Abstract
Cyanobacteria are photosynthetic microorganisms capable of using solar energy to convert CO2 and H2O into O2 and energy-rich organic compounds, thus enabling sustainable production of a wide range of bio-products. More and more strains of cyanobacteria are identified that show great promise as cell platforms for the generation of bioproducts. However, strain development is still required to optimize their biosynthesis and increase titers for industrial applications. This review describes the most well-known, newest and most promising strains available to the community and gives an overview of current cyanobacterial biotechnology and the latest innovative strategies used for engineering cyanobacteria. We summarize advanced synthetic biology tools for modulating gene expression and their use in metabolic pathway engineering to increase the production of value-added compounds, such as terpenoids, fatty acids and sugars, to provide a go-to source for scientists starting research in cyanobacterial metabolic engineering.
1. Introduction
Cyanobacteria are photosynthetic unicellular microorganisms with powerful biotechnological features. They are Gram-negative prokaryotes that belong to the bacterial domain and are considered one of the oldest and largest groups of bacteria on Earth. The oldest fossil dates back to the Archean era. Cyanobacteria were essential for forming the biosphere, creating oxygenating conditions by releasing O2 into the atmosphere [1]. They are also able to fix nitrogen, occupying a prominent role in the nitrogen cycle [2]. Given their long history, their ability to adapt to environmental changes on Earth is one of their principal characteristics. For instance, they have differentiated specialized nitrogen-fixing cell types, facilitating the dispersion of species [3] and are found in marine, freshwater and terrestrial environments. Cyanobacteria possess a typical prokaryotic cellular organization; however, they lack the cell wall usually found in bacteria. Plant chloroplasts are thought to be derived from endosymbiotic cyanobacteria [4], explaining the similarity of their photosynthetic apparatus embedded in the thylakoid membranes [5].
Despite being a relatively ‘young’ organism from a biotechnological point of view (compared to the well-characterized industrial chassis Escherichia coli and Saccharomyces cerevisiae), cyanobacteria’s capability to use solar energy for generating reducing power and energy along with their prokaryotic cellular organization make them attractive biotechnological agents to produce valuable compounds. Cyanobacteria convert inorganic carbon dioxide (CO2) and H2O into biomass and valuable products and some species can also fix molecular nitrogen. They have a clear advantage compared to the microorganisms, such as E. coli and S. cerevisiae, which are currently the preferred cell platforms in industrial biotechnology, but rely on reduced carbon and nitrogen sources, typically sugars and ammonia, increasing the production costs of the target compounds [6,7]. Their photosynthetic biomass production rate is also higher than that of plants [8,9,10]. Moreover, genetic modifications of cyanobacteria are faster and more efficient than in plants or algae [8,9]. Many molecules of commercial interest are natively produced by cyanobacteria, for instance: terpenoids, chlorophylls, fatty acids, sugar and amino acids [11,12]. Together, these traits make cyanobacteria ideal candidates for sustainable, low-cost biological production of high-value chemicals.
Various strategies have been implemented to optimize cyanobacteria for bioproduction, including flux enhancement through a determined pathway, removal of competitive pathways, or augmenting cell fitness against high product concentrations [13,14,15,16]. Most of the metabolic engineering approaches have led to scarce results in terms of productivity and titer in comparison to those achieved in heterotrophic microbes [10]. The lack of knowledge about cyanobacterial regulatory mechanisms, a gap in available genetic tools and the inconsistency in performance of characterized genomic parts across different cyanobacterial strains can explain this discrepancy. The increased number of cyanobacterial genome sequencing data (3858 cyanobacterial genome assemblies available in GenBank [17]) has greatly facilitated the integration of transcriptomics, proteomics and metabolomics studies and helped raise awareness beyond model organisms and identify new industrial relevant species [18]. The evolution of the synthetic biology paradigm as the systematic reconstitution of new standardized biological parts, modules and devices to produce a particular cellular output has further spurred the availability of well-characterized genetic parts (e.g., promoters, ribosome binding sites (RBS) and coding sequences) and improved methods for cyanobacteria metabolic engineering.
This review will introduce the principal cyanobacterial model organisms and lesser-known species with relevant biotechnological traits and applications. Secondly, we will focus on available genetic engineering and synthetic biology tools. Finally, we present the main achievements in terms of metabolic engineering of cyanobacteria, taking a closer look at the production of terpenoids, fatty acids and carbohydrates. We will also introduce several new engineering approaches still majorly unexplored in cyanobacteria and highlight some challenges and prospects of cyanobacterial cell factory development.
2. Model Organisms and Emerging Species
Due to the diverse ecological niches colonized by cyanobacteria, many studies focus on species with unique physiological features and have identified promising photosynthetic cell chassis candidates with industrially relevant traits. The main requirements for enabling metabolic engineering of an organism are the availability of an annotated genome sequence, amenability to genetic manipulation and rapid and efficient methods for generating mutants. Several cyanobacteria species fulfill these requirements (e.g., Synechocystis sp. PCC 6803, Synechococcus sp. PCC 7002, Synechococcus elongatus sp. PCC 7942 and Anabaena sp. PCC 7120) and have become model organisms in cyanobacteria physiology and metabolic engineering studies.
The freshwater cyanobacterium Synechocystis sp. PCC 6803 (Syn6803) is one of the most widespread cyanobacteria. This phototrophic organism was isolated in 1968 in Oakland, California [19]. Because of biochemical similarities with the chloroplast of green plants, it was used as a model for stress response and adaptation in higher plants [20] and is very well studied and understood. This is reflected by the 9380 scientific articles referring to this cyanobacterial strain [21]. Its genome was also the first completely sequenced cyanobacterial genome [22,23]. The strain possesses great potential for biotechnological applications, primarily because of its versatile carbon metabolism, compared to other photosynthetic organisms, and broad generation of valuable compounds. The transformation efficiency, genomic accessibility, the increasing number of synthetic biology tools available for genome editing and the ability to grow photo-heterotrophically at the expense of glucose make Syn6803 the most popular cyanobacterial model organism. More information about this organism can be found in the review from Yu et al. [24] and Table 1.
Synechococcus elongatus sp. PCC 7942 (Syn7942) (former Anacystis nidulans R2) is an obligate photoautotrophic freshwater cyanobacterium isolated for the first time in the San Francisco Bay area of California in the mid-1980s [25]. It was the first cyanobacterium transformed with exogenous genetic material [26]. Its physiology has been extensively studied, including carbon and nitrogen assimilation and regulation, and its adaptation and fitness under various environmental stresses has been investigated [27]. Syn7942 has been considered the main organism for exploring the prokaryotic circadian clock system [28]. Syn7942 is genetically tractable as it possesses a minimal genome (Table 1) [29] and a noticeable number of synthetic biology tools are available for modulating its protein expression. Thus, Syn7942 has been extensively used as a cell chassis for heterologous production of a plethora of compounds [30,31,32,33,34], as summarized in Table 1 and [25,35,36].
Synechococcus elongatus sp. PCC 7002 (Syn7002) is a unicellular marine cyanobacterium, first isolated on the Magueyes Island of Puerto Rico in 1963 [37]. Syn7002 has a remarkable halophilic and high-light tolerance [38] and is considered one of the fast-growing cyanobacteria (Table 1) [39]. Under high-light conditions, Syn7002 reduces antenna size and redistributes carbon flows to fuel biomass formation while increasing cell volume on average by about 3-fold [39]. Together with established techniques for protein overproduction and a wide range of synthetic biology tools available [40], it is a compelling candidate for large-scale bioreactor cultivation, its only drawback being its obligate requirement for exogenous vitamin B12 (cobalamin); for further details, see Table 1 and [36,41].
Anabaena sp. PCC 7120 (Ana7120), formerly known as Nostoc muscorum, is a filamentous, non-branching, autotrophic, freshwater cyanobacterium [42,43]. It was first isolated in 1971 from the algal collection at Iowa State University [44]. It is characterized by differently colored cells, grows aerobically under various temperature and light intensity regimes and can fix atmospheric nitrogen through its nitrogenase complex. Under nitrogen deficiency, Ana7120 differentiates into non-photosynthetic cells called heterocysts, specialized in nitrogen fixation [45]. Ana7120 is therefore considered the model organism for studying heterocyst differentiation and multicellularity. Ana7120 also attracted attention for its native production of industrial-relevant secondary metabolites such as polyketides and the sesquiterpenoid geosmin [46]. Its genome has been completely sequenced and it can easily be manipulated with an array of genetic tools for gene expression control, genome editing and reporter fusions [47,48]; further details are given in Table 1 and [49,50].
New industrially relevant organisms—besides those four model organisms, some fast-growing cyanobacteria strains were recently discovered that overcome the disadvantages of Syn6803 and Syn7942 in terms of low productivity, slow growth, inability to grow at elevated temperatures and lack of stress tolerance.
Synechococcus elongatus UTEX 2973 (Syn2973) is closely related to Syn7942 as their genomes differ by only 55 single nucleotides [51]. This recently discovered strain is characterized by thermotolerance and can grow twice as fast as Syn7942 and Syn6803 (Table 1). However, in contrast to these model strains, it is not naturally transformable [51]. Despite its recent discovery, several proteomic, transcriptomic and metabolomic studies have already been conducted with this strain [52,53,54]. Under nitrogen-depleted conditions, Syn2973 can accumulate glycogen to over 50% of dry cell weight [55] and has been considered a valuable feedstock for yeast-based biofuel production [56]. Some of the most recent synthetic biology techniques were already successfully applied in Syn2973, greatly facilitating rational engineering and making it one of the most promising candidates to be developed into an efficient photosynthetic chassis [57].
The cyanobacterium Synechococcus elongatus sp. PCC 11801 (Syn11801), isolated in 2018 from water samples collected from Powai Lake, Mumbai, India [58], shares 83% homology with Syn7942. It displays ultra-fast growth at elevated temperatures and light intensity (Table 1). Further promising biotechnological attributes are its amenability for natural transformation and cultivation with seawater. Syn11801 was efficiently engineered to produce succinic acid, confirming its biotechnological potential [59]. Along Syn11801, other less characterized fast-growing strains displayed interesting biotechnological features, such as Synechococcus elongatus sp. PCC 11802 (Syn11802). This strain grows even faster than Syn11801 under unlimited growth conditions (Table 1). It has outstanding carbon fixation capabilities, probably because the Calvin cycle key enzymes are not repressed under elevated CO2 concentrations [60]. Excellent CO2 fixation performance is a key biotechnological feature for enhancing chemical production. Another fast-growing cyanobacterium is Synechococcus sp. PCC 11901 (Syn11901) (Table 1). This euryhaline organism can tolerate a wide range of salinities, which is economically advantageous because it can potentially avoid using freshwater and can decrease contamination risks. Moreover, engineered strains of Syn11901 showed the highest capacity of free fatty acid production reported in cyanobacteria [61].
Some less-known genera of cyanobacteria have attracted attention for their ability to produce structurally complex compounds. These strains are being screened for new metabolites with unusual bioactivities, in particular, cyanobacteria belonging to the genera Nostoc, Lyngbya and Microcystis, which are studied for the production of antivirals (e.g., Nostoflan), the UV-screening compound Scytonemin, anticancer compounds (e.g., Apratoxin A and Curacin A) and enzyme inhibitors (e.g., Micropeptins) [62].
Cyanobacteria, such as the filamentous Spirulina, have also been used for single-cell protein (SCP) production. For instance, the dried biomass of the oxygenic photosynthetic bacterium Arthrospira platensis is used as a protein and vitamin source in human diet supplements [63]. It was first discovered in 1844 near Montevideo, Uruguay [64] and is the only cyanobacterium listed as a generally recognized as safe (GRAS) organism by the U.S. Food and Drug Administration [65]. This cyanobacterium has also been investigated to elucidate the origin and biochemical and biophysics mechanisms of oxygenic photosynthesis. The first reported genomic modification of A. plantensis C1 has been performed by transposon mutagenesis (Table 1) [66]. Recently, Jester et al. engineered A. plantensis, by natural transformation, for stable, high-level expression of bioactive antibodies against campylobacter infections (Table 1) [67]. Furthermore, A. plantensis was engineered for the production of the pain reliever Acetaminophen (Table 1) [68]. These new rational engineering strategies may pave the way for future orally-delivered A. plantensis strains as human therapeutics.
Fremyella diplosiphon is a freshwater cyanobacterium isolated from a Connecticut lake in 1952, which possesses one of the largest genomes amongst bacteria (Table 1). It has attracted attention because of its exceptional chromatic acclimation capabilities. In this process, F. diplosiphon changes its pigment composition to maximally absorb the available photons of light to support photosynthesis [69]. It is equipped with one of the most intricate light-sensing systems composed of 27 different phytochrome superfamily members for light color sensing [70]. It has, therefore, become the model cyanobacterium organism for studying complementary chromatic adaptation.
Prochlorococcus is the smallest (spherical diameter from 0.5 to 0.7 μm) and most abundant photosynthetic organism in the ocean [71]. It was first isolated in 1988 from the bottom of the euphotic zone in the Sargasso Sea [72]. Distributed all over the Earth, it can be grouped into different ecotypes based on the habitats it colonizes [73]. Because of its abundance and adaptability, it has become a model for studying biological diversity across diverse scales, achieved by integrating multi-omics studies and global ecosystem modeling (Table 1) [74]. Moreover, Prochlorococcus possesses some unique features amongst cyanobacteria, such as a photosynthetic apparatus composed of the divinyl derivatives of chlorophyll a and b (Chl-a2 and Chl-b2), a photosystem closer to that present in green plants and algae [73] and the ability to generate the volatile hemiterpenoid isoprene [75]. Although it has attractive biotechnological features, it is recalcitrant to DNA transfer and to growing axenically on solid media [76,77].
Gloeobacter violaceus is a rod-shaped primordial cyanobacterium isolated for the first time from limestone rocks in Kernwald, Switzerland in 1974 [78]. It lacks the thylakoid membrane and instead shuttles protons from the cytoplasm to the periplasm. Its archaic photosystem is solely composed of menaquinone and it does not possess the common cyanobacterial phylloquinone [79]. It is thought to be the first cyanobacterium and is the object of studies on the evolution of cyanobacteria lineage and the development of anoxygenic photosynthesis [80]. The main characteristics are summarized in Table 1.
Thermosynechococcus elongatus is a thermophilic rod-shaped cyanobacterium with an optimal temperature of 55 °C isolated from a hot spring in Beppu, Kyushu, Japan, in 1978 [81]. It is capable of natural transformation [82] and its genome has been fully sequenced (Table 1) [83]. Because of the thermostability of the proteins of its photosynthetic complex, it has become the model cyanobacterium organism for studying the biophysics of photosynthesis and the structure of the photosystem. Several crystal structures of many components of this intricate system have been solved [83,84,85,86,87].

Table 1.
Main features of cyanobacteria strains.
Table 1.
Main features of cyanobacteria strains.
| Organism | Genome Size (Mbp) | Plasmids | Doubling Time (hours) * | Growth Temperature * | CO2 Partial Pressure (%) * | Light Intensity (µmole photons m−2 s−1) * | Transformation Methods | Metabolism | Features | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Syn6803 | 3.5 | 4 | 5.1 | 35 °C | 5 | 790 | NT ****, Conjugation, Electroporation | Mixotrophic, Autotrophic | Model organism, best studied cyanobacterium, numerous synthetic biology tools available | [22,24,88] |
| Syn7942 | 2.69 | 1 | 4.1 | 38 °C | 3 | 300 | NT ****, Conjugation, Electroporation | Autotrophic | Model organism, especially suited for circadian clock studies | [26,28,35] |
| Syn7002 | 3.0 | 7 | 2.5 2.5 ** | 30 °C 3 °C ** | 2 1 * | 760 250 ** | NT ****, Conjugation, Electroporation | Mixotrophic, Autotrophic | Model organisms, high cell doubling rate; resistant to salt and high light conditions | [39,89] |
| Ana7120 | 7.2 | 6 | 18–24 | 23–30 °C | 2 | 160 | Conjugation | Mixotrophic, Autotrophic | Model organism, nitrogen-fixing strain | [90,91,92] |
| Syn2973 | 2.69 | 2 | 2.1 | 41 °C | 3 | 500 | Conjugation, Electroporation | Autotrophic | Highest growth rate, numerous synthetic biology tools available | [51] |
| Syn11801 | 2.7 | / | 2.3 | 41 °C | 0.4 | 1000 | NT ****, Conjugation, Electroporation | Autotrophic | Fast-growing strain, thermophil | [58,59] |
| Syn11802 | 2.7 | / | 2.8 | 38 °C | 1 | 1000 | NT ****, Conjugation, Electroporation | Autotrophic | Fast-growing strain, thermophil | [60] |
| Syn11901 | 3.0 | 1 | 2.1 | 38 °C | 1 | 660 | NT ****, Conjugation | Mixotrophic, Autotrophic | Fast-growing strain, resistant to high-stress conditions | [61,93] |
| A. platensis C1 | 6.62 *** | / | ~60 | 30 °C | 0.3 | 108 | NT ****, Electroporation | Mixotrophic, Autotrophic | GRAS organism, production of proteins and pigments | [66,67,68,94,95] |
| F. diplosiphon | 9.9 *** | 14 | ~20 | 28–30 °C | N/A | 30 | Conjugation, Electroporation | Autotrophic | Suited for studying light absorption and photomorphogenesis | [96,97,98,99,100] |
| P. marinus | 1.6 | / | ~24 | 20 °C | N/A | 100 | / | Autotrophic | Suited as biomarker for ocean metabolism | [77,101,102] |
| G. violaceus 7421 | 4.6 | / | ~73 | 25 °C | N/A | 5 | Conjugation | Autotrophic | Suited for studying anoxygenic photosynthesis | [103,104,105,106] |
| T. elongatus BP-1 | 2.6 | / | 6 | 55 °C | 1.5 | 200 | Electroporation | Autotrophic | Thermophilic cyanobacterium, ideal for studying cyanobacterial photosystem | [83,107,108] |
* CO2%, temperature and light intensity correspond to the parameters used to achieve the fastest cell doubling rate for each cyanobacterial strain. ** Nitrogen-depleted conditions. *** Draft Genome Sequence. **** NT, natural transformation.
3. Current Methods for DNA Transfer into Cyanobacteria
Transferring heterologous or mutated genes through transformation is essential for rational engineering approaches to exploit cyanobacteria as a recombinant production system. Several methods exist and are described in the following.
3.1. Genomic Integration and Extrachromosomal DNA Insertion
Efficient transfer of exogenous DNA molecules into cyanobacteria can be achieved using “suicide” vectors for chromosomal gene transfer. This involves integrating the DNA fragment into cyanobacterial chromosomes by double homologous recombination and plasmid transformation using self-replicating plasmids [109]. Chromosome integration may be used to characterize endogenous genes using classic loss of function studies, where an antibiotic resistance cassette replaces the endogenous gene. For heterologous gene expression, genomic neutral sites are often targeted [110], which can be disrupted with no effect on cell metabolism and physiology [111,112] and allow stable expression of the introduced gene [111].
For maintaining a stable mutant genotype, the mutation must be present in all chromosomal copies to be retained stably and not be rejected [110]. This condition of complete genome segregation is time-consuming because most cyanobacteria are polyploid organisms with multiple chromosome copies, up to 53 in Syn6803, depending on growth phase and environmental parameters [111,113]. The individuation of positive mutants is achieved by antibiotic resistance selection. To enforce the mutation of all chromosomes, single positive colonies are sequentially re-streaked on a medium containing increasing antibiotic concentrations [114]. This process can take up to two months, depending on the strain used. Recently, Pope et al. showed that much quicker gene segregation could be achieved by reducing the phosphate concentration in the growth medium because low phosphate concentration increases the proportion of monoploid cells. However, this was accompanied by a 100-fold drop in transformation efficiency [115]. Analogously, Riaz et al. found out that the reduction of phosphate and agar concentration in the growth medium and high temperature are key parameters for reducing polyploidy and accelerating segregation process [116].
Self-replicating plasmids can alternatively be used for heterologous gene expression to avoid long segregation times. Shuttle vectors that replicate both in E. coli and cyanobacteria facilitate plasmid cloning and maintenance [117]. However, the major disadvantage of their use is the narrow host range in which they are available. Due to species-specific replicon systems, usability in different cyanobacterial species is complicated [118,119]. This drawback has been addressed by creating RSF1010-based plasmids with broader inter-species compatibility [117]. The popular cyanobacterial RSF1010 shuttle vector is the pPMQAK1 plasmid, which is supposed to replicate most consistently. However, the low copy number (on average 10–20 copies per E. coli cell) compromises its use, as transformation methods require high amounts of plasmid (3–4 µg) [119]. Several variants have been developed that show better transmissibility [120], are streamlined to follow the Standard European Vector Architecture (EVA) structure [121] and facilitate modular cloning [122].
Recent studies on the native plasmid replication systems of Syn6803 (Table 1) revealed the presence of two genetic elements (Slr7037 and ssr7036), in the plasmid pSYSA, necessary for plasmid replication and extrachromosomal maintenance [123]. The insertion of Slr7037 (encoding for a cyanobacterial replication initiator protein, CyRepA1) and ssr7036 (encoding for a RNase E-mediated cleavage protein) into pUC19 backbone, which is not functional in cyanobacteria, enabled independent replication in Syn6803 and Syn7942 [123]. Thus, it may serve as a shuttle vector between cyanobacteria and E. coli.
3.2. Transformation Methods
The transformation of cyanobacteria and its methodology have been intensely investigated [26,124,125,126,127]. Three main transformation methods are available for cyanobacteria: natural transformation, conjugation and electroporation (Table 1).
3.2.1. Conjugation
Conjugation is a widespread and often preferred method for transferring DNA into cyanobacteria [109] because of its high efficiency and broad applicability amongst different species [125]. In triparental mating, two different E. coli strains are used: a helper strain harboring a conjugative plasmid (carrying the mob genes necessary for mobilizing the genetic elements) and a donor strain carrying the cargo plasmid containing an origin of replication (oriT), the sequence necessary for conjugation, and the gene(s) of interest to be transferred into the recipient cyanobacterial cells. Some species require additional vectors for optimizing conjugation efficiency. For instance, Ana7120 is co-transformed with the helper plasmid pRL623, which encodes three methylases to prevent the digestion of the mobilizer plasmid [128].
3.2.2. Electroporation
Electroporation involves applying a high-intensity electrical field to generate pores in the cell membrane, allowing DNA entry. So far, electroporation has been established for only a few cyanobacterial species, including A. platensis, Anabaena sp., Synechococcus sp., Synechocystis sp., F. diplosiphon and the nitrogen-fixing Plectonema boryanum [68,127]. Optimal voltage and pulse duration for high-efficiency transformation are species-specific [129]. Besides plasmid transfer, electroporation has also been successfully used to transform Syn6803 and Syn7942 with linear PCR fragments [130,131,132]. Using linear DNA fragments instead of plasmids is advantageous as it reduces laborious cloning steps and, therefore, costs and time but is prone to nuclease degradation of the fragments [133]. Recently, the efficiency of inserting linear PCR fragments into Syn7942 was improved by the addition of EDTA, which inhibits DNase activity [130].
3.2.3. Natural Transformation
Natural transformation describes the uptake and maintenance of exogenous DNA by bacterial cells. This process is the basis of horizontal gene transfer between prokaryotic organisms, one of the leading forces contributing to the evolution of the prokaryotic domain [134]. In cyanobacteria, the retractile pilus T4P makes contact with the extracellular DNA and its retraction pulls the foreign DNA inside the cell. Several cyanobacteria are known to be naturally transformable such as the model organisms Syn6803, Syn7942 and Syn7002 [26,135,136]; the thermophilic T. elongatus sp. PCC BP-1 [82], the fast-growing strains Syn11801, Syn11802 and Syn11901 [58,60,61], the bloom-forming cyanobacterium Microcystis aeruginosa sp. PCC 7806 [137] and the filamentous Phormidium lacuna and A. platensis [67,68,138]. It is also possible to enable natural transformation through genetic engineering. The introduction of the pilN gene from Syn7942 into Syn2973 led to the acquisition of natural transformation competency, although with lower efficiency than Syn7942 [139]. The uptake efficiency relies on the concentration and length of the DNA, the cyanobacterial strain used and the growth phase [129]. Syn7002′s and Syn6803′s competency drastically decreases from exponential to stationary phase [140]. The circadian clock also seems to play a role in cyanobacterial transformation efficiency; the pili genes are overexpressed in darkness or low light conditions. Accordingly, the competency of cyanobacteria cells dramatically increases during darkness [140].
4. Tools for Manipulating Gene Expression in Cyanobacteria
The generation of predictable cellular output from utilizing standardized biological parts stands at the base of synthetic biology. Availability and reproducible behavior of well-characterized parts are essential for building functional genetic circuits and efficient microbial engineering. Most synthetic biology designs, fully operative in heterotrophic organisms such as E. coli, cannot be translated into cyanobacteria. For example, promoters and RBS that strongly modulate gene expression in E. coli do not perform well in cyanobacteria [141]. To date, the number of molecular tools for engineering cyanobacteria is far from that developed for E. coli and S. cerevisiae, but significant progress has been made in expanding and improving the cyanobacterial toolbox.
4.1. Increasing Gene Expression by Promoter Engineering
The most common way to overexpress genes in cyanobacteria is to use strong constitutive native promoters, but only a few have been described to date. In this category, the most common promoters control the expression of genes encoding components of the photosynthetic apparatus and are often light-regulated. Their application will be described in the following section.
4.1.1. Strong Native Promoters
Amongst the endogenous promoters controlling the expression of the genes of the photosynthetic apparatus, PpsbA2 is a light-regulated promoter that natively drives the expression of the D1 protein of the photosystem II apparatus in Syn6803 [142]. It has been used for the light-inducible, heterologous expression of an isoprene synthase gene in Syn6803 [143]. PcpcB is another strong constitutive promoter that controls the expression of the C-phycocyanin operon (cpc), which was used to engineer Syn6803 for the heterologous production of ketone bodies [144]. Different cyanobacterial variants of the constitutive promoter PrbcL, which natively powers the expression of ribulose 1,5-diphosphate carboxylase/oxygenase (RuBisCO), are also frequently used [40,145,146].
The drawbacks of using strong native promoters are that strong gene expression is often limited to specific cyanobacteria species and that expression strength often oscillates with the circadian rhythm [147]. One example is PrbcL from Ana7120; the promoter was successfully deployed to drive fatty acids and ethanol production in Syn6803 [148,149] but showed a lower expression strength than PsbaA2 and the heterologous, inducible promoter Ptrc1O. Similarly, it displayed a lower activity in Syn7002 compared to its native strong promoters, PA2520 and PA2579 [40].
4.1.2. Synthetic Inducible Promoters
Synthetic promoters lead to increased and consistent expression strength, as they function orthogonally to the cell’s native regulatory network. While not entirely synthetic, the systematic modification of native promoters has been central to creating new variants with improved activity profiles. By manipulating the consensus sequence of core promoter elements, Wegelius et al. created a minimal synthetic promoter for heterocyst-specific expression in Nostoc punctiforme ATCC 29133, resulting in a 10-fold higher reporter expression [150]. Truncation of the PcpcB promoter and the addition of multiple transcription factor binding sites led to the very strong Syn6803 promoter Pcpc560 [151]. Similarly, gene expression driven by a truncated version of PpsbA2 displayed a 4-fold increment compared to the wild type [152]. Synthetic promoters have also been created to function in diverse cyanobacteria. Promoters of the series J23 [153] were shown to perform strongly in Ana120, Syn6803, Syn7002, Syn7942 and Syn2973 and synthetic promoter libraries recently created by mutation of the strong promoters PcpcB and PrbcL from Syn7942 are applicable in Syn8001 and Syn8002 [47,48,139,154,155].
4.1.3. Chemically Inducible Promoters
Dynamic gene expression, which allows decoupling growth and production, can be helpful in cases where the target product is toxic for the host. Such a dynamic regime can be achieved with promoters regulated by environmental conditions, such as oxygen, CO2 partial pressure and light intensity [156], or by chemical inducers that can either activate or repress expression (Figure 1a). The current cyanobacterial engineering toolbox provides several inducible promoters.
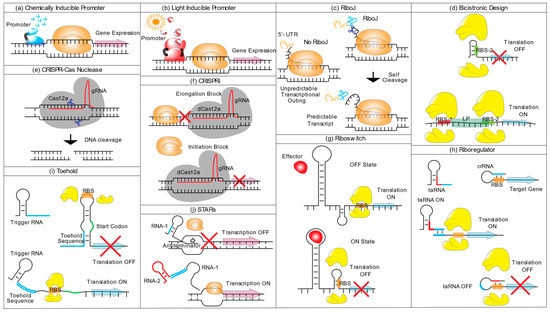
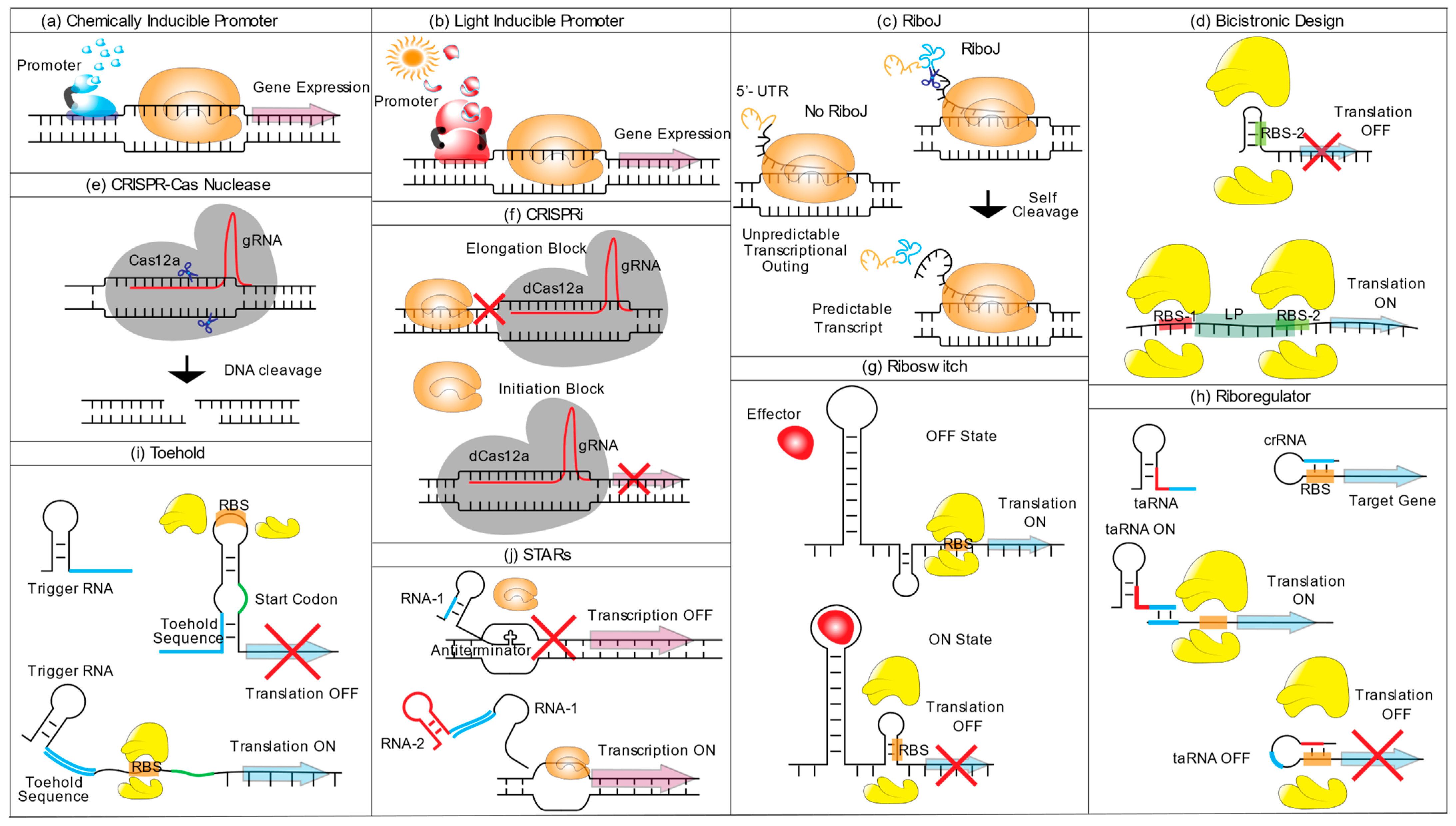
Figure 1.
Schematic representation of synthetic biology tools available for cyanobacteria. (a) Chemically inducible promoter, upon binding of a small chemical inducer (blue shapes), the activator binds to the promoter leading to transcription activation (violet arrow) through the activity of RNA polymerase II (orange shape). At the same time, a repressor dissociates from the DNA upon inducer binding (not shown). (b) Light inducible promoter, light-sensing proteins (red shapes) are (de)activated upon light induction (red sun) and induce a cascade that results in transcription activation (grey arrow) or repression. (c) RiboJ, the self-cleavage activity of RiboJ (blue line), cleaves the variable 5′-UTR sequence upstream of the ribozyme (orange line), leaving a shorter mRNA (black) with standardized 5′-UTR (d) Bicistronic design; to avoid translation obstruction through RNA secondary structures of the RBS (RBS-2) (upper figure), a short leader peptide (LP) sequence with its own RBS (RBS-1) is placed upstream of the GOI. During translation of LP, the helicase activity of the ribosome resolves possible RNA secondary structures present in the RBS-2, embedded in the LP region, permitting translation of the downstream gene. (e) CRISPR-Cas Nuclease, Cas12a nuclease (grey shape) identifies the target sequence based on complementarity to gRNA (red line) and cleaves the complementary DNA strand, creating staggered ends. (f) CRISPRi, guided by a gRNA, an inactive version of Cas12a, dCas12a, targets a specific gene and obstructs the transcription by interrupting RNA polymerase activity either by binding to an exonic region (Elongation block) or the promoter region (Initiation block). (g) Riboswitch, the presence or absence of a ligand effector defines the occurrence of translation and, therefore, the ON/OFF gene expression switch. In the absence of the ligand, the ribosome binds the RBS and initiates translation (Translation ON). In the presence of the effector, it hybridizes with the RNA leading to a conformational change that renders the RBS inaccessible to the ribosome and impedes translation (Translation OFF). (h) A Ribo-regulator is composed of a trans-activating RNA (taRNA) and a cis-repressed RNA (crRNA). When expressed alone, the crRNA is present in a conformation that precludes the RBS from binding to the ribosome (taRNA OFF). The expression of a taRNA (taRNA ON), containing a region complementary to the 5′ of the crRNA (coloured in blue), binds to the crRNA, restricting it to a conformational change that makes the RBS accessible for ribosome binding. (i) Toehold switch, the toehold RNA structure, is located upstream of the translation start site (TSS, coloured in red) and the toehold hairpin structure renders the RBS inaccessible to the ribosome, precluding the initiation of the translation. A second RNA molecule (trigger RNA), containing a sequence complementary to the toehold 5′ sequence of the toehold RNA structure (coloured in blue), binds to and stretches the toehold structure and frees up the RBS, allowing translation initiation. (j) Small transcription activating RNAs (STARs), an RNA molecule (RNA-1) containing an anti-terminator sequence, hybridizes upstream of a target gene. The presence of the anti-terminator forces the RNA polymerase to end transcription. The presence of a second regulatory RNA (RNA-2) capable of binding to a complementary region of RNA-1 (coloured in blue) forces a conformational change of RNA-1, preventing hairpin formation and enabling transcription.
Ptrc is a popular inducible promoter for the metabolic engineering of E. coli derived from the lactose operon system (lac operon). The system is induced by isopropyl ß-D-1-thiogalactopyranoside (IPTG), a molecular mimic of the natural lactose inducer and repressed in its absence [157]. To modulate expression strength, synthetic variants were created by modification of the spacer sequence between the -35 and -10 boxes and tested both in Syn7942 and Syn6803 [150,158]. Albers et al. placed lacI (lac repressor) under the control of the housekeeping gene sigA encoding the Sigma factor A, ensuring strong constitutive expression of the repressor [159]. Combined with the Psca6–2 promoter, a modified version of Ptrc, they achieved a 10-fold induction ratio after adding IPTG [159].
In E. coli, the transcription factor NanR can recognize and bind to specific DNA sequences “GGTATA”, called nan box and located within the promoter region, downstream of the RNA polymerase binding site [160]. Thus, it can repress the downstream gene expression. N-acetylneuraminic acid (Neu5Ac), a common sugar moiety, can reduce the NanR affinity towards nan box sites and favour gene expression [160]. Sun et al. generated, in Syn2973 and Syn7942, a controllable switch sensing machinery for gene expression that responds to Neu5Ac inducer [161]. Expression of lacZ gene (encoding β-galactosidase) is driven by the synthetic promoter PJ23119H10, containing two nan box sequences. Expression of lacZ is inhibited by the activity of NanR, whose synthesis is driven by Ptrc. Efficient dose-response expression of lacZ is achieved upon application of different concentrations of Neu5Ac.
The phage-derived TetR-regulated promoter PLtetO-1, inducible by anhydrotetracycline (aTc), was modified to obtain a variant applicable for Syn6803. Alterations of only a few base pairs upstream of the -10 consensus sequence resulted in variant PL03, which displayed a wide dynamic range in Syn6803 (290-fold induction upon aTc induction under red light) [162].
Metal native inducible promoters were also tested in Syn6803 but with limited success [163,164]. The nickel-inducible PnrsB efficiently promoted heterologous ethanol production upon Ni2+ addition, but cell viability defects compromised its use under high concentrations of Ni2+ [165]. The cobalt inducible promoter PcoaT has successfully been employed for heterologous triterpenoid biosynthesis in Syn6803, but the need to work in Co2+ depleted media hampered cell growth [166].
Arabinose inducible promoters such as PBAD are the most effective inducible promoters in cyanobacteria and are functional in both Syn6803 and Syn7942 [167,168]. PBAD works in concert with the repressor AraC. In the absence of L-arabinose, the transcriptional regulator AraC is active and represses transcription from PBAD, while it is deactivated in the presence of L-arabinose, resulting in the induction of PBAD controlled gene expression. This system enables tight gene expression control in Syn7942. It was applied for photomixotrophic growth with arabinose utilization in the dark to improve bioproduction robustness under diurnal conditions [168].
Further examples of inducible promoters are the rhamnose RhaS-regulated promoter PrhaBAD [169] and PvanCC, originating from Caulobacter crescentus [170], both showing tight regulation in the absence of the inducer, a linear response to inducer titration and an excellent dynamic range in Syn7942 and Syn6803 and Syn6803, respectively.
However, for large-scale industrial bioprocesses, the use of external chemical inducers is unpreferred because it significantly adds to the production costs and poses environmental risks, for instance, due to the usage of heavy metals [153]. Promoters tunable by cheap, non-toxic compounds or environmental/physical signals may overcome this problem (Figure 1b).
4.1.4. Environmentally Inducible Promoters
Several promoters activated by specific environmental inputs, such as light and oxygen, have been tested in cyanobacteria [156,171,172,173]. A common problem in strain engineering is the competition for precursors or cofactors required for production with native pathways. If the native pathway is essential for growth and cannot be deleted, this is addressed by downregulation of the competing activity [174]. This is achieved by exchanging native promoters with weaker ones or using promoters whose activity can be controlled by inducer or repressor titration [174].
In Syn6803, differential expression of the yellow fluorescent protein (YFP) was achieved by creating a dark inducible gene expression system based on EnvZ/OmpR two-component system of E. coli [175]. The dark inducible sensor (Cph8) is a chimeric protein composed of Syn6803’s phytochrome Cph1 and the histidine kinase domain EnvZ-OmpR. Dark conditions are sensed by Cph8 and lead to the dephosphorylation of EnvZ and phosphorylation of its corresponding regulator protein OmpR. The subsequent binding of the phosphorylated OmpR (OmpR-P) to the promoter ompC activates the promoter, prompting gene expression [175]. This tunable expression system represents an elegant strategy that can be exploited to produce light-sensitive proteins.
By contrast, Abe et al. exploited the cyanobacterial light-harvesting antenna, the phycobilisome, to build a light-sensing gene expression device composed of the green light receptor CcaS, the response sensor CcaR and the PcpcG2 naturally controlling the phycobilisome linker gene cpcG2 [176]. They regulated gene expression by shifting from red to green-light illumination and achieved expression levels comparable to Ptrc with an optimized system (Figure 1b) [171]. The same system was combined with the T7 phage RNA polymerase expression system, largely used in E. coli for heterologous protein expression. In this system, the PcpcG2 controls the expression of T7 polymerase, which transcribes T7 promoter (PT7) controlled genes. Tight control of gene expression was achieved, and the expression level was close to that achieved with the super-strong promoter Pcpc560 [172]. T7 RNA polymerase-based transcriptional systems have also been successfully applied in Syn7002 [177], Syn7942 [178] and Syn2973 [139] for strong gene expression.
The fumarate and nitrate-reducing system (FNR), which acts as a global gene expression regulator in E. coli under anaerobic conditions [179], was adapted for Syn6803, creating an oxygen-responsive system based on FNR-activated promoter PFNR [173]. This new device was used to create a logic AND gate, a two-level tunable gene expression system, in Syn6803 by pairing it with a Ptet promoter and the Salmonella typhimurium pathogenicity island 1 type III secretion system, which is required for virulence during intestinal infection [173]. It consists of promoter PsicA activated when the chaperone SicA and the transcriptional regulator InvF form a complex [180]. The transcription of sicA is boosted under anaerobic conditions, while InvF is under the control of Ptet and only formed in the presence of aTc. In the presence of both physical and chemical inducers, SicA and InvF form a complex that actives PsicA and, therefore, the downstream target gene, with limited leakiness and conspicuous dynamic range [173].
Given the multiple stress conditions and parameters to be evaluated when designing an efficient photosynthetic microbial cell factory, physical and chemical inducible promoters would potentially keep gene expression in tune with physiological needs dictated by the environment. Although understudied, the development of these promoters and their characterization in different cyanobacteria species increases rapidly, showing great promise as tunable gene expression systems.
4.2. Designing Ribosomal Binding Sites for Improving Gene Expression
While promoters act on the transcription rate, the translation rate heavily depends on the ribosome binding site (RBS). Translation initiation is fundamental for controlling protein expression; therefore, the design of short sequences with elevated ribosome binding affinity enables a significant increase in translation initiation.
4.2.1. Ribosome Binding Site (RBS)
The RBS, also known as Shine-Dalgarno (SD) sequence, is a relatively short sequence (around 6 bp) that is located at the 5′-untranslated region (5′-UTR) of the mRNA generally 7 to 11 bp upstream from the AUG start codon. The 16S ribosomal RNA (rRNA), part of the 30S small ribosomal subunit in prokaryotes, binds to the RBS by a complementary 3′ anti-SD sequence forming the primary complex for translation initiation. The SD/anti-SD complementarity, presence of secondary structures and the distance between RBS and the start codon can determine the overall RBS strength [181]. Despite a standard anti-SD sequence (CCUCC), the typical RBS of E. coli, 5′-GGAGG-3′, is less preferred in cyanobacteria. This sequence is only found in the UTR of 26% of all Syn6803 genes (compared to 57% in E. coli) and it is typically located 9–11 bp upstream of the start codon (7–9 bp in E. coli) [182]. This apparent difference in UTR structure suggests a limited reusability of standardized parts characterized in E. coli and profuse efforts were made to characterize alternative cyanobacterial RBSs. Nevertheless, Englund et al. found that a test set of 11 different RBSs resulted in similar relative translation rates in E. coli and Syn6803, in stark contrast to the results obtained from a study of the same group testing promoter function in these two species [152]. In the same strain, 20 endogenous RBSs were characterized by inserting the RBS sequences upstream of the strong promoter Ptrc1O [141]. RBS-ndhJ (Subunit J of the type 1 NADH dehydrogenase) and RBS-psaF (Subunit F of photosystem 1) accounted for the highest activities for translation initiation, while RBS-cpcB, RBS-rbcL and RBS-psbA2 failed to promote translation. The discrepancy in translation efficiency in the native and engineered setting may be attributable to the genetic context [139], pointing to the importance of taking the up- and downstream regions into account when designing RBSs.
Several synthetic RBSs designed using the RBS calculator [183] have been characterized in cyanobacteria species, including Syn7002, Syn7942, Ana7120, Syn6803 and Leptolyngbya sp. PCC BL0902 (Lep0902), adding to the synthetic biology toolbox of these organisms [117,154]. The efficacy of rationally designed RBSs was then efficiently exploited for the heterologous ethylene production in Syn6803 [184,185].
One of the potential drawbacks that can affect gene expression is the formation of secondary RNA structures at the level of the 5′-UTR-GOI junction, hampering RBS accessibility for the 30S ribosome and therefore obstructing translation initiation [186]. Additionally, regulatory DNA sequences downstream of the transcription start site, contained in many synthetic promoters, are transcribed, resulting in the accidental incorporation of nucleotides at the 5′ untranslated regions of the transcript [187]. These insertions may alter RNA stability and reduce translation efficiency and predictability [188]. The use of genetic insulators to leapfrog these events is discussed below.
4.2.2. RiboJ
RiboJ is formed by the sTRSV-ribozyme26 of the satellite tobacco ringspot virus RNA [189] and a 23-nucleotide synthetic hairpin [190]. When placed upstream of an RBS, sTRV cleaves the 5′-UTR of the mRNA [191]. This leads to a standardized 5′-UTR of the transcript that is “insulated” from the genetic context and provides more reliable expression (Figure 1c) and was also found to improve expression by improving ribosome access to the RBS [190,191]. This approach was successfully applied to engineer Syn6803 for 1-butanol production. A RiboJ insulator sequence was placed between the Ptrccore promoter and the genes npht7 and phaB (encoding for aceto-acetyl CoA synthase and aceto-acetyl-CoA reductase, respectively), leading to a 5.3-fold increase in 1-butanol production [192].
RiboJ cleavage was also tested in conjunction with a synthetic RBS ([A/G]AAGGAGGT, see above) and found to increase translation of the YFP reporter in Ana7120, Syn6803 and Lep0902 but not in Syn7942 [117]. The difference in RiboJ efficiency between different bacterial strains was explained by a single nucleotide polymorphism (SNP) near the catalytic RiboJ site [188].
4.2.3. Bicistronic Design (BCD)
The BCD (Figure 1d) is characterized by a short leader peptide (LP) and two RBSs, one located upstream of the LP (RBS-1) and a second (RBS-2) embedded within the LP sequence, just upstream of the gene of interest (GOI). The helicase activity of the ribosome translating the LP sequence resolves possible secondary RNA structures present in the RBS-2 that could otherwise hinder translation initiation of the downstream GOI (Figure 1 d) [193]. Both RiboJ and BCD were alternatively used to increase translation efficiency of proteins of the methylerythritol 4-phosphate (MEP) pathway in Syn6803 [194], thus showing the applicability in metabolic pathway engineering. Furthermore, the BCD design was recently ported to Syn6803 to improve translation efficiency of a hydrogenase for heterologous hydrogen production [195], demonstrating the practicality of this simple synthetic tool for expressing complex proteins. This system has been so far used only in Syn6803 and more applications in different species will need to be set up to demonstrate its general efficacy in cyanobacteria.
4.3. Using CRISPR for Gene Expression Regulation and Markerless Genome Editing
Due to the cyanobacterial polyploid genome organization, genome editing requires extended segregation periods to reach homogeneous populations. The clustered regularly interspaced short palindromic repeats (CRISPR) and its associated protein Cas (CRISPR/Cas) facilitate the genomic mutation frequency and speed up the segregation process. The Cas nuclease, directed by a guide RNA to a specific locus downstream of a protospacer adjacent motif (PAM), can precisely create double-stranded DNA cleavage that can be repaired by homology-directed repair, thus easing precise genome editing (Figure 1e). CRISPR/Cas was first applied as a genome editing tool in 2012 [196] but only exploited in cyanobacteria in 2016 and then established for genome modification in several cyanobacterial species [18]. Wendt et al. coupled the CRISPR system with the nuclease Cas9 from Streptococcus pyogenes [57]. While they successfully deleted the nblA gene responsible for phycobilisome degradation, they also experienced a toxic effect of Cas9 when constitutively expressed in Syn2973. This can be circumnavigated by employing inducible expression or weaker promoters [57,197] or the expression of Cas12a from Francisella novicida. The expression of this enzyme was reported to exert much lesser toxicity and it was efficiently used for markerless knock-ins, knock-outs and point mutations in Syn6803, Syn2973 and Ana7120, with about 20% correct genome edits [47].
More recent work reported a base editing tool based on CRISPR-Cas and deamination, which was successfully employed to introduce premature stop codons in genes of the glycogen synthesis pathways in Syn7942, thereby enabling the redirection of carbon into target products [198].
For essential genes which cannot be deleted, CRISPR interference (CRISPRi) that utilizes an enzymatically inactive “dead” Cas protein (dCas) can enable the downregulation of gene expression (Figure 1f). Experiments with inducible expression of dCas9 showcased applicability as a tunable gene repression system [199]. Using a CRISPR system, particularly CRISPRi, offers a substantial advantage for dynamic regulation of biosynthetic pathways to redirect the carbon flux toward a targeted chemical. Lactate production was propelled in Syn7002 upon overexpressing the lactate dehydrogenase (LDH) of Bacillus subtilis combined with downregulation of the glutamine synthetase I gene glnA through CRISPRi, which resulted in decreased ammonium uptake and an augmented level of the LDH substrate pyruvate [200]. A modest increase in succinic acid titer was achieved in Syn7942 by silencing glucose-1-phosphate adenylyl transferase (glgC) responsible for glycogen synthesis and the knock-in of two enzymes of the tricarboxylic acid cycle (TCA), phosphoenolpyruvate carboxylase (pepC) and citrate synthase (gltA) [155]. More recent work targeted additional genes via CRISPRi, resulting in succinic acid production of almost 9 g/L [201].
One of the great advantages of CRISPRi is the possibility of including different gRNAs in a single expression cassette, simultaneously targeting several DNA loci. This multiplexing nature of CRISPRi was exploited to simultaneously repress six lipid biosynthesis pathway genes, increasing the fatty acid production in Syn6803 [202]. Recently, CRISPRi/Cas12a mediated multiple gene interference was used in Syn7942 to partition carbon assimilation toward the triterpenoid squalene [203]. Both examples demonstrate the applicability for the metabolic engineering of cyanobacteria.
The use of CRISPRi as a modular and flexible gene regulation system that responds to different stimuli was recently showcased by combining it with two physically and chemically inducible promoters [204]. The synthetic systems used the circadian clock as a blueprint for a genetic circuit that computes an NGATE logic. In Syn 7942, the circadian rhythm is controlled by the kaiABC operon, which encodes a posttranslational oscillator complex that responds to environmental stimuli [205]. It works together with a two-component system, in which SasA (Synechococcus adaptive sensor A) intercepts with the complex and transmits the information to the second component RpaA (regulator of phycobilisome association A), whose phosphorylation state influences the expression of circadian clock genes [205]. The designed genetic circuit was composed of YFP controlled by the IPTG-inducible promoter Ptrc, a sgRNA targeting Ptrc, driven by a dark-induced promoter Purf and a dCas9 controlled by the aTc-inducible promoter Ptet. In the presence of IPTG, downregulation of YFP is only achieved in the presence of the two inputs: darkness and aTc addition; light conditions, instead, lead to the deactivation of Purf and derepression of YFP [204].
In 2020, multiple metabolic engineering targets, such as high-light and CO2 stress acclimation regulator genes, were identified in Syn6803 by creating the first cyanobacterial CRISPRi library comprising 10,498 clones [200]. Such libraries are a promising resource for identifying target genes relevant to biotechnological applications.
CRISPR-based techniques are particularly attractive for cyanobacterial strain engineering as no interspecies efficacy differences have been observed, in contrast to the other synthetic biology tools available for cyanobacteria. Overall, the use of Cas12a for generating markerless mutants may be better suited than Cas9. It does not exhibit toxicity issues; it requires only a single pre-crRNA array to target multiple genes and a 42 nucleotide RNA is sufficient and significantly shorter than the 100 nucleotides required for the Cas9 system, rendering all experiments cheaper. Moreover, the YTN (CTN or TTN) PAM sequence necessary for Cas12a is more often present than the canonical NGG PAM sequence proper of Cas9, opening the doors to more target sequences.
4.4. RNA-based Regulatory System
Post-transcriptional gene expression can be modulated by engineering mRNA stability and translation efficiency. This is often achieved by small regulatory RNAs (sRNAs) and a variety of synthetic variants of these RNA-based devices have been developed to aid metabolic engineering [206,207]. The use of RNA in synthetic biology confers several advantages in (a) terms of specificity since the cis-element can act on a specific RNA substrate; (b) programmability as the alteration of the RNA structure can be easily controlled; (c) orthogonality, because of their high degree of insularity that can prevent obstruction with other cellular processes; and (d) cell economy because of the lack of translation processes [207]. Some promising regulatory RNA elements applied in cyanobacteria include riboswitches (Figure 1g), riboregulators (Figure 1h), toehold switches (Figure 1i) and small transcription activating RNAs (STARs) (Figure 1j), are described in the following section.
4.4.1. Riboswitches
Riboswitches are cis-acting RNA elements that operate at the 5′-UTR of mRNA [208,209]. They are composed of two partially overlapping domains, an aptamer domain recognized by an effector molecule, whose binding creates a conformational change of the RNA structure, and an expression platform domain that can modulate gene expression by altering the transcription termination or by repressing the translation initiation (Figure 1g) [210]. Riboswitches are widespread in both Eukaryotic and Prokaryotic organisms because of their implication in regulating many metabolic pathways [211]. The best-characterized cyanobacterial riboswitch is a theophylline-dependent riboswitch. A synthetic version of this riboswitch was developed for Syn7942 and led to a 190-fold induction of a luciferase reporter [212]. The same device was successfully adapted for altering the circadian clock in Syn7942 by putting kaiC expression (part of the kaiABC operon, see above) under control of the theophylline inducible riboswitch [212]. A set of six different synthetic theophylline riboswitches, previously tested in various bacterial species, were adapted for use in Syn7942, Lep0902, Ana7120 and Synechocystis sp. strain WHSyn [213]. All riboswitches showed a wide dynamic range of induction in these cyanobacterial species and tight expression of the fluorescent protein YFP with minimal leakage [213]. Another theophylline riboswitch was developed for Syn6803. Its characterization showed its ability to tightly control the expression of a green fluorescent protein (GFP) reporter and further disclosed the importance of the N-terminal sequence of the riboswitch, whose secondary structures can affect translation efficiency [214].
A tunable gene expression system has been engineered by coupling the aTc-induced PL03 promoter (see above), the theophylline-riboswitch and CRISPR-dCas9, to control gene repression of glnA, a gene essential for nitrogen assimilation, in Ana7120 [48]. Using a L03 promoter alone to drive the expression of dCas9 was insufficient to ensure tight repression of glnA. However, the insertion of theophylline-riboswitch between the PL03 promoter and dCas9, in the presence of aTc and theophylline, robustly repressed glnA expression [48], demonstrating its utility for strictly controlling dCas9 expression for titratable regulation of genes.
Another elegant approach for controlling spatio-temporal gene induction was created in Ana7120 by coupling an adapted version of the Bacillus subtilis transcriptional ON riboswitch theo/pbuE* and pbuE/pbuE*, responding to theophylline or adenine respectively, with the heterocyst-specific promoter PnifB and a vegetative cell-specific promoter PrbcL [215]. Both riboswitches were able to achieve gene induction depending on cellular differentiation.
Two riboswitches endogenous to cyanobacteria have been tested for heterologous gene expression control with differing success. The cobalamin riboswitch, which regulates the production of vitamin B12 in Synechococcus elongatus 73,109 (Syn73109), was tested in Syn7002 and showed 6-fold induction of gene reporter expression [216]. In contrast, the glutamine riboswitch displayed poor binding affinity and consequentially low gene expression control [217].
Riboswitches have shown efficient and strict regulation of translation in several cyanobacterial strains. Given that they require only an aptamer-ligand interaction, they represent a simple and reliable synthetic biology tool for engineering cyanobacteria.
4.4.2. Riboregulators
Other RNA cis-acting elements for independent control of gene expression are riboregulators. They are composed of a cis-repressed RNA (crRNA) and a trans-activating RNA (ta-RNA). The crRNA hybridizes with the RBS, thereby blocking ribosome binding. The taRNA contains a sequence complementary to the internal loop region of the crRNA; taRNA binds to crRNA, constraining its ability to hybridize to the RBS and alleviating translation inhibition of the target gene (Figure 1h). Abe et al. [218] were the first to report the application of a ribo-regulator in Synechocystis. They used a mutant of the E. coli crR12-taR12 ribo-regulator (crR*2/taR*2), in which crRNA and taRNA expression were controlled by the constitutive Ptrc and the Ni2+-inducible nrsB promoter, respectively. With this configuration, the circuit is constitutively in an OFF state and translation (ON state) is only induced upon the addition of Ni2+. In Syn6803 this system led to a 13-fold increment in the GFP signal [218,219].
Follow-up work improved the gene expression and structure stability by inserting a sequence for binding RNA chaperone Hfq (a protein that increases the stability of sRNAs by protecting them from endo-nucleolytic cleavage) and a rho-independent transcription terminator sequence. These modifications promote correct RNA hybridization, including accurate and efficient gene regulation ability [220].
Sun et al. generated a binary regulation of target gene (lacZ) by coupling the NanR/Neu5Ac biosensor system (see above) to a theophylline-inducible ribo-regulator [161]. The system is composed by three different expression cassettes encoding for the chaperonin Hfq, NanR and a riboregulatory synthetic RNA sequence (sRNA) complementary to the target gene, regulated by Ptrc, Pcpc560 and PJ23119H10, respectively. Expression of lacZ is controlled by a theophylline inducible riboswitch (see above), while its expression level can be altered by sRNA regulatory element, which, in turn, is controlled by NanR/Neu5Ac switch. Tunable gene expression level was achieved depending on the concentrations of the two inducers, theophylline and Neu5Ac.
While applications of ribo-regulator technology in cyanobacteria are still scarcely investigated, significant improvements were accomplished, as demonstrated by the 78-fold increment in dynamic range using an optimized version of the taR*2/crR*2 [219]. Furthermore, the use of this system to repress the cyAbrA2 encoding a global transcriptional regulator in Syn6803 allowed a tunable ON/OFF device for regulating glycogen production, highlighting the applicability of riboregulators as a metabolic engineering tool in cyanobacteria [221].
4.4.3. Toehold Switches and Small Transcription Activating RNAs (STARs)
Other classes of riboregulators such as the Toehold switches and Small Transcription Activating RNAs (STARs) (Figure 1i,j) account only for a few applications in cyanobacteria so far. Toehold switches are composed of a hairpin loop containing an RBS and a start codon upstream of the coding sequence. The hairpin loop precludes the ribosome attachment to the RBS. When a trigger RNA binds to a complementary sequence upstream of the hairpin (Toehold sequence), the RNA unfolds, and translation is activated [222] (Figure 1i).
STARs are gene regulatory systems consisting of two components: a GOI and an upstream target RNA that, after transcription, folds into an intrinsic terminator that hampers the downstream gene transcription. The second component is an RNA that binds the target RNA, prohibiting hairpin formation and allowing gene transcription [223] (Figure 1j). In a study comparing STARs and Toehold switch performance in Ana7120 by placing the trans-element under the control of the aTc inducible L03 promoter and with LacZ as reporter, STARs showed substantial leakage activity while the Toehold ensured tight gene expression control [48].
Riboregulators can find their applicability in the future as effective multiple gene knockdown technology. In contrast to CRISPR, they do not rely on the presence of a PAM sequence, which makes the design more flexible. Compared to riboswitches, they allow a more robust regulation of gene expression; the possibility to be controlled independently from the promoter of the target gene makes them a very reliable gene regulatory system to be further exploited in cyanobacteria.
5. Metabolic Engineering Approaches for Increasing Cyanobacterial Production
Metabolic engineering can be defined as a strategy for perturbing cellular metabolic networks to improve the production of selected molecules [224]. Thus, metabolic engineering is a key approach for developing microbial platforms for the biosynthesis of molecules with valuable industrial properties. The potential of generating industrial-relevant molecules using light and CO2 as energy and carbon sources makes cyanobacteria attractive agents for sustainable bioproduction and they have been widely engineered to produce a vast array of different chemicals [10]. Here, we describe several metabolic engineering approaches adopted in cyanobacteria for increasing the production of fatty acids, carbohydrates and terpenoids. Industrially, the alkyl monoesters of fatty acids represent the starting material for biodiesel [225]; sugars such as glycogen may be used as a feedstock for bioethanol production [226,227] and terpenoids have broad industrial applications, from medical and agricultural to flavor and the fragrance industry [228,229].
5.1. Fatty Acid Production Engineering
Microbial fatty acid production aims to replace fossil fuel and environmentally destructive monocultures such as palm trees as sustainable sources of oleochemicals. However, microbial production needs to become more economically viable to be realized at scale. Here we review recent advances in metabolic engineering of fatty acid production in cyanobacteria. Fatty acid (FA) biosynthesis is highly conserved across prokaryotes and eukaryotes and highly regulated (Figure 2) [230]. Achieving a high fatty acid production in any microorganism requires its decoupling from growth either by metabolic engineering or environmental pressure as naturally achieved by oleaginous organisms, including some cyanobacteria [230,231]. Cyanobacteria present themselves as promising chassis for fatty acid production. Besides low feedstock requirements, one of the main advantages of cyanobacteria is their potential to secrete fatty acids into the media.
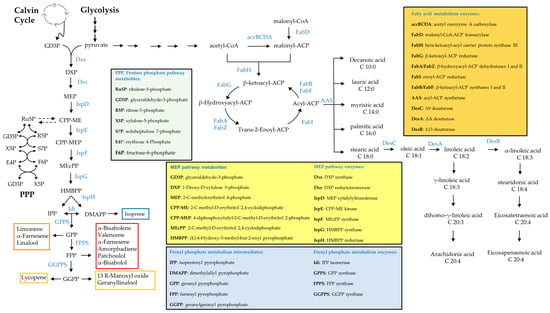
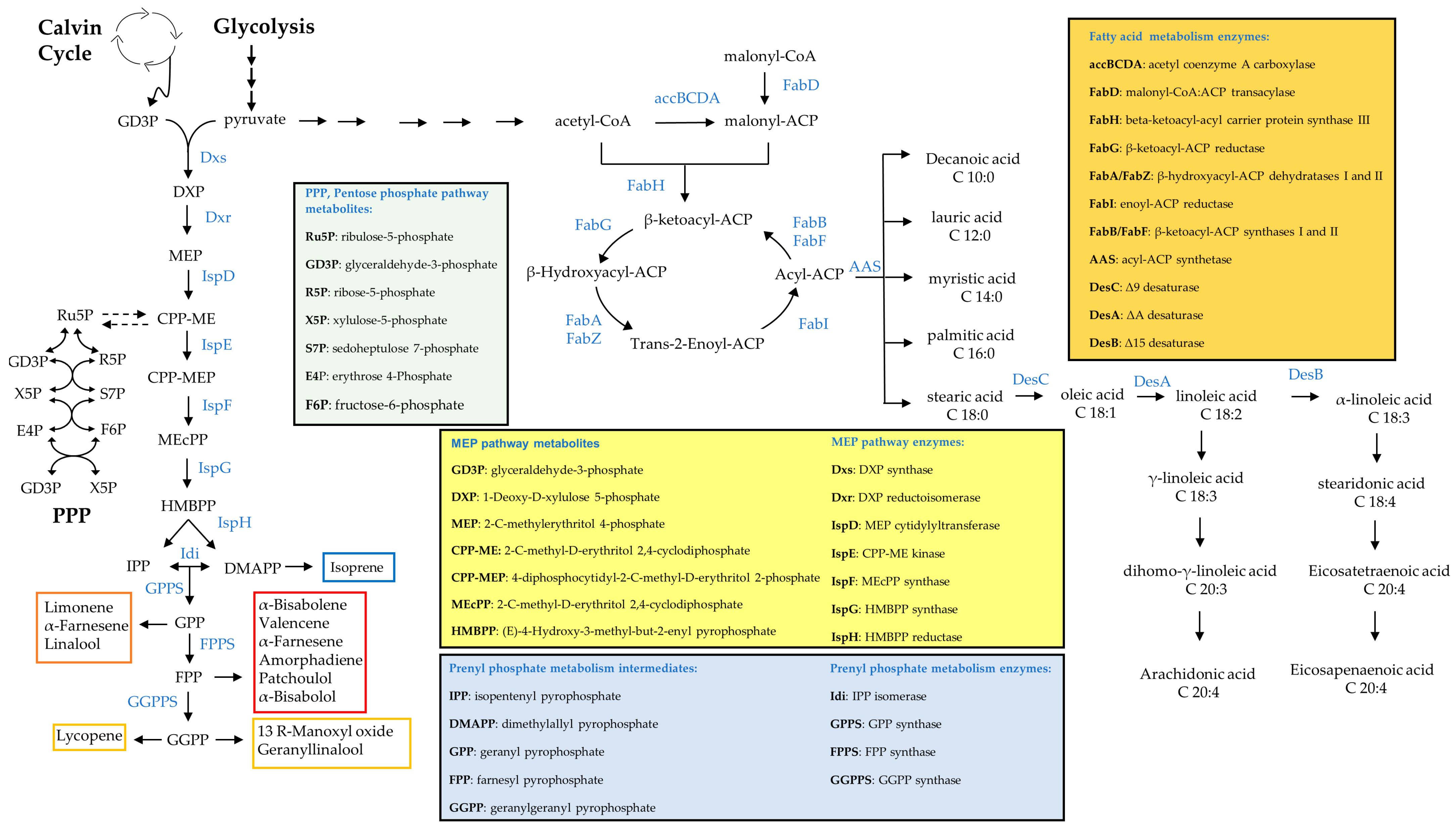
Figure 2.
Schematic representation of biosynthetic routes for terpenoids and fatty acids.
Liu et al. engineered Syn6803 to produce and secrete fatty acids to circumvent the biomass separation and extracting steps needed in other cases, producing a majority of 43% of C16:0 and a secretion yield of 197 mg L−1 of culture (Table 2) (Figure 2) [232]. They boosted the free fatty acid (FFA) level by sequentially introducing multiple copies of acyl-acyl carrier protein thio-esterase and acetyl-CoA carboxylase (ACC) genes, encoding rate-controlling enzymes in fatty acid synthesis (Figure 2). They also knocked out polyhydroxy-butyrate (PHB) synthesis to achieve the highest production levels (Table 2) [232]. In order to facilitate secretion, they also weakened the peptidoglycan layer by knocking out peptidoglycan assembly proteins [232]. However, a noticeable issue was that the cells became fragile and membrane damage occurred at low cell density [232]. Kato et al. were able to triple FFA secretion by using a two-phase culture, which alleviated lipid stress on the non-secreting strain dAS1T derived from Syn7942. Their system reached 0.64 mg L−1 FFA in the medium [233]. More recently, a non-model cyanobacterium, Syn11901, was found to produce an even higher titer of FFA [61], reaching 1.5 g L−1. Fatty acid alcohol synthesis is not naturally present in cyanobacteria but was engineered into Syn6803 by expressing a fatty acyl-CoA reductases (FAR) from mouse, jojoba and Arabidopsis, leading to a low initial product titer of around 10 µg L−1 [148]. Yao et al. picked another strategy and knocked out two enzymes involved in alkane synthesis in cyanobacteria and overexpressed a FAR from a marine bacterium, obtaining a slightly higher titer of 2.8 mg per gram cell dry weight (g CDW). The strain mainly produced hexadecanol and octa-decanol, but the availability of FAR with different chain-length specificities can be utilized to tailor the fatty alcohol profiles in cyanobacteria [234].
Santos-Merino et al. enabled alpha-linoleic acid (ALA), C18:3, production in Syn7942 via the introduction of two desaturases, deletion of fadD and overexpression of fadF, two genes involved in the fatty acid synthesis pathway of cyanobacteria (Figure 2), which enriched ALA to 22% of the total FAs pool. They also studied the role of each fad gene by deleting and overexpressing single genes and analyzing the fatty acid profile [235]. Thus, they demonstrated great versatility of the fatty acid metabolism in cyanobacteria and the possibility of making more complex fatty acids.
More recently, a higher amount of polyunsaturated fatty acids (PUFA) was produced in two engineered cyanobacteria strains: Lep0902, known to naturally produce a high amount of FA and Syn7002. Both strains produced ALA, but also stearidonic acid (SDA), C18:4 and eicosatetraenoic acid, C20:4, up to 40% of total FAs (Table 2 and Figure 2). To achieve this, Poole et al. overexpressed desaturase genes and a thylakoid membrane-promoting protein vipp1. The latter manipulation increases the formation of thylakoid membrane to which the desaturase reactions are localized [236].
Despite these advancements, maximal titers of various fatty acids are typically 10–50 times lower than those obtained with heterotrophs [230], a limitation that may be overcome with increasing knowledge on fatty acid synthesis in cyanobacteria [235]. The potential to tune the production of fatty acid-derived molecules in cyanobacteria, particularly for higher-value unsaturated PUFA with pharmaceutical applications, is promising [236].
5.2. Carbohydrate Production Engineering
Cyanobacteria naturally keep a high carbohydrate content as part of their osmotic regulatory system and accumulate them under stress conditions. Engineering high carbohydrate-producing strains has, therefore, the potential for enhancement via metabolic engineering. The primary use for high carbohydrate-producing cyanobacteria strains is as feedstock for growing industrially relevant microorganisms [56].
The cyanobacterium Syn7942 has been engineered to become a sucrose producer and exporter at rates competing with terrestrial equivalents such as sugar cane (Table 2). Relying on the natural production of sucrose, Ducat et al. overexpressed a sucrose permease gene cscB and knocked out invertase and an ADP-glucose pyro-phosphorylase [237] to reach a production of 36.1 mg L−1 h−1. The same phenotype was created in the UTEX 2973 strain (Table 1), which has a doubling time significantly superior to other cyanobacteria. Song et al. also turned Syn2973 into a high sucrose producer/exporter, reaching the same level as Syn7942 [55]. As Syn2973 is a significantly faster-growing strain and is more tolerant to abiotic stresses (Table 1), it could be a better choice to produce sucrose in an industrial setting [55]. Most recent work by Lin et al. doubled the sucrose production in Syn2973 by introducing the same scsB and additional overexpression of sps and spp, respectively, encoding for sucrose phosphate synthase and a sucrose phosphate phosphatase, reaching 79 mg L−1 h−1. They also managed to remove the need for high salt content in the media to induce sucrose production [238].
Besides sucrose, trehalose production is currently another attractive production target for cyanobacteria. Currently made by semi-enzymatic methods, it is an industrially relevant compound needed in the cosmetics industry but also useful for protection during organ transplant [239]. Recently Qiao et al. [239] have engineered a stress-tolerant strain of Syn7942 for trehalose production by blocking the sucrose production mechanism, introducing in place of sps a two-step pathway expressing a maltooligosyl trehalose synthase (MTS) and a maltooligosyl trehalose trehalo-hydrolase (MTH). They also expressed the insect trehalose transporter 1 TRET1 to secrete the sugar and engineered the glycogen metabolism to reach a titer of 5.7 g L−1 (Table 2).
Another exciting potential of cyanobacterial carbohydrates relies on valorizing their natural extracellular polymeric substances (EPS) as ingredients for the cosmetics and food industry as adjuvants and texture modifiers. With that aim in mind, Arias and Uggetti fully characterized a polysaccharide produced by Cyanothece sp. CCY 0110, named ‘cyanoflan’ (a biomaterial with potential use for wound healing) [240,241] and achieved 1.8 g L−1 of secreted EPS (Table 2).
Although not inherently highly valuable, cyanobacterial carbohydrate production has clear potential for various industrial applications, both for biofuel production and as a cosmetic ingredient and pharmaceutical additive production.

Table 2.
Compound titers and relative cyanobacterial strains.
Table 2.
Compound titers and relative cyanobacterial strains.
| Compounds | Organism | Titer | Reference |
|---|---|---|---|
| Free Fatty acids (secreted) | Syn6803 Syn7942 | 197 mg L−1 0.64 g L−1 | [232] [233] |
| Free Fatty acids | Syn11901 | 1.5 g L−1 | [61] |
| Fatty alcohol | Syn6803 Syn6803 | 9.7 μg OD730−1 L−1 2.8 mg gCDW−1 | [148] [234] |
| Alpha linoleic acid (C18:3) | Syn7942 | 22% of total FA | [235] |
| Alpha linoleic acid (C18:3), Stearidonic acid (C18:4), Eicosatetraenoic acid (C20:4) | Lep0902, Syn7002 | 40% of total FA | [236] |
| Total fatty acids | Syn7942 + R. glutinis | 39 mg L−1 | [242] |
| Sucrose | Syn7942 Syn2973 Syn2973 | 36.1 mg L−1 h−1 35.5 mg L−1 h−1 79 mg L−1 h−1 | [237] [55] [238] |
| Trehalose | Syn7942 | 5.7 g L−1 | [239] |
| Extracellular polysaccharide ‘cyanoflan’ | Cyanothece sp. CCY 0110 | 1.8 g L−1 | [240] |
| Isoprene (C5) | Syn7942 Syn6803 | 1.26 g L−1 12.3 mg gCDW−1 | [30] [243] |
| Limonene (C10) | Syn2973 Syn6803 Ana7120 Syn7002 | 16.4 mg L−1 6.7 mg L−1 3.6 μg L−1 4 mg L−1 | [244] [245] [246] [247] |
| (S)-linalool | Syn6803 | 11.4 mg L−1 | [248] |
| β-phellandrene | Syn6803 | 10 mg gCDW−1 | [249] |
| (E)-α-bisabolene (C15) | Syn6803 Syn7002 | 180 mg L−1 0.6 mg L−1 | [250] [247] |
| α-Farnesene | Ana7120 Syn7942 | 305.4 μg L−1 12.99 mg L−1 | [251] [252] |
| Amorphadiene | Syn7942 | 19.8 mg L−1 | [253] |
| Valencene | Syn6803 | 9.6 mg L−1 | [248] |
| β-Caryophyllene | Syn6803 | N.R.* | [254] |
| Patchoulol | Syn6803 | 17.3 mg L−1 | [255] |
| α-bisabolol | Syn6803 | 96.3 mg L−1 | [255] |
| 13R-manoyl oxide (C20) | Syn6803 | 2 mg L−1 | [256] |
| Geranyllinalool (Floating and intracellular) | Syn6803 | 390 μg gCDW−1 | [257] |
| Squalene (C30) | Syn7942 Syn6803 | 9.5 mg L−1 5.1 mg L−1 | [258] [259] |
| Lycopene (C40) | Syn6803 | 1.5 mg gCDW−1 | [260] |
5.3. Terpenoid Production Engineering
Unicellular, photosynthetic organisms are in the spotlight of microbial terpenoid production because these organisms possess traits that might benefit terpenoid production. Cyanobacteria synthesize terpenoids via the MEP pathway, of which a complete set of homologous genes has been identified. The flux through the terpenoid synthesis pathways is assumed to be higher, as in heterotrophs, because of the demand for chlorophyll and carotenoids to sustain photosynthesis and combat oxidative stress. Microalgae deliver the reducing equivalents strongly demanded by terpenoid production via photosynthesis while having a simpler cellular architecture and being more straightforwardly genetically manipulated than higher plants [228]. Despite these promises, terpenoid titers in engineered cyanobacteria or microalgae reported to date are very low, most often in the lower single-digit per mL range (Table 2). We here focus on some outstanding examples of terpene production from cyanobacteria.
Gao et al. [30] produced above 1 g L−1 of isoprene within three weeks in Syn7942 through upregulation of the native methylerythritol 4-phosphate (MEP) pathway (Figure 2). The key limiting steps identified in this study were 1-deoxy-D-xylulose 5-phosphate synthase (Dxs), 4-hydroxy-3-methylbut-2-enyl-diphosphate synthase (IspG) and isopentenyl pyrophosphate isomerase (Idi) (Figure 2), which were relieved by the integration of a second copy of the native dxs and ispG gene and a codon-optimized idi gene from S. cerevisiae under control of the strong, inducible Ptrc promoter. While dxs and ispG overexpression targeted kinetic bottlenecks of the MEP pathway (Figure 2), the heterologous isopentenyl isomerase was expressed to shift the ratio of the two hemiterpenes IPP and DMAPP towards the isoprene precursor DMAPP (Figure 2). Quantification of IPP and DMAPP, starting molecules of the prenyl phosphate metabolism (Figure 2), showed that this strategy successfully increased the DMAPP/IPP ratio by 130-fold. An interesting observation in this study was that the high specific isoprene production did not decrease the biomass yield but was balanced by an increased carbon fixation rate [30].
Following these results, Englund et al. [194] observed increased isoprene production in Syn6803 upon overexpression of either dxs or fni (encoding the native isopentenyl pyrophosphate isomerase). However, single overexpression of ispG did not result in higher isoprene production. Likely, the HMBPP synthase activity is not the primary limiting step of the Synechococcus MEP pathway and this strategy only becomes effective when other flux-constraining enzyme activities are upregulated [194]. Production of the sesquiterpenoids farnesene and amorphadiene and the triterpene squalene was likewise improved by lifting the metabolic bottlenecks given by Dxs and IPP isomerase activity (Figure 2) [251,253,261].
Chaves and Melis addressed low productivity by fusing the isoprene synthase characterized by low catalytic efficiency to the β-subunit of the highly expressed photosynthesis pigment phycocyanin encoded by cpcB. In the engineered strain, the abundance of the synthase was significantly increased and, in combination with the expression of a heterologous isopentenyl isomerase, resulted in a 62-fold improvement of the isoprene titer [243]. The group also successfully employed the same fusion strategy to improve a β-phellandrene synthase activity to produce this monoterpenoid [249].
Besides the canonical MEP Pathway, cyanobacteria are assumed to operate a shunt that channels intermediates of the pentose phosphate pathway into the lower MEP pathway under phototrophic conditions (Figure 2). This hypothesis is supported by the insensitivity of the MEP pathway in this organism to MEP pathway precursors and intermediates and its stimulation by some phosphorylated sugars [262,263].
This shunt might fuel the MEP pathway with intermediates from the pentose phosphate pathway (PPP), thereby bypassing the rate-limiting Dxs step and may have contributed to the increased limonene synthesis observed in Lin et al. [245]. The authors used a genome-scale metabolic model of Syn6803 to simulate the effect of the hypothetical connection between the Calvin-Benson-Cycle/PPP and the lower MEP pathway (Figure 2). The model extended with this shunt predicted that increased flux through this shortcut could increase limonene production. The model prediction was verified in vivo by overexpression of the PPP genes rpi and rpe, which pushed the limonene titers to more than 6 mg L−1 when combined with the simultaneous upregulation of geranyl pyrophosphate synthase (GPPS) activity. It remains to be elucidated if this increase is caused by a higher flux through the proposed PPP-MEP shunt (Figure 2).
As described above for E. coli, the recombinant expression of a mevalonate pathway likewise resulted in increased terpenoid production [264].
5.4. Nanocompartments Engineering
Bacterial nano-compartments (BNC) are intracellular structures composed of a selective proteinaceous scaffold that enclose a segment of a metabolic pathway [265]. Similarly to eukaryotic organelles, the external structure shields the internal enzymatic core from the cytosolic environment [265]. These structures may be exploited as cargo carriers for the delivery of molecules, as bioactive compounds with pharmacological properties and as agrochemicals and biomolecules within the scope of phyto-nanotechnology [266,267].
Exploring and gaining knowledge about cyanobacterial nano-compartments and their roles, is an interesting avenue for enhancing stress resistance and engineering novel metabolic pathways in nano-compartments.
One of the most commonly studied cyanobacterial nano-compartments are carboxysomes. Caroboxysomes were discovered in cyanobacteria in 1956 by Drew and Niklowitz; these protein compartments concentrate the CO2 to obtain the highest possible activity of the RuBisCO enzyme, which is essential for carbon fixation [268]. Plant scientists have obviously raised intense interest in this compartment and the engineering of agricultural plants for increasing plant carbon fixation efficiency with cyanobacterial carboxysomes has been attempted [269]. Delivery of enzymes or metabolic pathways into plant systems would ensure the transient expression of biomolecules, thus avoiding complicated transgene integration processes and the transfer of modified traits to the subsequent generations [266,267].
Stable integration of carboxysomes genes in the chloroplast is notoriously quite challenging [270]. Fang et al. report the first heterologous expression of Syn7942 β-carboxysome in E. coli using 19 genes split onto two synthetic plasmids [271]. Carboxysomes have an essential role in carbon fixation; therefore, gaining a better understanding of their assembly and function is essential in order one day to perhaps use them as a target to optimize cyanobacterial growth and to be able to eventually engineer better carbon fixation [272].
Besides carboxysomes, other nano-compartments exist in prokaryotic organisms, such as encapsulins [273]. Many different types of encapsulins have been discovered and genome mining studies were able to discover the presence of potentially dozens of novel families [274] involved in various domains, redox stress and iron mineralization, amongst others. Nichols et al. have investigated a nano-compartment encapsulating a cysteine desulfurase enzyme in Syn7942, which is upregulated upon sulfate starvation in cyanobacteria [275]. Besides their potential role in stress resistance, encapsulins could be engineered to encapsulate any enzyme or enzymatic pathway of interest. Nano-compartments can offer protease protection and isolate unstable intermediates in reaction mechanisms. Cyanobacterial encapsulins clearly represent an untapped and understudied area that could be promising for engineering more robust cyanobacteria and improving the efficiency of metabolic engineering efforts.
5.5. An Ecosystem Approach for a More Sustainable Production
The direct use of cyanobacteria as feedstock involves mixing the cells with other microorganisms, allowing them to either feed on the biomass and/or ferment the carbohydrates. Another strategy is integrating both cultures into a synthetic ecosystem to achieve production of the final compound of interest. Ideally the organisms of such a consortium would interact with and be mutually dependent on each other. Such a symbiotic culture could, for instance, be realized using cyanobacteria as the photosynthetic producer of carbon food sources used by an established industrial biotechnology workhorse such as S. cerevisiae.
Recent work has explored the use of cyanobacteria in fully characterized synthetic consortia of microorganisms symbiotically interacting to produce various compounds of interest, including the biopolymer polyhydroxy-butyrate (PHB) alpha-amylase [276] and has highlighted the challenges in establishing such systems. Hays et al. combined Syn7942, engineered for high sucrose production and export [237] with Bacillus, E. coli and Saccharomyces strains. The stability of each consortium over extended periods was assessed, as well as their response to various stresses and, finally, their ability to produce alpha-amylase and PHB. Challenges encountered were that heterotrophs needed a higher amount of sucrose than what was produced by the cyanobacteria to sustain growth after 24 h and the fact that a high optical density (OD) of cyanobacteria led to inhibition of the heterotrophs independently from the level of sucrose [253]. Potential partnering with a third microorganism might help to mitigate oxygen-deleterious side effects on the heterotroph. An interesting finding was that co-culturing cyanobacteria with other heterotrophs seemed to stimulate their growth [276], which could be related to oxygen removal that can be toxic for cyanobacteria at increased levels.
In another consortium involving a sucrose-producing cyanobacterium and three yeast strains, a similar issue of growth inhibition due to the presence of reactive oxygen species (ROS) was observed and alleviated by using a catalase enzyme [242]. The co-culture of Syn7942 (engineered to overexpress the proton/sucrose symporter gene, cscB, of E. coli) and the carotenogenic yeast Rhodotorula glutinis was able to achieve a higher rate of linoleic acid and palmitoleic acids compared to the yeast alone. Similarly, a positive impact of the heterotroph on the cyanobacteria growth was noticed [242].
Other systems have been studied involving a semi-controlled ecosystem growing cyanobacteria on wastewater, leading to high production of the biopolymer poly-hydroxy-alkanoate (PHA), a natural product made by some cyanobacteria when under stress, reviewed by Arias and Uggetti [277].
Although this consortium approach appears as a seducing alternative offering a sustainable and integrated system, harnessing various microorganisms’ strengths and combining them needs careful optimization. Optimizing such complex systems will probably require advanced metabolic engineering to design perfect partnerships. The potential for a photoproduction system involving cyanobacteria associated with one or multiple heterotroph microorganisms is vast and ecosystem engineering could allow tuning and controlling metabolite production at another level. Nevertheless, studies exploring the potential of these ecosystem approaches highlighted that, rather than focusing purely on metabolic engineering, product yield can also significantly be optimized by intentionally applying stress or other external factors; sometimes aiming for slowing growth purposefully or tuning down the expression to balance metabolic activities can lead to the best production.
6. Conclusions
This review covered the current state of cyanobacterial biotechnology, including cyanobacteria with industrially relevant traits, the synthetic biology tools available to control gene expression and a description of several metabolic engineering approaches applied for photosynthetic bioproduction.
We anticipate that more synthetic biology tools for the efficient development of cyanobacterial cell factories will be rapidly developed. Combined with large-scale efforts to generate genetic libraries (overexpression and deletion mutants), such as those available for E. coli, to improve understanding of cyanobacterial metabolism and physiology and to develop advanced photobioreactors, these capabilities will enable the full potential of cyanobacteria and sustainable and photosynthetic bioproduction to be realized.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schopf, J.W.; Packer, B.M. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73. [Google Scholar] [CrossRef]
- Wagner, S.C. Biological Nitrogen Fixation. Nat. Educ. Knowl. 2011, 3, 15. [Google Scholar]
- Tandeau de Marsac, N. Adaptation of cyanobacteria to environmental stimuli: New steps towards molecular mechanisms. FEMS Microbiol. Lett. 1993, 104, 119–189. [Google Scholar] [CrossRef]
- Jensen, P.E.; Leister, D. Chloroplast evolution, structure and functions. F1000prime Rep. 2014, 6, 40. [Google Scholar] [CrossRef]
- Nelson, N. Photosystems and global effects of oxygenic photosynthesis. Biochim. Biophys. Acta 2011, 1807, 856–863. [Google Scholar] [CrossRef]
- Buschke, N.; Schafer, R.; Becker, J.; Wittmann, C. Metabolic engineering of industrial platform microorganisms for biorefinery applications--optimization of substrate spectrum and process robustness by rational and evolutive strategies. Bioresour. Technol. 2013, 135, 544–554. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Suzuki, I.; Hanai, T. Optimization of isopropanol production by engineered cyanobacteria with a synthetic metabolic pathway. J. Biosci. Bioeng. 2015, 119, 585–590. [Google Scholar] [CrossRef]
- Dismukes, G.C.; Carrieri, D.; Bennette, N.; Ananyev, G.M.; Posewitz, M.C. Aquatic phototrophs: Efficient alternatives to land-based crops for biofuels. Curr. Opin. Biotechnol. 2008, 19, 235–240. [Google Scholar] [CrossRef]
- Lau, N.S.; Matsui, M.; Abdullah, A.A. Cyanobacteria: Photoautotrophic Microbial Factories for the Sustainable Synthesis of Industrial Products. Biomed. Res. Int. 2015, 2015, 754934. [Google Scholar] [CrossRef]
- Vavitsas, K.; Kugler, A.; Satta, A.; Hatzinikolaou, D.G.; Lindblad, P.; Fewer, D.P.; Lindberg, P.; Toivari, M.; Stensjo, K. Doing synthetic biology with photosynthetic microorganisms. Physiol. Plant. 2021, 173, 624–638. [Google Scholar] [CrossRef]
- Vranova, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Shabestary, K.; Hudson, E.P. Computational metabolic engineering strategies for growth-coupled biofuel production by Synechocystis. Metab. Eng. Commun. 2016, 3, 216–226. [Google Scholar] [CrossRef]
- Santos-Merino, M.; Singh, A.K.; Ducat, D.C. New Applications of Synthetic Biology Tools for Cyanobacterial Metabolic Engineering. Front. Bioeng. Biotechnol. 2019, 7, 33. [Google Scholar] [CrossRef]
- Hitchcock, A.; Hunter, C.N.; Canniffe, D.P. Progress and challenges in engineering cyanobacteria as chassis for light-driven biotechnology. Microb. Biotechnol. 2020, 13, 363–367. [Google Scholar] [CrossRef]
- Mukherjee, B.; Madhu, S.; Wangikar, P.P. The role of systems biology in developing non-model cyanobacteria as hosts for chemical production. Curr. Opin. Biotechnol. 2020, 64, 62–69. [Google Scholar] [CrossRef]
- NIH GenBank Sequence Database. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 20 November 2022).
- Gale, G.A.R.; Schiavon Osorio, A.A.; Mills, L.A.; Wang, B.; Lea-Smith, D.J.; McCormick, A.J. Emerging Species and Genome Editing Tools: Future Prospects in Cyanobacterial Synthetic Biology. Microorganisms 2019, 7, 409. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Los, D.A.; Zorina, A.; Sinetova, M.; Kryazhov, S.; Mironov, K.; Zinchenko, V.V. Stress sensors and signal transducers in cyanobacteria. Sensors 2010, 10, 2386–2415. [Google Scholar] [CrossRef]
- Google Scholar. Available online: https://scholar.google.com (accessed on 20 November 2022).
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136. [Google Scholar] [CrossRef]
- Kaneko, T.; Tanaka, A.; Sato, S.; Kotani, H.; Sazuka, T.; Miyajima, N.; Sugiura, M.; Tabata, S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. I. Sequence features in the 1 Mb region from map positions 64% to 92% of the genome. DNA Res. 1995, 2, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; You, L.; Liu, D.; Hollinshead, W.; Tang, Y.J.; Zhang, F. Development of Synechocystis sp. PCC 6803 as a phototrophic cell factory. Mar. Drugs. 2013, 11, 2894–2916. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.S. The international journeys and aliases of Synechococcus elongatus. N. Z. J. Bot. 2019, 57, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Shestakov, S.V.; Khyen, N.T. Evidence for genetic transformation in blue-green alga Anacystis nidulans. Mol. Gen. Genet. 1970, 107, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.S.; Johnson, C.H.; Kondo, T. The cyanobacterial circadian system: A clock apart. Curr. Opin. Microbiol. 1998, 1, 669–673. [Google Scholar] [CrossRef]
- Clerico, E.M.; Ditty, J.L.; Golden, S.S. Specialized Techniques for Site-Directed Mutagenesis in Cyanobacteria. In Circadian Rhythms: Methods and Protocols; Rosato, E., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 155–171. [Google Scholar]
- Gao, X.; Gao, F.; Liu, D.; Zhang, H.; Nie, X.; Yang, C. Engineering the methylerythritol phosphate pathway in cyanobacteria for photosynthetic isoprene production from CO2. Energy Environ. Sci. 2016, 9, 1400–1411. [Google Scholar] [CrossRef]
- Lan, E.I.; Chuang, D.S.; Shen, C.R.; Lee, A.M.; Ro, S.Y.; Liao, J.C. Metabolic engineering of cyanobacteria for photosynthetic 3-hydroxypropionic acid production from CO2 using Synechococcus elongatus PCC 7942. Metab. Eng. 2015, 31, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Dempo, Y.; Fukusaki, E.; Hanai, T. Metabolic engineering for isopropanol production by an engineered cyanobacterium, Synechococcus elongatus PCC 7942, under photosynthetic conditions. J. Biosci. Bioeng. 2017, 123, 39–45. [Google Scholar] [CrossRef]
- Sarnaik, A.; Abernathy, M.H.; Han, X.; Ouyang, Y.; Xia, K.; Chen, Y.; Cress, B.; Zhang, F.; Lali, A.; Pandit, R.; et al. Metabolic engineering of cyanobacteria for photoautotrophic production of heparosan, a pharmaceutical precursor of heparin. Algal Res. 2019, 37, 57–63. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Matsuo, S.; Hamada, H.; Matsuda, F.; Hanai, T. Metabolic engineering of Synechococcus elongatus PCC 7942 for improvement of 1,3-propanediol and glycerol production based on in silico simulation of metabolic flux distribution. Microb. Cell Fact. 2017, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Holtman, C.K.; Chen, Y.; Sandoval, P.; Gonzales, A.; Nalty, M.S.; Thomas, T.L.; Youderian, P.; Golden, S.S. High-throughput functional analysis of the Synechococcus elongatus PCC 7942 genome. DNA Res. 2005, 12, 103–115. [Google Scholar] [CrossRef]
- Broddrick, J.T.; Rubin, B.E.; Welkie, D.G.; Du, N.; Mih, N.; Diamond, S.; Lee, J.J.; Golden, S.S.; Palsson, B.O. Unique attributes of cyanobacterial metabolism revealed by improved genome-scale metabolic modeling and essential gene analysis. Proc. Natl. Acad. Sci. USA 2016, 113, E8344–E8353. [Google Scholar] [CrossRef]
- Van Baalen, C. Studies on Marine Blue-Green Algae. Bot. Mar. 1962, 4, 129–139. [Google Scholar] [CrossRef]
- Nomura, C.T.; Sakamoto, T.; Bryant, D.A. Roles for heme-copper oxidases in extreme high-light and oxidative stress response in the cyanobacterium Synechococcus sp. PCC 7002. Arch. Microbiol. 2006, 185, 471–479. [Google Scholar] [CrossRef]
- Bernstein, H.C.; McClure, R.S.; Hill, E.A.; Markillie, L.M.; Chrisler, W.B.; Romine, M.F.; McDermott, J.E.; Posewitz, M.C.; Bryant, D.A.; Konopka, A.E.; et al. Unlocking the Constraints of Cyanobacterial Productivity: Acclimations Enabling Ultrafast Growth. mBio 2016, 7, e00949-16. [Google Scholar] [CrossRef] [PubMed]
- Ruffing, A.M.; Jensen, T.J.; Strickland, L.M. Genetic tools for advancement of Synechococcus sp. PCC 7002 as a cyanobacterial chassis. Microb. Cell Fact. 2016, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Hill, E.A.; Kucek, L.A.; Konopka, A.E.; Beliaev, A.S.; Reed, J.L. Computational evaluation of Synechococcus sp. PCC 7002 metabolism for chemical production. Biotechnol. J. 2013, 8, 619–630. [Google Scholar] [CrossRef]
- Kumar, K.; Mella-Herrera, R.A.; Golden, J.W. Cyanobacterial heterocysts. Cold Spring Harb Perspect. Biol. 2010, 2, a000315. [Google Scholar] [CrossRef]
- Muro-Pastor, A.M.; Hess, W.R. Heterocyst differentiation: From single mutants to global approaches. Trends Microbiol. 2012, 20, 548–557. [Google Scholar] [CrossRef]
- Adolph, K.W.; Haselkorn, R. Isolation and characterization of a virus infecting the blue-green alga Nostoc muscorum. Virology 1971, 46, 200–208. [Google Scholar] [CrossRef]
- Flores, E.; Herrero, A. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 2010, 8, 39–50. [Google Scholar] [CrossRef]
- Kultschar, B.; Llewellyn, C. Secondary Metabolites in Cyanobacteria; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Ungerer, J.; Pakrasi, H.B. Cpf1 Is A Versatile Tool for CRISPR Genome Editing Across Diverse Species of Cyanobacteria. Sci. Rep. 2016, 6, 39681. [Google Scholar] [CrossRef]
- Higo, A.; Isu, A.; Fukaya, Y.; Ehira, S.; Hisabori, T. Application of CRISPR Interference for Metabolic Engineering of the Heterocyst-Forming Multicellular Cyanobacterium Anabaena sp. PCC 7120. Plant Cell Physiol. 2018, 59, 119–127. [Google Scholar] [CrossRef]
- Norena-Caro, D.A.; Zuniga, C.; Pete, A.J.; Saemundsson, S.A.; Donaldson, M.R.; Adams, A.J.; Dooley, K.M.; Zengler, K.; Benton, M.G. Analysis of the cyanobacterial amino acid metabolism with a precise genome-scale metabolic reconstruction of Anabaena sp. UTEX 2576. Biochem. Eng. J. 2021, 171, 108008. [Google Scholar] [CrossRef]
- Cumino, A.C.; Marcozzi, C.; Barreiro, R.; Salerno, G.L. Carbon cycling in Anabaena sp. PCC 7120. Sucrose synthesis in the heterocysts and possible role in nitrogen fixation. Plant Physiol. 2007, 143, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liberton, M.; Cliften, P.F.; Head, R.D.; Jacobs, J.M.; Smith, R.D.; Koppenaal, D.W.; Brand, J.J.; Pakrasi, H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO(2). Sci. Rep. 2015, 5, 8132. [Google Scholar] [CrossRef]
- Tan, X.; Hou, S.; Song, K.; Georg, J.; Klahn, S.; Lu, X.; Hess, W.R. The primary transcriptome of the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Biotechnol. Biofuels 2018, 11, 218. [Google Scholar] [CrossRef]
- Cui, J.; Sun, T.; Li, S.; Xie, Y.; Song, X.; Wang, F.; Chen, L.; Zhang, W. Improved Salt Tolerance and Metabolomics Analysis of Synechococcus elongatus UTEX 2973 by Overexpressing Mrp Antiporters. Front. Bioeng. Biotechnol. 2020, 8, 500. [Google Scholar] [CrossRef]
- Nagarajan, A.; Zhou, M.; Nguyen, A.Y.; Liberton, M.; Kedia, K.; Shi, T.; Piehowski, P.; Shukla, A.; Fillmore, T.L.; Nicora, C.; et al. Proteomic Insights into Phycobilisome Degradation, A Selective and Tightly Controlled Process in The Fast-Growing Cyanobacterium Synechococcus elongatus UTEX 2973. Biomolecules 2019, 9, 374. [Google Scholar] [CrossRef]
- Song, K.; Tan, X.; Liang, Y.; Lu, X. The potential of Synechococcus elongatus UTEX 2973 for sugar feedstock production. Appl. Microbiol. Biotechnol. 2016, 100, 7865–7875. [Google Scholar] [CrossRef] [PubMed]
- Mollers, K.B.; Cannella, D.; Jorgensen, H.; Frigaard, N.U. Cyanobacterial biomass as carbohydrate and nutrient feedstock for bioethanol production by yeast fermentation. Biotechnol. Biofuels 2014, 7, 64. [Google Scholar] [CrossRef]
- Wendt, K.E.; Ungerer, J.; Cobb, R.E.; Zhao, H.; Pakrasi, H.B. CRISPR/Cas9 mediated targeted mutagenesis of the fast growing cyanobacterium Synechococcus elongatus UTEX 2973. Microb. Cell Fact. 2016, 15, 115. [Google Scholar] [CrossRef]
- Jaiswal, D.; Sengupta, A.; Sohoni, S.; Sengupta, S.; Phadnavis, A.G.; Pakrasi, H.B.; Wangikar, P.P. Genome Features and Biochemical Characteristics of a Robust, Fast Growing and Naturally Transformable Cyanobacterium Synechococcus elongatus PCC 11801 Isolated from India. Sci. Rep. 2018, 8, 16632. [Google Scholar] [CrossRef]
- Sengupta, S.; Jaiswal, D.; Sengupta, A.; Shah, S.; Gadagkar, S.; Wangikar, P.P. Metabolic engineering of a fast-growing cyanobacterium Synechococcus elongatus PCC 11801 for photoautotrophic production of succinic acid. Biotechnol. Biofuels 2020, 13, 89. [Google Scholar] [CrossRef]
- Jaiswal, D.; Sengupta, A.; Sengupta, S.; Madhu, S.; Pakrasi, H.B.; Wangikar, P.P. A Novel Cyanobacterium Synechococcus elongatus PCC 11802 has Distinct Genomic and Metabolomic Characteristics Compared to its Neighbor PCC 11801. Sci. Rep. 2020, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, A.; Selao, T.T.; Norling, B.; Nixon, P.J. Newly discovered Synechococcus sp. PCC 11901 is a robust cyanobacterial strain for high biomass production. Commun. Biol. 2020, 3, 215. [Google Scholar] [CrossRef] [PubMed]
- Thuan, N.H.; An, T.T.; Shrestha, A.; Canh, N.X.; Sohng, J.K.; Dhakal, D. Recent Advances in Exploration and Biotechnological Production of Bioactive Compounds in Three Cyanobacterial Genera: Nostoc, Lyngbya and Microcystis. Front. Chem. 2019, 7, 604. [Google Scholar] [CrossRef]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in clinical practice: Evidence-based human applications. Evid. Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- Wan, D.; Wu, Q.; Kuča, K. Spirulina. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 569–583. [Google Scholar]
- Food and Drug Administration. Spirulina, the Dried Biomass of Arthrospira Platensis. Agency Response Letter GRAS Notice No. GRN000127. 10 April 2003. Available online: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=127 (accessed on 4 February 2023).
- Jeamton, W.; Dulsawat, S.; Tanticharoen, M.; Vonshak, A.; Cheevadhanarak, S. Overcoming Intrinsic Restriction Enzyme Barriers Enhances Transformation Efficiency in Arthrospira platensis C1. Plant Cell Physiol. 2017, 58, 822–830. [Google Scholar] [CrossRef]
- Jester, B.W.; Zhao, H.; Gewe, M.; Adame, T.; Perruzza, L.; Bolick, D.T.; Agosti, J.; Khuong, N.; Kuestner, R.; Gamble, C.; et al. Development of spirulina for the manufacture and oral delivery of protein therapeutics. Nat. Biotechnol. 2022, 40, 956–964. [Google Scholar] [CrossRef]
- Hilzinger, J.M.; Friedline, S.; Sivanandan, D.; Cheng, Y.-F.; Yamazaki, S.; Clark, D.S.; Skerker, J.M.; Arkin, A.P. Acetaminophen production in the edible, filamentous cyanobacterium Arthrospira platensis. bioRxiv 2022, 2022.06.2030.498297. [Google Scholar] [CrossRef]
- Kumar, V.; Maurya, P.K.; Mondal, S.; Sinha, R.P.; Singh, S.P. Photomorphogenesis in the Cyanobacterium Fremyella diplosiphon Improves Photosynthetic Efficiency. In Cyanobacteria; Mishra, A.K., Tiwari, D.N., Rai, A.N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 131–143. [Google Scholar]
- Haney, A.M.; Kehoe, D.M. Fremyella diplosiphon. Trends Microbiol. 2019, 27, 562–563. [Google Scholar] [CrossRef] [PubMed]
- Biller, S.J.; Berube, P.M.; Lindell, D.; Chisholm, S.W. Prochlorococcus: The structure and function of collective diversity. Nat. Rev. Microbiol. 2015, 13, 13–27. [Google Scholar] [CrossRef]
- Palenik, B.; Haselkorn, R. Multiple evolutionary origins of prochlorophytes, the chlorophyll b-containing prokaryotes. Nature 1992, 355, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Partensky, F.; Hess, W.R.; Vaulot, D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol. Mol. Biol. Rev. 1999, 63, 106–127. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Chisholm, S.W. Code and context: Prochlorococcus as a model for cross-scale biology. Trends Microbiol. 2007, 15, 398–407. [Google Scholar] [CrossRef]
- Shaw, S.L.; Chisholm, S.W.; Prinn, R.G. Isoprene production by Prochlorococcus, a marine cyanobacterium and other phytoplankton. Mar. Chem. 2003, 80, 227–245. [Google Scholar] [CrossRef]
- Bourgade, B.; Stensjo, K. Synthetic biology in marine cyanobacteria: Advances and challenges. Front. Microbiol. 2022, 13, 994365. [Google Scholar] [CrossRef]
- Laurenceau, R.; Bliem, C.; Osburne, M.S.; Becker, J.W.; Biller, S.J.; Cubillos-Ruiz, A.; Chisholm, S.W. Toward a genetic system in the marine cyanobacterium Prochlorococcus. Access. Microbiol. 2020, 2, acmi000107. [Google Scholar] [CrossRef]
- Rippka, R.; Waterbury, J.; Cohen-Bazire, G. A cyanobacterium which lacks thylakoids. Arch. Microbiol. 1974, 100, 419–436. [Google Scholar] [CrossRef]
- Bernat, G.; Schreiber, U.; Sendtko, E.; Stadnichuk, I.N.; Rexroth, S.; Rogner, M.; Koenig, F. Unique properties vs. common themes: The atypical cyanobacterium Gloeobacter violaceus PCC 7421 is capable of state transitions and blue-light-induced fluorescence quenching. Plant Cell Physiol. 2012, 53, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Montejano, G.; Becerra-Absalón, I.; Gold-Morgan, M.; Osorio-Santos, K. Gloeobacter violaceus: Primitive reproductive scheme and its significance. Plant Syst. Evol. 2018, 304, 1221–1229. [Google Scholar] [CrossRef]
- Yamaoka, T.; Satoh, K.; Katoh, S. Photosynthetic activities of a thermophilic blue-green alga. Plant Cell Physiol. 1978, 19, 943–954. [Google Scholar] [CrossRef]
- Onai, K.; Morishita, M.; Kaneko, T.; Tabata, S.; Ishiura, M. Natural transformation of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1: A simple and efficient method for gene transfer. Mol. Genet. Genom. 2004, 271, 50–59. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kaneko, T.; Sato, S.; Ikeuchi, M.; Katoh, H.; Sasamoto, S.; Watanabe, A.; Iriguchi, M.; Kawashima, K.; Kimura, T.; et al. Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. DNA Res. 2002, 9, 123–130. [Google Scholar] [CrossRef]
- Elli, A.F.; Jelezko, F.; Tietz, C.; Studier, H.; Brecht, M.; Bittl, R.; Wrachtrup, J. Red pool chlorophylls of photosystem I of the cyanobacterium Thermosynechococcus elongatus: A single-molecule study. Biochemistry 2006, 45, 1454–1458. [Google Scholar] [CrossRef]
- Falke, S.; Feiler, C.; Chapman, H.; Sarrou, I. Crystal structures of native cytochrome c(6) from Thermosynechococcus elongatus in two different space groups and implications for its oligomerization. Acta Cryst. F Struct. Biol. Commun. 2020, 76, 444–452. [Google Scholar] [CrossRef]
- Kern, J.; Loll, B.; Luneberg, C.; DiFiore, D.; Biesiadka, J.; Irrgang, K.D.; Zouni, A. Purification, characterisation and crystallisation of photosystem II from Thermosynechococcus elongatus cultivated in a new type of photobioreactor. Biochim. Biophys. Acta 2005, 1706, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kupitz, C.; Grotjohann, I.; Conrad, C.E.; Roy-Chowdhury, S.; Fromme, R.; Fromme, P. Microcrystallization techniques for serial femtosecond crystallography using photosystem II from Thermosynechococcus elongatus as a model system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130316. [Google Scholar] [CrossRef]
- Zavřel, T.; Sinetova, M.A.; Búzová, D.; Literáková, P.; Červený, J. Characterization of a model cyanobacteriumSynechocystissp. PCC 6803 autotrophic growth in a flat-panel photobioreactor. Eng. Life Sci. 2015, 15, 122–132. [Google Scholar] [CrossRef]
- Ludwig, M.; Bryant, D.A. Synechococcus sp. Strain PCC 7002 Transcriptome: Acclimation to Temperature, Salinity, Oxidative Stress and Mixotrophic Growth Conditions. Front. Microbiol. 2012, 3, 354. [Google Scholar] [CrossRef] [PubMed]
- Videau, P.; Cozy, L.M. Anabaena sp. strain PCC 7120: Laboratory Maintenance, Cultivation and Heterocyst Induction. Curr. Protoc. Microbiol. 2019, 52, e71. [Google Scholar] [CrossRef]
- Prasanna, R.; Kumar, R.; Sood, A.; Prasanna, B.M.; Singh, P.K. Morphological, physiochemical and molecular characterization of Anabaena strains. Microbiol. Res. 2006, 161, 187–202. [Google Scholar] [CrossRef]
- Berberoğlu, H.; Jay, J.; Pilon, L. Effect of nutrient media on photobiological hydrogen production by Anabaena variabilis ATCC 29413. Int. J. Hydrog. Energy 2008, 33, 1172–1184. [Google Scholar] [CrossRef]
- Selão, T.T.; Włodarczyk, A.; Nixon, P.J.; Norling, B. Growth and selection of the cyanobacterium Synechococcus sp. PCC 7002 using alternative nitrogen and phosphorus sources. Metab. Eng. 2019, 54, 255–263. [Google Scholar] [CrossRef] [PubMed]
- El Baky, H.H.A.; El Baroty, G.S.; Mostafa, E.M. Optimization Growth of Spirulina (Arthrospira) Platensis in Photobioreactor Under Varied Nitrogen Concentration for Maximized Biomass, Carotenoids and Lipid Contents. Recent Pat. Food Nutr. Agric. 2020, 11, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Qin, S.; Hu, Y.; Song, Z.; Ying, J.; Li, P.; Dong, W.; Zhao, F.; Yang, H.; Bao, Q. Whole genomic DNA sequencing and comparative genomic analysis of Arthrospira platensis: High genome plasticity and genetic diversity. DNA Res. 2016, 23, 325–338. [Google Scholar] [CrossRef]
- Agostoni, M.; Lucker, B.F.; Smith, M.A.Y.; Kanazawa, A.; Blanchard, G.J.; Kramer, D.M.; Montgomery, B.L. Competition-based phenotyping reveals a fitness cost for maintaining phycobilisomes under fluctuating light in the cyanobacterium Fremyella diplosiphon. Algal Res. 2016, 15, 110–119. [Google Scholar] [CrossRef]
- Quest, B.; Hubschmann, T.; Sharda, S.; Tandeau de Marsac, N.; Gartner, W. Homologous expression of a bacterial phytochrome. The cyanobacterium Fremyella diplosiphon incorporates biliverdin as a genuine, functional chromophore. FEBS J. 2007, 274, 2088–2098. [Google Scholar] [CrossRef]
- Tabatabai, B.; Fathabad, S.G.; Bonyi, E.; Rajini, S.; Aslan, K.; Sitther, V. Nanoparticle-mediated Impact on Growth and Fatty Acid Methyl Ester Composition in the Cyanobacterium Fremyella diplosiphon. Bioenergy Res. 2019, 12, 409–418. [Google Scholar] [CrossRef]
- Tabatabai, B.; Adusei, A.; Shrivastava, A.K.; Singh, P.K.; Sitther, V. Nitrogen Deprivation in Fremyella diplosiphon Augments Lipid Production without Affecting Growth. Energies 2020, 13, 5769. [Google Scholar] [CrossRef]
- Tabatabai, B.; Arumanayagam, A.S.; Enitan, O.; Mani, A.; Natarajan, S.S.; Sitther, V. Overexpression of hlyB and mdh genes confers halotolerance in Fremyella diplosiphon, a freshwater cyanobacterium. Enzym. Microb. Technol. 2017, 103, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Tetu, S.G.; Sarker, I.; Schrameyer, V.; Pickford, R.; Elbourne, L.D.H.; Moore, L.R.; Paulsen, I.T. Plastic leachates impair growth and oxygen production in Prochlorococcus, the ocean’s most abundant photosynthetic bacteria. Commun. Biol. 2019, 2, 184. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A.; Salanoubat, M.; Partensky, F.; Artiguenave, F.; Axmann, I.M.; Barbe, V.; Duprat, S.; Galperin, M.Y.; Koonin, E.V.; Le Gall, F.; et al. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc. Natl. Acad. Sci. USA 2003, 100, 10020–10025. [Google Scholar] [CrossRef]
- Guo, H.; Xu, X. Broad host range plasmid-based gene transfer system in the cyanobacterium Gloeobacter violaceus which lacks thylakoids *. Prog. Nat. Sci. 2004, 14, 31–35. [Google Scholar] [CrossRef]
- Koyama, K.; Suzuki, H.; Noguchi, T.; Akimoto, S.; Tsuchiya, T.; Mimuro, M. Oxygen evolution in the thylakoid-lacking cyanobacterium Gloeobacter violaceus PCC 7421. Biochim. Biophys. Acta 2008, 1777, 369–378. [Google Scholar] [CrossRef]
- Kula-Maximenko, M.; Zielinski, K.J.; Slesak, I. The Role of Selected Wavelengths of Light in the Activity of Photosystem II in Gloeobacter violaceus. Int. J. Mol. Sci. 2021, 22, 4021. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kaneko, T.; Sato, S.; Mimuro, M.; Miyashita, H.; Tsuchiya, T.; Sasamoto, S.; Watanabe, A.; Kawashima, K.; Kishida, Y.; et al. Complete genome structure of Gloeobacter violaceus PCC 7421, a cyanobacterium that lacks thylakoids. DNA Res. 2003, 10, 137–145. [Google Scholar] [CrossRef]
- Anne Knight, R.; Brand, J.J.; Alper, H.S. Coordinated Response and Regulation of Carotenogenesis in Thermosynechococcus elongatus (BP-1): Implications for Commercial Application. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2014. [Google Scholar]
- Sacko, O.; Barnes, C.L.; Greene, L.H.; Lee, J.W. Survivability of Wild-Type and Genetically Engineered Thermosynechococcus elongatus BP1 with Different Temperature Conditions. Appl. Biosaf. 2020, 25, 104–117. [Google Scholar] [CrossRef]
- Elhai, J.; Wolk, C.P. Conjugal transfer of DNA to cyanobacteria. Methods Enzym. 1988, 167, 747–754. [Google Scholar] [CrossRef]
- Arora, N.; Jaiswal, D.; Sengupta, S.; Wangikar, P.P. Metabolic engineering of cyanobacteria for production of platform chemicals: A synthetic biology approach. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 127–145. [Google Scholar]
- Ng, A.H.; Berla, B.M.; Pakrasi, H.B. Fine-Tuning of Photoautotrophic Protein Production by Combining Promoters and Neutral Sites in the Cyanobacterium Synechocystis sp. Strain PCC 6803. Appl. Environ. Microbiol. 2015, 81, 6857–6863. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.; Pacheco, C.C.; Oliveira, P.; Montagud, A.; Landels, A.; Couto, N.; Wright, P.C.; Urchueguía, J.F.; Tamagnini, P. Improving a Synechocystis-based photoautotrophic chassis through systematic genome mapping and validation of neutral sites. DNA Res. 2015, 22, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Zerulla, K.; Ludt, K.; Soppa, J. The ploidy level of Synechocystis sp. PCC 6803 is highly variable and is influenced by growth phase and by chemical and physical external parameters. Microbiology 2016, 162, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Lea-Smith, D.J.; Vasudevan, R.; Howe, C.J. Generation of Marked and Markerless Mutants in Model Cyanobacterial Species. J. Vis. Exp. 2016, 111, 54001. [Google Scholar] [CrossRef]
- Pope, M.A.; Hodge, J.A.; Nixon, P.J. An Improved Natural Transformation Protocol for the Cyanobacterium Synechocystis sp. PCC 6803. Front. Plant Sci. 2020, 11, 372. [Google Scholar] [CrossRef]
- Riaz, S.; Jiang, Y.; Xiao, M.; You, D.; Klepacz-Smółka, A.; Rasul, F.; Daroch, M. Generation of miniploid cells and improved natural transformation procedure for a model cyanobacterium Synechococcus elongatus PCC 7942. Front. Microbiol. 2022, 13, 959043. [Google Scholar] [CrossRef]
- Taton, A.; Unglaub, F.; Wright, N.E.; Zeng, W.Y.; Paz-Yepes, J.; Brahamsha, B.; Palenik, B.; Peterson, T.C.; Haerizadeh, F.; Golden, S.S.; et al. Broad-host-range vector system for synthetic biology and biotechnology in cyanobacteria. Nucleic Acids Res. 2014, 42, e136. [Google Scholar] [CrossRef]
- Chen, Y.; Taton, A.; Go, M.; London, R.E.; Pieper, L.M.; Golden, S.S.; Golden, J.W. Self-replicating shuttle vectors based on pANS, a small endogenous plasmid of the unicellular cyanobacterium Synechococcus elongatus PCC 7942. Microbiology (Reading) 2016, 162, 2029–2041. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Y.; Idoine, A.; Bhaya, D. Construction of a Shuttle Vector Using an Endogenous Plasmid From the Cyanobacterium Synechocystis sp. PCC6803. Front. Microbiol. 2018, 9, 1662. [Google Scholar] [CrossRef]
- Bishe, B.; Taton, A.; Golden, J.W. Modification of RSF1010-Based Broad-Host-Range Plasmids for Improved Conjugation and Cyanobacterial Bioprospecting. iScience 2019, 20, 216–228. [Google Scholar] [CrossRef]
- Ferreira, E.A.; Pacheco, C.C.; Pinto, F.; Pereira, J.; Lamosa, P.; Oliveira, P.; Kirov, B.; Jaramillo, A.; Tamagnini, P. Expanding the toolbox for Synechocystis sp. PCC 6803: Validation of replicative vectors and characterization of a novel set of promoters. Synth. Biol. 2018, 3, ysy014. [Google Scholar] [CrossRef]
- Behle, A.; Axmann, I.M. pSHDY: A New Tool for Genetic Engineering of Cyanobacteria. In Plant Synthetic Biology: Methods and Protocols; Zurbriggen, M.D., Ed.; Springer US: New York, NY, USA, 2022; pp. 67–79. [Google Scholar]
- Kaltenbrunner, A.; Reimann, V.; Hoffmann, U.A.; Aoyagi, T.; Sakata, M.; Nimura-Matsune, K.; Watanabe, S.; Steglich, C.; Wilde, A.; Hess, W.R. Regulation of pSYSA defense plasmid copy number in Synechocystis through RNase E and a highly transcribed asRNA. bioRxiv 2022, 2022.11.30.518505. [Google Scholar] [CrossRef]
- Vioque, A. Transformation of cyanobacteria. Adv. Exp. Med. Biol. 2007, 616, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Koksharova, O.A.; Wolk, C.P. Genetic tools for cyanobacteria. Appl. Microbiol. Biotechnol. 2002, 58, 123–137. [Google Scholar] [CrossRef]
- Eaton-Rye, J.J. The Construction of Gene Knockouts in the Cyanobacterium Synechocystis sp. PCC 6803. In Photosynthesis Research Protocols; Carpentier, R., Ed.; Humana Press: Totowa, NJ, USA, 2004; pp. 309–324. [Google Scholar]
- Al-Haj, L.; Lui, Y.T.; Abed, R.M.; Gomaa, M.A.; Purton, S. Cyanobacteria as Chassis for Industrial Biotechnology: Progress and Prospects. Life 2016, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Elhai, J.; Vepritskiy, A.; Muro-Pastor, A.M.; Flores, E.; Wolk, C.P. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 1997, 179, 1998–2005. [Google Scholar] [CrossRef]
- Wendt, K.E.; Pakrasi, H.B. Genomics Approaches to Deciphering Natural Transformation in Cyanobacteria. Front. Microbiol. 2019, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.V.; Martens, S.B.B.; Lanes, C.F.C.; Marins, L.F. Improved genetic transformation of Synechococcus elongatus PCC 7942 using linear DNA fragments in association with a DNase inhibitor. Biotechnol. Res. Innov. 2017, 1, 123–128. [Google Scholar] [CrossRef]
- Kufryk, G.I.; Sachet, M.; Schmetterer, G.; Vermaas, W.F. Transformation of the cyanobacterium Synechocystis sp. PCC 6803 as a tool for genetic mapping: Optimization of efficiency. FEMS Microbiol. Lett. 2002, 206, 215–219. [Google Scholar] [CrossRef]
- Nagarajan, A.; Winter, R.; Eaton-Rye, J.; Burnap, R. A synthetic DNA and fusion PCR approach to the ectopic expression of high levels of the D1 protein of photosystem II in Synechocystis sp. PCC 6803. J. Photochem. Photobiol. B 2011, 104, 212–219. [Google Scholar] [CrossRef]
- Stucken, K.; Koch, R.; Dagan, T. Cyanobacterial defense mechanisms against foreign DNA transfer and their impact on genetic engineering. Biol. Res. 2013, 46, 373–382. [Google Scholar] [CrossRef]
- Hall, R.J.; Whelan, F.J.; McInerney, J.O.; Ou, Y.; Domingo-Sananes, M.R. Horizontal Gene Transfer as a Source of Conflict and Cooperation in Prokaryotes. Front. Microbiol. 2020, 11, 1569. [Google Scholar] [CrossRef]
- Stevens, S.E.; Porter, R.D. Transformation in Agmenellum quadruplicatum. Proc. Natl. Acad. Sci. USA 1980, 77, 6052–6056. [Google Scholar] [CrossRef]
- Grigorieva, G.; Shestakov, S. Transformation in the cyanobacterium Synechocystis sp. 6803. FEMS Microbiol. Lett. 1982, 13, 367–370. [Google Scholar] [CrossRef]
- Dittmann, E.; Neilan, B.A.; Erhard, M.; von Döhren, H.; Börner, T. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol. Microbiol. 1997, 26, 779–787. [Google Scholar] [CrossRef]
- Nies, F.; Mielke, M.; Pochert, J.; Lamparter, T. Natural transformation of the filamentous cyanobacterium Phormidium lacuna. PLoS ONE 2020, 15, e0234440. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, T.; Xu, C.; Chen, L.; Zhang, W. Development and optimization of genetic toolboxes for a fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Metab Eng. 2018, 48, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Schirmacher, A.M.; Hanamghar, S.S.; Zedler, J.A.Z. Function and Benefits of Natural Competence in Cyanobacteria: From Ecology to Targeted Manipulation. Life 2020, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pakrasi, H.B. Exploring native genetic elements as plug-in tools for synthetic biology in the cyanobacterium Synechocystis sp. PCC 6803. Microb. Cell Fact. 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Jansson, C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol. Biol. 1989, 13, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, P.; Park, S.; Melis, A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 2010, 12, 70–79. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Zhang, Y.; Li, Y.; Ma, Y. Designing and creating a modularized synthetic pathway in cyanobacterium Synechocystis enables production of acetone from carbon dioxide. Metab. Eng. 2012, 14, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Camsund, D.; Lindblad, P.; Heidorn, T. Design and characterization of molecular tools for a Synthetic Biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res. 2010, 38, 2577–2593. [Google Scholar] [CrossRef]
- Deng, M.D.; Coleman, J.R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Piechura, J.R.; Amarnath, K.; O’Shea, E.K. Natural changes in light interact with circadian regulation at promoters to control gene expression in cyanobacteria. Elife 2017, 6, e32032. [Google Scholar] [CrossRef]
- Tan, X.; Yao, L.; Gao, Q.; Wang, W.; Qi, F.; Lu, X. Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab. Eng. 2011, 13, 169–176. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, H.; Li, Z.; Tan, X.; Lu, X. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ. Sci. 2012, 5, 9857–9865. [Google Scholar] [CrossRef]
- Wegelius, A.; Li, X.; Turco, F.; Stensjo, K. Design and characterization of a synthetic minimal promoter for heterocyst-specific expression in filamentous cyanobacteria. PLoS ONE 2018, 13, e0203898. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Meng, H.; Zhu, Y.; Bao, G.; Zhang, Y.; Li, Y.; Ma, Y. Discovery of a super-strong promoter enables efficient production of heterologous proteins in cyanobacteria. Sci. Rep. 2014, 4, 4500. [Google Scholar] [CrossRef]
- Englund, E.; Liang, F.; Lindberg, P. Evaluation of promoters and ribosome binding sites for biotechnological applications in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2016, 6, 36640. [Google Scholar] [CrossRef]
- Camsund, D.; Lindblad, P. Engineered transcriptional systems for cyanobacterial biotechnology. Front. Bioeng. Biotechnol. 2014, 2, 40. [Google Scholar] [CrossRef]
- Markley, A.L.; Begemann, M.B.; Clarke, R.E.; Gordon, G.C.; Pfleger, B.F. Synthetic biology toolbox for controlling gene expression in the cyanobacterium Synechococcus sp. strain PCC 7002. ACS Synth. Biol. 2015, 4, 595–603. [Google Scholar] [CrossRef]
- Li, H.; Shen, C.R.; Huang, C.H.; Sung, L.Y.; Wu, M.Y.; Hu, Y.C. CRISPR-Cas9 for the genome engineering of cyanobacteria and succinate production. Metab Eng. 2016, 38, 293–302. [Google Scholar] [CrossRef]
- Sengupta, A.; Madhu, S.; Wangikar, P.P. A Library of Tunable, Portable and Inducer-Free Promoters Derived from Cyanobacteria. ACS Synth. Biol. 2020, 9, 1790–1801. [Google Scholar] [CrossRef]
- Gilbert, W.; Muller-Hill, B. Isolation of the lac repressor. Proc. Natl. Acad. Sci. USA 1966, 56, 1891–1898. [Google Scholar] [CrossRef]
- Niederholtmeyer, H.; Wolfstadter, B.T.; Savage, D.F.; Silver, P.A.; Way, J.C. Engineering cyanobacteria to synthesize and export hydrophilic products. Appl. Environ. Microbiol. 2010, 76, 3462–3466. [Google Scholar] [CrossRef]
- Albers, S.C.; Gallegos, V.A.; Peebles, C.A. Engineering of genetic control tools in Synechocystis sp. PCC 6803 using rational design techniques. J. Biotechnol. 2015, 216, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.; De Paepe, B.; De Wannemaeker, L.; Duchi, D.; Maertens, J.; Lammertyn, J.; De Mey, M. Development of N-acetylneuraminic acid responsive biosensors based on the transcriptional regulator NanR. Biotechnol. Bioeng. 2018, 115, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, S.; Zhang, F.; Sun, T.; Chen, L.; Zhang, W. Development of a N-Acetylneuraminic Acid-Based Sensing and Responding Switch for Orthogonal Gene Regulation in Cyanobacterial Synechococcus Strains. ACS Synth. Biol. 2021, 10, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Lindblad, P. Wide-dynamic-range promoters engineered for cyanobacteria. J. Biol. Eng. 2013, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Cavet, J.S.; Borrelly, G.P.; Robinson, N.J. Zn, Cu and Co in cyanobacteria: Selective control of metal availability. FEMS Microbiol. Rev. 2003, 27, 165–181. [Google Scholar] [CrossRef]
- Peca, L.; Kós, P.B.; Vass, I. Characterization of the activity of heavy metal-responsive promoters in the cyanobacterium Synechocystis PCC 6803. Acta Biol. Hung. 2007, 58, 11–22. [Google Scholar] [CrossRef]
- Roussou, S.; Albergati, A.; Liang, F.; Lindblad, P. Engineered cyanobacteria with additional overexpression of selected Calvin-Benson-Bassham enzymes show further increased ethanol production. Metab. Eng. Commun. 2021, 12, e00161. [Google Scholar] [CrossRef] [PubMed]
- Loeschcke, A.; Dienst, D.; Wewer, V.; Hage-Hulsmann, J.; Dietsch, M.; Kranz-Finger, S.; Huren, V.; Metzger, S.; Urlacher, V.B.; Gigolashvili, T.; et al. The photosynthetic bacteria Rhodobacter capsulatus and Synechocystis sp. PCC 6803 as new hosts for cyclic plant triterpene biosynthesis. PLoS ONE 2017, 12, e0189816. [Google Scholar] [CrossRef]
- Oliver, J.W.; Atsumi, S. Metabolic design for cyanobacterial chemical synthesis. Photosynth. Res. 2014, 120, 249–261. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Li, Q.; Xia, P.F.; Wei, L.J.; Guo, N.; Li, J.W.; Wang, S.G. AraBAD Based Toolkit for Gene Expression and Metabolic Robustness Improvement in Synechococcus elongatus. Sci. Rep. 2017, 7, 18059. [Google Scholar] [CrossRef]
- Kelly, C.L.; Taylor, G.M.; Hitchcock, A.; Torres-Mendez, A.; Heap, J.T. A Rhamnose-Inducible System for Precise and Temporal Control of Gene Expression in Cyanobacteria. ACS Synth. Biol. 2018, 7, 1056–1066. [Google Scholar] [CrossRef]
- Behle, A.; Saake, P.; Germann, A.T.; Dienst, D.; Axmann, I.M. Comparative Dose-Response Analysis of Inducible Promoters in Cyanobacteria. ACS Synth. Biol. 2020, 9, 843–855. [Google Scholar] [CrossRef]
- Abe, K.; Miyake, K.; Nakamura, M.; Kojima, K.; Ferri, S.; Ikebukuro, K.; Sode, K. Engineering of a green-light inducible gene expression system in Synechocystis sp. PCC6803. Microb. Biotechnol. 2014, 7, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Shono, C.; Ariyanti, D.; Abe, K.; Sakai, Y.; Sakamoto, I.; Tsukakoshi, K.; Sode, K.; Ikebukuro, K. A Green Light-Regulated T7 RNA Polymerase Gene Expression System for Cyanobacteria. Mar. Biotechnol. 2021, 23, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Immethun, C.M.; Ng, K.M.; DeLorenzo, D.M.; Waldron-Feinstein, B.; Lee, Y.C.; Moon, T.S. Oxygen-responsive genetic circuits constructed in Synechocystis sp. PCC 6803. Biotechnol. Bioeng. 2016, 113, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Davy, A.M.; Kildegaard, H.F.; Andersen, M.R. Cell Factory Engineering. Cell Syst. 2017, 4, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Immethun, C.M.; DeLorenzo, D.M.; Focht, C.M.; Gupta, D.; Johnson, C.B.; Moon, T.S. Physical, chemical and metabolic state sensors expand the synthetic biology toolbox for Synechocystis sp. PCC 6803. Biotechnol. Bioeng. 2017, 114, 1561–1569. [Google Scholar] [CrossRef]
- Hirose, Y.; Shimada, T.; Narikawa, R.; Katayama, M.; Ikeuchi, M. Cyanobacteriochrome CcaS is the green light receptor that induces the expression of phycobilisome linker protein. Proc. Natl. Acad. Sci. USA 2008, 105, 9528–9533. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Korosh, T.C.; Nielsen, D.R.; Pfleger, B.F. Optimization of a T7-RNA polymerase system in Synechococcus sp. PCC 7002 mirrors the protein overproduction phenotype from E. coli BL21(DE3). Appl. Microbiol. Biotechnol. 2021, 105, 1147–1158. [Google Scholar] [CrossRef]
- Azevedo, R.; Lopes, J.L.; de Souza, M.M.; Quirino, B.F.; Cançado, L.J.; Marins, L.F. Synechococcus elongatus as a model of photosynthetic bioreactor for expression of recombinant β-glucosidases. Biotechnol. Biofuels 2019, 12, 174. [Google Scholar] [CrossRef]
- Kang, Y.; Weber, K.D.; Qiu, Y.; Kiley, P.J.; Blattner, F.R. Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function. J. Bacteriol. 2005, 187, 1135–1160. [Google Scholar] [CrossRef]
- Moon, T.S.; Lou, C.; Tamsir, A.; Stanton, B.C.; Voigt, C.A. Genetic programs constructed from layered logic gates in single cells. Nature 2012, 491, 249–253. [Google Scholar] [CrossRef]
- Heidorn, T.; Camsund, D.; Huang, H.-H.; Lindberg, P.; Oliveira, P.; Stensjö, K.; Lindblad, P. Chapter Twenty-Four—Synthetic Biology in Cyanobacteria: Engineering and Analyzing Novel Functions. In Methods in Enzymology; Voigt, C., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 497, pp. 539–579. [Google Scholar]
- Ma, J.; Campbell, A.; Karlin, S. Correlations between Shine-Dalgarno sequences and gene features such as predicted expression levels and operon structures. J. Bacteriol. 2002, 184, 5733–5745. [Google Scholar] [CrossRef]
- Salis, H.M.; Mirsky, E.A.; Voigt, C.A. Automated design of synthetic ribosome binding sites to control protein expression. Nat. Biotechnol. 2009, 27, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Morgan, J.A.; Ungerer, J.; Wang, B.; Maness, P.-C.; Yu, J. The plasticity of cyanobacterial metabolism supports direct CO2 conversion to ethylene. Nat. Plants 2015, 1, 15053. [Google Scholar] [CrossRef]
- Wang, B.; Eckert, C.; Maness, P.C.; Yu, J. A Genetic Toolbox for Modulating the Expression of Heterologous Genes in the Cyanobacterium Synechocystis sp. PCC 6803. ACS Synth. Biol. 2018, 7, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Kosuri, S.; Goodman, D.B.; Cambray, G.; Mutalik, V.K.; Gao, Y.; Arkin, A.P.; Endy, D.; Church, G.M. Composability of regulatory sequences controlling transcription and translation in Escherichia coli. Proc. Natl. Acad. Sci. USA 2013, 110, 14024–14029. [Google Scholar] [CrossRef] [PubMed]
- Clifton, K.P.; Jones, E.M.; Paudel, S.; Marken, J.P.; Monette, C.E.; Halleran, A.D.; Epp, L.; Saha, M.S. The genetic insulator RiboJ increases expression of insulated genes. J. Biol. Eng. 2018, 12, 23. [Google Scholar] [CrossRef]
- Vlkova, M.; Morampalli, B.R.; Silander, O.K. Efficiency of the synthetic self-splicing RiboJ ribozyme is robust to cis- and trans-changes in genetic background. Microbiologyopen 2021, 10, e1232. [Google Scholar] [CrossRef]
- Buzayan, J.M.; Gerlach, W.L.; Bruening, G. Satellite tobacco ringspot virus RNA: A subset of the RNA sequence is sufficient for autolytic processing. Proc. Natl. Acad. Sci. USA 1986, 83, 8859–8862. [Google Scholar] [CrossRef]
- Lou, C.; Stanton, B.; Chen, Y.J.; Munsky, B.; Voigt, C.A. Ribozyme-based insulator parts buffer synthetic circuits from genetic context. Nat. Biotechnol. 2012, 30, 1137–1142. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.; Xu, H.; Guo, J.; Ma, Q.; Mu, X.; Luo, Y. A method for Absolute Protein Expression Quantity Measurement Employing Insulator RiboJ. Engineering 2018, 4, 881–887. [Google Scholar] [CrossRef]
- Liu, X.; Miao, R.; Lindberg, P.; Lindblad, P. Modular engineering for efficient photosynthetic biosynthesis of 1-butanol from CO2 in cyanobacteria. Energy Environ. Sci. 2019, 12, 2765–2777. [Google Scholar] [CrossRef]
- Mutalik, V.K.; Guimaraes, J.C.; Cambray, G.; Lam, C.; Christoffersen, M.J.; Mai, Q.A.; Tran, A.B.; Paull, M.; Keasling, J.D.; Arkin, A.P.; et al. Precise and reliable gene expression via standard transcription and translation initiation elements. Nat. Methods 2013, 10, 354–360. [Google Scholar] [CrossRef]
- Englund, E.; Shabestary, K.; Hudson, E.P.; Lindberg, P. Systematic overexpression study to find target enzymes enhancing production of terpenes in Synechocystis PCC 6803, using isoprene as a model compound. Metab. Eng. 2018, 49, 164–177. [Google Scholar] [CrossRef]
- Wegelius, A.; Khanna, N.; Esmieu, C.; Barone, G.D.; Pinto, F.; Tamagnini, P.; Berggren, G.; Lindblad, P. Generation of a functional, semisynthetic [FeFe]-hydrogenase in a photosynthetic microorganism. Energy Environ. Sci. 2018, 11, 3163–3167. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Cengic, I.; Cañadas, I.C.; Minton, N.P.; Hudson, E.P. Inducible CRISPR/Cas9 Allows for Multiplexed and Rapidly Segregated Single-Target Genome Editing in Synechocystis Sp. PCC 6803. ACS Synth. Biol. 2022, 11, 3100–3113. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Li, X.; Wang, S.-G.; Xia, P.-F. Base editing for reprogramming cyanobacterium Synechococcus elongatus. Metab. Eng. 2023, 75, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.C.; Korosh, T.C.; Cameron, J.C.; Markley, A.L.; Begemann, M.B.; Pfleger, B.F. CRISPR interference as a titratable, trans-acting regulatory tool for metabolic engineering in the cyanobacterium Synechococcus sp. strain PCC 7002. Metab. Eng. 2016, 38, 170–179. [Google Scholar] [CrossRef]
- Yao, L.; Shabestary, K.; Bjork, S.M.; Asplund-Samuelsson, J.; Joensson, H.N.; Jahn, M.; Hudson, E.P. Pooled CRISPRi screening of the cyanobacterium Synechocystis sp PCC 6803 for enhanced industrial phenotypes. Nat. Commun. 2020, 11, 1666. [Google Scholar] [CrossRef]
- Lai, M.J.; Tsai, J.C.; Lan, E.I. CRISPRi-enhanced direct photosynthetic conversion of carbon dioxide to succinic acid by metabolically engineered cyanobacteria. Bioresour. Technol. 2022, 366, 128131. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarzyk, D.; Cengic, I.; Yao, L.; Hudson, E.P. Diversion of the long-chain acyl-ACP pool in Synechocystis to fatty alcohols through CRISPRi repression of the essential phosphate acyltransferase PlsX. Metab. Eng. 2018, 45, 59–66. [Google Scholar] [CrossRef]
- Knoot, C.J.; Biswas, S.; Pakrasi, H.B. Tunable Repression of Key Photosynthetic Processes Using Cas12a CRISPR Interference in the Fast-Growing Cyanobacterium Synechococcus sp. UTEX 2973. ACS Synth. Biol. 2020, 9, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Woo, H.M. A Logic NAND Gate for Controlling Gene Expression in a Circadian Rhythm in Cyanobacteria. ACS Synth. Biol. 2020, 9, 3210–3216. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.E.; Golden, S.S. Circadian Rhythms in Cyanobacteria. Microbiol. Mol. Biol. Rev. 2015, 79, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Breaker, R.R. The structural and functional diversity of metabolite-binding riboswitches. Annu. Rev. Biochem. 2009, 78, 305–334. [Google Scholar] [CrossRef]
- Apura, P.; Domingues, S.; Viegas, S.C.; Arraiano, C.M. Reprogramming bacteria with RNA regulators. Biochem. Soc. Trans. 2019, 47, 1279–1289. [Google Scholar] [CrossRef]
- Winkler, W.; Nahvi, A.; Breaker, R.R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 2002, 419, 952–956. [Google Scholar] [CrossRef]
- Winkler, W.C.; Cohen-Chalamish, S.; Breaker, R.R. An mRNA structure that controls gene expression by binding FMN. Proc. Natl. Acad. Sci. USA 2002, 99, 15908–15913. [Google Scholar] [CrossRef]
- Garst, A.D.; Edwards, A.L.; Batey, R.T. Riboswitches: Structures and mechanisms. Cold Spring Harb. Perspect. Biol. 2011, 3, a003533. [Google Scholar] [CrossRef]
- Serganov, A.; Nudler, E. A decade of riboswitches. Cell 2013, 152, 17–24. [Google Scholar] [CrossRef]
- Nakahira, Y.; Ogawa, A.; Asano, H.; Oyama, T.; Tozawa, Y. Theophylline-dependent riboswitch as a novel genetic tool for strict regulation of protein expression in Cyanobacterium Synechococcus elongatus PCC 7942. Plant Cell Physiol. 2013, 54, 1724–1735. [Google Scholar] [CrossRef]
- Ma, A.T.; Schmidt, C.M.; Golden, J.W. Regulation of gene expression in diverse cyanobacterial species by using theophylline-responsive riboswitches. Appl. Environ. Microbiol. 2014, 80, 6704–6713. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, R.; Akai, H.; Yoshikawa, H.; Hess, W.R.; Watanabe, S. A tightly inducible riboswitch system in Synechocystis sp. PCC 6803. J. Gen. Appl. Microbiol. 2016, 62, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Higo, A.; Isu, A.; Fukaya, Y.; Hisabori, T. Spatio-Temporal Gene Induction Systems in the Heterocyst-Forming Multicellular Cyanobacterium Anabaena sp. PCC 7120. Plant Cell Physiol. 2018, 59, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.A.; Liu, Z.; Rodionov, D.A.; Li, Z.; Bryant, D.A. Complementation of Cobalamin Auxotrophy in Synechococcus sp. Strain PCC 7002 and Validation of a Putative Cobalamin Riboswitch In Vivo. J. Bacteriol. 2016, 198, 2743–2752. [Google Scholar] [CrossRef]
- Klahn, S.; Bolay, P.; Wright, P.R.; Atilho, R.M.; Brewer, K.I.; Hagemann, M.; Breaker, R.R.; Hess, W.R. A glutamine riboswitch is a key element for the regulation of glutamine synthetase in cyanobacteria. Nucleic Acids Res. 2018, 46, 10082–10094. [Google Scholar] [CrossRef]
- Abe, K.; Sakai, Y.; Nakashima, S.; Araki, M.; Yoshida, W.; Sode, K.; Ikebukuro, K. Design of riboregulators for control of cyanobacterial (Synechocystis) protein expression. Biotechnol. Lett. 2014, 36, 287–294. [Google Scholar] [CrossRef]
- Sakamoto, I.; Abe, K.; Kawai, S.; Tsukakoshi, K.; Sakai, Y.; Sode, K.; Ikebukuro, K. Improving the induction fold of riboregulators for cyanobacteria. RNA Biol. 2018, 15, 353–358. [Google Scholar] [CrossRef]
- Sakai, Y.; Abe, K.; Nakashima, S.; Ellinger, J.J.; Ferri, S.; Sode, K.; Ikebukuro, K. Scaffold-fused riboregulators for enhanced gene activation in Synechocystis sp. PCC 6803. Microbiologyopen 2015, 4, 533–540. [Google Scholar] [CrossRef]
- Ueno, K.; Sakai, Y.; Shono, C.; Sakamoto, I.; Tsukakoshi, K.; Hihara, Y.; Sode, K.; Ikebukuro, K. Applying a riboregulator as a new chromosomal gene regulation tool for higher glycogen production in Synechocystis sp. PCC 6803. Appl. Microbiol. Biotechnol. 2017, 101, 8465–8474. [Google Scholar] [CrossRef]
- To, A.C.; Chu, D.H.; Wang, A.R.; Li, F.C.; Chiu, A.W.; Gao, D.Y.; Choi, C.H.J.; Kong, S.K.; Chan, T.F.; Chan, K.M.; et al. A comprehensive web tool for toehold switch design. Bioinformatics 2018, 34, 2862–2864. [Google Scholar] [CrossRef]
- Chappell, J.; Westbrook, A.; Verosloff, M.; Lucks, J.B. Computational design of small transcription activating RNAs for versatile and dynamic gene regulation. Nat. Commun. 2017, 8, 1051. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Prasad, S. Metabolic engineering of bacteria. Indian J. Microbiol. 2011, 51, 403–409. [Google Scholar] [CrossRef]
- Mund, N.K.; Liu, Y.; Chen, S. Advances in metabolic engineering of cyanobacteria for production of biofuels. Fuel 2022, 322, 124117. [Google Scholar] [CrossRef]
- Aikawa, S.; Joseph, A.; Yamada, R.; Izumi, Y.; Yamagishi, T.; Matsuda, F.; Kawai, H.; Chang, J.-S.; Hasunuma, T.; Kondo, A. Direct conversion of Spirulina to ethanol without pretreatment or enzymatic hydrolysis processes. Energy Environ. Sci. 2013, 6, 1844–1849. [Google Scholar] [CrossRef]
- Harun, R.; Jason, W.S.Y.; Cherrington, T.; Danquah, M.K. Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl. Energy 2011, 88, 3464–3467. [Google Scholar] [CrossRef]
- Vavitsas, K.; Fabris, M.; Vickers, C.E. Terpenoid Metabolic Engineering in Photosynthetic Microorganisms. Genes 2018, 9, 520. [Google Scholar] [CrossRef]
- Satta, A.; Esquirol, L.; Ebert, B.E.; Newman, J.; Peat, T.S.; Plan, M.; Schenk, G.; Vickers, C.E. Molecular characterization of cyanobacterial short-chain prenyltransferases and discovery of a novel GGPP phosphatase. FEBS J. 2022, 289, 6672–6693. [Google Scholar] [CrossRef]
- Krishnan, A.; McNeil, B.A.; Stuart, D.T. Biosynthesis of Fatty Alcohols in Engineered Microbial Cell Factories: Advances and Limitations. Front. Bioeng. Biotechnol. 2020, 8, 610936. [Google Scholar] [CrossRef] [PubMed]
- Slocombe, S.P.; Zhang, Q.; Ross, M.; Anderson, A.; Thomas, N.J.; Lapresa, A.; Rad-Menendez, C.; Campbell, C.N.; Black, K.D.; Stanley, M.S.; et al. Unlocking nature’s treasure-chest: Screening for oleaginous algae. Sci. Rep. 2015, 5, 9844. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, J.; Curtiss, R., 3rd. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 6899–6904. [Google Scholar] [CrossRef]
- Kato, A.; Takatani, N.; Ikeda, K.; Maeda, S.I.; Omata, T. Removal of the product from the culture medium strongly enhances free fatty acid production by genetically engineered Synechococcus elongatus. Biotechnol. Biofuels 2017, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Qi, F.; Tan, X.; Lu, X. Improved production of fatty alcohols in cyanobacteria by metabolic engineering. Biotechnol. Biofuels 2014, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Santos-Merino, M.; Garcillan-Barcia, M.P.; de la Cruz, F. Engineering the fatty acid synthesis pathway in Synechococcus elongatus PCC 7942 improves omega-3 fatty acid production. Biotechnol. Biofuels 2018, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B.; Parsonage, D.; Sergeant, S.; Miller, L.R.; Lee, J.; Furdui, C.M.; Chilton, F.H. Acyl-lipid desaturases and Vipp1 cooperate in cyanobacteria to produce novel omega-3 PUFA-containing glycolipids. Biotechnol. Biofuels 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Ducat, D.C.; Avelar-Rivas, J.A.; Way, J.C.; Silver, P.A. Rerouting carbon flux to enhance photosynthetic productivity. Appl. Environ. Microbiol. 2012, 78, 2660–2668. [Google Scholar] [CrossRef]
- Lin, P.C.; Zhang, F.; Pakrasi, H.B. Enhanced production of sucrose in the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Sci. Rep. 2020, 10, 390. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, W.; Lu, X. Engineering cyanobacteria as cell factories for direct trehalose production from CO(2). Metab. Eng. 2020, 62, 161–171. [Google Scholar] [CrossRef]
- Mota, R.; Guimaraes, R.; Buttel, Z.; Rossi, F.; Colica, G.; Silva, C.J.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R.; et al. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef]
- Mota, R.; Vidal, R.; Pandeirada, C.; Flores, C.; Adessi, A.; De Philippis, R.; Nunes, C.; Coimbra, M.A.; Tamagnini, P. Cyanoflan: A cyanobacterial sulfated carbohydrate polymer with emulsifying properties. Carbohydr. Polym. 2020, 229, 115525. [Google Scholar] [CrossRef]
- Li, T.; Li, C.T.; Butler, K.; Hays, S.G.; Guarnieri, M.T.; Oyler, G.A.; Betenbaugh, M.J. Mimicking lichens: Incorporation of yeast strains together with sucrose-secreting cyanobacteria improves survival, growth, ROS removal and lipid production in a stable mutualistic co-culture production platform. Biotechnol. Biofuels 2017, 10, 55. [Google Scholar] [CrossRef]
- Chaves, J.E.; Melis, A. Biotechnology of cyanobacterial isoprene production. Appl. Microbiol. Biotechnol. 2018, 102, 6451–6458. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Zhang, F.; Pakrasi, H.B. Enhanced limonene production in a fast-growing cyanobacterium through combinatorial metabolic engineering. Metab Eng. Commun. 2021, 12, e00164. [Google Scholar] [CrossRef]
- Lin, P.C.; Saha, R.; Zhang, F.; Pakrasi, H.B. Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocysti s sp. PCC 6803. Sci. Rep. 2017, 7, 17503. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, C.; Gu, L.; Zhou, R. Engineering cyanobacteria for the production of a cyclic hydrocarbon fuel from CO2 and H2O. Green Chem. 2014, 16, 3175–3185. [Google Scholar] [CrossRef]
- Davies, F.K.; Work, V.H.; Beliaev, A.S.; Posewitz, M.C. Engineering Limonene and Bisabolene Production in Wild Type and a Glycogen-Deficient Mutant of Synechococcus sp. PCC 7002. Front. Bioeng. Biotechnol. 2014, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Matsudaira, A.; Hoshino, Y.; Uesaka, K.; Takatani, N.; Omata, T.; Usuda, Y. Production of glutamate and stereospecific flavors, (S)-linalool and (+)-valencene, by Synechocystis sp. PCC6803. J. Biosci. Bioeng. 2020, 130, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Formighieri, C.; Melis, A. Sustainable heterologous production of terpene hydrocarbons in cyanobacteria. Photosynth. Res. 2016, 130, 123–135. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; Lindberg, P. Metabolic engineering of Synechocystis sp. PCC 6803 for improved bisabolene production. Metab Eng. Commun. 2021, 12, e00159. [Google Scholar] [CrossRef]
- Halfmann, C.; Gu, L.; Gibbons, W.; Zhou, R. Genetically engineering cyanobacteria to convert CO(2), water and light into the long-chain hydrocarbon farnesene. Appl. Microbiol. Biotechnol. 2014, 98, 9869–9877. [Google Scholar] [CrossRef]
- Pattharaprachayakul, N.; Lee, H.J.; Incharoensakdi, A.; Woo, H.M. Evolutionary Engineering of Cyanobacteria to Enhance the Production of alpha-Farnesene from CO(2). J. Agric. Food Chem. 2019, 67, 13658–13664. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, H.J.; Choi, J.; Kim, J.; Sim, S.J.; Um, Y.; Kim, Y.; Lee, T.S.; Keasling, J.D.; Woo, H.M. Photosynthetic conversion of CO2 to farnesyl diphosphate-derived phytochemicals (amorpha-4,11-diene and squalene) by engineered cyanobacteria. Biotechnol. Biofuels 2016, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Reinsvold, R.E.; Jinkerson, R.E.; Radakovits, R.; Posewitz, M.C.; Basu, C. The production of the sesquiterpene beta-caryophyllene in a transgenic strain of the cyanobacterium Synechocystis. J. Plant Physiol. 2011, 168, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Dienst, D.; Wichmann, J.; Mantovani, O.; Rodrigues, J.S.; Lindberg, P. High density cultivation for efficient sesquiterpenoid biosynthesis in Synechocystis sp. PCC 6803. Sci. Rep. 2020, 10, 5932. [Google Scholar] [CrossRef]
- Vavitsas, K.; Rue, E.O.; Stefansdottir, L.K.; Gnanasekaran, T.; Blennow, A.; Crocoll, C.; Gudmundsson, S.; Jensen, P.E. Responses of Synechocystis sp. PCC 6803 to heterologous biosynthetic pathways. Microb. Cell Fact. 2017, 16, 140. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. Heterologous synthesis of geranyllinalool, a diterpenol plant product, in the cyanobacterium Synechocystis. Appl. Microbiol. Biotechnol. 2017, 101, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Woo, H.M. CRISPRi-dCas12a: A dCas12a-Mediated CRISPR Interference for Repression of Multiple Genes and Metabolic Engineering in Cyanobacteria. ACS Synth. Biol. 2020, 9, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, B.; Englund, E.; Nolte, N.; Lindberg, P. Introduction of a green algal squalene synthase enhances squalene accumulation in a strain of Synechocystis sp. PCC 6803. Metab Eng. Commun. 2020, 10, e00125. [Google Scholar] [CrossRef]
- Taylor, G.M.; Hitchcock, A.; Heap, J.T. Combinatorial assembly platform enabling engineering of genetically stable metabolic pathways in cyanobacteria. Nucleic Acids Res. 2021, 49, e123. [Google Scholar] [CrossRef]
- Englund, E.; Pattanaik, B.; Ubhayasekera, S.J.; Stensjo, K.; Bergquist, J.; Lindberg, P. Production of squalene in Synechocystis sp. PCC 6803. PLoS ONE 2014, 9, e90270. [Google Scholar] [CrossRef]
- Ershov, Y.V.; Gantt, R.R.; Cunningham, F.X., Jr.; Gantt, E. Isoprenoid biosynthesis in Synechocystis sp. strain PCC6803 is stimulated by compounds of the pentose phosphate cycle but not by pyruvate or deoxyxylulose-5-phosphate. J. Bacteriol. 2002, 184, 5045–5051. [Google Scholar] [CrossRef]
- Poliquin, K.; Ershov, Y.V.; Cunningham, F.X., Jr.; Woreta, T.T.; Gantt, R.R.; Gantt, E. Inactivation of sll1556 in Synechocystis strain PCC 6803 impairs isoprenoid biosynthesis from pentose phosphate cycle substrates in vitro. J. Bacteriol. 2004, 186, 4685–4693. [Google Scholar] [CrossRef]
- Bentley, F.K.; Zurbriggen, A.; Melis, A. Heterologous expression of the mevalonic acid pathway in cyanobacteria enhances endogenous carbon partitioning to isoprene. Mol. Plant 2014, 7, 71–86. [Google Scholar] [CrossRef]
- Kerfeld, C.A.; Aussignargues, C.; Zarzycki, J.; Cai, F.; Sutter, M. Bacterial microcompartments. Nat. Rev. Microbiol. 2018, 16, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Murmu, G.; Mukherjee, K.; Saha, S.; Maity, D. A comprehensive overview of nanotechnology in sustainable agriculture. J. Biotechnol. 2022, 355, 21–41. [Google Scholar] [CrossRef]
- Jiang, M.; Song, Y.; Kanwar, M.K.; Ahammed, G.J.; Shao, S.; Zhou, J. Phytonanotechnology applications in modern agriculture. J. Nanobiotechnol. 2021, 19, 430. [Google Scholar] [CrossRef] [PubMed]
- Niklowitz, W.; Drews, G. Beiträge zur Cytologie der Blaualgen. Arch. Für Mikrobiol. 1957, 27, 150–165. [Google Scholar] [CrossRef]
- Occhialini, A.; Lin, M.T.; Andralojc, P.J.; Hanson, M.R.; Parry, M.A. Transgenic tobacco plants with improved cyanobacterial Rubisco expression but no extra assembly factors grow at near wild-type rates if provided with elevated CO2. Plant J. 2016, 85, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.R.; Lin, M.T.; Carmo-Silva, A.E.; Parry, M.A. Towards engineering carboxysomes into C3 plants. Plant J. 2016, 87, 38–50. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, F.; Faulkner, M.; Jiang, Q.; Dykes, G.F.; Yang, M.; Liu, L.N. Engineering and Modulating Functional Cyanobacterial CO(2)-Fixing Organelles. Front. Plant Sci. 2018, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, X.; Aigner, H.; Bracher, A.; Nguyen, N.D.; Hee, W.Y.; Long, B.M.; Price, G.D.; Hartl, F.U.; Hayer-Hartl, M. Rubisco condensate formation by CcmM in beta-carboxysome biogenesis. Nature 2019, 566, 131–135. [Google Scholar] [CrossRef]
- Sutter, M.; Boehringer, D.; Gutmann, S.; Gunther, S.; Prangishvili, D.; Loessner, M.J.; Stetter, K.O.; Weber-Ban, E.; Ban, N. Structural basis of enzyme encapsulation into a bacterial nanocompartment. Nat. Struct. Mol. Biol. 2008, 15, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Tracey, J.C.; Coronado, M.; Giessen, T.W.; Lau, M.C.Y.; Silver, P.A.; Ward, B.B. The Discovery of Twenty-Eight New Encapsulin Sequences, Including Three in Anammox Bacteria. Sci. Rep. 2019, 9, 20122. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.J.; LaFrance, B.; Phillips, N.R.; Radford, D.R.; Oltrogge, L.M.; Valentin-Alvarado, L.E.; Bischoff, A.J.; Nogales, E.; Savage, D.F. Discovery and characterization of a novel family of prokaryotic nanocompartments involved in sulfur metabolism. Elife 2021, 10, e59288. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.G.; Yan, L.L.W.; Silver, P.A.; Ducat, D.C. Synthetic photosynthetic consortia define interactions leading to robustness and photoproduction. J. Biol. Eng. 2017, 11, 4. [Google Scholar] [CrossRef]
- Arias, D.M.; Garcia, J.; Uggetti, E. Production of polymers by cyanobacteria grown in wastewater: Current status, challenges and future perspectives. N. Biotechnol. 2020, 55, 46–57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).