Application of Magnetite-Nanoparticles and Microbial Fuel Cell on Anaerobic Digestion: Influence of External Resistance
Abstract
1. Introduction
2. Materials and Methods
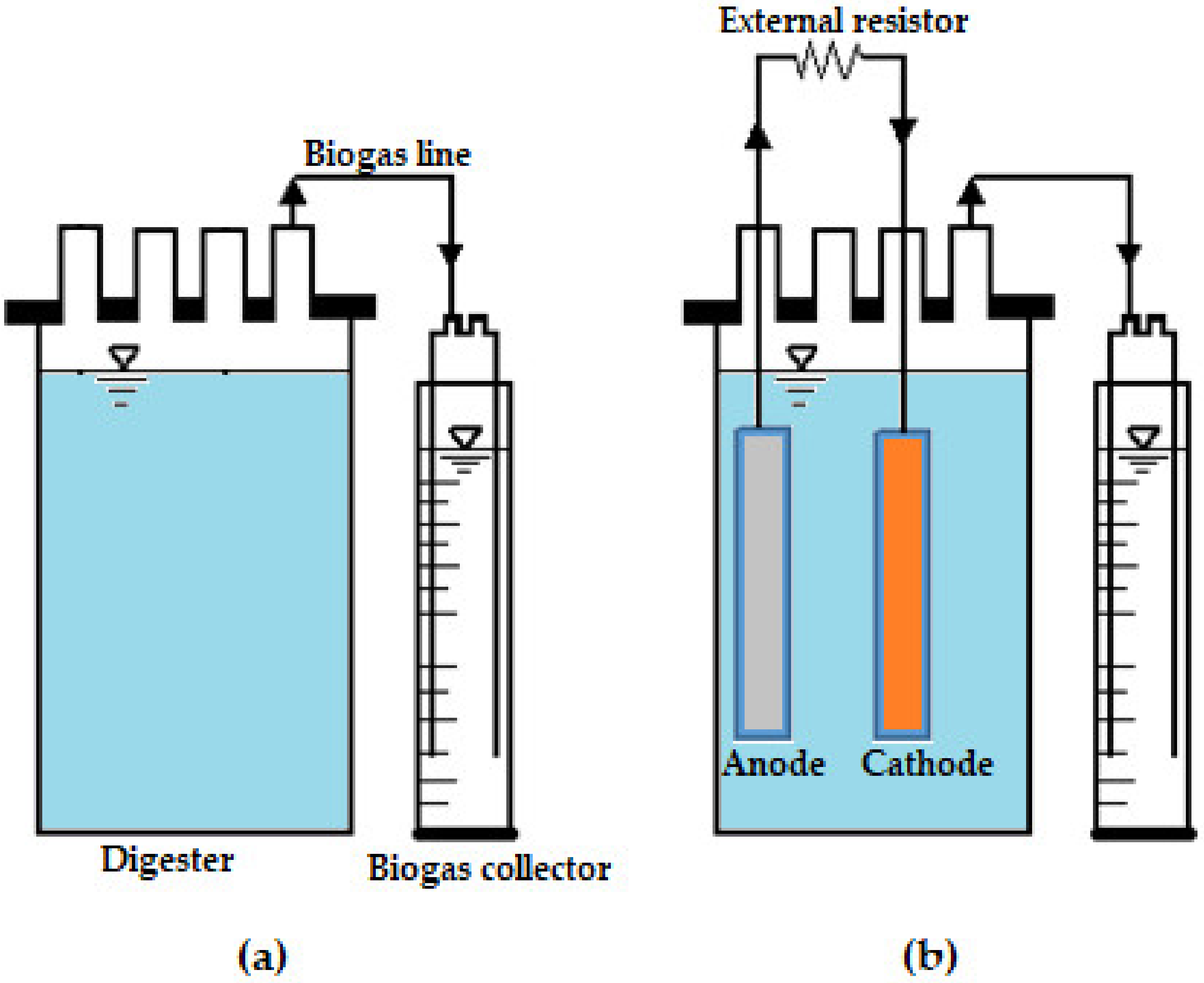
2.1. Equipment Set-Up and Operation
2.2. Analyses and Substrates
2.3. Synthesis of Magnetite-Nanoparticles and Chemical Reagents
3. Results and Discussions
3.1. Biogas Accumulation
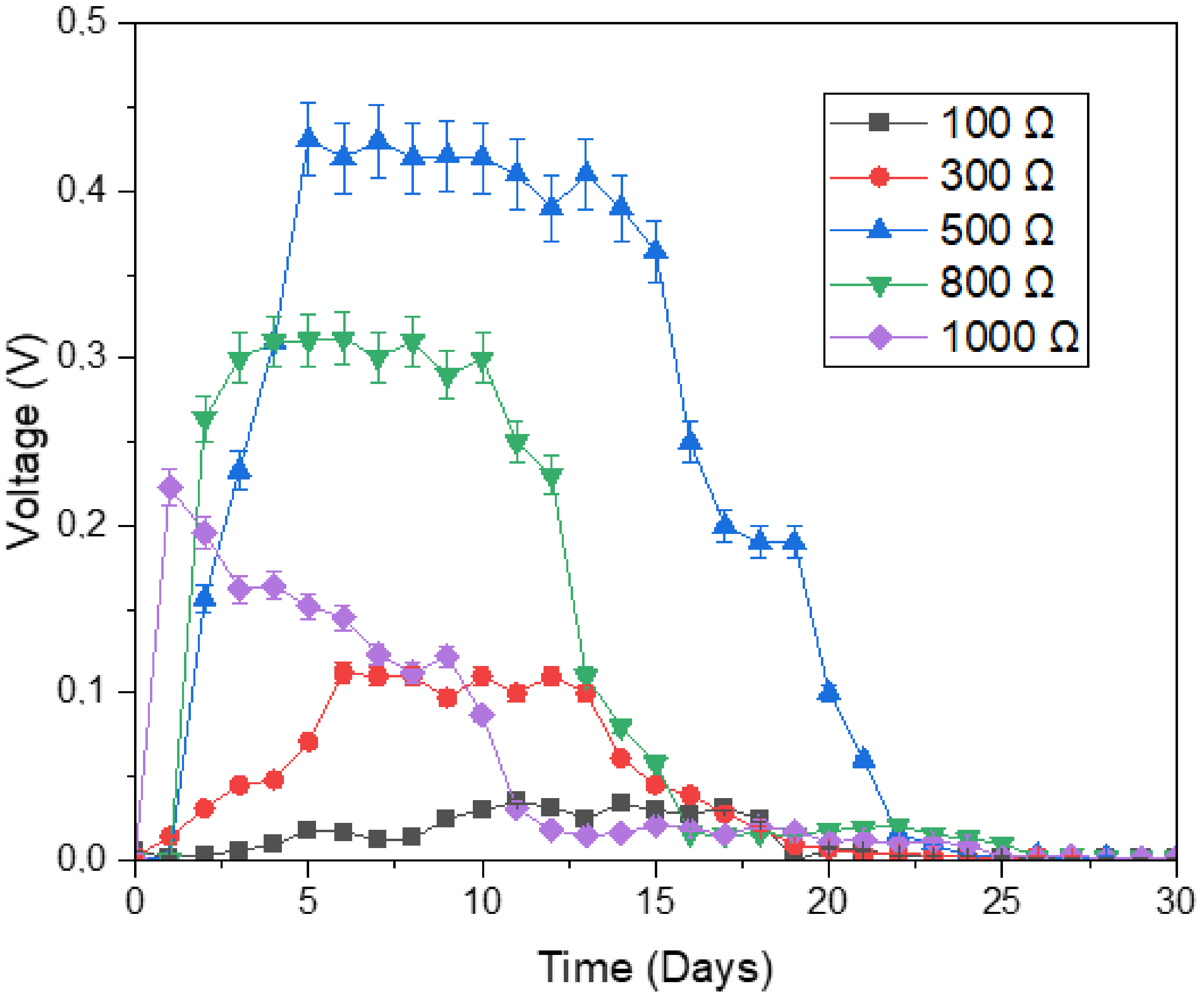
3.2. Current, Resistance and Voltage
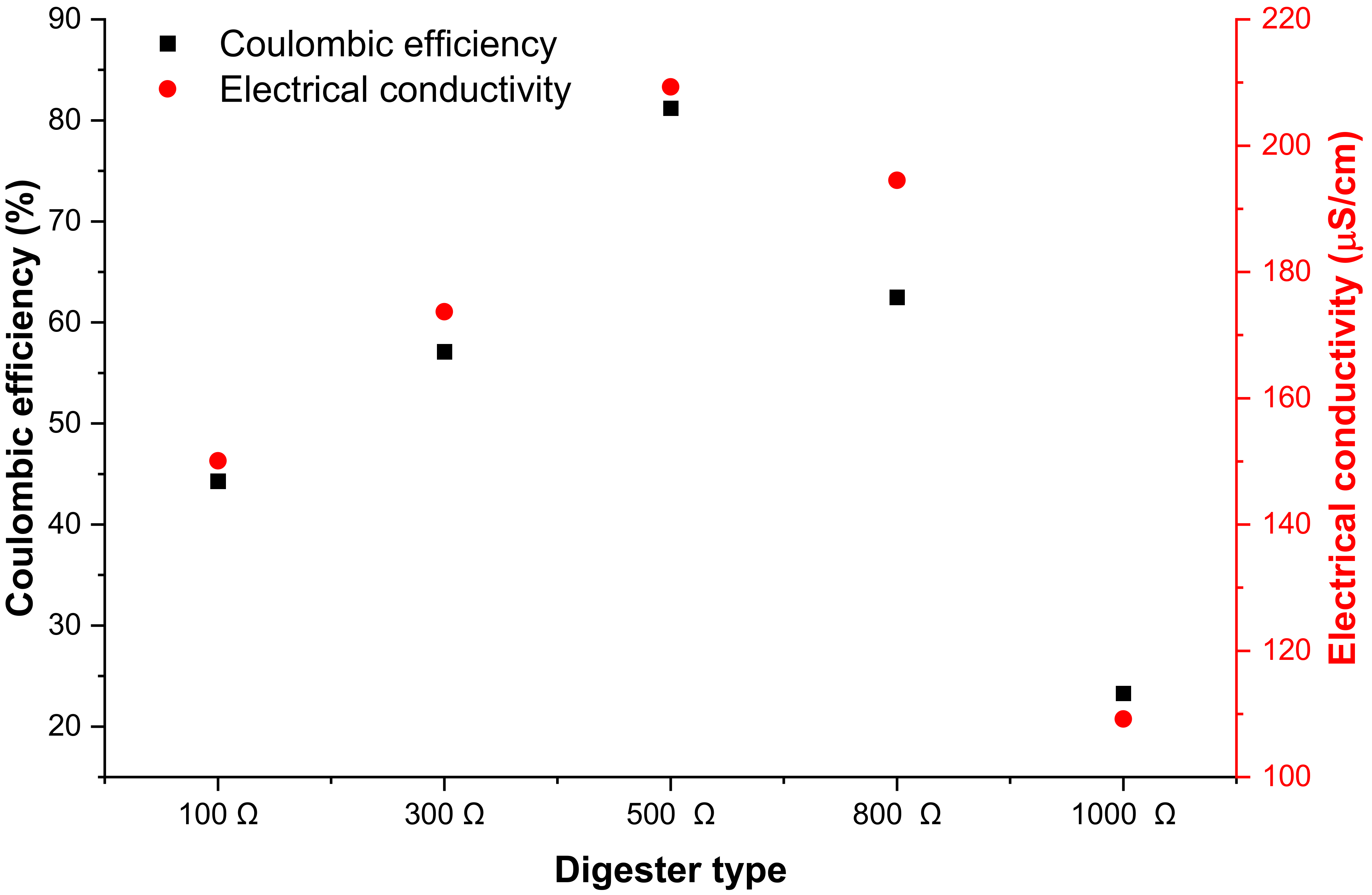
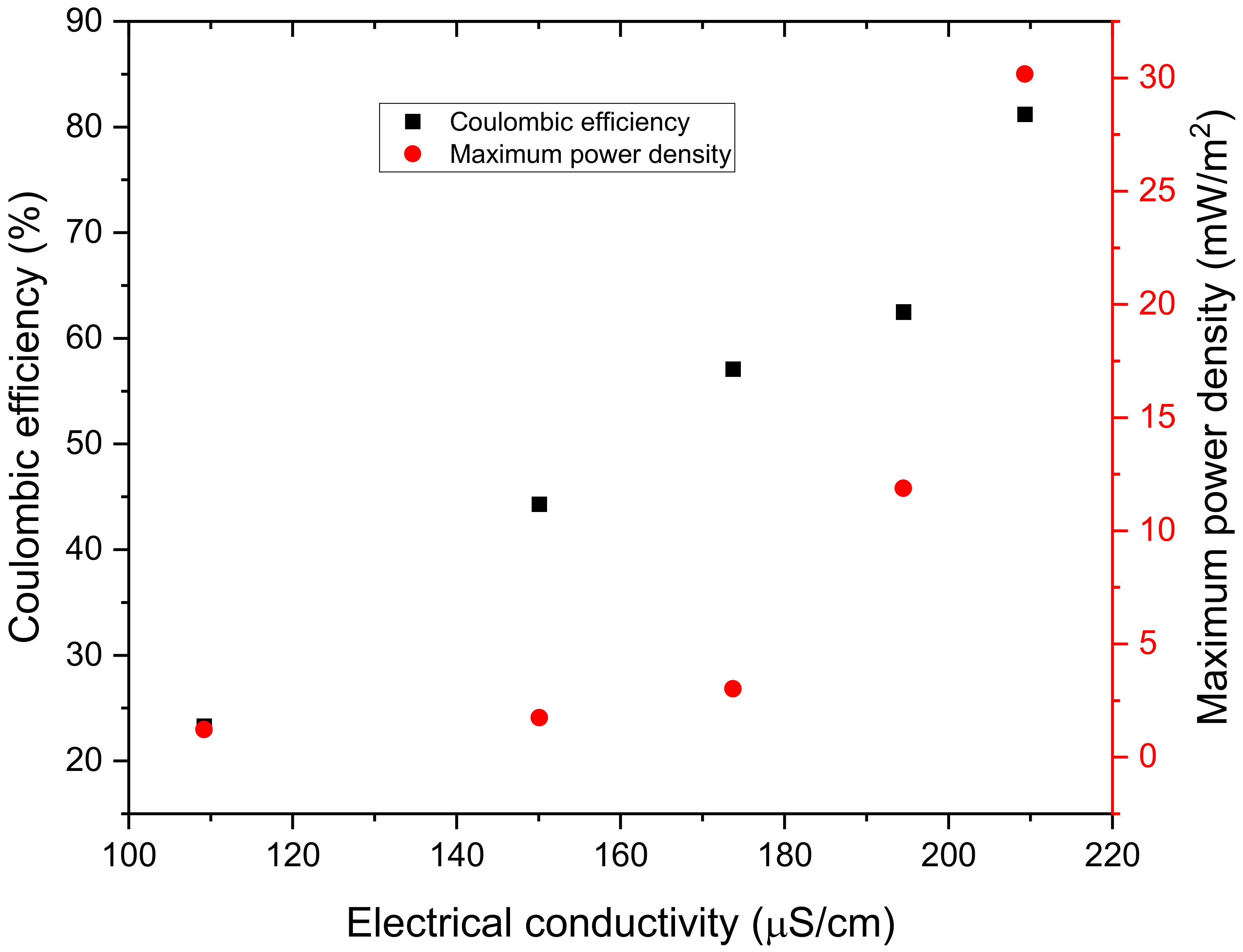
3.3. Electrochemical Efficiencies
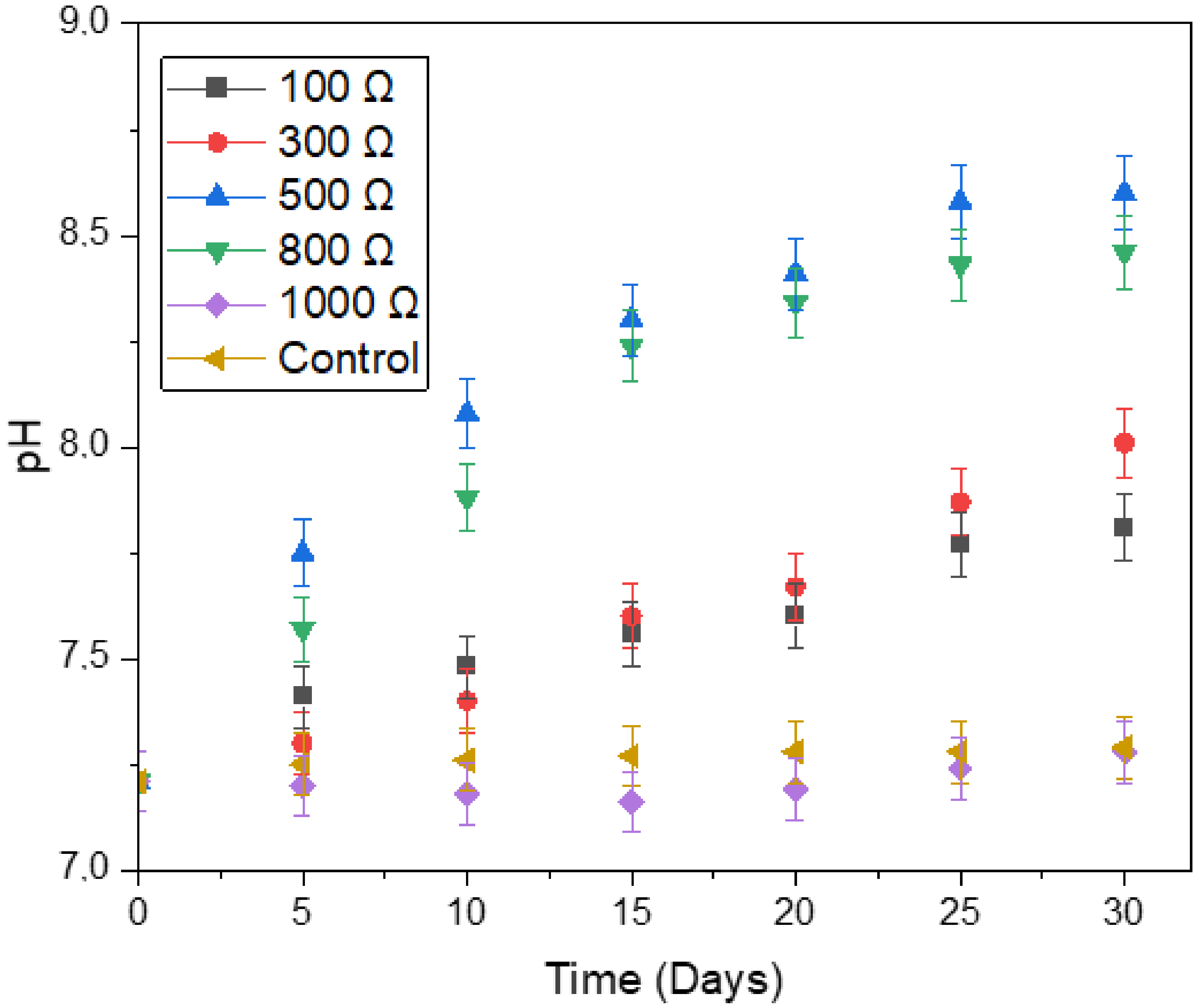
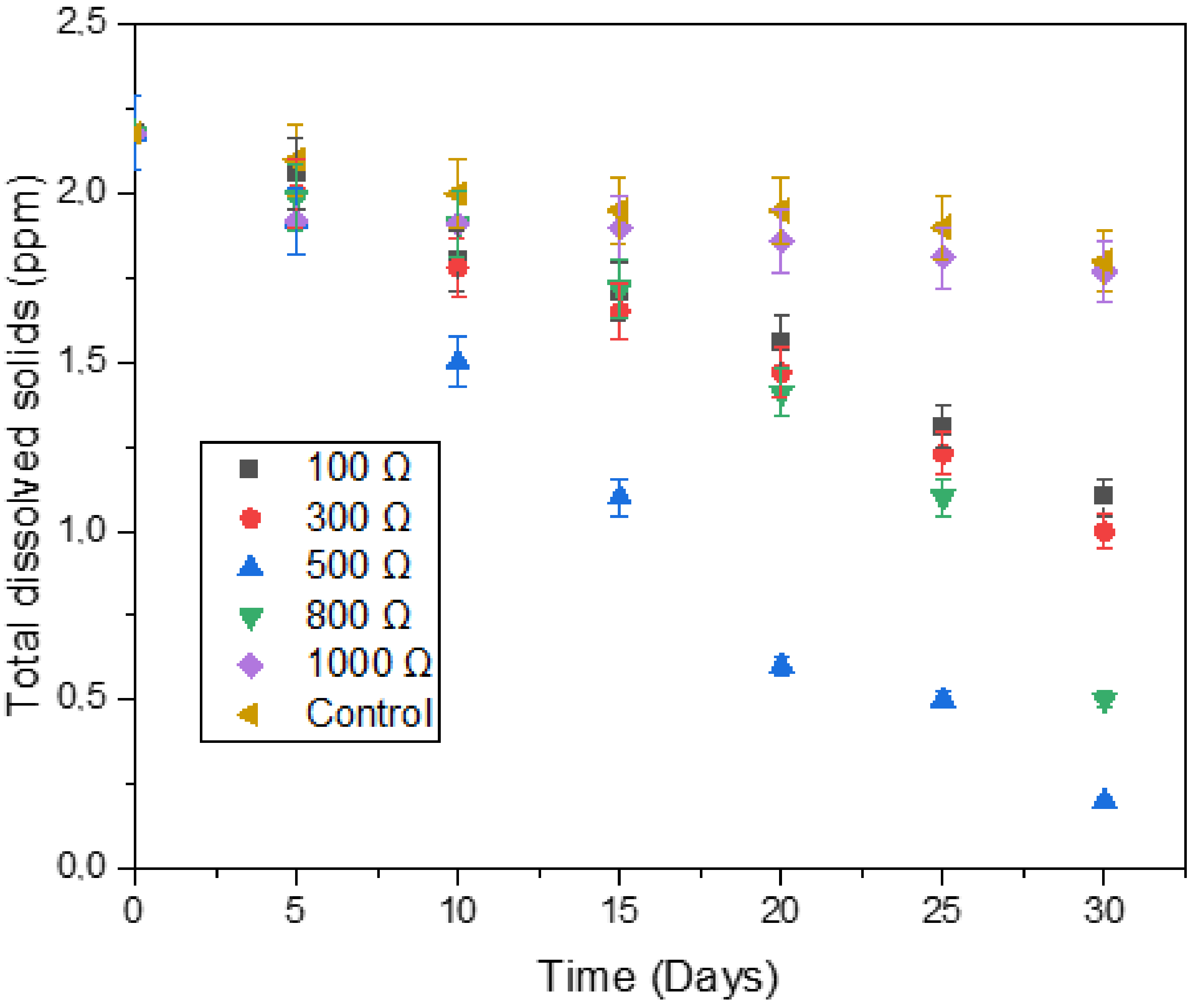
3.4. Process Stability Indicator
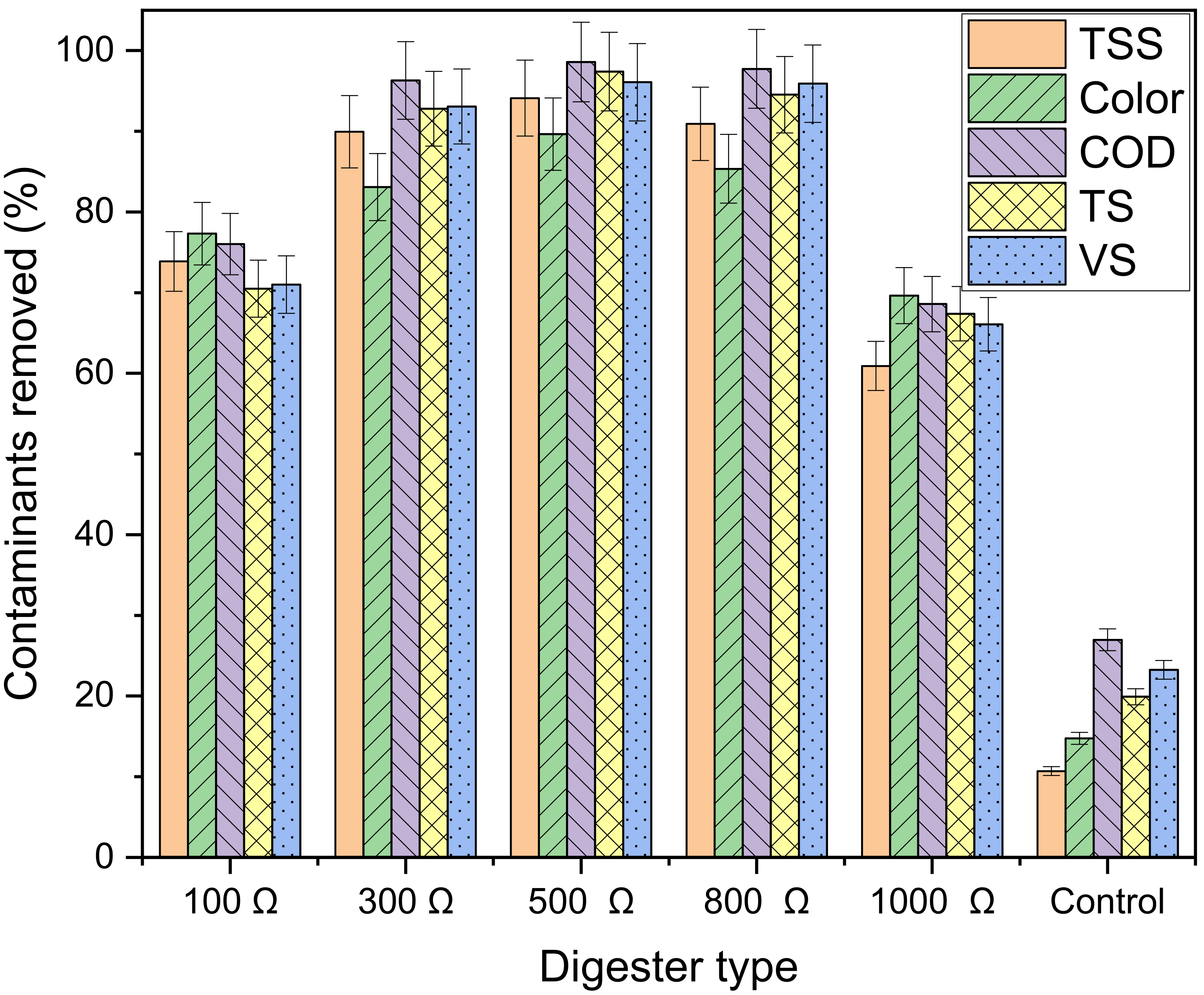
3.5. Decontamination
3.6. Economic and Energetic Viability of the Systems
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, A.; Radulescu, M.; Cismaș, L.M.; Cismaș, C.-M.; Chandio, A.A.; Simoni, S. Renewable Energy, Urbanization, Fossil Fuel Consumption, and Economic Growth Dilemma in Romania: Examining the Short- and Long-Term Impact. Energies 2022, 15, 7180. [Google Scholar] [CrossRef]
- Ben-Salha, O.; Hakimi, A.; Zaghdoudi, T.; Soltani, H.; Nsaibi, M. Assessing the Impact of Fossil Fuel Prices on Renewable Energy in China Using the Novel Dynamic ARDL Simulations Approach. Sustainability 2022, 14, 10439. [Google Scholar] [CrossRef]
- Strielkowski, W.; Civín, L.; Tarkhanova, E.; Tvaronavičienė, M.; Petrenko, Y. Renewable Energy in the Sustainable Development of Electrical Power Sector: A Review. Energies 2021, 14, 8240. [Google Scholar] [CrossRef]
- Zupancic, M.; Mozic, V.; Moze, M.; Cimerman, F.; Golobic, I. Current Status and Review of Waste-to-Biogas Conversion for Selected European Countries and Worldwide. Sustainability 2022, 14, 1823. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 25, 38–50. [Google Scholar] [CrossRef]
- Kumar, M. Social, Economic, and Environmental Impacts of Renewable Energy Resources. In Wind Solar Hybrid Renewable Energy System; Okedu, K.E., Tahour, A., Aissaou, A.G., Eds.; IntechOpen: London, UK, 2020; p. 252. [Google Scholar]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for Biogas Upgrading to Biomethane: A Review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef]
- Pavičić, J.; Novak Mavar, K.; Brkić, V.; Simon, K. Biogas and Biomethane Production and Usage: Technology Development, Advantages and Challenges in Europe. Energies 2022, 15, 2940. [Google Scholar] [CrossRef]
- Guo, P.; Zhou, J.; Ma, R.; Yu, N.; Yuan, Y. Biogas Production and Heat Transfer Performance of a Multiphase Flow Digester. Energies 2019, 12, 1960. [Google Scholar] [CrossRef]
- König, R.; Cuomo, M.; Pianta, E.; Buetti, A.; Mauri, F.; Tanadini, M.; Principi, P. Addition of Conductive Materials to Support Syntrophic Microorganisms in Anaerobic Digestion. Fermentation 2022, 8, 354. [Google Scholar] [CrossRef]
- Yang, S.; Han, S.; Yun, Y.-M.; Kang, S. Stimulation of Biomethane Productivity in Anaerobic Digestion Using ElectroConductive Carbon-Nanotube Hollow-Fiber Media. Minerals 2021, 11, 179. [Google Scholar] [CrossRef]
- Bajracharya, S.; Srikanth, S.; Mohanakrishna, G.; Zacharia, R.; Strik, D.P.B.T.B.; Pant, D. Biotransformation of carbon dioxide in bioelectrochemical systems: State of the art and future prospects. J. Power Sources 2017, 356, 256–273. [Google Scholar] [CrossRef]
- Wang, H.M.; Ren, Z.Y.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Factors Affecting the Effectiveness of Bioelectrochemical System Applications: Data Synthesis and Meta-Analysis. Batteries 2018, 4, 34. [Google Scholar] [CrossRef]
- Simeon, I.M.; Weig, A.; Freitag, R. Optimization of soil microbial fuel cell for sustainable bio-electricity production: Combined efects of electrode material, electrode spacing, and substrate feeding frequency on power generation and microbial community diversity. Biotechnol. Biofuels Bioprod. 2022, 15, 124. [Google Scholar] [CrossRef]
- Yasin, A.; Yasin, A.R.; Saqib, M.B.; Zia, S.; Riaz, M.; Nazir, R.; Abdalia, R.A.E.; Bajwa, S. Fuel Cell Voltage Regulation Using Dynamic Integral Sliding Mode Control. Electronics 2022, 11, 2922. [Google Scholar] [CrossRef]
- Tsekouras, G.J.; Deligianni, P.M.; Kanellos, F.D.; Kontargyri, V.T.; Kontaxis, P.A.; Manousakis, N.M.; Elias, C.N. Microbial Fuel Cell for Wastewater Treatment as Power Plant in Smart Grids: Utopia or Reality? Fron. Energy Res. 2022, 10, 843768. [Google Scholar] [CrossRef]
- Yanuka-Golub, K.; Dubinsky, V.; Korenblum, E.; Reshef, L.; Ofek-Lalzar, M.; Rishpon, J.; Gophnaa, U. Anode Surface Bioaugmentation Enhances Deterministic Biofilm Assembly in Microbial Fuel Cells. Appl. Environ. Sci. 2021, 12, e03629-20. [Google Scholar] [CrossRef]
- Logan, B.E.; Murano, C.; Scott, K.; Gray, N.D.; Head, I.M. Electricity generation from cysteine in a microbial fuel cell. Water Res. 2005, 39, 942–952. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Mirbagheri, S.A. A review of the operating parameters on the microbial fuel cell for wastewater treatment and electricity generation. Water Sci. Technol. 2021, 84, 1309. [Google Scholar] [CrossRef]
- Liu, T.; Yu, Y.Y.; Li, D.; Song, H.; Yan, X.; Chen, W.N. The effect of external resistance on biofilm formation and internal resistance formation in Shewanella inoculated microbial fuel cells. RSC Adv. 2016, 6, 20317–20323. [Google Scholar] [CrossRef]
- Paucar, N.E.; Sato, C. Microbial Fuel Cell for Energy Production, Nutrient Removal and Recovery from Wastewater: A Review. Processes 2021, 9, 1318. [Google Scholar] [CrossRef]
- Clauwaert, P.; Aelterman, P.; Pham, T.H.; De Schamphelaire, L.; Carballa, M.; Rabaey, K.; Verstraete, W. Minimizing losses in bioelectrochemical systems: The road to applications. Appl. Microbiol. Biotechnol. 2008, 79, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B.A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial fuel cells and their electrified biofilms. Biofilm 2021, 3, 100057. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.P.; Srinivasan, B.; Guiot, S.R.; Tartakovsky, B. The effect of real-time external resistance optimization on microbial fuel cell performance. Water Res. 2011, 45, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, S.; León-Fernández, L.F.; Nieznanski, J.; Karkosinski, D.; Fernandez-Morales, F.J. The Influence of External Load on the Performance of Microbial Fuel Cells. Energies 2021, 14, 612. [Google Scholar] [CrossRef]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion. Bioengineering 2021, 8, 198. [Google Scholar] [CrossRef]
- Madondo, N.I.; Rathilal, S.; Bakare, B.F. Utilization of Response Surface Methodology in Optimization and Modelling of a Microbial Electrolysis Cell for Wastewater Treatment Using Box–Behnken Design Method. Catalysts 2022, 12, 1052. [Google Scholar] [CrossRef]
- Morosini, C.; Conti, F.; Torretta, V.; Rada, E.C.; Passamani, G.; Schiavon, M.; Cioca, L.I.; Ragazzi, M. Biochemical methane potential assays to test the biogas production from the anaerobic digestion of sewage sludge and other organic matrices. WIT Trans. Ecol. Environ. 2016, 205, 235–244. [Google Scholar]
- WRC. Equipment for Measurement of Gas Production at Low Rates of Flow; Technical Memorandum TM104—Water Research Centre: Swindon, UK, 1975. [Google Scholar]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook, 6th ed.; McGraw-Hill Inc.: New York, NY, USA, 1984. [Google Scholar]
- Gregg, L.W. Water Analysis Handbook; H.A.C.H Company: Loveland, CO, USA, 1989; pp. 33–39. [Google Scholar]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Application of Bioelectrochemical System and Magnetite Nanoparticles on the Anaerobic Digestion of Sewage Sludge: Effect of Electrode Configuration. Catalysts 2022, 12, 642. [Google Scholar] [CrossRef]
- Amo-Duodu, G.; Tetteh, E.K.; Rathilal, S.; Chollom, M. Synthesis and characterization of magnetic nanoparticles: Biocatalytic effects on wastewater treatment. Mater. Today Proc. 2022, 62, S79–S84. [Google Scholar] [CrossRef]
- Molognoni, D.; Puig, S.; Balaguer, M.D.; Liberale, A.; Capodaglio, A.G.; Callegari, A.; Colprim, J. Reducing start-up time and minimizing energy losses of microbial fuel cells using maximum power point tracking strategy. J. Power Sources 2014, 269, 403–411. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Zhu, X.; Ye, D.; Fu, Q.; Liao, Q. Startup performance and anodic biofilm distribution in continuous-flow microbial fuel cells with serpentine flow fields: Effects of external resistance. Ind. Eng. Chem. Res. 2017, 56, 3767–3774. [Google Scholar] [CrossRef]
- Suzuki, K.; Kato, Y.; Yui, A.; Yamamoto, S.; Ando, S.; Rubaba, O.; Tashiro, Y.; Futamata, H. Bacterial communities adapted to higher external resistance can reduce the onset potential of anode in microbial fuel cells. J. Biosci. Bioeng. 2018, 125, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.P.; Pandit, S. Important Factors Influencing Microbial Fuel Cell Performance. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 377–406. [Google Scholar]
- Lyon, D.Y.; Buret, F.; Vogel, T.M.; Monier, J.M. Is resistance futile? Changing external resistance does not improve microbial fuel cell performance. Bioelectrochemistry 2010, 78, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yan, H.; Wang, W.; Mench, M.M.; Regan, J.M. Characterization of microbial fuel cells at microbially and electrochemically meaningful time scales. Environ. Sci. Technol. 2011, 45, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Ghangrekar, M.M.; Shinde, V.B. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour. Technol 2007, 98, 2879–2885. [Google Scholar] [CrossRef]
- Cai, W.F.; Geng, J.F.; Pu, K.B.; Ma, Q.; Jing, D.W.; Wang, Y.H.; Chen, Q.Y.; Liu, H. Investigation of a two-dimensional model on microbial fuel cell with different biofilm porosities and external resistances. Chem. Eng. J. 2018, 333, 572–582. [Google Scholar] [CrossRef]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8901. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Christy, A.D.; Carver, S.M.; Yu, Z.T.; Dehority, B.A.; Tuovinen, O.H. Effect of external resistance on bacterial diversity and metabolism in cellulose-fed microbial fuel cells. Bioresour. Technol. 2011, 102, 278–283. [Google Scholar] [CrossRef]
- Madondo, N.I.; Kweinor Tetteh, E.; Rathilal, S.; Bakare, B.F. Effect of an Electromagnetic Field on Anaerobic Digestion: Comparing an Electromagnetic System (ES), a Microbial Electrolysis System (MEC), and a Control with No External Force. Molecules 2022, 27, 3372. [Google Scholar] [CrossRef]
- Kook, L.; Nemestothy, N.; Belafi-Bako, K.; Bakonyi, P. Investigating the specific role of external load on the performance versus stability trade-off in microbial fuel cells. Bioresour. Technol. 2020, 309, 123313. [Google Scholar] [CrossRef] [PubMed]
- Kamau, J.M.; Mbui, D.N.; Mwaniki, J.M.; Mwaura, F.B.; Kamau, G.N. Microbial Fuel Cells: Influence of External Resistors on Power, Current and Power Density. J. Thermodyn. Catal. 2017, 8, 182. [Google Scholar] [CrossRef]
- Cordova-Bautista, Y.; Paraguay-Delgado, F.; Perez Hernandez, B.; Perez Hernandez, G.; Martinez Pereyra, G.; Ramirez Morales, E. Influence of external resistance and anodic pH on power density in microbial fuel cell operated with b. subtilis BSC-2 strain. Appl. Ecol. Environ. Res. 2018, 16, 1983–1997. [Google Scholar] [CrossRef]
- Carreon-Bautista, S.; Erbay, C.; Han, A.; Sanchez-Sinecio, E. Power Management System with Integrated Maximum Power Extraction Algorithm for Microbial Fuel Cells. IEE Trans. Energy Convers. 2015, 30, 262–272. [Google Scholar] [CrossRef]
- Zhang, X.; He, W.; Ren, L.; Stager, J.; Evans, P.J.; Logan, B.E. COD removal characteristics in air cathode microbial fuel cells. Bioresour. Technol. 2015, 176, 23–31. [Google Scholar] [CrossRef]
- Hashsham, S.A.; Fernandez, A.S.; Dollhopf, S.L.; Dazzo, F.B.; Hickey, R.F.; Tiedje, J.M.; Criddle, C.S. Parallel Processing of Substrate Correlates with Greater Functional Stability in Methanogenic Bioreactor Communities Perturbed by Glucose. Appl. Environ. Microbiol. 2000, 66, 4050–4057. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Li, J.; Qiang, L.; Ye, D. Biofilm formation and electricity generation of a microbial fuel cell started up under different external resistances. J. Power Sources 2011, 196, 6029–6035. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Sohaili, J.; Muda, K.; Sillanpaa, M. Magnetic Field Application and its Potential in Water and Wastewater Treatment Systems. Sep. Purif. Rev. 2014, 43, 206–240. [Google Scholar] [CrossRef]
- Zielinski, M.; Debowski, M.; Kazimierowicz, J. The Effect of Static Magnetic Field on Methanogenesis in the Anaerobic Digestion of Municipal Sewage Sludge. Energies 2021, 14, 590. [Google Scholar] [CrossRef]
- Alabdraba, W.S.; Albayati, M.B.A.; Radeef, A.Y.; Rejab, M.M. Influence of Magnetic Field on The Efficiency of The Coagulation Process to Remove Turbidity from Water. Int. Rev. Chem. Eng. 2013, 5, 8. [Google Scholar]
- Noveriansyah; Haryati, S.; Bustan, M.D. Effect of Acidity and Electromagnetic Field Strengths on Raw Water Treatment (Turbidity and Color). Int. J. Sci. Technol. Res. 2020, 9, 491–495. [Google Scholar]
- Herrero, M.; Laca, A.; Diaz, M. Application of life cycle assessment to food industry wastes. Food Ind. Wastes 2020, 1, 331–353. [Google Scholar]
- Kiran, E.U.; Stamatelatou, K.; Antonopoulou, G.; Lyberatos, G. Chapter 10: Production of biogas via anaerobic digestion. In Handbook of Biofuels Production: Processes and Technologies, 2nd ed.; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Sawston, UK, 2016; Volume 1, pp. 259–301. [Google Scholar]






| Parameter | Unit | Amount |
|---|---|---|
| pH | - | 7.2 ± 0.3 |
| VS | mg/L | 45.6 ± 1.1 |
| TS | mg/L | 53.3 ± 5.3 |
| Color | Pt.Co. | 254.3 ± 6.3 |
| COD | mg/L | 2451.2 ± 200 |
| TSS | mg/L | 39.4 ± 1 |
| Digester Type | pH | Maximum Power Density (mW/m2) |
|---|---|---|
| 1000 Ω | 7.28 ± 0.27 | 0.83 ± 0.08 |
| 100 Ω | 7.81 ± 0.15 | 3.17 ± 0.12 |
| 300 Ω | 8.01 ± 0.14 | 4.27 ± 0.13 |
| 800 Ω | 8.46 ± 0.10 | 11.88 ± 0.11 |
| 500 Ω | 8.60 ± 0.11 | 30.17 ± 0.10 |
| Type | Unit | 100 Ω | 300 Ω | 500 Ω | 800 Ω | 1000 Ω | Control |
|---|---|---|---|---|---|---|---|
| Energy content of methane | m3/h | 0.00149 | 0.00252 | 0.0062 | 0.0042 | 0.00253 | 0.00011 |
| Energy generated by methane (EG) | kWh | 0.00119 | 0.00202 | 0.0050 | 0.0034 | 0.00202 | 0.00035 |
| Energy used by the water bath (EB) | kWh | 0.00014 | 0.00014 | 0.00014 | 0.00014 | 0.00014 | 0.00014 |
| Energy produced by the MFC (EE) | kW/h | 0.00380 | 0.00512 | 0.03620 | 0.01426 | 0.00099 | 0.00000 |
| Total energy (ET) | kWh | 0.00486 | 0.00700 | 0.04102 | 0.01750 | 0.00288 | 0.00021 |
| Daily energy profit (3.22 ZAR/kWh) | ZAR/kWh | 0.01564 | 0.02255 | 0.13209 | 0.05636 | 0.00926 | 0.00068 |
| Daily net energy profit (0.23 USD/kWh) | USD/kWh | 0.00112 | 0.00161 | 0.00944 | 0.00403 | 0.00066 | 0.00005 |
| Annual net energy profit | ZAR/kWh | 5.71 | 8.23 | 48.22 | 20.57 | 3.38 | 2.50 |
| Annual net energy profit | USD/kWh | 0.41 | 0.59 | 3.45 | 1.46 | 0.24 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madondo, N.I.; Rathilal, S.; Bakare, B.F.; Tetteh, E.K. Application of Magnetite-Nanoparticles and Microbial Fuel Cell on Anaerobic Digestion: Influence of External Resistance. Microorganisms 2023, 11, 643. https://doi.org/10.3390/microorganisms11030643
Madondo NI, Rathilal S, Bakare BF, Tetteh EK. Application of Magnetite-Nanoparticles and Microbial Fuel Cell on Anaerobic Digestion: Influence of External Resistance. Microorganisms. 2023; 11(3):643. https://doi.org/10.3390/microorganisms11030643
Chicago/Turabian StyleMadondo, Nhlanganiso Ivan, Sudesh Rathilal, Babatunde Femi Bakare, and Emmanuel Kweinor Tetteh. 2023. "Application of Magnetite-Nanoparticles and Microbial Fuel Cell on Anaerobic Digestion: Influence of External Resistance" Microorganisms 11, no. 3: 643. https://doi.org/10.3390/microorganisms11030643
APA StyleMadondo, N. I., Rathilal, S., Bakare, B. F., & Tetteh, E. K. (2023). Application of Magnetite-Nanoparticles and Microbial Fuel Cell on Anaerobic Digestion: Influence of External Resistance. Microorganisms, 11(3), 643. https://doi.org/10.3390/microorganisms11030643









