In-Package Atmospheric Cold Plasma Treatment and Storage Effects on Membrane Integrity, Oxidative Stress, and Esterase Activity of Listeria monocytogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ham Sample Preparation
2.2. Bacterial Culture Preparation
2.3. Inoculation and Packaging
2.4. Cold Plasma Treatment
2.5. Total Viable Cell Counts
2.6. Flow Cytometry Analysis
2.7. Membrane Integrity
2.8. Esterase Activity and Membrane Permeabilization
2.9. Oxidative Stress and Membrane Permeabilization
2.10. Statistical Analysis
3. Results
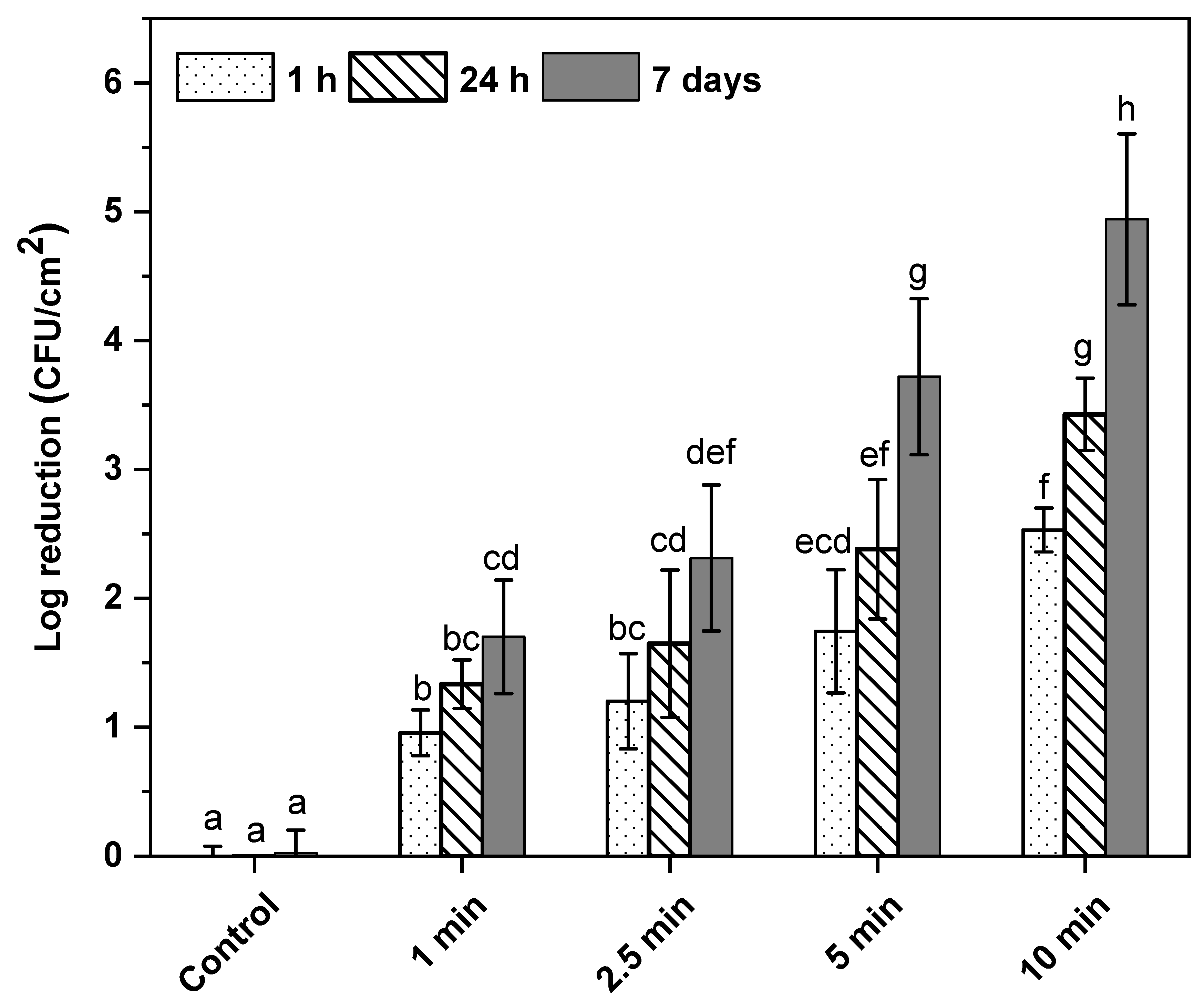
3.1. Effect of Post-ACP-Treatment Storage Time on Total Viable Cell Counts
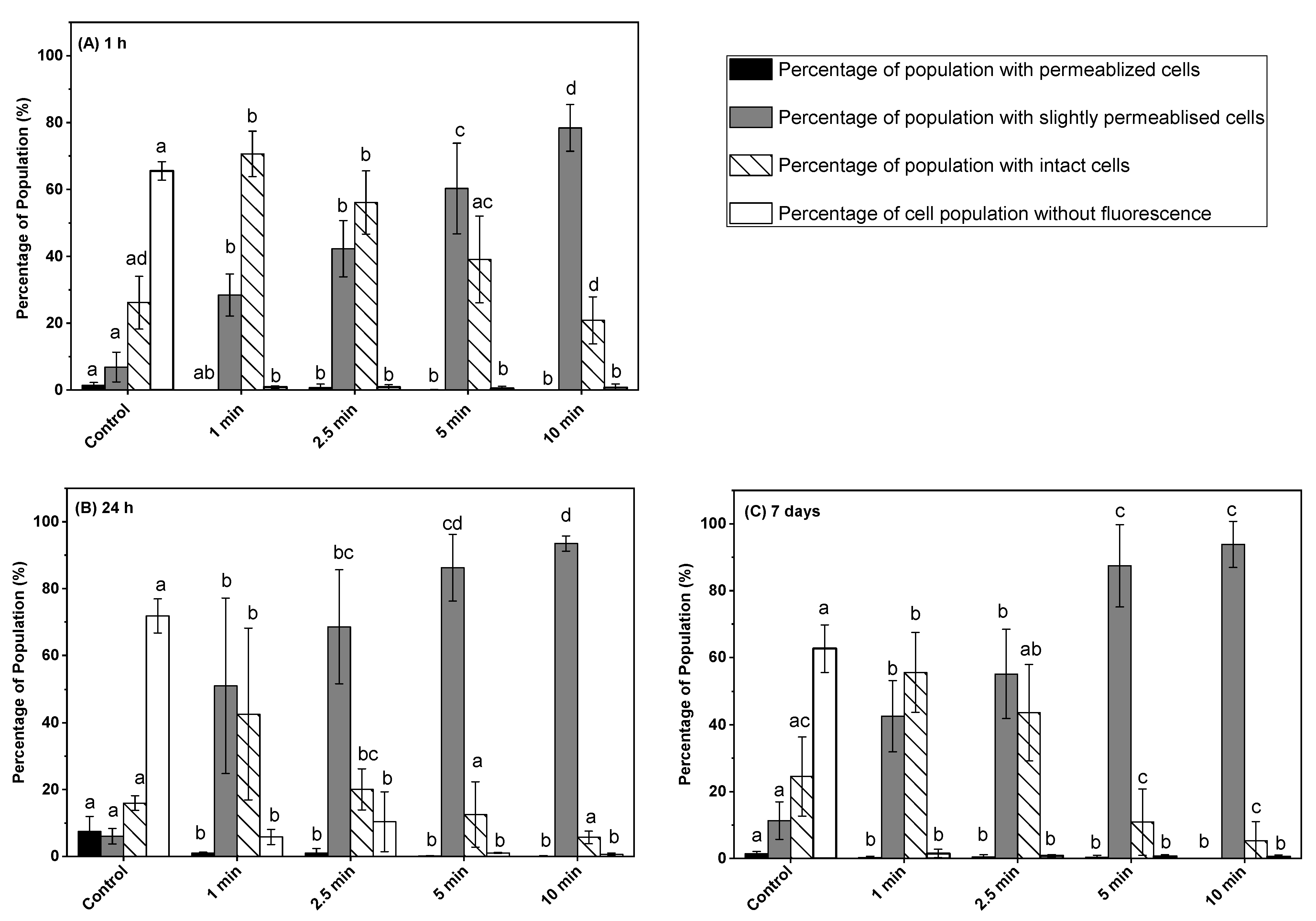
3.2. Effect of Post-ACP-Treatment Storage Time on Membrane Integrity
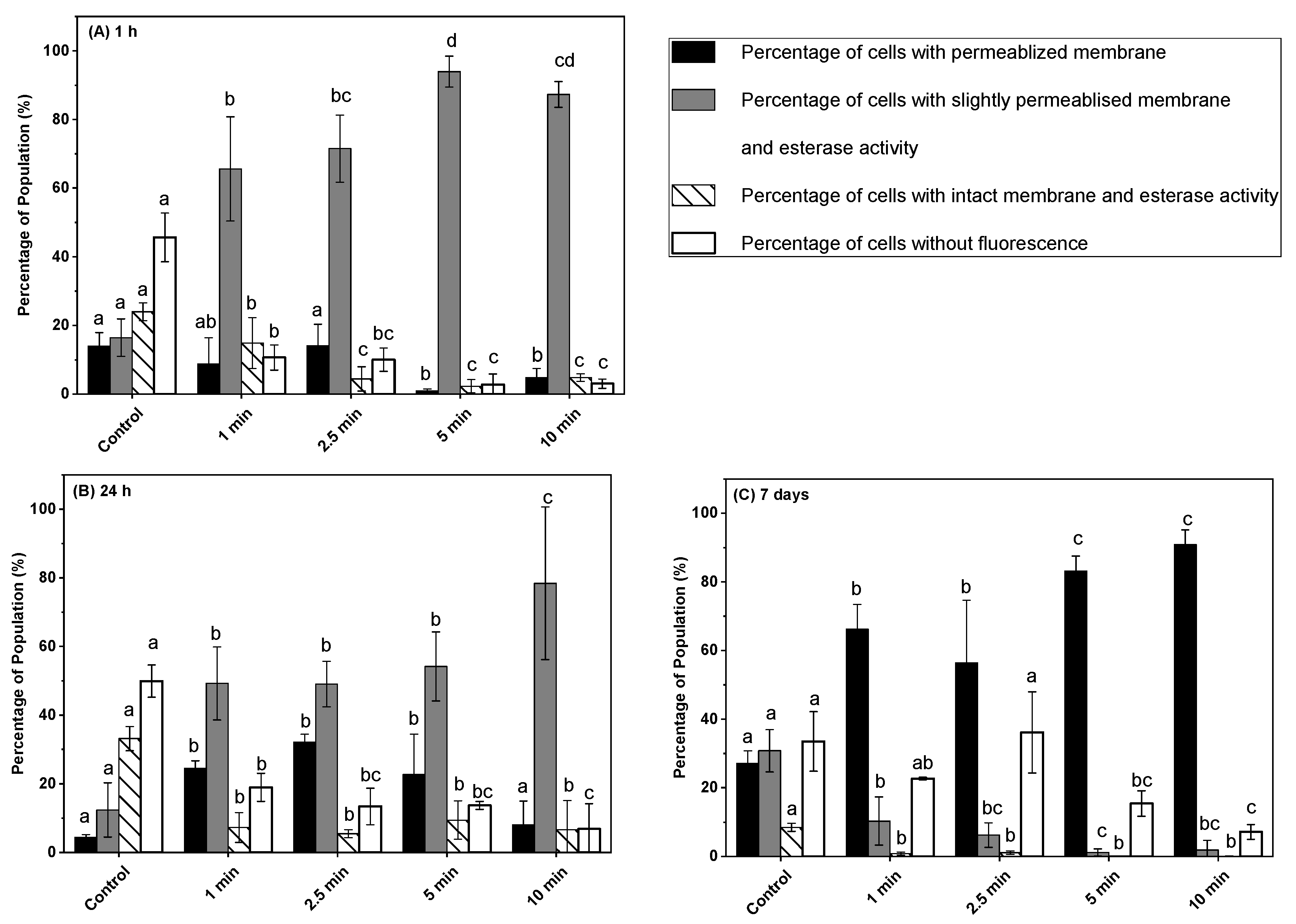
3.3. Effect of Post-ACP Treatment Storage Time on Esterase Activity
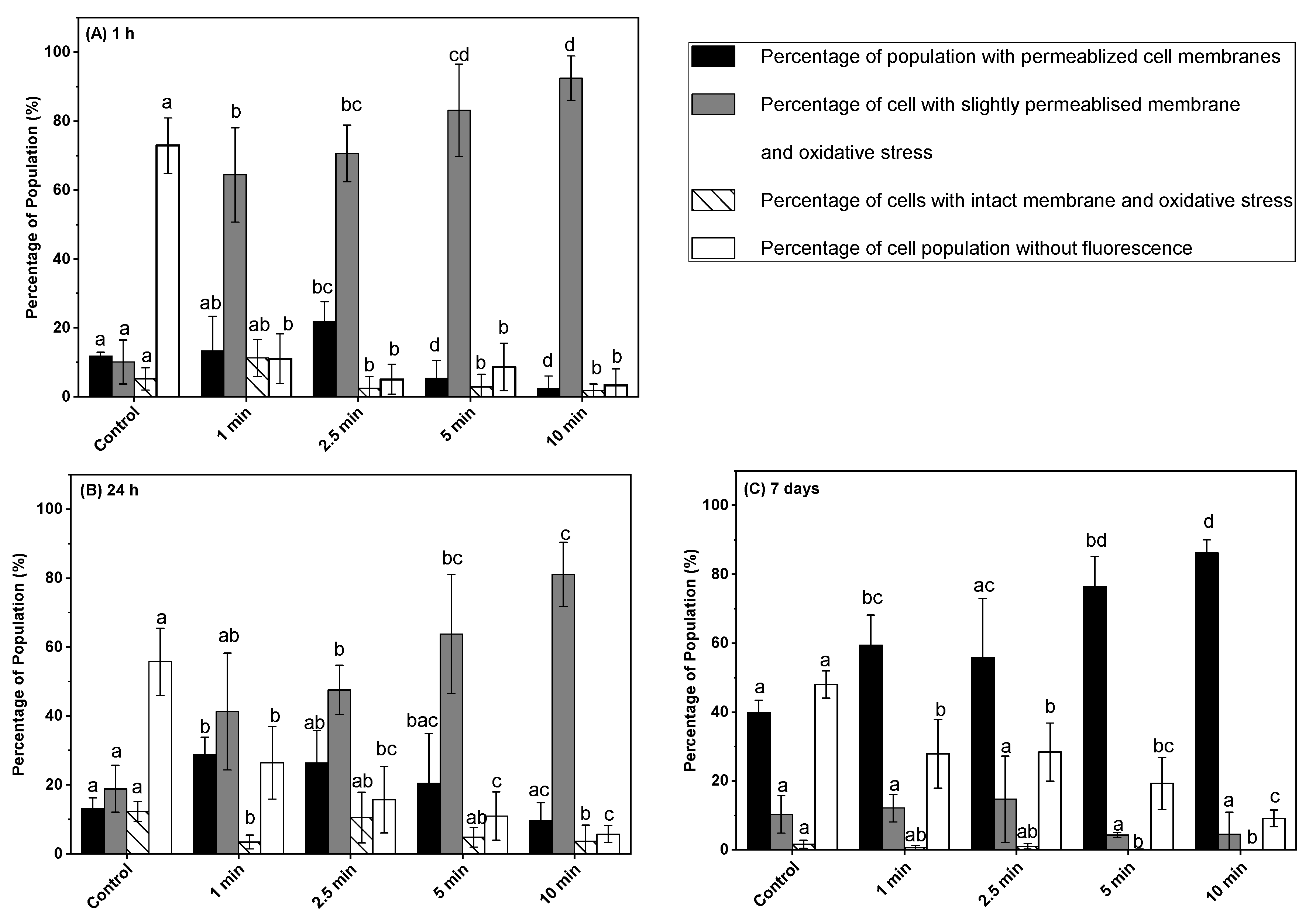
3.4. Effect of Post-ACP Treatment Storage Time on Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Health Canada. Food-Related Illnesses, Hospitalizations and Deaths in Canada. Public Health Agency of Canada. Available online: https://www.canada.ca/en/public-health/services/publications/food-nutrition/infographic-food-related-illnesses-hospitalizations-deaths-in-canada.html (accessed on 12 April 2018).
- Hernandez-Milian, A.; Payeras-Cifre, A. What Is New in Listeriosis? Biomed. Res. Int. 2014, 2014, 358051. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Murray, R.; Flockhart, L.; Pintar, K.; Fazil, A.; Nesbitt, A.; Marshall, B.; Tataryn, J.; Pollari, F. Estimates of Foodborne Illness–Related Hospitalizations and Deaths in Canada for 30 Specified Pathogens and Unspecified Agents. Foodborne Pathog. Dis. 2015, 12, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Policy on Listeria Monocytogenes in Ready-to-Eat Foods; Bureau of Microbial Hazards Food Directorate Health Products and Food Branch: Ottawa, ON, Canada, 2011; pp. 1–49. [Google Scholar]
- Zhu, M.; Du, M.; Cordray, J.; Ahn, D.U. Control of Listeria monocytogenes Contamination in Ready-to-Eat Meat Products. Compr. Rev. Food Sci. Food Saf. 2005, 4, 34–42. [Google Scholar] [CrossRef] [PubMed]
- CDC. Foodborne Diseases Active Surveillance Network FoodNet 2014 Surveillance Report; CDC: Atlanta, GA, USA, 2014. [Google Scholar]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria Monocytogenes Contamination of Ready-to-eat Foods and the Risk for Human Health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar] [CrossRef]
- Rød, S.K.; Hansen, F.; Leipold, F.; Knøchel, S. Cold Atmospheric Pressure Plasma Treatment of Ready-to-Eat Meat: Inactivation of Listeria Innocua and Changes in Product Quality. Food Microbiol. 2012, 30, 233–238. [Google Scholar] [CrossRef]
- Beresford, M.R.; Andrew, P.W.; Shama, G. Listeria Monocytogenes Adhers to Many Materials Found in Food-Processing Environments. J. Appl. Microbiol. 2001, 90, 1000–1005. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.S.; Ebaid, E.M.S.M.; Khalel, K.H.M.; Imre, K.; Morar, A.; Herman, V.; EL-Nawawi, F.A.M. Decontamination of Chicken Meat Using Dielectric Barrier Discharge Cold Plasma Technology: The Effect on Microbial Quality, Physicochemical Properties, Topographical Structure, and Sensory Attributes. LWT 2022, 165, 113739. [Google Scholar] [CrossRef]
- Gök, V.; Aktop, S.; Özkan, M.; Tomar, O. The Effects of Atmospheric Cold Plasma on Inactivation of Listeria Monocytogenes and Staphylococcus Aureus and Some Quality Characteristics of Pastırma—A Dry-Cured Beef Product. Innov. Food Sci. Emerg. Technol. 2019, 56, 102188. [Google Scholar] [CrossRef]
- Albertos, I.; Martín-Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Ojha, S.K.; Bourke, P.; Álvarez, C.; Rico, D. Effects of Dielectric Barrier Discharge (DBD) Generated Plasma on Microbial Reduction and Quality Parameters of Fresh Mackerel (Scomber Scombrus) Fillets. Innov. Food Sci. Emerg. Technol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of Cold Plasma Technology for Microbiological Safety in Meat Industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.W.; Yong, H.I.; Lee, H.J.; Jo, C.; Jung, S. Use of Atmospheric Pressure Cold Plasma for Meat Industry. Korean J. Food Sci. Anim. Resour. 2017, 37, 477–485. [Google Scholar] [CrossRef]
- Song, H.P.; Kim, B.; Choe, J.H.; Jung, S.; Moon, S.Y.; Choe, W.; Jo, C. Evaluation of Atmospheric Pressure Plasma to Improve the Safety of Sliced Cheese and Ham Inoculated by 3-Strain Cocktail Listeria Monocytogenes. Food Microbiol. 2009, 26, 432–436. [Google Scholar] [CrossRef]
- Yadav, B.; Spinelli, A.C.; Govindan, B.N.; Tsui, Y.Y.; McMullen, L.M.; Roopesh, M.S. Cold Plasma Treatment of Ready-to-Eat Ham: Influence of Process Conditions and Storage on Inactivation of Listeria Innocua. Food Res. Int. 2019, 123, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.I.; Lee, H.; Park, S.; Park, J.; Choe, W.; Jung, S.; Jo, C. Flexible Thin-Layer Plasma Inactivation of Bacteria and Mold Survival in Beef Jerky Packaging and Its Effects on the Meat’s Physicochemical Properties. Meat Sci. 2017, 123, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Fröhling, A.; Durek, J.; Schnabel, U.; Ehlbeck, J.; Bolling, J.; Schlüter, O. Indirect Plasma Treatment of Fresh Pork: Decontamination Efficiency and Effects on Quality Attributes. Innov. Food Sci. Emerg. Technol. 2012, 16, 381–390. [Google Scholar] [CrossRef]
- Han, L.; Boehm, D.; Amias, E.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Atmospheric Cold Plasma Interactions with Modified Atmosphere Packaging Inducer Gases for Safe Food Preservation. Innov. Food Sci. Emerg. Technol. 2016, 38, 384–392. [Google Scholar] [CrossRef]
- Yadav, B.; Spinelli, A.C.; Misra, N.N.; Tsui, Y.Y.; McMullen, L.M.; Roopesh, M.S. Effect of In-Package Atmospheric Cold Plasma Discharge on Microbial Safety and Quality of Ready-to-Eat Ham in Modified Atmospheric Packaging during Storage. J. Food Sci. 2020, 85, 1203–1212. [Google Scholar] [CrossRef]
- Lis, K.A.; Boulaaba, A.; Binder, S.; Li, Y.; Kehrenberg, C.; Zimmermann, J.L.; Klein, G.; Ahlfeld, B. Inactivation of Salmonella Typhimurium and Listeria Monocytogenes on Ham with Nonthermal Atmospheric Pressure Plasma. PLoS ONE 2018, 13, e0197773. [Google Scholar] [CrossRef]
- Schottroff, F.; Fröhling, A.; Zunabovic-Pichler, M.; Krottenthaler, A.; Schlüter, O.; Jäger, H. Sublethal Injury and Viable but Non-Culturable (VBNC) State in Microorganisms during Preservation of Food and Biological Materials by Non-Thermal Processes. Front. Microbiol. 2018, 9, 2773. [Google Scholar] [CrossRef]
- Ramamurthy, T.; Ghosh, A.; Pazhani, G.P.; Shinoda, S. Current Perspectives on Viable but Non-Culturable (VBNC) Pathogenic Bacteria. Front. Public Health 2014, 2, 103. [Google Scholar] [CrossRef]
- Fröhling, A.; Schlüter, O. Flow Cytometric Evaluation of Physico-Chemical Impact on Gram-Positive and Gram-Negative Bacteria. Front. Microbiol. 2015, 6, 939. [Google Scholar] [CrossRef]
- Lang, E.; Guyot, S.; Peltier, C.; Alvarez-Martin, P.; Perrier-Cornet, J.M.; Gervais, P. Cellular Injuries in Cronobacter Sakazakii CIP 103183T and Salmonella Enterica Exposed to Drying and Subsequent Heat Treatment in Milk Powder. Front. Microbiol. 2018, 9, 475. [Google Scholar] [CrossRef]
- Fröhling, A.; Baier, M.; Ehlbeck, J.; Knorr, D.; Schlüter, O. Atmospheric Pressure Plasma Treatment of Listeria Innocua and Escherichia Coli at Polysaccharide Surfaces: Inactivation Kinetics and Flow Cytometric Characterization. Innov. Food Sci. Emerg. Technol. 2012, 13, 142–150. [Google Scholar] [CrossRef]
- Carré, G.; Charpentier, E.; Audonnet, S.; Terryn, C.; Boudifa, M.; Doliwa, C.; Belgacem, Z.B.; Gangloff, S.C.; Gelle, M.P. Contribution of Fluorescence Techniques in Determining the Efficiency of the Non-Thermal Plasma Treatment. Front. Microbiol. 2018, 9, 2171. [Google Scholar] [CrossRef]
- Surowsky, B.; Fröhling, A.; Gottschalk, N.; Schlüter, O.; Knorr, D. Impact of Cold Plasma on Citrobacter Freundii in Apple Juice: Inactivation Kinetics and Mechanisms. Int. J. Food Microbiol. 2014, 174, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miller, P.; Basu, U.; Mcmullen, L.M. Sodium Chloride-Induced Filamentation and Alternative Gene Expression of Fts, MurZ, and Gnd in Listeria Monocytogenes 08-5923 on Vacuum-Packaged Ham. FEMS Microbiol. Lett. 2014, 360, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.U.; Egli, T. Assessment and Interpretation of Bacterial Viability by Using the LIVE/DEAD BacLight Kit in Combination with Flow Cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Braschi, G.; Patrignani, F.; Siroli, L.; Lanciotti, R.; Schlueter, O.; Froehling, A. Flow Cytometric Assessment of the Morphological and Physiological Changes of Listeria Monocytogenes and Escherichia Coli in Response to Natural Antimicrobial Exposure. Front. Microbiol. 2018, 9, 2783. [Google Scholar] [CrossRef]
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-Package Cold Plasma Technologies. J. Food Eng. 2018, 244, 21–31. [Google Scholar] [CrossRef]
- Toyokawa, Y.; Yagyu, Y.; Misawa, T.; Sakudo, A. A New Roller Conveyer System of Non-Thermal Gas Plasma as a Potential Control Measure of Plant Pathogenic Bacteria in Primary Food Production. Food Control 2017, 72, 62–72. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhuang, H.; Lawrence, K.; Zhang, J.H. Disinfection of Chicken Fillets in Packages with Atmospheric Cold Plasma: Effects of Treatment Voltage and Time. J. Appl. Microbiol. 2018, 124, 1212–1219. [Google Scholar] [CrossRef]
- Al-Abduly, A.; Christensen, P. An in Situ and Downstream Study of Non-Thermal Plasma Chemistry in an Air Fed Dielectric Barrier Discharge (DBD). Plasma Sources Sci. Technol. 2015, 24, 065006. [Google Scholar] [CrossRef]
- Moiseev, T.; Misra, N.N.; Patil, S.; Cullen, P.J.; Bourke, P.; Keener, K.M.; Mosnier, J.P. Post-Discharge Gas Composition of a Large-Gap DBD in Humid Air by UV-Vis Absorption Spectroscopy. Plasma Sources Sci. Technol. 2014, 23, 065033. [Google Scholar] [CrossRef]
- Boxhammer, V.; Morfill, G.E.; Jokipii, J.R.; Shimizu, T.; Klämpfl, T.; Li, Y.-F.; Köritzer, J.; Schlegel, J.; Zimmermann, J.L. Bactericidal Action of Cold Atmospheric Plasma in Solution. New J. Phys. 2012, 14, 113042. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation Mechanisms of Non-Thermal Plasma on Microbes: A Review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Yadav, B.; Roopesh, M.S. In-Package Atmospheric Cold Plasma Inactivation of Salmonella in Freeze-Dried Pet Foods: Effect of Inoculum Population, Water Activity, and Storage. Innov. Food Sci. Emerg. Technol. 2020, 66, 102543. [Google Scholar] [CrossRef]
- Patange, A.; O’Byrne, C.; Boehm, D.; Cullen, P.J.; Keener, K.; Bourke, P. The Effect of Atmospheric Cold Plasma on Bacterial Stress Responses and Virulence Using Listeria Monocytogenes Knockout Mutants. Front. Microbiol. 2019, 10, 2841. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, B.; Roopesh, M.S. In-Package Atmospheric Cold Plasma Treatment and Storage Effects on Membrane Integrity, Oxidative Stress, and Esterase Activity of Listeria monocytogenes. Microorganisms 2023, 11, 682. https://doi.org/10.3390/microorganisms11030682
Yadav B, Roopesh MS. In-Package Atmospheric Cold Plasma Treatment and Storage Effects on Membrane Integrity, Oxidative Stress, and Esterase Activity of Listeria monocytogenes. Microorganisms. 2023; 11(3):682. https://doi.org/10.3390/microorganisms11030682
Chicago/Turabian StyleYadav, Barun, and M. S. Roopesh. 2023. "In-Package Atmospheric Cold Plasma Treatment and Storage Effects on Membrane Integrity, Oxidative Stress, and Esterase Activity of Listeria monocytogenes" Microorganisms 11, no. 3: 682. https://doi.org/10.3390/microorganisms11030682
APA StyleYadav, B., & Roopesh, M. S. (2023). In-Package Atmospheric Cold Plasma Treatment and Storage Effects on Membrane Integrity, Oxidative Stress, and Esterase Activity of Listeria monocytogenes. Microorganisms, 11(3), 682. https://doi.org/10.3390/microorganisms11030682





