Effect of Yeast Saccharomyces cerevisiae as a Probiotic on Diet Digestibility, Fermentative Metabolites, and Composition and Functional Potential of the Fecal Microbiota of Dogs Submitted to an Abrupt Dietary Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Experimental Diets and Groups
2.3. Diet Digestibility
2.4. Fecal Fermentative Products Analysis
2.5. Microbiome and Functional Analysis
2.6. Calculations and Statistical Analysis
ME (kcal/kg) = {kcal/g GE intake − kcal/g GE fecal excretion − [(g CP intake − g CP
fecal excretion) × 1.25kcal/g]}/g of feed intake.
3. Results
3.1. Diet Intake and Digestibility
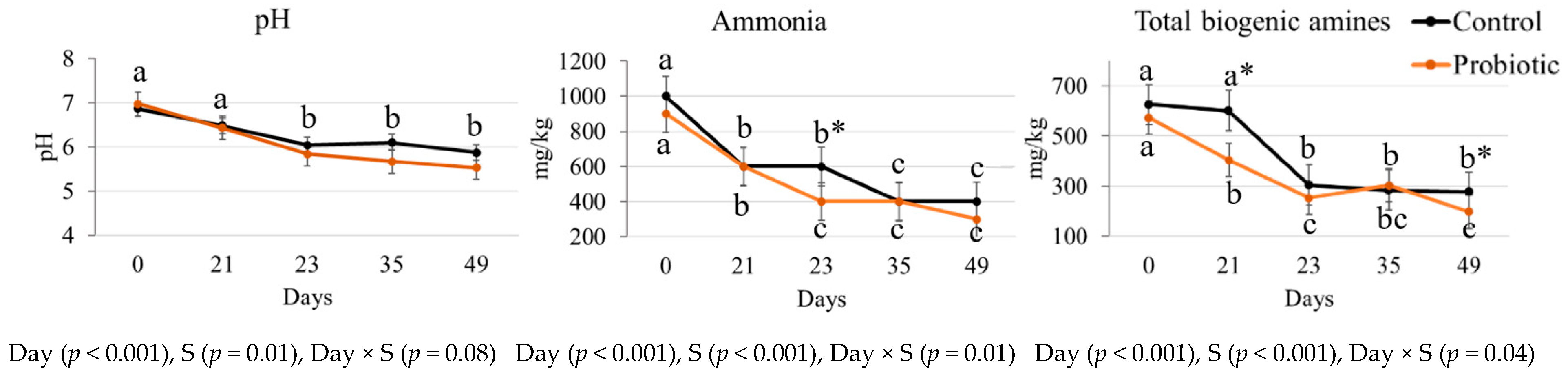
3.2. Fecal Fermentative Metabolites
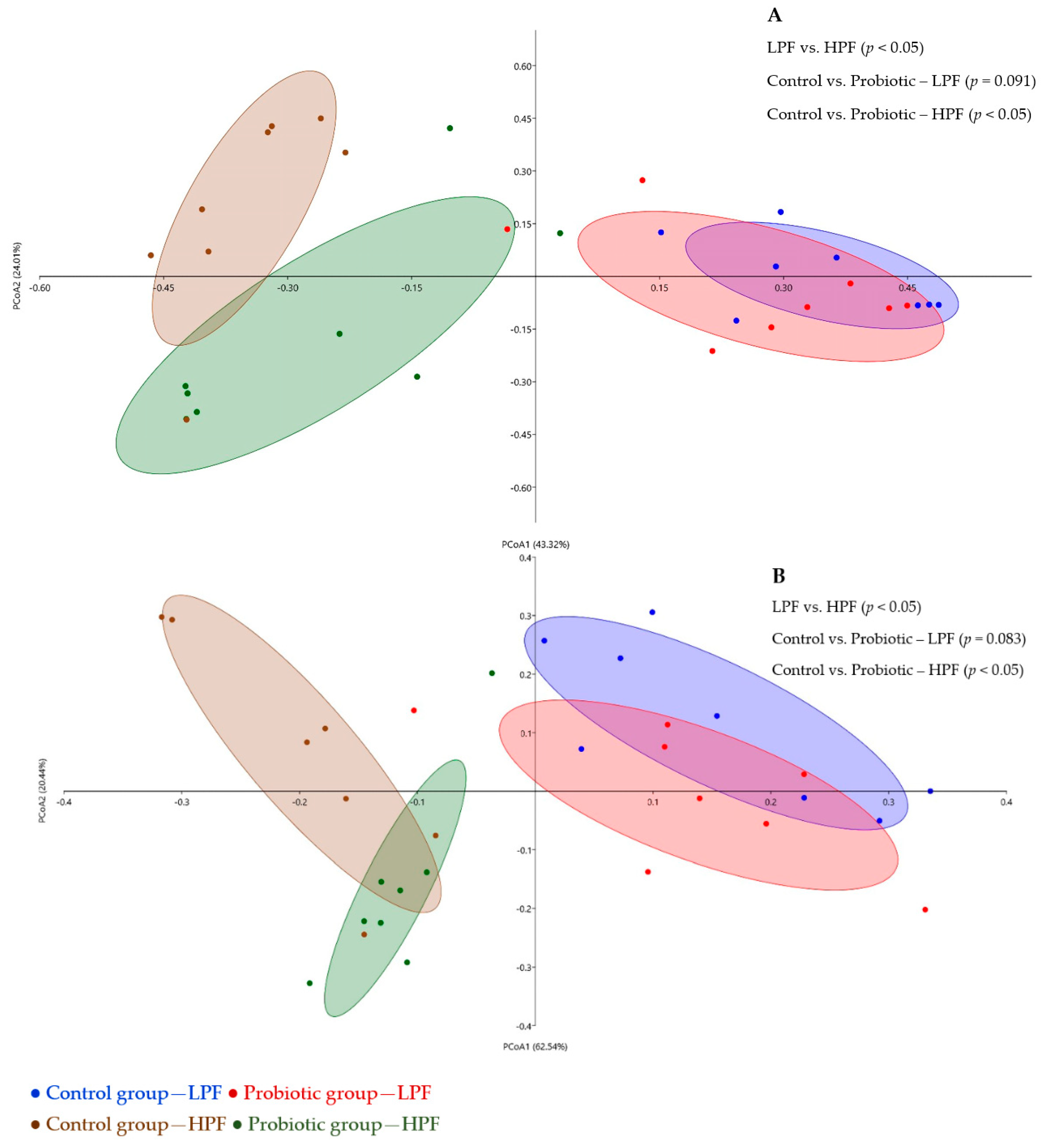
3.3. Fecal Microbiome and Functional Profiling
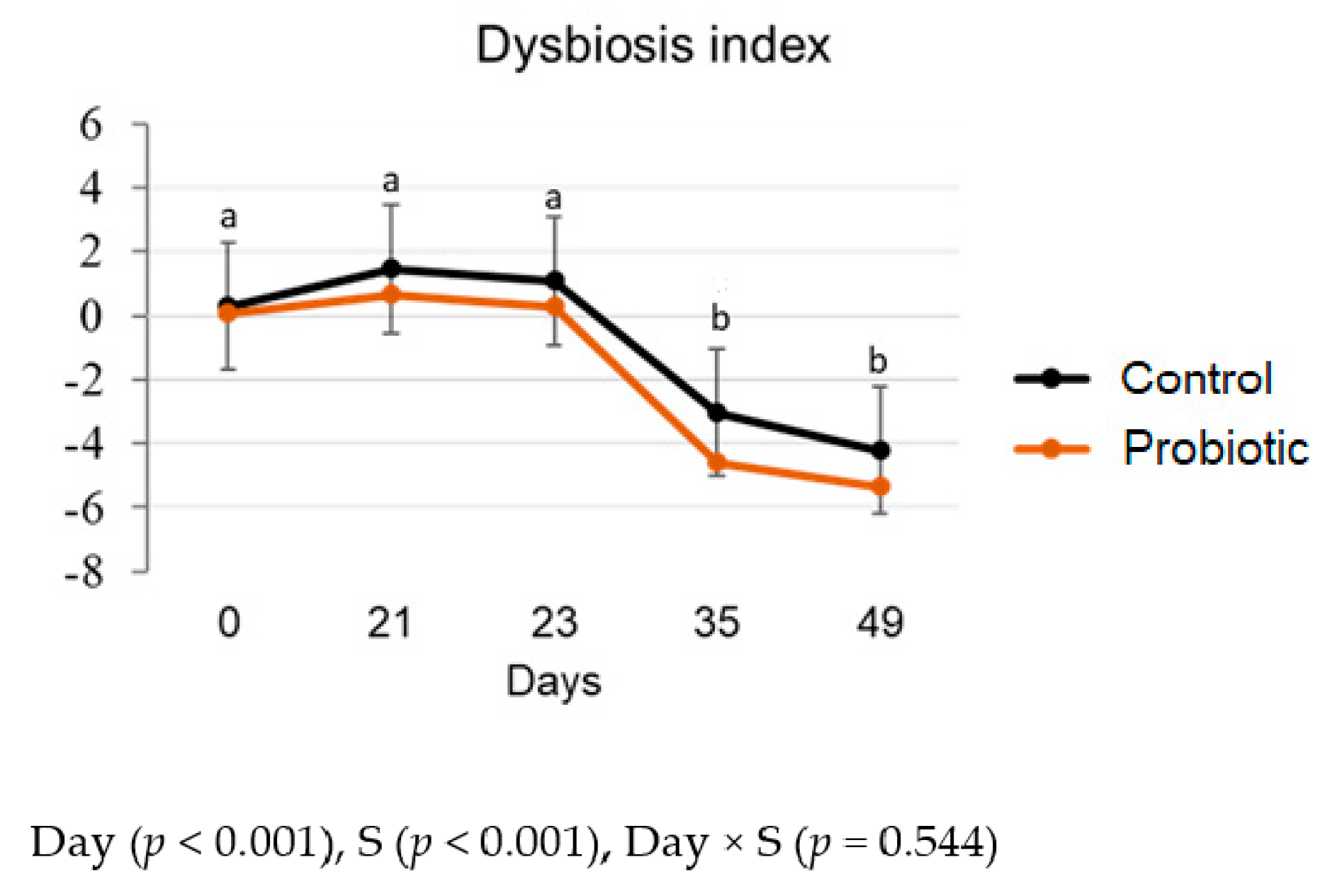
3.3.1. Dysbiosis Index and qPCR
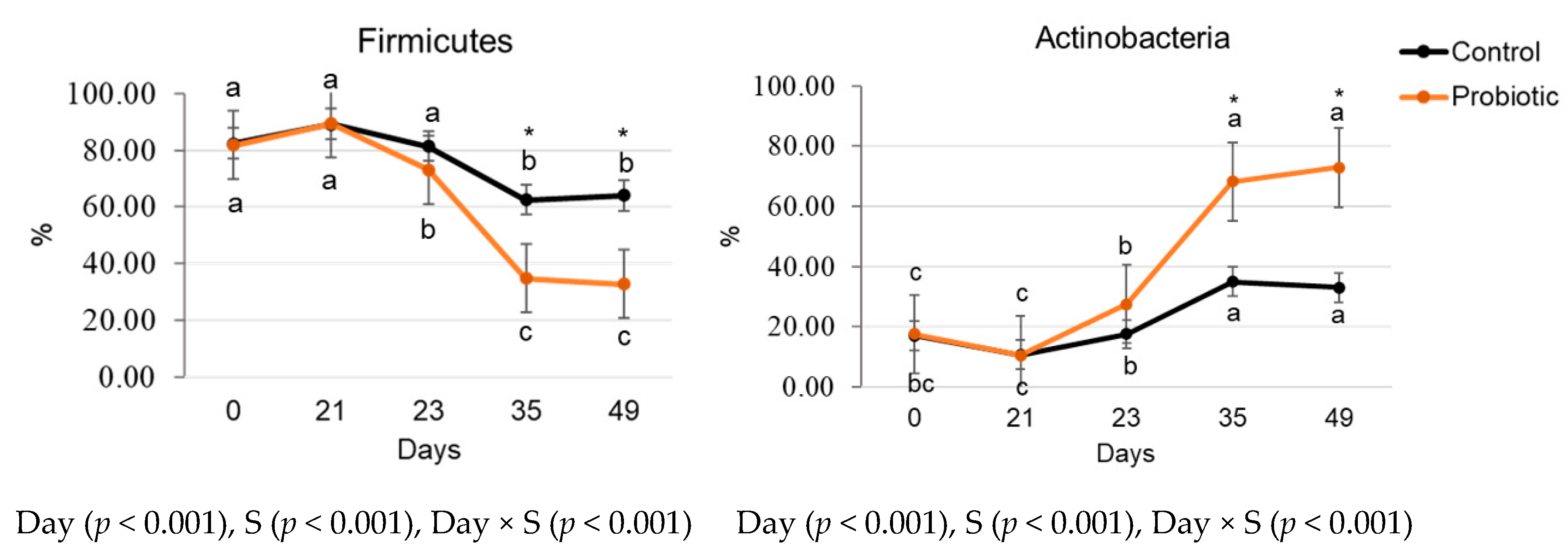
3.3.2. Relative Microbial Abundance and Diversity Indexes
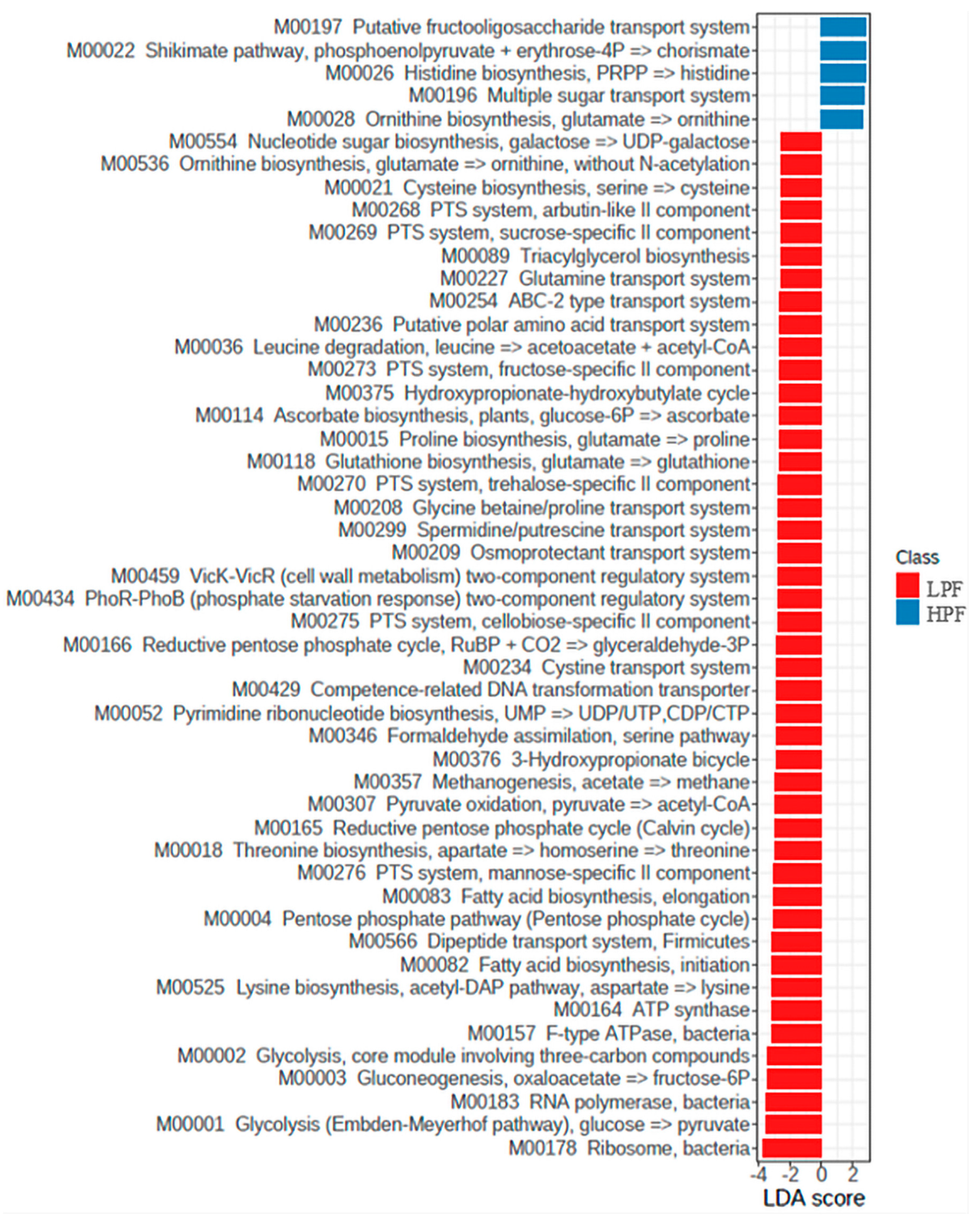
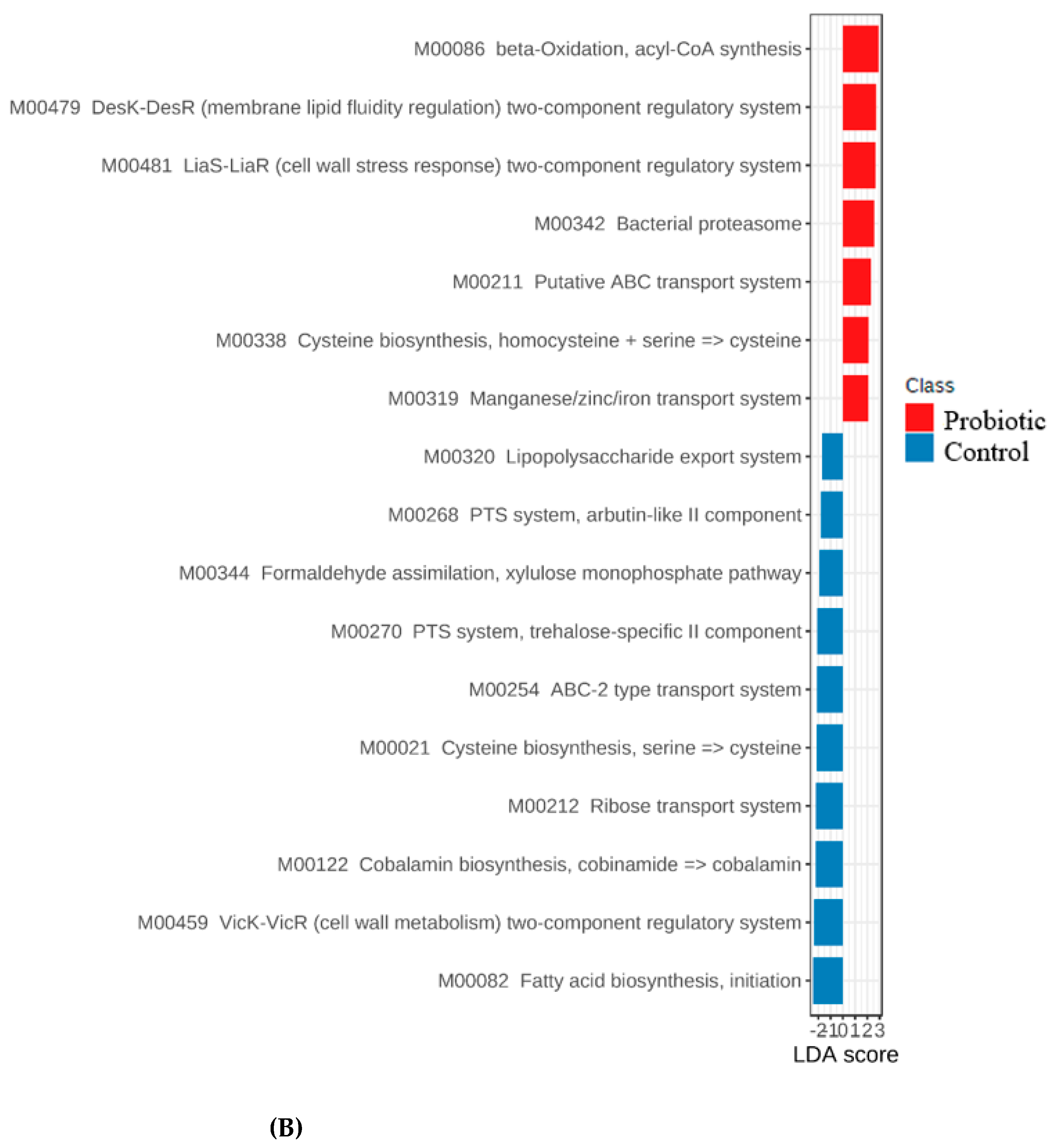
3.4. Functional Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.Y.; Carroll, M.Q.; Miller, M.J.; Rabot, R.; Swanson, K.S. Supplementation of yeast Cell Wall fraction tends to improve intestinal health in adult dogs undergoing an abrupt diet transition. Front. Vet. Sci. 2020, 7, 597939. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Swanson, K.S. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113 (Suppl. 1), S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.S.; Sakomura, N.K.; Souza, D.F.; Filho, F.O.R.; Gomes, M.O.S.; Vasconcellos, R.S.; Carciofi, A.C. Brewer’s yeast and sugarcane yeast as protein sources for dogs. J. Anim. Physiol. Anim. Nutr. 2013, 98, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Stercova, E.; Kumprechtova, D.; Auclair, E.; Novakova, J. Effects of live yeast dietary supplementation on nutrient digestibility and fecal microflora in beagle dogs. J. Anim. Sci. 2016, 94, 2909–2918. [Google Scholar] [CrossRef]
- Kiros, T.G.; Derakhshani, H.; Pinloche, E.; D’inca, R.; Marshall, J.; Auclair, E.; Van Kessel, A. Effect of live yeast Saccharomyces cerevisiae (Actisaf Sc 47) supplementation on the performance and hindgut microbiota composition of weanling pigs. Sci. Rep. 2018, 8, 5315. [Google Scholar] [CrossRef]
- Kiros, T.G.; Luise, D.; Derakhshani, H.; Petri, R.; Trevisi, P.; D’Inca, R.; Van Kessel, A.G. Effect of live yeast Saccharomyces cerevisiae supplementation on the performance and cecum microbial profile of suckling piglets. PLoS ONE 2019, 14, e0219557. [Google Scholar] [CrossRef]
- Delima, M.D.; Lopes, I.M.G.; da Silva, K.F.; Miranda, H.A.F.; de Almeida, A.C.; Duarte, E.R. Uso de aditivos em dietas para leitões em fase de creche: Uma revisão. Res. Soc. Dev. 2020, 9, e26491211081. [Google Scholar]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- Laflamme, D.R.P.C. Development and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- NRC—National Research Council. Nutrient Requirements of Dogs and Cats; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- AOAC—Association of the Official Analytical Chemists. Official Methods and Tentative Methods of Analysis, 16th ed.; AOAC International: Rockville, MD, USA, 1995. [Google Scholar]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; DeVries, J.W.; Furda, I. Determination of insoluble, soluble, and total dietary fiber in foods and food products: Interlaboratory study. J. Assoc. Off. Anal. Chem. 1988, 71, 1017–1023. [Google Scholar] [CrossRef]
- Brito, C.B.M.; Félix, A.P.; Jesus, R.M.; França, M.I.; Oliveira, S.G.; Krabbe, E.L.; Maiorka, A. Digestibility and palatability of dog foods containing different moisture levels, and the inclusion of a mould inhibitor. Anim. Feed Sci. Technol. 2010, 159, 150–155. [Google Scholar] [CrossRef]
- Urrego, M.I.G.; Matheus, L.F.D.O.; Santos, K.M.; Ernandes, M.C.; Monti, M.; Souza, D.F.; Brunetto, M.A. Effects of different protein sources on fermentation metabolites and nutrient digestibility of brachycephalic dogs. J. Nutr. Sci. 2017, 6, E43. [Google Scholar] [CrossRef] [PubMed]
- Carciofi, A.C.; de-Oliveira, L.D.; Valério, A.G.; Borges, L.L.; de Carvalho, F.M.; Brunetto, M.A.; Vasconcellos, R.S. Comparison of micronized whole soybeans to common protein sources in dry dog and cat diets. Anim. Feed Sci. Technol. 2009, 151, 251–260. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Pruitt, K.D. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable, and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- AAFCO—Association of American Feed Control Officials. Dog and Cat Nutrient Profiles. In Official Publications of the Association of American Feed Control Officials Incorporated; AAFCO: Oxford, IN, USA, 2016. [Google Scholar]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Lin, C.Y.; Jha, A.R.; Oba, P.M.; Yotis, S.M.; Shmalberg, J.; Honaker, R.W.; Swanson, K.S. Longitudinal fecal microbiome and metabolite data demonstrate rapid shifts and subsequent stabilization after an abrupt dietary change in healthy adult dogs. Anim. Microb. 2022, 4, 46. [Google Scholar] [CrossRef]
- Lin, C.Y.; Alexander, C.; Steelman, A.J.; Warzecha, C.M.; De Godoy, M.R.; Swanson, K.S. Effects of a Saccharomyces cerevisiae fermentation product on fecal characteristics, nutrient digestibility, fecal fermentative end-products, fecal microbial populations, immune function, and diet palatability in adult dogs. J. Anim. Sci. 2019, 97, 1586–1599. [Google Scholar] [CrossRef]
- Kröger, S.; Vahjen, W.; Zentek, J. Influence of lignocellulose and low or high levels of sugar beet pulp on nutrient digestibility and the fecal microbiota in dogs. J. Anim. Sci. 2017, 95, 1598–1605. [Google Scholar] [CrossRef]
- Sabchuk, T.T.; Lowndes, F.G.; Scheraiber, M.; Silva, L.P.; Félix, A.P.; Maiorka, A.; Oliveira, S.G. Effect of soya hulls on diet digestibility, palatability, and intestinal gas production in dogs. Anim. Feed Sci. Technol. 2017, 225, 134–142. [Google Scholar] [CrossRef]
- Ephraim, E.; Cochrane, C.Y.; Jewell, D.E. Varying protein levels influence metabolomics and the gut microbiome in healthy adult dogs. Toxins 2020, 12, 517. [Google Scholar] [CrossRef]
- Tramontano, M.; Andrejev, S.; Pruteanu, M.; Klünemann, M.; Kuhn, M.; Galardini, M.; Jouhten, P.; Zelezniak, A.; Zeller, G.; Bork, P.; et al. Nutritional preferences of human gut bacteria reveal their metabolic idiosyncrasies. Nat. Microbiol. 2018, 3, 514–522. [Google Scholar] [CrossRef]
- Alexander, C.; Swanson, K.S.; Fahey Jr, G.C.; Garleb, K.A. Perspective: Physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation. Adv. Nutr. 2019, 10, 576–589. [Google Scholar] [CrossRef]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
- Long, S.; He, T.; Kim, S.W.; Shang, Q.; Kiros, T.; Mahfuz, S.U.; Wang, C.; Piao, X. Live Yeast or Live Yeast Combined with Zinc Oxide Enhanced Growth Performance, Antioxidative Capacity, Immunoglobulins and Gut Health in Nursery Pigs. Animals 2021, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Celi, P.; Verlhac, V.; Calvo, E.P.; Schmeisser, J.; Kluenter, A.M. Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim. Feed. Sci. Technol. 2019, 250, 9–31. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.F. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- O’Neill, I.; Schofield, Z.; Hall, L.J. Exploring the role of the microbiota member Bifidobacterium in modulating immune-linked diseases. Emerg. Top. Life Sci. 2017, 1, 333–349. [Google Scholar]
- Walker, A.W.; Lawley, T.D. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 2013, 69, 75–86. [Google Scholar] [CrossRef]
- Marsilio, S.; Pilla, R.; Sarawichitr, B.; Chow, B.; Hill, S.L.; Ackermann, M.R.; Suchodolski, J.S. Characterization of the fecal microbiome in cats with inflammatory bowel disease or alimentary small cell lymphoma. Sci. Rep. 2019, 9, 19208. [Google Scholar] [CrossRef] [PubMed]
- Isaiah, A.; Parambeth, J.C.; Steiner, J.M.; Lidbury, J.A.; Suchodolski, J.S. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe 2017, 45, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.E.; Nunnally, A.E.; Jimenez, V., Jr.; Cope, E.K.; Sahl, J.W.; Sheridan, K.; Hornstra, H.M.; Vinocur, J.; Settles, E.W.; Headley, K.C.; et al. Domestic canines do not display evidence of gut microbial dysbiosis in the presence of Clostridioides (Clostridium) difficile, despite cellular susceptibility to its toxins. Anaerobe 2019, 58, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Félix, A.P.; Souza, C.M.M.; de Oliveira, S.G. Biomarkers of gastrointestinal functionality in dogs: A systematic review and meta-analysis. Anim. Feed Sci. Technol. 2022, 283, 115183. [Google Scholar] [CrossRef]
- Suchodolski, J.S. Analysis of the gut microbiome in dogs and cats. Vet. Clin. Pathol. 2022, 50, 6–17. [Google Scholar] [CrossRef]
- Auclair, E. Yeast as an example of the mode of action of probiotics in monogastric and ruminant species. Feed Manuf. Mediterr. Reg. Improv. Saf. Feed Food 2001, 54, 45–53. [Google Scholar]
- Rai, V.; Yadav, B.; Lakhani, G.P. Applications of probiotic and prebiotic in animals production: A review. Environ. Ecol. 2013, 31, 873–876. [Google Scholar]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Healy, H.P.; Dawson, K.A.; Merchen, N.R.; Fahey, G.C., Jr. Effects of supplemental fructooligosaccharides plus mannanoligosaccharides on immune function and ileal and fecal microbial populations in adult dogs. Arch. Anim. Nutr. 2002, 56, 309–318. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Duysburgh, C.; Rakebrandt, M.; Marzorati, M. Dried yeast cell walls high in beta-glucan and mannan-oligosaccharides positively affect microbial composition and activity in the canine gastrointestinal tract in vitro. J. Anim. Sci. 2020, 98, skaa173. [Google Scholar] [CrossRef]
- Liu, M.; McClure, M.J.; Zhu, H.; Xie, G.; Lei, B. The Two-Component Regulatory System VicRK is Important to Virulence of Streptococcus equi Subspecies equi. Open Microbiol. J. 2008, 2, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ghattargi, V.C.; Gaikwad, M.A.; Meti, B.S.; Nimonkar, Y.S.; Dixit, K.; Prakash, O.; Shouche, Y.S.; Pawar, S.P.; Dhotre, D.P. Comparative genome analysis reveals key genetic factors associated with probiotic property in Enterococcus faecium strains. BMC Genom. 2018, 19, 652. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Treu, L.; Zhu, X.; Tian, H.; Basile, A.; Fotidis, I.A.; Campanaro, S.; Angelidaki, I. Insights into ammonia adaptation and methanogenic precursor oxidation by genome-centric analysis. Environ. Sci. Technol. 2020, 54, 12568–12582. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, H.; Zheng, L.; Li, J.; Hong, Y.; Liang, P.; Kwok, L.Y.; Zuo, Y.; Zhang, W.; Zhang, H. iProbiotics: A machine learning platform for rapid identification of probiotic properties from whole-genome primary sequences. Brief. Bioinform. 2022, 23, bbab477. [Google Scholar] [CrossRef]

| Item | LPF | HPF |
|---|---|---|
| Ash | 10.44 | 5.81 |
| Crude Protein | 20.42 | 27.52 |
| Ether Extract in Acid Hydrolysis | 10.25 | 8.02 |
| Total Dietary Fiber | 6.10 | 27.20 |
| Insoluble Fiber | 4.89 | 26.10 |
| Soluble Fiber | 1.21 | 1.80 |
| Calcium | 1.68 | 0.76 |
| Phosphorus | 1.42 | 0.63 |
| Metabolizable Energy (kcal/kg) * | 3.600 | 2.912 |
| Item | D | Supplementation (S) | SEM | p-Values | |||
|---|---|---|---|---|---|---|---|
| Control | Probiotic | D | S | D × S | |||
| Intake (g DM basis/dog/day) | |||||||
| DM | LPF | 181.6 | 181.7 | 3.66 | <0.001 | 0.302 | 0.291 |
| HPF | 244.0 | 233.5 | |||||
| CP | LPF | 37.1 | 37.1 | 2.67 | <0.001 | 0.110 | 0.110 |
| HPF | 68.0 | 64.2 | |||||
| EE | LPF | 18.6 | 18.6 | 0.24 | 0.122 | 0.245 | 0.235 |
| HPF | 19.8 | 18.8 | |||||
| IF | LPF | 8.9 | 8.9 | 0.97 | <0.001 | 0.092 | 0.092 |
| HPF | 63.7 | 60.9 | |||||
| SF | LPF | 2.2 | 2.2 | 0.47 | <0.001 | 0.078 | 0.073 |
| HPF | 4.4 | 4.2 | |||||
| ME | LPF | 708.4 | 713.4 | 42.37 | <0.001 | 0.296 | 0.201 |
| HPF | 858.2 | 811.4 | |||||
| ATTD | |||||||
| DM | LPF | 82.0 | 82.3 | 0.88 | <0.001 | 0.758 | 0.984 |
| HPF | 73.6 | 73.9 | |||||
| OM | LPF | 87.3 | 87.4 | 1.19 | <0.001 | 0.818 | 0.885 |
| HPF | 74.5 | 74.8 | |||||
| CP | LPF | 83.2 | 83.7 | 0.55 | <0.001 | 0.899 | 0.682 |
| HPF | 87.5 | 87.3 | |||||
| EE | LPF | 89.9 | 90.2 | 0.39 | <0.001 | 0.657 | 0.947 |
| HPF | 86.7 | 87.0 | |||||
| ME | LPF | 3899.5 | 3929.2 | 28.01 | <0.001 | 0.626 | 0.644 |
| HPF | 3472.1 | 3472.9 | |||||
| Fecal characteristics | |||||||
| Wet feces | LPF | 89.6 | 85.6 | 8.89 | <0.001 | 0.298 | 0.646 |
| HPF | 184.8 | 174.7 | |||||
| DM (%) | LPF | 36.9 | 37.4 | 0.56 | 0.046 | 0.855 | 0.828 |
| HPF | 34.9 | 34.9 | |||||
| Item | S | Day | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 21 | 23 | 35 | 49 | Day | S | Day × S | |||
| Spermidine | C | 148.4 a | 94.9 b | 119.8 b | 99.6 b | 104.1 b | 8.2 | <0.001 | 0.041 | 0.183 |
| P | 135.8 a | 94.8 b | 103.4 b | 91.2 b | 76.1 b | 8.4 | ||||
| Spermine | C | 185.0 a | 36.8 b | 49.1 b | 51.5 b | 35.5 b | 24.1 | <0.001 | 0.841 | 0.874 |
| P | 178.7 a | 33.5 b | 44.6 b | 59.3 b | 37.3 b | 23.1 | ||||
| Putrescine | C | 38.0 b | 143.8 a* | 81.5 ab | 82.9 ab | 116.9 a* | 15.1 | <0.001 | 0.222 | 0.022 |
| P | 52.8 a | 44.3 a* | 63.0 a | 100.8 a | 79.8 a* | 8.5 | ||||
| Tyramine | C | 254.8 a | 326.2 a | 53.6 b | 49.8 b | 19.4 b | 52.8 | <0.001 | 0.173 | 0.872 |
| P | 205.7 a | 232.0 a | 41.8 b | 52.7 b | 4.5 b | 39.2 | ||||
| Indole | C | 2.3 a | 2.1 a | 2.3 a | 1.5 b | 1.6 b | 0.1 | 0.021 | <0.001 | 0.081 |
| P | 2.0 a | 1.9 a | 0.3 a | 0.6 b | 0.6 b | 0.3 | ||||
| Phenols | C | 0.89 | 1.05 | 1.89 | 1.83 | 1.65 | 0.3 | 0.476 | 0.040 | 0.069 |
| P | 1.75 | 0.61 | 0.94 | 0.98 | 0.52 | 0.2 | ||||
| p-cresol | C | 0.85 a | 1.27 a | 0.77 b | 0.62 b | 1.61 a | 0.2 | 0.015 | <0.001 | 0.140 |
| P | 1.40 a | 0.93 a | 0.46 b | 0.54 b | 0.36 a | 0.2 | ||||
| Item | S | Day | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 21 | 23 | 35 | 49 | Day | S | Day × S | |||
| SCFA | ||||||||||
| Acetate | C | 156.8 b | 206.8 a | 131.9 b | 138.7 b | 133.5 b | 11.8 | <0.001 | 0.824 | 0.367 |
| P | 140.3 b | 200.7 a | 155.4 b | 155.8 b | 123.7 b | 10.8 | ||||
| Propionate | C | 50.3 b | 103.4 a | 58.5 b | 49.1 b | 46.7 b | 9.0 | <0.001 | 0.354 | 0.089 |
| P | 46.6 b | 85.8 a | 61.7 b | 58.6 b | 39.6 b | 6.3 | ||||
| Butyrate | C | 11.1 a | 11.3 a | 7.4 b | 5.9 b | 5.6 b | 1.0 | <0.001 | 0.040 | 0.149 |
| P | 13.4 a | 12.3 a | 7.4 b | 8.6 b | 6.0 b | 2.0 | ||||
| Valerate | C | 3.4 a | 2.6 ab | 2.0 b | 2.0 b | 1.9 b | 0.2 | <0.001 | 0.105 | 0.459 |
| P | 3.2 a | 2.9 ab | 2.2 b | 2.3 b | 1.9 b | 0.2 | ||||
| Total SCFA | C | 221.6 b | 324.1 a | 199.8 b | 195.7 b | 187.7 b | 21.3 | <0.001 | 0.990 | 0.329 |
| P | 204.5 b | 301.7 a | 226.7 b | 225.3 b | 171.2 b | 18.1 | ||||
| BCFA | ||||||||||
| Isovalerate | C | 1.0 ab | 1.2 a | 1.1 a | 0.9 ab | 0.9 b | 0.01 | <0.001 | 0.850 | 0.880 |
| P | 1.0 ab | 1.3 a | 1.1 a | 1.0 ab | 0.8 b | 0.01 | ||||
| Isobutyrate | C | 2.2 a | 2.0 a | 1.4 b | 1.3 b | 1.5 b | 0.1 | <0.001 | 0.048 | 0.647 |
| P | 2.3 a | 2.2 a | 1.7 b | 1.6 b | 1.5 b | 0.1 | ||||
| Total BCFA | C | 3.2 a | 3.3 a | 2.5 b | 2.3 b | 2.4 b | 0.2 | <0.001 | 0.028 | 0.338 |
| P | 3.2 a | 3.5 a | 2.8 b | 2.7 b | 2.3 b | 0.2 | ||||
| Item | S | Day | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 21 | 23 | 35 | 49 | Day | S | Day × S | |||
| Faecalibacterium | C | 4.5 | 4.2 | 4.5 | 4.5 | 4.5 | 0.47 | 0.544 | 0.929 | 0.971 |
| P | 4.3 | 4.3 | 4.7 | 4.6 | 4.5 | 0.06 | ||||
| Fusobacterium | C | 6.6 b | 6.5 b | 6.7 b | 6.7 b | 7.3 a | 0.11 | <0.001 | 0.238 | 0.757 |
| P | 6.6 b | 6.6 b | 6.6 b | 6.9 b | 7.5 a | 0.16 | ||||
| Blautia | C | 10.4 a | 9.8 b | 10.1 ab | 10.0 ab | 10.2 a | 0.08 | <0.001 | 0.163 | 0.436 |
| P | 10.3 a | 10.1 b | 10.1 ab | 10.2 ab | 10.3 a | 0.04 | ||||
| Turicibacter | C | 8.7 a | 8.6 a | 8.2 b | 8.6 a | 8.8 a | 0.08 | 0.017 | 0.028 | 0.952 |
| P | 9.1 a | 9.0 a | 8.4 b | 8.8 a | 8.9 a | 0.09 | ||||
| C. hiranonis | C | 6.1 a | 5.9 a | 6.0 a | 5.8 ab | 5.7 b | 0.06 | 0.008 | 0.311 | 0.711 |
| P | 6.1 a | 6.1 a | 5.9 a | 5.9 ab | 5.8 b | 0.06 | ||||
| Streptococcus | C | 7.8 a | 8.6 a | 8.1 a | 4.9 b | 3.7 b | 0.82 | <0.001 | 0.098 | 0.276 |
| P | 7.8 a | 8.6 a | 7.8 a | 3.9 b | 3.5 b | 0.91 | ||||
| E. coli | C | 4.3 a | 4.2 ab | 4.7 a | 3.4 b | 4.8 a | 0.20 | 0.030 | 0.043 | 0.256 |
| P | 4.2 a | 3.7 ab | 3.7 a | 3.7 b | 3.7 a | 0.11 | ||||
| Total bacteria | C | 10.9 a | 11.0 a | 10.9 a* | 10.5 b | 10.7 ab | 0.08 | <0.001 | 0.520 | 0.030 |
| P | 11.0 a | 10.9 a | 10.6 a* | 10.7 a | 10.9 a | 0.05 | ||||
| Item | S | Day (D) | SEM | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 21 | 23 | 35 | 49 | D | S | D × S | |||
| Chao1 | C | 408.9 a | 332.7 b | 422.8 a | 389.2 ab | 407.2 a | 9.36 | <0.001 | 0.521 | 0.208 |
| P | 415.8 a | 356.2 b | 462.8 a | 396.6 ab | 384.9 a | 11.00 | ||||
| OTUs | C | 346.4 a | 255.1 b | 348.9 a | 344.1 a | 359.0 a | 10.77 | <0.001 | 0.406 | 0.131 |
| P | 356.4 a | 295.6 b | 393.8 a | 335.3 a | 328.3 a | 10.06 | ||||
| Shannon | C | 3.2 ab | 2.7 b | 3.3 a | 3.3 a | 3.8 a | 0.11 | 0.030 | 0.186 | 0.691 |
| P | 3.3 ab | 3.0 b | 3.6 a | 3.6 a | 3.7 a | 0.07 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, T.S.; Souza, C.M.M.; Legendre, H.; Richard, N.; Pilla, R.; Suchodolski, J.S.; de Oliveira, S.G.; Lesaux, A.A.; Félix, A.P. Effect of Yeast Saccharomyces cerevisiae as a Probiotic on Diet Digestibility, Fermentative Metabolites, and Composition and Functional Potential of the Fecal Microbiota of Dogs Submitted to an Abrupt Dietary Change. Microorganisms 2023, 11, 506. https://doi.org/10.3390/microorganisms11020506
Bastos TS, Souza CMM, Legendre H, Richard N, Pilla R, Suchodolski JS, de Oliveira SG, Lesaux AA, Félix AP. Effect of Yeast Saccharomyces cerevisiae as a Probiotic on Diet Digestibility, Fermentative Metabolites, and Composition and Functional Potential of the Fecal Microbiota of Dogs Submitted to an Abrupt Dietary Change. Microorganisms. 2023; 11(2):506. https://doi.org/10.3390/microorganisms11020506
Chicago/Turabian StyleBastos, Taís Silvino, Camilla Mariane Menezes Souza, Héloïse Legendre, Nadège Richard, Rachel Pilla, Jan S. Suchodolski, Simone Gisele de Oliveira, Achraf Adib Lesaux, and Ananda Portella Félix. 2023. "Effect of Yeast Saccharomyces cerevisiae as a Probiotic on Diet Digestibility, Fermentative Metabolites, and Composition and Functional Potential of the Fecal Microbiota of Dogs Submitted to an Abrupt Dietary Change" Microorganisms 11, no. 2: 506. https://doi.org/10.3390/microorganisms11020506
APA StyleBastos, T. S., Souza, C. M. M., Legendre, H., Richard, N., Pilla, R., Suchodolski, J. S., de Oliveira, S. G., Lesaux, A. A., & Félix, A. P. (2023). Effect of Yeast Saccharomyces cerevisiae as a Probiotic on Diet Digestibility, Fermentative Metabolites, and Composition and Functional Potential of the Fecal Microbiota of Dogs Submitted to an Abrupt Dietary Change. Microorganisms, 11(2), 506. https://doi.org/10.3390/microorganisms11020506










