. Assoc. UK 1999, 79, 1017–1027. [Google Scholar] [CrossRef]
- Moens, T.; Traunspurger, W.; Bergtold, M. Feeding ecology of free-living benthic nematodes. In Freshwater Nematodes. Ecology and Taxonomy; Abebe, E., Traunspurger, W., Andrássy, I., Eds.; CAB International Publishing: Wallingford, UK, 2006; pp. 105–131. [Google Scholar]
- Zhou, Y.; Falck, J.R.; Rothe, M.; Schunck, W.H.; Menzel, R. Role of CYP-eicosanoids in the regulation of pharyngeal pumping and food uptake in C. elegans. J. Lipid Res. 2015, 56, 2110–2123. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qin, L.W.; Li, R.; Zhang, C.; Al-Sheikh, U.; Wu, Z.X. Reciprocal modulation of 5-HT and octopamine regulates pumping via feedforward and feedback circuits in C. elegans. Proc. Natl. Acad. Sci. USA 2019, 116, 7107–7112. [Google Scholar] [CrossRef]
- Fueser, H.; Rauchschwalbe, M.T.; Höss, S.; Traunspurger, W. Food bacteria and synthetic microparticles of similar size influence pharyngeal pumping of Caenorhabditis elegans. Aquat. Toxicol. 2021, 235, 105827. [Google Scholar] [CrossRef]
- Gaur, A.V.; Agarwal, R. Risperidone induced alterations in feeding and locomotion behavior of Caenorhabditis elegans. Curr. Res. Toxicol. 2021, 2, 367–374. [Google Scholar] [CrossRef]
- Chiang, J.T.A.; Steciuk, M.; Shtonda, B.; Avery, L. Evolution of pharyngeal behaviors and neuronal functions in free-living soil nematodes. J. Exp. Biol. 2006, 209, 1859–1873. [Google Scholar] [CrossRef]
- Bongers, T. De Nematoden van Nederland; Stichting Uitgeverij van de Koninklijke Natuurhistorische Vereniging: Utrecht, The Netherlands, 1994; pp. 1–408. [Google Scholar]
- Fang-Yen, C.; Avery, L.; Samuel, A.D. Two size-selective mechanisms specifically trap bacteria-sized food particles in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 20093–20096. [Google Scholar] [CrossRef]
- Song, B.M.; Avery, L. The pharynx of the nematode C. elegans: A model system for the study of motor control. Worm 2013, 2, e21833. [Google Scholar] [CrossRef]
- Ruess, L. Studies on the nematode fauna of an acid forest soil: Spatial distribution and extraction. Nematologica 1995, 41, 229–239. [Google Scholar] [CrossRef]
- Holovachov, O.; Boström, S. Identification of Plectida (Nematoda); EUMAINE, Gent and Nematology; UC Riverside: Riverside, CA, USA, 2010; Available online: http://www.nrm.se/download/18.9ff3752132fdaeccb6800015609/PLECTIDA%5B1%5D.pdf (accessed on 12 November 2020).
- Zell, H. Die Gattung Plectus Bastian, 1865 Sensu lato (Nematoda, Plectidae)–ein Beitrag zur Ökologie, Biogeographie, Phylogenie und Taxonomie der Plectidae; Staatliches Museum für Naturkunde Karlsruhe: Karlsruhe, Germany, 1993; pp. 1–172. [Google Scholar]
- Andrássy, I. Free-Living Nematodes of Hungary (Nematoda errantia), I; Hungarian Natural History Museum: Budapest, Hungary, 2005; pp. 1–518. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.W.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [PubMed]
- Charpentier, X.; Kay, E.; Schneider, D.; Shuman, H.A. Antibiotics and UV radiation induce competence for natural transformation in Legionella pneumophila. J. Bacteriol. 2011, 193, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.L.; Johnson, J.J.; Wang, S.; Sibley, M.H.; Gupta, M.C.; Kramer, J.M. Type IV collagen is detectable in most, but not all, basement membranes of Caenorhabditis elegans and assembles on tissues that do not express it. J. Cell Biol. 1997, 137, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Sileika, T.; Warta, R.; Cianciotto, N.P.; Packman, A. Role of bacterial adhesion in the microbial ecology of biofilms in cooling tower systems. Biofouling 2009, 25, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, L.; Tandoi, V.; Congestri, R.; Rossetti, S.; Di Pippo, F. Unravelling the core microbiome of biofilms in cooling tower systems. Biofouling 2017, 33, 793–806. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Majdi, N.; Traunspurger, W.; Fueser, H.; Gansfort, B.; Laffaille, P.; Maire, A. Effects of a broad range of experimental temperatures on the population growth and body-size of five species of free-living nematodes. J. Therm. Biol. 2019, 80, 21–36. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Schenk, J.; Traunspurger, W.; Ristau, K. Genetic diversity of widespread moss-dwelling nematode species in German beech forests. Eur. J. Soil Biol. 2016, 74, 23–31. [Google Scholar] [CrossRef]
- Shevchenko, V.L.; Zhylina, T.M. Taxonomic structure of nematode communities of epiphytic mosses in green plantations of Chernihiv, Ukraine. Вестнuк 3ooлoгuu 2016, 50, 477–482. [Google Scholar] [CrossRef]
- Gibbs, D.S.; Anderson, G.L.; Beuchat, L.R.; Carta, L.K.; Williams, P.L. Potential role of Diploscapter sp. strain LKC25, a bacterivorous nematode from soil, as a vector of food-borne pathogenic bacteria to preharvest fruits and vegetables. Appl. Environ. Microbiol. 2005, 71, 2433–2437. [Google Scholar] [CrossRef]
- Bergtold, M.; Mayr, G.; Traunspurger, W. Nematodes in wastewater biofilms—Appearance and density of species in three biofilter reactors. Water Res. 2007, 4, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Vadeboncoeur, Y.; Steinman, A.D. Periphyton function in lake ecosystems. Sci. World J. 2002, 2, 1449–1468. [Google Scholar] [CrossRef]
- Gubelit, Y.I.; Grossart, H.P. New Methods, New Concepts: What Can Be Applied to Freshwater Periphyton? Front. Microbiol. 2020, 11, 1275. [Google Scholar] [CrossRef] [PubMed]
- Żbikowska, E.; Kletkiewicz, H.; Walczak, M.; Burkowska, A. Coexistence of Legionella pneumophila bacteria and free-living amoebae in lakes serving as a cooling system of a power plant. Water Air Soil Pollut. 2014, 225, 2066. [Google Scholar] [CrossRef]
- Schwake, D.O.; Alum, A.; Abbaszadegan, M. Legionella Occurrence beyond Cooling Towers and Premise Plumbing. Microorganisms 2021, 9, 2543. [Google Scholar] [CrossRef]
- Declerck, P. Biofilms: The environmental playground of Legionella pneumophila. Environ. Microbiol. 2010, 12, 557–566. [Google Scholar] [CrossRef]
- Abu Khweek, A.; Amer, A.O. Factors mediating environmental biofilm formation by Legionella pneumophila. Front. Cell. Infect. Microbiol. 2018, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Gaudes, A.; Sabater, S.; Vilalta, E.; Muñoz, I. The nematode community in cyanobacterial biofilms in the river Llobregat, Spain. Nematology 2006, 8, 909–919. [Google Scholar]
- Croll, N.A.; Zullini, A. 1972. Observations on the bionomics of the freshwater nematode Chromadorina bioculata. J. Nematol. 1972, 4, 256–260. [Google Scholar]
- Majdi, N.; Traunspurger, W.; Boyer, S.; Mialet, B.; Tackx, M.; Fernandez, R.; Gehner, S.; Ten-Hage, L.; Buffan-Dubau, E. Response of biofilm-dwelling nematodes to habitat changes in the Garonne River, France: Influence of hydrodynamics and microalgal availability. Hydrobiologia 2011, 673, 229–244. [Google Scholar] [CrossRef]
- Abada, E.A.E.; Sung, H.; Dwivedi, M.; Park, B.J.; Lee, S.K.; Ahnn, J. C. elegans behavior of preference choice on bacterial food. Mol. Cells 2009, 28, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Lynch, D.J.; Lee, K.S.; Levine, E.; Biron, D. A scalable method for automatically measuring pharyngeal pumping in C. elegans. J. Neurosci. Methods 2016, 274, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Iwanir, S.; Kopito, R.B.; Scholz, M.; Calarco, J.A.; Biron, D.; Levine, E. Serotonin-dependent kinetics of feeding bursts underlie a graded response to food availability in C. elegans. Nat. Commun. 2017, 8, 14221. [Google Scholar] [CrossRef]
- Buse, H.Y.; Ashbolt, N.J. Counting Legionella cells within single amoeba host cells. Appl. Environ. Microbiol. 2012, 78, 2070–2072. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Monsalve, G.C.; Tse, V.; Saiki, R.; Weng, E.; Lee, L.; Srinivasan, C.; Frand, A.R.; Clarke, C.F. Delayed accumulation of intestinal coliform bacteria enhances life span and stress resistance in Caenorhabditis elegans fed respiratory deficient E. coli. BMC Microbiol. 2012, 12, 300. [Google Scholar] [CrossRef]
- Flavell, S.W.; Pokala, N.; Macosko, E.Z.; Albrecht, D.R.; Larsch, J.; Bargmann, C.I. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 2013, 154, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Shtonda, B.B.; Avery, L. Dietary choice behavior in Caenorhabditis elegans. J. Exp. Biol. 2006, 209, 89–102. [Google Scholar] [CrossRef]
- Ben Arous, J.; Laffont, S.; Chatenay, D. Molecular and sensory basis of a food related two-state behavior in C. elegans. PLoS ONE 2009, 4, e7584. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Jain, S.; Oloketuyi, S.F. Bacteria and bacterial products: Foe and friends to Caenorhabditis elegans. Microbiol. Res. 2018, 215, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Darby, C.; Cosma, C.L.; Thomas, J.H.; Manoil, C. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 15202–15207. [Google Scholar] [CrossRef] [PubMed]
- Jousset, A.; Rochat, L.; Péchy-Tarr, M.; Keel, C.; Scheu, S.; Bonkowski, M. Predators promote defence of rhizosphere bacterial populations by selective feeding on non-toxic cheaters. ISME J. 2009, 3, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Kern, T.; Wolf, S.; Struck, U.; Ruess, L. Trophic and non-trophic interactions in binary links affect carbon flow in the soil micro-food web. Soil Biol. Biochem. 2019, 135, 239–247. [Google Scholar] [CrossRef]
- Jousset, A. Ecological and evolutive implications of bacterial defences against predators. Environ. Microbiol. 2012, 14, 1830–1843. [Google Scholar] [CrossRef]
- Guo, J.; Jing, X.; Peng, W.L.; Nie, Q.; Zhai, Y.; Shao, Z.; Zheng, L.; Cai, M.; Li, G.; Zuo, H.; et al. Comparative genomic and functional analyses: Unearthing the diversity and specificity of nematicidal factors in Pseudomonas putida strain 1A00316. Sci. Rep. 2016, 6, 29211. [Google Scholar] [CrossRef]
- Lian, L.H.; Tian, B.Y.; Xiong, R.; Zhu, M.Z.; Xu, J.; Zhang, K.Q. Proteases from Bacillus: A new insight into the mechanism of action for rhizobacterial suppression of nematode populations. Lett. Appl. Microbiol. 2007, 45, 262–269. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Haas, D.; Heeb, S. Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root-knot nematode Meloidogyne incognita. Appl. Environ. Microbiol. 2005, 71, 5646–5649. [Google Scholar] [CrossRef]
- Brouse, L.; Brouse, R.; Brouse, D. Natural pathogen control chemistry to replace toxic treatment of microbes and biofilm in cooling towers. Pathogens 2017, 6, 14. [Google Scholar] [CrossRef]
- Badegewässer Landkreis Oder-Spree. Available online: https://www.landkreis-oder-spree.de/media/custom/2689_1595_1.PDF?1530869752 (accessed on 20 October 2022).
- Flughafensee: Wassertemperatur. Available online: https://wasserportal.berlin.de/station.php?anzeige=g&sgrafik=mw&thema=owt&station=5800303 (accessed on 20 October 2022).
- Badegewässer Landkreis Barnim. Available online: https://presse.barnim.de/documents/tabelle-badewasserqualitaet-80833 (accessed on 20 October 2022).
- Badewarnungen für drei Seen. Available online: https://hohen-neuendorf.de/de/stadt-leben/aktuelles/badewarnungen-fuer-drei-seen (accessed on 20 October 2022).
- Am Wolfsschluchtkanal: Wassertemperatur. Available online: https://wasserportal.berlin.de/station.php?anzeige=g&sgrafik=tw&thema=owt&station=5800107 (accessed on 20 October 2022).
- Plötzensee: Wassertemperatur. Available online: https://wasserportal.berlin.de/station.php?anzeige=g&sgrafik=mw&thema=owt&station=5800312 (accessed on 20 October 2022).
- Biesdorfer Baggersee: Wassertemperatur. Available online: https://wasserportal.berlin.de/station.php?anzeige=g&sgrafik=mw&thema=owt&station=5800317 (accessed on 20 October 2022).
- Nayhauss, A. Ab ins Wasser! Das sind die besen Badeseen in Berlin. Available online: https://www.morgenpost.de/berlin/article214942137/Sportlich-schick-romantisch-Badeseen-fuer-jeden-Typ.html (accessed on 20 October 2022).

| Trophic Group | Genus | c-p | Swimming Lakes (n = 9) | Cooling Towers (n = 7) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Surface, Algae | Read, Macrophytes | Submerged Stones, Litter | CT 1 | CT 2 | CT 3 | CT 4 | CT 5 | CT 6 | CT 7 | |||
| Plant feeders | Aphelenchoides | 2 | 1.5 ± 1.3 | 2.8 ± 4.0 | 1.7 ± 3.0 | - | - | - | - | - | - | - |
| Filenchus | 2 | - | - | 3.5 ± 6.0 | 21.5 | - | - | 25.6 | - | - | - | |
| Bacterial feeders | Acrobeloides | 2 | - | - | - | - | - | 28.6 | 76.9 | - | - | - |
| Alaimus | 4 | 1.5 ± 1.3 | 2.1 ± 3.6 | - | - | - | - | - | - | - | - | |
| Bastiania | 3 | 0.7 ± 1.2 | - | - | - | - | - | - | - | - | - | |
| Chromadorina | 3 | 63.3 ± 36.7 | 136.2 ± 64.2 | 68.2 ± 45.0 | - | - | - | - | - | - | - | |
| Diplogasteritus | 1 | - | - | - | - | - | - | - | 9.5 ± 16.5 | - | - | |
| Diploscapter | 1 | - | - | - | - | - | - | - | 161.9 ± 16.5 | - | - | |
| Eucephalobus | 2 | - | - | - | - | - | - | - | - | - | 28.6 | |
| Eumonhystera | 1 | 16.1 ± 17.3 | 6.6 ± 5.7 | 1.1 ± 0.9 | - | - | - | - | - | - | - | |
| Heterocephalobus | 2 | 0.4 ± 0.7 | - | - | 10.8 | - | - | - | - | - | - | |
| Monhystrella | 1 | 0.7 ± 1.2 | - | - | - | - | - | - | - | - | - | |
| Panagrolaimus | 1 | 2.2 ± 2.1 | - | - | - | - | - | - | - | - | - | |
| Paraphanolaimus | 3 | - | - | 2.4 ± 4.1 | - | - | - | - | - | - | - | |
| Plectus | 2 | 3.4 ± 4.2 | 1.5 ± 1.3 | 1.1 ± 0.9 | 1044.6 | - | 28.6 | 282.1 | - | 28.6 | - | |
| Rhabdolaimus | 3 | - | 18.8 ± 32.5 | 7.0 ± 9.2 | - | - | - | - | - | - | - | |
| Omnivores | Achromadora | 3 | 0.8 ± 1.3 | - | 6.1 ± 6.1 | - | - | - | - | - | - | - |
| Epidorylaimus | 4 | - | - | 1.2 ± 2.1 | - | - | - | - | - | - | - | |
| Eudorylaimus | 4 | 0.7 ± 1.2 | - | - | - | - | - | - | - | - | - | |
| Laimydorus | 4 | 7.7 ± 13.3 | 38.3 ± 64.2 | 1.0 ± 1.7 | - | - | - | - | - | - | - | |
| Mesodorylaimus | 4 | 6.0 ± 9.4 | 88.3 ± 150.8 | 4.2 ± 3.8 | - | - | - | - | - | - | - | |
| Predators | Ironus | 4 | - | 2.2 ± 3.6 | - | - | - | - | - | - | - | - |
| Mononchus | 4 | - | 0.8 ± 1.4 | - | - | - | - | - | - | - | - | |
| Tobrilus | 3 | 60.7 ± 49.2 | 12.4 ± 10.7 | 17.9 ± 25.0 | - | - | - | - | - | - | - | |
| Total number of nematode genera | 14 | 11 | 12 | 3 | 0 | 2 | 3 | 2 | 1 | 1 | ||
| Detection Legionella pneumophila | - | - | - | + | + | + | + | + | - 1 | + | ||
| Strain | Plectus similis | Plectus sp. | ||||
|---|---|---|---|---|---|---|
| Inactive | Active | Feeding | Inactive | Active | Feeding | |
| L. pneumophila | ||||||
| KV02mCherry | 46 ± 17 ab | 54 ± 17 ab | 9 ± 5 | 77 ± 9 | 23 ± 9 | 14 ± 2 |
| Corby wild-type | 59 ± 12 a | 41 ± 12 a | 7 ± 3 | 71 ± 24 | 29 ± 24 | 13 ± 1 |
| ΔproA | 33 ± 8 ab | 67 ± 8 ab | 7 ± 1 | 68 ± 16 | 32 ± 16 | 14 ± 2 |
| ΔproA proA | 17 ± 4 b | 83 ± 4 b | 16 ± 5 | 59 ± 3 | 41 ± 3 | 12 ± 0 |
| E. coli OP50 | ||||||
| OP50 | 12 ± 1 a | 88 ± 1 a | 87 ± 1 a | 52 ± 9 | 48 ± 9 | 39 ± 2 a |
| OP50 + KV02mCherry filtrate | 21 ± 9 ab | 79 ± 9 ab | 21 ± 2 ab | 53 ± 8 | 47 ± 8 | 11 ± 1 b |
| OP50 + ProA | 76 ± 3 b | 24 ± 3 a | 12 ± 3 b | 66 ± 8 | 34 ± 8 | 21 ± 2 ab |
| E. coli BL21 | ||||||
| BL21 pET22b(+)-proA | 46 ± 6 | 54 ± 6 | 51 ± 6 | 41 ± 4 | 59 ± 4 | 54 ± 6 |
| BL21 | 54 ± 2 | 46 ± 2 | 44 ± 3 | 60 ± 11 | 40 ± 11 | 38 ± 12 |
| A. castellanii | ||||||
| ATCC 30234 | 56 ± 7 | 44 ± 7 | 41 ± 6 | 38 ±12 | 62 ± 12 | 52 ± 18 |
| ATCC 30234 + KV02mCherry | 46 ± 10 | 54 ± 10 | 49 ± 11 | 31 ±4 | 69 ± 4 | 58 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemmerling, C.; Labrosse, A.; Ruess, L.; Steinert, M. Legionella pneumophila and Free-Living Nematodes: Environmental Co-Occurrence and Trophic Link. Microorganisms 2023, 11, 738. https://doi.org/10.3390/microorganisms11030738
Hemmerling C, Labrosse A, Ruess L, Steinert M. Legionella pneumophila and Free-Living Nematodes: Environmental Co-Occurrence and Trophic Link. Microorganisms. 2023; 11(3):738. https://doi.org/10.3390/microorganisms11030738
Chicago/Turabian StyleHemmerling, Christin, Aurélie Labrosse, Liliane Ruess, and Michael Steinert. 2023. "Legionella pneumophila and Free-Living Nematodes: Environmental Co-Occurrence and Trophic Link" Microorganisms 11, no. 3: 738. https://doi.org/10.3390/microorganisms11030738
APA StyleHemmerling, C., Labrosse, A., Ruess, L., & Steinert, M. (2023). Legionella pneumophila and Free-Living Nematodes: Environmental Co-Occurrence and Trophic Link. Microorganisms, 11(3), 738. https://doi.org/10.3390/microorganisms11030738


_Brussaard.png)



