Diversity of Bacterial Clones and Plasmids of NDM-1 Producing Escherichia coli Clinical Isolates in Central Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of blaNDM-1 Escerichia coli
2.2. Whole Genome Sequencing of blaNDM-1 Positive E. coli
2.3. Nucleotide Sequence Accession Numbers
2.4. Plasmid Analysis of blaNDM-1 Klebsiella Pneumoniae Strains
2.5. Data Management
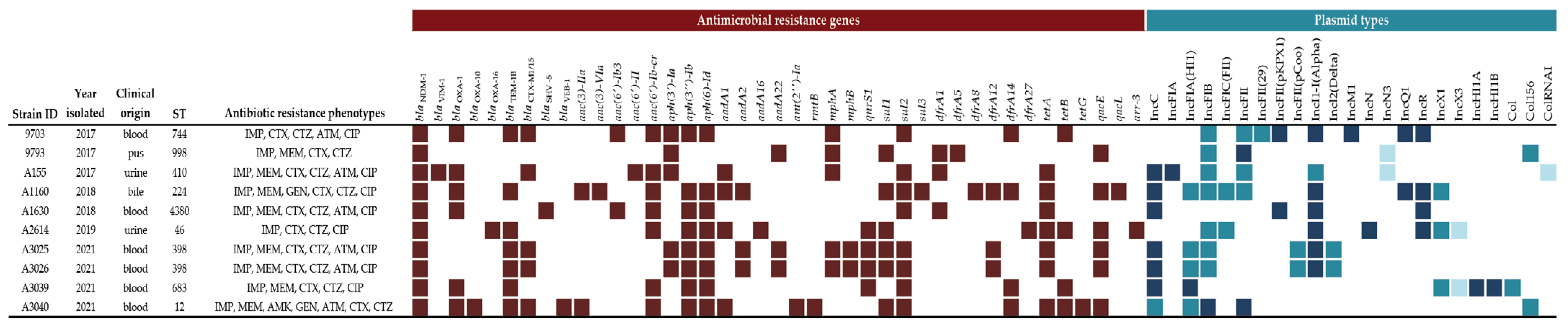
3. Results
3.1. Antimicrobial Susceptibility Profiles
3.2. Multi-Locus Sequence Typing (MLST)
3.3. Identification of Resistance Genes
3.4. Identification of Plasmids
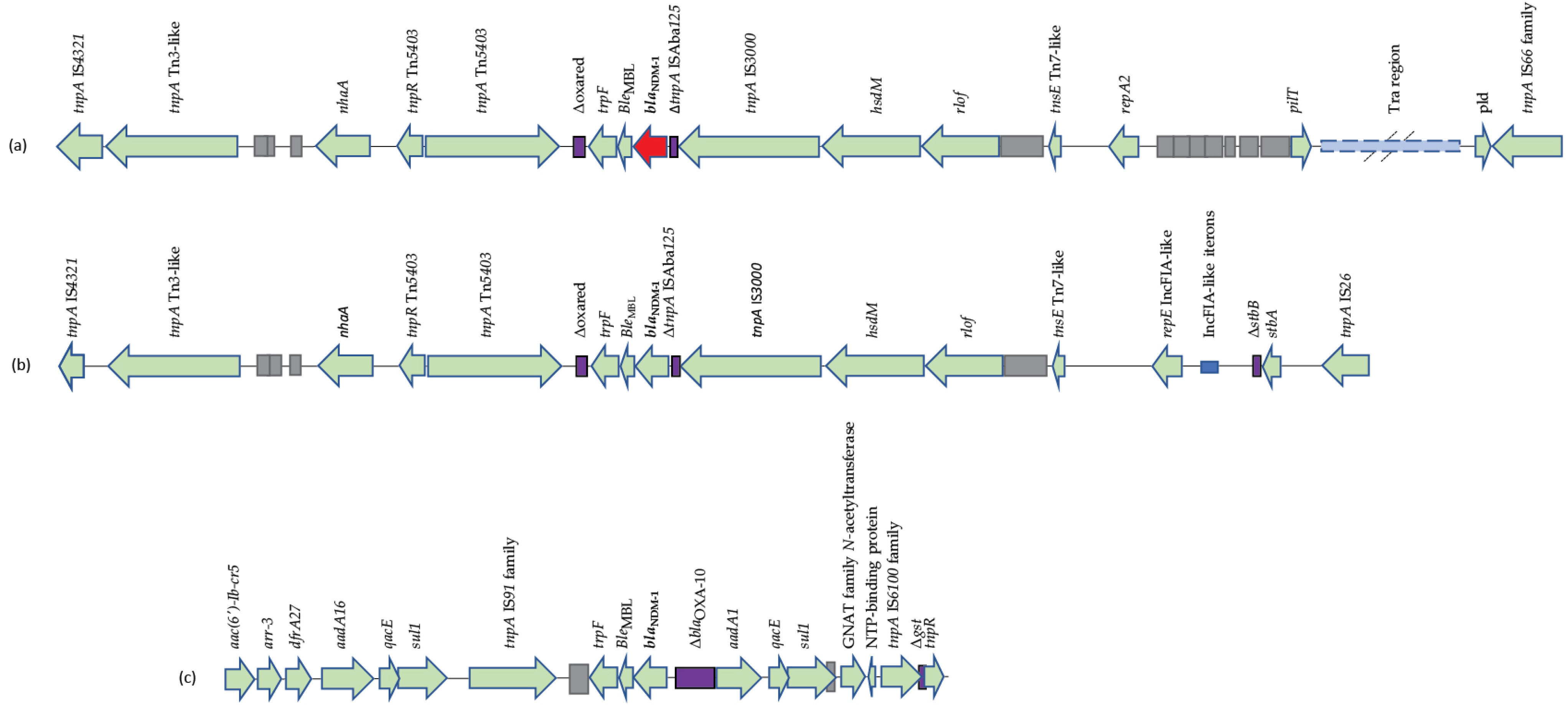
3.5. Genetic Environment of blaNDM-1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health. 2013, 10, 6235–6254. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J.; Chen, L.; Patel, G.; Gomez-Simmonds, A.; Weston, G.; Kim, A.C.; Seo, S.K.; Rosenthal, M.E.; Sperber, S.J.; Jenkins, S.G.; et al. Multicenter Clinical and Molecular Epidemiological Analysis of Bacteremia Due to Carbapenem-Resistant Enterobacteriaceae (CRE) in the CRE Epicenter of the United States. Antimicrob. Ag. Chemother. 2017, 61, e02349. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Bonomo, R.A. “Stormy waters ahead”: Global emergence of carbapenemases. Front Microbiol. 2013, 4, 48. [Google Scholar] [CrossRef]
- Ambler, R.P. The structure of beta-lactamases. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1980, 289, 321–331. [Google Scholar]
- Bush, K.; Jacoby, G.A. Updated functional classification of beta-lactamases. Antimicrob. Ag. Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Li, F.; Ye, K.; Li, X.; Ye, L.; Guo, L.; Wang, L.; Yang, J. Genetic characterization of carbapenem-resistant Escherichia coli from China, 2015-2017. BMC Microbiol. 2021, 21, 248. [Google Scholar] [CrossRef]
- Gondal, A.J.; Choudhry, N.; Bukhari, H.; Rizvi, Z.; Yasmin, N. Characterization of genomic diversity among carbapenem-resistant Escherichia coli clinical isolates and antibacterial efficacy of silver nanoparticles from Pakistan. Microorganisms 2022, 10, 2283. [Google Scholar] [CrossRef]
- Dadashi, M.; Yaslianifard, S.; Hajikhani, B.; Kabir, K.; Owlia, P.; Goudarzi, M.; Hakemivala, M.; Darban-Sarokhalil, D. Frequency distribution, genotypes and prevalent sequence types of New Delhi metallo-β-lactamase-producing Escherichia coli among clinical isolates around the world: A review. J. Glob. Antimicrob. Resist. 2019, 19, 284–293. [Google Scholar] [CrossRef]
- Tsilipounidaki, K.; Athanasakopoulou, Z.; Billinis, C.; Miriagou, V.; Petinaki, E. Importation of the first bovine ST361 New Delhi metallo-5 positive Escherichia coli in Greece. Microb. Drug Resist. 2022, 28, 386–387. [Google Scholar] [CrossRef]
- Papagiannitsis, C.C.; Bitar, I.; Malli, E.; Tsilipounidaki, K.; Hrabak, J.; Petinaki, E. IncC blaKPC-2-positive plasmid characterised from ST648 Escherichia coli. J. Glob. Antimicrob. Resist. 2019, 9, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Studentova, V.; Dobiasova, H.; Hedlova, D.; Dolejska, M.; Papagiannitsis, C.C.; Hrabak, J. Complete nucleotide sequences of two NDM-1-encoding plasmids from the same sequence type 11 Klebsiella pneumoniae strain. Antimicrob. Ag. Chemother. 2015, 59, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, T.L.; Lu, P.L.; Chen, Y.T.; Fung, C.P.; Chuang, Y.C.; Lin, J.C.; Siu, L.K. Closely related NDM-1-encoding plasmids from Escherichia coli and Klebsiella pneumoniae in Taiwan. PLoS ONE 2014, 9, e104899. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Ag. Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Berrazeg, M.; Diene, S.; Medjahed, L.; Parola, P.; Drissi, M.; Raoult, D.; Rolain, J. New Delhi metallo-beta-lactamase around the world: An eReview using Google Maps. EuroSurveillance 2014, 19, 20809. [Google Scholar] [CrossRef]
- Biez, L.; Bonnin, R.; Naas, T.; Dortet, L. Characterization of VIM-1, NDM-1 and OXA-48-producing Citrobacter freundii in France. J Antimicrob Chemother. 2022, 77, 1200–1202. [Google Scholar] [CrossRef]
- Protonotariou, E.; Meletis, G.; Chatzopoulou, F.; Malousi, A.; Chatzidimitriou, D.; Skoura, L. Emergence of Klebsiella pneumoniae ST11 co-producing NDM-1 and OXA-48 carbapenemases in Greece. J. Glob. Antimicrob. Resist. 2019, 19, 81–82. [Google Scholar] [CrossRef]
- Papagiannitsis, C.C.; Malli, E.; Florou, Z.; Sarrou, S.; Hrabak, J.; Mantzarlis, K.; Zakynthinos, E.; Petinaki, E. Emergence of sequence type 11 Klebsiella pneumoniae coproducing NDM-1 and VIM-1 metallo-β-lactamases in a Greek hospital. Diagn. Microbiol. Infect. Dis. 2017, 87, 295–297. [Google Scholar] [CrossRef]
- Meletis, G.; Chatzopoulou, F.; Chatzidimitriou, D.; Tsingerlioti, F.; Botziori, C.; Tzimagiorgis, G.; Skoura, L. Whole genome sequencing of NDM-1-producing ST11 Klebsiella pneumoniae isolated in a private laboratory in Greece. Microb. Drug. Resist. 2019, 25, 80–86. [Google Scholar] [CrossRef]
- Politi, L.; Gartzonika, K.; Spanakis, N.; Zarkotou, O.; Poulou, A.; Skoura, L.; Vrioni, G.; Tsakris, A. Emergence of NDM-1-producing Klebsiella pneumoniae in Greece: Evidence of a widespread clonal outbreak. J. Antimicrob. Chemother. 2019, 74, 2197–2202. [Google Scholar] [CrossRef]
- Wailan, A.M.; Paterson, D.L. The spread and acquisition of NDM-1: A multifactorial problem. Expert. Rev. Anti Infect. Ther. 2014, 12, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Villa, L.; Poirel, L.; Bonnin, R.A.; Nordmann, P. Evolution of IncA/C blaCMY-₂-carrying plasmids by acquisition of the blaNDM-₁ carbapenemase gene. Antimicrob. Ag. Chemother. 2012, 56, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Giufrè, M.; Errico, G.; Monaco, M.; Del Grosso, M.; Sabbatucci, M.; Pantosti, A.; Cerquetti, M.; Pagnotta, M.; Marra, M.; Carollo, M.; et al. Whole genome sequencing and molecular analysis of carbapenemase-producing Escherichia coli from intestinal carriage in elderly inpatients. Microorganisms 2022, 10, 1561. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; McNally, A.; Zong, Z. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin. Microbiol. Rev. 2019, 32, e00115. [Google Scholar] [CrossRef]
- Peirano, G.; Chen, L.; Nobrega, D.; Finn, T.J.; Kreiswirth, B.N.; DeVinney, R.; Pitout, J.D.D. Genomic Epidemiology of Global Carbapenemase-Producing Escherichia coli, 2015–2017. Emerg. Infect. Dis. 2022, 28, 924–931. [Google Scholar] [CrossRef]
- Azour, A.; Al-Bayssari, C.; Dagher, T.N.; Fajloun, F.; Fajloun, M.; Rolain, J.M. Clonal dissemination of plasmid-mediated carbapenem and colistin resistance in refugees living in overcrowded camps in North Lebanon. Antibiotics 2021, 10, 1478. [Google Scholar] [CrossRef]
- El-Herte, R.I.; Araj, G.F.; Matar, G.M.; Baroud, M.; Kanafani, Z.A.; Kanj, S.S. Detection of carbapenem-resistant Escherichia coli and Klebsiella pneumoniae producing NDM-1 in Lebanon. J. Infect. Dev. Ctries. 2012, 6, 457–461. [Google Scholar] [CrossRef]
- Baroud, M.; Dandache, I.; Araj, G.F.; Wakim, R.; Kanj, S.; Kanafani, Z.; Khairallah, M.; Sabra, A.; Shehab, M.; Dbaibo, G.; et al. Underlying mechanisms of carbapenem resistance in extended-spectrum β-lactamase-producing Klebsiella Pneumoniae and Escherichia Coli isolates at a tertiary care centre in Lebanon: Role of OXA-48 and NDM-1 carbapenemases. Int. J. Antimicrob. Ag. 2013, 41, 75–79. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, L.; Sun, S.; Yin, Y.; Wang, R.; Chen, F.; Wang, X.; Zhang, Y.; Hou, J.; Zhang, Y.; et al. Occurrence of high levels of cefiderocol resistance in carbapenem-resistant Escherichia coli before its approval in China: A report from China CRE-network. Microbiol. Spectr. 2022, 10, e0267021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsilipounidaki, K.; Florou, Z.; Skoulakis, A.; Fthenakis, G.C.; Miriagou, V.; Petinaki, E. Diversity of Bacterial Clones and Plasmids of NDM-1 Producing Escherichia coli Clinical Isolates in Central Greece. Microorganisms 2023, 11, 516. https://doi.org/10.3390/microorganisms11020516
Tsilipounidaki K, Florou Z, Skoulakis A, Fthenakis GC, Miriagou V, Petinaki E. Diversity of Bacterial Clones and Plasmids of NDM-1 Producing Escherichia coli Clinical Isolates in Central Greece. Microorganisms. 2023; 11(2):516. https://doi.org/10.3390/microorganisms11020516
Chicago/Turabian StyleTsilipounidaki, Katerina, Zoi Florou, Anargyros Skoulakis, George C. Fthenakis, Vivi Miriagou, and Efthymia Petinaki. 2023. "Diversity of Bacterial Clones and Plasmids of NDM-1 Producing Escherichia coli Clinical Isolates in Central Greece" Microorganisms 11, no. 2: 516. https://doi.org/10.3390/microorganisms11020516
APA StyleTsilipounidaki, K., Florou, Z., Skoulakis, A., Fthenakis, G. C., Miriagou, V., & Petinaki, E. (2023). Diversity of Bacterial Clones and Plasmids of NDM-1 Producing Escherichia coli Clinical Isolates in Central Greece. Microorganisms, 11(2), 516. https://doi.org/10.3390/microorganisms11020516








