A Simple In-Vivo Method for Evaluation of Antibiofilm and Wound Healing Activity Using Excision Wound Model in Diabetic Swiss Albino Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
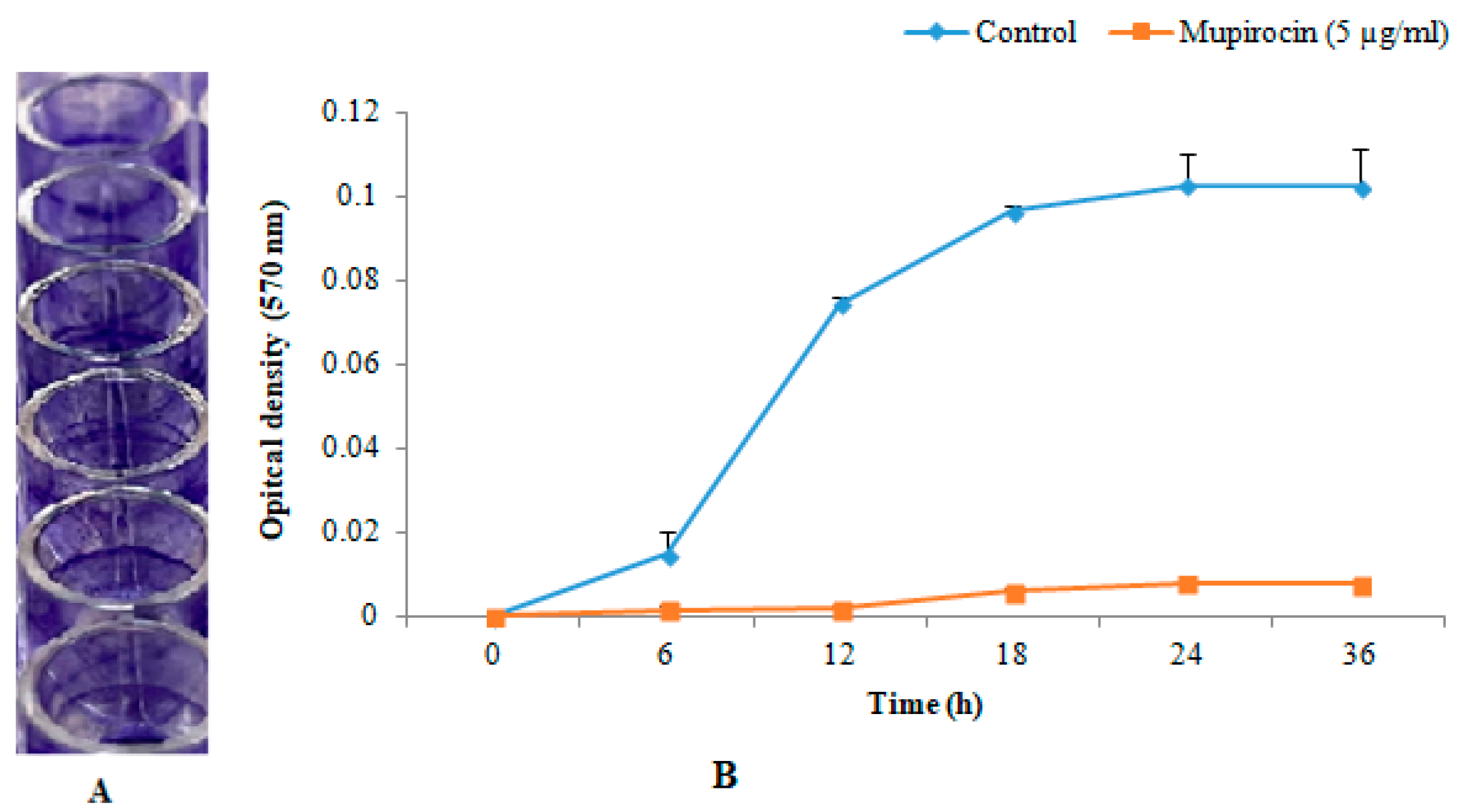
2.3. In-Vitro Biofilm Formation
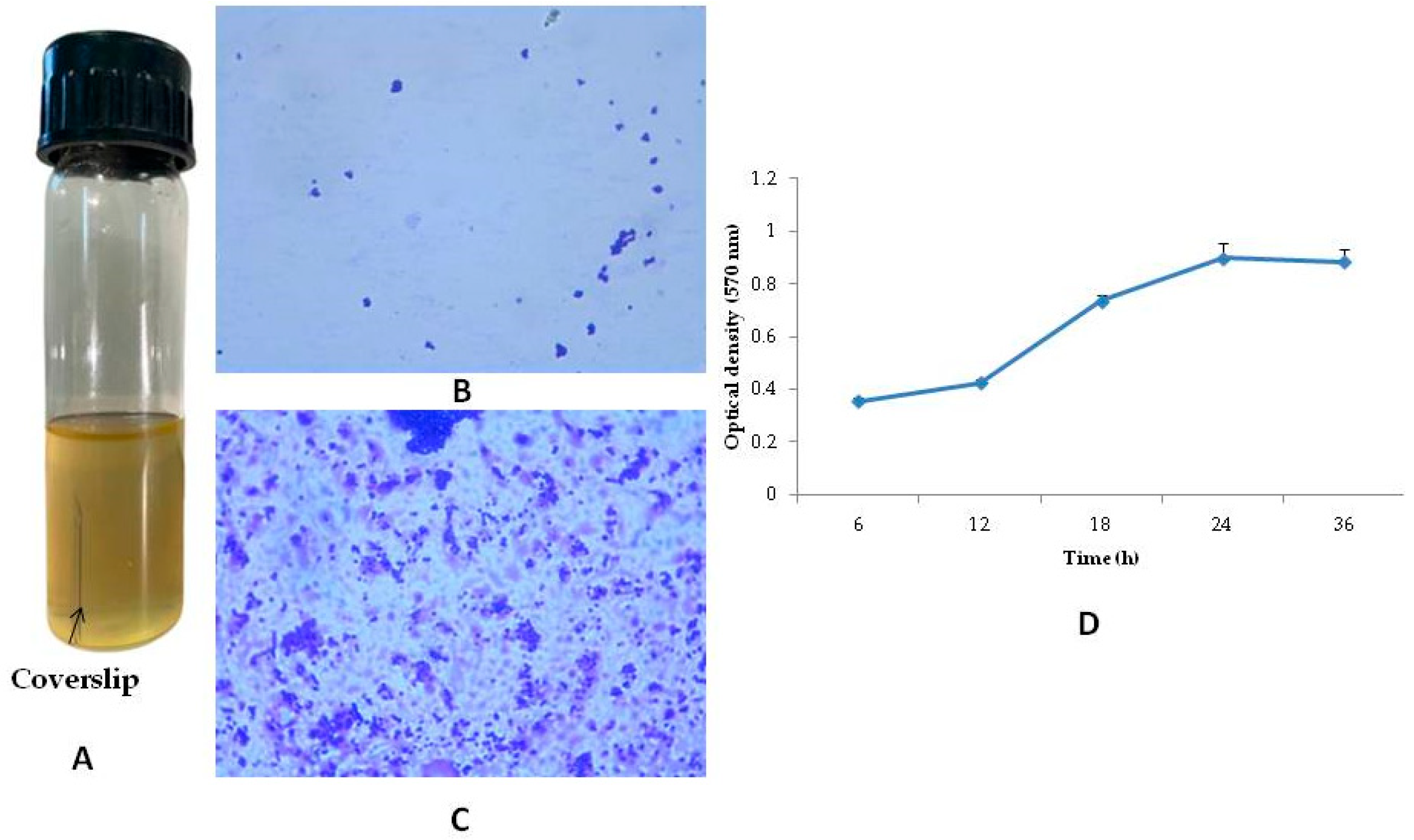
2.4. Preparation of Biofilm on the Coverslips and Its Determination
2.5. Induction of Type-II Diabetes in Mice
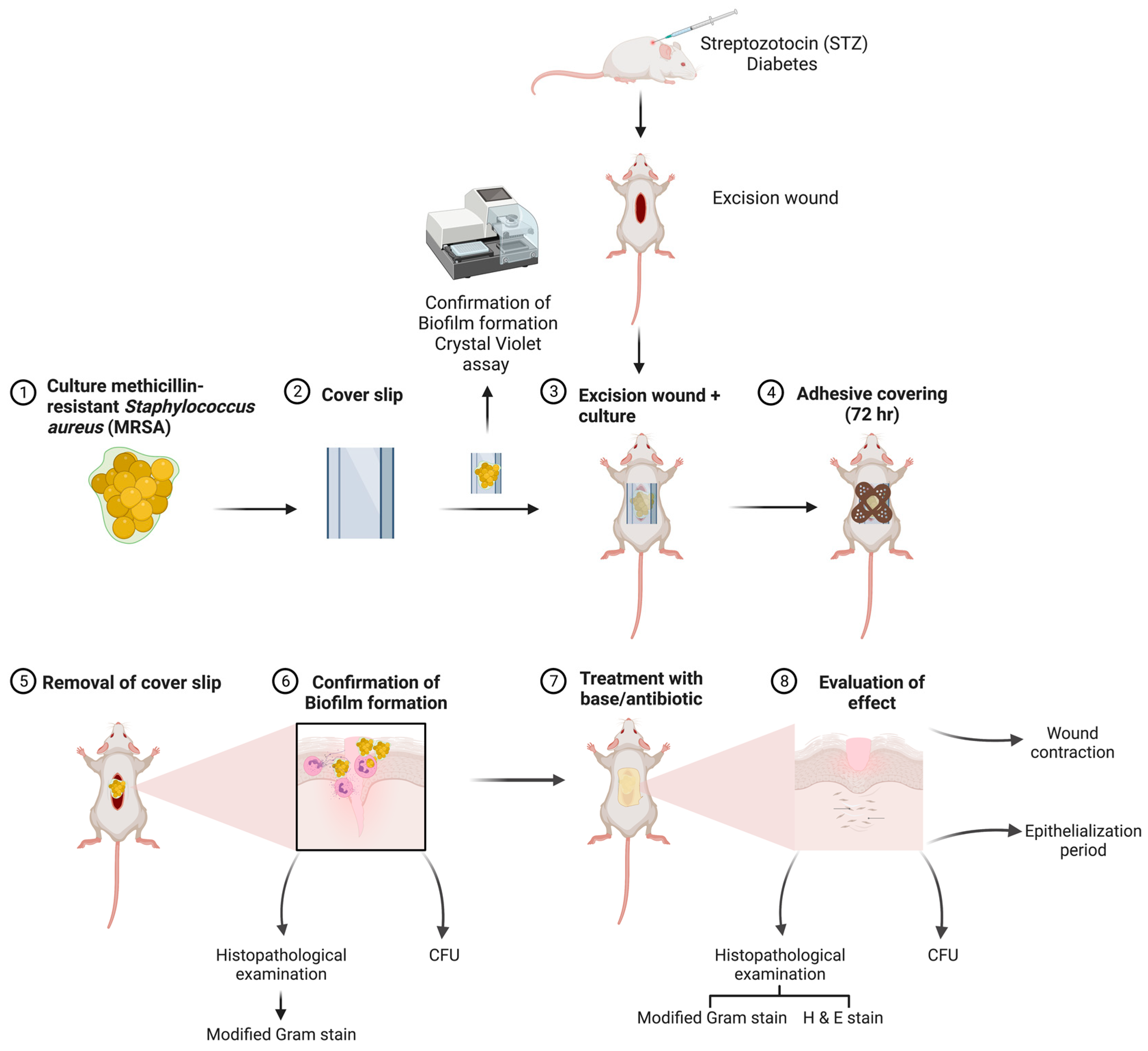
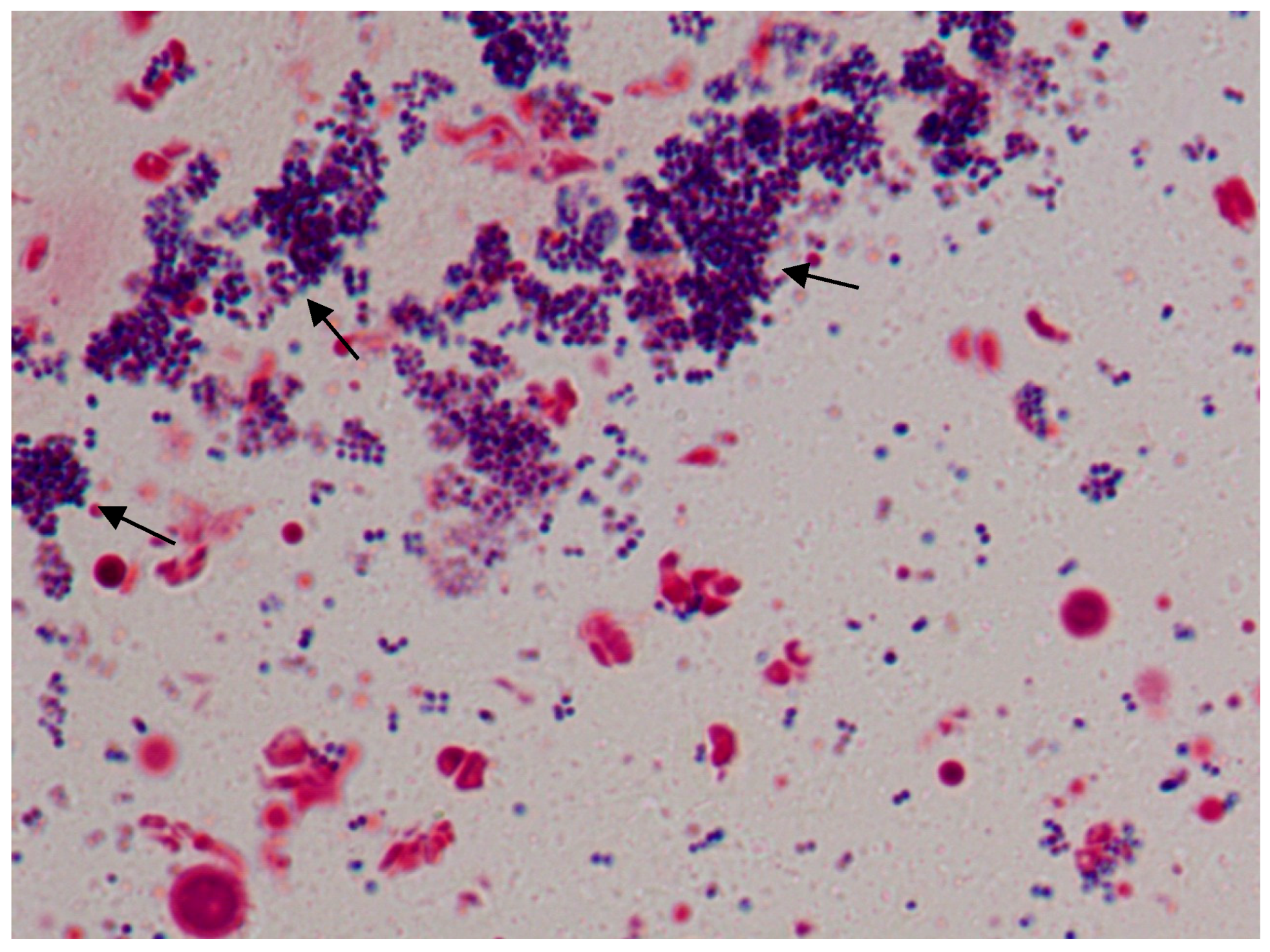
2.6. Excision Wound and Biofilm Formation
Staining
3. Results
3.1. In-Vitro Biofilm Formation
3.2. Biofilm Formation in Coverslip and Its Determination
3.3. Induction of Type-II Diabetes in Mice
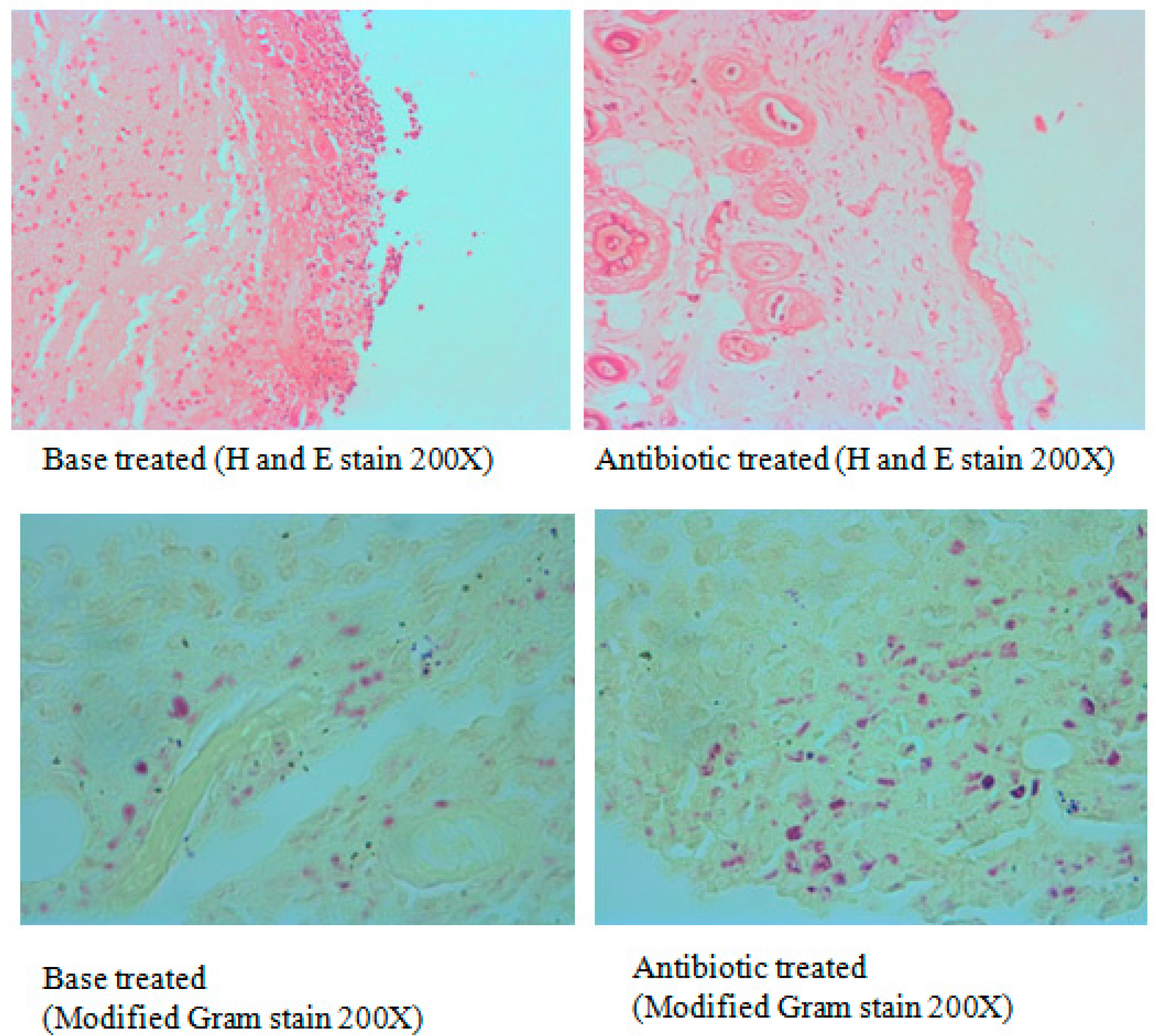
3.4. Excision Wound in Diabetic Animals
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 1106. [Google Scholar] [CrossRef]
- Durand, B.A.R.N.; Pouget, C.; Magnan, C.; Molle, V.; Lavigne, J.P.; Dunyach-Remy, C. Bacterial Interactions in the Context of Chronic Wound Biofilm: A Review. Microorganisms 2022, 10, 1500. [Google Scholar] [CrossRef]
- Su, Y.; Yrastorza, J.T.; Matis, M.; Cusick, J.; Zhao, S.; Wang, G.; Xie, J. Biofilms: Formation, Research Models, Potential Targets, and Methods for Prevention and Treatment. Adv. Sci. 2022, 9, 2203291. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Sinha, M.; Mathew-Steiner, S.S.; Das, A.; Roy, S.; Sen, C.K. Chronic Wound Biofilm Model. Adv. Wound Care 2015, 4, 382. [Google Scholar] [CrossRef]
- Vyas, H.K.N.; Xia, B.; Mai-Prochnow, A. Clinically Relevant in Vitro Biofilm Models: A Need to Mimic and Recapitulate the Host Environment. Biofilm 2022, 4, 100069. [Google Scholar] [CrossRef] [PubMed]
- Anju, V.T.; Busi, S.; Imchen, M.; Kumavath, R.; Mohan, M.S.; Salim, S.A.; Subhaswaraj, P.; Dyavaiah, M. Polymicrobial Infections and Biofilms: Clinical Significance and Eradication Strategies. Antibiotics 2022, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, K.; Kobayashi, O.; Kishi, K.; Shirai, R.; Østrup Jensen, P.; Heydorn, A.; Hentzer, M.; Calum, H.; Christophersen, L.; Høiby, N.; et al. Animal Models of Chronic and Recurrent Pseudomonas Aeruginosa Lung Infection: Significance of Macrolide Treatment. APMIS 2022, 130, 458–476. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle: Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-Resistant Staphylococcus Aureus (MRSA): Antibiotic-Resistance and the Biofilm Phenotype. Medchemcomm 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic Strategies To Counteract Antibiotic Resistance in MRSA Biofilm-Associated Infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus Aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Rizzetto, G.; Radi, G.; Molinelli, E.; Cirioni, O.; Giacometti, A.; Offidani, A. New Perspectives on Old and New Therapies of Staphylococcal Skin Infections: The Role of Biofilm Targeting in Wound Healing. Antibiotics 2021, 10, 1377. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Hamed, M.I.; Panitch, A.; Seleem, M.N. Targeting Methicillin-Resistant Staphylococcus Aureus with Short Salt-Resistant Synthetic Peptides. Antimicrob. Agents Chemother. 2014, 58, 4113. [Google Scholar] [CrossRef]
- Walker, J.N.; Horswill, A.R. A Coverslip-Based Technique for Evaluating Staphylococcus Aureus Biofilm Formation on Human Plasma. Front. Cell. Infect. Microbiol. 2012, 2, 39. [Google Scholar] [CrossRef]
- Yan, L.J. The Nicotinamide/Streptozotocin Rodent Model of Type 2 Diabetes: Renal Pathophysiology and Redox Imbalance Features. Biomolecules 2022, 12, 1225. [Google Scholar] [CrossRef]
- Anesthesia (Guideline)|Vertebrate Animal Research. Available online: https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia (accessed on 13 February 2022).
- Becerra, S.C.; Roy, D.C.; Sanchez, C.J.; Christy, R.J.; Burmeister, D.M. An Optimized Staining Technique for the Detection of Gram Positive and Gram Negative Bacteria within Tissue. BMC Res. Notes 2016, 9, 216. [Google Scholar] [CrossRef]
- SOULSBY, J. The New Ointment Bases of the British Pharmocopoeia, 1948. Br. J. Dermatol. Syph. 1949, 61, 206–210. [Google Scholar] [CrossRef]
- Conceição, T.; de Lencastre, H.; Aires-de-Sousa, M. Prevalence of Biocide Resistance Genes and Chlorhexidine and Mupirocin Non-Susceptibility in Portuguese Hospitals during a 31-Year Period (1985–2016). J. Glob. Antimicrob. Resist. 2021, 24, 169–174. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual Hematoxylin and Eosin Staining of Mouse Tissue Sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Revision of the ARRIVE Guidelines|NC3Rs. Available online: https://www.nc3rs.org.uk/our-portfolio/revision-arrive-guidelines (accessed on 28 January 2023).
- Schlafer, S.; Meyer, R.L. Confocal Microscopy Imaging of the Biofilm Matrix. J. Microbiol. Methods 2017, 138, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Watters, C.; Deleon, K.; Trivedi, U.; Griswold, J.A.; Lyte, M.; Hampel, K.J.; Wargo, M.J.; Rumbaugh, K.P. Pseudomonas Aeruginosa Biofilms Perturb Wound Resolution and Antibiotic Tolerance in Diabetic Mice. Med. Microbiol. Immunol. 2013, 202, 131–141. [Google Scholar] [CrossRef]
- Szkudelski, T. Streptozotocin-Nicotinamide-Induced Diabetes in the Rat. Characteristics of the Experimental Model. Exp. Biol. Med. 2012, 237, 481–490. [Google Scholar] [CrossRef]
- Müller-Graff, F.T.; Fitzner, B.; Jaster, R.; Vollmar, B.; Zechner, D. Impact of Hyperglycemia on Autoimmune Pancreatitis and Regulatory T-Cells. World J. Gastroenterol. 2018, 24, 3120–3129. [Google Scholar] [CrossRef] [PubMed]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front. Endocrinol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Dai, T.; Kharkwal, G.B.; Tanaka, M.; Huang, Y.Y.; Bil de Arce, V.J.; Hamblin, M.R. Animal Models of External Traumatic Wound Infections. Virulence 2011, 2, 296–315. [Google Scholar] [CrossRef]
- Zhao, G.; Hochwalt, P.C.; Usui, M.L.; Underwood, R.A.; Singh, P.K.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Delayed Wound Healing in Diabetic (Db/Db) Mice with Pseudomonas Aeruginosa Biofilm Challenge: A Model for the Study of Chronic Wounds. Wound Repair Regen. 2010, 18, 467–477. [Google Scholar] [CrossRef]
- Simola, N.; Morelli, M.; Mizuno, T.; Mitchell, S.H.; de Wit, H.; Curran, H.V.; Morgan, C.J.A.; Anagnostaras, S.G.; Sage, J.R.; Carmack, S.A.; et al. Db/Db Mouse. In Encyclopedia of Psychopharmacology; Springer: Cham, Switzerland, 2010; p. 365. [Google Scholar] [CrossRef]
- John, M.; Goldsworthy, H.; Burton, S.; Lappin-Scott, H. Gene Expression of Pseudomonas Aeruginosa and MRSA within a Catheter-Associated Urinary Tract Infection Biofilm Model. Biosci. Horizons Int. J. Student Res. 2008, 1, 28–37. [Google Scholar] [CrossRef]
- Agostinho Hunt, A.M.; Gibson, J.A.; Larrivee, C.L.; O’Reilly, S.; Navitskaya, S.; Busik, J.V.; Waters, C.M. Come to the Light Side: In Vivo Monitoring of Pseudomonas Aeruginosa Biofilm Infections in Chronic Wounds in a Diabetic Hairless Murine Model. J. Vis. Exp. 2017, 2017, e55991. [Google Scholar] [CrossRef]
- Rais, N.; Ved, A.; Ahmad, R.; Parveen, K.; Gautam, G.K.; Bari, D.G.; Shukla, K.S.; Gaur, R.; Singh, A.P. Model of Streptozotocin-Nicotinamide Induced Type 2 Diabetes: A Comparative Review. Curr. Diabetes Rev. 2022, 18, 58–69. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and Chronic Wound Infections: Microbiological, Immunological, Clinical and Therapeutic Distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Askri, Z.U.A.; Shahid, M.H.; Khan, A.Z.; Asif, S.; Ghumman, A.R.; Afzal, M.F. The Emergence of Multiple Antibiotic Resistance in Culture Sensitivities of Post-Surgical Patients in Lahore General Hospital, Lahore. Cureus 2022, 14, e23212. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic Wound Infections: The Role of Pseudomonas Aeruginosa and Staphylococcus Aureus. Expert Rev. Anti. Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Deeds, M.C.; Anderson, J.M.; Armstrong, A.S.; Gastineau, D.A.; Hiddinga, H.J.; Jahangir, A.; Eberhardt, N.L.; Kudva, Y.C. Single Dose Streptozotocin-Induced Diabetes: Considerations for Study Design in Islet Transplantation Models. Lab. Anim. 2011, 45, 131–140. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrouji, M.; Kuriri, F.A.; Alqasmi, M.H.; AlSudais, H.; Alissa, M.; Alsuwat, M.A.; Asad, M.; Joseph, B.; Almuhanna, Y. A Simple In-Vivo Method for Evaluation of Antibiofilm and Wound Healing Activity Using Excision Wound Model in Diabetic Swiss Albino Mice. Microorganisms 2023, 11, 692. https://doi.org/10.3390/microorganisms11030692
Alrouji M, Kuriri FA, Alqasmi MH, AlSudais H, Alissa M, Alsuwat MA, Asad M, Joseph B, Almuhanna Y. A Simple In-Vivo Method for Evaluation of Antibiofilm and Wound Healing Activity Using Excision Wound Model in Diabetic Swiss Albino Mice. Microorganisms. 2023; 11(3):692. https://doi.org/10.3390/microorganisms11030692
Chicago/Turabian StyleAlrouji, Mohammed, Fahd A. Kuriri, Mohammed Hussein Alqasmi, Hamood AlSudais, Mohammed Alissa, Meshari A. Alsuwat, Mohammed Asad, Babu Joseph, and Yasir Almuhanna. 2023. "A Simple In-Vivo Method for Evaluation of Antibiofilm and Wound Healing Activity Using Excision Wound Model in Diabetic Swiss Albino Mice" Microorganisms 11, no. 3: 692. https://doi.org/10.3390/microorganisms11030692
APA StyleAlrouji, M., Kuriri, F. A., Alqasmi, M. H., AlSudais, H., Alissa, M., Alsuwat, M. A., Asad, M., Joseph, B., & Almuhanna, Y. (2023). A Simple In-Vivo Method for Evaluation of Antibiofilm and Wound Healing Activity Using Excision Wound Model in Diabetic Swiss Albino Mice. Microorganisms, 11(3), 692. https://doi.org/10.3390/microorganisms11030692






