Abstract
Endometriosis affects approximately 6 to 10% of reproductive-age women globally. Despite much effort invested, the pathogenesis that promotes the development, as well as the progression of this chronic inflammatory disease, is poorly understood. The imbalance in the microbiome or dysbiosis has been implicated in a variety of human diseases, especially the gut microbiome. In the case of endometriosis, emerging evidence suggests that there may be urogenital-gastrointestinal crosstalk that leads to the development of endometriosis. Researchers may now exploit important information from microbiome studies to design endometriosis treatment strategies and disease biomarkers with the use of advanced molecular technologies and increased computational capacity. Future studies into the functional profile of the microbiome would greatly assist in the development of microbiome-based therapies to alleviate endometriosis symptoms and improve the quality of life of women suffering from endometriosis.
1. Introduction
According to a recent study, 196 million women between the ages of 12 and 52 have endometriosis, an uncomfortable condition that is estrogen-dependent and causes chronic pelvic and lower abdominal pain [1]. This gynecological illness, which is characterized by the growth of endometrial glands and stromal cells both inside and outside the pelvic cavity, has a detrimental effect on the quality of life of its patients. It affects approximately 6 to 10% of women of reproductive age globally [2,3,4]. Meanwhile, postmenopausal endometriosis occurs in approximately 2–5% of postmenopausal women and this form of the disease has been proposed to have a more convoluted pathophysiology than the premenopausal form [5,6,7,8]. Nonetheless, the illness frequently manifests as dyspareunia, infertility, dysmenorrhea, and excruciating pelvic discomfort [9]. Endometriosis has been the subject of innumerable scientific and clinical studies, but its origin remains unknown [10,11,12]. The most plausible hypothesis is Sampson’s theory, which states that the focus of the disease is caused by retrograde menstruation [13]. The gynecologist John Albertson Sampson suggested that the retrograde tubal flow plants menstrual endometrial tissues in the peritoneal cavity as well as other organs. In contrast to this finding, many scientists have hypothesized that additional factors, including genetic, anatomical, endocrine, inflammatory, and environmental factors, may affect tissue implantation. This is due to studies showing that while 90% of women had retrograde menstruation, only 10% acquire the condition [14,15,16,17,18,19,20,21]. Additionally, researchers discovered that the microbiome and endometriosis development are bidirectional related, implying that any change in the host’s microbiome can have a significant impact on the development and progression of endometriosis [22]. Therefore, it is of utmost importance to thoroughly investigate the link between the microbiome and endometriosis in order to better understand the condition and maybe develop medications that would alleviate the burden on sufferers.
The quantity of bacteria in the human body was once thought to be ten times greater than that of human cells [23,24]. However, a recent study has proposed revised estimations for the number of human and bacteria cells in a human adult (70 kg body weight) and the ratio of bacteria to human cells is now recorded as 1:1 ratio. These findings then highlight the potential impact bacteria cells can have on human health, on top of their “supporting” role in host metabolism and maturation of the immune system. The human gut microbiome, for instance, produces vitamin K and B12, which helps to maintain the integrity of the intestinal mucosa, repairs epithelial cells, stimulates angiogenesis, and modifies the immune system [25,26]. Furthermore, it has been established that the composition of the microbiome at a given site (i.e., gut, vaginal, nasal, etc.) varies between a healthy individual and a patient with specific diseases. A number of these diseases include autoimmune disease, cancer, metabolic cancer, inflammatory bowel disease, and many others [27,28]. There is growing evidence that endometriosis patients have higher levels of bacterial colonization in their endometrial tissues and menstrual blood than do women in the general population [16,29,30,31]. The use of animal models in endometriosis research is critical for understanding the pathophysiological mechanism underpinning the disease [32]. Recently, endometrial tissues have recently been transplanted to ectopic sites to create endometriosis models in tiny laboratory animals such as rodents. This method offers a more affordable alternative than using non-human primates, but it has certain drawbacks because rodents do not naturally contract the disease while some primates do [33]. Without a doubt, pre-clinical studies employing animal models allow researchers to comprehend the mechanics underlying disease and to test potential therapeutic or preventive agents, especially the degrees of experiment safety before carrying out clinical trials on humans. Thus, the aim of this narrative review is to summarize recent scientific and clinical findings on the relationship between endometriosis and microbiomes of different body sites including the female reproductive tract, gastrointestinal as well as the peritoneal region. Ultimately, these discoveries would provide a foundation for future research to carry on the fight against this agonizing ailment, particularly via biomarker discoveries and microbiome-based therapeutics.
2. How Common Is Endometriosis?
Before discussing possible important risk factors that could potentiate the development of endometriosis, it is important to understand the prevalence of the disease across the globe, which further enhances our knowledge of the disease burden among different countries. While it is accepted that endometriosis could affect women of any age, a cross-sectional online survey conducted in Canada estimated the prevalence of endometriosis in Canada to be 7.0% (2004 women of 28,532 women surveyed), with nearly half of the respondents (47.5%) aged 18–29 years old when they were diagnosed with endometriosis [34]. Interestingly, the study also reported that 84.1% of women had endometriosis symptoms prior to diagnosis, indicating a considerable clinical burden and perhaps unmet needs for earlier diagnosis.
Fuldeore and Soliman [35] surveyed women aged 18–49 years old in August–November 2012 and the team reported that the overall prevalence of diagnosed endometriosis was estimated at 6.1% (2922 out of 48,020), with a surprisingly large proportion of these respondents (86.2%) reported to have experience symptoms prior to diagnosis [36]. The primary analysis of the retrospective cohort study revealed a declining incidence rate (which includes participants aged 16 to 60 years during the selected study period, who had a uterus, were continuously enrolled for at least two years before the study was conducted, and had at least one healthcare utilization from 30.2 per 10,000 person-years in 2006 to 17.4 per 10,000 person-years in 2015. The incidence rate did not differ by race or ethnicity groups, but the highest incidence rate was observed among women aged 36 to 45 years old. On the other hand, a study in Spain reported prevalence data collected from the year 2009 to 2018 reflected the opposite trend, with the team observing an increased overall prevalence of endometriosis, reaching as high as 1.24% in 2018. The median incidence rates were reported as 94.9 (range: 92.6–102.9) per 100,000 women-years. As reported by Christ et al. [36], Medina-Perucha et al. reported that the incidence of endometriosis was highest among women aged 35–44 years old [37]. Additionally, Yamamoto et al. were interested to understand if ethnicity/race contributes to the endometriosis prevalence among IVF patients, and they discovered a significantly higher prevalence among Asian women compared to Caucasians (15.7 vs. 5.8%, p < 0.01) [38]. Asian women had 2.96 times the odds of being diagnosed with endometriosis (95% CI 1.65, 5.31; p = 0.0003), whereas Hispanic women and other races/ethnicities did not differ significantly from Caucasians. A systematic review published by Bougie et al. in 2019 found that Asian women were more likely to have the diagnosis of endometriosis (OR 1.63, 95% CI 1.03–2.58, based on 10 articles which explored the likelihood of endometriosis diagnosis in Asian women) [39].
Spanning over to Africa continent, a 10-year retrospective cohort study (from June 2003 to November 2014) at Nordica Fertility Center Lagos, Nigeria explained that 2.69% of participants (61/2265) were diagnosis with endometriosis via laparoscopy, with the highest proportion (43/61) of endometriosis patient in the age group of 31–40 years old [40]. These findings undoubtedly raised concerns about the assumption that endometriosis is a “rare” disease among indigenous African women. Another prospective cross-sectional study conducted at Kenyatta National Hospital reported that 4.6% of indigenous Africans had histologically proven endometriosis (95% CI 0.5–18.4) [41]. Several research groups emphasized the potential of underdiagnosis that leads to low reporting of endometriosis cases in Africa continent [41,42,43].
How prevalent is endometriosis in the Asia Pacific region? Back in 1976, Miyazawa observed an interesting phenomenon in Hawaii whereby clinicians reported a high incidence of endometriosis among Oriental women. The study by Miyazawa analyzed the data of gynecologic admission and endometriosis diagnosis from three hospitals (two in Hawaii and one in Japan) which led to the conclusion that indeed there is a higher incidence among the Japanese population [44]. Approximately 10% of gynecologic admission was reported to be associated with endometriosis. Almost 30 years later, another study conducted in Japan attempted to gauge the prevalence of endometriosis in the country via the Japan Nurses’ Health Study [45]. Out of 15,019 participants, 6.8% of them (n = 1025) had a self-reported history of endometriosis. Subsequent analysis of the data collected from 862 participants showed that the mean age of endometriosis diagnosis was 32 years old with the highest proportion observed among those aged 32 to 44 years (150/330, 45.5%). Conversely, a study by Feng and the team estimated the trends in endometriosis incidence from 1990 to 2019 were estimated using join point regression [46]. When compared to data from North and South America continents, their results reported a significant age-related effect on endometriosis incidence at a relatively young age: increased risk was seen in the age groups ranging from 15 to 19 to 20 to 24 years, with the latter group having the highest relative risk of 2.54 (95% CI: 2.45, 2.64). Nonetheless, the study reported declining age-standardized incidence rates (ASIR) by nearly 30% in 2019 and that the overall ASIR was −1.2% (95% CI: −1.20, −1.10). A recent study from China that recruited patients between September 2021 and February 2022 reported that endometriosis remains to be one of the most prevalent gynecological diseases [47]. This study found a slightly lower prevalence of endometriosis (4.09%) than studies from other countries. However, the study also outlines additional research is needed to substantiate their findings given that the majority of the participants (97.95%) belonged to the Han race and a nationwide study is required to properly comprehend the disease burden in the country. Rowlands and the team investigated the prevalence and incidence of endometriosis among Australian women by studying national hospital data, along with three administrative health databases [48]. The team discovered that one in nine women had clinically confirmed or suspected endometriosis by the age of 44 after retrieving the health records of 13,508 Australian women who were born between the years of 1973 and 1978 over a period of 20 years. The cumulative prevalence of clinically diagnosed endometriosis was 6.0% (95% CI 5.8–6.2%) at age 40–44 years, which was higher than the other studies.
In 1992, a study in Southeast Asia that was completed by Yamamoto et al. in the United States reported findings that were in line with their findings [38,49]. Arumugam and Templeton described that endometriosis was more prominent among infertile women from Kuala Lumpur, Malaysia than in the United Kingdom (51% vs. 22%, p < 0.001). According to a study from Thailand, 30.5% (101/331) of the women who underwent surgery for the benign gynecological disease were found to have endometriosis (mean age: 39.4 ± 17.4 years old) [50]. One would generally assume that comparable incidence rates were reported with data from the Asian continent, given the multi-ethnicity background in this region. Yet, the data on the epidemiology of endometriosis within this region is scarce and more studies are still required to clearly understand the disease burden and any potential “modifiable” risk factors (e.g., body mass index, parity, etc.) that could contribute to the development of endometriosis [51]. Nonetheless, there are emerging working groups in several Southeast Asia countries including Thailand and Malaysia, that are actively advocating early diagnosis of endometriosis, along with knowledge dissemination and support systems for endometriosis patients [52,53]. While improving general health and empowering the local community, these efforts collectively would allow researchers to understand the etiology of endometriosis before developing an effective management plan to tackle this multifactorial disease.
3. Diagnosis and Management of Endometriosis
Despite the fact that endometriosis can manifest itself in various ways, there are suggested diagnostic procedures that clinicians, including gynecologists and general practitioners, should follow [54,55]. One of the ways is observing the patient’s clinical signs and symptoms. An individual with endometriosis may exhibit one or more symptoms, including persistent pelvic discomfort, infertility, dysmenorrhea, dyspareunia, hematuria, dysuria, and dyschezia [56]. Along with pain in the chest and beneath the shoulder blade, other signs that the disease may be present include catamenial pneumothorax, cyclical cough, and cyclical scar edema [57,58,59,60]. During a pelvic examination, clinicians typically feel for pelvic masses, noduling uterosacral ligaments, retroverted fixtures, uterine or adnexal discomfort, and other probable endometriosis symptoms [61,62]. Even though imaging technology such as magnetic resonance and ultrasound make preoperative disease detection possible, it is crucial to understand that a negative imaging result does not necessarily rule out the presence of the disease, particularly if it is a superficial peritoneal disease [63,64]. Transvaginal ultrasound is preferred to gain a clear view of the endometrial and uterine cavity, as well as to identify ovarian endometriotic cysts without ruling out the possibility of deep-infiltrating endometriosis, peritoneal endometriosis, and adhesions related to endometriosis [61,65,66,67,68,69]. Transvaginal ultrasound’s maximum sensitivity is limited to the diagnosis of endometriomas. Nevertheless, with current technological advancements and sufficient training, it may be possible to raise the detection sensitivity for other phenotypes of the disease [70,71]. The laparoscopic examination was previously thought to be the gold standard for diagnosis, but according to a recent European Society of Human Reproduction and Embryology (ESHRE) recommendation state that it should only be used in cases where imaging fails to clearly demonstrate the presence of pathology in suspected patients [72]. Although histopathological confirmation is excellent, it has limitations in terms of its sensitivity because the definitions of the syndrome have been static for decades, which is mostly true for younger women with the illness [72,73]. In fact, up to 40% of laparoscopies performed for pelvic pain were unable to discover any pathology, necessitating the use of alternative non-invasive methods such as biomarkers by clinicians to aid in the early detection of the disease [74]. In order to maximize the likelihood of a correct diagnosis, a combination of numerous diagnostic techniques is clearly required. It is essential that the medical management team can use these diagnostic tools effectively to suggest the best course of treatment to alleviate the patients’ pain.
Endometriosis management calls for an individualized, multimodal, and interdisciplinary strategy [58]. This may include surgical excision of lesions, non-drug therapies, analgesics, hormonal and non-hormonal therapies, or any of those approaches in combination [56,58,75,76]. Table 1 lists treatments for pain while Table 2 lists treatments for endometriosis-related infertility as per recommendations from international guidelines such as the ESHRE Guideline and National Institute for Health and Care Excellence (NICE) Guideline, along with the national consensus or guidelines such as the Thailand Interest Group for Endometriosis (TIGE), the Obstetrical and Gynecological Society of Malaysia (OGSM), the American Society of Reproductive Medicine (ASRM) [52,56,57,77,78]. Researchers highlighted the lack of reliable information regarding the effects of laparoscopic surgery for the contravention of the recommendations, which has led to many doubts and uncertainties in providing patients with a better quality of life and, most importantly, minimizing pain [79]. Non-drug therapies frequently employ dietary intervention, physical therapy, and psychological intervention. Dietary interventions have been shown to have a positive impact on endometriosis patients’ symptoms. In a study, it was shown that omega-3 polyunsaturated fatty acids (o-PUFAs) were found to lower patients’ pain scores [80], demonstrating that they can both lower inflammation and lessen pain [81,82]. Additionally, cognitive behavior therapy underwent trials to determine its efficacy in creating pain coping mechanisms for a variety of chronic pain illnesses with varied degrees of severity [74,83,84]. Another popular non-drug treatment is pelvic physiotherapy, where physiotherapists accompany patients by supervising rehabilitation activities and offer supplementary treatments such as massages to ease their symptoms. The disadvantage of this approach is proving the efficacy of physiotherapy alone in treating the illness, as the majority of studies have evaluated the therapy in conjunction with psychological and/or medicinal therapy [85]. Patient preference, cost, and side effects are routinely considered in the pharmaceutical management of endometriosis. The initial line of treatment is typically analgesics such as non-steroidal anti-inflammatory drugs and hormonal treatment, such as low-dose combination oral contraceptives such as ethyl estradiol and progestins [86]. Moreover, several clinical studies have demonstrated that gonadotropin-releasing hormones (GnRH) antagonists offer potential therapeutic effects in the management of pain [87,88,89,90]. Lastly, non-hormonal therapies such as anticonvulsants, analgesic tricyclic antidepressants, and selective serotonin uptake inhibitors are occasionally prescribed by clinical practitioners to relieve endometriosis patients’ discomfort. The drawback is that little study has been completed to back it up [91]. This information then indicates that there is still much space for improvement in the management of the disease. More study should be completed to ascertain the efficacy of the current treatments to enhance and develop more effective ones.

Table 1.
Examples of endometriosis guidelines and their level of evidence in treating pain (GPP: good practice points) [52,56,58,77,78].

Table 2.
Examples of Endometriosis Guidelines and its level of evidence in treating infertility [56,58,77,78].
4. The Intricate Relationship between the Female Reproductive Tract Microbiome and Gut Microbiome in the Development and Progression of Endometriosis
The term “microbiome” refers to a collective group of microorganisms that live in a habitat or specific site. Therefore, it is understandable that the microbiome across the human body may demonstrate differences in the microbial population [92]. The classic example would be the gastrointestinal (GI) system (which harbors the largest microbes in the human body)—pH and oxygen availability changes throughout the GI tract which then poses selective pressure on microbes [93,94]. The gastric microbiome of a healthy human adult would consist of a microbial population that could tolerate the low pH in the environment, which include those belonging to the Prevotella, Streptococcus, Veillonella, Rothia, and Haemophilus genus [94,95]. In contrast, the colon microbiome of a healthy human adult is predominantly colonized by members of bacteria belonging to the genera of Lactobacillus, Akkermansia, Enterobacter, Lachnospiraceae, Prevotella, and several more [96]. It is important to note that while microbiome structure may vary between individuals, microbial functions are pretty much conserved which allows researchers to exploit them as disease biomarkers or even therapeutic targets as part of the microbiome-therapeutics strategy. The primary function of the human gastrointestinal system was thought to support the host’s metabolism, including digesting, and absorbing ingested nutrients, and excreting waste products of digestion. However, a growing body of evidence is accentuating the role of the gastrointestinal system as an organ of immunity, particularly in maintaining immune system homeostasis [97]. The gastrointestinal-associated lymphoid tissue acts as the “control center” to manage the immune system in response to massive antigen exposure in the gut and activate adaptive immune responses such as B cell maturation [98].
The role of the gut microbiome in the etiology and pathogenesis of the human disease remains one of the top research areas for the past few decades [99,100]. Nevertheless, emerging evidence highlighted the potential of crosstalk between microbiomes of different sites in several human diseases, including the urogenital and gut microbiome given their anatomical proximity [101,102,103]. Comparable to the continuum observed in the gut microbiome, different parts of the female reproductive tract (FRT) display different distributions of microbes [104,105]. In fact, FRT is categorized into a higher part which consists of the endocervix and uterus proper, and a lower part which comprises the vaginal canal and ectocervix (Figure 1). The lower FRT is dominated by Lactobacillus spp. and these microbes protect the host against pathogens by creating a low pH environment and production of bacteriocins as well as hydrogen peroxide. The vaginal community state type (CST) classification system describes a total of five CSTs, whereby CST I, II, III, and V are dominated by Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii, respectively [106,107]. These four CSTs are associated with a healthy vaginal microbiome, whereas CST IV, which presents higher proportions of strictly anaerobic bacteria (e.g., Prevotella, Dialister, Atopobium, Gardnerella, Megasphaera, Peptoniphilus, Sneathia, Eggerthella, Aerococcus, Finegoldia, and Mobiluncus), is suggested to be linked with inflammation or dysbiosis in the vagina. Additionally, it is important to note that vaginal CSTs can change throughout women’s lifetimes. Given that some microbes can stimulate the immune system to trigger inflammation while a portion of them helps to maintain homeostasis in the host by the production of antimicrobial compounds or even immunomodulatory compounds, researchers are now exploring the possibility of microbiome involvement in the development and progression of endometriosis.
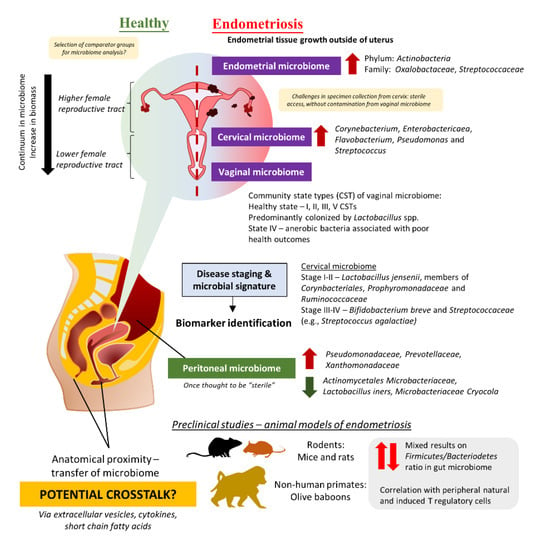
Figure 1.
The potential role of the microbiome in endometriosis.
4.1. Evidence from Clinical Studies: Are There Any Distinct Microbiome Changes in the Vaginal Microbiome?
The majority of the clinical studies investigating endometriosis and microbiome changes were derived from Asia including Japan, China, Taiwan, Korea, and Turkey (Table 3, Supplementary Table S1) [21,108,109,110,111,112,113,114,115,116,117,118]. There were also some studies from the United States, Brazil, Sweden, and Canada [119,120,121,122,123]. Almost all the clinical trials conducted diagnosed endometriosis cases via laparoscopy or histology tests and scored based on the criteria described in the Revised American Society for Reproductive Medicine (r-ASRM) classification of endometriosis. The study by Akiyama et al. in 2019 reflected that even though Lactobacillus spp. dominated the cervical microbiome of endometriosis patients, they still presented a higher abundance of Corynebacterium, Enterobactericaea, Flavobacterium, Pseudomonas, and Streptococcus as compared to control (without endometriosis) [21]. Subsequent quantification of bacteria using real-time PCR confirms the finding from next-generation sequencing, in which Enterobacteriaceae and Streptococcus abundance were statistically different between endometriosis and non-endometriosis control (p > 0.05). Similarly, another team in Taiwan described that not only the cervical microbiome of endometriosis patients was different from healthy women, but there were also some differences between endometriosis patients in Stage I and II as compared to those in Stage III and IV [115]. The team has suggested that potential microbial biomarkers for different stages: (a) Stage I–II: L. jensenii or members in Corynbacteriales, Porphyromonadaceae, and Ruminococcaceae, (b) Stage III–IV: Bifidobacterium breve and Streptococcaceae members (e.g., Streptococcus agalactiae).

Table 3.
Clinical studies reporting changes in the gut, peritoneal and urogenital microbiome (AMEM, adenomyosis-endometriosis; CST, community state type; DIE, Deep Infiltrating Endometriosis; N.A, not available; OCP, oral contraceptives).
Given the challenges in obtaining cervical specimens without cervicovaginal contamination and the nature of biomass in the upper FRT, several teams have attempted to study the differences in the microbiome of the lower FRT. For instance, three studies in Brazil and China studied the vaginal swab or fluid obtained from patients and observed a lower abundance of Lactobacillus in the endometriosis group as compared to the control [114,118,119]. Besides that, the study by Ata et al. discussed the differences in vaginal samples obtained from Stage III or IV endometriosis patients as compared to healthy women [108]. At the genus level, Gemella and Atopobium spp. was absent in the vaginal samples obtained from the endometriosis group. A similar approach was taken by Perrotta et al., but the team took a broader approach to look at the vaginal CST rather than looking at just a specific group of microbes [120]. These data then allowed the team to build a random forest-based classification model with machine-learning methods on microbiota composition to predict r-ASRM stages of endometriosis. Analyzing the changes during follicular and menstrual phases yielded highly predictive taxa which can be used to predict either stage I-II or stage III-IV endometriosis—the genus Anaerococcus (phylum Firmicutes).
4.2. Beyond the Female Reproductive Tract: Connections between Gut Microbiome, Peritoneal Microbiome, and Endometriosis
On the contrary, Chen et al. were unable to identify microbial signatures from FRT microbiome for use as a biomarker but a prediction of metagenome functions using bioinformatic tools indicated a higher proportion of microbes involved in general metabolism, lipid metabolism as well as synthesis and degradation of ketone bodies [109]. Furthermore, Huang et al. conducted a study in China, that recruited patients from June 2019–October 2019, and reported that there are no significant differences in cervical microbiome observed between women without endometriosis and endometriosis patients [112]. In spite of this, the team uncovered differences in fecal microbiome composition between women with endometriosis and those without. Analysis of the fecal microbiome showed depletion of ten taxa including Clostridia Clostridiales, Lachnospiraceae Ruminococcus, Clostridiales Lachnospiraceae, and Ruminococcaceae Ruminococcus, along with an increased abundance of Eggerthella lenta and Eubacterium dolicum in endometriosis patients as compared to women without endometriosis. Likewise, another report by Shan et al. increased Firmicutes/Bacteriodetes ratio in endometriosis group, with enrichment of Actinobacteria, Cynaobacteria, Saccharibacteria, Fusobacteria, and Acidobacteria (p < 0.05) as compared to the control [113]. In essence, these results imply the involvement of the gut microbiome in the development of endometriosis.
Apart from the FRT and gut microbiome, there is another special microbiome that has been investigated to understand the development of the progression of endometriosis—the peritoneal microbiome. Once thought to be “sterile”, the peritoneal microbiome is found to be associated with the etiology of diseases such as end-stage kidney disease and cancer [125,126,127]. As such, it is certainly logical to investigate the changes in the peritoneal microbiome among endometriosis patients, given that the lesion may involve the peritoneum [110,116,128]. In 2022, Yuan et al. reported that there was a total of 276 operational taxonomic units (OTUs) detected in peritoneal fluid collected from endometriosis patients (as compared to 211 OTUs in the control group); out of which, 120 of them were unique to endometriosis group [117]. At the genus level, there was a significantly higher abundance of Acidovorax (p = 0.01), Devosia (p = 0.03), Methylobacterium (p = 0.03), Phascolarctobacterium (p = 0.03), and Streptococcoccus (p = 0.04) in endometriosis group than the control group. In order to investigate potential crosstalk between different microbiomes, a research group in Korea decided to study the extracellular vesicles in the peritoneal fluid of endometriosis patients in comparison to those without endometriosis [111]. The team successfully characterized microbes present in the extracellular vesicles and reported that there was a significant decrease in Actinobacteria at the phylum level among endometriosis patients.
4.3. Establishment of Animal Model for Endometriosis
Over the past few years, researchers have developed animal models for endometriosis to study the changes in the microbiome, specifically to understand how microbes are involved in the pathogenesis of endometriosis as well as the influence on disease progression. Based on the published literature, the majority of studies reporting microbiome changes established the endometriosis model via three main ways: (a) homologous (syngeneic) transplantation, (b) autologous transplantation, or (c) heterologous transplantation of uterine or endometrial tissue [33]. Homologous transplantation requires a donor animal of the same species which typically receives hormonal treatment (e.g., estradiol benzoate) prior to sacrifice. Uterine tissue from the donor will then be injected or sutured into the peritoneal cavity of the recipient animal to the development of endometriosis in humans [129]. On another note, autologous transplantation produces comparable endometriosis phenotypes as homologous transplantation, but this process involves surgical intervention and transplantation within the same animal. In simple terms, the endometrial tissue will be recovered from one of the uterine horns and processed before being injected intraperitoneally into the same animal. In fact, the autologous endometriosis model using rats was initially described in 1985 by Vernon and Wilson, where they compared four types of surgical techniques in producing pathophysiologic features that are consistent with endometriosis [130]. In their study, the team discovered that rats that underwent autologous transplantation had higher peritoneal adhesion, with the implants developed into ellipsoidal cystic structures which consisted of both endometrial glands and stroma. The success in developing the endometriosis model created great opportunities for subsequent studies to investigate the role of the microbiome in endometriosis (Table 4) [131,132,133,134,135,136].

Table 4.
Studies reporting microbiome changes in animal models of endometriosis.
Similar to the findings from clinical studies, animal models of endometriosis reflected potential impact on different microbiomes. Most of the studies which attempted to search for microbial signatures in endometriosis were focusing on a specific locality—the gut microbiome which harbors the most microbes in the human body. Interestingly, an earlier study by Yuan and the team in 2018 reported enrichment of Firmicutes and Actinobacteria phylum in mice with endometriosis induced via intraperitoneal injection of endometrial segments, along with an elevated level of Bifidobactericeae and Alcaligenceae [131]. Consistent findings were also reported by Ni and the team in 2021 where they discovered an increased abundance of Clostridium_sensu_stricto_1, Bifidobacterium, and Candidatus_Saccharimonas, on top of elevated Lactobacillus abundance in the gut microbiome of endometriosis mice [136]. Their team conducted a linear discriminant analysis that further explains the differences in microbial composition between the endometriosis and control group which arises from 17 genera in the former and 11 genera in the latter group.
Furthermore, the ratio of Firmicutes/Bacteroidetes has been used as a general indicator of a healthy gut microbiome, given that these two populations constitute a large proportion of microbes in the gut. Results from endometriosis studies involving the use of mice reported heterogeneity in results. While most of the studies reported an increase in Firmicutes and a reduction in Bacteroidetes abundance, there were two studies reporting the opposite effect, observing a drop in Firmicutes abundance and an elevated abundance of Bacteroidetes [132,134]. Even so, these findings might be attributed to sampling methods as most of the studies collected fecal pellets, rather than feces from the cecum segment. On top of that, findings presented by Chadchan et al. suggested that the administration of metronidazole in mice with induced endometriosis reduces endometriotic lesion growth, which in turn suggests the possible role of metronidazole-sensitive microbes in the disease progression and warranted further studies to devise better management plan for endometriosis [132].
Additionally, the interest in identifying the key microbiome changes that contribute to the pathogenesis and progression of endometriosis continues to encourage scientists to explore animal models other than mice. In fact, a study in China adopted the autologous transplantation methods used in mice to create endometriotic lesions in six-to-eight weeks-old female Sprague Dawley (SD) rats [135]. The results were comparable to the mice model where they reported an increased Firmicutes/Bacteroidetes ratio, with a decrease in abundance of another bacterial family known as Ruminococcaceae. Decreased Ruminoccoccaceae abundance has been proposed to have a negative impact, given that these strictly anaerobic bacteria can produce anti-inflammatory short-chain fatty acids such as butyrate and contributes substantially to maintaining the general health of the gut [137,138,139,140]. While many studies have also reported the diminished Ruminococcaceae abundance in the gut microbiome in autoimmune diseases such as inflammatory bowel disease, several reports emphasized significant inverse correlations of Ruminococcaceae and its member, Ruminococcus with the inflammatory cytokine IL-6 [141,142].
Another recent study on non-human primates, olive baboons (Papio Anubis) in reproductive age also indicated the involvement of T regulatory cells as they investigated changes in the gastrointestinal and urogenital microbiome [121]. Similar to findings from the rodents’ model of endometriosis, the team reported dynamic changes in the gut microbiome of these olive baboons throughout their 15-months study. For instance, the abundance of genera Succinivibrio, Prevotella, Megasphaera, Lactobacillus, and CF231 was decreased in fecal samples at three months post-induction of endometriosis; out of these genera, three of them—Succinivibrio, Prevotella, and CF231 abundance increased throughout the disease progression from six to nine months post-induction. Among the literature retrieved from databases reporting endometriosis in animal studies, this is the only study that implies the relationship between gastrointestinal, urogenital, and peritoneal microbiome. In the peritoneal cavity, it was reported that a group of unclassified bacteria dominated the peritoneal microbiome followed by Proteobacteria. The team reported that both the peritoneal and vaginal microbiome were diminished upon disease induction and failed to be restored throughout the progression of the disease. An in-depth correlation study on microbial species and peripheral immune cells after induction of endometriosis identified that certain microbial populations in the GI tract displayed a positive correlation with immune cell populations (i.e., natural T regulatory cells and T helper 17 cells) at different study time points, but not the peritoneal and vaginal microbiome. Altogether, the authors concurred that additional analysis needed to be conducted to elucidate the exact mechanisms of these bacterial species in modulating host immune response. These exciting findings further strengthen the rationale to exploit microbiomes as treatment targets, especially their potential in modulating the host immune system and potentially alleviating pelvic inflammation which is commonly seen in endometriosis patients.
5. Potential Benefits of Probiotics in the Management of Endometriosis
While pharmacotherapy remains critical in the symptomatic management of endometriosis patients, microbiome-based therapeutics may potentially be used in the nearest future to restore the balance in microbiomes and to alleviate chronic inflammation that is commonly observed among endometriosis patients [118,143,144,145]. For instance, L. gasseri OLL2809 was found to be able to inhibit the development of ectopic endometrial cells in the peritoneal cavity via activation of natural killer cells in a rodent model of endometriosis, which most likely occurred via the induction of interleukin-12 production [144]. In the subsequent placebo-controlled study, Itoh et al. also found that taking L. gasseri OLL2809 tablets for three months significantly reduces pain intensity on the visual analog scale (VAS) and dysmenorrhea on the verbal rating scales (VRS) in the active group, recording at −3.28 ± 0.36 and −1.44 ± 0.17, respectively, as compared to the −2.00 ± 0.29 and −1.03 ± 0.16 recorded in the placebo group [146]. Correspondingly, a different study conducted in Japan supported the use of L. gasseri OLL2809 in the treatment of the condition. In this study, endometriosis volume was significantly different between the active group of rats and the control and dienogest-treated groups, with a significant difference in log value of p < 0.05 recorded after four weeks of treatment [147]. Another pilot placebo-controlled randomized clinical trial in Iran evaluated the effects of a multi-strain probiotic capsule known as LactoFem® capsule (containing 109 colony of Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus fermentum, and L. gasseri) among Stage III-IV endometriosis patients. The team noted a significant drop in dysmenorrhea scores after 8 weeks of treatment in the probiotic group—from 6.53 ± 2.88 to 3.07 ± 2.49 as compared to 5.60 ± 2.06 to 4.47 ± 2.13 (p = 0.018). Then again, more studies should be conducted to evaluate the actual changes in different microbiomes post-administration of probiotics to monitor the microbial dynamics throughout the period as well as test different administration routes to ensure optimal results from microbiome-based therapeutics. Given that the methods of probiotics preparation differ between administration routes, considerations should also be given to its stability to ensure optimal delivery to the targeted site [148,149]. Along with that, the actions of probiotics on microbiome stability are equally important to counter dysbiosis and subsequently provide beneficial effects in a long-term manner. To date, there is still a lack of guidelines outlining or supporting the standard use of probiotics in the management of endometriosis. Thus, additional investigations into these aspects would enhance the understanding of disease etiology as well as strengthen the rationale for using microbiome-based therapeutics in endometriosis management.
6. Future Recommendations and Conclusions
In the past few decades, the scientific community witnessed the advancements in molecular techniques that allow in-depth investigations on host biology as well as the involvement of microbes in human diseases. As a matter of fact, understanding a complex disease such as endometriosis requires more than just perseverance and collective efforts from experts in different fields. Based on the gathered literature, there were some levels of changes in microbial signatures present among endometriosis patients; interestingly, these changes were not only restricted to the FRT but also at other sites including the gut as well as the peritoneal region. Yet, it is evident that the search for microbial-derived disease biomarkers for endometriosis is still in the infancy stage, especially for its implementation in clinical applications. Further studies would need to be conducted to resolve the heterogeneity issue observed in different clinical studies, given that these differences in data may arise from several factors including ethnicity, diet, or specimen quality [150,151,152]. Functional analysis of the microbiome using whole genome shotgun metagenomic sequencing can possibly provide more insights into the functional characteristics of specific microbes in the specimens.
Another essential point to consider in the crosstalk of microbiome and host processes is the estrogen-microbiome axis [121,124,153,154,155]. While studies have noted the relationship between the microbiome and immune response, emerging data is reflecting that alterations in the estrobolome brought upon by dysbiosis can trigger estrogen-mediated pathologies, including endometriosis as well as endometrial cancer. On top of identifying key microbial signatures that could be used as biomarkers to predict disease development or progression, understanding the functional role of these microbes would indefinitely assist in the design of an effective management plan for endometriosis. All in all, there is still much to be explored but global cooperative efforts between government authorities, researchers, clinicians, and the public will pave the way in tackling a chronic, “neglected” disease such as endometriosis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11020360/s1, Table S1: Study characteristics reporting microbiome changes in endometriosis patients.
Author Contributions
Conceptualization, H.-L.S. and R.A.R.A.; literature search, H.-L.S. and S.-J.A.Y.; data analysis, H.-L.S. and S.-J.A.Y.; writing—original draft preparation, H.-L.S. and S.-J.A.Y.; writing—review and editing, M.N.S., N.M.M. and R.A.R.A.; funding acquisition, H.-L.S., N.M.M. and R.A.R.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and immune dysfunction in endometriosis. Biomed. Res. Int. 2015, 2015, 795976. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhou, Y.; Zhang, X.; Xue, M.; Sun, P.; Leng, J.; Chapron, C. Factors associated with deep infiltrating endometriosis versus ovarian endometrioma in China: A subgroup analysis from the FEELING study. BMC Womens Health 2018, 18, 205. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, B.D.; Bertschi, D.; Bersinger, N.A.; Mueller, M.D. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol. Metab. 2015, 26, 1–10. [Google Scholar] [CrossRef]
- de Almeida Asencio, F.; Ribeiro, H.A.; Ayrosa Ribeiro, P.; Malzoni, M.; Adamyan, L.; Ussia, A.; Gomel, V.; Martin, D.C.; Koninckx, P.R. Symptomatic endometriosis developing several years after menopause in the absence of increased circulating estrogen concentrations: A systematic review and seven case reports. Gynecol. Surg. 2019, 16, 1–11. [Google Scholar] [CrossRef]
- Ladanyi, C.; Boyd, S.; Sticco, P.; Mohling, S. Postmenopausal endometriosis, where are we now? Curr. Opin. Obs. Gynecol. 2019, 31, 267–278. [Google Scholar] [CrossRef]
- Secosan, C.; Balulescu, L.; Brasoveanu, S.; Balint, O.; Pirtea, P.; Dorin, G.; Pirtea, L. Endometriosis in Menopause-Renewed Attention on a Controversial Disease. Diagnostics 2020, 10, 134. [Google Scholar] [CrossRef]
- Giannella, L.; Marconi, C.; Di Giuseppe, J.; Delli Carpini, G.; Fichera, M.; Grelloni, C.; Giuliani, L.; Montanari, M.; Insinga, S.; Ciavattini, A. Malignant Transformation of Postmenopausal Endometriosis: A Systematic Review of the Literature. Cancers 2021, 13, 4026. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X.; Xue, M.; Zhou, Y.; Sun, P.; Leng, J. Not having been breastfed may protect Chinese women from developing deep infiltrating endometriosis: Results from subgroup analyses of the FEELING study. Reprod. Sci. 2019, 26, 1158–1167. [Google Scholar] [CrossRef]
- Attar, E.; Bulun, S.E. Aromatase and other steroidogenic genes in endometriosis: Translational aspects. Hum. Reprod. Update 2006, 12, 49–56. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Immunopathogenesis of pelvic endometriosis: Role of hepatocyte growth factor, macrophages and ovarian steroids. Am. J. Reprod. Immunol. 2008, 60, 383–404. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110, 143. [Google Scholar]
- Bellelis, P.; Frediani Barbeiro, D.; Gueuvoghlanian-Silva, B.Y.; Kalil, J.; Abrao, M.S.; Podgaec, S. Interleukin-15 and interleukin-7 are the major cytokines to maintain endometriosis. Gynecol. Obs. Investig. 2019, 84, 435–444. [Google Scholar] [CrossRef]
- Gueuvoghlanian-Silva, B.Y.; Bellelis, P.; Barbeiro, D.F.; Hernandes, C.; Podgaec, S. Treg and NK cells related cytokines are associated with deep rectosigmoid endometriosis and clinical symptoms related to the disease. J. Reprod. Immunol. 2018, 126, 32–38. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Hiraki, K.; Kitajima, M.; Nakashima, M.; Fushiki, S.; Kitawaki, J. Bacterial contamination hypothesis: A new concept in endometriosis. Reprod. Med. Biol. 2018, 17, 125–133. [Google Scholar] [CrossRef]
- Podgaec, S.; Dias, J.A., Jr.; Chapron, C.; Oliveira, R.M.; Baracat, E.C.; Abrao, M.S. Th1 and Th2 ummune responses related to pelvic endometriosis. Rev. Assoc. Med. Bras. 2010, 56, 92–98. [Google Scholar] [CrossRef]
- Podgaec, S.; Rizzo, L.V.; Fernandes, L.F.; Baracat, E.C.; Abrao, M.S. CD4(+) CD25(high) Foxp3(+) cells increased in the peritoneal fluid of patients with endometriosis. Am. J. Reprod. Immunol. 2012, 68, 301–308. [Google Scholar] [CrossRef]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014, 2014, 179515. [Google Scholar] [CrossRef]
- Zhang, T.; De Carolis, C.; Man, G.C.W.; Wang, C.C. The link between immunity, autoimmunity and endometriosis: A literature update. Autoimmun. Rev. 2018, 17, 945–955. [Google Scholar] [CrossRef]
- Akiyama, K.; Nishioka, K.; Khan, K.N.; Tanaka, Y.; Mori, T.; Nakaya, T.; Kitawaki, J. Molecular detection of microbial colonization in cervical mucus of women with and without endometriosis. Am. J. Reprod. Immunol. 2019, 82, e13147. [Google Scholar] [CrossRef] [PubMed]
- Jiang, I.; Yong, P.J.; Allaire, C.; Bedaiwy, M.A. Intricate Connections between the Microbiota and Endometriosis. Int. J. Mol. Sci. 2021, 22, 5644. [Google Scholar] [CrossRef] [PubMed]
- Nih Hmp Working Group. Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Ramakrishna, B.S. Role of the gut microbiota in human nutrition and metabolism. J. Gastroenterol. Hepatol. 2013, 28, 9–17. [Google Scholar] [CrossRef]
- Schirbel, A.; Kessler, S.; Rieder, F.; West, G.; Rebert, N.; Asosingh, K.; McDonald, C.; Fiocchi, C. Pro-angiogenic activity of TLRs and NLRs: A novel link between gut microbiota and intestinal angiogenesis. Gastroenterology 2013, 144, 613–623.e619. [Google Scholar] [CrossRef]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef]
- Hasain, Z.; Raja Ali, R.A.; Abdul Razak, S.; Azizan, K.A.; El-Omar, E.; Razalli, N.H.; Mokhtar, N.M. Gut microbiota signature among Asian post-gestational diabetes women linked to macronutrient intakes and metabolic phenotypes. Front. Microbiol. 2021, 12, 680622. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Kitajima, M.; Hiraki, K.; Nakashima, M.; Masuzaki, H. Intra-uterine microbial colonization and occurrence of endometritis in women with endometriosisdagger. Hum. Reprod. 2014, 29, 2446–2456. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Masumoto, H.; Muto, H.; Kitajima, M.; Masuzaki, H.; Kitawaki, J. Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur. J. Obs. Gynecol. Reprod. Biol. 2016, 199, 69–75. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Yamaguchi, N.; Katamine, S.; Matsuyama, T.; Nakashima, M.; Fujishita, A.; Ishimaru, T.; Masuzaki, H. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil. Steril. 2010, 94, 2860–2863. [Google Scholar] [CrossRef]
- Grummer, R. Animal models in endometriosis research. Hum. Reprod. Update 2006, 12, 641–649. [Google Scholar] [CrossRef]
- Lagana, A.S.; Garzon, S.; Franchi, M.; Casarin, J.; Gullo, G.; Ghezzi, F. Translational animal models for endometriosis research: A long and windy road. Ann. Transl. Med. 2018, 6, 431. [Google Scholar] [CrossRef]
- Singh, S.; Soliman, A.M.; Rahal, Y.; Robert, C.; Defoy, I.; Nisbet, P.; Leyland, N. Prevalence, Symptomatic Burden, and Diagnosis of Endometriosis in Canada: Cross-Sectional Survey of 30,000 Women. J. Obs. Gynaecol. Can. 2020, 42, 829–838. [Google Scholar] [CrossRef]
- Fuldeore, M.J.; Soliman, A.M. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: National estimates from a cross-sectional survey of 59,411 women. Gynecol. Obs. Investig. 2017, 82, 453–461. [Google Scholar] [CrossRef]
- Christ, J.P.; Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Reed, S.D. Incidence, prevalence, and trends in endometriosis diagnosis: A United States population-based study from 2006 to 2015. Am. J. Obs. Gynecol. 2021, 225, 500.e1. [Google Scholar] [CrossRef]
- Medina-Perucha, L.; Pistillo, A.; Raventos, B.; Jacques-Avino, C.; Munros-Feliu, J.; Martinez-Bueno, C.; Valls-Llobet, C.; Carmona, F.; Lopez-Jimenez, T.; Pujolar-Diaz, G.; et al. Endometriosis prevalence and incidence trends in a large population-based study in Catalonia (Spain) from 2009 to 2018. Womens Health 2022, 18, 17455057221130566. [Google Scholar] [CrossRef]
- Yamamoto, A.; Johnstone, E.B.; Bloom, M.S.; Huddleston, H.G.; Fujimoto, V.Y. A higher prevalence of endometriosis among Asian women does not contribute to poorer IVF outcomes. J. Assist. Reprod. Genet. 2017, 34, 765–774. [Google Scholar] [CrossRef]
- Bougie, O.; Yap, M.I.; Sikora, L.; Flaxman, T.; Singh, S. Influence of race/ethnicity on prevalence and presentation of endometriosis: A systematic review and meta-analysis. BJOG Int. J. Obs. Gynaecol. 2019, 126, 1104–1115. [Google Scholar] [CrossRef]
- Ajayi, A.B.; Ajayi, V.D.; Biobaku, O.; Oyetunji, I.; Aikhuele, H.; Atiba, A.; Afolabi, B.M. A 10-year study of endometriosis in an indigenous Black African population. J. Endometr. Pelvic Pain Disord. 2016, 8, 157–166. [Google Scholar] [CrossRef]
- Gichuhi, J.; Ogengo, J.; Gichangi, P. Laparoscopic diagnosis of endometriosis at Kenyatta National Hospital, Kenya. East Afr. Med. J. 2021, 98, 4038–4046. [Google Scholar]
- Labinjo, T. A review of the prevalence of endometriosis in African women. J. Womens Health Issues Care 9 2020, 4, 2. [Google Scholar]
- Farland, L.V.; Horne, A.W. Disparity in endometriosis diagnoses between racial/ethnic groups. BJOG Int. J. Obs. Gynaecol. 2019, 126, 1115–1116. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K. Incidence of endometriosis among Japanese women. Obs. Gynecol. 1976, 48, 407–409. [Google Scholar]
- Yasui, T.; Hayashi, K.; Nagai, K.; Mizunuma, H.; Kubota, T.; Lee, J.S.; Suzuki, S. Risk profiles for endometriosis in Japanese women: Results from a repeated survey of self-reports. J. Epidemiol. 2015, 25, 194–203. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, S.; Chen, J.; Yang, J.; Zhu, J. Long-term trends in the incidence of endometriosis in China from 1990 to 2019: A joinpoint and age-period-cohort analysis. Gynecol. Endocrinol. 2021, 37, 1041–1045. [Google Scholar] [CrossRef]
- Bigambo, F.M.; Wang, D.; Zhang, Y.; Mzava, S.M.; Dai, R.; Wang, X. Current situation of menstruation and gynecological diseases prevalence among Chinese women: A cross-sectional study. BMC Womens Health 2022, 22, 270. [Google Scholar] [CrossRef]
- Rowlands, I.J.; Abbott, J.A.; Montgomery, G.W.; Hockey, R.; Rogers, P.; Mishra, G.D. Prevalence and incidence of endometriosis in Australian women: A data linkage cohort study. BJOG Int. J. Obs. Gynaecol. 2021, 128, 657–665. [Google Scholar] [CrossRef]
- Arumugam, K.; Templeton, A.A. Endometriosis and race. Aust. New Zealand J. Obs. Gynaecol. 1992, 32, 164–165. [Google Scholar] [CrossRef]
- Tanmahasamut, P.; Noothong, S.; Sanga-Areekul, N.; Silprasit, K.; Dangrat, C. Prevalence of endometriosis in women undergoing surgery for benign gynecologic diseases. J. Med. Assoc. Thai. 2014, 97, 147–152. [Google Scholar]
- Yen, C.F.; Kim, M.R.; Lee, C.L. Epidemiologic Factors Associated with Endometriosis in East Asia. Gynecol. Minim. Invasive Ther. 2019, 8, 4–11. [Google Scholar] [CrossRef]
- Wisawasukmongchol, W.; Chalermchockcharoenkit, A.; Panyakhamlerd, K.; Ratchanon, S.; Luanratanakorn, S.; Sophonsritsuk, A.; Rungruxsirivorn, T.; Choksuchat, C.; Lertvikool, S.; Pantasri, T. Thai Interest Group for Endometriosis (TIGE) consensus statement on endometriosis-associated pain. J. Obs. Gynaecol. 2022, 42, 1607–1612. [Google Scholar] [CrossRef]
- Wilson, S.; Mogan, S.; Kaur, K. Understanding the role of Facebook to support women with endometriosis: A Malaysian perspective. Int. J. Nurs. Pract. 2020, 26, e12833. [Google Scholar] [CrossRef]
- Capezzuoli, T.; Clemenza, S.; Sorbi, F.; Campana, D.; Vannuccini, S.; Chapron, C.; Petraglia, F. Classification/staging systems for endometriosis: The state of the art. Gynecol. Reprod. Endocrinol. Metab. 2020, 1, 14–22. [Google Scholar]
- Vermeulen, N.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Johnson, N.P.; Lee, T.T.M.; Missmer, S.; Petrozza, J.; Tomassetti, C.; Zondervan, K.T.; et al. Endometriosis Classification, Staging and Reporting Systems: A Review on the Road to a Universally Accepted Endometriosis Classification. J. Minim. Invasive Gynecol. 2021, 28, 1822–1848. [Google Scholar] [CrossRef]
- Kuznetsov, L.; Dworzynski, K.; Davies, M.; Overton, C.; Guideline, C. Diagnosis and management of endometriosis: Summary of NICE guidance. BMJ 2017, 358, j3935. [Google Scholar] [CrossRef]
- Ballard, K.D.; Seaman, H.E.; de Vries, C.S.; Wright, J.T. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG Int. J. Obs. Gynaecol. 2008, 115, 1382–1391. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Eskenazi, B.; Warner, M.; Bonsignore, L.; Olive, D.; Samuels, S.; Vercellini, P. Validation study of nonsurgical diagnosis of endometriosis. Fertil. Steril. 2001, 76, 929–935. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Hummelshoj, L.; Kennedy, S.H.; Jenkinson, C.; Zondervan, K.T.; World Endometriosis Research Foundation Women’s Health Symptom Survey. Developing symptom-based predictive models of endometriosis as a clinical screening tool: Results from a multicenter study. Fertil. Steril. 2012, 98, 692–701.e695. [Google Scholar] [CrossRef]
- Bazot, M.; Lafont, C.; Rouzier, R.; Roseau, G.; Thomassin-Naggara, I.; Darai, E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil. Steril. 2009, 92, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obs. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.P.C.; Ribeiro, H.; Bernardo, W.M.; Simoes, R.; Torres, U.S.; D’Ippolito, G.; Bazot, M.; Ribeiro, P. Accuracy of transvaginal sonography versus magnetic resonance imaging in the diagnosis of rectosigmoid endometriosis: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0214842, Erratum in PLoS ONE 2019, 14, e0221499. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Thomassin, I.; Hourani, R.; Cortez, A.; Darai, E. Diagnostic accuracy of transvaginal sonography for deep pelvic endometriosis. Ultrasound Obs. Gynecol. 2004, 24, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Dessole, S.; Farina, M.; Rubattu, G.; Cosmi, E.; Ambrosini, G.; Nardelli, G.B. Sonovaginography is a new technique for assessing rectovaginal endometriosis. Fertil. Steril. 2003, 79, 1023–1027. [Google Scholar] [CrossRef]
- Guerriero, S.; Martinez, L.; Gomez, I.; Pascual, M.A.; Ajossa, S.; Pagliuca, M.; Alcazar, J.L. Diagnostic accuracy of transvaginal sonography for detecting parametrial involvement in women with deep endometriosis: Systematic review and meta-analysis. Ultrasound Obs. Gynecol. 2021, 58, 669–676. [Google Scholar] [CrossRef]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E.; Endometriosis, E.S.I.G.f.; et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef]
- Moore, J.; Copley, S.; Morris, J.; Lindsell, D.; Golding, S.; Kennedy, S. A systematic review of the accuracy of ultrasound in the diagnosis of endometriosis. Ultrasound Obs. Gynecol. 2002, 20, 630–634. [Google Scholar] [CrossRef]
- Guerriero, S.; Condous, G.; van den Bosch, T.; Valentin, L.; Leone, F.P.; Van Schoubroeck, D.; Exacoustos, C.; Installe, A.J.; Martins, W.P.; Abrao, M.S.; et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: A consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obs. Gynecol. 2016, 48, 318–332. [Google Scholar] [CrossRef]
- Leonardi, M.; Uzuner, C.; Mestdagh, W.; Lu, C.; Guerriero, S.; Zajicek, M.; Dueckelmann, A.; Filippi, F.; Buonomo, F.; Pascual, M.A.; et al. Diagnostic accuracy of transvaginal ultrasound for detection of endometriosis using International Deep Endometriosis Analysis (IDEA) approach: Prospective international pilot study. Ultrasound Obs. Gynecol. 2022, 60, 404–413. [Google Scholar] [CrossRef]
- Taylor, H.S.; Adamson, G.D.; Diamond, M.P.; Goldstein, S.R.; Horne, A.W.; Missmer, S.A.; Snabes, M.C.; Surrey, E.; Taylor, R.N. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int. J. Gynaecol. Obs. 2018, 142, 131–142. [Google Scholar] [CrossRef]
- Watkins, J.C.; DiVasta, A.D.; Vitonis, A.F.; Crum, C.P.; Laufer, M.R.; Terry, K.L.; Howitt, B.E.; Missmer, S.A. A Clinical and Pathologic Exploration of Suspected Peritoneal Endometriotic Lesions. Int. J. Gynecol. Pathol. 2021, 40, 602–610. [Google Scholar] [CrossRef]
- Lamvu, G.; Carrillo, J.; Ouyang, C.; Rapkin, A. Chronic Pelvic Pain in Women: A Review. JAMA 2021, 325, 2381–2391. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef]
- Abdul Karim, A.K.; Abd Aziz, N.H.; Md Zin, R.R.; Mohd Mokhtar, N.; Shafiee, M.N. The Effect of Surgical Intervention of Endometriosis to CA-125 and Pain. Malays. J. Med. Sci. 2020, 27, 7–14. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of endometriosis: A review with comparison of 8 guidelines. BMC Womens Health 2021, 21, 397. [Google Scholar] [CrossRef]
- Subramaniam, R.S.; Damodaran, S.E.; Tham, S.W. Clinical Guidelines for the Management of Endometriosis 2016. OGSM: 2016. Available online: http://www.ogsm.org.my (accessed on 6 December 2022).
- Bafort, C.; Beebeejaun, Y.; Tomassetti, C.; Bosteels, J.; Duffy, J.M. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 2020, 10, CD011031. [Google Scholar] [CrossRef]
- Huijs, E.; Nap, A. The effects of nutrients on symptoms in women with endometriosis: A systematic review. Reprod. Biomed. Online 2020, 41, 317–328. [Google Scholar] [CrossRef]
- Abokhrais, I.M.; Denison, F.C.; Whitaker, L.H.R.; Saunders, P.T.K.; Doust, A.; Williams, L.J.; Horne, A.W. Correction: A two-arm parallel double-blind randomised controlled pilot trial of the efficacy of Omega-3 polyunsaturated fatty acids for the treatment of women with endometriosis-associated pain (PurFECT1). PLoS ONE 2020, 15, e0230055. [Google Scholar] [CrossRef]
- Nodler, J.L.; DiVasta, A.D.; Vitonis, A.F.; Karevicius, S.; Malsch, M.; Sarda, V.; Fadayomi, A.; Harris, H.R.; Missmer, S.A. Supplementation with vitamin D or omega-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2020, 112, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Williams, A.C.C.; Fisher, E.; Hearn, L.; Eccleston, C. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2020, 8, CD007407. [Google Scholar] [CrossRef] [PubMed]
- Loving, S.; Nordling, J.; Jaszczak, P.; Thomsen, T. Does evidence support physiotherapy management of adult female chronic pelvic pain? A systematic review. Scand. J. Pain. 2012, 3, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Zito, G.; Luppi, S.; Giolo, E.; Martinelli, M.; Venturin, I.; Di Lorenzo, G.; Ricci, G. Medical treatments for endometriosis-associated pelvic pain. Biomed. Res. Int. 2014, 2014, 191967. [Google Scholar] [CrossRef]
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 2010, CD008475. [Google Scholar] [CrossRef]
- Donnez, J.; Taylor, H.S.; Taylor, R.N.; Akin, M.D.; Tatarchuk, T.F.; Wilk, K.; Gotteland, J.P.; Lecomte, V.; Bestel, E. Treatment of endometriosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone-antagonist: A randomized clinical trial. Fertil. Steril. 2020, 114, 44–55. [Google Scholar] [CrossRef]
- Osuga, Y.; Seki, Y.; Tanimoto, M.; Kusumoto, T.; Kudou, K.; Terakawa, N. Relugolix, an oral gonadotropin-releasing hormone receptor antagonist, reduces endometriosis-associated pain in a dose-response manner: A randomized, double-blind, placebo-controlled study. Fertil. Steril. 2021, 115, 397–405. [Google Scholar] [CrossRef]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
- Coxon, L.; Horne, A.W.; Vincent, K. Pathophysiology of endometriosis-associated pain: A review of pelvic and central nervous system mechanisms. Best Pr. Res. Clin. Obs. Gynaecol. 2018, 51, 53–67. [Google Scholar] [CrossRef]
- Blum, H.E. The human microbiome. Adv. Med. Sci. 2017, 62, 414–420. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Franceschi, F.; Gasbarrini, A. The gastrointestinal microbiome—Functional interference between stomach and intestine. Best Pr. Res. Clin. Gastroenterol. 2014, 28, 995–1002. [Google Scholar] [CrossRef]
- Noto, J.M.; Peek, R.M., Jr. The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer. PLoS Pathog. 2017, 13, e1006573. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef]
- Mason, K.L.; Huffnagle, G.B.; Noverr, M.C.; Kao, J.Y. Overview of gut immunology. Adv. Exp. Med. Biol. 2008, 635, 1–14. [Google Scholar] [CrossRef]
- Morbe, U.M.; Jorgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Nie, M.; Wen, L.; Zou, B.; Zhang, L.; Xie, J.; Ser, H.L.; Lee, L.H.; Wang, S.; et al. Salt-Sensitive Ileal Microbiota Plays a Role in Atrial Natriuretic Peptide Deficiency-Induced Cardiac Injury. Nutrients 2022, 14, 3129. [Google Scholar] [CrossRef]
- Ser, H.-L.; Wong, J.Y.-J.; Goh, B.-H.; Reginald, K. IDDF2022-ABS-0236 Healing the GUT with probiotics: Can probiotics help relieve allergic rhinitis? Gut 2022, 71, A63–A64. [Google Scholar]
- Joseph, R.J.; Ser, H.L.; Kuai, Y.H.; Tan, L.T.; Arasoo, V.J.T.; Letchumanan, V.; Wang, L.; Pusparajah, P.; Goh, B.H.; Ab Mutalib, N.S.; et al. Finding a Balance in the Vaginal Microbiome: How Do We Treat and Prevent the Occurrence of Bacterial Vaginosis? Antibiotics 2021, 10, 719. [Google Scholar] [CrossRef]
- Mestrovic, T.; Matijasic, M.; Peric, M.; Cipcic Paljetak, H.; Baresic, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Ser, H.-L.; Wong, J.Y.J.; Letchumanan, V.; Law, J.W.-F.; Tan, L.T.-H.; Lee, L.-H. IDDF2021-ABS-0132 Moving beyond the gastrointestinal tract: The involvement of gut microbiome in endometriosis. Gut 2021, 70, A46–A47. [Google Scholar]
- Plesniarski, A.; Siddik, A.B.; Su, R.C. The Microbiome as a Key Regulator of Female Genital Tract Barrier Function. Front. Cell. Infect. Microbiol. 2021, 11, 790627. [Google Scholar] [CrossRef] [PubMed]
- Punzon-Jimenez, P.; Labarta, E. The impact of the female genital tract microbiome in women health and reproduction: A review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schutte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra152. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Ata, B.; Yildiz, S.; Turkgeldi, E.; Brocal, V.P.; Dinleyici, E.C.; Moya, A.; Urman, B. The endobiota study: Comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Chen, S.; Gu, Z.; Zhang, W.; Jia, S.; Wu, Y.; Zheng, P.; Dai, Y.; Leng, J. Microbiome of the lower genital tract in Chinese women with endometriosis by 16s-rRNA sequencing technique: A pilot study. Ann. Transl. Med. 2020, 8, 1440. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, X.; Tang, H.; Zeng, L.; Wu, R. Microbiota composition and distribution along the female reproductive tract of women with endometriosis. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 15. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, J.C.; Kim, S.H.; Oh, Y.S.; Chae, H.D.; Seo, H.; Kang, C.S.; Shin, T.S. Altered Composition of Microbiota in Women with Ovarian Endometrioma: Microbiome Analyses of Extracellular Vesicles in the Peritoneal Fluid. Int. J. Mol. Sci. 2021, 22, 4608. [Google Scholar] [CrossRef]
- Huang, L.; Liu, B.; Liu, Z.; Feng, W.; Liu, M.; Wang, Y.; Peng, D.; Fu, X.; Zhu, H.; Cui, Z.; et al. Gut Microbiota Exceeds Cervical Microbiota for Early Diagnosis of Endometriosis. Front. Cell. Infect. Microbiol. 2021, 11, 788836. [Google Scholar] [CrossRef]
- Shan, J.; Ni, Z.; Cheng, W.; Zhou, L.; Zhai, D.; Sun, S.; Yu, C. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch. Gynecol. Obs. 2021, 304, 1363–1373. [Google Scholar] [CrossRef]
- Chao, X.; Liu, Y.; Fan, Q.; Shi, H.; Wang, S.; Lang, J. The role of the vaginal microbiome in distinguishing female chronic pelvic pain caused by endometriosis/adenomyosis. Ann. Transl. Med. 2021, 9, 771. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chiang, A.J.; Lai, M.T.; Yan, M.J.; Tseng, C.C.; Lo, L.C.; Wan, L.; Li, C.J.; Tsui, K.H.; Chen, C.M.; et al. A more diverse cervical microbiome associates with better clinical outcomes in patients with endometriosis: A pilot study. Biomedicines 2022, 10, 174. [Google Scholar] [CrossRef]
- Oishi, S.; Mekaru, K.; Tanaka, S.E.; Arai, W.; Ashikawa, K.; Sakuraba, Y.; Nishioka, M.; Nakamura, R.; Miyagi, M.; Akamine, K. Microbiome analysis in women with endometriosis: Does a microbiome exist in peritoneal fluid and ovarian cystic fluid? Reprod. Med. Biol. 2022, 21, e12441. [Google Scholar] [CrossRef]
- Yuan, W.; Wu, Y.; Chai, X.; Wu, X. The colonized microbiota composition in the peritoneal fluid in women with endometriosis. Arch. Gynecol. Obs. 2022, 305, 1573–1580. [Google Scholar] [CrossRef]
- Lu, F.; Wei, J.; Zhong, Y.; Feng, Y.; Ma, B.; Xiong, Y.; Wei, K.; Tan, B.; Chen, T. Antibiotic Therapy and Vaginal Microbiota Transplantation Reduce Endometriosis Disease Progression in Female Mice via NF-kappaB Signaling Pathway. Front. Med. 2022, 9, 831115. [Google Scholar] [CrossRef]
- Hernandes, C.; Silveira, P.; Rodrigues Sereia, A.F.; Christoff, A.P.; Mendes, H.; Valter de Oliveira, L.F.; Podgaec, S. Microbiome Profile of Deep Endometriosis Patients: Comparison of Vaginal Fluid, Endometrium and Lesion. Diagnostics 2020, 10, 163. [Google Scholar] [CrossRef]
- Perrotta, A.R.; Borrelli, G.M.; Martins, C.O.; Kallas, E.G.; Sanabani, S.S.; Griffith, L.G.; Alm, E.J.; Abrao, M.S. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: A pilot study. Reprod. Sci. 2020, 27, 1064–1073. [Google Scholar] [CrossRef]
- Le, N.; Cregger, M.; Fazleabas, A.; Braundmeier-Fleming, A. Effects of endometriosis on immunity and mucosal microbial community dynamics in female olive baboons. Sci. Rep. 2022, 12, 1590. [Google Scholar] [CrossRef]
- Svensson, A.; Brunkwall, L.; Roth, B.; Orho-Melander, M.; Ohlsson, B. Associations between endometriosis and gut microbiota. Reprod. Sci. 2021, 28, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.M.; Domínguez, M.A.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. Endometrial microbiota is more diverse in people with endometriosis than symptomatic controls. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Cregger, M.; Brown, V.; Loret de Mola, J.; Bremer, P.; Nguyen, L.; Groesch, K.; Wilson, T.; Diaz-Sylvester, P.; Braundmeier-Fleming, A. Association of microbial dynamics with urinary estrogens and estrogen metabolites in patients with endometriosis. PLoS ONE 2021, 16, e0261362. [Google Scholar] [CrossRef] [PubMed]
- Simoes-Silva, L.; Araujo, R.; Pestana, M.; Soares-Silva, I.; Sampaio-Maia, B. Peritoneal Microbiome in End-Stage Renal Disease Patients and the Impact of Peritoneal Dialysis Therapy. Microorganisms 2020, 8, 173. [Google Scholar] [CrossRef]
- Gilbreath, J.J.; Semino-Mora, C.; Friedline, C.J.; Liu, H.; Bodi, K.L.; McAvoy, T.J.; Francis, J.; Nieroda, C.; Sardi, A.; Dubois, A.; et al. A core microbiome associated with the peritoneal tumors of pseudomyxoma peritonei. Orphanet J. Rare Dis. 2013, 8, 105. [Google Scholar] [CrossRef]
- Semino-Mora, C.; Liu, H.; McAvoy, T.; Nieroda, C.; Studeman, K.; Sardi, A.; Dubois, A. Pseudomyxoma peritonei: Is disease progression related to microbial agents? A study of bacteria, MUC2 AND MUC5AC expression in disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. Ann. Surg. Oncol. 2008, 15, 1414–1423. [Google Scholar] [CrossRef]
- Ferrero, S.; Vellone, V.G.; Barra, F. Pathophysiology of pain in patients with peritoneal endometriosis. Ann. Transl. Med. 2019, 7, S8. [Google Scholar] [CrossRef]
- Taniguchi, F.; Wibisono, H.; Mon Khine, Y.; Harada, T. Animal models for research on endometriosis. Front. Biosci. 2021, 13, 37–53. [Google Scholar] [CrossRef]
- Vernon, M.W.; Wilson, E.A. Studies on the surgical induction of endometriosis in the rat. Fertil. Steril. 1985, 44, 684–694. [Google Scholar] [CrossRef]
- Yuan, M.; Li, D.; Zhang, Z.; Sun, H.; An, M.; Wang, G. Endometriosis induces gut microbiota alterations in mice. Hum. Reprod. 2018, 33, 607–616. [Google Scholar] [CrossRef]
- Chadchan, S.B.; Cheng, M.; Parnell, L.A.; Yin, Y.; Schriefer, A.; Mysorekar, I.U.; Kommagani, R. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum. Reprod. 2019, 34, 1106–1116. [Google Scholar] [CrossRef]
- Hantschel, J.; Weis, S.; Schafer, K.H.; Menger, M.D.; Kohl, M.; Egert, M.; Laschke, M.W. Effect of endometriosis on the fecal bacteriota composition of mice during the acute phase of lesion formation. PLoS ONE 2019, 14, e0226835. [Google Scholar] [CrossRef]
- Ni, Z.; Sun, S.; Bi, Y.; Ding, J.; Cheng, W.; Yu, J.; Zhou, L.; Li, M.; Yu, C. Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am. J. Reprod. Immunol. 2020, 84, e13307. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, C.; Jia, Y.; Xu, D.; Yu, Y. Letrozole and the traditional chinese medicine, Shaofu Zhuyu decoction, reduce endometriotic disease progression in rats: A potential role for gut microbiota. Evid. Based Complement. Altern. Med. 2020, 2020, 3687498. [Google Scholar] [CrossRef]
- Ni, Z.; Ding, J.; Zhao, Q.; Cheng, W.; Yu, J.; Zhou, L.; Sun, S.; Yu, C. Alpha-linolenic acid regulates the gut microbiota and the inflammatory environment in a mouse model of endometriosis. Am. J. Reprod. Immunol. 2021, 86, e13471. [Google Scholar] [CrossRef]
- Higuchi, B.S.; Rodrigues, N.; Gonzaga, M.I.; Paiolo, J.C.C.; Stefanutto, N.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Matheucci, E., Jr.; Mariano, V.S.; et al. Intestinal Dysbiosis in Autoimmune Diabetes Is Correlated With Poor Glycemic Control and Increased Interleukin-6: A Pilot Study. Front. Immunol. 2018, 9, 1689. [Google Scholar] [CrossRef]
- Leite, A.Z.; Rodrigues, N.C.; Gonzaga, M.I.; Paiolo, J.C.C.; de Souza, C.A.; Stefanutto, N.A.V.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Matheucci, E., Jr.; et al. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella copri and Bacteroides vulgatus in the Feces of Type 2 Diabetes Patients. Front. Immunol. 2017, 8, 1107. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Meng, J.; Banerjee, S.; Zhang, L.; Sindberg, G.; Moidunny, S.; Li, B.; Robbins, D.J.; Girotra, M.; Segura, B.; Ramakrishnan, S.; et al. Opioids Impair Intestinal Epithelial Repair in HIV-Infected Humanized Mice. Front. Immunol. 2019, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, S.; Mohammadbeigi, R.; Khaledi, M.; Mesdaghinia, L.; Sharifzadeh, F.; Nasiripour, S.; Gorginzadeh, M. Beneficial Effects of Oral Lactobacillus on Pain Severity in Women Suffering from Endometriosis: A Pilot Placebo-Controlled Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Sashihara, T.; Hosono, A.; Kaminogawa, S.; Uchida, M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 2011, 63, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Ali, R.A.R.; Manaf, M.R.A.; Ahmad, N.; Tajurruddin, F.W.; Qin, W.Z.; Desa, S.H.M.; Ibrahim, N.M. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PLoS ONE 2020, 15, e0244680. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Uchida, M.; Sashihara, T.; Ji, Z.S.; Li, J.; Tang, Q.; Ni, S.; Song, L.; Kaminogawa, S. Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: Randomized, double-blind, placebo-controlled study. Cytotechnology 2011, 63, 153–161. [Google Scholar] [CrossRef]
- Uchida, M.; Kobayashi, O. Effects of Lactobacillus gasseri OLL2809 on the induced endometriosis in rats. Biosci. Biotechnol. Biochem. 2013, 77, 1879–1881. [Google Scholar] [CrossRef]
- Del Piano, M.; Carmagnola, S.; Ballare, M.; Sartori, M.; Orsello, M.; Balzarini, M.; Pagliarulo, M.; Tari, R.; Anderloni, A.; Strozzi, G.P.; et al. Is microencapsulation the future of probiotic preparations? The increased efficacy of gastro-protected probiotics. Gut Microbes 2011, 2, 120–123. [Google Scholar] [CrossRef]
- Sanders, M.E.; Klaenhammer, T.R.; Ouwehand, A.C.; Pot, B.; Johansen, E.; Heimbach, J.T.; Marco, M.L.; Tennila, J.; Ross, R.P.; Franz, C.; et al. Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Ann. New York Acad. Sci. 2014, 1309, 1–18. [Google Scholar] [CrossRef]
- Su, K.-Y.; Koh Kok, J.-Y.; Chua, Y.-W.; Ong, S.-D.; Ser, H.L.; Pusparajah, P.; San Saw, P.; Goh, B.H.; Lee, W.-L. Bacterial extracellular vesicles in biofluids as potential diagnostic biomarkers. Expert Rev. Mol. Diagn. 2023, 22, 1057–1062. [Google Scholar] [CrossRef]
- Anderson, G. Endometriosis Pathoetiology and Pathophysiology: Roles of Vitamin A, Estrogen, Immunity, Adipocytes, Gut Microbiome and Melatonergic Pathway on Mitochondria Regulation. Biomol. Concepts 2019, 10, 133–149. [Google Scholar] [CrossRef]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef]
- Kaliannan, K.; Robertson, R.C.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Hao, L.; Bhan, A.K.; Kang, J.X. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018, 6, 205. [Google Scholar] [CrossRef]
- Karim, A.K.A.; Shafiee, M.N.; Abd Aziz, N.H.; Omar, M.H.; Zin, R.R.M.; Mokhtar, N.M. Perturbations in progesterone receptor isoforms in endometriosis. J. Reprod. Med. 2019, 64, 275–281. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).