Changing Rhizosphere Microbial Community and Metabolites with Developmental Stages of Coleus barbatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. DNA Isolation from Soil Samples
2.3. Preparation of Libraries
2.4. Cluster Generation and Sequencing
2.5. Computational Analysis
2.5.1. QIIME Analysis
2.5.2. Diversity Comparison
2.6. Metabolite Quantification
2.6.1. Preparation of Extracts for Phytochemical Quantification
2.6.2. Flavonoid Quantification
2.6.3. Phenolic Quantification
2.6.4. Terpenoid Quantification
2.6.5. Alkaloid Quantification
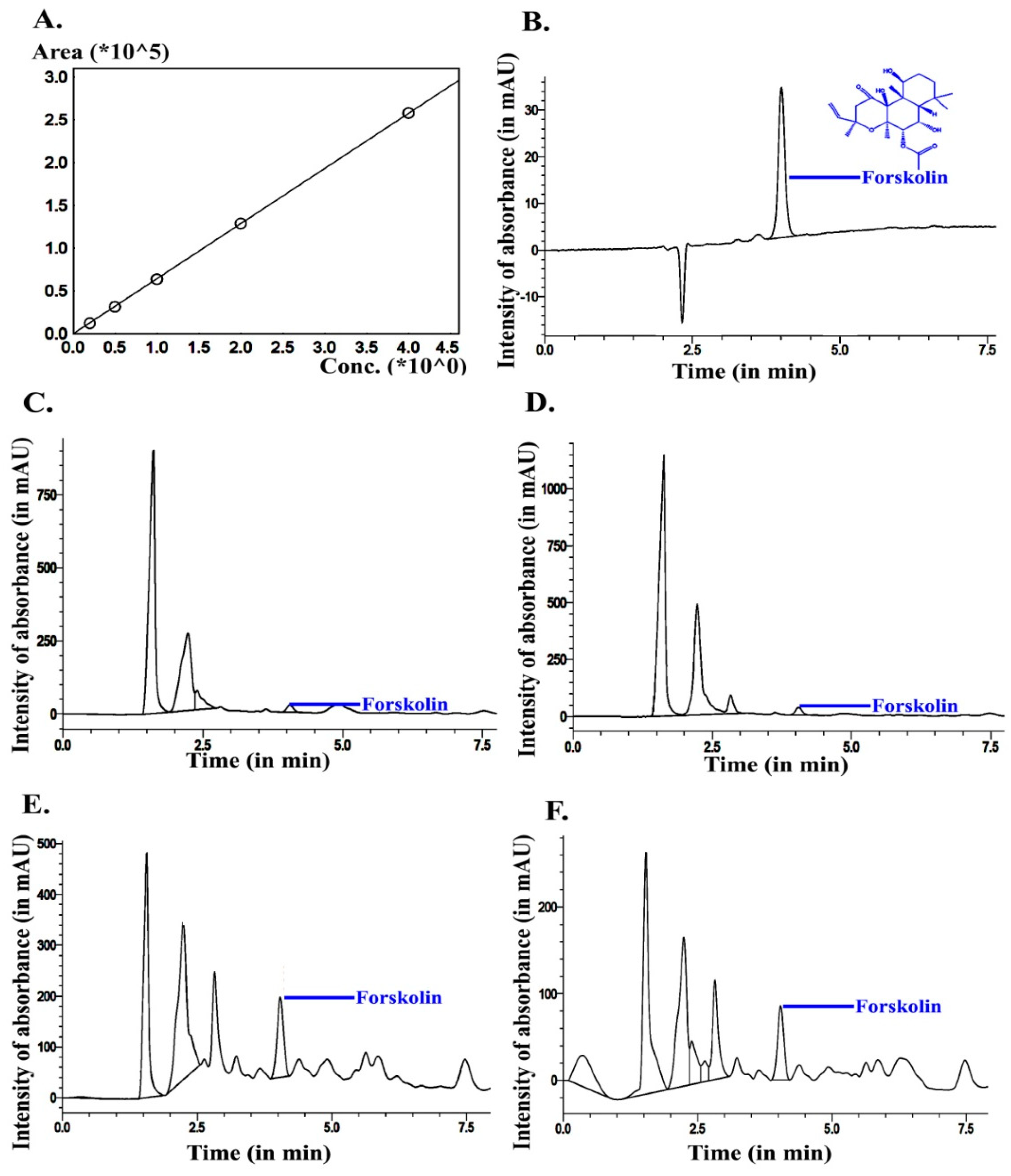
2.6.6. Forskolin Quantification
3. Results
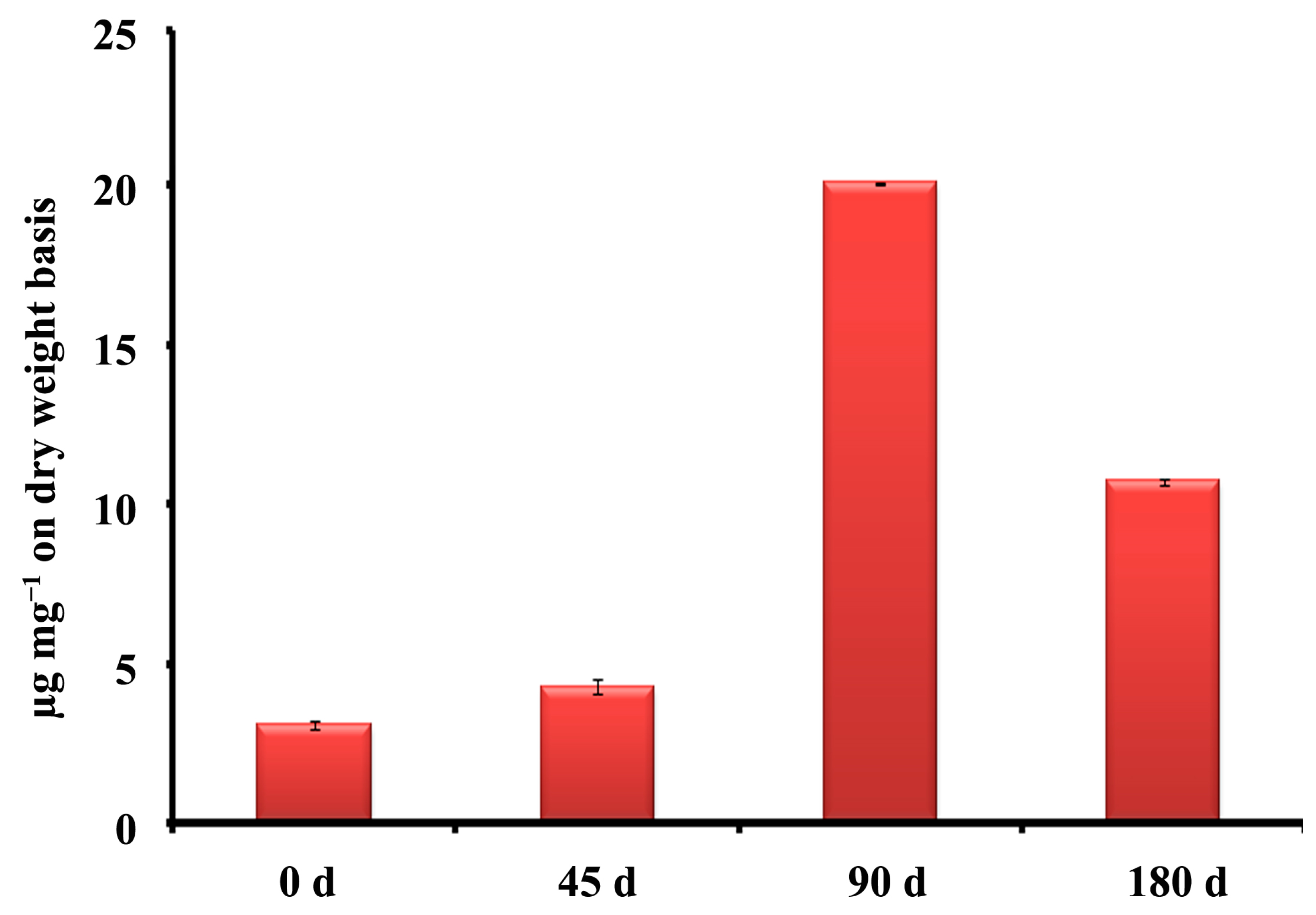
3.1. Quantification of Bulk Phytochemicals and Forskolin at Different Developmental Stages
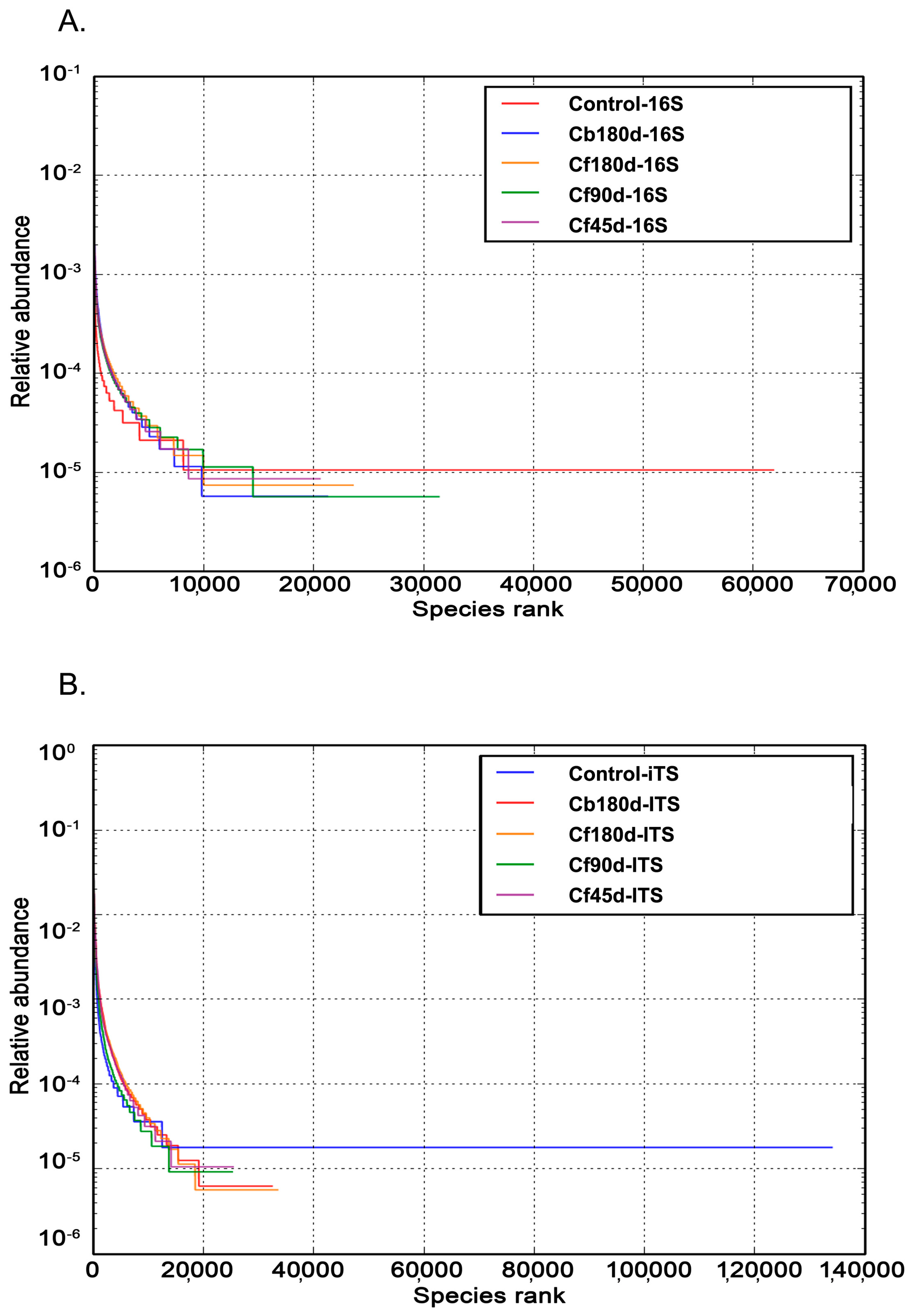
3.2. Sequencing of Rhizospheric DNA
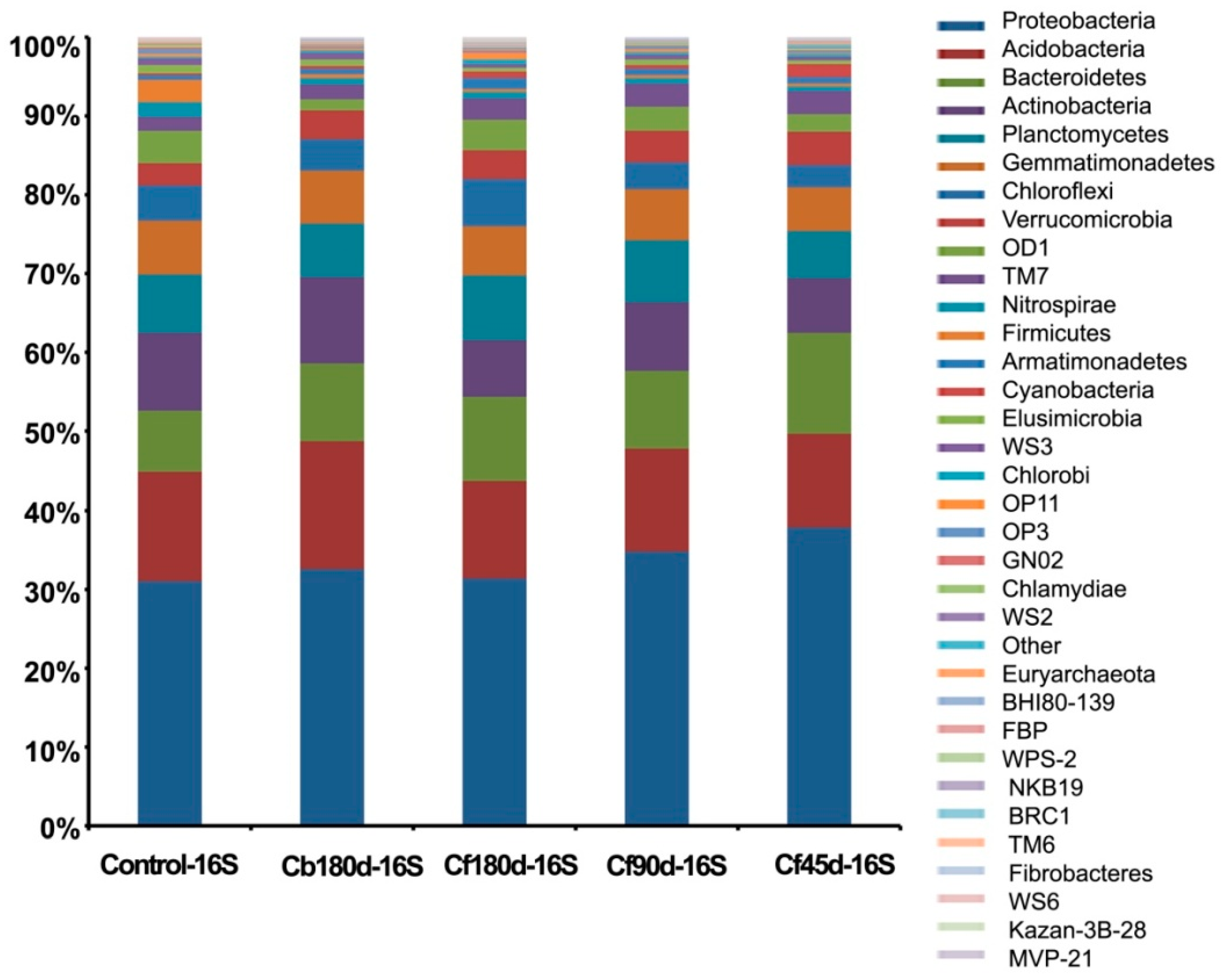
3.3. Taxonomic Composition of Rhizosphere
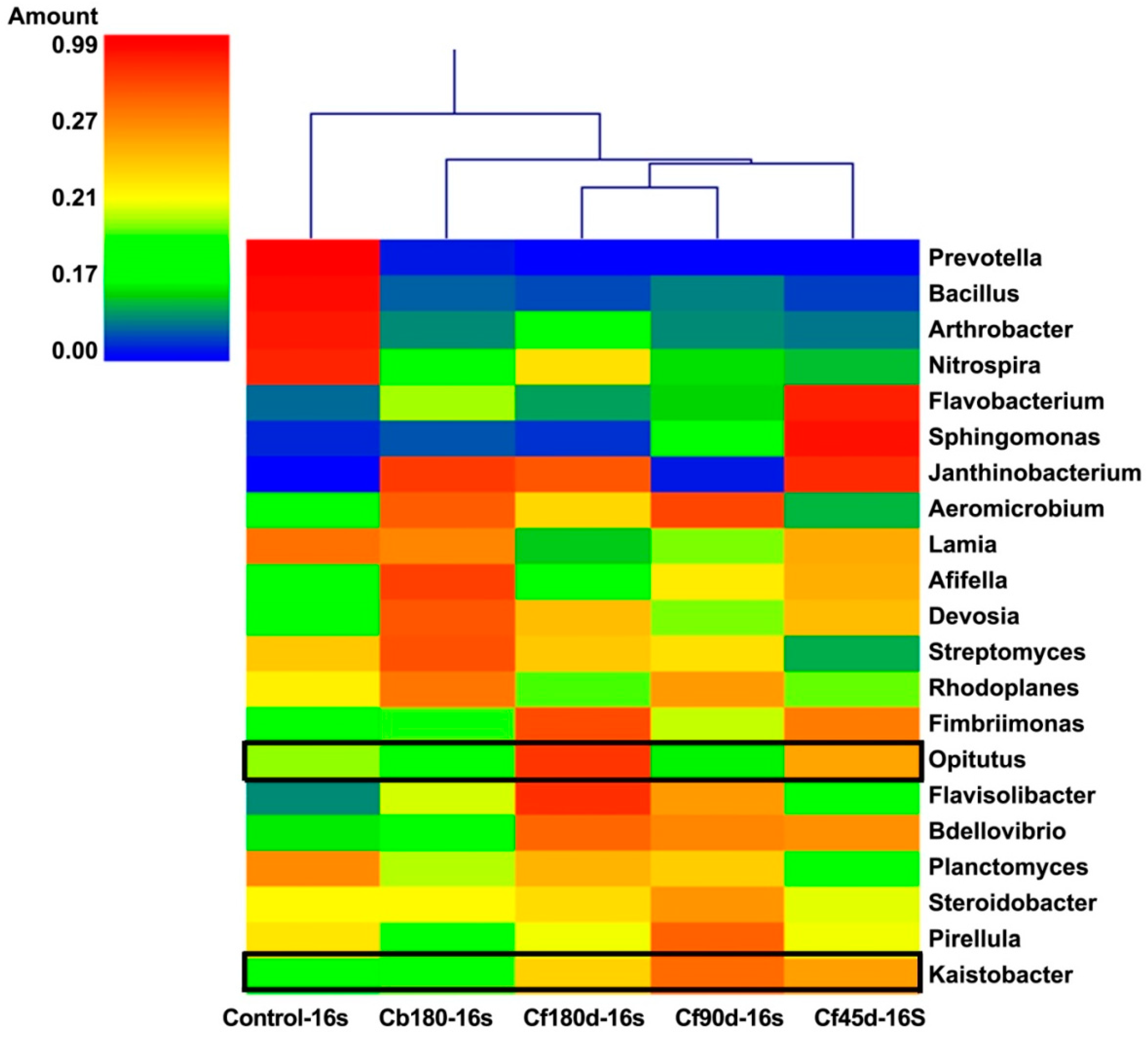
3.3.1. Bacterial Diversity
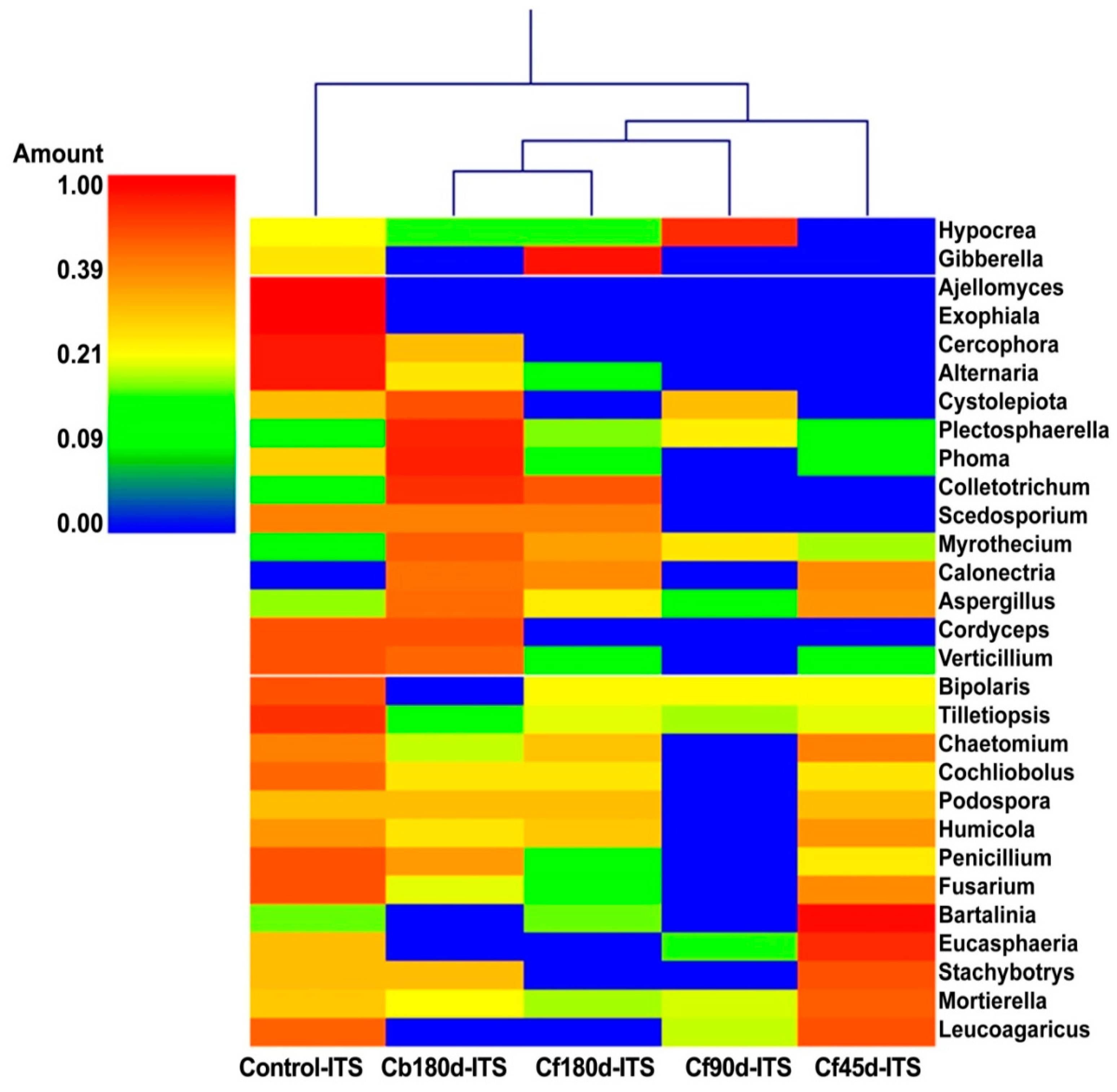
3.3.2. Fungal Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hinsinger, P.; Plassard, C.; Jaillard, B. Rhizosphere: A New Frontier for Soil Biogeochemistry. J. Geochem. Explor. 2006, 88, 210–213. [Google Scholar] [CrossRef]
- Rovira, A.D. Interactions between Plant Roots and Soil Microorganisms. Annu. Rev. Microbiol. 1965, 19, 241–266. [Google Scholar] [CrossRef] [PubMed]
- De Boer, W.; Kowalchuk, G.A.; Van Veen, J.A. ‘Root-Food’and the Rhizosphere Microbial Community Composition. New Phytol. 2006, 170, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.; Leeflang, P.; Glandorf, B.; Dirk van Elsas, J.; Wernars, K. Analysis of Fungal Diversity in the Wheat Rhizosphere by Sequencing of Cloned PCR-Amplified Genes Encoding 18S RRNA and Temperature Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 1999, 65, 2614–2621. [Google Scholar] [CrossRef] [PubMed]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE Database for Molecular Identification of Fungi--Recent Updates and Future Perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- van Elsas, J.D.; Duarte, G.F.; Keijzer-Wolters, A.; Smit, E. Analysis of the Dynamics of Fungal Communities in Soil via Fungal-Specific PCR of Soil DNA Followed by Denaturing Gradient Gel Electrophoresis. J. Microbiol. Methods 2000, 43, 133–151. [Google Scholar] [CrossRef]
- Mougel, C.; Offre, P.; Ranjard, L.; Corberand, T.; Gamalero, E.; Robin, C.; Lemanceau, P. Dynamic of the Genetic Structure of Bacterial and Fungal Communities at Different Developmental Stages of Medicago Truncatula Gaertn. Cv. Jemalong Line J5. New Phytol. 2006, 170, 165–175. [Google Scholar] [CrossRef]
- Baudoin, E.; Benizri, E.; Guckert, A. Impact of Growth Stage on the Bacterial Community Structure along Maize Roots, as Determined by Metabolic and Genetic Fingerprinting. Appl. Soil Ecol. 2002, 19, 135–145. [Google Scholar] [CrossRef]
- Houlden, A.; Timms-Wilson, T.M.; Day, M.J.; Bailey, M.J. Influence of Plant Developmental Stage on Microbial Community Structure and Activity in the Rhizosphere of Three Field Crops. FEMS Microbiol. Ecol. 2008, 65, 193–201. [Google Scholar] [CrossRef]
- Huang, X.-F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere Interactions: Root Exudates, Microbes, and Microbial Communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere Microbiome Assemblage Is Affected by Plant Development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, J.; Zhang, L.; Cheng, R.; Wei, G.; Su, H.; Yang, J.; Qian, J.; Xu, R.; Chen, S. Rhizospheric Microbial Communities Are Driven by Panax Ginseng at Different Growth Stages and Biocontrol Bacteria Alleviates Replanting Mortality. Acta Pharm. Sin. B 2018, 8, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Baca, C.P.; Rivers, A.R.; Kim, W.; Iwata, R.; McClung, A.M.; Roberts, D.P.; Reddy, V.R.; Barnaby, J.Y. Changes in Rhizosphere Soil Microbial Communities across Plant Developmental Stages of High and Low Methane Emitting Rice Genotypes. Soil Biol. Biochem. 2021, 156, 108233. [Google Scholar] [CrossRef]
- Dibner, R.R.; Weaver, A.M.; Brock, M.T.; Custer, G.F.; Morrison, H.G.; Maignien, L.; Weinig, C. Time Outweighs the Effect of Host Developmental Stage on Microbial Community Composition. FEMS Microbiol. Ecol. 2021, 97, fiab102. [Google Scholar] [CrossRef] [PubMed]
- Buyer, J.S.; Roberts, D.P.; Russek-Cohen, E. Soil and Plant Effects on Microbial Community Structure. Can. J. Microbiol. 2002, 48, 955–964. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The Diversity and Biogeography of Soil Bacterial Communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Grayston, S.J.; Wang, S.; Campbell, C.D.; Edwards, A.C. Selective Influence of Plant Species on Microbial Diversity in the Rhizosphere. Soil Biol. Biochem. 1998, 30, 369–378. [Google Scholar] [CrossRef]
- Costa, R.; Götz, M.; Mrotzek, N.; Lottmann, J.; Berg, G.; Smalla, K. Effects of Site and Plant Species on Rhizosphere Community Structure as Revealed by Molecular Analysis of Microbial Guilds. FEMS Microbiol. Ecol. 2006, 56, 236–249. [Google Scholar] [CrossRef]
- De Vries, F.T.; Manning, P.; Tallowin, J.R.B.; Mortimer, S.R.; Pilgrim, E.S.; Harrison, K.A.; Hobbs, P.J.; Quirk, H.; Shipley, B.; Cornelissen, J.H.C. Abiotic Drivers and Plant Traits Explain Landscape-scale Patterns in Soil Microbial Communities. Ecol. Lett. 2012, 15, 1230–1239. [Google Scholar] [CrossRef]
- Lareen, A.; Burton, F.; Schäfer, P. Plant Root-Microbe Communication in Shaping Root Microbiomes. Plant Mol. Biol. 2016, 90, 575–587. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant Species and Soil Type Cooperatively Shape the Structure and Function of Microbial Communities in the Rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, Variation, and Assembly of the Root-Associated Microbiomes of Rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef]
- Robinson, R.J.; Fraaije, B.A.; Clark, I.M.; Jackson, R.W.; Hirsch, P.R.; Mauchline, T.H. Endophytic Bacterial Community Composition in Wheat (Triticum Aestivum) Is Determined by Plant Tissue Type, Developmental Stage and Soil Nutrient Availability. Plant Soil 2016, 405, 381–396. [Google Scholar] [CrossRef]
- Essarioui, A.; LeBlanc, N.; Kistler, H.C.; Kinkel, L.L. Plant Community Richness Mediates Inhibitory Interactions and Resource Competition between Streptomyces and Fusarium Populations in the Rhizosphere. Microb. Ecol. 2017, 74, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of Root System Architecture on Rhizosphere and Root Microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Contreras, J.; Hupp, B.M.; Lindenberger, J.H.; Chen, D.; Zhang, Q.; Wang, C.; Twigg, P.; Saleem, M. Root Microbiome Changes with Root Branching Order and Root Chemistry in Peach Rhizosphere Soil. Rhizosphere 2020, 16, 100249. [Google Scholar] [CrossRef]
- Rasche, F.; Hödl, V.; Poll, C.; Kandeler, E.; Gerzabek, M.H.; Van Elsas, J.D.; Sessitsch, A. Rhizosphere Bacteria Affected by Transgenic Potatoes with Antibacterial Activities Compared with the Effects of Soil, Wild-Type Potatoes, Vegetation Stage and Pathogen Exposure. FEMS Microbiol. Ecol. 2006, 56, 219–235. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Chen, T.; Wang, W.; Liu, G.; Zhang, W.; Li, S.; Wang, M.; Zhao, C.; Zhou, H.; et al. Plant Phenotypic Traits Eventually Shape Its Microbiota: A Common Garden Test. Front. Microbiol. 2018, 9, 2479. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, G.; Yu, Z.; Ding, H.; Xu, Y.; Zhang, Z. Effect of Drought Stress and Developmental Stages on Microbial Community Structure and Diversity in Peanut Rhizosphere Soil. Int. J. Mol. Sci. 2019, 20, 2265. [Google Scholar] [CrossRef]
- Ammon, H.P.; Kemper, F.H. Ayurveda: 3000 years of Indian traditional medicine. Med. Welt 1982, 33, 148–153. [Google Scholar]
- Fradkin, J.E.; Cook, G.H.; Kilhoffer, M.C.; Wolff, J. Forskolin Stimulation of Thyroid Adenylate Cyclase and Cyclic 3′,5′-Adenosine Monophosphate Accumulation. Endocrinology 1982, 111, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Birnbaumer, L.; Stengel, D.; Desmier, M.; Hanoune, J. Forskolin Regulation of Liver Membrane Adenylyl Cyclase. Eur. J. Biochem. 1983, 136, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kelecom, A. Isolation, Structure Determination, and Absolute Configuration of Barbatusol, a New Bioactive Diterpene with a Rearranged Abietane Skeleton from the Labiate Coleus Barbatus. Tetrahedron 1983, 39, 3603–3608. [Google Scholar] [CrossRef]
- Seamon, K.B.; Daly, J.W.; Metzger, H.; de Souza, N.J.; Reden, J. Structure-Activity Relationships for Activation of Adenylate Cyclase by the Diterpene Forskolin and Its Derivatives. J. Med. Chem. 1983, 26, 436–439. [Google Scholar] [CrossRef]
- Costa, R.M.; Silva, A.J. Mouse Models of Neurofibromatosis Type I: Bridging the GAP. Trends Mol. Med. 2003, 9, 19–23. [Google Scholar] [CrossRef]
- Matu, E.N.; van Staden, J. Antibacterial and Anti-Inflammatory Activities of Some Plants Used for Medicinal Purposes in Kenya. J. Ethnopharmacol. 2003, 87, 35–41. [Google Scholar] [CrossRef]
- Runyoro, D.K.; Matee, M.I.; Ngassapa, O.D.; Joseph, C.C.; Mbwambo, Z.H. Screening of Tanzanian Medicinal Plants for Anti-Candida Activity. BMC Complement Altern Med. 2006, 6, 11. [Google Scholar] [CrossRef]
- Lindner, E.; Dohadwalla, A.N.; Bhattacharya, B.K. Positive Inotropic and Blood Pressure Lowering Activity of a Diterpene Derivative Isolated from Coleus Forskohli: Forskolin. Arzneimittelforschung 1978, 28, 284–289. [Google Scholar]
- Dubey, M.P.; Srimal, R.C.; Nityanand, S.; Dhawan, B.N. Pharmacological Studies on Coleonol, a Hypotensive Diterpene from Coleus Forskohlii. J. Ethnopharmacol. 1981, 3, 1–13. [Google Scholar] [CrossRef]
- Reichling, J.; Nolkemper, S.; Stintzing, F.C.; Schnitzler, P. Impact of Ethanolic Lamiaceae Extracts on Herpesvirus Infectivity in Cell Culture. Forsch Komplementmed 2008, 15, 313–320. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic Acid and Its Derivatives: Biotechnology and Applications. Crit Rev. Biotechnol. 2012, 32, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Szmigielska, A.M.; Van Rees, K.C.J.; Cieslinski, G.; Huang, P.M. Low Molecular Weight Dicarboxylic Acids in Rhizosphere Soil of Durum Wheat. J. Agric. Food Chem. 1996, 44, 1036–1040. [Google Scholar] [CrossRef]
- Cieśliński, G.; Van Rees, K.C.J.; Szmigielska, A.M.; Krishnamurti, G.S.R.; Huang, P.M. Low-Molecular-Weight Organic Acids in Rhizosphere Soils of Durum Wheat and Their Effect on Cadmium Bioaccumulation. Plant Soil 1998, 203, 109–117. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Kõljalg, U.; Larsson, K.H.; Abarenkov, K.; Nilsson, R.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; et al. UNITE: A Database Providing Web-Based Methods for the Molecular Identification of Ectomycorrhizal Fungi. New Phytol. 2005, 166, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Env. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a Unified Paradigm for Sequence-Based Identification of Fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, H.R.; Schigel, D.; Tedersoo, L.; Larsson, K.H.; May, T.W.; Taylor, A.F.S.; Jeppesen, T.S.; Frøslev, T.G.; Lindahl, B.D.; et al. The Taxon Hypothesis Paradigm-On the Unambiguous Detection and Communication of Taxa. Microorganisms 2020, 8, 1910. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE Database for Molecular Identification of Fungi: Handling Dark Taxa and Parallel Taxonomic Classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, Y.; Zhang, J.; Lu, X.; Wei, G. Naturally Selected Dominant Weeds as Heavy Metal Accumulators and Excluders Assisted by Rhizosphere Bacteria in a Mining Area. Chemosphere 2020, 243, 125365. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, P.; Mahajan, V.; Jamwal, V.L.; Kapoor, N.; Rasool, S.; Bedi, Y.S.; Gandhi, S.G. Cloning and Expression Analysis of Chalcone Synthase Gene from Coleus Forskohlii. J. Genet. 2016, 95, 647–657. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Nunez, M.J. Extraction of Antioxidant Phenolics from Almond Hulls (Prunus Amygdalus) and Pine Sawdust (Pinus Pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of Total Terpenoids Concentration in Plant Tissues Using a Monoterpene, Linalool as Standard Reagent. Res. Sq. 2012, 5. [Google Scholar] [CrossRef]
- Ajanal, M.; Gundkalle, M.B.; Nayak, S.U. Estimation of Total Alkaloid in Chitrakadivati by UV-Spectrophotometer. Anc. Sci. Life 2012, 31, 198. [Google Scholar] [CrossRef]
- Foster, S.D.; Dunstan, P.K. The Analysis of Biodiversity Using Rank Abundance Distributions. Biometrics 2010, 66, 186–195. [Google Scholar] [CrossRef]
- Wawrik, B.; Kerkhof, L.; Kukor, J.; Zylstra, G. Effect of Different Carbon Sources on Community Composition of Bacterial Enrichments from Soil. Appl. Env. Microbiol. 2005, 71, 6776–6783. [Google Scholar] [CrossRef]
- Schimel, J.P.; Gulledge, J.M.; Clein-Curley, J.S.; Lindstrom, J.E.; Braddock, J.F. Moisture Effects on Microbial Activity and Community Structure in Decomposing Birch Litter in the Alaskan Taiga. Soil Biol. Biochem. 1999, 31, 831–838. [Google Scholar] [CrossRef]
- Stachel, S.E.; Nester, E.W. The Genetic and Transcriptional Organization of the Vir Region of the A6 Ti Plasmid of Agrobacterium Tumefaciens. EMBO J. 1986, 5, 1445–1454. [Google Scholar] [CrossRef]
- Hess, K.M.; Dudley, M.W.; Lynn, D.G.; Joerger, R.D.; Binns, A.N. Mechanism of Phenolic Activation of Agrobacterium Virulence Genes: Development of a Specific Inhibitor of Bacterial Sensor/Response Systems. Proc. Natl. Acad. Sci. USA 1991, 88, 7854–7858. [Google Scholar] [CrossRef] [PubMed]
- Fanaro, S.; Chierici, R.; Guerrini, P.; Vigi, V. Intestinal Microflora in Early Infancy: Composition and Development. Acta Paediatr. Suppl. 2003, 91, 48–55. [Google Scholar] [CrossRef]
- Ding, T.; Schloss, P.D. Dynamics and Associations of Microbial Community Types across the Human Body. Nature 2014, 509, 357–360. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Hegde, L.; Kumar, T.V.; Himabindu, K. Evaluation of Coleus Forskohlii Accessions for Tuber and Forskolin Yield. In Proceedings of the III WOCMAP Congress on Medicinal and Aromatic Plants-Volume 1: Bioprospecting and Ethnopharmacology, Chiang Mai, Thailand, 3–7 February 2003; Volume 675, pp. 217–219. [Google Scholar]
- Singh, R.; Kalra, A.; Ravish, B.S.; Divya, S.; Parameswaran, T.N.; Srinivas, K.; Bagyaraj, D.J. Effect of Potential Bioinoculants and Organic Manures on Root-rot and Wilt, Growth, Yield and Quality of Organically Grown Coleus Forskohlii in a Semiarid Tropical Region of Bangalore (India). Plant Pathol. 2012, 61, 700–708. [Google Scholar] [CrossRef]
- Mastan, A.; Rane, D.; Dastager, S.G.; Vivek Babu, C.S. Plant Probiotic Bacterial Endophyte, Alcaligenes Faecalis, Modulates Plant Growth and Forskolin Biosynthesis in Coleus Forskohlii. Probiotics Antimicrob. Proteins 2020, 12, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Dohrmann, A.B.; Küting, M.; Jünemann, S.; Jaenicke, S.; Schlüter, A.; Tebbe, C.C. Importance of Rare Taxa for Bacterial Diversity in the Rhizosphere of Bt- and Conventional Maize Varieties. ISME J. 2013, 7, 37–49. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Guo, X.; Zhang, Y.; Ye, B. Comparative Analysis of Bacterial Community Structure in the Rhizosphere of Maize by High-Throughput Pyrosequencing. PLoS ONE 2017, 12, e0178425. [Google Scholar] [CrossRef]
- Latif, S.; Bibi, S.; Kouser, R.; Fatimah, H.; Farooq, S.; Naseer, S.; Kousar, R. Characterization of Bacterial Community Structure in the Rhizosphere of Triticum aestivum L. Genomics 2020, 112, 4760–4768. [Google Scholar] [CrossRef]
- Qi, X.; Wang, E.; Xing, M.; Zhao, W.; Chen, X. Rhizosphere and Non-Rhizosphere Bacterial Community Composition of the Wild Medicinal Plant Rumex Patientia. World J. Microbiol. Biotechnol. 2012, 28, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Zu, M.; Liu, L.; Song, X.; Yuan, Y. Composition and Diversity of Bacterial Communities in the Rhizosphere of the Chinese Medicinal Herb Dendrobium. BMC Plant Biol. 2021, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Del Rio, T.G.; et al. Defining the Core Arabidopsis Thaliana Root Microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Roy, K.S.; Das, M.; Ray, S.; Balachandar, D.; Karthikeyan, S.; Nayak, A.K.; Mohapatra, T. Elucidation of Rice Rhizosphere Metagenome in Relation to Methane and Nitrogen Metabolism under Elevated Carbon Dioxide and Temperature Using Whole Genome Metagenomic Approach. Sci. Total Env. 2016, 542, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Wang, F.; Zhang, J.; Chen, Y.; Zhang, C.; Liu, G.; Zhang, H.; Ma, C.; Zhang, J. The Variation in the Rhizosphere Microbiome of Cotton with Soil Type, Genotype and Developmental Stage. Sci. Rep. 2017, 7, 3940. [Google Scholar] [CrossRef] [PubMed]
- Felestrino, É.B.; Santiago, I.F.; Freitas, L.D.; Rosa, L.H.; Ribeiro, S.P.; Moreira, L.M. Plant Growth Promoting Bacteria Associated with Langsdorffia Hypogaea-Rhizosphere-Host Biological Interface: A Neglected Model of Bacterial Prospection. Front. Microbiol. 2017, 8, 172. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Panjiar, N.; Kumar, S.; Saxena, A.K.; Suman, A. Assessment of Genetic Diversity and Plant Growth Promoting Attributes of Psychrotolerant Bacteria Allied with Wheat (Triticum Aestivum) from the Northern Hills Zone of India. Ann. Microbiol. 2015, 65, 1885–1899. [Google Scholar] [CrossRef]
- Yadav, A.N.; Sachan, S.G.; Verma, P.; Saxena, A.K. Prospecting Cold Deserts of North Western Himalayas for Microbial Diversity and Plant Growth Promoting Attributes. J. Biosci. Bioeng. 2015, 119, 683–693. [Google Scholar] [CrossRef]
- Yadav, A.N.; Verma, P.; Kumar, S.; Kumar, V.; Kumar, M.; Sugitha, T.C.K.; Singh, B.P.; Saxena, A.K.; Dhaliwal, H.S. Actinobacteria from Rhizosphere: Molecular Diversity, Distributions, and Potential Biotechnological Applications. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 13–41. [Google Scholar]
- Gray, C.M.; Helmig, D.; Fierer, N.; Zak, D.R.; Groffman, P.M. Bacteria and Fungi Associated with Isoprene Consumption in SoilSoil Microbes Associated with Isoprene Consumption. Elem. Sci. Anthr. 2015, 3, 53. [Google Scholar] [CrossRef]
- Geml, J.; Gravendeel, B.; van der Gaag, K.J.; Neilen, M.; Lammers, Y.; Raes, N.; Semenova, T.A.; de Knijff, P.; Noordeloos, M.E. The Contribution of DNA Metabarcoding to Fungal Conservation: Diversity Assessment, Habitat Partitioning and Mapping Red-Listed Fungi in Protected Coastal Salix Repens Communities in the Netherlands. PLoS ONE 2014, 9, e99852. [Google Scholar] [CrossRef]
- Praeg, N.; Illmer, P. Microbial Community Composition in the Rhizosphere of Larix Decidua under Different Light Regimes with Additional Focus on Methane Cycling Microorganisms. Sci. Rep. 2020, 10, 22324. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Cui, Y.; Li, H.; Kuang, A.; Li, X.; Wei, Y.; Ji, X. Rhizospheric Soil and Root Endogenous Fungal Diversity and Composition in Response to Continuous Panax Notoginseng Cropping Practices. Microbiol. Res. 2017, 194, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Ponder, M.M.; Garcia-Diaz, J. Phoma Infections: Classification, Potential Food Sources, and Its Clinical Impact. Microorganisms 2018, 6, 58. [Google Scholar] [CrossRef]
- Miao, Z.; Li, S.; Liu, X.; Chen, Y.; Li, Y.; Wang, Y.; Guo, R.; Xia, Z.; Zhang, K. The Causal Microorganisms of Panax Notoginseng Root Rot Disease. Sci. Agric. Sin. 2006, 39, 1371–1378. [Google Scholar]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of Plant-Borne Flavonoids into the Rhizosphere and Their Role in Plant Nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Mwendwa, J.M.; Weston, P.A.; Weidenhamer, J.D.; Fomsgaard, I.S.; Wu, H.; Gurusinghe, S.; Weston, L.A. Metabolic Profiling of Benzoxazinoids in the Roots and Rhizosphere of Commercial Winter Wheat Genotypes. Plant Soil 2021, 466, 467–489. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Z.; Feng, X.; Wang, M.; Luo, Y.; Wang, Y.; Xu, P. L-Theanine Exuded from Camellia Sinensis Roots Regulates Element Cycling in Soil by Shaping the Rhizosphere Microbiome Assembly. Sci. Total Environ. 2022, 837, 155801. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Berg, M.P.; Staudt, M.; Holmstrup, M.; Glasius, M.; Ellers, J.; Tomiolo, S.; Madsen, R.B.; Slotsbo, S.; Penuelas, J. Plant Secondary Compounds in Soil and Their Role in Belowground Species Interactions. Trends Ecol. Evol. 2020, 35, 716–730. [Google Scholar] [CrossRef]
- da Silva Lima, L.; Olivares, F.L.; Rodrigues de Oliveira, R.; Vega, M.R.G.; Aguiar, N.O.; Canellas, L.P. Root Exudate Profiling of Maize Seedlings Inoculated with Herbaspirillum Seropedicaeand Humic Acids. Chem. Biol. Technol. Agric. 2014, 1, 1–18. [Google Scholar] [CrossRef]
- Lobón, N.C.; de la Cruz, I.F.; Gallego, J.C.A. Autotoxicity of Diterpenes Present in Leaves of Cistus ladanifer L. Plants 2019, 8, 27. [Google Scholar] [CrossRef]
- Chen, F.; Ro, D.-K.; Petri, J.; Gershenzon, J.; Bohlmann, J.; Pichersky, E.; Tholl, D. Characterization of a Root-Specific Arabidopsis Terpene Synthase Responsible for the Formation of the Volatile Monoterpene 1, 8-Cineole. Plant Physiol. 2004, 135, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Yoshimura, H. Monoterpenes of Salvia Leucophylla. Curr. Bioact. Compd. 2012, 8, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Linhart, Y.B.; Gauthier, P.; Keefover-Ring, K.; Thompson, J.D. Variable Phytotoxic Effects of Thymus Vulgaris (Lamiaceae) Terpenes on Associated Species. Int. J. Plant Sci. 2015, 176, 20–30. [Google Scholar] [CrossRef]


| Primer Name | Primer Sequence (5′ to 3′) | Length of Primer | |
|---|---|---|---|
| 16S | V3-Forward | CCTACGGGNGGCWGCAG | 17 |
| V4-Reverse | GACTACHVGGGTATCTAATCC | 21 | |
| ITS | ITS2-Forward | GCATCGATGAAGAACGCAGC | 20 |
| ITS2-Reverse | TCCTCCGCTTATTGATATGC | 20 |
| Sample Name | No. of Reads |
|---|---|
| Control-16S | 229,680 |
| Cb180d-16S | 350,216 |
| Cf180d-16S | 362,838 |
| Cf90d-16S | 352,793 |
| Cf45d-16S | 323,549 |
| Control-ITS | 253,061 |
| Cb180d-ITS | 106,639 |
| Cf180d-ITS | 196,431 |
| Cf90d-ITS | 198,977 |
| Cf45d-ITS | 140,556 |
| Sample Name | Shannon Alpha Diversity | Observed Species |
|---|---|---|
| Control-16S | 15.03 | 61,832 |
| Cb180d-16S | 11.82 | 21,231 |
| Cf180d-16S | 12.39 | 23,554 |
| Cf90d-16S | 12.60 | 31,371 |
| Cf45d-16S | 11.93 | 20,555 |
| Control-ITS | 8.57 | 13,396 |
| Cb180d-ITS | 7.23 | 2531 |
| Cf180d-ITS | 7.12 | 3241 |
| Cf90d-ITS | 7.04 | 3342 |
| Cf45d-ITS | 6.47 | 2516 |
| Control-16S | Cb180d-16S | Cf180d-16S | Cf90d-16S | Cf45d-16S | |
| Control-16S | 0 | 6322.79 | 3594.24 | 5601.30 | 4720.02 |
| Cb180d-16S | 6322.79 | 0 | 7289.51 | 8856.55 | 7668.94 |
| Cf180d-16S | 3594.24 | 7289.51 | 0 | 6092.86 | 5962.04 |
| Cf90d-16S | 5601.30 | 8856.55 | 6092.86 | 0 | 7949.65 |
| Cf45d-16S | 4720.02 | 7668.94 | 5962.04 | 7949.65 | 0 |
| Control-ITS | Cb180d-ITS | Cf180d-ITS | Cf90d-ITS | Cf45d-ITS | |
| Control-ITS | 0 | 14,457.61 | 33,958.67 | 29,702.54 | 23,094.09 |
| Cb180d-ITS | 14,457.61 | 0 | 33,013.18 | 32,609.14 | 24,629.59 |
| Cf180d-ITS | 33,958.67 | 33,013.18 | 0 | 43,308.69 | 38,896.66 |
| Cf90d-ITS | 29,702.54 | 32,609.14 | 43,308.69 | 0 | 34,828.69 |
| Cf45d-ITS | 23,094.09 | 24,629.59 | 38,896.66 | 34,828.69 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamwal, V.L.; Rather, I.A.; Ahmed, S.; Kumar, A.; Gandhi, S.G. Changing Rhizosphere Microbial Community and Metabolites with Developmental Stages of Coleus barbatus. Microorganisms 2023, 11, 705. https://doi.org/10.3390/microorganisms11030705
Jamwal VL, Rather IA, Ahmed S, Kumar A, Gandhi SG. Changing Rhizosphere Microbial Community and Metabolites with Developmental Stages of Coleus barbatus. Microorganisms. 2023; 11(3):705. https://doi.org/10.3390/microorganisms11030705
Chicago/Turabian StyleJamwal, Vijay Lakshmi, Irshad Ahmad Rather, Sajad Ahmed, Amit Kumar, and Sumit G. Gandhi. 2023. "Changing Rhizosphere Microbial Community and Metabolites with Developmental Stages of Coleus barbatus" Microorganisms 11, no. 3: 705. https://doi.org/10.3390/microorganisms11030705
APA StyleJamwal, V. L., Rather, I. A., Ahmed, S., Kumar, A., & Gandhi, S. G. (2023). Changing Rhizosphere Microbial Community and Metabolites with Developmental Stages of Coleus barbatus. Microorganisms, 11(3), 705. https://doi.org/10.3390/microorganisms11030705








