The Oral Microbiome as Mediator between Oral Hygiene and Its Impact on Nasopharyngeal Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data and Saliva Sample Collection
2.3. Oral Hygiene Assessment
2.4. 16S rRNA Gene Sequencing and Bioinformatics Analysis
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Associations of Oral Hygiene Factors with the Risk of NPC
3.3. Links between the Oral Microbiome Composition and NPC
3.4. Influence of Dental Fillings on Oral Microbiome Composition
3.5. Associations between the Oral Microbiome Composition and Oral Hygiene Score
3.6. Mediation Effects of Oral Microbiome on the Associations of Oral Hygiene with NPC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Kar, A.; Thakur, S.; Rao, V.U.S. Periodontitis and oral Cancer-A striking link. Oral Oncol. 2020, 106, 104630. [Google Scholar] [CrossRef] [PubMed]
- Ndegwa, N.; Ploner, A.; Liu, Z.W.; Roosaar, A.; Axell, T.; Ye, W.M. Association between poor oral health and gastric cancer: A prospective cohort study. Int. J. Cancer 2018, 143, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.G.; Luu, H.N.; Cai, H.; Xiang, Y.B.; Steinwandel, M.; Gao, Y.T.; Hargreaves, M.; Zheng, W.; Blot, W.J.; Long, J.R.; et al. Oral health and risk of colorectal cancer: Results from three cohort studies and a meta-analysis. Ann. Oncol. 2016, 27, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann. Oncol. 2017, 28, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Wen, W.; Long, J.; Zheng, W.; Blot, W.J.; Cai, Q. Association of oral health with lung cancer risk in a low-income population of African Americans and European Americans in the Southeastern United States. Lung. Cancer 2019, 127, 90–95. [Google Scholar] [CrossRef]
- Guha, N.; Boffetta, P.; Wunsch Filho, V.; Eluf Neto, J.; Shangina, O.; Zaridze, D.; Curado, M.P.; Koifman, S.; Matos, E.; Menezes, A.; et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: Results of two multicentric case-control studies. Am. J. Epidemiol. 2007, 166, 1159–1173. [Google Scholar] [CrossRef]
- Chang, J.S.; Lo, H.I.; Wong, T.Y.; Huang, C.C.; Lee, W.T.; Tsai, S.T.; Chen, K.C.; Yen, C.J.; Wu, Y.H.; Hsueh, W.T.; et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013, 49, 1010–1017. [Google Scholar] [CrossRef]
- Hashim, D.; Sartori, S.; Brennan, P.; Curado, M.P.; Wunsch-Filho, V.; Divaris, K.; Olshan, A.F.; Zevallos, J.P.; Winn, D.M.; Franceschi, S.; et al. The role of oral hygiene in head and neck cancer: Results from International Head and Neck Cancer Epidemiology (INHANCE) consortium. Ann. Oncol. 2016, 27, 1619–1625. [Google Scholar] [CrossRef]
- Vu, H.; Shin, Y.J.; Kong, M.S.; Kim, H.D. Smoking and Drinking Adjusted Association between Head and Neck Cancers and Oral Health Status Related to Periodontitis: A Meta-Analysis. J. Korean Med. Sci. 2021, 36, e98. [Google Scholar] [CrossRef]
- Tang, L.L.; Chen, Y.P.; Chen, C.B.; Chen, M.Y.; Chen, N.Y.; Chen, X.Z.; Du, X.J.; Fang, W.F.; Feng, M.; Gao, J.; et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun. 2021, 41, 1195–1227. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.W.; Hui, E.P.; Lo, K.W.; Lam, W.K.J.; Johnson, D.; Li, L.; Tao, Q.; Chan, K.C.A.; To, K.F.; King, A.D.; et al. Nasopharyngeal carcinoma: An evolving paradigm. Nat. Rev. Clin. Oncol. 2021, 18, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Abdulamir, A.S.; Hafidh, R.R.; Abdulmuhaimen, N.; Abubakar, F.; Abbas, K.A. The distinctive profile of risk factors of nasopharyngeal carcinoma in comparison with other head and neck cancer types. BMC Public Health 2008, 8, 400. [Google Scholar] [CrossRef]
- Xu, F.H.; Xiong, D.; Xu, Y.F.; Cao, S.M.; Xue, W.Q.; Qin, H.D.; Liu, W.S.; Cao, J.Y.; Zhang, Y.; Feng, Q.S.; et al. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein-Barr virus activation. J. Natl. Cancer Inst. 2012, 104, 1396–1410. [Google Scholar] [CrossRef]
- Cao, S.M.; Chen, S.H.; Qian, C.N.; Liu, Q.; Xia, Y.F. Familial nasopharyngeal carcinomas possess distinguished clinical characteristics in southern China. Chin. J. Cancer Res. 2014, 26, 543–549. [Google Scholar] [CrossRef]
- Bakkalci, D.; Jia, Y.; Winter, J.R.; Lewis, J.E.; Taylor, G.S.; Stagg, H.R. Risk factors for Epstein Barr virus-associated cancers: A systematic review, critical appraisal, and mapping of the epidemiological evidence. J. Glob. Health 2020, 10, 010405. [Google Scholar] [CrossRef]
- Liu, Z.; Chang, E.T.; Liu, Q.; Cai, Y.; Zhang, Z.; Chen, G.; Xie, S.H.; Cao, S.M.; Shao, J.Y.; Jia, W.H.; et al. Oral Hygiene and Risk of Nasopharyngeal Carcinoma-A Population-Based Case-Control Study in China. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1201–1207. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharm. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharm. 2018, 99, 883–893. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, R.J.; Barrionuevo, A.M.P.; Shigdel, R.; Lie, S.A.; Lin, H.; Real, F.G.; Ringel-Kulka, T.; Astrom, A.N.; Svanes, C. Association of oral bacteria with oral hygiene habits and self-reported gingival bleeding. J. Clin. Periodontol. 2022, 49, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Relvas, M.; Regueira-Iglesias, A.; Balsa-Castro, C.; Salazar, F.; Pacheco, J.J.; Cabral, C.; Henriques, C.; Tomas, I. Relationship between dental and periodontal health status and the salivary microbiome: Bacterial diversity, co-occurrence networks and predictive models. Sci. Rep. 2021, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Kageyama, S.; Furuta, M.; Tsuboi, H.; Takeuchi, K.; Shibata, Y.; Shimazaki, Y.; Akifusa, S.; Ninomiya, T.; Kiyohara, Y.; et al. Bacterial diversity in saliva and oral health-related conditions: The Hisayama Study. Sci. Rep. 2016, 6, 22164. [Google Scholar] [CrossRef]
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef]
- Banas, J.A.; Drake, D.R. Are the mutans streptococci still considered relevant to understanding the microbial etiology of dental caries? BMC Oral Health 2018, 18, 129. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol. 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Xu, Y.; Teng, F.; Huang, S.; Lin, Z.M.; Yuan, X.; Zeng, X.W.; Yang, F. Changes of saliva microbiota in nasopharyngeal carcinoma patients under chemoradiation therapy. Arch. Oral Biol. 2014, 59, 176–186. [Google Scholar] [CrossRef]
- Debelius, J.W.; Huang, T.T.; Cai, Y.L.; Ploner, A.; Barrett, D.; Zhou, X.Y.; Xiao, X.; Li, Y.C.; Liao, J.; Zheng, Y.M.; et al. Subspecies Niche Specialization in the Oral Microbiome Is Associated with Nasopharyngeal Carcinoma Risk. Msystems 2020, 5, e00065-20. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, J.B.; Lu, L.X.; Jia, Y.J.; Zheng, M.Q.; Debelius, J.W.; He, Y.Q.; Wang, T.M.; Deng, C.M.; Tong, X.T.; et al. Oral Microbiota Alteration and Roles in Epstein-Barr Virus Reactivation in Nasopharyngeal Carcinoma. Microbiol. Spectr. 2023, 11, e0344822. [Google Scholar] [CrossRef]
- Richiardi, L.; Bellocco, R.; Zugna, D. Mediation analysis in epidemiology: Methods, interpretation and bias. Int. J. Epidemiol. 2013, 42, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Menni, C.; Louca, P.; Berry, S.E.; Vijay, A.; Astbury, S.; Leeming, E.R.; Gibson, R.; Asnicar, F.; Piccinno, G.; Wolf, J.; et al. High intake of vegetables is linked to lower white blood cell profile and the effect is mediated by the gut microbiome. BMC Med. 2021, 19, 37. [Google Scholar] [CrossRef]
- Fu, J.; Xu, K.; Ni, X.; Li, X.; Zhu, X.; Xu, W. Habitual Dietary Fiber Intake, Fecal Microbiota, and Hemoglobin A1c Level in Chinese Patients with Type 2 Diabetes. Nutrients 2022, 14, 1003. [Google Scholar] [CrossRef]
- Wu, H.; Dong, C.; Xiao, W.; Wei, H.; Shao, Y.; Chen, T.; Xia, Y. Associations between PM2.5 exposure and infant growth: A mediation analysis of oral microbiota. Sci. Total Environ. 2022, 823, 153688. [Google Scholar] [CrossRef] [PubMed]
- Turkoz, F.P.; Celenkoglu, G.; Dogu, G.G.; Kalender, M.E.; Coskun, U.; Alkis, N.; Ozkan, M.; Turk, H.M.; Arslan, U.Y. Risk factors of nasopharyngeal carcinoma in Turkey-an epidemiological survey of the Anatolian Society of Medical Oncology. Asian Pac. J. Cancer Prev. 2011, 12, 3017–3021. [Google Scholar] [PubMed]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar] [CrossRef]
- Kanady, J.A.; Aruni, A.W.; Ninnis, J.R.; Hopper, A.O.; Blood, J.D.; Byrd, B.L.; Holley, L.R.; Staker, M.R.; Hutson, S.; Fletcher, H.M.; et al. Nitrate reductase activity of bacteria in saliva of term and preterm infants. Nitric Oxide 2012, 27, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Tagaino, R.; Washio, J.; Abiko, Y.; Tanda, N.; Sasaki, K.; Takahashi, N. Metabolic property of acetaldehyde production from ethanol and glucose by oral Streptococcus and Neisseria. Sci. Rep. 2019, 9, 10446. [Google Scholar] [CrossRef]
- Kato, A.; Imai, K.; Ochiai, K.; Ogata, Y. Prevalence and quantitative analysis of Epstein-Barr virus DNA and Porphyromonas gingivalis associated with Japanese chronic periodontitis patients. Clin. Oral Investig. 2015, 19, 1605–1610. [Google Scholar] [CrossRef]
- Kato, A.; Imai, K.; Sato, H.; Ogata, Y. Prevalence of Epstein-Barr virus DNA and Porphyromonas gingivalis in Japanese peri-implantitis patients. BMC Oral Health 2017, 17, 148. [Google Scholar] [CrossRef]
- Imai, K.; Inoue, H.; Tamura, M.; Cueno, M.E.; Inoue, H.; Takeichi, O.; Kusama, K.; Saito, I.; Ochiai, K. The periodontal pathogen Porphyromonas gingivalis induces the Epstein-Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie 2012, 94, 839–846. [Google Scholar] [CrossRef]
- Dzutsev, A.; Badger, J.H.; Perez-Chanona, E.; Roy, S.; Salcedo, R.; Smith, C.K.; Trinchieri, G. Microbes and Cancer. Annu. Rev. Immunol. 2017, 35, 199–228. [Google Scholar] [CrossRef]
- Chen, J.; Domingue, J.C.; Sears, C.L. Microbiota dysbiosis in select human cancers: Evidence of association and causality. Semin. Immunol. 2017, 32, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Li, H.; Wen, X.; Tan, X.; Yang, C.; Liu, N. Multi-Omics Integration Reveals the Crucial Role of Fusobacterium in the Inflammatory Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Microbiol. Spectr. 2022, 10, e0106822. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tian, W.; Wei, Q.; Xu, J. Involvement of Fusobacterium nucleatum in malignancies except for colorectal cancer: A literature review. Front. Immunol. 2022, 13, 968649. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Tan, X.R.; Li, H.; Li, J.Y.; Chen, X.Z.; Li, Y.Q.; Li, W.F.; Tang, L.L.; Zhou, G.Q.; Zhang, Y.; et al. Association of Intratumoral Microbiota With Prognosis in Patients With Nasopharyngeal Carcinoma From 2 Hospitals in China. JAMA Oncol. 2022, 8, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, A.; Li, J.; Zhang, F.; Zhong, P.; Li, Y.; Li, Y. Combined Non-Invasive Prediction and New Biomarkers of Oral and Fecal Microbiota in Patients With Gastric and Colorectal Cancer. Front. Cell. Infect. Microbiol. 2022, 12, 830684. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dou, L.; Zhang, Y.; He, S.; Zhao, D.; Hao, C.; Song, G.; Zhang, W.; Liu, Y.; Wang, G. Characterization of the Oral and Esophageal Microbiota in Esophageal Precancerous Lesions and Squamous Cell Carcinoma. Front. Cell. Infect. Microbiol. 2021, 11, 714162. [Google Scholar] [CrossRef]
- Nomburg, J.; Bullman, S.; Nasrollahzadeh, D.; Collisson, E.A.; Abedi-Ardekani, B.; Akoko, L.O.; Atkins, J.R.; Buckle, G.C.; Gopal, S.; Hu, N.; et al. An international report on bacterial communities in esophageal squamous cell carcinoma. Int. J. Cancer 2022, 151, 1947–1959. [Google Scholar] [CrossRef]
- Wirth, R.; Maroti, G.; Liptak, L.; Mester, M.; Al Ayoubi, A.; Pap, B.; Madlena, M.; Minarovits, J.; Kovacs, K.L. Microbiomes in supragingival biofilms and saliva of adolescents with gingivitis and gingival health. Oral Dis. 2022, 28, 2000–2014. [Google Scholar] [CrossRef]
- Barbagallo, G.; Santagati, M.; Guni, A.; Torrisi, P.; Spitale, A.; Stefani, S.; Ferlito, S.; Nibali, L. Microbiome differences in periodontal, peri-implant, and healthy sites: A cross-sectional pilot study. Clin. Oral Investig. 2022, 26, 2771–2781. [Google Scholar] [CrossRef]
- Yang, J.; He, P.; Zhou, M.; Li, S.; Zhang, J.; Tao, X.; Wang, A.; Wu, X. Variations in oral microbiome and its predictive functions between tumorous and healthy individuals. J. Med. Microbiol. 2022, 71, 001568. [Google Scholar] [CrossRef]
- Urbaniak, C.; Lorenzi, H.; Thissen, J.; Jaing, C.; Crucian, B.; Sams, C.; Pierson, D.; Venkateswaran, K.; Mehta, S. The influence of spaceflight on the astronaut salivary microbiome and the search for a microbiome biomarker for viral reactivation. Microbiome 2020, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Abedini, F.; Azimzadeh Jamalkandi, S.; Shariati, P.; Ahmadi, A.; Gholami Fesharaki, M. The composition of lung microbiome in lung cancer: A systematic review and meta-analysis. BMC Microbiol. 2021, 21, 315. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Aoki, Y.; Tamahara, T.; Goto, M.; Matsui, H.; Kawashima, J.; Danjoh, I.; Hozawa, A.; Kuriyama, S.; Suzuki, Y.; et al. Oral Microbiome Analysis in Prospective Genome Cohort Studies of the Tohoku Medical Megabank Project. Front. Cell. Infect. Microbiol. 2020, 10, 604596. [Google Scholar] [CrossRef]
- Ansiliero, R.; Gelinski, J.; Samistraro, Q.L.; Baratto, C.M.; Almeida, C.A.; Locatelli, C. Pathogenic Microbial Profile and Antibiotic Resistance Associated with Periodontitis. Indian J. Microbiol. 2021, 61, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Vashishta, A.; Jimenez-Flores, E.; Klaes, C.K.; Tian, S.; Miralda, I.; Lamont, R.J.; Uriarte, S.M. Putative Periodontal Pathogens, Filifactor Alocis and Peptoanaerobacter Stomatis, Induce Differential Cytokine and Chemokine Production by Human Neutrophils. Pathogens 2019, 8, 59. [Google Scholar] [CrossRef]
- Miralda, I.; Uriarte, S.M. Periodontal Pathogens’ strategies disarm neutrophils to promote dysregulated inflammation. Mol. Oral Microbiol. 2021, 36, 103–120. [Google Scholar] [CrossRef]
- Hall, V.; Collins, M.D.; Lawson, P.A.; Falsen, E.; Duerden, B.I. Actinomyces dentalis sp. nov., from a human dental abscess. Int. J. Syst. Evol. Microbiol. 2005, 55, 427–431. [Google Scholar] [CrossRef]
- Vielkind, P.; Jentsch, H.; Eschrich, K.; Rodloff, A.C.; Stingu, C.S. Prevalence of Actinomyces spp. in patients with chronic periodontitis. Int. J. Med. Microbiol. 2015, 305, 682–688. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Chen, X.; Jiang, W.; Wang, S.; Xu, L.; Tu, Y.; Zheng, P.; Wang, Y.; Lin, X.; et al. Profiling of Oral Microbiota in Early Childhood Caries Using Single-Molecule Real-Time Sequencing. Front. Microbiol. 2017, 8, 2244. [Google Scholar] [CrossRef]
- Skelly, E.; Johnson, N.W.; Kapellas, K.; Kroon, J.; Lalloo, R.; Weyrich, L. Response of Salivary Microbiota to Caries Preventive Treatment in Aboriginal and Torres Strait Islander Children. J. Oral Microbiol. 2020, 12, 1830623. [Google Scholar] [CrossRef]
- Han, X.Y.; Hong, T.; Falsen, E. Neisseria bacilliformis sp. nov. isolated from human infections. J. Clin. Microbiol. 2006, 44, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, Y.; Xie, H.; Wang, X.; Wu, J.; Long, J.; Courtney, R.; Shu, X.O.; Zheng, W.; Blot, W.J.; et al. Association of oral microbiota with lung cancer risk in a low-income population in the Southeastern USA. Cancer Causes Control 2021, 32, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Q.; Shu, X.O.; Steinwandel, M.D.; Blot, W.J.; Zheng, W.; Long, J. Prospective study of oral microbiome and colorectal cancer risk in low-income and African American populations. Int. J. Cancer 2019, 144, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Soergel, D.A.; Repo, S.; Brenner, S.E. Association of gut microbiota with post-operative clinical course in Crohn’s disease. BMC Gastroenterol. 2013, 13, 131. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Lee, E.; Park, S.; Um, S.; Kim, S.; Lee, J.; Jang, J.; Jeong, H.O.; Shin, J.; Kang, J.; Lee, S.; et al. Microbiome of Saliva and Plaque in Children According to Age and Dental Caries Experience. Diagnostics 2021, 11, 1324. [Google Scholar] [CrossRef]
- Ren, W.; Xun, Z.; Wang, Z.; Zhang, Q.; Liu, X.; Zheng, H.; Zhang, Q.; Zhang, Y.; Zhang, L.; Wu, C.; et al. Tongue Coating and the Salivary Microbial Communities Vary in Children with Halitosis. Sci. Rep. 2016, 6, 24481. [Google Scholar] [CrossRef]
- Ozbey, G.; Sproston, E.; Hanafiah, A. Helicobacter pylori Infection and Gastric Microbiota. Euroasian J. Hepato-Gastroenterol. 2020, 10, 36–41. [Google Scholar] [CrossRef]
- Kang, X.; Lu, B.; Xiao, P.; Hua, Z.; Shen, R.; Wu, J.; Wu, J.; Wu, Z.; Cheng, C.; Zhang, J. Microbial Characteristics of Common Tongue Coatings in Patients with Precancerous Lesions of the Upper Gastrointestinal Tract. J. Health Eng. 2022, 2022, 7598427. [Google Scholar] [CrossRef]
- Mougeot, J.C.; Beckman, M.F.; Langdon, H.C.; Lalla, R.V.; Brennan, M.T.; Bahrani Mougeot, F.K. Haemophilus pittmaniae and Leptotrichia spp. Constitute a Multi-Marker Signature in a Cohort of Human Papillomavirus-Positive Head and Neck Cancer Patients. Front. Microbiol. 2021, 12, 794546. [Google Scholar] [CrossRef]
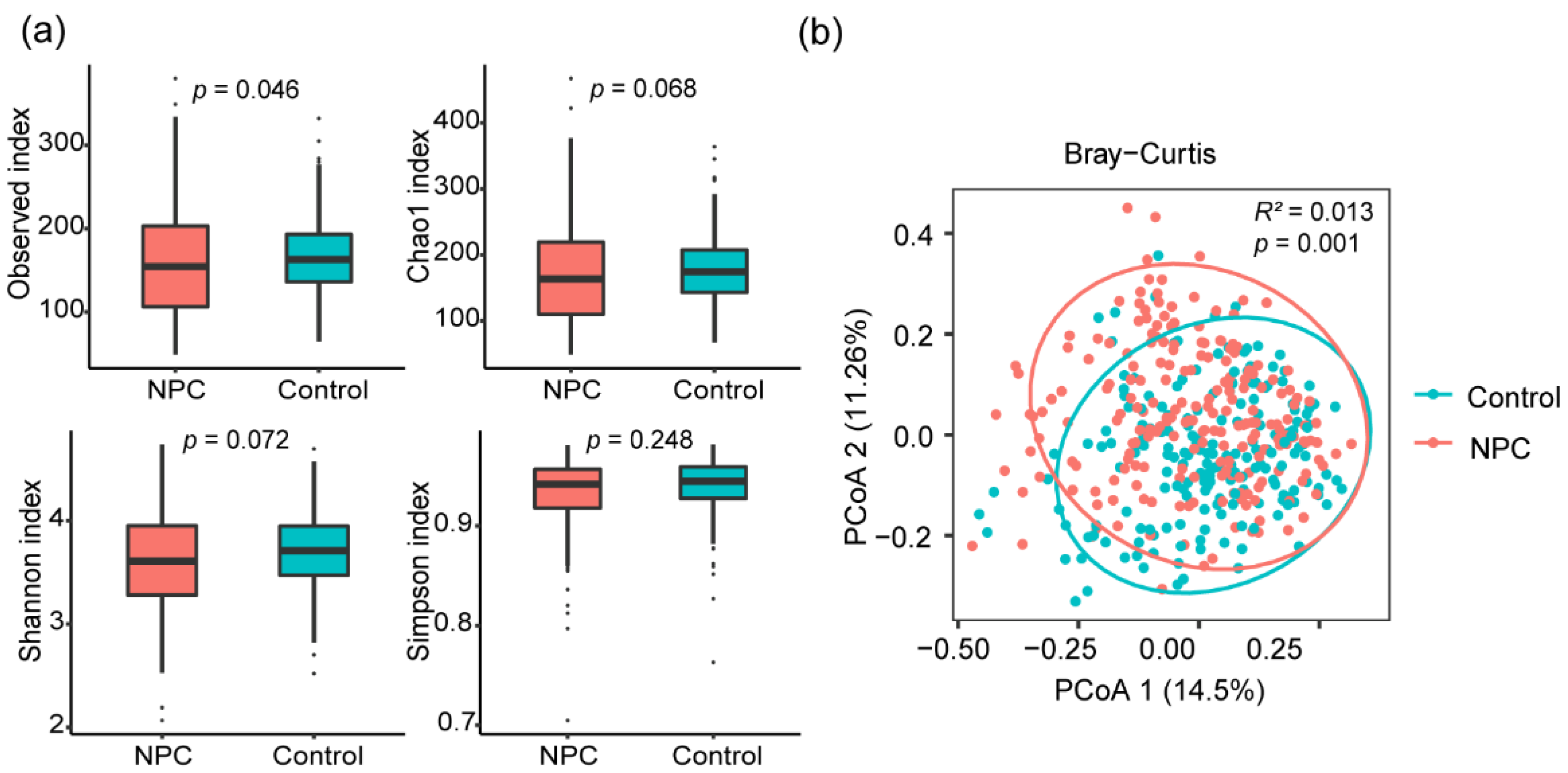
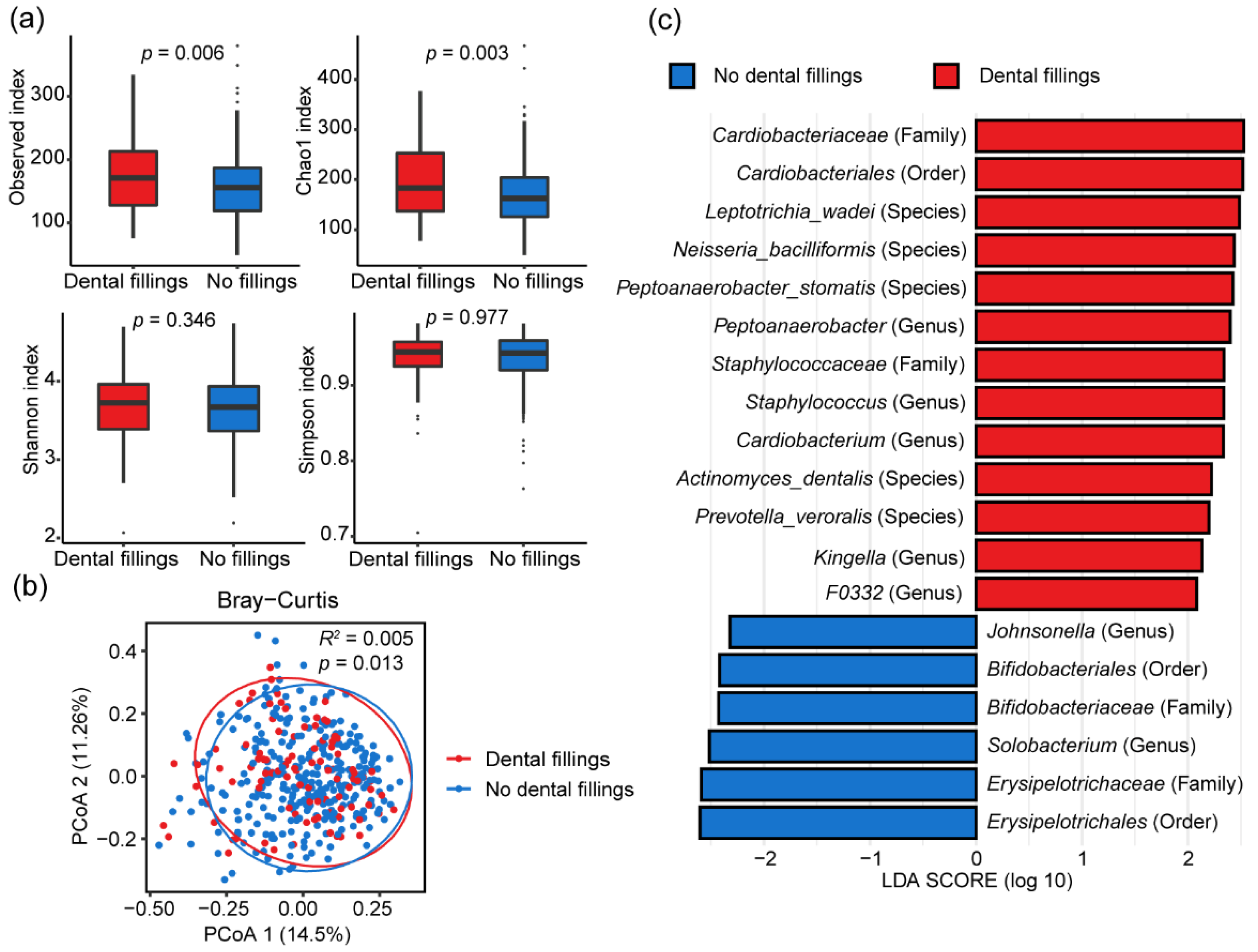

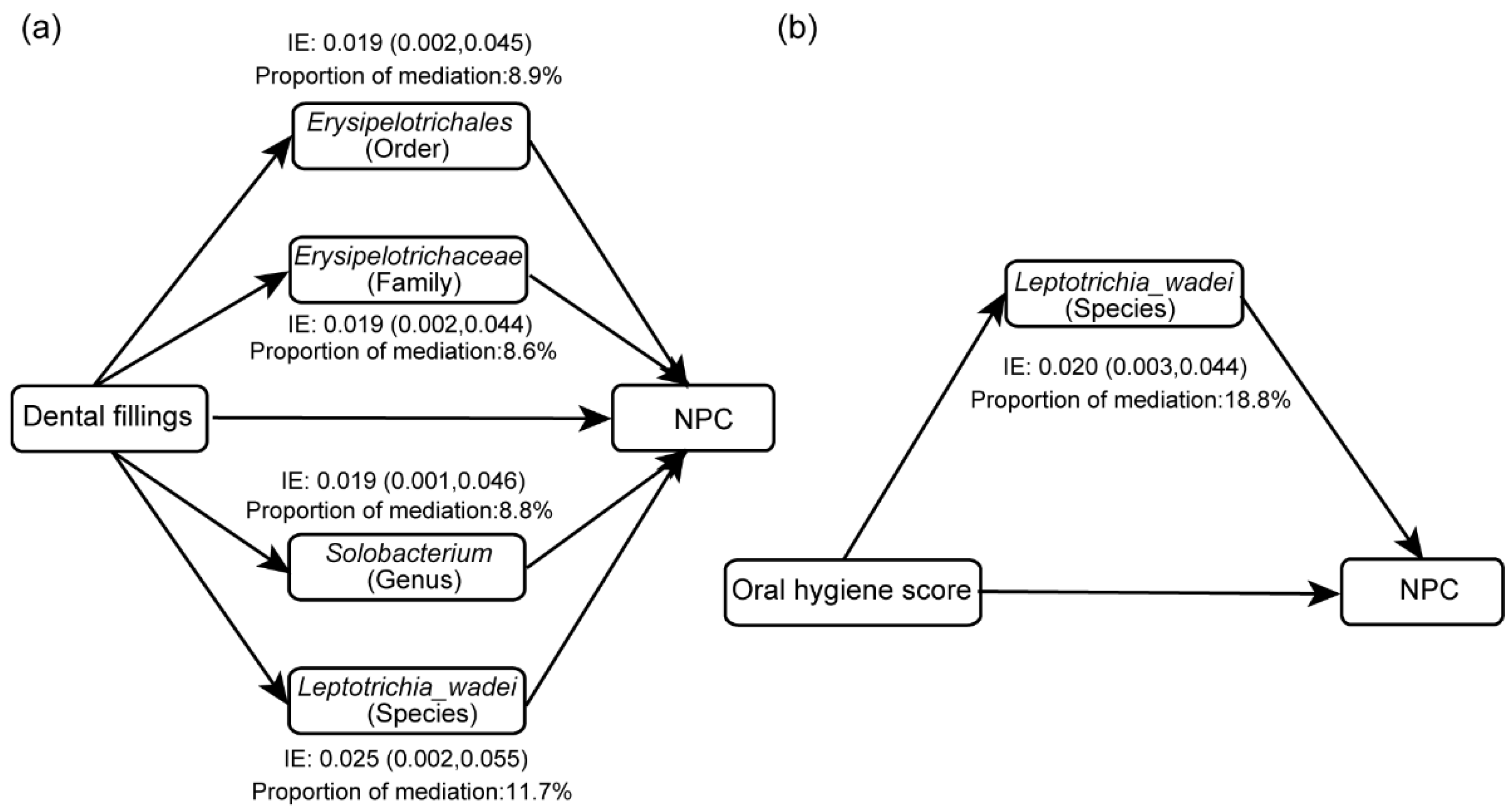
| Characteristics | NPC (n = 218) | Control (n = 192) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 48.33 (10.07) | 46.53 (10.33) | 0.075 a |
| Sex, n (%) | 0.876 b | ||
| Male | 157 (72.02) | 136 (70.83) | |
| Female | 61 (27.98) | 56 (29.17) | |
| Educational level, n (%) c | 0.009 b | ||
| <High school | 178 (81.65) | 134 (70.16) | |
| ≥High school | 40 (18.35) | 57 (29.84) | |
| Cigarette smoking, n (%) | 0.119 b | ||
| Never | 85 (38.99) | 89 (46.35) | |
| Former | 32 (14.68) | 17 (8.85) | |
| Current | 101 (46.33) | 86 (44.79) | |
| Alcohol drinking, n (%) | 0.915 b | ||
| Non-drinker | 152 (69.72) | 132 (68.75) | |
| Drinker | 66 (30.28) | 60 (31.25) |
| Oral Hygiene | NPC n (%) | Controls n (%) | OR (95% CI) a | p-Value a | OR (95% CI) b | p-Value b |
|---|---|---|---|---|---|---|
| Tooth loss | 0.042 | 0.207 | ||||
| No | 95 (43.58) | 103 (53.65) | 1.00 | 1.00 | ||
| Yes | 123 (56.42) | 89 (46.35) | 1.50 (1.02, 2.22) | 1.32 (0.86, 2.02) | ||
| Dental fillings | 0.008 | <0.001 | ||||
| No | 155 (71.10) | 158 (82.29) | 1.00 | 1.00 | ||
| Yes | 63 (28.90) | 34 (17.71) | 1.89 (1.18,3.05) | 2.51 (1.52, 4.25) | ||
| Tooth brushing | 0.091 | 0.442 | ||||
| ≥2 times per day | 71 (32.57) | 78 (40.62) | 1.00 | 1.00 | ||
| <2 times per day | 147 (67.43) | 114 (59.38) | 1.42 (0.95, 2.12) | 1.18 (0.77, 1.81) | ||
| Oral hygiene score | 0.013 | 0.040 | ||||
| Good (0–1) | 106 (48.62) | 117 (60.94) | 1.00 | 1.00 | ||
| Poor (2–3) | 112 (51.38) | 75 (39.06) | 1.65 (1.11, 2.45) | 1.54 (1.02, 2.33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.-Y.; Liao, Y.; Wu, Y.-X.; Diao, H.; Du, Y.; Chen, Y.-W.; Xie, J.-R.; Xue, W.-Q.; He, Y.-Q.; Wang, T.-M.; et al. The Oral Microbiome as Mediator between Oral Hygiene and Its Impact on Nasopharyngeal Carcinoma. Microorganisms 2023, 11, 719. https://doi.org/10.3390/microorganisms11030719
Liu Q-Y, Liao Y, Wu Y-X, Diao H, Du Y, Chen Y-W, Xie J-R, Xue W-Q, He Y-Q, Wang T-M, et al. The Oral Microbiome as Mediator between Oral Hygiene and Its Impact on Nasopharyngeal Carcinoma. Microorganisms. 2023; 11(3):719. https://doi.org/10.3390/microorganisms11030719
Chicago/Turabian StyleLiu, Qiao-Yun, Ying Liao, Yan-Xia Wu, Hua Diao, Yan Du, Yi-Wei Chen, Jin-Ru Xie, Wen-Qiong Xue, Yong-Qiao He, Tong-Min Wang, and et al. 2023. "The Oral Microbiome as Mediator between Oral Hygiene and Its Impact on Nasopharyngeal Carcinoma" Microorganisms 11, no. 3: 719. https://doi.org/10.3390/microorganisms11030719
APA StyleLiu, Q.-Y., Liao, Y., Wu, Y.-X., Diao, H., Du, Y., Chen, Y.-W., Xie, J.-R., Xue, W.-Q., He, Y.-Q., Wang, T.-M., Zheng, X.-H., & Jia, W.-H. (2023). The Oral Microbiome as Mediator between Oral Hygiene and Its Impact on Nasopharyngeal Carcinoma. Microorganisms, 11(3), 719. https://doi.org/10.3390/microorganisms11030719







