Comparative Genomics of Legionella pneumophila Isolates from the West Bank and Germany Support Molecular Epidemiology of Legionnaires’ Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of L. pneumophila Strains and Reference Genomes
2.2. Illumina HiSeq and Pacific Biosciences Genome Sequencing, Assembly and Annotation
2.3. Genome Analyses
2.4. Detection and Identification of Plasmids and Phages
3. Results
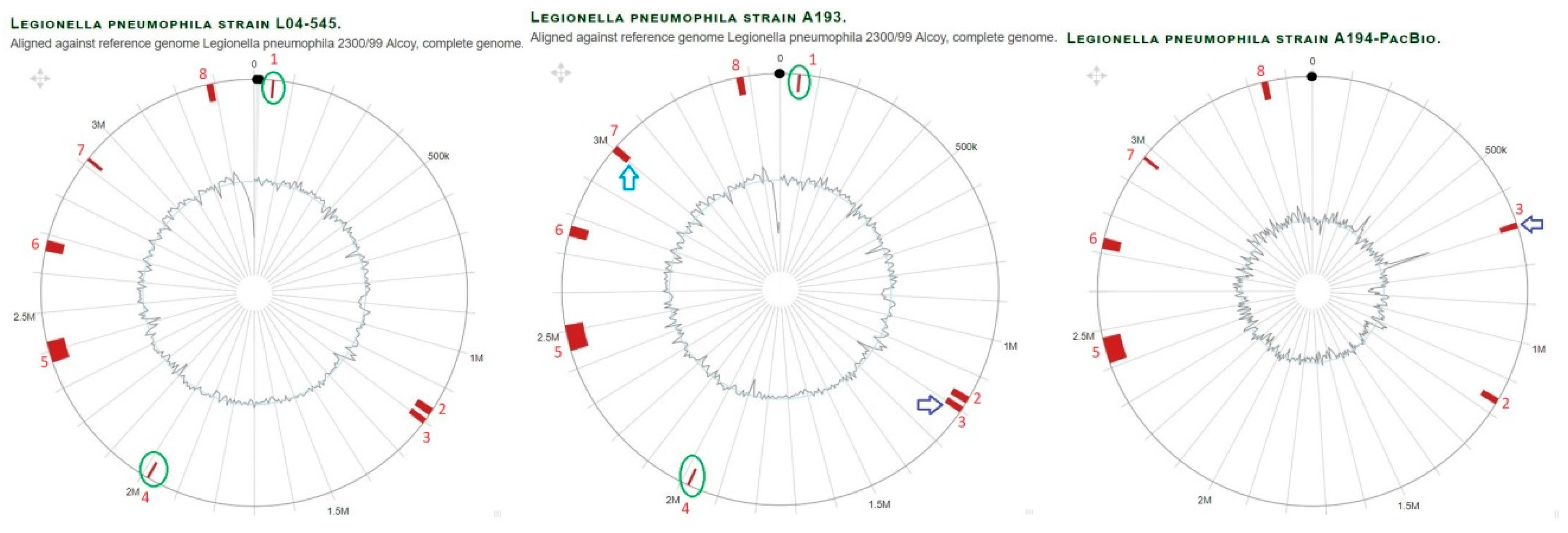
3.1. General Features of Genomes from L. pneumophila Isolates
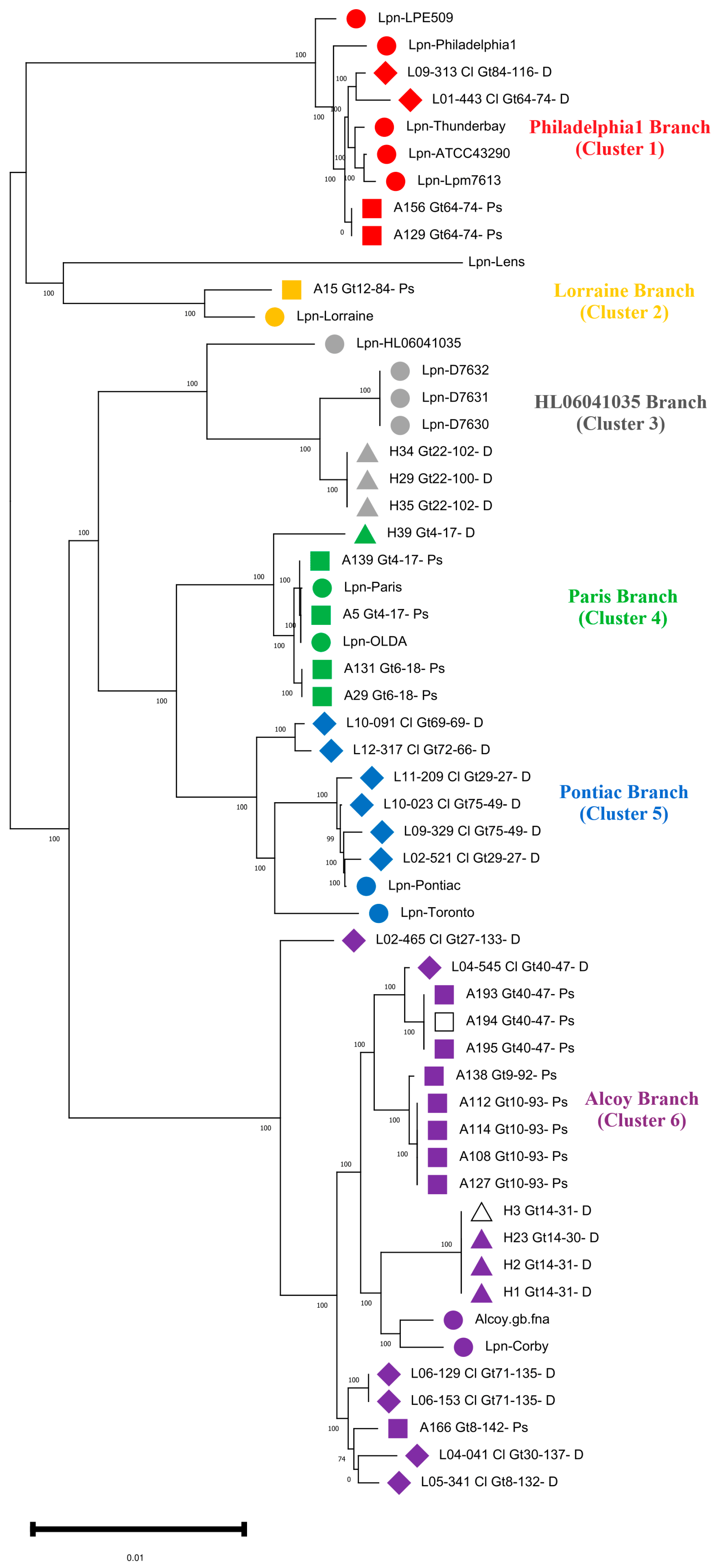
3.2. Phylogenomic and SNP Analysis of L. pneumophila Isolates
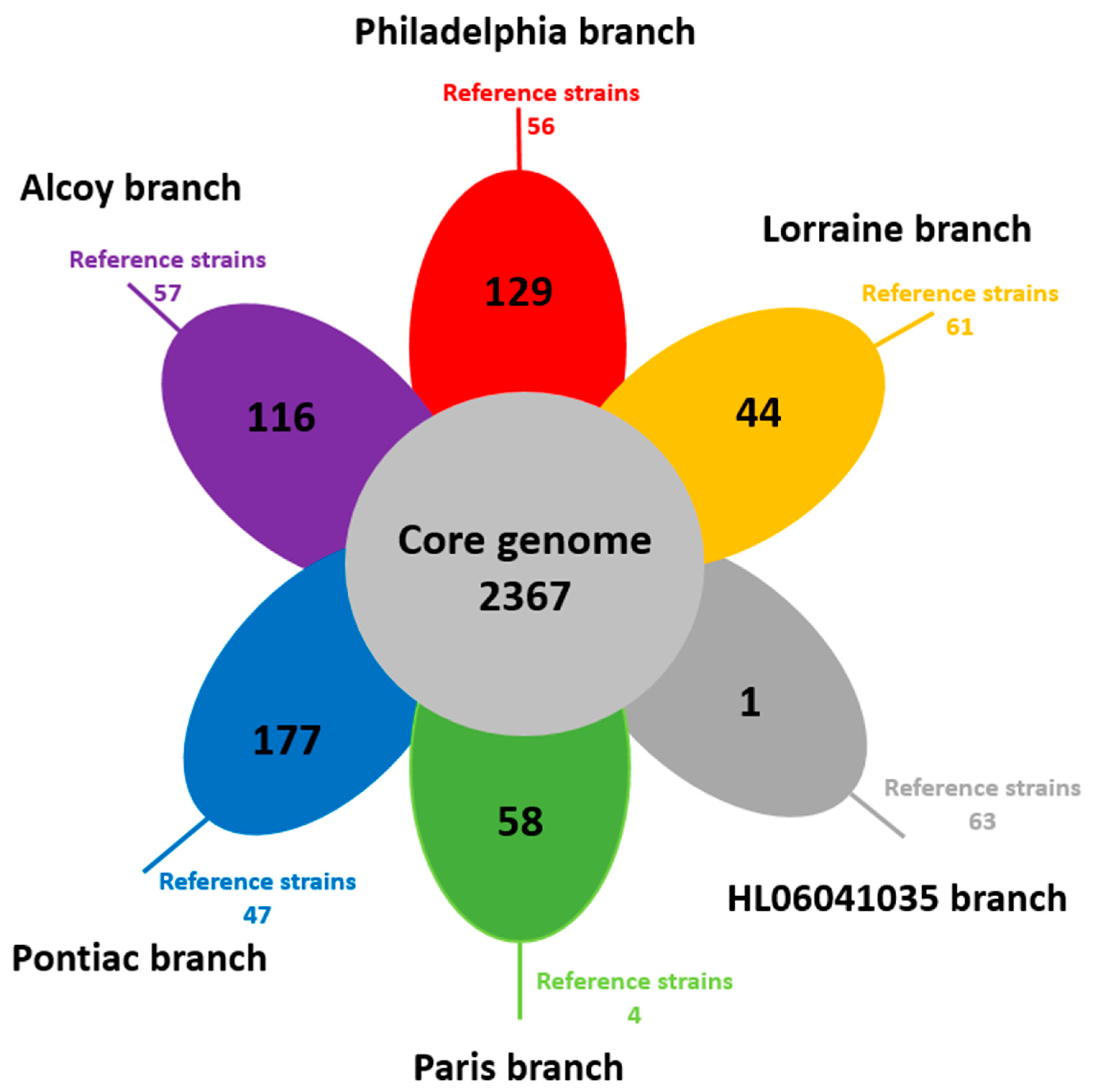
3.3. Identification of Orthologous Genes in L. pneumophila Isolates
3.4. Comparison of MLVA Genotyping and Whole Genome SNP Analysis
3.5. Detailed Analysis of Individual SNPs
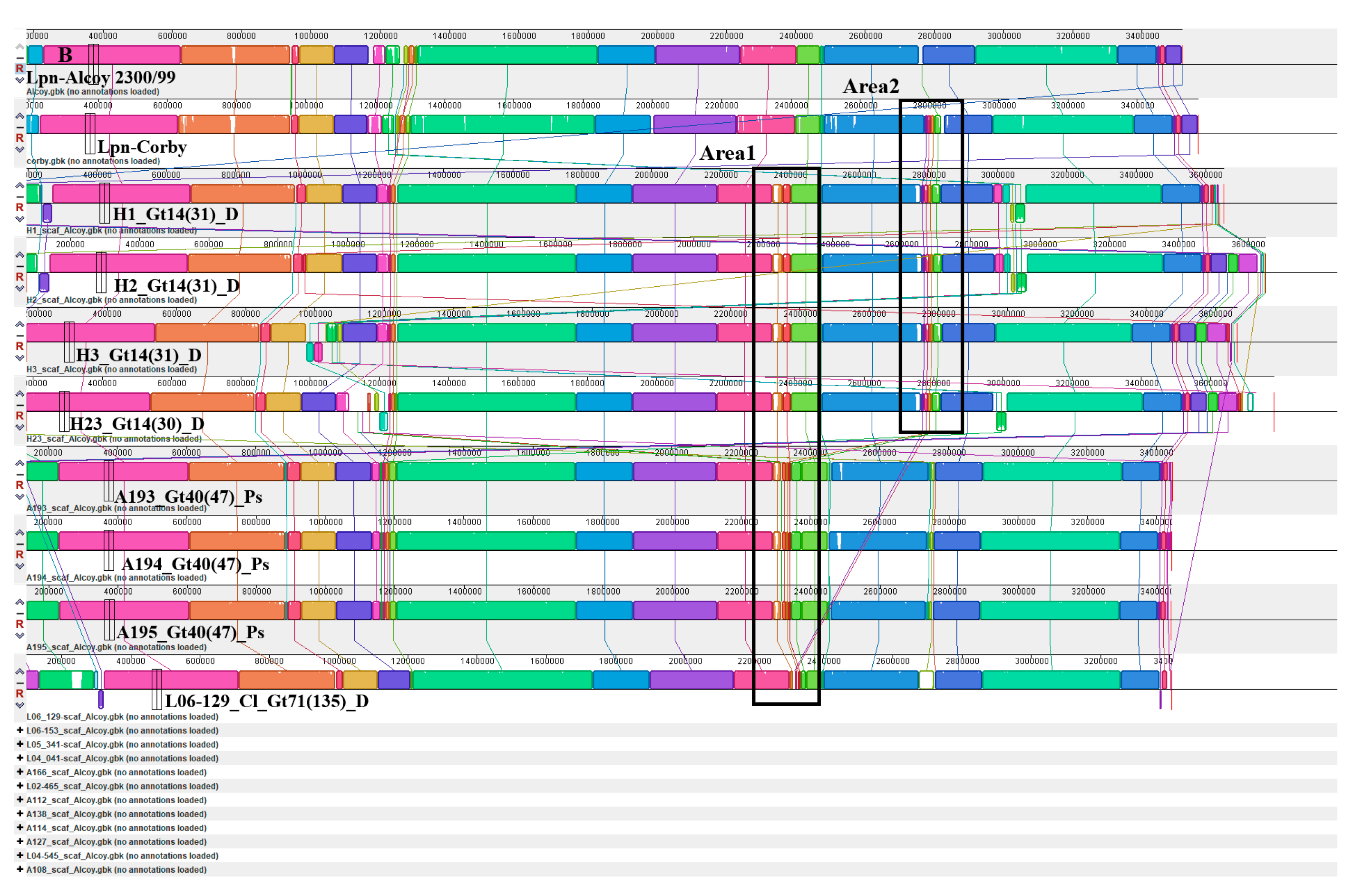
3.6. Identification of Mobile Elements in the Alcoy Branch
3.7. Identification and Comparison of Genomic Islands in L. pneumophila Isolates
3.8. L. pneumophila Isolates Encode Proteins from Different Eukaryotic Domains
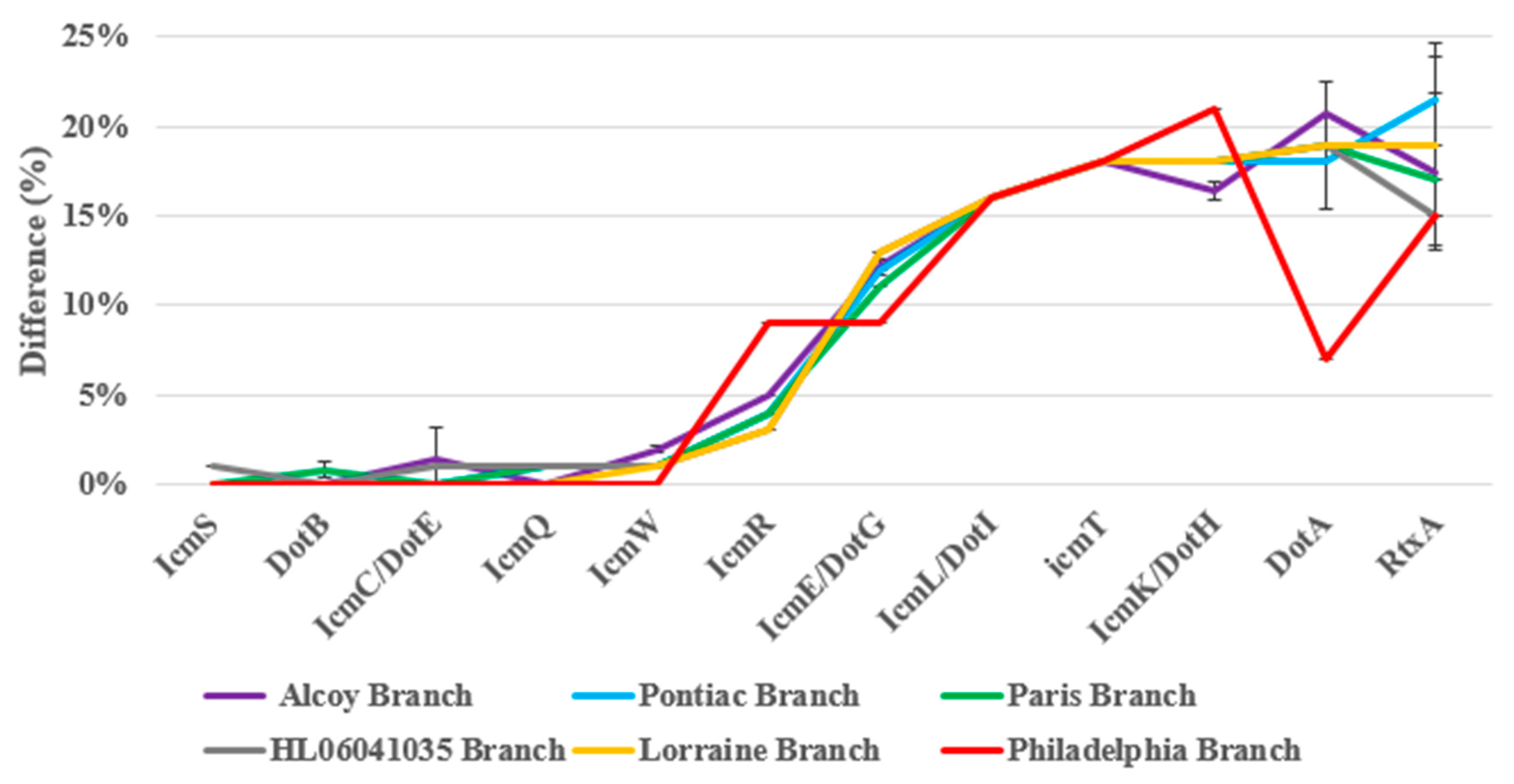
3.9. Identification of Pore-Forming Genes Mediating Cytotoxicity in L. pneumophila Isolates
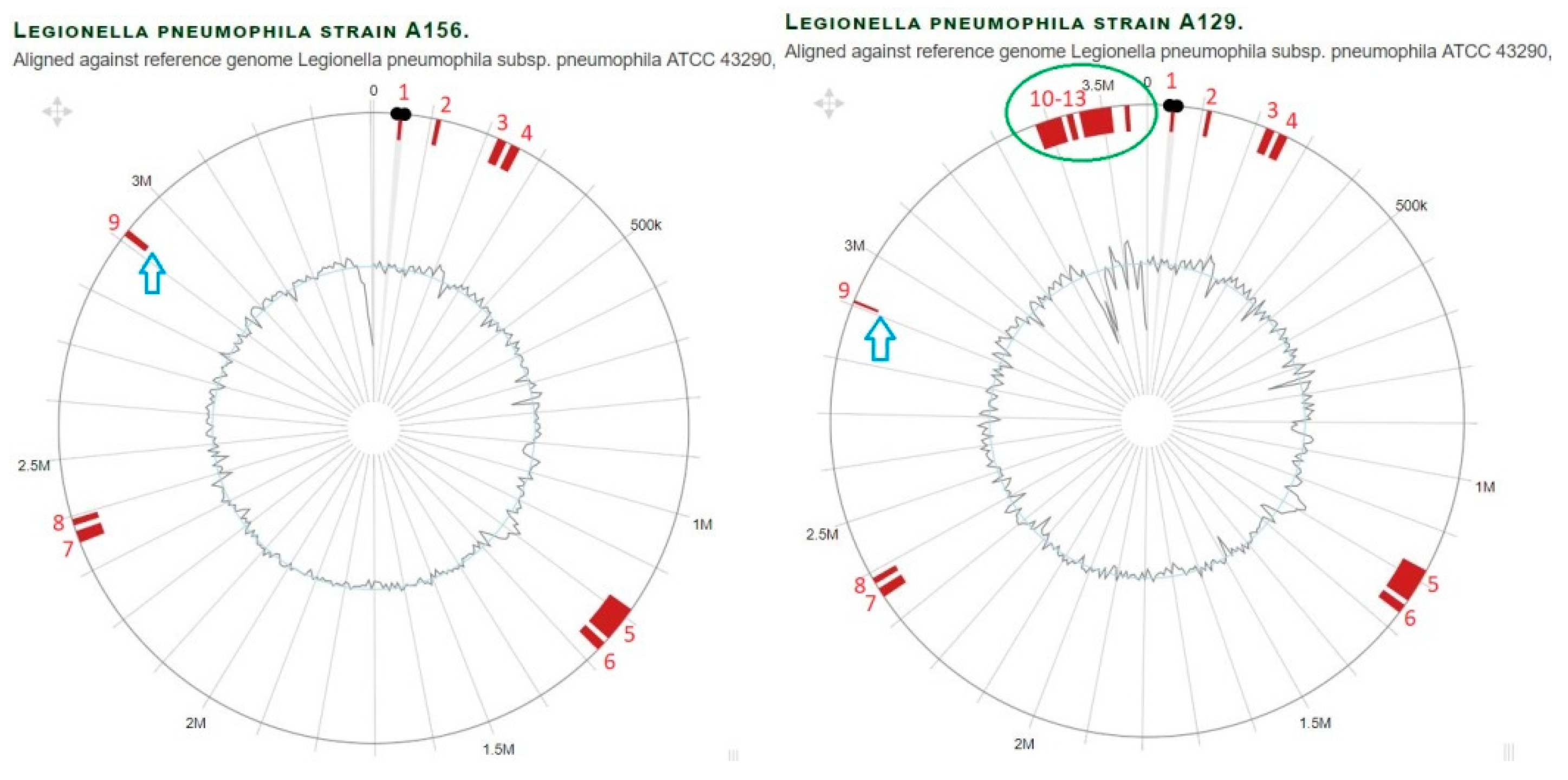
3.10. Identification of Plasmids and Phages in L. pneumophila Isolates
4. Discussion
4.1. Comparison of Taxonomic Resolution of MLVA Genotyping and SNP Analysis
4.2. Analysis of Pore-Forming and Virulence Genes
4.3. Evolutionary Scenarios Explaining Levels of Diversity Observed in Virulence and Other Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef] [PubMed]
- Fry, N.K.; Alexiou-Daniel, S.; Bangsborg, J.M.; Bernander, S.; Pastoris, M.C.; Etienne, J.; Forsblom, B.; Gaia, V.; Helbig, J.H.; Lindsay, D.; et al. A multicenter evaluation of genotypic methods for the epidemiologic typing of Legionella pneumophila serogroup 1: Results of a pan-European study. Clin. Microbiol. Infect. 1999, 5, 462–477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luck, C.; Fry, N.K.; Helbig, J.H.; Jarraud, S.; Harrison, T.G. Typing methods for legionella. Methods Mol. Biol. 2013, 954, 119–148. [Google Scholar] [PubMed]
- Pourcel, C.; Visca, P.; Afshar, B.; D’Arezzo, S.; Vergnaud, G.; Fry, N.K. Identification of variable-number tandem-repeat (VNTR) sequences in Legionella pneumophila and development of an optimized multiple-locus VNTR analysis typing scheme. J. Clin. Microbiol. 2007, 45, 1190–1199. [Google Scholar] [CrossRef]
- Zayed, A.; Butmeh, S.; Pecellin, M.; Salah, A.; Alalam, H.; Steinert, M.; Höfle, M.; Bitar, D.; Brettar, I. Biogeography and Environmental Drivers of Legionella pneumophila Abundance and Genotype Composition Across the West Bank: Relevance of a Genotype-Based Ecology for Understanding Legionella Occurrence. Pathogens 2020, 9, 1012. [Google Scholar] [CrossRef]
- Sobral, D.; Le Cann, P.; Gerard, A.; Jarraud, S.; Lebeau, B.; Loisy-Hamon, F.; Vergnaud, G.; Pourcel, C. High-throughput typing method to identify a non-outbreak-involved Legionella pneumophila strain colonizing the entire water supply system in the town of Rennes, France. Appl. Environ. Microbiol. 2011, 77, 6899–6907. [Google Scholar] [CrossRef]
- Visca, P.; D’Arezzo, S.; Ramisse, F.; Gelfand, Y.; Benson, G.; Vergnaud, G.; Fry, N.K.; Pourcel, C. Investigation of the population structure of Legionella pneumophila by analysis of tandem repeat copy number and internal sequence variation. Microbiology 2011, 157 Pt 9, 2582–2594. [Google Scholar] [CrossRef][Green Version]
- Underwood, A.P.; Jones, G.; Mentasti, M.; Fry, N.K.; Harrison, T.G. Comparison of the Legionella pneumophila population structure as determined by sequence-based typing and whole genome sequencing. BMC Microbiol. 2013, 13, 302. [Google Scholar] [CrossRef]
- Bosch, T.; Euser, S.M.; Landman, F.; Bruin, J.P.; Ijzerman, E.P.; Boer, J.W.D.; Schouls, L.M. Whole-Genome Mapping as a Novel High-Resolution Typing Tool for Legionella pneumophila. J. Clin. Microbiol. 2015, 53, 3234–3238. [Google Scholar] [CrossRef]
- Chien, M.; Morozova, I.; Shi, S.; Sheng, H.; Chen, J.; Gomez, S.M.; Asamani, G.; Hill, K.; Nuara, J.; Feder, M.; et al. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 2004, 305, 1966–1968. [Google Scholar] [CrossRef]
- Cazalet, C.; Rusniok, C.; Brüggemann, H.; Zidane, N.; Magnier, A.; Ma, L.; Tichit, M.; Jarraud, S.; Bouchier, C.; Vandenesch, F.; et al. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 2004, 36, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Valero, L.; Buchrieser, C. Genome dynamics in Legionella: The basis of versatility and adaptation to intracellular replication. Cold Spring Harb. Perspect. Med. 2013, 3, a009993. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Harrison, T.G.; Koser, C.U.; Ellington, M.J.; Smith, G.P.; Parkhill, J.; Peacock, S.J.; Bentley, S.D.; Török, M.E. A pilot study of rapid whole-genome sequencing for the investigation of a Legionella outbreak. BMJ Open 2013, 3, e002175. [Google Scholar] [CrossRef]
- Sánchez-Busó, L.; Guiral, S.; Crespi, S.; Moya, V.; Camaró, M.L.; Olmos, M.P.; Adrián, F.; Morera, V.; González-Morán, F.; Vanaclocha, H.; et al. Genomic Investigation of a Legionellosis Outbreak in a Persistently Colonized Hotel. Front. Microbiol. 2015, 6, 1556. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Rusniok, C.; Mentasti, M.; Gomez-Valero, L.; Harris, S.R.; Lechat, P.; Lees, J.; Ginevra, C.; Glaser, P.; Ma, L.; et al. Multiple major disease-associated clones of Legionella pneumophila have emerged recently and independently. Genome Res. 2016, 26, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Zhang, W.; Liu, W.; Zhou, H.; Ren, H.; Shao, Z.; Lan, R.; Xu, J. Population structure and minimum core genome typing of Legionella pneumophila. Sci. Rep. 2016, 6, 21356. [Google Scholar] [CrossRef][Green Version]
- Gaia, V.; Fry, N.K.; Harrison, T.G.; Peduzzi, R. Sequence-based typing of Legionella pneumophila serogroup 1 offers the potential for true portability in legionellosis outbreak investigation. J. Clin. Microbiol. 2003, 41, 2932–2939. [Google Scholar] [CrossRef]
- Pecellín, M. Structure and Virulence of Legionella pneumophila Populations from Freshwater Systems in Germany and Middle East. Ph.D. Thesis, Technische Universität Braunschweig, Braunschweig, Germany, 2016. [Google Scholar]
- Zayed, A.; Pecellin, M.; Salah, A.; Alalam, H.; Butmeh, S.; Steinert, M.; Lesnik, R.; Brettar, I.; Höfle, M.; Bitar, D. Characterization of Legionella pneumophila Populations by Multilocus Variable Number of Tandem Repeats (MLVA) Genotyping from Drinking Water and Biofilm in Hospitals from Different Regions of the West Bank. Pathogens 2020, 9, 862. [Google Scholar] [CrossRef]
- Zayed, A.R.; Pecellin, M.; Jaber, L.; Butmeh, S.; Bahader, S.A.; Steinert, M.; Höfle, M.G.; Brettar, I.; Bitar, D.M. Cytotoxicity, Intracellular Replication, and Contact-Dependent Pore Formation of Genotyped Environmental Legionella pneumophila Isolates from Hospital Water Systems in the West Bank, Palestine. Pathogens 2021, 10, 417. [Google Scholar] [CrossRef]
- Zayed, A.R.; Bunk, B.; Jaber, L.; Abu-Teer, H.; Ali, M.; Steinert, M.; Höfle, M.G.; Brettar, I.; Bitar, D.M. Whole-genome sequencing of the clinical isolate of Legionella pneumophila ALAW1 from the West Bank allows high-resolution typing and determination of pathogenicity mechanisms. Eur. Clin. Respir. J. 2023, 10, 2168346. [Google Scholar] [CrossRef]
- Steinert, M.; Heuner, K.; Buchrieser, C.; Albert-Weissenberger, C.; Glockner, G. Legionella pathogenicity: Genome structure, regulatory networks and the host cell response. Int. J. Med. Microbiol. 2007, 297, 577–587. [Google Scholar] [CrossRef]
- D’Auria, G.; Jimenez-Hernandez, N.; Peris-Bondia, F.; Moya, A.; Latorre, A. Legionella pneumophila pangenome reveals strain-specific virulence factors. BMC Genom. 2010, 11, 181. [Google Scholar] [CrossRef]
- Khan, M.A.; Knox, N.; Prashar, A.; Alexander, D.; Abdel-Nour, M.; Duncan, C.; Tang, P.; Amatullah, H.; Dos Santos, C.C.; Tijet, N.; et al. Comparative Genomics Reveal That Host-Innate Immune Responses Influence the Clinical Prevalence of Legionella pneumophila Serogroups. PLoS ONE 2013, 8, e67298. [Google Scholar] [CrossRef] [PubMed]
- Mercante, J.W.; Morrison, S.S.; Raphael, B.H.; Winchell, J.M. Complete Genome Sequences of the Historical Legionella pneumophila Strains OLDA and Pontiac. Genome Announc. 2016, 4, e00866-16. [Google Scholar] [CrossRef] [PubMed]
- Amaro, F.; Gilbert, J.A.; Owens, S.; Trimble, W.; Shuman, H.A. Whole-genome sequence of the human pathogen Legionella pneumophila serogroup 12 strain 570-CO-H. J. Bacteriol. 2012, 194, 1613–1614. [Google Scholar] [CrossRef]
- Ma, J.; He, Y.; Hu, B.; Luo, Z.Q. Genome Sequence of an Environmental Isolate of the Bacterial Pathogen Legionella pneumophila. Genome Announc. 2013, 1, e00320-13. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef]
- Quail, M.A.; Smith, M.; Coupland, P.; Otto, T.D.; Harris, S.R.; Connor, T.R.; Bertoni, A.; Swerdlow, H.P.; Gu, Y. A tale of three next generation sequencing platforms: Comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genom. 2012, 13, 341. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Goker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Hahnke, R.L.; Petersen, J.; Scheuner, C.; Michael, V.; Fiebig, A.; Rohde, C.; Rohde, M.; Fartmann, B.; Goodwin, L.A.; et al. Complete genome sequence of DSM 30083(T), the type strain (U5/41(T)) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand. Genom. Sci. 2014, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef]
- Darling, A.C.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef]
- Darling, A.E.; Treangen, T.J.; Messeguer, X.; Perna, N.T. Analyzing patterns of microbial evolution using the mauve genome alignment system. Methods Mol. Biol. 2007, 396, 135–152. [Google Scholar] [PubMed]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Simon Fraser University Research Computing Group; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef]
- Lechner, M.; Findeiss, S.; Steiner, L.; Marz, M.; Stadler, P.F.; Prohaska, S.J. Proteinortho: Detection of (co-)orthologs in large-scale analysis. BMC Bioinform. 2011, 12, 124. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Gomez-Valero, L.; Rusniok, C.; Jarraud, S.; Vacherie, B.; Rouy, Z.; Barbe, V.; Medigue, C.; Etienne, J.; Buchrieser, C. Extensive recombination events and horizontal gene transfer shaped the Legionella pneumophila genomes. BMC Genom. 2011, 12, 536. [Google Scholar] [CrossRef]
- David, S.; Mentasti, M.; Tewolde, R.; Aslett, M.; Harris, S.R.; Afshar, B.; Underwood, A.; Fry, N.K.; Parkhill, J.; Harrison, T.G. Evaluation of an Optimal Epidemiological Typing Scheme for Legionella pneumophila with Whole-Genome Sequence Data Using Validation Guidelines. J. Clin. Microbiol. 2016, 54, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Petzold, M.; Prior, K.; Moran-Gilad, J.; Harmsen, D.; Luck, C. Epidemiological information is key when interpreting whole genome sequence data—Lessons learned from a large Legionella pneumophila outbreak in Warstein, Germany, 2013. Eurosurveillance 2017, 22, 17-00137. [Google Scholar] [CrossRef]
- Segal, G.; Shuman, H.A. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect. Immun. 1997, 65, 5057–5066. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.H.; Isberg, R.R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 1993, 7, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Zink, S.D.; Pedersen, L.; Cianciotto, N.P.; Abu-Kwaik, Y. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 2002, 70, 1657–1663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Segal, G.; Shuman, H.A. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 1999, 67, 2117–2124. [Google Scholar] [CrossRef]
- Cirillo, S.L.; Bermudez, L.E.; El-Etr, S.H.; Duhamel, G.E.; Cirillo, J.D. Legionella pneumophila entry gene rtxA is involved in virulence. Infect. Immun. 2001, 69, 508–517. [Google Scholar] [CrossRef]
- Broich, M.; Rydzewski, K.; McNealy, T.L.; Marre, R.; Flieger, A. The global regulatory proteins LetA and RpoS control phospholipase A, lysophospholipase A, acyltransferase, and other hydrolytic activities of Legionella pneumophila JR32. J. Bacteriol. 2006, 188, 1218–1226. [Google Scholar] [CrossRef]
- Molmeret, M.; Alli, O.A.; Zink, S.; Flieger, A.; Cianciotto, N.P.; Kwaik, Y.A. icmT is essential for pore formation-mediated egress of Legionella pneumophila from mammalian and protozoan cells. Infect. Immun. 2002, 70, 69–78. [Google Scholar] [CrossRef]
- Bitar, D.M.; Molmeret, M.; Kwaik, Y.A. Structure-function analysis of the C-terminus of IcmT of Legionella pneumophila in pore formation-mediated egress from macrophages. FEMS Microbiol. Lett. 2005, 242, 177–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Molmeret, M.; Bitar, D.M.; Han, L.; Kwaik, Y.A. Cell biology of the intracellular infection by Legionella pneumophila. Microbes Infect. 2004, 6, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.E.; Vogel, J.P.; Andrews, H.L.; Isberg, R.R. Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol. 1998, 27, 323–336. [Google Scholar] [CrossRef]
- Krysinska, M.; Baranowski, B.; Deszcz, B.; Pawlowski, K.; Gradowski, M. Pan-kinome of Legionella expanded by a bioinformatics survey. Sci. Rep. 2022, 12, 21782. [Google Scholar] [CrossRef]
- Moran-Gilad, J.; Prior, K.; Yakunin, E.; Harrison, T.G.; Underwood, A.; Lazarovitch, T.; Valinsky, L.; Lück, C.; Krux, F.; Agmon, V.; et al. Design and application of a core genome multilocus sequence typing scheme for investigation of Legionnaires’ disease incidents. Eurosurveillance 2015, 20, 21186. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.M.; Doyle, C.J.; Jennison, A.V. Real-time investigation of a Legionella pneumophila outbreak using whole genome sequencing. Epidemiol. Infect. 2014, 142, 2347–2351. [Google Scholar] [CrossRef]
- Mercante, J.W.; Morrison, S.S.; Desai, H.P.; Raphael, B.H.; Winchell, J.M. Genomic Analysis Reveals Novel Diversity among the 1976 Philadelphia Legionnaires’ Disease Outbreak Isolates and Additional ST36 Strains. PLoS ONE 2016, 11, e0164074. [Google Scholar] [CrossRef]
- Khodr, A.; Kay, E.; Gomez-Valero, L.; Ginevra, C.; Doublet, P.; Buchrieser, C.; Jarraud, S. Molecular epidemiology, phylogeny and evolution of Legionella. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 43, 108–122. [Google Scholar] [CrossRef]
- Morozova, I.; Qu, X.; Shi, S.; Asamani, G.; Greenberg, J.E.; Shuman, H.A.; Russo, J.J. Comparative sequence analysis of the icm/dot genes in Legionella. Plasmid 2004, 51, 127–147. [Google Scholar] [CrossRef]
- Gomez-Valero, L.; Rusniok, C.; Cazalet, C.; Buchrieser, C. Comparative and functional genomics of legionella identified eukaryotic like proteins as key players in host-pathogen interactions. Front. Microbiol. 2011, 2, 208. [Google Scholar] [CrossRef]
- Roy, C.R.; Berger, K.H.; Isberg, R.R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 1998, 28, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Tiago, I.; Da Costa, M.S.; Verissimo, A. Molecular evolution of Legionella pneumophila dotA gene, the contribution of natural environmental strains. Environ. Microbiol. 2010, 12, 2711–2729. [Google Scholar] [CrossRef] [PubMed]
- Dumenil, G.; Montminy, T.P.; Tang, M.; Isberg, R.R. IcmR-regulated membrane insertion and efflux by the Legionella pneumophila IcmQ protein. J. Biol. Chem. 2004, 279, 4686–4695. [Google Scholar] [CrossRef]
- Gomez-Valero, L.; Rusniok, C.; Rolando, M.; Neou, M.; Dervins-Ravault, D.; Demirtas, J.; Rouy, Z.; Moore, R.J.; Chen, H.; Petty, N.K.; et al. Comparative analyses of Legionella species identifies genetic features of strains causing Legionnaires’ disease. Genome Biol. 2014, 15, 505. [Google Scholar]
- Bellanger, X.; Payot, S.; Leblond-Bourget, N.; Guedon, G. Conjugative and mobilizable genomic islands in bacteria: Evolution and diversity. FEMS Microbiol. Rev. 2014, 38, 720–760. [Google Scholar] [CrossRef] [PubMed]
- Glockner, G.; Albert-Weissenberger, C.; Weinmann, E.; Jacobi, S.; Schunder, E.; Steinert, M.; Hacker, J.; Heuner, K. Identification and characterization of a new conjugation/type IVA secretion system (trb/tra) of Legionella pneumophila Corby localized on two mobile genomic islands. Int. J. Med. Microbiol. 2008, 298, 411–428. [Google Scholar] [CrossRef]
- Andersson, D.I.; Jerlstrom-Hultqvist, J.; Nasvall, J. Evolution of new functions de novo and from preexisting genes. Cold Spring Harb. Perspect. Biol. 2015, 7, a017996. [Google Scholar] [CrossRef]
- Lesnik, R.; Brettar, I.; Hofle, M.G. Legionella species diversity and dynamics from surface reservoir to tap water: From cold adaptation to thermophily. ISME J. 2016, 10, 1064–1080. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayed, A.R.; Bitar, D.M.; Steinert, M.; Lück, C.; Spröer, C.; Brettar, I.; Höfle, M.G.; Bunk, B. Comparative Genomics of Legionella pneumophila Isolates from the West Bank and Germany Support Molecular Epidemiology of Legionnaires’ Disease. Microorganisms 2023, 11, 449. https://doi.org/10.3390/microorganisms11020449
Zayed AR, Bitar DM, Steinert M, Lück C, Spröer C, Brettar I, Höfle MG, Bunk B. Comparative Genomics of Legionella pneumophila Isolates from the West Bank and Germany Support Molecular Epidemiology of Legionnaires’ Disease. Microorganisms. 2023; 11(2):449. https://doi.org/10.3390/microorganisms11020449
Chicago/Turabian StyleZayed, Ashraf R., Dina M. Bitar, Michael Steinert, Christian Lück, Cathrin Spröer, Ingrid Brettar, Manfred G. Höfle, and Boyke Bunk. 2023. "Comparative Genomics of Legionella pneumophila Isolates from the West Bank and Germany Support Molecular Epidemiology of Legionnaires’ Disease" Microorganisms 11, no. 2: 449. https://doi.org/10.3390/microorganisms11020449
APA StyleZayed, A. R., Bitar, D. M., Steinert, M., Lück, C., Spröer, C., Brettar, I., Höfle, M. G., & Bunk, B. (2023). Comparative Genomics of Legionella pneumophila Isolates from the West Bank and Germany Support Molecular Epidemiology of Legionnaires’ Disease. Microorganisms, 11(2), 449. https://doi.org/10.3390/microorganisms11020449






