Evaluation of the Toxic Activity of the Graphene Oxide in the Ex Vivo Model of Human PBMC Infection with Mycobacterium tuberculosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Graphene Oxide Characterization
2.2. Bacterial Manipulation
2.3. hPBMC Isolation
2.4. hPBMC In Vitro Infection Model
2.5. GO Toxicity Evaluation on hPBMCs
2.6. Statistical Analysis
3. Results
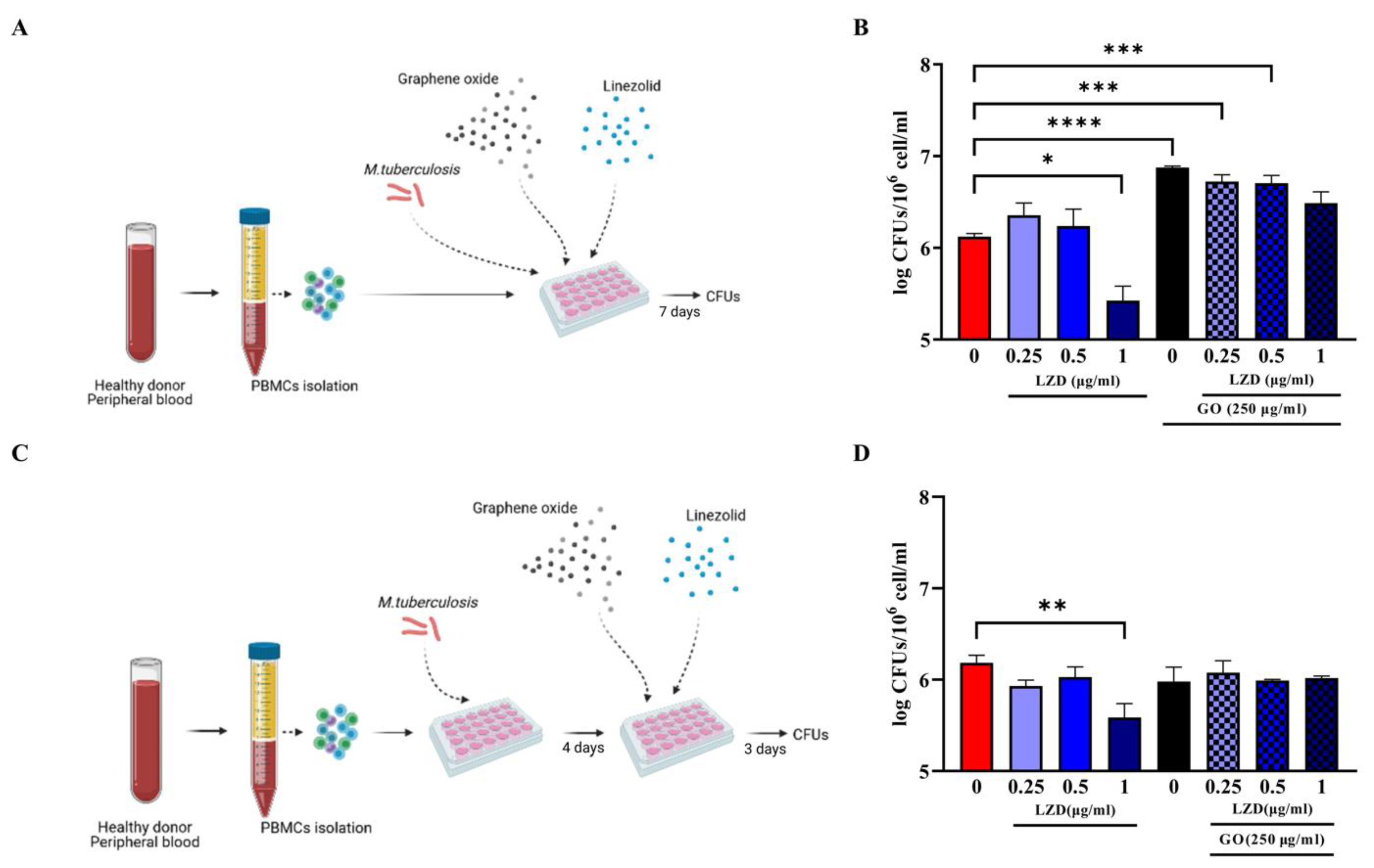
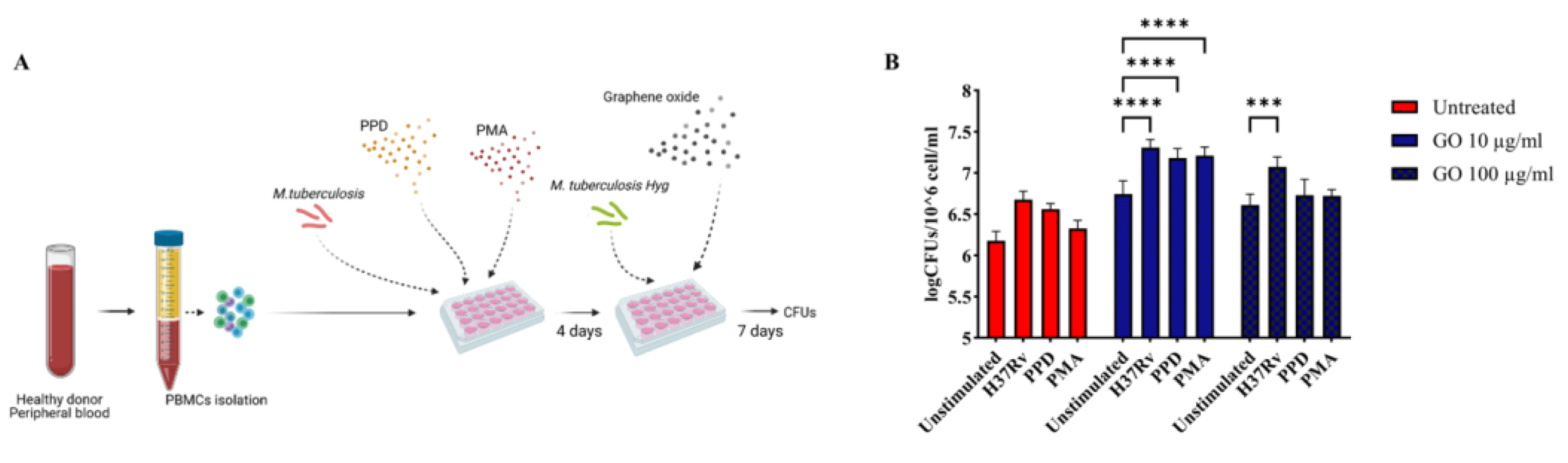
3.1. Combined Administration of GO with LZD Does Not Enhance the Anti-Mtb Activity of hPBMC
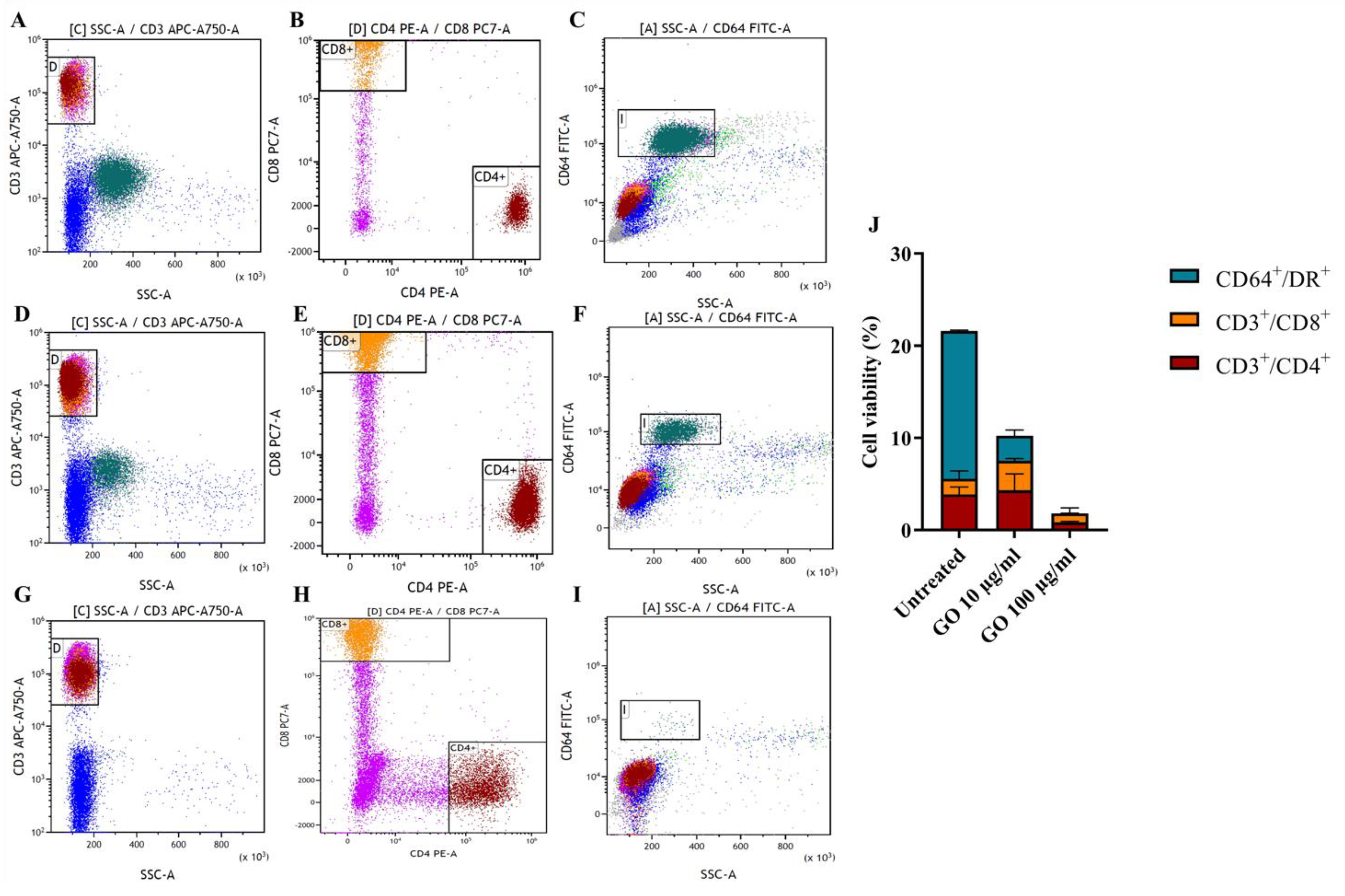
3.2. hPBMCs Incubated with GO Show a Reduction of Not Differentiated Monocytes
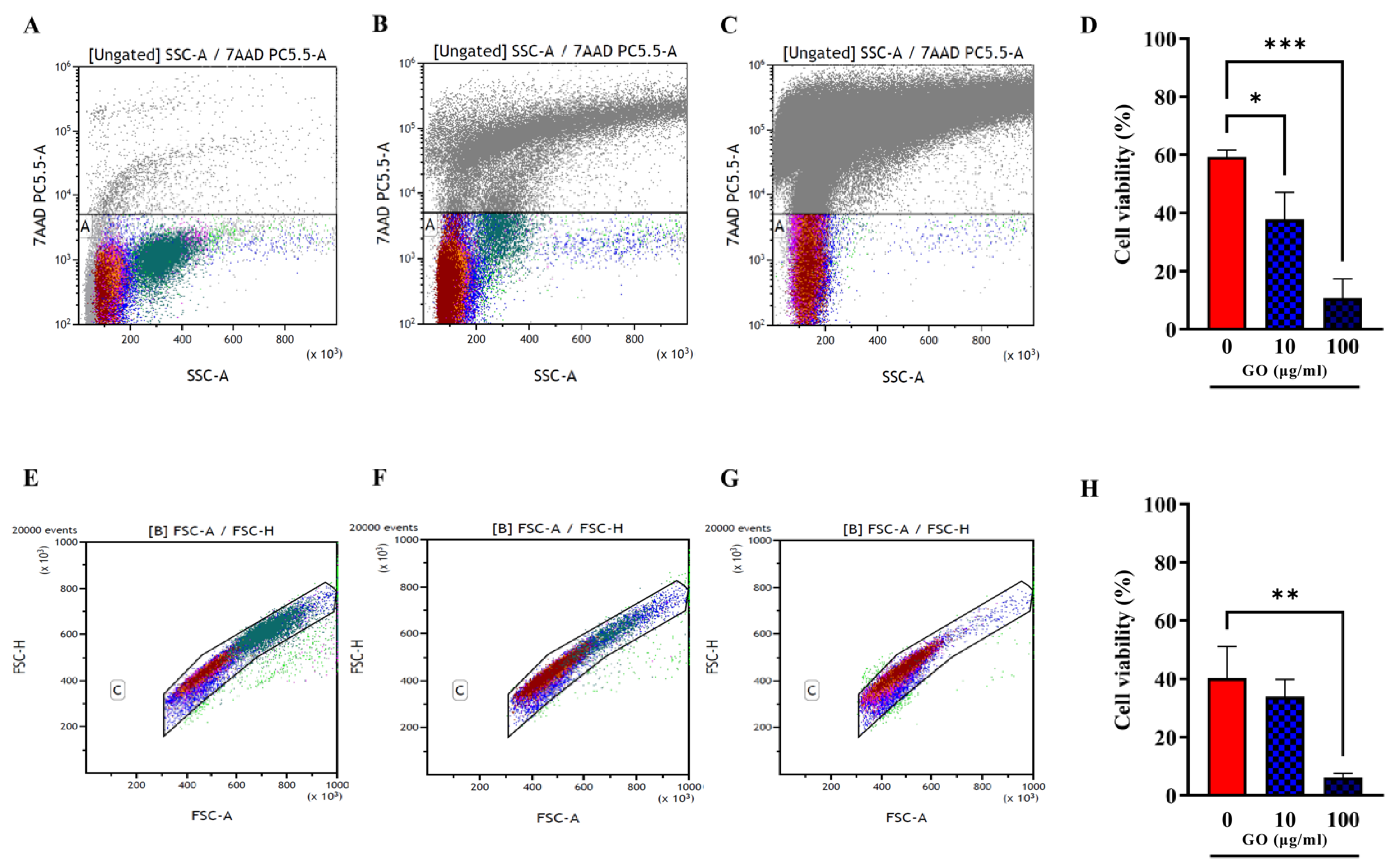
3.3. GO Exerts a Generalized Toxic Effect on Human Immune Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022.
- WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment-Drug-Susceptible Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2022.
- Abbasi, M.A. Guidelines for the treatment of drug resistant Tuberculosis: The 2018 revision. J. Ayub Med. Coll. Abbottabad 2018, 30, 493–494. [Google Scholar]
- WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2019.
- De Maio, F.; Bianco, D.M.; Delogu, G. The dark side of the COVID-19 treatments on mycobacterium tuberculosis infection. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022021. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.; Palmieri, V.; De Spirito, M.; Delogu, G.; Papi, M. Carbon nanomaterials: A new way against tuberculosis. Expert Rev. Med. Devices 2019, 16, 863–875. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Bugli, F.; Cacaci, M.; Palmieri, V.; Di Santo, R.; Torelli, R.; Ciasca, G.; Di Vito, M.; Vitali, A.; Conti, C.; Sanguinetti, M.; et al. Curcumin-loaded graphene oxide flakes as an effective antibacterial system against methicillin-resistant Staphylococcus aureus. Interface Focus 2018, 8, 20170059. [Google Scholar] [CrossRef]
- Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial Activity of Carbon-Based Nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef]
- Bosi, S.; Da Ros, T.; Castellano, S.; Banfi, E.; Prato, M. Antimycobacterial activity of ionic fullerene derivatives. Bioorganic Med. Chem. Lett. 2000, 10, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Konwarh, R.; Barua, N.; Buragohain, A.K.; Karak, N. Bio-based hyperbranched poly(ester amide)–MWCNT nanocomposites: Multimodalities at the biointerface. Biomater. Sci. 2013, 2, 192–202. [Google Scholar] [CrossRef]
- Han, W.; Niu, W.-Y.; Sun, B.; Shi, G.-C.; Cui, X.-Q. Biofabrication of polyphenols stabilized reduced graphene oxide and its anti-tuberculosis activity. J. Photochem. Photobiol. B Biol. 2016, 165, 305–309. [Google Scholar] [CrossRef]
- Saifullah, B.; Chrzastek, A.; Maitra, A.; Naeemullah, B.; Fakurazi, S.; Bhakta, S.; Hussein, M.Z. Novel Anti-Tuberculosis Nanodelivery Formulation of Ethambutol with Graphene Oxide. Molecules 2017, 22, 1560. [Google Scholar] [CrossRef]
- Bugli, F.; Posteraro, B.; Papi, M.; Torelli, R.; Maiorana, A.; Paroni Sterbini, F.; Posteraro, P.; Sanguinetti, M.; De Spirito, M. In Vitro Interaction between Alginate Lyase and Amphotericin B against Aspergillus fumigatus Biofilm Determined by Different Methods. Antimicrob. Agents Chemother. 2013, 57, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Perini, G.; De Spirito, M.; Papi, M. Graphene oxide touches blood: In vivo interactions of bio-coronated 2D materials. Nanoscale Horiz. 2019, 4, 273–290. [Google Scholar] [CrossRef]
- De Maio, F.; Palmieri, V.; Salustri, A.; Perini, G.; Sanguinetti, M.; De Spirito, M.; Delogu, G.; Papi, M. Graphene oxide prevents mycobacteria entry into macrophages through extracellular entrapment. Nanoscale Adv. 2019, 1, 1421–1431. [Google Scholar] [CrossRef]
- De Maio, F.; Palmieri, V.; Santarelli, G.; Perini, G.; Salustri, A.; Palucci, I.; Sali, M.; Gervasoni, J.; Primiano, A.; Ciasca, G.; et al. Graphene Oxide-Linezolid Combination as Potential New Anti-Tuberculosis Treatment. Nanomaterials 2020, 10, 1431. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.; Battah, B.; Palmieri, V.; Petrone, L.; Corrente, F.; Salustri, A.; Palucci, I.; Bellesi, S.; Papi, M.; Rubino, S.; et al. PE_PGRS3 of Mycobacterium tuberculosis is specifically expressed at low phosphate concentration, and its arginine-rich C-terminal domain mediates adhesion and persistence in host tissues when expressed in Mycobacterium smegmatis. Cell Microbiol. 2018, 20, e12952. [Google Scholar] [CrossRef]
- Battah, B.; Chemi, G.; Butini, S.; Campiani, G.; Brogi, S.; Delogu, G.; Gemma, S. A Repurposing Approach for Uncovering the Anti-Tubercular Activity of FDA-Approved Drugs with Potential Multi-Targeting Profiles. Molecules 2019, 24, 4373. [Google Scholar] [CrossRef]
- De Maio, F.; Cingolani, A.; Bianco, D.M.; Salustri, A.; Palucci, I.; Sanguinetti, M.; Delogu, G.; Sali, M. First description of the katG gene deletion in a Mycobacterium tuberculosis clinical isolate and its impact on the mycobacterial fitness. Int. J. Med. Microbiol. 2021, 311, 151506. [Google Scholar] [CrossRef] [PubMed]
- Fusco, L.; Orecchioni, M.; Reina, G.; Bordoni, V.; Fuoco, C.; Gurcan, C.; Guo, S.; Zoccheddu, M.; Collino, F.; Zavan, B.; et al. Lateral dimension and amino-functionalization on the balance to assess the single-cell toxicity of graphene on fifteen immune cell types. Nanoimpact 2021, 23, 100330. [Google Scholar] [CrossRef]
- Russier, J.; Treossi, E.; Scarsi, A.; Perrozzi, F.; Dumortier, H.; Ottaviano, L.; Meneghetti, M.; Palermo, V.; Bianco, A. Evidencing the mask effect of graphene oxide: A comparative study on primary human and murine phagocytic cells. Nanoscale 2013, 5, 11234–11247. [Google Scholar] [CrossRef]
- Shipkova, M.; Wieland, E. Surface markers of lymphocyte activation and markers of cell proliferation. Clin. Chim. Acta Int. J. Clin. Chem. 2012, 413, 1338–1349. [Google Scholar] [CrossRef]
- Sava, F.; Toldi, G.; Treszl, A.; Hajdú, J.; Harmath, Á.; Tulassay, T.; Vásárhelyi, B. Expression of lymphocyte activation markers of preterm neonates is associated with perinatal complications. BMC Immunol. 2016, 17, 19. [Google Scholar] [CrossRef]
- Palmieri, V.; Barba, M.; Di Pietro, L.; Gentilini, S.; Braidotti, M.C.; Ciancico, C.; Bugli, F.; Ciasca, G.; Larciprete, R.; Lattanzi, W.; et al. Reduction and shaping of graphene-oxide by laser-printing for controlled bone tissue regeneration and bacterial killing. 2D Mater. 2017, 5, 015027. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Park, E.-J.; Lee, G.-H.; Han, B.S.; Lee, B.-S.; Lee, S.; Cho, M.-H.; Kim, J.-H.; Kim, D.-W. Toxic response of graphene nanoplatelets in vivo and in vitro. Arch. Toxicol. 2014, 89, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-Functionalized Graphene Oxide as a Nanocarrier for Drug and Gene Delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, W.-J.; Zhang, X.; Zheng, J.; Li, L.; Zhu, X.; Yang, Q.; Zhang, M.; Liu, H.; et al. Analysis of the Antigenic Properties of Membrane Proteins of Mycobacterium tuberculosis. Sci. Rep. 2019, 9, 3042. [Google Scholar] [CrossRef]
- Ahmad, F.; Rani, A.; Alam, A.; Zarin, S.; Pandey, S.; Singh, H.; Hasnain, S.E.; Ehtesham, N.Z. Macrophage: A Cell With Many Faces and Functions in Tuberculosis. Front. Immunol. 2022, 13, 747799. [Google Scholar] [CrossRef]
- Saunders, B.M.; Frank, A.A.; Orme, I.M.; Cooper, A.M. CD4 is required for the development of a protective granulomatous response to pulmonary tuberculosis. Cell Immunol. 2002, 216, 65–72. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Z.; Ma, H.; Chen, Y. In Vitro Hemocompatibility and Toxic Mechanism of Graphene Oxide on Human Peripheral Blood T Lymphocytes and Serum Albumin. ACS Appl. Mater. Interfaces 2014, 6, 19797–19807. [Google Scholar] [CrossRef]
- Orecchioni, M.; Jasim, D.A.; Pescatori, M.; Manetti, R.; Fozza, C.; Sgarrella, F.; Bedognetti, D.; Bianco, A.; Kostarelos, K.; Delogu, L.G. Molecular and Genomic Impact of Large and Small Lateral Dimension Graphene Oxide Sheets on Human Immune Cells from Healthy Donors. Adv. Health Mater. 2015, 5, 276–287. [Google Scholar] [CrossRef]
- Jia, P.-P.; Sun, T.; Junaid, M.; Yang, L.; Ma, Y.-B.; Cui, Z.-S.; Wei, D.-P.; Shi, H.-F.; Pei, D.-S. Nanotoxicity of different sizes of graphene (G) and graphene oxide (GO) in vitro and in vivo. Environ. Pollut. 2019, 247, 595–606. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Kostarelos, K.; Fadeel, B. Cytokine Profiling of Primary Human Macrophages Exposed to Endotoxin-Free Graphene Oxide: Size-Independent NLRP3 Inflammasome Activation. Adv. Healthc. Mater. 2017, 7, 1700815. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Sánchez-Jiménez, F.M. Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000, 32, 157–170. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Sadanandan, A.R.; Ashokan, A.; Chandran, P.; Girish, C.M.; Menon, D.; Nair, S.V.; Rao, C.N.R.; Koyakutty, M. Hemocompatibility and Macrophage Response of Pristine and Functionalized Graphene. Small 2012, 8, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, L.; Huang, C.-C.; Lung, S.-C.C.; Yang, L.; Wang, W.-C.; Lin, P.-H.; Suo, G.; Lin, C.-H. Consecutive evaluation of graphene oxide and reduced graphene oxide nanoplatelets immunotoxicity on monocytes. Colloids Surf. B Biointerfaces 2017, 153, 300–309. [Google Scholar] [CrossRef]
- Dudek, I.; Skoda, M.; Jarosz, A.; Szukiewicz, D. The Molecular Influence of Graphene and Graphene Oxide on the Immune System Under In Vitro and In Vivo Conditions. Arch. Immunol. Ther. Exp. 2015, 64, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yan, X.; Tang, L.; Dai, G.; Luo, C. A Nanoparticle-Conjugated Anti-TBK1 siRNA Induces Autophagy-Related Apoptosis and Enhances cGAS-STING Pathway in GBM Cells. Evid. -Based Complement. Altern. Med. 2021, 2021, 6521953. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Capes-Davis, A.; Davis, J.M.; Downward, J.; Freshney, R.I.; Knezevic, I.; Lovell-Badge, R.; Masters, J.R.W.; Meredith, J.; Stacey, G.N.; et al. Guidelines for the use of cell lines in biomedical research. Br. J. Cancer 2014, 111, 1021–1046. [Google Scholar] [CrossRef]
- Lorsch, J.R.; Collins, F.S.; Lippincott-Schwartz, J. Fixing problems with cell lines. Science 2014, 346, 1452–1453. [Google Scholar] [CrossRef]
- Pan, C.; Kumar, C.; Bohl, S.; Klingmüller, U.; Mann, M. Comparative Proteomic Phenotyping of Cell Lines and Primary Cells to Assess Preservation of Cell Type-specific Functions. Mol. Cell. Proteom. 2009, 8, 443–450. [Google Scholar] [CrossRef]
- Tudose, M.; Anghel, E.M.; Culita, D.; Somacescu, S.; Calderon-Moreno, J.; Tecuceanu, V.; Dumitrascu, F.D.; Dracea, O.; Popa, M.; Marutescu, L.; et al. Covalent coupling of tuberculostatic agents and graphene oxide: A promising approach for enhancing and extending their antimicrobial applications. Appl. Surf. Sci. 2019, 471, 553–565. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, E.; Wang, Y.; Zhao, Y.; Yang, Z.; Zheng, B.; Mu, X.; Deng, X.; Shen, H.; Rong, H.; et al. In vivo toxicity assessment of four types of graphene quantum dots (GQDs) using mRNA sequencing. Toxicol. Lett. 2022, 363, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Raja, I.S.; Molkenova, A.; Kang, M.S.; Lee, S.H.; Lee, J.E.; Kim, B.; Han, D.-W.; Atabaev, T.S. Differential Toxicity of Graphene Family Nanomaterials Concerning Morphology. In Differential Toxicity of Graphene Family Nanomaterials Concerning Morphology; Han, D.W., Hong, S.W., Eds.; Springer: Singapore, 2022; Volume 1351, pp. 23–39. [Google Scholar] [CrossRef]
- Vasyukova, I.A.; Zakharova, O.V.; Kuznetsov, D.V.; Gusev, A.A. Synthesis, Toxicity Assessment, Environmental and Biomedical Applications of MXenes: A Review. Nanomaterials 2022, 12, 1797. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salustri, A.; De Maio, F.; Palmieri, V.; Santarelli, G.; Palucci, I.; Mercedes Bianco, D.; Marchionni, F.; Bellesi, S.; Ciasca, G.; Perini, G.; et al. Evaluation of the Toxic Activity of the Graphene Oxide in the Ex Vivo Model of Human PBMC Infection with Mycobacterium tuberculosis. Microorganisms 2023, 11, 554. https://doi.org/10.3390/microorganisms11030554
Salustri A, De Maio F, Palmieri V, Santarelli G, Palucci I, Mercedes Bianco D, Marchionni F, Bellesi S, Ciasca G, Perini G, et al. Evaluation of the Toxic Activity of the Graphene Oxide in the Ex Vivo Model of Human PBMC Infection with Mycobacterium tuberculosis. Microorganisms. 2023; 11(3):554. https://doi.org/10.3390/microorganisms11030554
Chicago/Turabian StyleSalustri, Alessandro, Flavio De Maio, Valentina Palmieri, Giulia Santarelli, Ivana Palucci, Delia Mercedes Bianco, Federica Marchionni, Silvia Bellesi, Gabriele Ciasca, Giordano Perini, and et al. 2023. "Evaluation of the Toxic Activity of the Graphene Oxide in the Ex Vivo Model of Human PBMC Infection with Mycobacterium tuberculosis" Microorganisms 11, no. 3: 554. https://doi.org/10.3390/microorganisms11030554
APA StyleSalustri, A., De Maio, F., Palmieri, V., Santarelli, G., Palucci, I., Mercedes Bianco, D., Marchionni, F., Bellesi, S., Ciasca, G., Perini, G., Sanguinetti, M., Sali, M., Papi, M., De Spirito, M., & Delogu, G. (2023). Evaluation of the Toxic Activity of the Graphene Oxide in the Ex Vivo Model of Human PBMC Infection with Mycobacterium tuberculosis. Microorganisms, 11(3), 554. https://doi.org/10.3390/microorganisms11030554









