Oleaginous Yeast Extracts and Their Possible Effects on Human Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of Microbial Cells and Their Processing
2.2. Preparation of Microbial Extracts
2.2.1. Rehydrated Biomass
2.2.2. Folch Extract Preparation
2.3. Characterization of Yeast Extracts
2.3.1. Antioxidant Activity
2.3.2. Antimicrobial Activity
2.3.3. Methods of Analysis of Yeast Metabolites
HPLC Analysis of Carotenoids, Sterols, and Ubiquinone
Analysis of Lipids and Fatty Acids
Analysis of Other Metabolites
2.4. Cytotoxicity Tests/Interaction with Human Cells
2.4.1. Cell Cultures
2.4.2. Tetrazolium Blue Assay-MTT Test
- DAY: seed 1 × 104 cell/100µL/1 well in a 96 well plate, incubate for 24 h
- DAY: add 100 µL of extract, mixed with medium for a specific concentration
- DAY: add 20 µL of MTT (concentration 2.5 mg/mL PBS)After 3 h add the stop solution, which is 10% SDS (sodium dodecyl sulphate) in PBS (10 g/100 mL PBS), 100µL in each well, and leave it in the dark at room temperature for 20 h.
- DAY: read the plates on Elisa reader at 540 λ = nm
2.4.3. Apoptosis
2.4.4. Statistical Analysis
3. Results
3.1. Growth and Production of Biomass
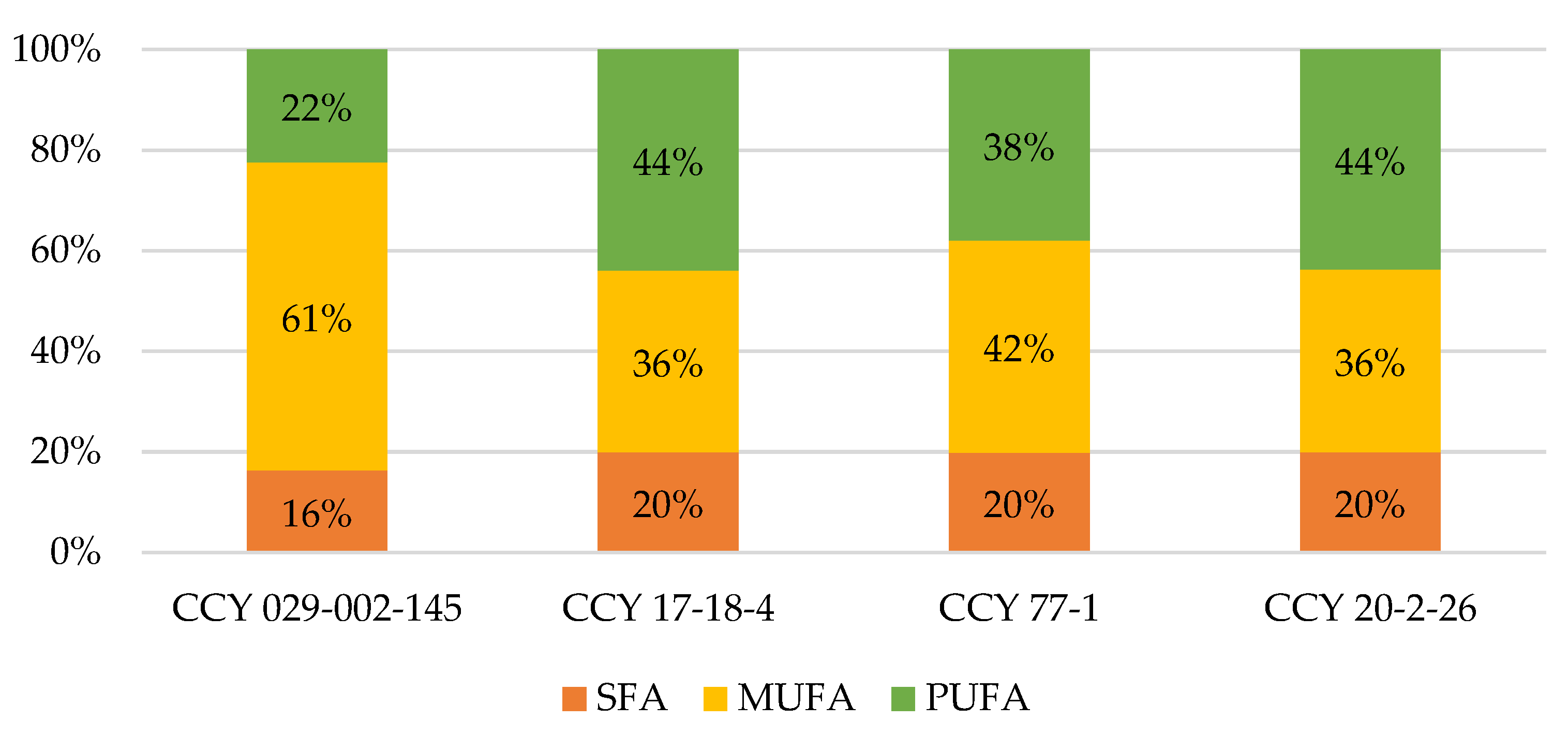
3.2. Bioactive Compounds in Biomass
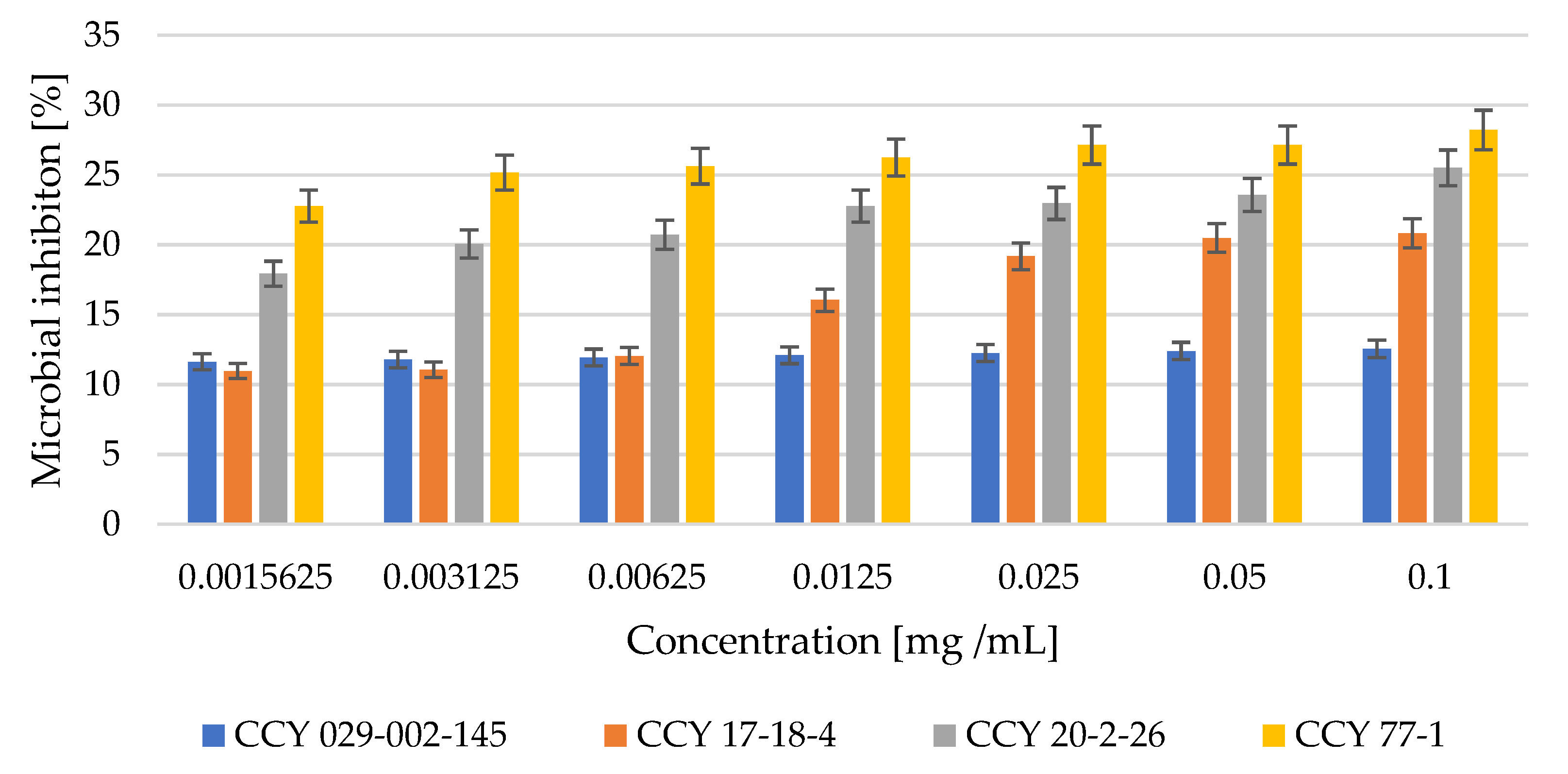
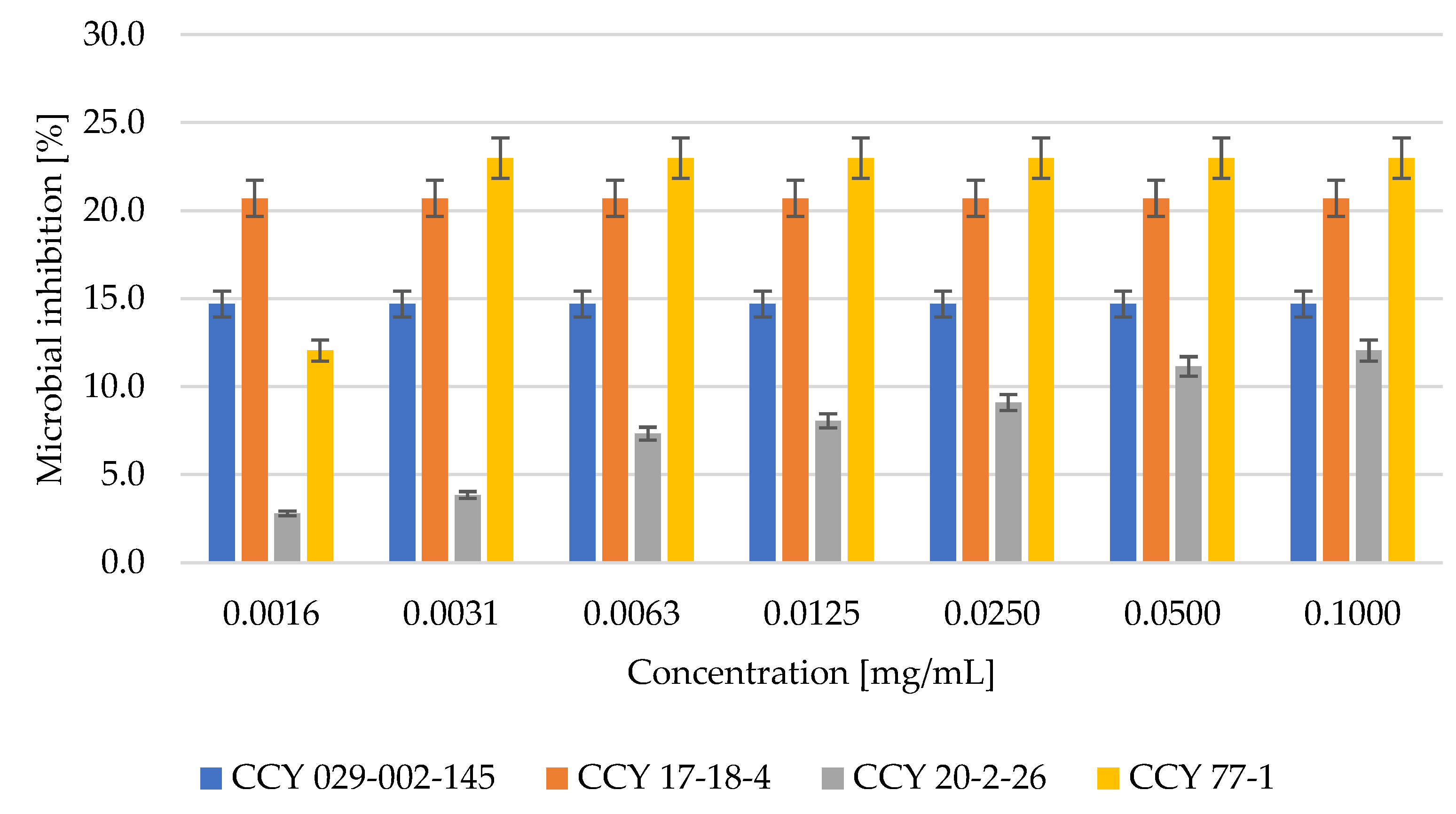
3.3. Antioxidant and Antimicrobial Activity
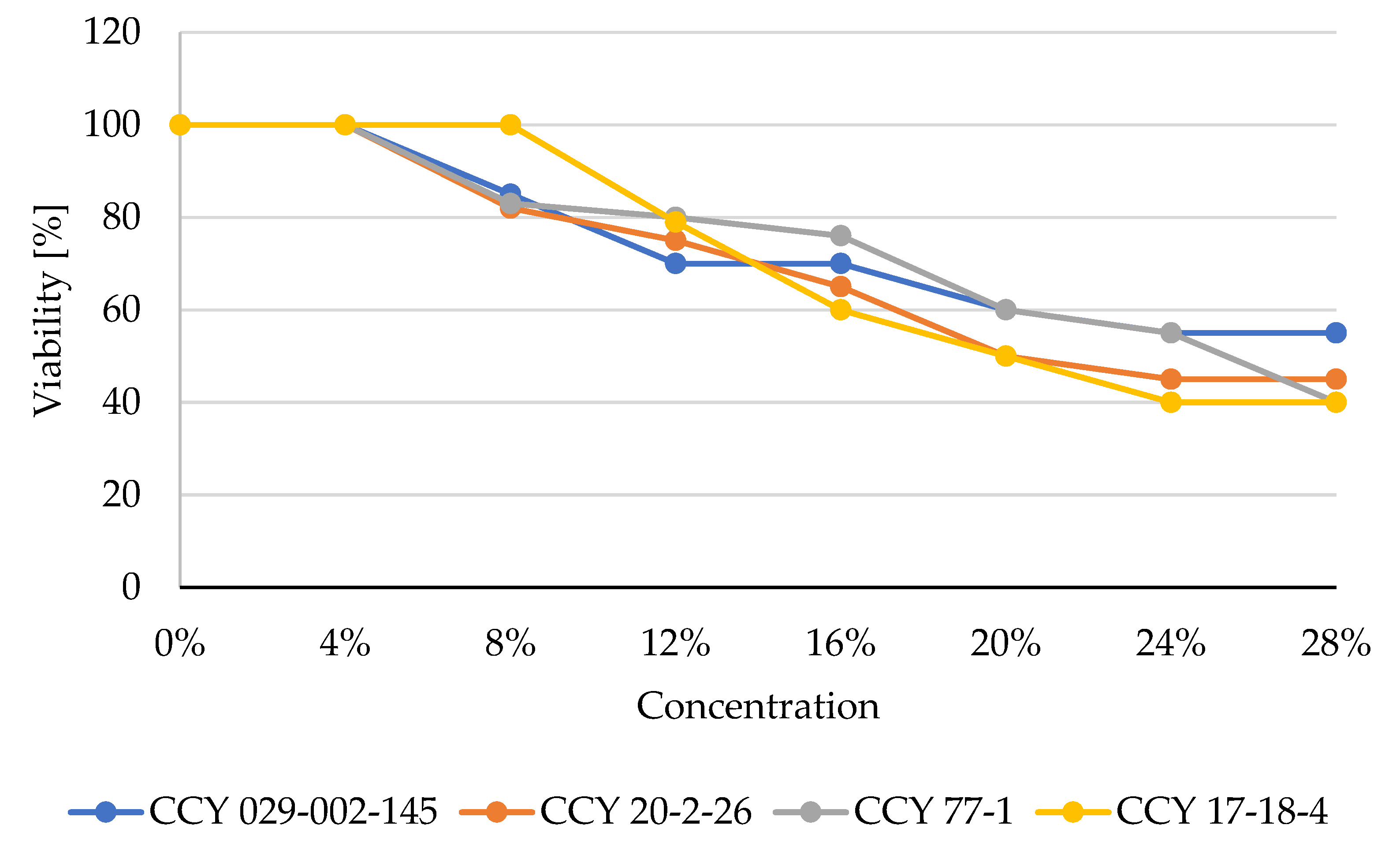
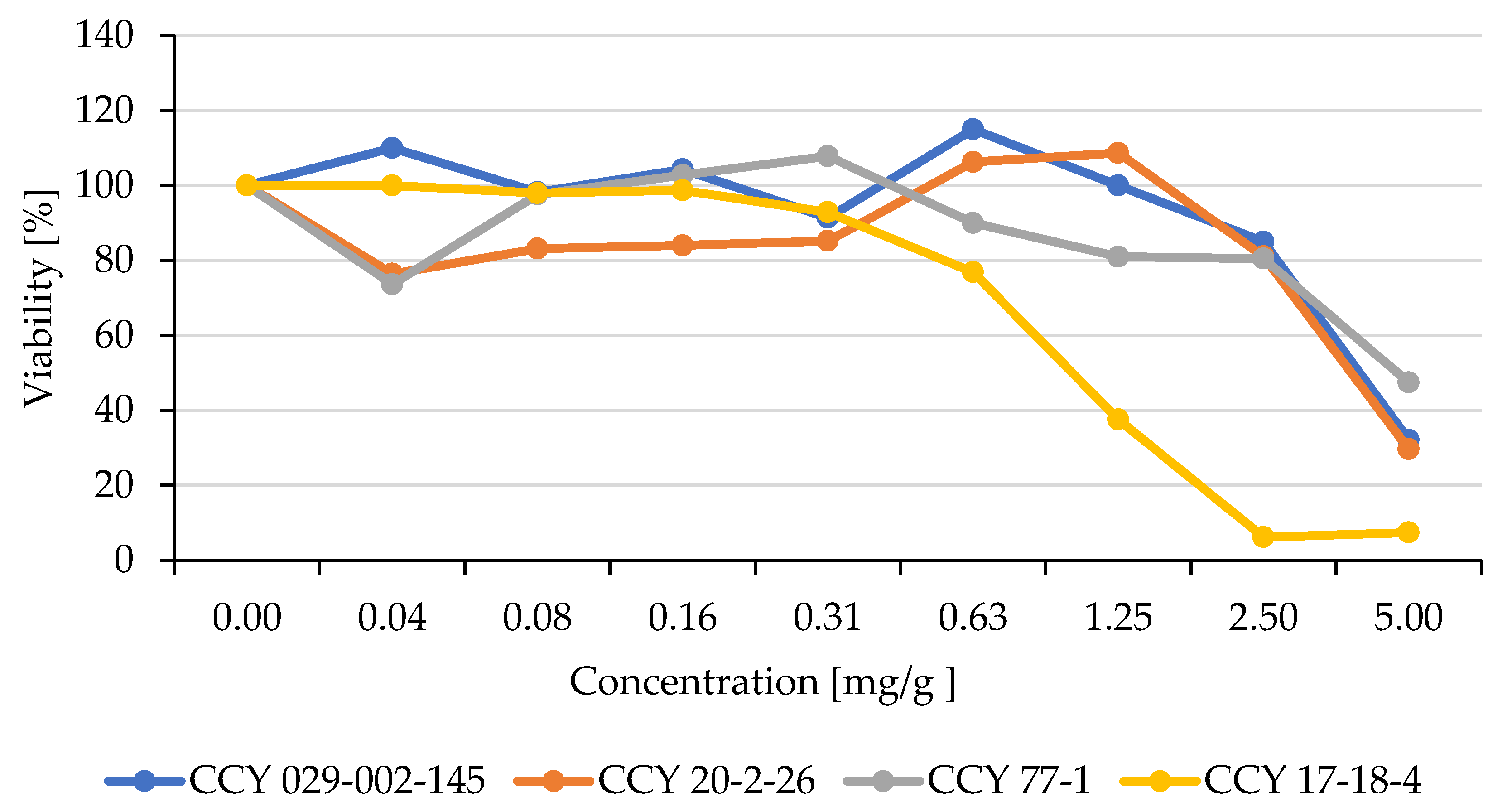
3.4. Influence of Yeast Extracts on the Viability of Human Cell Lines
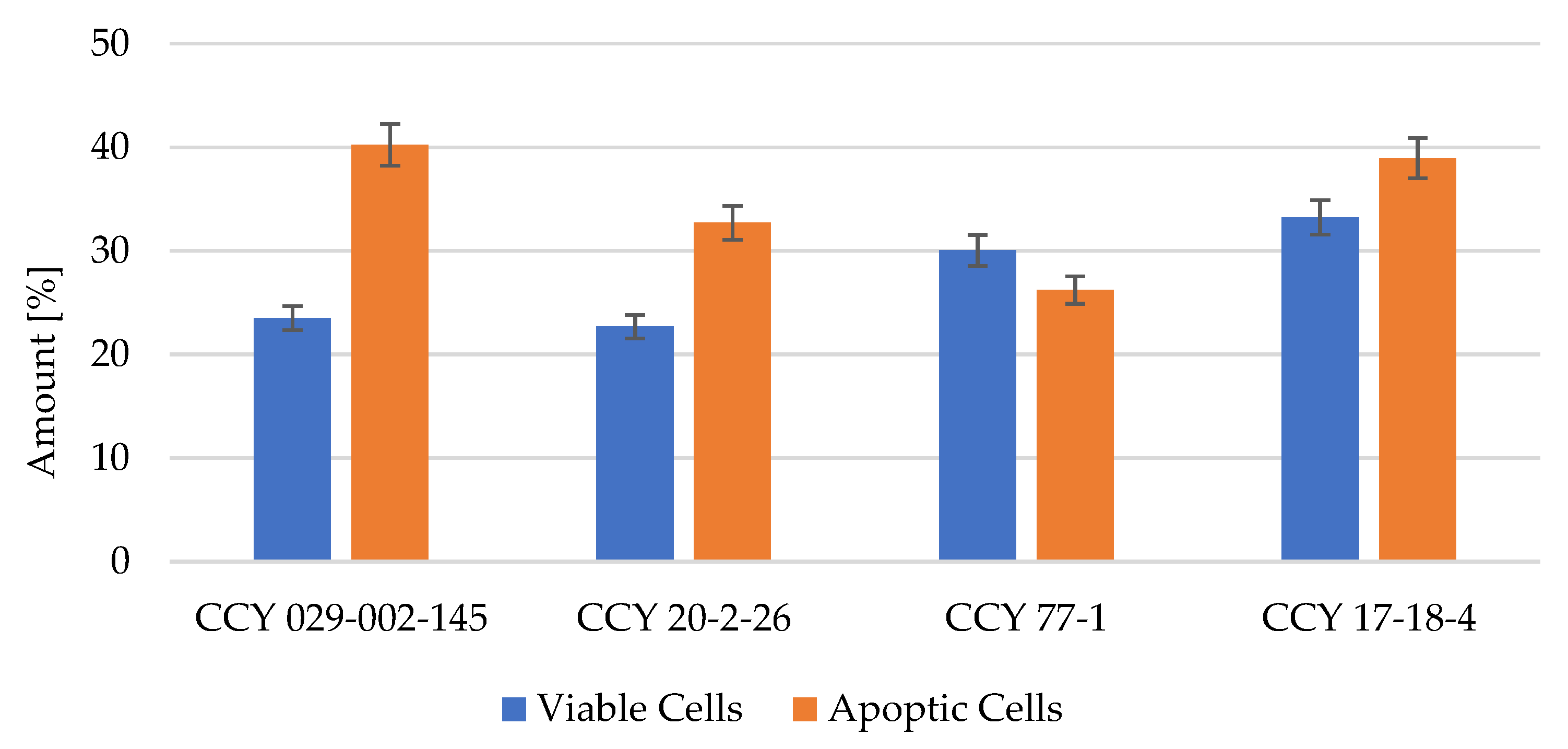
3.5. Apoptosis Testing
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Adedayo, M.R.; Ajiboye, E.A.; Akintudne, J.K.; Odaibo, A. Single cell proteins: As nutritional enhancer. Adv. Appl. Sci. Res. 2011, 2, 396–409. [Google Scholar]
- Reed, G.; Nagodawithana, T.W. Technology of Yeast Usage in Winemaking. Am. J. Enol. Vitic. 1988, 39, 83–90. [Google Scholar] [CrossRef]
- Ferreira, I.; Pinho, O.; Vieira, E.; Tavarela, J. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014, 2014, 476207. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as a valuable source of bioactive compounds. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- Groenewald, M.; Boekhout, T.; Neuveglise, C.; Gaillardin, C.; Van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206. [Google Scholar] [CrossRef]
- Ramos, O.L.; Santos, A.C.; Leao, M.V.; Pereira, J.O.; Silva, S.I.; Fernandes, J.C.; Franco, M.I.; Pintado, M.E.; Malcata, F.X. Antimicrobial activity of edible coatings prepared from whey protein isolate and formulated with various antimicrobial agents. Int. Dairy J. 2012, 25, 132–141. [Google Scholar] [CrossRef]
- Monnet, C.; Bleicher, A.; Neuhaus, K.; Sarthou, A.-S.; Leclercq-Perlat, M.-N.; Irlinger, F. Assessment of the anti-listerial activity of microfloras from the surface of smear-ripened cheeses. Food Microbiol. 2010, 27, 302–310. [Google Scholar] [CrossRef]
- Knutsen, A.K.; Robert, V.; Poot, G.A.; Epping, W.; Figge, M.; Holst-Jensen, A.; Skaar, I.; Smith, M.T. Polyphasic re-examination of Yarrowia lipolytica strains and the description of three novel Candida species: Candida oslonensis sp. nov., Candida alimentaria sp. nov. and Candida hollandica sp. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 2426–2435. [Google Scholar] [CrossRef]
- Leme, F.C.O.; Negreiros, M.M.D.B.; Koga, F.A.; Bosco, S.D.M.G.; Bagagli, E.; Haddad, V., Jr. Evaluation of pathogenic fungi occurrence in traumatogenic structures of freshwater fish. Rev. Soc. Brasil. Med. Trop. 2011, 44, 182–185. [Google Scholar] [CrossRef]
- Stratford, M. Food and beverage spoilage yeasts. In The Yeast Handbook: Yeasts in Food and Beverages; Querol, A., Fleet, G.H., Eds.; Springer: Berlin, Germany, 2006; pp. 335–379. [Google Scholar]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Micro. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Wirth, F.; Goldani, L.Z. Epidemiology of Rhodotorula: An Emerging Pathogen. Interdisciplinar. Persp. Infect. Dis. 2012, 2012, 465717. [Google Scholar] [CrossRef]
- Senses-Ergul, S.; Agoston, R.; Belak, A.; Deak, T. Characterization of some yeasts isolated from foods by traditional and molecular tests. Int. J. Food Micro. 2006, 108, 120–124. [Google Scholar] [CrossRef]
- Zoz, L.; Carvalho, J.C.; Soccol, V.T.; Casagrande, T.C.; Cardoso, L. Torularhodin and Torulene: Bioproduction, Properties and Prospective Applications in Food and Cosmetics—A Review. Brazil. Arch. Biol. Technol. 2015, 58, 278–288. [Google Scholar] [CrossRef]
- Byrtusova, D.; Szotkowski, M.; Kurowska, K.; Shapaval, V.; Marova, I. Rhodotorula kratochvilovae CCY 20-2-26—The Source of Multifunctional Metabolites. Microorganisms 2021, 9, 1280. [Google Scholar] [CrossRef]
- Szotkowski, M.; Byrtusova, D.; Haronikova, A.; Vysoka, M.; Rapta, M.; Shapaval, V.; Marova, I. Study of Metabolic Adaptation of Red Yeasts to Waste Animal Fat Substrate. Microorganisms 2019, 7, 578. [Google Scholar] [CrossRef]
- Szotkowski, M.; Holub, J.; Simansky, S.A.; Hubacova, K.; Hladka, D.; Nemcova, A.; Marova, I. Production of Enriched Sporidiobolus sp. Yeast Biomass Cultivated on Mixed Coffee Hydrolyzate and Fat/Oil Waste Materials. Microorganisms 2021, 9, 1848. [Google Scholar] [CrossRef]
- Byrtusova, D.; Shapaval, V.; Holub, J.; Simansky, S.; Rapta, M.; Szotkowski, M.; Kohler, A.; Marova, I. Revealing the Potential of Lipid and β-Glucans Coproduction in Basidiomycetes Yeast. Microorganisms 2020, 8, 1034. [Google Scholar] [CrossRef]
- Li, X.; Turánek, J.; Knötigová, P.; Kudláčková, H.; Masek, J.; Parkin, S.; Rankin, S.; Knutson, B.L.; Lehmler, H.-J. Hydrophobic tail length, degree of fluorination and headgroup stereochemistry are determinants of the biocompatibility of (fluorinated) carbohydrate surfactants. Colloids Surf. B Biointerfaces 2009, 73, 65–74. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; He, Q.; Wu, Y.; Lu, Z.; Sun, J.; Liu, Z.; Shao, Y.; Wang, A. Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci. Rep. 2017, 7, 11277. [Google Scholar] [CrossRef]
- Moona, H.S.; Batirel, S.; Mantzoros, C.S. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism 2014, 63, 1447–14454. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Gad, M.Z. Omega-9 fatty acids: Potential roles in infammation and cancer management. J. Gen. Eng. Biotec. 2022, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Westheim, J.F.; Stoffels, L.M.; Dubois, L.J.; van Bergenhenegouwen, J.; van Helvoort, A.; Langen, R.C.J.; Shiri-Sverdlov, R.; Theys, J. Fatty Acids as a Tool to Boost Cancer Immunotherapy Efficacy. Front. Nutr. 2022, 9, 868436. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Vital Biomass | Imortalized Biomass |
|---|---|---|
| Proteins in dry biomass (mg/g) | 250–338 | 247–345 |
| Glucans in dry biomass (mg/g) | 110–245 | 118–220 |
| Lipids in dry biomass (mg/g) | 124–598 | 143–589 |
| Beta-carotene (mg/g d.w.) | 0.4–2.10 | 0.90–4.25 |
| Total carotenoids (mg/g d.w.) | 0.61–3.90 | 1.07–5.42 |
| Ubiquinone (mg/g d.w.) | 0.59–3.10 | 0.80–2.92 |
| Ergosterol (mg/g d.w.) | 0.22–3.25 | 0.42–3.87 |
| Metschnikowia Pulcherrima | Cystofilobasidium Infirmominiatum | Phaffia Rhodozyma | Rhodotorula Kratochvilovae | |
|---|---|---|---|---|
| Ergosterol | 0.210 ± 0.010 | 0.940 ± 0.012 | 1.121 ± 0.013 | 2.955 ± 0.012 |
| Ubiquinon | 0.469 ± 0.020 | 1.350 ± 0.021 | 1.548 ± 0.009 | 2.335 ± 0.013 |
| Torularhodin | - | - | 0.856 ± 0.009 | 1.063 ± 0.009 |
| Lycopen | - | - | - | 0.051 ± 0.001 |
| Torulen | - | - | 0.058 ± 0.002 | 0.103 ± 0.002 |
| β- carotene | - | 0.006 ± 0.001 | 0.024 ± 0.001 | 0.066 ± 0.001 |
| Total carotenoids | - | 0.007 ± 0.001 | 1.250 ± 0.004 | 1.374 ± 0.008 |
| Antioxidant Activity [mg of Trolox Equivalent/g] | ||
|---|---|---|
| Rehydrated biomass | Folch extract | |
| CCY 029-002-145 | 4.435 ± 0.05 | 4.107 ± 0.01 |
| CCY 20-2-26 | 5.807 ± 0.08 | 5.002 ± 0.02 |
| CCY 77-1 | 4.215 ± 0.02 | 4.321 ± 0.03 |
| CCY 17-18-4 | 4.536 ± 0.07 | 4.612 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vysoka, M.; Szotkowski, M.; Slaninova, E.; Dzuricka, L.; Strecanska, P.; Blazkova, J.; Marova, I. Oleaginous Yeast Extracts and Their Possible Effects on Human Health. Microorganisms 2023, 11, 492. https://doi.org/10.3390/microorganisms11020492
Vysoka M, Szotkowski M, Slaninova E, Dzuricka L, Strecanska P, Blazkova J, Marova I. Oleaginous Yeast Extracts and Their Possible Effects on Human Health. Microorganisms. 2023; 11(2):492. https://doi.org/10.3390/microorganisms11020492
Chicago/Turabian StyleVysoka, Marie, Martin Szotkowski, Eva Slaninova, Lucia Dzuricka, Paulina Strecanska, Jana Blazkova, and Ivana Marova. 2023. "Oleaginous Yeast Extracts and Their Possible Effects on Human Health" Microorganisms 11, no. 2: 492. https://doi.org/10.3390/microorganisms11020492
APA StyleVysoka, M., Szotkowski, M., Slaninova, E., Dzuricka, L., Strecanska, P., Blazkova, J., & Marova, I. (2023). Oleaginous Yeast Extracts and Their Possible Effects on Human Health. Microorganisms, 11(2), 492. https://doi.org/10.3390/microorganisms11020492






